Abstract
Background
A proportion of coronavirus disease 2019 (COVID-19) survivors experience persistent dyspnoea without measurable impairments in lung function. We performed a systematic review and meta-analysis to determine relationships between dyspnoea and imaging abnormalities over time in post-COVID-19 patients.
Methods
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we analysed studies published prior to 15 September 2022 and indexed by Google Scholar, PubMed and LitCOVID which assessed chest imaging in adults ≥3 months after COVID-19. Demographic, chest imaging, spirometric and post-COVID-19 symptom data were extracted. The relationships between imaging abnormalities and dyspnoea, sex and age were determined using a random effects model and meta-regression.
Results
47 studies were included in the meta-analysis (n=3557). The most prevalent computed tomography (CT) imaging abnormality was ground-glass opacities (GGOs) (44.9% (95% CI 37.0–52.9%) at any follow-up time-point). Occurrence of reticulations significantly decreased between early and late follow-up (p=0.01). The prevalence of imaging abnormalities was related to the proportion of patients with dyspnoea (p=0.012). The proportion of females was negatively correlated with the presence of reticulations (p=0.001), bronchiectasis (p=0.001) and consolidations (p=0.025). Age was positively correlated with imaging abnormalities across all modalities (p=0.002) and imaging abnormalities present only on CT (p=0.001) (GGOs (p=0.004) and reticulations (p=0.001)). Spirometric values improved during follow-up but remained within the normal range at all time-points.
Conclusions
Imaging abnormalities were common 3 months after COVID-19 and their occurrence was significantly related to the presence of dyspnoea. This suggests that CT imaging is a sensitive tool for detecting pulmonary abnormalities in patients with dyspnoea, even in the presence of normal spirometric measurements.
Tweetable abstract
This systematic review found chest imaging abnormalities persist in patients ≥3 months after COVID-19. The prevalence of imaging abnormalities is significantly related to the proportion of patients with dyspnoea, despite normal lung function measurements. https://bit.ly/3MVrkgl
Introduction
Over 500 million people worldwide have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Among survivors, at least 10–20% exhibit “post-acute sequelae of SARS-CoV-2 infection” (PASC) [2, 3]. This often consists of symptoms such as dyspnoea or “brain fog” that persist (≥12 weeks) following an acute bout of coronavirus disease 2019 (COVID-19) [4–7]. For many patients, symptoms may last over 1 year [4, 6, 8, 9]. A recent meta-analysis showed that more than half of hospitalised individuals and about one-third of non-hospitalised patients report persistent symptoms post-COVID-19 [10]. As the most common cause of hospitalisation and emergency room visits is COVID-19 pneumonia, respiratory symptoms such as cough, chest pain or dyspnoea are frequent among patients with PASC [6, 9, 11]. However, in many cases, pulmonary function tests (PFTs) may be completely normal, suggesting that these measurements lack sufficient sensitivity in detecting relevant physiological changes in the lung of patients with PASC [12].
Thoracic imaging tools, such as chest radiography, computed tomography (CT), ultrasound or magnetic resonance imaging (MRI), can be used to non-invasively assess the impact of SARS-CoV-2 infection on the lungs. A number of post-COVID-19 cohort studies have performed pulmonary imaging using a range of modalities, yet there is mixed evidence regarding the frequency and type of imaging abnormalities in post-COVID-19 patients [6, 10]. The primary purpose of this systematic review and meta-analysis was to determine the prevalence of chest imaging abnormalities in COVID-19 patients at follow-up. Specifically, we addressed the following research questions: 1) What is the prevalence of chest imaging abnormalities among COVID-19 survivors 3 months post-infection and beyond? 2) Which specific abnormalities are most common? 3) Does the occurrence of imaging abnormalities relate to patient symptoms such as dyspnoea?
Methods
Search strategy and study selection
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was prospectively registered in the Prospective Register of Systematic Reviews (PROSPERO; CRD42021291415) [13, 14]. The study inclusion criteria were: adult population (≥18 years of age); lung imaging performed at least 3 months (mean or median follow-up of ≥85 days with first quartile or minus one standard deviation of ≥75 days) post-acute COVID-19; cohort studies, case series or case–control studies; and published in a peer-reviewed journal. The exclusion criteria were: paediatric/adolescent populations; studies with uncertain follow-up times; post-mortem or autopsy studies; studies that did not specifically report lung imaging findings; studies that were not available in English; preprints/unpublished studies; and studies that included patients with a secondary pulmonary infection (e.g. fungal infections).
We searched PubMed, Google Scholar and LitCOVID using the terms ((“Post COVID” OR “Long COVID”) AND (“Pulmonary imaging” OR “CT” OR “HRCT” OR “MRI” OR “scintigraphy” OR “ultrasound” OR “radiograph”)). The initial search was performed on 31 October 2021 and was updated on 15 September 2022. Additional records were found by searching the bibliographies of included studies and previously published systematic reviews. All records were managed through Covidence review software [15].
Title/abstract screening, full-text review and data extraction were performed independently and in parallel by two reviewers (E.G. and F.V.G.). Predetermined variables for data extraction included the imaging modality, number of participants imaged, time from COVID-19 diagnosis or hospital discharge, number of participants with imaging abnormalities, types of imaging abnormalities, symptoms and PFT results. We identified potentially overlapping populations based on the study location and recruitment period. In these instances, we included only the study with the most comprehensive imaging data (i.e. both overall and specific imaging abnormalities reported). If both papers were equally comprehensive in their reporting of imaging data, the study with the more comprehensive patient characteristics (e.g. disease severity or lung function) was selected. Conflicts and data discrepancies were resolved by consensus or by referees (R.L.E. and H.L.) when a consensus could not be reached.
The risk of bias in the included studies was assessed using the Clinical Advances through Research and Information Translation (CLARITY) group tool by the authors (E.G. and F.V.G.) [16]. For the purpose of implementing this tool, we considered COVID-19 to be the “exposure” and imaging abnormalities to be the “outcome”. Publication bias was assessed using funnel plots and an Egger's test was used to evaluate funnel plot symmetry [17].
Meta-analysis
We extracted the proportion of study participants with imaging abnormalities at various time-points during follow-up: 1) overall (i.e. at any time-point), 2) early (i.e. 3 months) and 3) late (i.e. a composite of all time-points beyond 3 months of follow-up). In instances where multiple time-points were reported in a study, only the latest time-point was used. In some cases, only participants with an imaging abnormality at an early time-point were re-imaged at a later time-point; since this would bias the prevalence estimates, only the early time-point was included. We used the 3-month time-point as the cut-off based on the World Health Organization (WHO)'s definition of post-COVID-19 condition, which “occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset [of COVID-19]” [4].
To estimate the prevalence of imaging abnormalities among post-COVID-19 patients, we first transformed the individual proportions to better approximate a normal distribution, with normalised and stable variance, using the Freeman–Tukey (double arcsine) transformation [18]. Next, we performed a meta-analysis of proportions using the R package metafor [19]. This type of meta-analysis allows estimation of the prevalence of events while accounting for differences in study sample size and is thus a robust way of determining the overall prevalence of an event [20]. We quantified interstudy heterogeneity with the I2 metric and residual heterogeneity between studies with a Cochran's Q-test [21]. No threshold for I2 was selected as meta-analyses of proportions generally exhibit high heterogeneity owing to differences across studies in design, follow-up time, severity of the acute episode, treatment effects, patient case mix, access to the healthcare system and many other factors [20]. Notwithstanding, to determine the impact of study heterogeneity on the findings, we performed a sensitivity analysis by removing studies in a stepwise fashion until heterogeneity (measured by Cochran's Q-test) was no longer statistically significant. These results are detailed in the supplementary material.
The primary end-point was the prevalence of imaging abnormality during follow-up (overall, early and late). Secondary end-points included the prevalence of specific imaging abnormalities and the change in the prevalence of the imaging abnormality from early to late follow-up, as determined using a restricted maximum likelihood (REML) random effects model in metafor, with the heterogeneity attributed to follow-up time quantified as the model R2 [19]. We also performed subgroup analyses wherein only CT imaging data were included. Meta-regressions were performed to further evaluate the relationship between the occurrence of imaging abnormalities and the proportion of patients with dyspnoea, the proportion of female participants and the average cohort age. If the average cohort age was reported as a median with an interquartile range, this was converted to mean and standard deviation using methods provided by Wan et al. [22]. This approach was also used to convert PFT values, as needed.
We also performed meta-regression to ascertain the impact of follow-up time on the percentage predicted forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio, FEV1, FVC and diffusing capacity of the lung for carbon monoxide (DLCO), using the same definitions of early and late follow-up. These results are presented in the supplementary material (supplementary table S3).
Results
Search
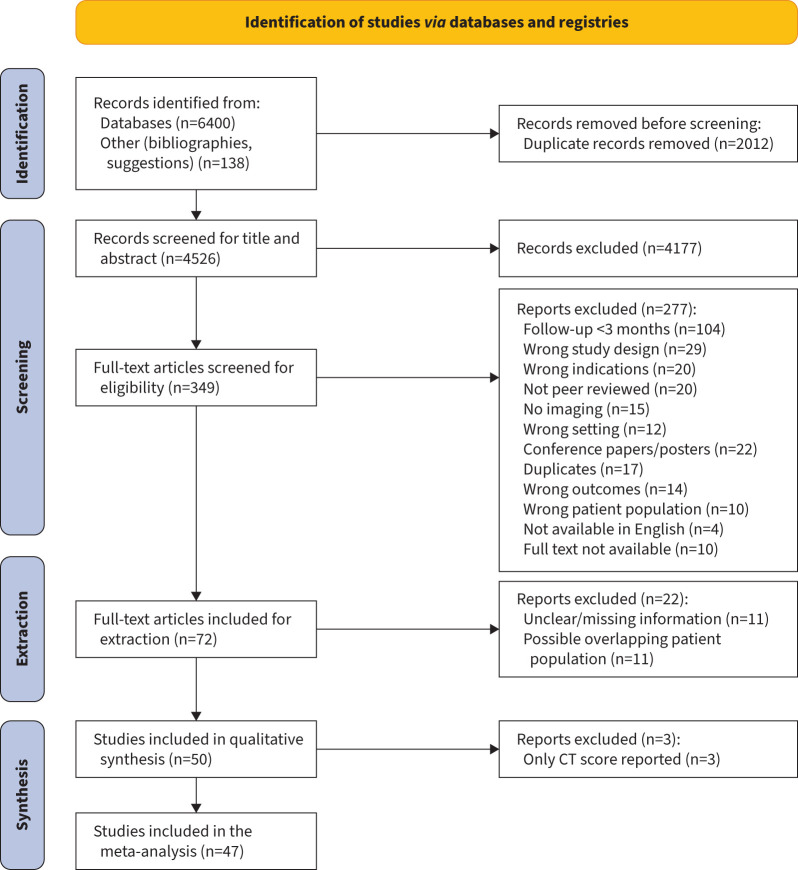
Our search yielded 6538 studies (figure 1). The full text was assessed in 349 studies and 72 were selected for data extraction. After reviewing these papers, 50 were deemed eligible for qualitative assessment [8, 23–71] and 47 were included in the meta-analysis [8, 23, 24, 26–57, 59–63, 65–71]. 11 studies which had potentially overlapping patient populations [72–83] were removed. A summary of the included studies is shown in supplementary table S1.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. CT: computed tomography.
Characteristics of the included studies
Most of the included studies were from Europe (n=28) or Asia (n=17). Generally, studies had more male (56%) participants than females (44%). All studies included hospitalised patients and four studies included non-hospitalised individuals [37, 41, 57, 66]. If severity of the acute infection was reported, it was typically done using qualitative classifiers: “moderate”, “severe” and “critical”, although the definitions varied between studies [37, 39, 45, 62, 65, 66].
23 studies imaged patients in early (3 months) follow-up and 24 studies imaged patients at late (>3 months) follow-up. Only four studies used non-CT-based technology (ultrasound, MRI, plain radiography or scintigraphy) [30, 36, 53, 71]. The study that employed scintigraphy also performed CT in the same patients [53]. The studies used various definitions for imaging abnormalities, but many used the Fleischner Society guidelines and associated terminology for CT abnormalities [84]. PFT data were available in 26 studies; however, only seven studies reported the relationship between PFT measurements and imaging abnormalities [23, 31, 46, 48, 63, 65, 72].
Prevalence of imaging abnormalities at follow-up
38 studies reported the total number of patients with any abnormal imaging findings (supplementary table S1). The prevalence of imaging abnormalities was: 63.9% (95% CI 56.9–70.6%) overall (i.e. at any time-point), 67.2% (95% CI 56.8–76.8%) in early follow-up and 60.8% (95% CI 51.3–70.0%) in late follow-up (table 1). The early follow-up time-point had a significant Egger's test (p=0.047), raising the possibility of publication bias. There was significant interstudy heterogeneity for all three analyses (I2=93.43%, 93.11% and 93.55%, respectively; Cochrane's Q-test p<0.001) (supplementary figure S2). Although the prevalence estimates decreased between early and late follow-up, this was not significant in the REML model (p=0.37) and 0.0% of the heterogeneity was attributable to the follow-up time (table 1).
TABLE 1.
Total and specific chest imaging abnormalities during follow-up
| Abnormality | Patients evaluated at each time-point, n | Prevalence of imaging abnormalities, % (95% CI) | Change from early to late follow-up, p-value + | Heterogeneity attributable to follow-up time § , % | ||
| Overall | Early follow-up # | Late follow-up ¶ | ||||
| Any abnormality | 3160 (total) 1867 (early follow-up) 1295 (late follow-up) |
63.9 (56.9–70.6) | 67.2 (56.8–76.8) | 60.8 (51.3–70.0) | 0.37 | 0.0 |
| Any abnormality (CT imaging subgroup) | 2817 (total) 1036 (early follow-up) 1781 (late follow-up) |
62.8 (55.9–69.4) | 68.1 (58.4–77.1) | 57.9 (48.5–67.0) | 0.13 | 3.2 |
| Ground-glass opacities | 2659 (total) 938 (early follow-up) 1721 (late follow-up) |
44.9 (37.0–52.9) | 52.8 (38.8–66.5) | 37.5 (30.7–44.6) | 0.049 | 8.5 |
| Reticulations | 1897 (total) 394 (early follow-up) 1503 (late follow-up) |
21.2 (12.9–30.9) | 38.2 (24.4–53.0) | 14.3 (6.63–24.2) | 0.01 | 22 |
| Bronchiectasis | 1732 (total) 602 (early follow-up) 1070 (late follow-up) |
16.8 (9.10–26.1) | 27.0 (9.78–48.7) | 11.6 (5.29–19.7) | 0.09 | 9.8 |
| Consolidations | 1752 (total) 574 (early follow-up) 1178 (late follow-up) |
6.50 (3.76–9.83) | 7.57 (3.61–12.6) | 5.70 (2.23–10.4) | 0.48 | 0.0 |
| Fibrosis | 2091 (total) 1016 (early follow-up) 1075 (late follow-up) |
27.8 (17.9–39.0) | 22.9 (10.9–37.6) | 33.6 (18.3–50.8) | 0.33 | 0.01 |
CT: computed tomography. #: 3 months since acute infection; ¶: >3 months since acute infection; +: p-value from random effects model; §: R2 from random effects model.
In subgroup analyses with only CT imaging data [8, 23, 24, 26–29, 34, 35, 38–42, 45–51, 53, 55–57, 59–63, 65, 66, 68, 70], the prevalence of any imaging abnormalities was: 62.8% (95% CI 55.9–69.4%) overall, 68.1% (95% CI 58.4–77.1%) in early follow-up and 57.9% (95% CI 48.5–67.0%) in late follow-up (table 1). There was significant heterogeneity between the studies (I2=91.97%, 90.23% and 93.05%, respectively; Cochran's Q-test p<0.001 for each) (supplementary figure S5). There was no significant decrease in the prevalence of CT imaging abnormalities from early to late follow-up (p=0.13) and 3.2% of the heterogeneity in the findings was attributable to the follow-up time (table 1).
Prevalence of specific imaging abnormalities at follow-up
The most commonly reported imaging abnormalities were ground-glass opacities (GGOs) (37 studies), reticulations (22 studies), consolidations (22 studies), fibrosis (21 studies) and bronchiectasis (19 studies) (supplementary table S1).
The specific imaging abnormality with the highest prevalence was GGOs. The overall prevalence was 44.9% (95% CI 37.0–52.9%), the prevalence at early follow-up was 52.8% (95% CI 38.8–66.5%) and the prevalence at late follow-up was 37.5% (95% CI 30.7–44.6%) (table 1), with significant interstudy heterogeneity (I2=94.22%, 95.954% and 88.11%, respectively; Cochran's Q-test p<0.01 for each) (supplementary figure S3). The change in prevalence over time was significant (p=0.049) and the heterogeneity attributed to follow-up time was 8.5% (table 1).
The specific imaging abnormality with the greatest change from early to late follow-up was reticulation. The overall prevalence was relatively low (21.2% (95% CI 12.9–30.9%)) (table 1). However, there was a relatively high prevalence at early follow-up of 38.2% (95% CI 24.4–53.0%), which decreased by more than half at late follow-up to 14.3% (95% CI 6.63–24.2%) (table 1): the REML model was significant (p=0.01) and follow-up time accounted for 22% of the heterogeneity (supplementary figure S4). However, there was significant interstudy heterogeneity in each of the analyses (I2=95.15%, 93.05% and 95.75%, respectively; Cochran's Q-test p<0.001 for each) (supplementary figure S4).
In contrast to reticulations, the occurrence of fibrosis did not significantly change over time: overall prevalence 27.8% (95% CI 17.9–39.0%), early follow-up prevalence 22.9% (95% CI 10.9–37.6%) and late follow-up prevalence 33.6% (95% CI 18.3–50.8%); REML model p=0.33 and 0.01% of the heterogeneity was attributable to the follow-up time (table 1). Both the 3-month follow-up and the total proportion of patients with fibrosis had significant Egger's test values (p=0.0068 and 0.0047, respectively), which may indicate considerable publication bias. Heterogeneity was significant by Cochrane's Q-test (p<0.01) and I2=96.52%, 96.14% and 96.87%, respectively (supplementary figure S7). The changes in prevalence for bronchiectasis and consolidations were not significant, and their changes in proportion, I2 and R2 are reported in table 1 and supplementary figures S4 and S5. Consolidations had significant publication bias (Egger's test p=0.0342) at the 3-month follow-up, which may result in an inflated proportion.
Relationship between the prevalence of imaging abnormalities and dyspnoea
16 studies reported dyspnoea [23, 26, 27, 30, 32, 35, 36, 51, 53, 57, 61–63, 67, 68, 70]. Of these, 14 also reported patients with any imaging abnormalities, across all modalities (supplementary table S1). The mixed effects model of these studies demonstrated I2=92.15%; 28% of the heterogeneity in the proportion of patients with imaging abnormalities was attributable to the occurrence of dyspnoea, and this relationship was significant and positive (p=0.012) (figure 2). Furthermore, 14 studies that included dyspnoea also reported the occurrence of GGOs [23, 26, 27, 32, 35, 51, 53, 57, 61–63, 67, 68, 70] (supplementary table S1). The residual heterogeneity was I2=93.97% with R2=0.0% (supplementary table S2). This relationship was not significant (p=0.47). Fewer than 10 papers reported on the presence of bronchiectasis, consolidations, reticulations and fibrosis alongside dyspnoea, thus we did not run the model for these abnormalities.
FIGURE 2.
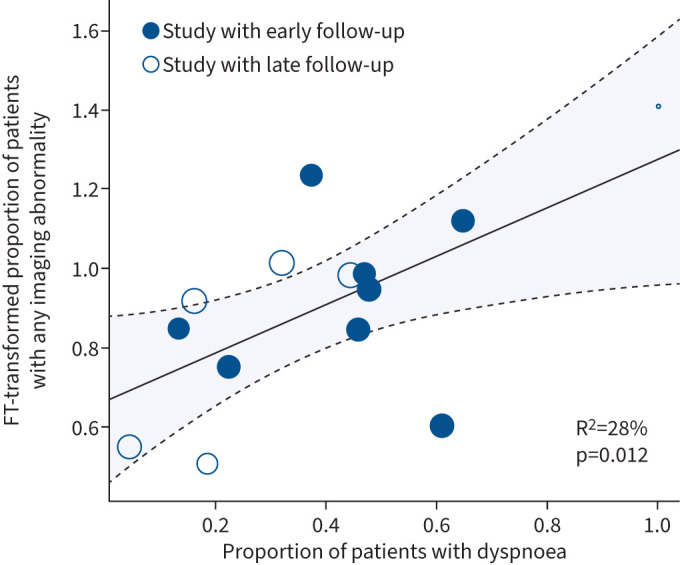
Meta-regression between proportion of subjects with any imaging abnormalities and proportion of subjects with dyspnoea. FT: Freeman–Tukey. The size of the circle is proportional to the size of the patient cohort.
Spirometric values remained within the normal range at all time-points (supplementary material and supplementary table S3). Moreover, neither FEV1 nor DLCO significantly correlated with dyspnoea (supplementary material).
Relationship between the prevalence of imaging abnormalities and sex
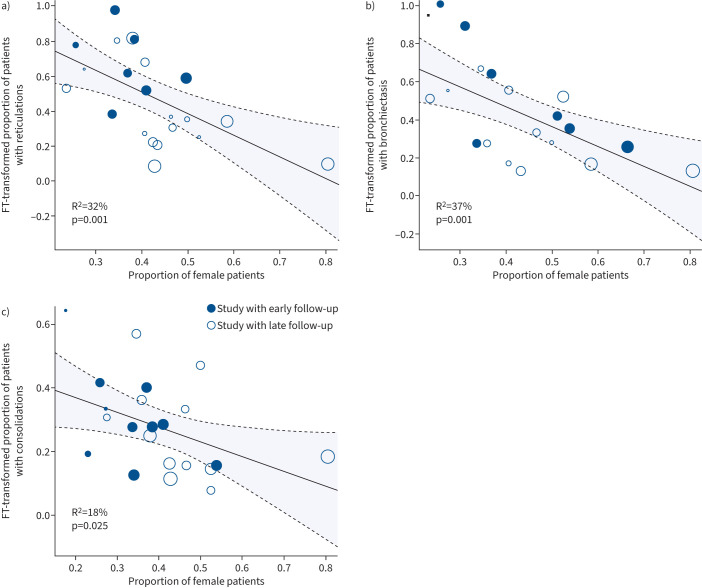
All studies reported the number of female participants in their cohort. The proportion of female participants was not significantly related to the occurrence of any abnormalities or CT-specific abnormalities such as GGOs and fibrosis (supplementary table S2). However, the occurrence of reticulations, bronchiectasis and consolidations was negatively correlated with the percentage of female participants in a cohort (figure 3a–c). The interstudy heterogeneity was high, with I2 ranging between 80% and 97%.
FIGURE 3.
Meta-regression between proportion of subjects with imaging abnormalities and proportion of female subjects. Meta-regression analysis between female sex in cohort and proportion of patients with a) reticulations, b) bronchiectasis and c) consolidations. FT: Freeman–Tukey. The size of the circle is proportional to the size of the patient cohort.
Relationship between the prevalence of imaging abnormalities and age
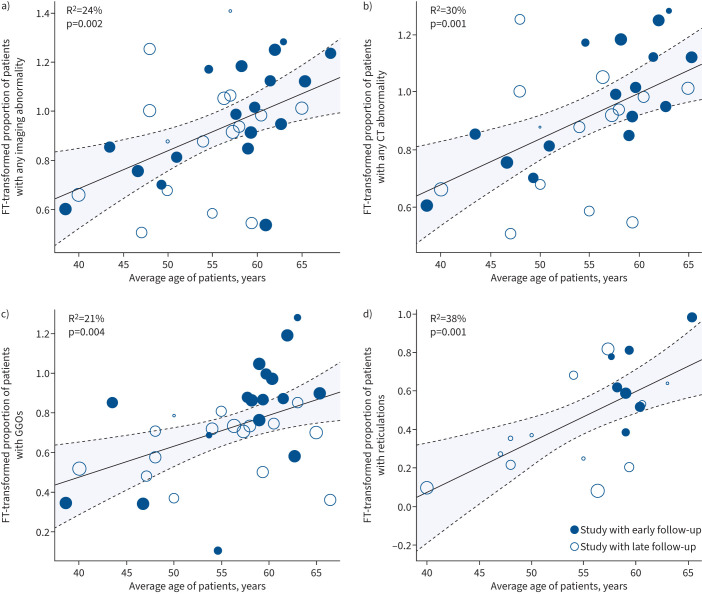
While all studies reported age, only a subset (41 studies) reported the age of the entire cohort. Of these 41 studies, 34 reported the proportion of patients with any abnormality. The interstudy heterogeneity was I2=92.36% and R2=24% (supplementary table S2). The relationship between age and any abnormalities across all modalities was significant (p=0.002) (figure 4a). This was also observed when we restricted the analysis to the 30 studies that used CT imaging (figure 4b). The interstudy heterogeneity was I2=93.56% and R2=30% (supplementary table S2). The relationship was significant and positive (p=0.001).
FIGURE 4.
Meta-regression between proportion of subjects with imaging abnormalities and mean age of cohort. Meta-regression analysis between mean age in cohort and proportion of patients with a) any abnormality, b) only computed tomography (CT) abnormalities, c) ground-glass opacities (GGOs), d) reticulations. FT: Freeman–Tukey. The size of the circle is proportional to the size of the patient cohort.
For specific abnormalities, 33 studies reported mean age along with GGOs, 19 with reticulations, 20 with consolidations, 19 with fibrosis and 15 with bronchiectasis. The interstudy heterogeneity (I2) ranged between 80% and 97%. Both GGOs and reticulations were significantly correlated with mean age of the study, with a respective R2 of 21% and 38% (p=0.004 and 0.001, respectively) (supplementary table S2, and figure 4c and d). The other abnormalities were not significantly correlated with mean cohort age.
Bias assessment
Using the CLARITY bias assessment tool, most studies were found to be at a moderate risk of bias (supplementary figure S1). To confirm the diagnosis of COVID-19 (i.e. the exposure), many studies relied on the widely used real-time PCR. When assessing the outcome of interest (imaging abnormalities), only a few studies excluded patients with pre-existing pulmonary disease (such as asthma or COPD) [23, 31, 43, 45, 50, 55, 62, 63]. This is a potential source of bias since the outcome of interest may have been present before the exposure (i.e. imaging abnormalities may be related to comorbid pulmonary disease, not COVID-19). Additionally, treatments (co-interventions) and comorbidities were not always reported and often varied across patients. Most of this information was obtained from electronic medical records. Images were typically interpreted by two or more experts, usually radiologists, often blinded to the clinical data [8, 23, 26–28, 31, 32, 34, 40, 43, 46–49, 52, 54, 55, 60–63, 66]; 14 studies used computer software for interpretation in addition to human expertise [23, 27, 32, 35, 37, 39–41, 44, 46, 47, 55, 66, 70]. Most of the abnormalities did not exhibit significant publication bias. Funnel plots are presented in supplementary figure S9.
Discussion
Even as vaccination rates increase, the risk of symptoms continuing well past the clearance of the acute SARS-CoV-2 infection remains to be evaluated [1]. Post-COVID-19 condition affects 10–20% of individuals depending on the population studied and the definition used, with ∼12% of these patients not being able to return to work after a SARS-CoV-2 infection [2, 3, 40]. Our systematic review demonstrates that imaging abnormalities are common among those previously infected with SARS-CoV-2. Additionally, we found that while some abnormalities resolved with time, others did not. The persistence of these imaging abnormalities (especially GGOs) was significantly related to the presence of post-COVID-19 pulmonary symptoms such as dyspnoea over time. Notably, we found that FEV1 and DLCO improved significantly over time following a SARS-CoV-2 infection and were often within the normal range in post-COVID-19 patients, which reduces their clinical or diagnostic utility.
Our analysis included any imaging modality at up to two time-points across over 40 studies. In contrast, previous systematic reviews on this topic have focused only on thoracic CT findings [85, 86]. Our findings are in keeping with a recent systematic review by Fabbri et al. [86], which found that combined “inflammatory” imaging abnormalities (defined as GGOs or consolidations) decreased in prevalence over time, while combined “fibrotic” imaging abnormalities (defined as reticulations, lung architectural distortion, interlobular septal thickening, traction bronchiectasis or honeycombing) did not. We extend these findings by showing that reticulations, which were grouped as part of “fibrotic” changes in Fabbri et al. [86], showed the greatest reduction in prevalence over time, suggesting that “reticulations” are not precursors to fibrosis or part of its spectrum in the context of post-acute COVID-19. Moreover, unlike the previous review by Fabbri et al. [86], our study included both hospitalised and non-hospitalised patients, which better reflects the broad spectrum of disease severity in the community. We also extend the findings of the previous reviews by demonstrating a significant link between the occurrence of CT changes (especially GGOs) and the prevalence of dyspnoea across studies. These data raise the possibility that these CT changes may be a biomarker for post-COVID-19 disease, especially among those who have persistent dyspnoea. Interestingly, female sex, which is considered a risk factor for post-COVID-19 disease, was negatively associated with certain specific imaging abnormalities, notably reticulations, bronchiectasis and consolidations [6, 74]. In contrast, older age was significantly correlated with imaging abnormalities. Both of these features (male sex and older age) are associated with increased disease severity [87]. We therefore posit that increased disease severity may be a factor in certain imaging abnormalities. It also indicates that older males with dyspnoea may be prioritised for CT imaging after COVID-19. However, it should be noted that CT exposes patients to ionising radiation, thus a cost–benefit assessment for each individual should be performed before use [88]. Further studies will be required to elucidate the molecular and histological features of these imaging abnormalities and their importance in the pathogenesis of post-COVID-19 disease.
Our study had several limitations. First, our search terms were very specific and may have unintentionally excluded studies which did not use this exact terminology. Second, we included studies even if they had included patients with pre-existing pulmonary diseases even though they were often unaccounted for in the determination of the prevalence of imaging abnormalities. Additionally, many studies included both long COVID (symptomatic) and non-long COVID patients and did not stratify imaging findings based on symptoms. Therefore, we could not assess imaging in long COVID patients exclusively and could only use the post-COVID-19 designation. There was also a scarcity of non-hospitalised patients in our cohort due to data availability and our overall prevalence estimates are thus more driven predominantly by the hospitalised population. Of the four studies that reported on mild patients, only one provided data on dyspnoea symptoms as well; thus, we could not evaluate the relationship between dyspnoea and disease severity [57]. Moreover, the distribution of time-points was heavily skewed towards the earlier time-point (3 months). For this reason, we did not perform a meta-regression based on follow-up time and chose to split our data as early (3 months) and late (>3 months). While our study looked at all imaging modalities, there was under-representation of less common imaging methods, such as lung ultrasound and scintigraphy. Thus, we were unable to assess changes in time or risk factors associated with abnormalities for those specific modalities. Finally, interstudy heterogeneity (I2) was high at all time-points, as expected. The heterogeneity likely comes from the fact that the data were collected by different study centres, many with different protocols, and which experienced different outcomes of the COVID-19 pandemic. The impact of heterogeneity on the overall findings was mitigated by using a random effects model. Moreover, we performed a sensitivity analysis by removing in a stepwise fashion studies that contributed significantly to the heterogeneity and found that our findings did not materially change [20].
In summary, we show here that: 1) imaging abnormalities are relatively common in post-COVID-19 patients; 2) some imaging abnormalities may resolve with time while others may persist; 3) the prevalence of imaging abnormalities is significantly linked to the proportion of patients with dyspnoea; 4) older age and male sex are significant risk factors for persistent imaging abnormalities after COVID-19; and 5) while spirometric values generally improve during follow-up, they are typically within the normal range at all time-points, which reduces their clinical or diagnostic utility. These findings indicate that persistent pulmonary symptoms post-acute COVID-19 may be related to GGOs and other changes in thoracic imaging. Future work is needed to understand the pathophysiology and clinical relevance of these imaging changes on chest imaging. Moreover, with the growing number of patients with PASC in the community, there is a pressing need to understand the mechanisms of dyspnoea in the post-COVID-19 setting and the impact of both acute and chronic treatments on patient symptoms and their imaging abnormalities.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0253-2022.SUPPLEMENT (2.4MB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Availability of data and materials: All underlying data analysed in this work were extracted from published materials. Full summary data will be made available immediately following publication to any investigators and for any purpose, upon reasonable request. Proposals should be made directly to the corresponding author.
Author contributions: E. Guinto, F.V. Gerayeli, H. Lee and R.L. Eddy participated in the systematic review process. E. Guinto and F.V. Gerayeli extracted the data. E. Guinto performed the analysis. R.L. Eddy, S. Milne and D.D. Sin reviewed and interpreted results. E. Guinto wrote the first draft of the manuscript. R.L. Eddy verified the underlying data. All authors reviewed and edited the manuscript and approved the final draft. All authors had full access to the data.
Conflict of interest: R.L. Eddy receives personal consulting fees from VIDA Diagnostics Inc., and lecture honoraria from AstraZeneca, outside the submitted work. S. Milne reports grants from British Columbia Lung Association, CHEST Foundation, Genome British Columbia, Mitacs and Michael Smith Health Research BC, and financial support from Chiesi Australia, outside the submitted work. D.D. Sin has received an honorarium for speaking engagements for COPD from GSK, AstraZeneca and Boehringer Ingelheim, and chairs a DSMB for an NHLBI-sponsored clinical trial in COPD, outside the submitted work. E. Guinto, F.V. Gerayeli and H. Lee do not declare any conflicts of interest.
Support statement: R.L. Eddy is supported by a Michael Smith Health Research BC Trainee Award and Canadian Respiratory Research Network fellowship. S. Milne is supported by Mitacs and a Michael Smith Health Research BC Trainee Award. F.V. Gerayeli is supported by Mitacs. E. Guinto is supported by Mitacs. D.D. Sin holds a Tier 1 Canada Research Chair in COPD and the de Lazzari Family Chair at the Centre for Heart Lung Innovation.
References
- 1.World Health Organization . WHO coronavirus (COVID-19) dashboard. 2022. https://covid19.who.int Date last accessed: 5 July 2022.
- 2.Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. 2022. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6may2022 Date last accessed: 16 July 2022.
- 3.World Health Organization . Coronavirus disease (COVID-19): post COVID-19 condition. 2022. www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition Date last accessed: 16 July 2022.
- 4.Soriano JB, Murthy S, Marshall JC, et al. . A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022; 22: e102–e107. doi: 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen NN, Hoang VT, Dao TL, et al. . Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: a systematic review. Eur J Clin Microbiol Infect Dis 2022; 41: 515–545. doi: 10.1007/s10096-022-04417-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelen M, Manoharan L, Elkheir N, et al. . Characterising long COVID: a living systematic review. BMJ Glob Health 2021; 6: e005427. doi: 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. . Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health 2021; 18: 2621. doi: 10.3390/ijerph18052621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Yang C, An X, et al. . Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis 2021; 112: 173–182. doi: 10.1016/j.ijid.2021.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crook H, Raza S, Nowell J, et al. . Long covid – mechanisms, risk factors, and management. BMJ 2021; 374: n1648. doi: 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Haupert SR, Zimmermann L, et al. . Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis 2022; 226: jiac136. doi: 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George PM, Barratt SL, Condliffe R, et al. . Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020; 75: 1009–1016. doi: 10.1136/thoraxjnl-2020-215314 [DOI] [PubMed] [Google Scholar]
- 12.Akbarialiabad H, Taghrir MH, Abdollahi A, et al. . Long COVID, a comprehensive systematic scoping review. Infection 2021; 49: 1163–1186. doi: 10.1007/s15010-021-01666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q 2019; 38: 171–180. doi: 10.1080/02763869.2019.1588072 [DOI] [PubMed] [Google Scholar]
- 15.Covidence . Better systematic review management. 2022. www.covidence.org Date last accessed: 16 May 2022.
- 16.CLARITY Group at McMaster University . Tool to assess risk of bias in case control studies. 2017. www.evidencepartners.com/wp-content/uploads/2017/09/Tool-to-Assess-Risk-of-Bias-in-Case-Control-Studies.pdf Date last accessed: 20 June 2023.
- 17.Egger M, Smith GD, Schneider M, et al. . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat 1978; 32: 138–138. doi: 10.1080/00031305.1978.10479283 [DOI] [Google Scholar]
- 19.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 20.Barker TH, Migliavaca CB, Stein C, et al. . Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol 2021; 21: 189. doi: 10.1186/s12874-021-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10: 101–129. doi: 10.2307/3001666 [DOI] [Google Scholar]
- 22.Wan X, Wang W, Liu J, et al. . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balbi M, Conti C, Imeri G, et al. . Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients. Eur J Radiol 2021; 138: 109676. doi: 10.1016/j.ejrad.2021.109676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardakci MI, Ozturk EN, Ozkarafakili MA, et al. . Evaluation of long-term radiological findings, pulmonary functions, and health-related quality of life in survivors of severe COVID-19. J Med Virol 2021; 93: 5574–5581. doi: 10.1002/jmv.27101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellan M, Baricich A, Patrucco F, et al. . Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci Rep 2021; 11: 22666. doi: 10.1038/s41598-021-01215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao J, Zheng X, Wei W, et al. . Three-month outcomes of recovered COVID-19 patients: prospective observational study. Ther Adv Respir Dis 2021; 15: 17534666211009410. doi: 10.1177/17534666211009410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso D, Guido G, Zerunian M, et al. . Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology 2021; 301: E396–E405. doi: 10.1148/radiol.2021210834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Ding C, Yu L, et al. . One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med 2021; 19: 191. doi: 10.1186/s12916-021-02056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai S, Zhao B, Liu D, et al. . Follow-up study of the cardiopulmonary and psychological outcomes of COVID-19 survivors six months after discharge in Sichuan, China. Int J Gen Med 2021; 14: 7207–7217. doi: 10.2147/IJGM.S337604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortini A, Torrigiani A, Sbaragli S, et al. . COVID-19: persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection 2021; 49: 1007–1015. doi: 10.1007/s15010-021-01638-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frija-Masson J, Debray MP, Boussouar S, et al. . Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: the post Covid M3 study. Respir Med 2021; 184: 106435. doi: 10.1016/j.rmed.2021.106435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froidure A, Mahsouli A, Liistro G, et al. . Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir Med 2021; 181: 106383. doi: 10.1016/j.rmed.2021.106383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamberini L, Mazzoli CA, Prediletto I, et al. . Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study. Respir Med 2021; 189: 106665. doi: 10.1016/j.rmed.2021.106665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianella P, Rigamonti E, Marando M, et al. . Clinical, radiological and functional outcomes in patients with SARS-CoV-2 pneumonia: a prospective observational study. BMC Pulm Med 2021; 21: 136. doi: 10.1186/s12890-021-01509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez J, Benitez ID, Carmona P, et al. . Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest 2021; 160: 187–198. doi: 10.1016/j.chest.2021.02.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grist JT, Chen M, Collier GJ, et al. . Hyperpolarized 129Xe MRI abnormalities in dyspneic patients 3 months after COVID-19 pneumonia: preliminary results. Radiology 2021; 301: E353–E360. doi: 10.1148/radiol.2021210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guler SA, Ebner L, Aubry-Beigelman C, et al. . Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellemons ME, Huijts S, Bek LM, et al. . Persistent health problems beyond pulmonary recovery up to 6 months after hospitalization for COVID-19: a longitudinal study of respiratory, physical, and psychological outcomes. Ann Am Thorac Soc 2021; 19: 551–561. doi: 10.1513/AnnalsATS.202103-340OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C, Zeng JP, Peng K, et al. . Clinical features and temporal lung radiographic changes in 25 patients recovering from COVID-19 pneumonia: a retrospective case-control study. Med Sci Monit 2021; 27: e933381. doi: 10.12659/MSM.933381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2023; 401: E21–E33. doi: 10.1016/S0140-6736(23)00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnsen S, Sattler SM, Miskowiak KW, et al. . Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res 2021; 7: 00205-2021. doi: 10.1183/23120541.00205-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labarca G, Henriquez-Beltran M, Lastra J, et al. . Analysis of clinical symptoms, radiological changes and pulmonary function data 4 months after COVID-19. Clin Respir J 2021; 15: 992–1002. doi: 10.1111/crj.13403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerum TV, Aalokken TM, Bronstad E, et al. . Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021; 75: 2003448. doi: 10.1183/13993003.03448-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Shen C, Wang L, et al. . Pulmonary fibrosis and its related factors in discharged patients with new corona virus pneumonia: a cohort study. Respir Res 2021; 22: 203. doi: 10.1186/s12931-021-01798-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao T, Meng D, Xiong L, et al. . Long-term effects of COVID-19 on health care workers 1-year post-discharge in Wuhan. Infect Ther 2021; 11: 145–163. doi: 10.1007/s40121-021-00553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Lv F, Huang Y, et al. . Follow-up study of the chest CT characteristics of COVID-19 survivors seven months after recovery. Front Med Lausanne 2021; 8: 636298. doi: 10.3389/fmed.2021.636298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luger AK, Sonnweber T, Gruber L, et al. . Chest CT of lung injury 1 year after COVID-19 pneumonia: the CovILD study. Radiology 2022; 304: 211670. doi: 10.1148/radiol.211670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGroder CF, Zhang D, Choudhury MA, et al. . Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021; 76: 1242–1245. doi: 10.1136/thoraxjnl-2021-217031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miwa M, Nakajima M, Kaszynski RH, et al. . Abnormal pulmonary function and imaging studies in critical COVID-19 survivors at 100 days after the onset of symptoms. Respir Investig 2021; 59: 614–621. doi: 10.1016/j.resinv.2021.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohr A, Dannerbeck L, Lange TJ, et al. . Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidiscip Respir Med 2021; 16: 732. doi: 10.4081/mrm.2021.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mumoli N, Bonaventura A, Colombo A, et al. . Lung function and symptoms in post-COVID-19 patients: a single-center experience. Mayo Clin Proc Innov Qual Outcomes 2021; 5: 907–915. doi: 10.1016/j.mayocpiqo.2021.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nabahati M, Ebrahimpour S, Khaleghnejad Tabari R, et al. . Post-COVID-19 pulmonary fibrosis and its predictive factors: a prospective study. Egypt J Radiol Nucl Med 2021; 52: 248. doi: 10.1186/s43055-021-00632-9 [DOI] [Google Scholar]
- 53.Noel-Savina E, Viatge T, Faviez G, et al. . Severe SARS-CoV-2 pneumonia: clinical, functional and imaging outcomes at 4 months. Respir Med Res 2021; 80: 100822. doi: 10.1016/j.resmer.2021.100822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Núñez-Fernández M, Ramos-Hernández C, García-Río F, et al. . Alterations in respiratory function test three months after hospitalisation for COVID-19 pneumonia: value of determining nitric oxide diffusion. J Clin Med 2021; 10: 2119. doi: 10.3390/jcm10102119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remy-Jardin M, Duthoit L, Perez T, et al. . Assessment of pulmonary arterial circulation 3 months after hospitalization for SARS-CoV-2 pneumonia: dual-energy CT (DECT) angiographic study in 55 patients. EClinicalMedicine 2021; 34: 100778. doi: 10.1016/j.eclinm.2021.100778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robey RC, Kemp K, Hayton P, et al. . Pulmonary sequelae at 4 months after COVID-19 infection: a single-centre experience of a COVID follow-up service. Adv Ther 2021; 38: 4505–4519. doi: 10.1007/s12325-021-01833-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skala M, Svoboda M, Kopecky M, et al. . Heterogeneity of post-COVID impairment: interim analysis of a prospective study from Czechia. Virol J 2021; 18: 73. doi: 10.1186/s12985-021-01546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stylemans D, Smet J, Hanon S, et al. . Evolution of lung function and chest CT 6 months after COVID-19 pneumonia: real-life data from a Belgian university hospital. Respir Med 2021; 182: 106421. doi: 10.1016/j.rmed.2021.106421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Truffaut L, Demey L, Bruyneel AV, et al. . Post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res 2021; 22: 29. doi: 10.1186/s12931-021-01625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Gassel RJJ, Bels JLM, Raafs A, et al. . High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med 2020; 203: 371–374. doi: 10.1164/rccm.202010-3823LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vijayakumar B, Tonkin J, Devaraj A, et al. . CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology 2021; 303: 444–454. doi: 10.1148/radiol.2021211746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Q, Zhong L, Li H, et al. . A follow-up study of lung function and chest computed tomography at 6 months after discharge in patients with coronavirus disease 2019. Can Respir J 2021; 2021: 6692409. doi: 10.1155/2021/6692409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu X, Liu X, Zhou Y, et al. . 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med 2021; 9: 747–754. doi: 10.1016/S2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, Zhou M, Luo P, et al. . Plasma metabolomic profiling of patients recovered from coronavirus disease 2019 (COVID-19) with pulmonary sequelae 3 months after discharge. Clin Infect Dis 2021; 73: 2228–2239. doi: 10.1093/cid/ciab147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang S, Bai W, Yue J, et al. . Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep 2021; 11: 13854. doi: 10.1038/s41598-021-93191-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou M, Xu J, Liao T, et al. . Comparison of residual pulmonary abnormalities 3 months after discharge in patients who recovered from COVID-19 of different severity. Front Med 2021; 8: 682087. doi: 10.3389/fmed.2021.682087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martino GP, Benfaremo D, Bitti G, et al. . 6 and 12 month outcomes in patients following COVID-19-related hospitalization: a prospective monocentric study. Intern Emerg Med 2022; 17: 1641–1649. doi: 10.1007/s11739-022-02979-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jutant EM, Meyrignac O, Beurnier A, et al. . Respiratory symptoms and radiological findings in post-acute COVID-19 syndrome. ERJ Open Res 2022; 8: 00479-2021. doi: 10.1183/23120541.00479-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muhammad S, Solangi FA, Salam A, et al. . Analysis of predictive factors of post-COVID-19 associated pulmonary fibrosis: a longitudinal study. Pak J Med Health Sci 2022; 16: 1178. doi: 10.53350/pjmhs221651178 [DOI] [Google Scholar]
- 70.Holdsworth DA, Chamley R, Barker-Davies R, et al. . Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLoS One 2022; 17: e0267392. doi: 10.1371/journal.pone.0267392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gurbani N, Acosta-Sorensen M, Díaz-Pérez D, et al. . Clinical outcomes and lung ultrasound findings in COVID-19 follow up: calm comes after the storm? Respir Med Res 2022; 82: 100907. doi: 10.1016/j.resmer.2022.100907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.The Writing Committee for the COMEBAC Study Group . Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325: 1525–1534. doi: 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han X, Fan Y, Alwalid O, et al. . Six-month follow-up Chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299: E177–E186. doi: 10.1148/radiol.2021203153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang L, Yao Q, Gu X, et al. . 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398: 747–758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang L, Yang B, Jiang N, et al. . Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35: e418. doi: 10.3346/jkms.2020.35.e418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu T, Wu D, Yan W, et al. . Twelve-month systemic consequences of coronavirus disease 2019 (COVID-19) in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis 2022; 74: 1953–1965. doi: 10.1093/cid/ciab703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan F, Yang L, Liang B, et al. . Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology 2022; 302: 709–719. doi: 10.1148/radiol.2021211199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sonnweber T, Sahanic S, Pizzini A, et al. . Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J 2021; 57: 2003481. doi: 10.1183/13993003.03481-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou M, Yin Z, Xu J, et al. . Inflammatory profiles and clinical features of coronavirus 2019 survivors 3 months after discharge in Wuhan, China. J Infect Dis 2021; 224: 1473–1488. doi: 10.1093/infdis/jiab181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonzalez J, Zuil M, Benitez ID, et al. . One year overview and follow-up in a post-COVID consultation of critically ill patients. Front Med 2022; 9: 897990. doi: 10.3389/fmed.2022.897990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerget B, Çelik E, Kerget F, et al. . Evaluation of 3-month follow-up of patients with postacute COVID-19 syndrome. J Med Virol 2022; 94: 2026–2034. doi: 10.1002/jmv.27579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang L, Li X, Gu X, et al. . Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022; 10: 863–876. doi: 10.1016/S2213-2600(22)00126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corsi A, Caroli A, Bonaffini PA, et al. . Structural and functional pulmonary assessment in severe COVID-19 survivors at 12 months after discharge. Tomography 2022; 8: 2588–2603. doi: 10.3390/tomography8050216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hansell DM, Bankier AA, MacMahon H, et al. . Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 85.Watanabe A, So M, Iwagami M, et al. . One-year follow-up CT findings in COVID-19 patients: a systematic review and meta-analysis. Respirology 2022; 27: 605–616. doi: 10.1111/resp.14311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fabbri L, Moss S, Khan FA, et al. . Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax 2022; 78: 191–201. doi: 10.1136/thoraxjnl-2021-218275 [DOI] [PubMed] [Google Scholar]
- 87.Gao YD, Ding M, Dong X, et al. . Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 2021; 76: 428–455. doi: 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 88.Sarma A, Heilbrun ME, Conner KE, et al. . Radiation and chest CT scan examinations: what do we know? Chest 2012; 142: 750–760. doi: 10.1378/chest.11-2863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0253-2022.SUPPLEMENT (2.4MB, pdf)