Abstract
Fish skin grafting as a new skin substitute is currently being used in clinical applications. Acceleration of the wound healing, lack of disease transmission, and low cost of the production process can introduce fish skin as a potential alternative to other grafts. An appropriate decellularization process allows the design of 3D acellular scaffolds for skin regeneration without damaging the morphology and extracellular matrix content. Therefore, the role of decellularization processes is very important to maintain the properties of fish skin. In this review article, recent studies on various decellularization processes as well as biological, physical, and mechanical properties of fish skin and its applications with therapeutic effects in wound healing were investigated.
Keywords: biological and physical & mechanical properties, decellularization, fish skin, in‐vivo studies, wound healing
1. INTRODUCTION
Burns are a common critical problem that is the most traumatic and devastating physical injury that affects almost every organ system and results in significant morbidity and mortality. About 300 000 burn deaths are recorded worldwide each year. 1 Burn injuries are classified as first, second, third, and fourth degree depending on the depth and intensity of their penetration to the skin surface. Superficial damage (first degree) involves only the epidermis of the skin. Superficial partial thickness (grade II or 1B) involves the superficial dermis, while deep partial thickness (grade II or 2B) involves the more inner parts of the dermis. A complete burn of the skin and subcutaneous structures is known as a third‐degree burn injury, and if the severity of the burn increases, it may also damage the underlying muscles, tendons, and bones, which is referred to as a fourth‐degree burn. 1 , 2 , 3 Various studies have shown that acute and chronic wounds lead to the inflammatory response of the host body and the formation of scar tissue. Therefore, a better understanding of the inflammatory response can play an important role in the healing and regeneration process regardless of the type and severity of the wound. Wound healing is a systematic process that includes four phases: homeostasis (coagulation), inflammation (infiltration of mononuclear cells), proliferation (epithelialization, fibroplasia, angiogenesis, and granulation tissue formation), and maturation (deposition collagen) or remodelling process (Figure 1). Based on the understanding of these mechanisms, therapeutic methods were also developed. The traditional treatment of burn wounds is based on the removal of debridement and the use of sterile gas with local antimicrobial compounds or dressings containing silver such as Silvadene Sulfamylon, and Acticoat. 4 , 5 , 6 New methods such as tissue engineering have opened a new functional solution to the repair and regeneration of tissues. The main goal of tissue engineering is to use biological sciences and engineering to create tissue substitutes that can cause repair and improvement or regeneration of damaged tissues and organs. 7 The three main elements of tissue engineering are cells, scaffolds, and factors. 8
FIGURE 1.
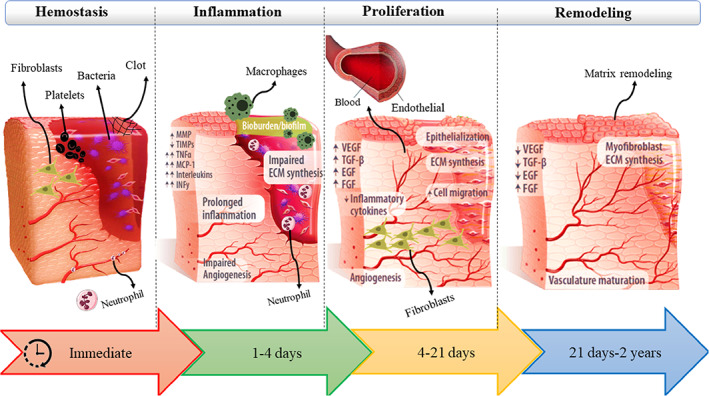
Illustration of the process of skin wound healing.
Scaffolds are structures composed of synthetic or natural biomaterials that are used to mimic the natural environment of cells, known as the extracellular matrix (ECM), to aid growth and proliferation, and cell organisation. 9 These substances provide a suitable environment to support cells and stimulate the synthesis of ECM by cells. Different methods for making scaffolds have been proposed to be able to produce suitable microenvironments for tissue production in the in‐vivo. Natural and synthetic materials that make up scaffolds have advantages and disadvantages. The most important characteristics of natural polymers include better biocompatibility, cheapness, greater cell adhesion, and inherent structural similarity to body tissues. On the other hand, their main drawbacks include limited tunability, immune response, unpredictable degradation rate, microbial contamination (eg, endotoxins), swelling, and low mechanical strength. Compared with natural polymers, synthetic polymers have advantages such as adjustable properties, better shaping or manufacturability, and higher mechanical properties. One of the most important defects of synthetic biomaterials is their inappropriate cell adhesion, hence chemical and physical modification can work to improve this performance. In addition, some synthetic biomaterials require toxic solvents for their formation, which can cause biocompatibility problems. 9 , 10 , 11 , 12 Decellularized matrix (DM) is one of the natural biological materials that can be used as an ideal scaffold in tissue engineering. DM with suitable biodegradability can provide a suitable space for tissue growth, as well as it has low immunogenicity and can facilitate tissue repair by preserving bioactive molecules. DM may provide a biomimetic microenvironment that promotes cell adhesion and proliferation. 13 Recently, decellularized tissues from non‐mammalian sources have received special attention because of fewer ethical restrictions and low risk of infection transmission (foot‐and‐mouth disease, swine influenza, and bovine spongiform encephalopathy). 14 Avoiding the risk of disease transmission and abundant access to disposable wastes of fish such as fish skin have attracted the attention of many researchers, and a variety of decellularization methods have been used to design an efficient product as a biological scaffold. 15 , 16 Fish skin is a versatile tissue that performs various essential functions such as sensory function, chemical and physical protection, and hormonal metabolism. Moreover, it serves as a significant first line of defence against disease as fish are continually exposed to a variety of microbes in the aquatic environment. Therefore, this tissue has structural similarities with mammalian tissue. The epidermis of fish, similar to the epidermis of terrestrial vertebrates such as mice or humans, has a multi‐layered tissue that is separated from the dermis by a distinct basement membrane. 17 In addition to the antimicrobial nature of fish skin, it also shows high biocompatibility and low immunogenicity, which accelerates the wound‐healing process. They do not have the α‐Gal antigen and also have a low risk of prion and/or viral infection. 18 In recent years, there has been increasing interest in a fish skin‐derived acellular dermal matrix (ADM) and its extracted collagen for tissue engineering applications. 18 According to the mentioned cases, ADM can be used as a suitable scaffold in the field of tissue engineering, especially in wound healing. To use fish skin, tissue decellularization must be performed. In the process of decellularization, host cells that can cause the stimulation of the body's immune system must be removed, but during the process, the overall structure of the matrix should not undergo fundamental changes. On the other hand, the mechanical and physical properties of the DM such as degradability and mechanical properties can be changed. To solve this problem, different chemical and physical modification methods such as crosslinkers can be used. 19 In this review article, the different types of decellularization protocols performed on fish skin, as well as their biological, physical, and mechanical properties and in vivo applications in the treatment of skin wounds will be discussed.
2. DECELLULARIZATION PROCESS
Decellularization methods are classically classified into three groups: chemical, physical, and enzymatic. 20 To reduce the negative effects of each technique, usually, a combination of decellularization methods has been used in different studies. In addition, the decellularization method chosen for different tissues differs from each other. 21 Table 1 shows some decellularization protocols and their application on fish tissue.
TABLE 1.
Different decellularization methods of fish tissues.
| Type of fish tissue | Fish specie | Method of decellularization | Results | Reference |
|---|---|---|---|---|
| Air bladder | Silver carp (Aristichthys nobilis) |
Chemical: Triton 0.2% X − 100 + 24 mM sodium deoxycholate in 0.2% EDTA |
|
22 |
| Swim bladder |
Goldfish (Carassius auratus) |
Chemical: CHAP buffer + SDS |
|
23 |
| Cartilage | Sturgeon fish |
Chemical + Enzymatic: SDS + DNAase1 |
|
24 |
| Scale | Crisp flesh grass carps |
Chemical + Enzymatic: Hypotonic tris buffer solution containing protease inhibitor and 1% Triton X‐100 solution + DNase and RNase |
|
25 |
| Scale | Tilapia |
Chemical + Physical: 2% SDS + 4 cycles of freezing and thawing |
|
26 |
| Skin | Tilapia |
Chemical: 3% sodium bicarbonate (NaHCO3) + 3% hydrogen peroxide (H2O2) |
|
27 |
| Skin | Grass carp |
Chemical + Physical: Hypertonic and hypotonic solution along with Triton 0.5% + freezing and thawing |
|
28 |
| Skin | Grass Carp |
Chemical + Physical: Hypertonic and hypotonic solution along with Triton 0.5% + freezing and thawing |
|
16 |
| Skin | Tilapia |
Chemical: NaOH + Triton X‐ 100 |
In compare with native skin dECM retained:
|
18 |
| Skin | Nile tilapia (Oreochromis niloticus) |
Chemical + Enzymatic: SDS + dispase and nuclease enzyme |
In compare with native skin: dECM retained:
|
14 |
| Skin | Baza fish (Pangasius bocourti) |
Chemical + Physical: NaOH + 3 cycles of freezing and thawing |
|
29 |
2.1. Chemical methods
In the chemical method of decellularization, ionic (sodium dodecyl sulfate [SDS]) and non‐ionic (Triton X‐100) solutions, zwitterionic (3‐[(3‐Chola‐isopropyl) dimethylammonio]‐1‐ propane sulfonate (CHAPS)) surfactants, as well as acidic (peracetic acid) and basic (ammonium hydroxide; NH4OH) solutions are used to destroy the membrane and remove the cells. 30 Surfactants lyse cells by rearranging phospholipids in the cell membrane. Ionic SDS is one of the most widely used surfactants because of its effectiveness in removing cells and removing at least 90% of DNA. Nevertheless, it has been shown that SDS especially thin tissues can change the basic mechanical properties of the tissues and prevent the re‐aggregation of cells in the tissue by damaging the structure of proteins and structural and signalling components. 31 , 32 , 33 Khajovi et al. investigated the effectiveness of SDS on the decellularization of sturgeon cartilage. In this study, after the decellularization process, the high amount of hydroxyproline indicated the preservation of collagen fibres. In addition, the effectiveness of this method in removing nucleic acids was determined. However, the results showed that the number of glycosaminoglycans (GAGs) in the cell matrix decreased. 24 Cytotoxicity is another problem with SDS, and tissue must be washed after decellularization. Considering that SDS is an ionic surfactant, washing it with solutions such as phosphate‐buffered saline (PBS) presents its challenges. As a result, a careful washing process is necessary. 33
For tilapia skin decellularization, the samples were immersed in 1% SDS and 0.25% trypsin solution and then soaked in 0.1% NaOH and 3% H2O2 and finally freeze‐dried. Scanning electron microscope (SEM) images showed that two sides of the untreated skin have a dense structure, whereas for the decellularized skin sample, one side was dense and smooth and the other side was loose and porous because of the removal of the epidermis. The collagen fibres in the skin had an orderly and tight structure. The decellularized skin sample swelled after exposure to the aqueous environment, which loosened the structure and eventually created gaps between the collagen layers. The results of haematoxylin and eosin staining also confirmed this morphological change. The results of HE staining showed that this decellularization method is well able to remove fish skin cells and leave only the acellular matrix. 34
To aid in the washing process, non‐ionic surfactants such as Triton X‐100 are often mixed with SDS. 33 Triton X‐100 is used to delete the remaining fats by disrupting the connections between lipids and proteins. 35 The effectiveness of the decellularization method based on Triton X‐100 has been investigated on the silver carp air bladder and grass carp fish skin. The results showed that the created scaffold has a porous structure and causes cells to grow and differentiate. The significant reduction of cells and fat, as well as the preservation of collagen fibres as the most important component of the ECM, showed the high efficiency of this method. 22 , 28 The remaining DNA concentration of 0.8 ng/mg, which is two times lower than the generally known limit (50 ng/mg of dry weight), can show the efficiency of this technique. 36 According to the amount of remaining DNA and the lack of nuclear structures after Triton X‐100 treatment, which indicates the successful removal of cells, but a decrease in the concentration of tissue GAGs and as a result, a change in the mechanical strength of the tissue was observed. 33 By using their inherent electrical charges, bases like sodium hydroxide and acids like peracetic acid destroy nuclear material and the cell membrane. Proteins are denatured by acids and bases, and cells are ruptured as a result of the solubilisation of cell components. They do not have preferences; therefore, they also alter ECM components, including collagen, GAGs, and growth factors. 37 It seems that the combined use of acids and bases along with other decellularization agents has better results. For instance, in the decellularization of tilapia and basa fish, NaOH along with Triton X‐100 and 3 cycles of freeze and thaw were used respectively. In both cases, images of 4′,6‐diamidino‐2‐phenylindole (DAPI) and hematoxylin and eosin H&E staining showed that nuclei were absent in the obtained matrix and the majority of cells were removed. 29 In the skin of tilapia, the residual DNA content of decellularized skin with NaOH and Triton X‐100 was 1.4 ± 0.7 ng per mg dry weight, which was much less than the limit of 50 ng/mg recognised by the medical industry. 18 In addition, the efficiency of the decellularization method in tilapia skin is comparable to the method used by Lau et al. 14 which used SDS in combination with a nuclease. The combined use of decellularizing detergents seems to have better results. In a study, seven different combination protocols were used for the decellularization of fresh carp skin. 16 , 28 Protocols such as freezing and thawing, hypertonic and hypotonic solutions, Triton X‐100, and freeze‐drying at different concentrations and times were used on fish skin (Figure 2). The cells were well removed from the ECM with the help of non‐ionic detergents and physical and chemical methods. The results showed that the fibres maintained their arrangement and structural order during the decellularization process. High biocompatibility and low toxicity, cell adhesion, and suitable physical and mechanical properties were the characteristics of decellularized skin. The best sample of decellularized skin in all respects related to the combination of hypertonic and hypotonic solutions and Triton X‐100 (0.5%) was obtained. The maximum Young's modulus and tensile stress were 284.14 MPa and 32.77 MPa, respectively, for the fish skin sample with a thickness of 0.12 mm, whereas Young's modulus and tensile stress were about 192.15 MPa and 21 MPa respectively for the decellularized skin sample. The results indicate that despite the low thickness, the fish skin can withstand tensile pressures, and the matrix mainly maintained its structure and entanglement after decellularization.
FIGURE 2.
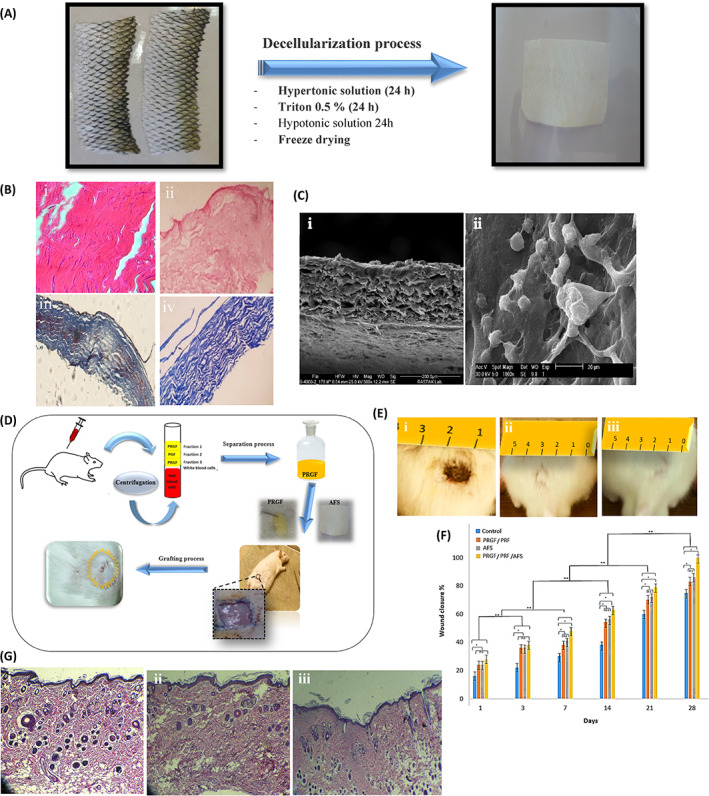
(A) The decellularization method. (B) H&E and Trichrome Masson staining of (i, ii) native skin; (iii, iv) decellularized skin. (C) SEM images of decellularized (I) and recellularized fish skin (ii). (D) Animal model for evaluating skin regeneration by acellular fish skin and Plasma Rich in Growth Factor PRGF gel (E) Macroscopic evaluation of woumd (i) on the first day, (ii) treated wound in the 28th day with acellular fish skin and (iii) treated wound with acellular fish skin and PRGF gel in 28th. (F) Wound closure for all samples on the 28th day after grafting (G) H&E images of (i) native skin, (ii) treated with acellular fish skin, and (iii) treated with acellular fish skin and PRGF gel in the 28th. 16 , 28
Boal et al. used two detergents (Triton X‐100, SDS) with different concentrations (0.1%, 1.0%) and different temperature conditions (4°C, room temperature) to decellularize milkfish skin, and then compared the decellularized skins. The results showed that the morphology of fish skin was better preserved under the treatment with the Triton X‐100 agent, while SDS showed the power to remove more cellular components, especially at a concentration of 1% and a temperature of 4°C. Of course, the two parameters of temperature and concentration had a significant effect on the physical and mechanical properties of decellularized skins. 38 In general, the chemical methods mentioned above have advantages and disadvantages. For example, the simplicity of the method of decellularization of biological tissues by detergents is one of the advantages of their use, but it has negative aspects such as a long period of treatment, changed mechanical properties, and toxicity. 39 SDS as a detergent agent can lead to complete cell removal and destroy up to 90% of DNA, but may also reduce ECM glycosaminoglycan and growth factor content. Compared with SDS, Triton X‐100 showed fewer destructive effects, but the ability to remove cell components was lower. 8 Therefore, combined methods to both remove cells and minimise harmful effects on the physical structure of the ECM can be more efficient. 32 Tilapia skin was decellularized using 3% sodium bicarbonate (NaHCO3), 0.1 M sodium hydroxide (NaOH), 1 M sodium chloride (NaCl), and 3% hydrogen peroxide (H2O2) solutions at different times. The results showed that the collagen type I structure was preserved during the process. The decellularized skin showed good mechanical strength, biodegradability, and biocompatibility, which can be a suitable substrate for cell penetration, adhesion, and growth. In the rat skin wound healing model, the result indicated good biological activity of the acellular skin. The acellular skin was well able to induce the expression of biological factors related to skin repair. 27
2.2. Physical methods
Physical methods include freeze–thaw, force and pressure, and non‐thermal irreversible electroporation, which reduce the content of vital cells. 40 , 41 Freeze–thaw involves periodic freezing temperatures (~ −80°C) with biological temperatures (~37°C) for an optimised number of cycles, although protocols can be optimised by the number of freeze–thaw cycles and/or modifying the temperature. One cycle of freezing and thawing might lessen unfavourable immunological reactions such as leukocyte infiltration in avascular ECM scaffolds. Several freeze–thaw cycles might be used to decellularize a tissue. 33 , 42 Although this method cannot completely remove cells, it preserves the mechanical strength and structure of the matrix. Freeze–thaw is typically used in conjunction with other techniques and chemical reagents because it is unsuccessful on its own at eliminating cells. 33 This method has some limitations including, destruction of ECM, decreased mechanical strength, neovascularization, and inflammation. 42 , 43 As mentioned above multiple freeze–thaw cycles along with other agents are used for the decellularization of tissues. To decellularize fish scales from tilapia, 4 cycles of the freeze–thaw method with SDS were used. Based on the results of Sirius Red and Mason staining, the collagen fibres were organised and scattered on the surface layer. A significant reduction in the number of GAGs was one of the good results of this method. These results indicate that processed fish scales can still provide bioactive GAGs and may provide the best conditions for cell growth. 26
2.3. Enzymatic methods
Proteolytic enzymes such as trypsin and nucleases (DNase, RNase, and Benzoase) have been used to facilitate the removal of nucleotides after cell lysis in most cases. 44 Despite the advantages of enzymatic digestion, these methods are challenging to replicate. In addition, they greatly increase the possibility of changing the structural and proteomic structure of the ECM. 30 Nucleases such as DNase, RNase, and Benzonase are usually used in the steps after chemical and physical decellularization methods. These enzymes degrade nuclear material to effectively remove biological components from the matrix. 30 For example, DNase and RNase along with chemical methods were used in the decellularization of scales of flesh grass carp. With this protocol, collagen type I and hydroxyapatite remained in the scaffold of decellularized fish scales. 25 When it comes to enzymes, a benzoate homologous nuclease, a genetically modified endonuclease, can efficiently degrade nucleic acids without showing proteolytic activity and is easily removed by repeated washing.
Dispase, which was previously reported as a harmful agent to the human amniotic membrane in an isotonic solution, has unexpectedly been characterised as a beneficial addition to skin decellularization. After applying a gentle dispase treatment (0.56 U/mL, 4 _C in isotonic solution), followed by exposures to SDS and gentle trypsin, on porcine skin, excellent cell clearance was achieved without causing any negative effects on the ECM after 4 and 12‐h treatments. These findings indicate that decreasing temperature and concentration have a softening impact on the disease treatment. 45 Instead of using a straightforward isotonic solution, Dulbecco's modified eagle medium (DMEM) cell culture media with a variety of additives may change enzyme function. In the decellularization of the fish skin, dispase can use to separate the epidermis. In a study, the simultaneous application of enzymes with SDS caused, the retaining of 69.3% of the native collagen content. Moreover, the crisscrossing pattern was preserved in the decellularized skin, but the collagen fibres became looser. 14
3. ACELLULAR FISH SKIN
3.1. Biological properties
Fish waste is an abundant source of important proteins like collagen, minerals, vitamins, and other bioactive substances. 46 , 47 As a part of a fibrous structural protein in the connective tissue, ECM, and the skin, collagen is an essential protein in the body. It has attracted significant attention from biomedical researchers in recent years because of its improved physicochemical properties, stability, mechanical strength, high water absorption capacity, biodegradability, low immunogenicity, superior biocompatibility, easy processing, high porosity, ability to penetrate a lipid‐free interface, and native ability to combine with other materials. Furthermore, collagen can influence the proliferation of fibroblasts and other cells. Collagen is a crucial protein that is used in the cosmetics, pharmaceutical, and biomedical industries because of all of these characteristics. Collagen I, the most plentiful protein in the human body, is abundant in fish skin. It not only acts as an ECM and offers fibroblast cells the perfect environment to grow, but also accelerates the wound‐healing process. 27 , 46 , 48 Extracting collagen from fish is a simpler and more economical process. 28 Most importantly, unlike bovine and porcine sources, this type of collagen is free of disease transmission and poses no serious risk to human health. 46 , 47 , 48 The results of the comparison between tilapia acellular dermal matrix (TADM) and crosslinked electrospun collagen (extracted from tilapia skin) indicated that both TADM and crosslinked electrospun collagen promoted cellular metabolic activity, differentiation, and mineralization of murine osteogenic MC3T3‐E1 cells. In addition, the amount of metabolic activity in the cultured cells on the collagen scaffold was higher than on the decellularized scaffold. Such conditions also existed for mineralization. By contrast, the osteogenic differentiation of cells was higher in the decellularized scaffold than in the collagen scaffold. In general, collagen scaffold showed better biological properties than decellularized scaffold but has a restriction of poor mechanical strength and fast degradation. The in vivo results demonstrated that neither scaffold induced hyperacute rejections. In addition, in comparison to the sham control, they improved bone repair in the critical defect. According to this study, scaffolds made from tilapia have many potential in tissue engineering. 14
As mentioned, to reduce the risk of transmission of viruses or prions, xenograft materials often require a relatively complex preparation process. Chemical reagents can also denature tissue proteins and dissolve their soluble components. But there is no requirement to inactivate the virus by complex processes in the fish skin. These gentler processes make it possible to preserve the structural integrity and molecular components of the skin, such as omega‐3 unsaturated fatty acids, soluble collagen, elastin, laminin, and glycoproteins. Numerous studies have shown that fatty acids, such as long‐chain n‐3 polyunsaturated fatty acids (n‐3 LCPUFAs), icosapentaenoic acid (C20:5n‐3, EPA), and docosahexaenoic acid (C22:6n‐3, DHA), are present in both edible and typically discarded parts of fish, such as subcutaneous tissue, belly flaps, and muscles. 47 , 49 , 50 Because of their vital physiological functions and the inability of the human body to synthesise these compounds, these fish oils are often prescribed as treatments for problems such as hypertriglyceridemia. 51 EPA and DHA are the most prominent among several extracted bioactive substances. The abundance of omega‐3 polyunsaturated fatty acids in fish skin, EPA, and DHA minimises inflammatory reactions and promotes pro‐inflammatory cytokines that promote wound healing. Therefore, exit from the inflammatory phase of wound healing can be facilitated by an ADM rich in these omega‐3 polyunsaturated fatty acids. Recent studies have shown promising results in the treatment of chronic diabetic foot ulcers (DFUs), calciphylaxis wounds, necrotic angiodermatitis, iatrogenic calcinosis cutis, and even neovaginoplasty in people with Mayer‐Rokitansky‐Küster‐Hauser syndrome with ADM rich in omega‐3. 49 , 50 Dorweiler et al. indicated that the omega‐3 allows the growth of keratinocytes and fibroblasts. One of the advantages of this type of treatment is the possibility of outpatient treatment, which includes weekly visits and dressing changes, as well as follow‐up of the wound matrix if necessary. In addition, local anti‐inflammatory and analgesic effects of the wound matrix are other benefits. 52 The omega‐3/ matrix increases angiogenesis and also facilitates the formation of granulation tissue in the wound healing process. Therapy with the omega‐3/ matrix as opposed to the porcine matrix (Oasis) results in no seroconversions of autoantibodies and significantly accelerates healing. Application of the omega‐3/ matrix in acute complex wounds results in a 50% mean reduction in wound area in the early stages of healing. Furthermore, the omega‐3/ matrix is a promising material in treating chronic wounds, including DFUs. ADM grafts also have an immunomodulatory impact, regarding in vitro research, by preventing macrophages from secreting the pro‐inflammatory cytokine IL‐1β. Furthermore, these fish skin‐derived grafts healed faster than dHACM (dehydrated human amnion/chorion membrane), acute biopsy wounds treated with fish skin grafts. 50
In another study when foetal bovine skin acellular dermal matrix (FBADM) was used as the control group in a haemolysis test on New Zealand white rabbits, the results showed that TADM has haemolytic properties. In addition, the cells in the TADM group grew well in comparison to the control group, and there was no significant difference in the number of dead cells, indicating that the TADM scaffold is non‐toxic. TADM is generally permeable to L929 cells and they can bind to it. 27 Studies have shown that ADM can accelerate wound healing and reduce the need for dressing changes and treatment costs, all of which are important factors in the management of burn wounds. By reducing inflammatory reactions and increasing pro‐inflammatory cytokines that promote wound healing, ADM would serve as an ideal skin substitute. 47 , 49 , 50
Bioactive nitrogenous compounds and protein of fish skin are other biological features of fish skin. Both bacterial fermentation and enzymatic hydrolysis can transform these compounds into peptides. Low‐cost peptides can serve as antioxidants, antihypertensive, anticoagulant, and immunomodulatory agents in the pharmaceutical sector, among other parts. These peptides have opioid‐like anticancer, anti‐hypertensive, antibacterial, antithrombotic, antioxidant, and immunomodulatory properties. 53 As fish are always in the vicinity of their aquatic environment, they are constantly exposed to various types of external hazards (such as aerobic and anaerobic bacteria, parasites and viruses, and pollutants). Mucus acts as a protective barrier between the environment and the fish and repels harmful microbes. The continuous mucous membrane that surrounds fish is their first physical, chemical, and biological defence against infection, as well as the first point of contact with pathogens. The mucus composition is quite complicated and includes immunoglobulins, lectins, lysins, and lysozymes. These elements play a crucial function in protecting fish against invasive infections. In fish, epidermal goblet cells, or mucus cells, create the majority of the epidermal mucus, which is mostly made up of water and macromolecules that form gels. Mucins and other glycoproteins are among them. Lectins, which make up a significant portion of mucus and are neither antibodies nor enzymes but play crucial roles in both innate and adaptive immunity, and are carbohydrate‐binding proteins. 54 , 55 According to research, omega‐3 fatty acids support both antibacterial and antiviral effects against herpes simplex, human immunodeficiency virus, and Gram‐positive and Gram‐negative bacteria. 50 , 56
3.2. Physicomechanical properties
The porous microstructure of decellularized skin allows the ingrowth of dermal cells and capillaries. 52 In addition, the decellularized fish skin is porous and spongy which simplifies cell adhesion and is friendly to cell growth. 27 The scaffold obtained from decellularized fish Grass Carp skin showed a porous structure (pore sizes about 20 to 100 μm) which can be a suitable environment for cell adhesion and proliferation. 28 Because of its highly porous and interconnected structure, ability to allow nutrients and oxygen to pass through the membrane, high‐density cell seeding, and least immune response compared with other naturally derived biomaterials, ADM has demonstrated high potential in clinical applications. 46 The process of decellularization of fish skin causes cells to come out and create a porous structure, and this process and its effect on the morphology of the structure can change the mechanical properties of the skin. It has been established that the tilapia fish skin has and maximum tensile stress of 35.4 MPa and Young's modulus of 143.8 MPa. After decellularization, the maximum tensile stress drop to 24 and Young's modulus to 56.2 MPa. 14 This shows that depending on the application of fish skin, it may be necessary to use other materials or crosslinkers to improve these properties.
The skin of most fish shows a low denaturation temperature because of the low concentration of glycine, proline, and hydroxyproline. Consequently, the low denaturation temperature of fish‐derived collagen is a significant disadvantage. For example, shark collagen denatures at 30°C, but salmon collagen is stable below 19°C. This temperature is not suitable for using fish collagen at the physiological temperature of the human body. However, chemical cross‐linking can increase the thermal stability of collagen scaffolds produced from fish. 46 Among the types of fish, tilapia has its advantages. It has indicated that the hydroxyproline content of acellular tilapia and salmon skin is as high as 9.1 ± 0.2%, and as low as 6.0 ± 0.1% respectively. Therefore, from this point of view, it is beneficial to use tilapia fish skin as ADM raw material. Another benefit of tilapia skin is its GAG content. GAG removal may affect the viscoelastic properties of the DM. The GAG content of tilapia skin is 0.1 ± 0.6 μg per mg of dry weight. However, the use of an alkaline solution of 0.1 M NaOH for decellularization probably resulted in higher GAG clearance. 18 As mentioned above, the stability of decellularized ECM (dECM) of fish skin and materials extracted from it can improve by cross‐linking. Crosslinking is a fundamental step in tissue engineering because it is essential for maintaining the scaffold's ideal design and structural integrity by generating a strong network in the polymeric matrix. The process of crosslinking scaffolds is crucial for their use in tissue engineering because it improves the material's physical characteristics, such as its water contact angle, performance at higher temperatures, resistance to enzymatic and chemical degradation, roughness, pore size, and viscoelasticity. The cross‐linking process renders the matrix insoluble in water and increases the matrix's thermal and mechanical stability under a physiological environment. In addition, the scaffold's pore size may be modified via this method. Active functional groups on the biomaterial often engage with the crosslinkers during the crosslinking process to produce a 3D network. 57 , 58 Crosslinking is a process that involves joining two or more molecules chemically to stabilise the final products. Because of the absence of movement, it will produce an ionic or covalent connection that connects one polymer chain to another, forming a network structure that is more stable and less reactive. 59
Many studies have used different types of crosslinkers to improve the physicomechanical properties of fish skin. Ma et al. modified Basa acellular dermal matrix (BADM) by carbodiimide (EDC), oxidised chitosan, and glutaraldehyde crosslinkers. According to the results of a scanning electron microscope, BADM was made up of unorganised collagen fibres. The BADM microfiber morphology before and after crosslinking remained approximately unchanged. In addition, BADM exhibited a porous fibre architecture with pores that ranged in size from 10 to 200 μm. The high porosity stimulated the growth and adherence of the cells. The mechanical properties of the crosslinked scaffold increased significantly after crosslinking and the thermal stability of wet and dried EDC‐BADM increased compared with that of non‐crosslinked BADM. The EDC‐BADM enhanced mechanical strength, thermal stability, biocompatibility, and cytocompatibility while preserving the structural integrity of the original BADM. Furthermore, the area around the implanted EDC‐BADM in the rat's spine showed no macroscopic symptoms of inflammation. The rats tolerated the EDC‐BADM well throughout the 28 days, and no abnormalities were seen. 29 Alginate dialdehyde, lactose heat‐treated at 105°C, and Glutaraldehyde were used to crosslink fish‐derived gelatin. In all studies, the mechanical strength of crosslinked gelatin increased significantly. Moreover, alginate dialdehyde increased the antioxidant capacity, and the mechanism of lactose and heat‐treated at 105°C methods was affecting the pores and reducing the porosity and increasing the size of the pores. 60 , 61 , 62 In addition, the crosslinking of gelatin with citric acid showed that by increasing the pH from 1.8 to 3.7, fibres maintain their morphology. 63 In another study, the crosslinking of ε‐PLL‐fortified fish gelatin/chitosan composite with Glutaraldehyde and Cinnamaldehyde showed that the films crosslinked by these crosslinkers had smooth and rough surfaces, respectively. In addition, Cinnamaldehyde induced very small pores in the cross‐section of the composite film and higher antibacterial activity was measured in the matrices crosslinked by Cinnamaldehyde than in Glutaraldehyde. 64 Considering that the reason for the high strength of collagen in the body environment is because of covalent bonds. In a study, to create these covalent bonds between the collagens of a cold fish called Hoki (Macruronus neovaezelandiae), citric acid was used as a chemical crosslinker. Citric acid was able to increase mechanical strength by creating the mentioned links. 65 In addition, the short time of UV irradiation may lead to crosslinking of collagen molecules. 66 To evaluate the dielectric properties of fish collagen, this material was crosslinked with dehydrothermal treatment and EDC/NHS (Ethyl [dimethyl aminopropyl]carbodiimide/N‐hydroxysuccinimide). The results demonstrated that collagen crosslinked with EDC/NHS has better conductivity and also higher mechanical strength than dehydrothermal treatment. 67 In addition, EDC/NHS has been used for crosslinking hydrogel derived from tilapia fish collagen and hyaluronic acid. The resulting hydrogel had a highly ordered network and its mass almost doubled in PBS because of swelling. 68 Cell migration is enabled by interconnected pores with diameters between 10 and 100 μm. It has been found that by adding EDC/NHS to the collagen obtained from tilapia fish, the stiffness of the collagen will be similar to the stiffness of the brain, and this condition can cause the differentiation of stem cells into nerve cells. 69 Diogo et al. used a mixture of collagen derived from shark skin (Prionace glauca) and calcium phosphates derived from the teeth of two different species of shark (Prionace glauca and Isurus oxyrinchus) to create a scaffold for bone. The obtained scaffold was modified with two different types of binders, namely EDC/NHS and hexamethylene diisocyanate (HMDI). The findings of this research showed that the created composites cause adhesion and growth of osteoblast‐like cells. 70 According to the studies, it can be concluded that modification of fish skin and its derived products is one of the key steps in using it for therapeutic uses. Because these materials have fast degradation and do not have adequate physical and mechanical properties for body tissue regeneration, therefore, its modification can cause better performance.
3.3. In‐vivo applications
Fish skin contains collagen I and III, omega‐3 polyunsaturated fatty acids, notably eicosapentaenoic acid (EPA), and DHA. DHA and EPA have antibacterial and antioxidant activities. As a result, fish skin is unlikely to be an infectious illness source. Because of the many mentioned benefits of fish skin, it can be used as a natural substance in the treatment of burns and wounds. Fish skin dressing accelerates wound healing and lessens the need for painkillers. 71 , 72
The thawed tilapia skin was decellularized using 0.1% Triton X‐100 solutions and then 0.1 M sodium hydroxide (NaOH) solution at the specified temperature and time. The tilapia‐skin acellular dermal matrix (TS‐ADM) sample was evaluated in terms of physical properties, biocompatibility, pre‐clinical safety, and wound healing. Histopathological results and DNA quantification showed acceptable removal of tilapia skin nuclear components. Compared with the commercial porcine acellular dermal matrix (DC‐ADM), the acellular tilapia skin sample showed better morphology, higher thermal stability, degradability, and more suitable water vapour transmission. The degradability of TS‐ADM was shown to be more appropriate than DC‐ADM in vitro and in vivo studies. As shown in Figure 3, the ability to repair and regenerate decellularized tilapia skin (DTFS) was evaluated on two rat and mini‐pig wound models. The significant increase in granulation growth, collagen deposition, angiogenesis, and reepithelialization with the TS‐ADM skin sample may be because of the high expression of transforming growth factor‐beta 1 (TGF‐β1), alpha‐smooth muscle actin (α‐SMA) and CD31. 18
FIGURE 3.
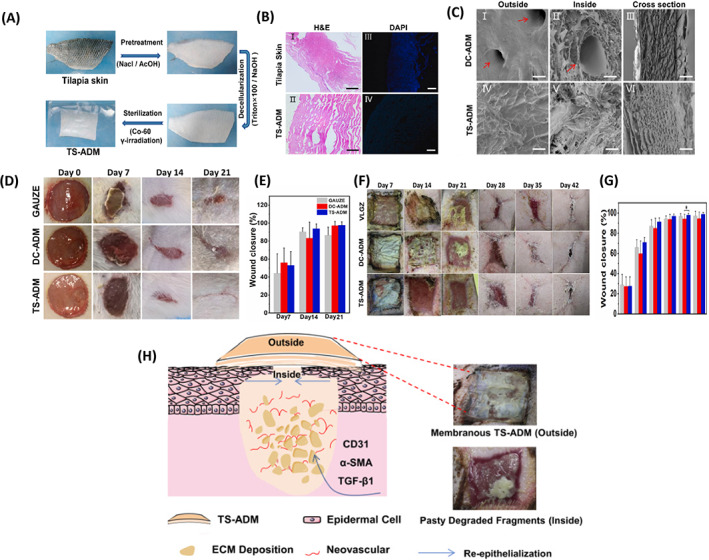
(A) design of tilapia skin acellular dermal matrix (TS‐ADM), (B) histological assays by H&E (I and II) and DAPI (III and IV) staining of TS‐ADM (Scale bar:100 μm), (C) scanning electron microscopy of TS‐ADM and DC‐ADM, (D) wound healing process on a rat model with TS‐ADM within 21 days, (E) wound closure on days 7, 14 and 21 (rat model), (F) wound healing process on porcine model with TS‐ADM on days 7, 14, 21, 28, 35, 42 and 180 postoperation, (G) wound closure on days 7, 14 and 21 (porcine model), (H) Schematic diagram: the partially degraded TS‐ADM promotes extracellular matrix deposition, angiogenesis, and reepithelialization by forming a microenvironment conducive to the expression of TGF‐β1, α‐SMA and CD31. 18
One of the clinical challenges is the poor regeneration ability of damaged tendon tissues. The use of acellular porcine and bovine ECM with stem cells can be promising. But for the reasons mentioned, such as ease of access, less biological risk, and also for religious reasons, the use of fish skin can be more efficient. Liu et al used DTFS to solve this challenge. The samples had regular collagen fibres, a structure with natural pores, a smooth and dense outer layer, and an inner layer with a soft structure and uneven surface. To improve the mechanical properties, the samples were cross‐linked with 1‐ethyl‐3‐(3‐dimethyl aminopropyl)‐carbodiimide (EDC) and N‐hydroxysuccinimide (NHS). The acellular skin sample modified with crosslinker did not show toxicity and also increased migration and tonic differentiation of tendon‐derived stem cells (TDSCs). The viability, differentiation, and proliferation of TDSCs seeded into an acellular matrix were demonstrated. The results of the in vivo study on the rat Achilles tendon defect model showed that the DTFS with cultured cells can help regenerate the tendon tissue. Therefore, the authors claimed that DTFS with TDSCs is a promising therapeutic option for tendon tissue regeneration. 73 Cao et al. designed a hybrid skin patch consisting of acellular tilapia fish skin (AFS) and chitosan (CS), for wound healing. Pregel CS containing vascular endothelial growth factor (VEGF) was linked to the acellular skin matrix. The hybrid patch was evaluated on a rat model with a full‐thickness skin defect (diameter 1.5 cm). Mice were divided into different groups (PBS [control group], AFS, and AFS + CS + VEGF) and the wound healing status was evaluated at different times (0, 3, 5, 7, and 9 days). The results of HE staining showed that new granulation tissues appeared in the wound area after 9 days, and the speed of wound closure was significantly accelerated especially in the AFS + CS + VEGF sample. The results of immunohistochemical staining on day 9 by measuring the expression of the two typical inflammatory cytokines, interleukin‐6 (IL‐6) and tumour necrosis factor‐α (TNF‐α), showed that the control group had the highest expression of inflammatory factors, whereas the AFS group CS + VEGF showed the least inflammation because of the antimicrobial activity of CS. More collagen deposition was also observed in samples treated with AFS + CS + VEGF, which was attributed to the antimicrobial function of CS. Angiogenesis as another important index was evaluated by double immunofluorescence staining of CD31 and α‐smooth muscle actin (α‐SMA). Because of the high inflammatory level in the control sample, few new blood vessels were observed, whereas the density of new blood vessels in the AFS + CS + VEGF group was relatively higher because of the presence of growth factor. The statistical analysis showed that the combination of angiogenesis stimulating agent, antimicrobial and inflammatory agent chitosan on the substrate of acellular fish skin caused the most collagen deposition, angiogenesis formation, and tissue regeneration. 34 Since the FDA has approved fish skin (cellular/tissue‐based therapy [CTP]) for wound dressings, this innovative skin substitute has been widely used in clinical applications. Fish skin CTPs also demonstrate promising outcomes in the management of DFUs, venous leg ulcers (VLUs), and other acute and chronic wounds. 74 In comparison with other animal‐derived skin graft materials, fish skin CTPs are a promising material for accelerating wound healing and have been demonstrated to have antiviral and antibacterial qualities. For instance, Fish skin grafts cause quicker healing than pig intestinal submucosa and dehydrated amnion chorionic membrane in an acute wound model. 75 In contrast to often used mammalian grafts, the omega‐3 fatty acid content of CTP is particularly high in DHA and EPA, with 1.4% and 1.1% of the total lipid content, respectively. As mentioned above, the EPA and DHA act as antibacterial agents and control the acute inflammatory response (Figure 4). Decellularized fish skin CTPs have been demonstrated to be a suitable choice for individuals who may be prone to hypersensitivity responses to foreign materials. 74 , 75 , 76 Ideally, the CTP would generate a structural matrix that would promote epidermal and dermal regeneration. One of the common applications of CTPs is their use in the treatment of DFUs.
FIGURE 4.
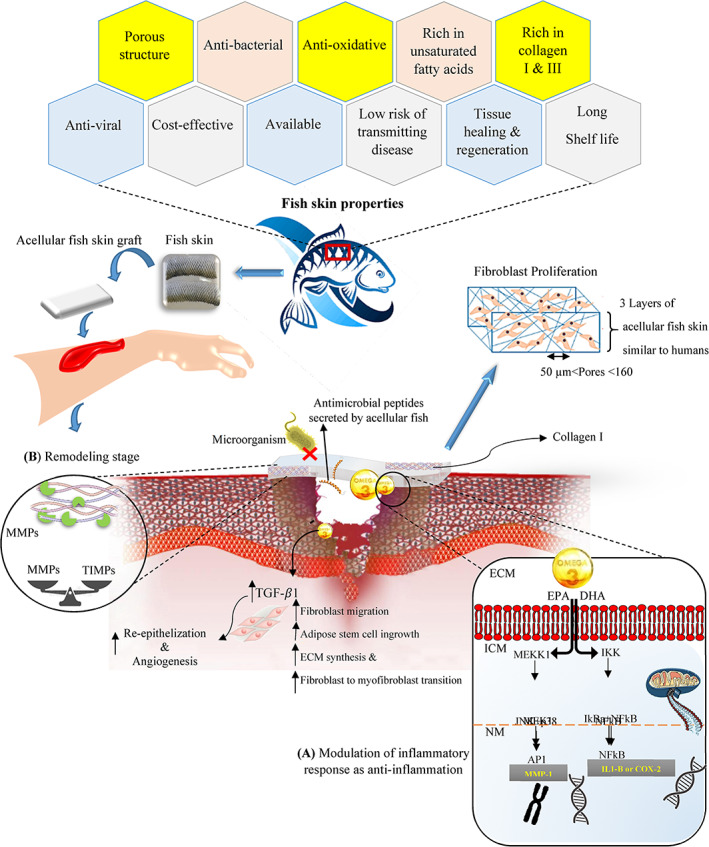
Acellular fish skin properties and the effect of Omega‐3 polyunsaturated fatty acids on signalling activity for inflammation stage of wound healing as well as mediating tissue remodelling. (A) Mechanism of action for Omega‐3 polyunsaturated fatty acids such eicosatetraenoic acid and docosahexaenoic acid (AP1, activator protein 1; COX‐2, cyclooxygenase‐2; DHA, docosahexaenoic acid; ECM, extracellular matrix; EPA, eicosatetraenoic acid; ICM, intracellular matrix; IkBa, NFkB inhibitor a; IKK, IkB kinase; IL1‐B, interleukin 1‐B; JNK, c‐Jun N‐terminal kinase; MEKK1, MAPK kinase 1; MEK1, MAPK kinases 1; MMP1, Matrix metallopeptidase 1; NM, nuclear matrix). 74 (B) The enzymatic activities of MMPs by acting as an attachment site for breakdown of scaffold rather than developing host tissue and ECM components; so, it causes the balance between MMPs and tissue inhibitors of metalloproteinases (TIMPs) and finally the new ECM is formed and the inflammation time reduces.
According to research, two‐thirds of people with diabetes develop peripheral neuropathy, which causes discomfort, abnormal foot structure, and loss of sensation. A quarter of these people may also have leg ulcers. A fifth of them have to be amputated, and more than half of them need to be hospitalised. Diabetes and foot issues are already putting a significant strain on health care resources. It was shown that the size of the wound in most patients decreased by 50% after 4 weeks and 30‐9% after 12 weeks. 50 , 77 In this context, the role of cell and tissue‐based therapies in the treatment of chronic wounds is increasing day by day. 76 ADM products are commonly used to treat diabetic and VLUs, which are among the most difficult chronic ulcers. According to research, by using decellularized fish skin, more than 100 patient wounds have been treated without negative side effects. 78 Fish skin grafts which are high in omega‐3, have been found to hasten the healing of full‐thickness wounds. To handle treatment‐resistant DFUs, which are defined as superficial ulcers not touching tendon capsules or bone, Lullove et al. compared the fish skin transplant with the collagen/alginate standard dressing. The final sample size for analysis was 49 patients. The wounds of 8 of 25 patients (32%) were completely closed with standard dressing and 16 of 24 patients (67%) with fish skin after 12 weeks. The results of this study support the use of fish skin grafts to improve DFUs that do not improve after receiving standard care. 79 In another study, acellular fish skin grafts were used once weekly for 6 weeks to treat postoperative diabetic foot lesions to evaluate the potential benefits of fish skin grafts (Kerecis Omega 3). The findings showed that regardless of size, the average percentage of wound surface reduction after 6 weeks was more than 84.9%. Skin reactions or infections did not occur in any case. The findings of this study support the idea that ADM has the potential to repair DFU. 77 Omega 3‐rich fish skin was effectively used to treat a patient with haemophilia who suffered from a chronic ulcer that did not respond to previous treatments. In this case report study, the haemostatic properties of fish skin were used to treat the wound of a haemophilia patient. A 56‐year‐old man with an infected forefoot ulcer with cellulitis and osteomyelitis healed after 14 weeks using a fish skin graft. The wound was completely healed after 1 year and 10 months. This study showed that fish skin graft treatment is a promising option for a wide range of patients, where the risk of bleeding or hematoma formation is increased. This case demonstrates the effectiveness of the product to achieve haemostasis and helping wound closure in a high‐risk patient with diabetes and haemophilia. 80 Comparing the use of decellularized fish skin with other repair methods shows the superiority of this material in many parameters related to wound repair. As shown in Figure 5, the use of this material has been more effective in the treatment of various acute and chronic wounds compared with bovine foetal collagen and amniotic membrane‐based matrix. 50 , 52 , 76
FIGURE 5.
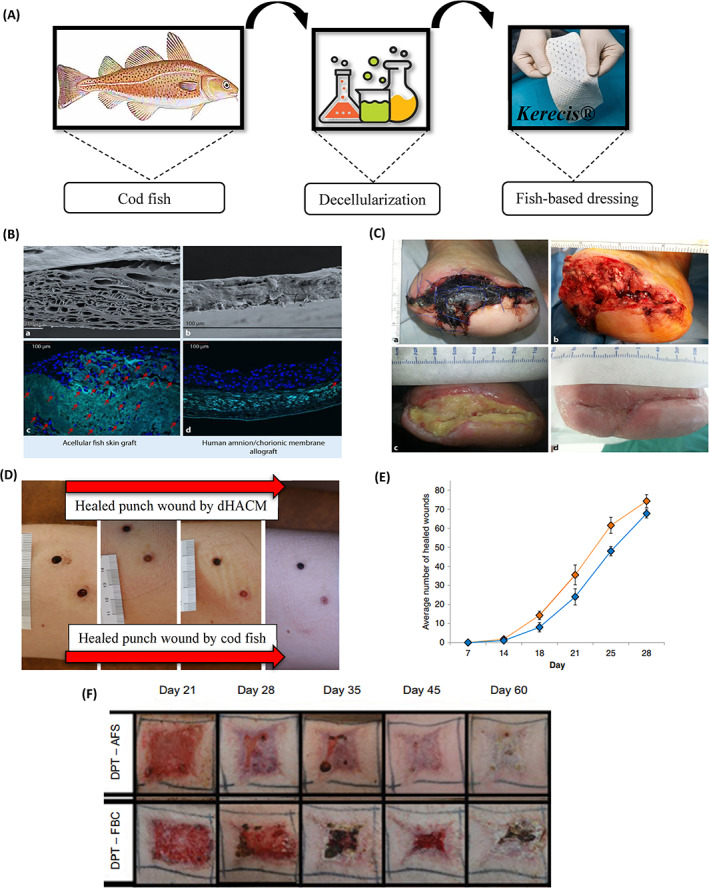
(A) Kerecis® product representative of acellular Atlantic cod fish skin, (B) SEM of (a) Acellular dermal matrix derived from fish skin and (b) decellularized amnion matrix. Stem cells were cultured for 12 days in an acellular dermal matrix derived from fish skin (c) and amnion (d). The stem cells stained blue have migrated into the fish skin matrix, while the stem cells on the amniotic matrix have settled on the surface of the matrix, (C) Healed wound (wound area 29 cm2) following a total treatment duration of 33 week using acellular Cod fish skin, 52 (D) Followed up to the healing of punch wounds that wounds on the bottom side of figure was treated with Cod fish skin graft, while the top wound was treated with dehydrated human amnion/chorion membrane or dHACM, (E) Clinical use of antibiotics before and after the application of AFS in a cohort of chronic wound patients, 50 (F) Progression of porcine burn wound closure when treated with AFS with and without skin grafting. (AFS, acellular cod fish skin; DPT, deep partial thickness; FBC, fetal bovine collagen; SEM, Scanning electron microscope). 76
Various studies have also shown the potential of ADM in healing acute wounds such as burn wounds. 81 Burns is the seventh most frequent non‐fatal paediatric injury cause globally. According to the World Health Organization, the rate of child burn mortality is presently more than seven times higher in low‐ and middle‐income nations, where the frequency is significantly higher than in high‐income nations. Therefore, more research must be performed to provide less expensive burn treatment options. 82 The degree, source, and extent of the burn, as well as the patient's age, general health, and any underlying medical conditions all affect how quickly burn wounds heal. Early wound closure reduces the risk of infection and fluid loss, as well as mortality and length of hospital stay. However, treatment recommendations should be tailored to each patient's needs. The ideal biological dressing prevents bacterial contamination and hydro electrolytic losses, promotes epithelization, and accelerates the formation of granular tissue in grafting cases. Considering that fish skin has almost all these features, various studies have recommended its use. 82 , 83 ADM plays an important role in mouse skin wound healing by producing biological components involved in healing. 27 The presence of peptides with antimicrobial functions in ADM can cause its use in the healing of acute wounds. Tilapia skin has been shown to have a non‐infectious microbiota and morphological structure similar to human skin, with an even greater composition of type I collagen. These factors support its clinical application as a biomaterial for burn treatment. 82 , 83 Thirty kids with superficial partial thickness burns between the ages of 2 and 12 received tilapia skin in an open‐label, monocentric, randomised phase II pilot research. As a result of the strong adhesion of tilapia fish skin to the wound bed, fewer dressing changes and fewer anaesthetic drugs were needed, which was associated with a reduction in the workload of patients and medical staff. The duration of treatment, the total amount of analgesics required, the burn improvement on the day of dressing removal, and the amount of pain experienced were all compared with traditional treatment with silver sulfadiazine. The results showed that tilapia skin is an efficient and low‐cost tool in the treatment of superficial partial‐thickness burns in children. 49 In a case report study by Costa et al. A 3‐year‐old child with burns on the left side of the face, neck, chest, abdomen, and left arm was treated with tilapia skin. It was found that tilapia skin adheres well to the wound bed. After a total of 10 days and epithelialization, the patient was discharged. In another study, treatment with tilapia fish skin was performed on a 33‐year‐old woman with 10% body surface burns, and the results showed that fish skin can be effective as a flexible xenograft by adhering to the wound bed. Non‐toxicity and also as a barrier against microorganisms along with epithelialization in 10 days showed successful burn treatment. 83 In addition, the repair of a deep partial thickness burn on the left upper limb and a superficial partial thickness burn on the right upper limb of a 23‐year‐old male patient with tilapia skin graft proves the benefits of using a fish skin graft. In this case, complete reepithelialization occurred at two sites at 12 and 17 days after treatment, respectively. 81 In a retrospective case–control study, 12 patients with superficial or deep dermal burn wounds were treated with three different skin grafts that is, fish skin graft, Suprathel® (PolyMedics Innovations GmbH, Denkendorf, Germany) and split‐thickness skin graft (STSG). Depending on the depth of the wound, burn wounds were coated after enzymatic debridement using NexoBrid. Suprathel was used to treat superficial partial‐thickness burns. To treat deep partial‐thickness burns, either a fish skin transplant or an autologous STSG was used (0.2 mm, meshed 1:1.5). Fish skin wound dressing resulted in faster wound healing, water retention ability, and significant pain management. In addition, improved functional and aesthetic results were demonstrated in terms of skin elasticity, thickness, and pigmentation. Compared with wounds treated with STSG, patients treated with fish skin reported less pain and itching on the Patient and Observer Scar Assessment Scale (POSAS). Most importantly, wounds treated with fish skin had much better sebum production and skin elasticity than wounds treated with Supratel. 84
4. CONCLUSION
As shown in this article and other studies, fish skin and its derivatives can be a suitable substitute for the regeneration of damaged tissues for many reasons. The results of clinical trials show significant progress in the field of skin tissue engineering. However, more studies are needed in the field of decellularization methods as well as the use of crosslinkers and modifiers to improve properties. The results have shown that a combination of different decellularization protocols (physical, chemical, and enzymatic) can create ideal conditions for obtaining a biological scaffold. In addition, the use of technologies such as bio‐printing with bio‐inks made from fish skin contents can improve the performance of the scaffold with pre‐designed structural morphology. Although some decellularization protocols can disrupt the morphology of the scaffold. In general, fish skin can be a cheap and accessible biological scaffold or a high‐performance scaffold in the process of repairing and regenerating damaged tissues.
FUNDING INFORMATION
This research received no specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
Esmaeili A, Biazar E, Ebrahimi M, Heidari Keshel S, Kheilnezhad B, Saeedi Landi F. Acellular fish skin for wound healing. Int Wound J. 2023;20(7):2924‐2941. doi: 10.1111/iwj.14158
This study was carried out in accordance with the relevant guidelines and regulations.
Contributor Information
Esmaeil Biazar, Email: e.biazar@toniau.ac.ir, Email: kia_esm@yahoo.com.
Saeed Heidari Keshel, Email: saeed.heidari@sbmu.ac.ir.
DATA AVAILABILITY STATEMENT
Supplementary material is available on the publisher'sweb site along with the published article
REFERENCES
- 1. Yao Y, Zhang A, Yuan C, Chen X, Liu Y. Recent trends on burn wound care: hydrogel dressings and scaffolds. Biomater Sci. 2021;9(13):4523‐4540. [DOI] [PubMed] [Google Scholar]
- 2. Markiewicz‐Gospodarek A, Kozioł M, Tobiasz M, Baj J, Radzikowska‐Büchner E, Przekora A. Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. Int J Environ Res Public Health. 2022;19(3):1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaddoura I, Abu‐Sittah G, Ibrahim A, Karamanoukian R, Papazian N. Burn injury: review of pathophysiology and therapeutic modalities in major burns. Ann Burns Fire Disasters. 2017;30:95‐102. [PMC free article] [PubMed] [Google Scholar]
- 4. Cartotto R. Topical antimicrobial agents for pediatric burns. Burn Trauma. 2017;5:33. doi: 10.1186/s41038-017-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khadjibayev AM, Fayazov AD, Djabriyev DA, Kamilov UR. Surgical treatment of deep burns. Ann Burns Fire Disasters. 2008;21:150‐152. [PMC free article] [PubMed] [Google Scholar]
- 6. Gacto‐Sanchez P. Surgical treatment and management of the severely burn patient: review and update. Med Intensiva. 2017;41:356‐364. doi: 10.1016/j.medin.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 7. Inci I, Norouz Dizaji A, Ozel C, Morali U, Dogan Guzel F, Avci H. Decellularized inner body membranes for tissue engineering: a review. J Biomater Sci Polym ed. 2020;31(10):1287‐1368. [DOI] [PubMed] [Google Scholar]
- 8. Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int. 2017;2017:9831534‐9831513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reddy MS, Ponnamma D, Choudhary R, Sadasivuni KK. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers (Basel). 2021;13(7):1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vats A, Tolley NS, Polak JM, Gough JE. Scaffolds and biomaterials for tissue engineering: a review of clinical applications. Clin Otolaryngol Allied Sci. 2003;28(3):165‐172. [DOI] [PubMed] [Google Scholar]
- 11. Kiradzhiyska DD, Mantcheva RD. Overview of biocompatible materials and their use in medicine. Folia Med (Plovdiv). 2019;61(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 12. Chaudhari AA, Vig K, Baganizi DR, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci. 2016;17(12):1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu W, Cao L, Song C, Pang Z, Jiang H, Guo C. Cell‐derived decellularized extracellular matrix scaffolds for articular cartilage repair. Int J Artif Organs. 2021;44(4):269‐281. [DOI] [PubMed] [Google Scholar]
- 14. Lau CS, Hassanbhai A, Wen F, et al. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J Tissue Eng Regen Med. 2019;13(10):1779‐1791. [DOI] [PubMed] [Google Scholar]
- 15. Sun L, Li B, Song W, Zhang K, Fan Y, Hou H. Comprehensive assessment of Nile tilapia skin collagen sponges as hemostatic dressings. Mater Sci Eng C Mater Biol Appl. 2020;109:110532. [DOI] [PubMed] [Google Scholar]
- 16. Kamalvand M, Biazar E, Daliri‐Joupari M, Montazer F, Rezaei‐Tavirani M, Heidari‐Keshel S. Design of a decellularized fish skin as a biological scaffold for skin tissue regeneration. Tissue Cell. 2021;71:101509. [DOI] [PubMed] [Google Scholar]
- 17. Rakers S, Gebert M, Uppalapati S, et al. 'Fish matters': the relevance of fish skin biology to investigative dermatology. Exp Dermatol. 2010;19(4):313‐324. [DOI] [PubMed] [Google Scholar]
- 18. Li D, Sun WQ, Wang T, et al. Evaluation of a novel tilapia‐skin acellular dermis matrix rationally processed for enhanced wound healing. Mater Sci Eng C Mater Biol Appl. 2021;127:112202. [DOI] [PubMed] [Google Scholar]
- 19. Lehmann N, Christ T, Daugs A, Bloch O, Holinski S. EDC cross‐linking of decellularized tissue: a promising approach? Tissue Eng Part A. 2017;23(13–14):675‐682. [DOI] [PubMed] [Google Scholar]
- 20. Dussoyer M, Michopoulou A, Rousselle P. Decellularized scaffolds for skin repair and regeneration. Appl Sci (Basel). 2020;10(10):3435. [Google Scholar]
- 21. Moffat D, Ye K, Jin S. Decellularization for the retention of tissue niches. J Tissue Eng. 2022;13:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Chen J, Ran Y, et al. Utility of air bladder‐derived nanostructured ECM for tissue regeneration. Front Bioeng Biotechnol. 2020;8:553‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bai H, Sun P, Wu H, et al. The application of tissue‐engineered fish swim bladder vascular graft. Commun Biol. 2021;4(1):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khajavi M, Hajimoradloo A, Zandi M, Pezeshki‐Modaress M, Bonakdar S, Zamani A. Fish cartilage: a promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J Biomed Mater Res A. 2021;109(9):1737‐1750. [DOI] [PubMed] [Google Scholar]
- 25. Wu W, Zhou Z, Sun G, Liu Y, Zhang A, Chen X. Construction and characterization of degradable fish scales for enhancing cellular adhesion and potential using as tissue engineering scaffolds. Mater Sci Eng C Mater Biol Appl. 2021;122:111919. [DOI] [PubMed] [Google Scholar]
- 26. Lin X, Kong B, Zhu Y, Zhao Y. Bioactive fish scale scaffolds with MSCs‐loading for skin flap regeneration. Adv Sci (Weinh). 2022;9(21):e2201226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lv K, Wang L, He X, Li W, Han L, Qin S. Application of tilapia skin acellular dermal matrix to induce acute skin wound repair in rats. Front Bioeng Biotechnol. 2021;9:792344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biazar E, Heidari Keshel S, Rezaei Tavirani M, Kamalvand M. Healing effect of acellular fish skin with plasma rich in growth factor on full‐thickness skin defects. Int Wound J. 2022;19(8):2154‐2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma P, Wang Y, Li B, Hou H. Cross‐linking effects of carbodiimide, oxidized chitosan oligosaccharide and glutaraldehyde on acellular dermal matrix of basa fish (Pangasius bocourti). Int J Biol Macromol. 2020;164:677‐686. [DOI] [PubMed] [Google Scholar]
- 30. Miranda C, Leonel L, Cañada RR, et al. Effects of chemical and physical methods on decellularization of murine skeletal muscles. An Acad Bras Cienc. 2021;93(2):e20190942. [DOI] [PubMed] [Google Scholar]
- 31. Shevtsov A, Leybovich B, Artyuhov I, Maleev Y, Peregudov A. Production of organ extracellular matrix using a freeze‐thaw cycle employing extracellular cryoprotectants. Cryo Letters. 2014;35(5):400‐406. [PubMed] [Google Scholar]
- 32. Kawasaki T, Kirita Y, Kami D, et al. Novel detergent for whole organ tissue engineering. J Biomed Mater Res A. 2015;103(10):3364‐3373. [DOI] [PubMed] [Google Scholar]
- 33. García‐Gareta E, Abduldaiem Y, Sawadkar P, Kyriakidis C, Lali F, Greco KV. Decellularised scaffolds: just a framework? Current knowledge and future directions. J Tissue Eng. 2020;11:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao X, Sun L, Luo Z, Lin X, Zhao YJER. Aquaculture derived hybrid skin patches for wound healing. ER. 2023;4(1):28‐35. [Google Scholar]
- 35. Mohiuddin OA, Campbell B, Poche JN, et al. Decellularized adipose tissue: biochemical composition, in vivo analysis and potential clinical applications. Adv Exp Med Biol. 2020;1212:57‐70. [DOI] [PubMed] [Google Scholar]
- 36. Robinson PS, Tranquillo RT. Planar biaxial behavior of fibrin‐based tissue‐engineered heart valve leaflets. Tissue Eng Part A. 2009;15(10):2763‐2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Somuncu ÖS. Decellularization concept in regenerative medicine. Adv Exp Med Biol. 2020;6:71‐85. [DOI] [PubMed] [Google Scholar]
- 38. Bual R, Labares M Jr, Valle KD, et al. Characterization of decellularized extracellular matrix from milkfish (Chanos chanos) skin. Biomimetics (Basel). 2022;7(4):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Funamoto S, Nam K, Kimura T, et al. The use of high‐hydrostatic pressure treatment to decellularize blood vessels. Biomaterials. 2010;31(13):3590‐3595. [DOI] [PubMed] [Google Scholar]
- 40. Burk J, Erbe I, Berner D, et al. Freeze‐thaw cycles enhance decellularization of large tendons. Tissue Eng Part C Methods. 2014;20(4):276‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233‐3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Isidan A, Liu S, Li P, et al. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation. 2019;26(6):e12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Q, Wang H, Dai Z, Cao Y, Jin C. Preparation and biomechanical properties of an acellular porcine corneal stroma. Cornea. 2017;36(11):1343‐1351. [DOI] [PubMed] [Google Scholar]
- 44. Boccafoschi F, Botta M, Fusaro L, Copes F, Ramella M, Cannas M. Decellularized biological matrices: an interesting approach for cardiovascular tissue repair and regeneration. J Tissue Eng Regen Med. 2017;11(5):1648‐1657. [DOI] [PubMed] [Google Scholar]
- 45. Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25(13):2679‐2686. [DOI] [PubMed] [Google Scholar]
- 46. Subhan F, Hussain Z, Tauseef I, Shehzad A, Wahid F. A review on recent advances and applications of fish collagen. Crit Rev Food Sci Nutr. 2021;61(6):1027‐1037. [DOI] [PubMed] [Google Scholar]
- 47. Pateiro M, Domínguez R, Varzakas T, Munekata PES, Movilla Fierro E, Lorenzo JM. Omega‐3‐rich oils from marine side streams and their potential application in food. Mar Drugs. 2021;19(5):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kotronoulas A, de Lomana AL, Karvelsson ST, et al. Lipid mediator profiles of burn wound healing: acellular cod fish skin grafts promote the formation of EPA and DHA derived lipid mediators following seven days of treatment. Prostaglandins Leukot Essent Fatty Acids. 2021;175:102358. [DOI] [PubMed] [Google Scholar]
- 49. Luze H, Nischwitz SP, Smolle C, Zrim R, Kamolz LP. The use of acellular fish skin grafts in burn wound management‐a systematic review. Medicina (Kaunas). 2022;58(7):912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kirsner RS, Margolis DJ, Baldursson BT, et al. Fish skin grafts compared to human amnion/chorion membrane allografts: a double‐blind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020;28(1):75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ahmmed MK, Ahmmed F, Tian HS, Carne A, Bekhit AE. Marine omega‐3 (n‐3) phospholipids: a comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr Rev Food Sci Food Saf. 2020;19(1):64‐123. [DOI] [PubMed] [Google Scholar]
- 52. Dorweiler B, Trinh T, Dünschede F, et al. The marine Omega3 wound matrix for treatment of complicated wounds. Gefasschirurgie. 2018;23(2):46‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ucak I, Afreen M, Montesano D, et al. Functional and bioactive properties of peptides derived from marine side streams. Mar Drugs. 2021;19(2):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang ZH, Ma AJ, Lei JL. Progress in Study on the Skin Mucus Lectin in Fish. 2013. [PubMed]
- 55. Benhamed S, Guardiola FA, Mars M, Esteban M. Pathogen bacteria adhesion to skin mucus of fishes. Vet Microbiol. 2014;171(1–2):1‐12. [DOI] [PubMed] [Google Scholar]
- 56. Kim TH, Park JH, Jeong HG, Wee S. The utility of novel fish‐skin derived acellular dermal matrix (Kerecis) as a wound dressing material. JWMR. 2021;17(1):39‐47. [Google Scholar]
- 57. Thakur G, Rodrigues FC, Singh K. Crosslinking biopolymers for advanced drug delivery and tissue engineering applications. Cutting‐Edge Enabling Technologies for Regenerative Medicine. 2018;1078:213‐231. [DOI] [PubMed] [Google Scholar]
- 58. Indurkar A, Pandit A, Jain R, Dandekar P. Plant based cross‐linkers for tissue engineering applications. J Biomater Appl. 2021;36(1):76‐94. [DOI] [PubMed] [Google Scholar]
- 59. Utami Nike D, Md Fadilah NI, Sallehuddin N, et al. Genipin‐crosslinking effects on biomatrix development for cutaneous wound healing: a concise review. Front Bioeng Biotechnol. 2022;10:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Park J, Nam J, Yun H, Jin HJ, Kwak HW. Aquatic polymer‐based edible films of fish gelatin crosslinked with alginate dialdehyde having enhanced physicochemical properties. Carbohydr Polym. 2021;254:117317. [DOI] [PubMed] [Google Scholar]
- 61. Fan HY, Duquette D, Dumont MJ, Simpson BK. Salmon skin gelatin‐corn zein composite films produced via crosslinking with glutaraldehyde: optimization using response surface methodology and characterization. Int J Biol Macromol. 2018;120(Pt A):263‐273. [DOI] [PubMed] [Google Scholar]
- 62. Etxabide A, Ribeiro R, Guerrero P, et al. Lactose‐crosslinked fish gelatin‐based porous scaffolds embedded with tetrahydrocurcumin for cartilage regeneration. Int J Biol Macromol. 2018;117:199‐208. [DOI] [PubMed] [Google Scholar]
- 63. Liguori A, Uranga J, Panzavolta S, Guerrero P, de la Caba K, Focarete ML. Electrospinning of fish gelatin solution containing citric acid: an environmentally friendly approach to prepare crosslinked gelatin fibers. Materials (Basel). 2019;12(17):2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mousavi Z, Naseri M, Babaei S, Hosseini SM, Shekarforoush SS. The effect of cross‐linker type on structural, antimicrobial and controlled release properties of fish gelatin‐chitosan composite films incorporated with ε‐poly‐l‐lysine. Int J Biol Macromol. 2021;183:1743‐1752. [DOI] [PubMed] [Google Scholar]
- 65. Cumming MH, Leonard AR, LeCorre‐Bordes DS, Hofman K. Intra‐fibrillar citric acid crosslinking of marine collagen electrospun nanofibres. Int J Biol Macromol. 2018;114:874‐881. [DOI] [PubMed] [Google Scholar]
- 66. Sionkowska A, Lewandowska K, Adamiak K. The influence of UV light on rheological properties of collagen extracted from silver carp skin. Materials (Basel). 2020;13(19):4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Marzec E, Pietrucha K. Selecting the correct scaffold model for assessing of the dielectric response of collagen‐based biomaterials. Colloids Surf B Biointerfaces. 2018;171:506‐513. [DOI] [PubMed] [Google Scholar]
- 68. Menezes M, Ribeiro HL, Abreu F, Feitosa JPA, Filho M. Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid. Colloids Surf B Biointerfaces. 2020;189:110852. [DOI] [PubMed] [Google Scholar]
- 69. Iwashita M, Ohta H, Fujisawa T, et al. Brain‐stiffness‐mimicking tilapia collagen gel promotes the induction of dorsal cortical neurons from human pluripotent stem cells. Sci Rep. 2019;9(1):3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Diogo GS, Senra EL, Pirraco RP, et al. Marine collagen/apatite composite scaffolds envisaging hard tissue applications. Mar Drugs. 2018;16(8):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ibrahim A, Soliman M, Kotb S, Ali MM. Evaluation of fish skin as a biological dressing for metacarpal wounds in donkeys. BMC Vet Res. 2020;16(1):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ibrahim A, Hassan D, Kelany N, Kotb S, Soliman M. Validation of three different sterilization methods of tilapia skin dressing: impact on microbiological enumeration and collagen content. Front Vet Sci. 2020;7:597751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu Z, Yu MZ, Peng H, et al. Decellularized tilapia fish skin: a novel candidate for tendon tissue engineering. Mater Today Bio. 2022;17:100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fiakos G, Kuang Z, Lo E. Improved skin regeneration with acellular fish skin grafts. ER. 2020;1:95‐101. [Google Scholar]
- 75. Magnusson S, Baldursson BT, Kjartansson H, Rolfsson O, Sigurjonsson G. Regenerative and antibacterial properties of acellular fish skin grafts and human amnion/chorion membrane: implications for tissue preservation in combat casualty care. Mil Med. 2017;182(suppl_1):383‐388. [DOI] [PubMed] [Google Scholar]
- 76. Patel M, Lantis J. Fish skin acellular dermal matrix: potential in the treatment of chronic wounds. Wound Care Manag Res. 2019;6:59‐70. [Google Scholar]
- 77. Woodrow T, Chant T, Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega‐3: a prospective evaluation. J Wound Care. 2019;28(2):76‐80. [DOI] [PubMed] [Google Scholar]
- 78. Baldursson BT, Kjartansson H, Konrádsdóttir F, Gudnason P, Sigurjonsson GF, Lund SH. Healing rate and autoimmune safety of full‐thickness wounds treated with fish skin acellular dermal matrix versus porcine small‐intestine submucosa: a noninferiority study. Int J Low Extrem Wounds. 2015;14(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 79. Lullove EJ, Liden B, Winters C, McEneaney P, Raphael A, Lantis Ii JC. A multicenter, blinded, randomized controlled clinical trial evaluating the effect of Omega‐3‐rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers. Wounds. 2021;33(7):169‐177. [DOI] [PubMed] [Google Scholar]
- 80. Winters C, Partner C. Wound dehiscence on a diabetic patient with hemophilia and high risk of further amputation successfully healed with omega 3 rich fish skin: a case report. Diabet Foot. 2018;21(3):146‐206. [Google Scholar]
- 81. Lima‐Junior EM, de Moraes Filho MO, Costa BA, et al. Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. J Surg Case Rep. 2019;2019(6):rjz181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Costa BA, Lima Júnior EM, de Moraes Filho MO, et al. Use of tilapia skin as a xenograft for pediatric burn treatment: a case report. J Burn Care Res. 2019;40(5):714‐717. [DOI] [PubMed] [Google Scholar]
- 83. Júnior EM, de Moraes Filho MO, Costa BA, et al. Lyophilised tilapia skin as a xenograft for superficial partial thickness burns: a novel preparation and storage technique. J Wound Care. 2020;29(10):598‐602. [DOI] [PubMed] [Google Scholar]
- 84. Wallner C, Holtermann J, Drysch M, et al. The use of intact fish skin as a novel treatment method for deep dermal burns following enzymatic debridement: a retrospective case‐control study. Eur Burn j. 2022;3(1):43‐55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary material is available on the publisher'sweb site along with the published article


