Abstract
Background
Digital therapeutics are patient-facing digital health interventions that can significantly alter the health care landscape. Despite digital therapeutics being used to successfully treat a range of conditions, their uptake in health systems remains limited. Understanding the full spectrum of uptake factors is essential to identify ways in which policy makers and providers can facilitate the adoption of effective digital therapeutics within a health system, as well as the steps developers can take to assist in the deployment of products.
Objective
In this review, we aimed to map the most frequently discussed factors that determine the integration of digital therapeutics into health systems and practical use of digital therapeutics by patients and professionals.
Methods
A scoping review was conducted in MEDLINE, Web of Science, Cochrane Database of Systematic Reviews, and Google Scholar. Relevant data were extracted and synthesized using a thematic analysis.
Results
We identified 35,541 academic and 221 gray literature reports, with 244 (0.69%) included in the review, covering 35 countries. Overall, 85 factors that can impact the uptake of digital therapeutics were extracted and pooled into 5 categories: policy and system, patient characteristics, properties of digital therapeutics, characteristics of health professionals, and outcomes. The need for a regulatory framework for digital therapeutics was the most stated factor at the policy level. Demographic characteristics formed the most iterated patient-related factor, whereas digital literacy was considered the most important factor for health professionals. Among the properties of digital therapeutics, their interoperability across the broader health system was most emphasized. Finally, the ability to expand access to health care was the most frequently stated outcome measure.
Conclusions
The map of factors developed in this review offers a multistakeholder approach to recognizing the uptake factors of digital therapeutics in the health care pathway and provides an analytical tool for policy makers to assess their health system’s readiness for digital therapeutics.
Keywords: digital health, uptake, implementation, adoption, framework, digital therapeutics, scoping review, thematic analysis, digital medicine, policy
Introduction
Background
The digital health landscape encompasses a broad range of technologies that promote, improve, or support health system functioning and the delivery of health care, including electronic health records, telemedicine, mobile health apps, and health data analytics [1]. Digital therapeutics are a specific subset of the overarching digital health landscape that generate and deliver clinically validated medical interventions and are used as part of a clinical treatment pathway for various health conditions [2,3]. As a result, they can be regulated and prescribed as therapeutics or medical devices, which distinguishes them from more generic digital health applications in the well-being and lifestyle space [4].
Despite digital therapeutics being used to successfully treat a range of conditions, including insomnia, attention-deficit/hyperactivity disorder in children, and substance use disorder [5-9], as well as showing cost-reducing properties [8,10], there are several barriers that reduce their uptake. These include issues around reimbursing them [11,12] and a lack of standardization in evaluating the technologies and their outcome measures. This makes it difficult to use standard approaches to compare different interventions using traditional clinical and cost-effectiveness evaluations [1,11,13,14]. The resulting lack of data on the comparative effectiveness and cost-effectiveness of digital therapeutics can limit market access for innovative technologies [13,15] and present challenges for policy makers and providers when establishing reimbursement mechanisms. There are also factors that influence uptake at the patient level, including digital infrastructure and literacy [2,15-18], and at the health professional level, including lack of training, uncertainties surrounding accountability, and shifts in professional workflow [13,19]. Although the COVID-19 pandemic boosted the implementation of digital health across the health ecosystem [17,18,20], the uptake was not sustainable, as patients reverted back to traditional health services over time [21].
Objective
A number of studies have identified a range of barriers to the uptake of digital health products at different levels [15,19,22,23]. Understanding the full spectrum of uptake factors is essential to identify ways in which policy makers and providers can facilitate the adoption of effective digital therapeutics within a health system, as well as the steps developers can take to assist in the deployment of products [24,25]. In this review, we aimed to map the most frequently discussed uptake factors of digital therapeutics using a scoping review of the digital health literature.
Methods
Overview
We performed a scoping review with thematic synthesis in accordance with the scoping review framework developed by Arksey and O’Malley [26] and Levac [27]. This method allows for the rapid mapping of the key concepts underpinning a broad research area, which is particularly valuable for complex issues that have not been reviewed comprehensively to date [26-28]. In this specific context, our scoping review sought to identify articles with characteristics relevant to the uptake factors of digital therapeutics until additional articles no longer expand the argumentation and thematic saturation is reached instead of providing an exhaustive list of published literature [29]. As such, a representative sample of the digital health literature is sufficient in this context [29]. We followed Joanna Briggs Institute Manual for Evidence Synthesis for Scoping Reviews and reported the findings according to the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) guidelines (Table S1 in Multimedia Appendix 1) [30,31]. As is the case with most scoping reviews, no review protocol was published.
Eligibility Criteria
To be eligible for inclusion, publications had to discuss factors determining digital therapeutic uptake in the health care sector. Uptake, in this context, refers to both the integration of digital therapeutics into the health system and the acceptability and practical use of digital therapeutics by patients and health professionals. Publications from 2000 onward were considered, as this year marks the start of digitalization in health [1,17,21]. Only publications in English were considered. In terms of study designs, research protocols, conference abstracts, theses, preprints, news articles, and workshop proceedings were excluded. Publications that explicitly focused on electronic health records were also excluded. Given the nature of a scoping review, the inclusion criteria were kept broad to ensure that all aspects and dimensions of the uptake factors of digital therapeutics were covered.
Search Strategy and Data Collection
Scientific articles were systematically identified through 3 scientific databases (MEDLINE, Cochrane Database of Systematic Reviews, and Web of Science). These databases were chosen to cover both health-specific and interdisciplinary academic fields. The scientific search was supplemented with a nonsystematic search for gray literature using Google Scholar (first 200 hits) [32]. The full query for the scientific databases is shown in Table S2 in Multimedia Appendix 1. An information specialist at the London School of Economics and Political Science Library further validated the search strategy.
The initial search was conducted on December 12, 2022, and an updated search was conducted on March 9, 2023. The complete screening process (title or abstract and full-text screening) was performed by 1 reviewer in counterchronological order to ensure that recent evidence that might capture novel developments in the uptake of digital therapeutics is not overlooked. To further improve the robustness of the methodology, a subset (828/35,541, 2.33%) of the articles was also assessed by a second reviewer for verification, and interrater agreement scores were computed. Disagreements between the reviewers were resolved by consensus, and no third person was involved. The interrater agreement between the 2 reviewers was calculated using Cohen κ in R (version 4.1.2; R Foundation for Statistical Computing) [33,34]. Deduplication was performed using Endnote (version 22; Clarivate Plc), and screening was performed using Covidence (Veritas Health Innovation) [35]. Reference lists of the included articles were also screened for relevant articles.
Data Synthesis
A thematic analysis was used to extract data relevant to the uptake of digital therapeutics [36]. Salient factors were extracted by 1 author (RvK) and clustered post hoc into relevant domains of the Consolidated Framework for Implementation Research (CFIR) [37]: (1) outer and inner settings, (2) individuals, and (3) innovation. In this review, we decided to pool the outer and inner settings into 1 domain, seeing as their contents are entangled and not mutually exclusive in the context of digital therapeutics [38]. The clustering was reviewed and verified by other authors (AR-U, MA, IK, and EM). We also applied frequency categories to the salient factors to indicate how often a particular factor was reported in the included documents: very frequent was applied when >15% of the included documents mention a factor, frequent was applied when between 5% and 15% of the included documents reported a factor, and regular was applied when <5% of the included documents reported a factor that can affect the uptake of digital therapeutics. It is important to note that these frequency labels do not communicate the relative importance of the uptake factors. They only capture how often a factor was mentioned in the included documents. These frequency categories were translated into color codes in the development of the map of uptake factors, with red indicating the factors very frequently cited, yellow indicating the factors frequently cited, and blue indicating the factors infrequently cited.
The results were further clustered according to Donabedian quality framework, which makes a distinction among structure, process, and outcome measures [39]. In this review, structure measures refer to the uptake factors that describe the physical, institutional, or organizational context in which care is delivered (eg, policy context or demographic characteristics). Process measures capture the transactional uptake factors of digital therapeutics (eg, the preferences, perceptions, or attitudes of patients and professionals). Outcome measures capture all the direct and indirect effects of digital therapeutics on the health care ecosystem. The policy category exclusively includes structure factors. For the patient and health professional characteristics, as well as the properties of digital therapeutics, a subclassification between structure and process measures was made. The outcome category exclusively covers outcome measures, which were divided into 4 subcategories: patient-level, health system, public health, and data science outcomes.
Ethical Considerations
The results presented in this manuscript have no inherent ethical implications or considerations. No animals or human participants were involved in this research. No personal data were used in this research. Thus, we did not seek a review of our study design from an institutional review board.
Results
Search Results
Our search strategy yielded 49,192 results from academic database searches (n=35,541, 72.24% after deduplication) and 221 results from supplementary searches, amassing 35,762 unique records. Ultimately, out of 35,541 documents, we included 244 (0.69%) documents (n=234, 95.9% scientific articles; n=6, 2.5% organizational reports; n=2, 0.8% health technology assessment frameworks [although the included literature covered additional frameworks]; and n=2, 0.8% web pages) in this review (Figure 1). Out of 244 documents, our final sample comprised 60 (24.6%) literature reviews [19,40-98]; 49 (20.1%) viewpoints, commentaries, and editorials [11,13,17,99-144]; 43 (17.6%) cross-sectional studies [83,145-186]; 39 (16%) qualitative research articles [1,15,187-223]; 13 (5.3%) case reports [4,5,12,224-233]; 11 (4.5%) longitudinal studies [234-244]; 9 (3.7%) mixed methods studies [245-253]; 5 (2%) economic evaluations [254-258]; 5 (2%) randomized controlled trials [259-263]; 4 (1.6%) policy documents [264-267]; 3 (1.2%) preference studies [268-270]; 2 (0.8%) websites [271,272]; and 1 (0.4%) book chapter [273]. Of the 244 included studies, 23 (9.4%) were published before 2014 [41,66,71,96,98,116,122,124-126,129,130,132,158,163, 167,171,192,211,227,237,238,268]. The included articles covered 35 distinct countries from all continents. Articles representing the United States (67/244, 27.5%) constituted the largest share of the included articles, followed by those with no specific country focus (62/244, 25.4%) and those focusing on Germany (21/244, 8.6%). Figures S1 and S2 in Multimedia Appendix 1 show an overview of the article types published per geographic region. In the subset screening of the total sample by the second reviewer (828/35,541, 2.33%), we found a crude interrater agreement score of 88.6% (734/828 observations) between the 2 reviewers. We also accounted for the possibility of reaching interrater agreement by chance by computing Cohen κ (0.589), which indicated a moderate agreement between the observers.
Figure 1.
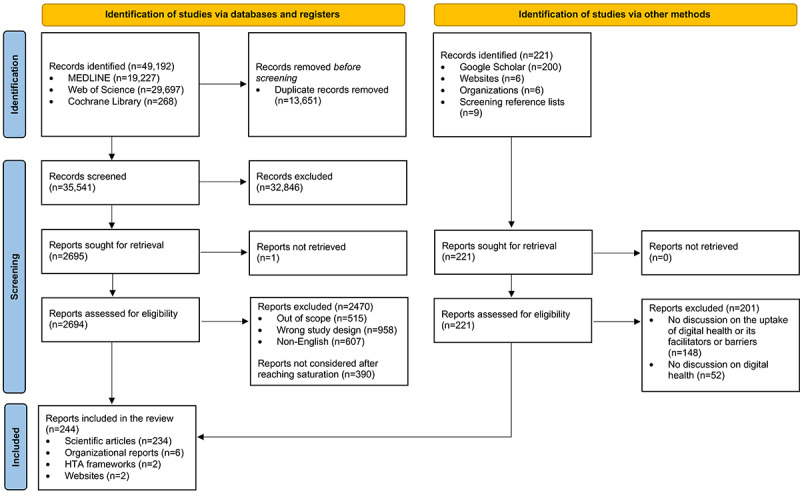
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart outlining the data collection process. HTA: health technology assessment.
Outer and Inner Setting Domains
An important uptake factor of digital therapeutics was the presence of a legal framework to (1) distinguish digital therapeutics from commercial lifestyle and well-being apps, accrediting the former as a reimbursable form of health care, and (2) protect patients’ privacy, confidentiality, and (health) data security [1,4,5,13,15,47,52,54-56,59,66,77,80,87,96, 99,108,109,112,116,120,123,133,139,148,149,157,161,166,167, 169,186,190,208,209,212,231]. A clear example of such a legal framework is the mHealthBelgium validation pyramid in Belgium, which consists of 3 levels: the first level determines the basic requirements, such as the need for Conformité Européene marking, which indicates that a product adheres to the European Union health, safety, and environmental protection standards; the second level is designed to ensure interoperability and connectivity with the broader health informatics system; and the third level regulates the funding of digital health apps by the National Institute of Health and Disability Insurance, which is acquired after a digital health app has passed the clinical review process [224]. In terms of evidence requirements, establishing different evidence tiers to distinguish digital therapeutics from other types of digital health applications (eg, those aimed at communication or administrative functions) was considered vital [5,12,93,123,157,161,203,212,224,231,265]. In practice, this is already observed in the United Kingdom, where the National Institute of Health and Care Excellence (NICE) has devised a 3-level evidence framework that distinguishes (1) digital tools with no patient-relevant outcomes, (2) digital tools that aid in communication and education, and (3) digital therapeutics that are designed to generate and deliver medical interventions [5,224,265]. These 3 evidence tiers also each have their respective evidence requirements (Table S3 in Multimedia Appendix 1), with the highest level requiring evidence of effectiveness for the claimed benefits of the digital therapeutic on top of the requirements for the second evidence tier. The establishment of a formal body to transparently assess and certify digital therapeutics also influenced uptake [15,121,166,174,213]. Practical examples include the NICE in the United Kingdom, the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte) in Germany, and the Finnish Coordinating Center for Health Technology Assessment [75,224,229,265]. The NICE has also established an early value assessment process for digital therapeutics, which can provide preliminary insights into how digital therapeutics can benefit the health system (particularly surrounding their ability to address unmet health needs) while also assessing what types of evidence are available and what types of evidence are missing [272].
The financing model for digital therapeutics was considered highly influential with respect to their uptake in the health care system [12,65,81,87,127,139,162,170,203,205,212,229, 230,232]. In the included literature, eleven distinct financing models for digital therapeutics were identified: (1) periodical subscription (Germany and the United Kingdom), (2) 1-time license fee (Germany), (3) part of bundle packages with other (nondigital) therapeutics (the United States), (4) financing through (innovation) grants, subsidies, or fundraisers (the Netherlands and Germany), (5) sponsor-based agreements in which the digital therapeutic is sponsored by a large platform or institution (eg, Alzheimer Netherlands sponsors a digital health intervention for Dutch patients with dementia at no charge to caregivers), (6) public-private partnerships (eg, a private actor helps develop the local digital health infrastructure on the condition that their digital therapeutic is added in the local health care inventory), (7) inclusion in data plans, (8) performance-based contracts (the United States), (9) risk-benefit sharing contracts (the United States), (10) part of employment benefit plans, and (11) pay-as-you-go arrangements [12,65,86,87,127,143,162,170,203,205,212,229,230,232,239]. In addition, some form of reimbursement parity (eg, service or payment parity) with traditional biomedical therapeutics was considered important [68,70,119,123,153,169,206,238]. Incentives for the uptake of digital therapeutics were also identified as an important means of bolstering the development of a reimbursement pathway for digital therapeutics (eg, dedicated funding or financial compensation for offsetting the high fixed costs of implementing digital therapeutics in practice) [1,19,62,87,122,126,133,140,143,145,169,174,187,188,200,218,225, 227,231]. An important aspect of these incentives is the development of reimbursement codes for digital therapeutics [2,53,99,148,153,169,170,212]. The United States created reimbursement codes for Medicare and Medicaid to be able to better reimburse digital health, but these payment codes are used for digital health as a whole and not specifically for digital therapeutics [53,160]. After certification, the uptake of digital therapeutics is facilitated by the ability of health care professionals or health insurers to identify accredited digital therapeutics through an openly accessible register or inventory [4,59,224,226,229,231,232]. These registers have already been established in Germany, Belgium, and France [15,224], whereas the National Health Service (NHS) App Library in England was decommissioned in December 2021 during a restructuring of the NHS website [271].
Another vital element is the development of funding options for digital therapeutics during and after the research phase (eg, innovation and research grants, pay-for-performance constructs, or payment/completed digital therapeutic use cycle). For the latter, it is important that the financial responsibility is not placed on patients to protect them from disproportionate out-of-pocket payments and ensure that digital therapeutics do not become an exclusive domain of higher socioeconomic groups [12,15,46,58,61,72,86,99,100,105,106,114,116,146,149,154, 164,172,190,192,197,200,202,226-228,249]. It was emphasized that there were benefits to allowing the procurement of digital therapeutics as locally as possible, as health purchasers (eg, Integrated Care Boards in the United Kingdom, insurance companies in the Netherlands, and employer groups in the United States) are best placed to assess the needs and preferences of their covered population, although this could translate into longer adoption times and higher uptake costs, as each individual insurer needs to invest in the digital infrastructure. There was a stated need to adjust or redesign the existing protocols and professional guidelines, commonly created for the provision of face-to-face health care, to create opportunities for digital therapeutics and address the liability and risk-sharing concerns with using digital therapeutics [19,51,52,57,63-66,81,87,106,110,112,114,118,121,126, 139,149,158,192,205,207,225,233]. The presence or development of a high-quality digital infrastructure that can be accessed by all population groups [1,5, 13,40,45,47,63,66,74,87,100,106,119,122,124, 134,135,137,140,153,162,199,201,209,218,238,270,273], the availability of testing environments so that health professionals can gain experience with digital therapeutics [129,201], and the inclusion of digital therapeutic modules in medical curricula were identified as facilitators of the uptake of digital therapeutics [52,58,61,62,70,78,80,81,87,105,110,115,134,149,190,197, 207,208]. A good practice example of this can be found in the United Kingdom with the establishment of the NHS Digital Academy, which trains health professionals according to a comprehensive framework on digital health competencies (Figure S3 in Multimedia Appendix 1) [97,267]. Finally, the included articles emphasized the importance of developing a formal license for health professionals to allow them to prescribe digital therapeutics [13,64,68,70,87,123,124,131].
Individuals Domain
Patients
In terms of the Donabedian framework structure measures, the characteristics of the population group targeted by a digital therapeutic are important uptake determinants, as they influence the impact that any digital therapeutic might have on different clinical pathways and different diseases. Demographic characteristics are associated with patients’ uptake of digital therapeutics. Younger age [5,19,40,43,49,52-54,63, 83,100,101,126,135,139,175,177,181,182,196,203,206,235,236,256,259], sex (male or female depending on the circumstances) [17,53,62,63,101,181,182,235,256], higher language skills [19,53,126], higher education level [5,17, 43,49,54,62,63,83,100,101,107,135,175,177,181,182], higher health literacy [40,47], being employed [5,17,62,100,101], higher income level [17,40,52,54,62,100,101,107, 152,153,171,175,269,270], and living in an urban environment (compared with living in a rural environment or being homeless) [17,40,53,54,62,100,101,139,156,170,171,181,258,269] were associated with increased uptake of digital therapeutics. Furthermore, cultural and social environments [49,62,71,82,86,115,135,142,159,193,195,235]; ethnic background [40,101,102,107,142,175,193,195,235]; being unhealthy or living with a disability [11,17,40,52,62,80,100-103,107,181,190,206,236,259,270]; patient-specific needs, attitudes, and habits [1,80,102,109,110,193,195]; distance to health services [152,171,185,236], risk of contracting a disease [165]; insurance status [175,270]; time since diagnosis [177]; and past health care experiences [217] can influence the uptake of digital therapeutics. The extent to which the target group can access the internet is an additional determinant of the uptake of digital therapeutics [17,19,52,53,62,63,76,77,80,88,100-102,115,137, 139,182,206,269], as well as the target group’s (perceived) digital skills and capabilities and ability to understand the risks associated with using digital tools [1,5, 13,17,40,45,49,50,52,62,63,80-82,100-102,105,107,115,135,139,147,175,177, 188,191,192,194,199-201,210,224,225,237,245,252].
For process measures, it was considered vital to understand how patients perceive the need to use the digital therapeutic (on its own as well as compared with in-person visits); their views, doubts, and comfort regarding the use of the digital therapeutic and its potential benefits; resilience to setbacks; waiting times for the health care in question; and the ease of using the digital therapeutic [13,15,19,42-44,48,50,52,62,82,83,88,107,133,141, 150,159,170,177,178,186,188,189,193,201-203,208,216, 220,221,245,247,251,252,254,269,270]. Articles from the United States and Germany indicate that individuals who rate their health status as being lower favor the use of digital tools to aid them in their health care [43,50,195], although none of these articles focused on digital therapeutics specifically. Nevertheless, patients’ preferences for using digital therapeutics instead or alongside of traditional care form a core part of whether digital therapeutics are used in practice [42,50,62,73,82,165,199,209,246,247].
Health Professionals
As with the target patient group, health professionals’ perceptions are also key factors of the uptake of digital therapeutics. Relevant factors include how easy the digital therapeutic is to use [13,42,44,202,218], how health professionals perceive the digital capabilities and risk profile of the patient and the quality of the digital therapeutic [90,145,172,184,211,218], how the technology will affect organizational workflow, and professional responsibilities and autonomy [13,42,54,62,110,116,133,155,189,197,198, 202,207,211,221,273]. Barriers to implementation (eg, financial, human resource, or time- and workload-related barriers) were also frequently cited [42,43,62, 63,90,104,115,139,145,155,156,199,200,202,218].
With respect to structure measures, the demographic characteristics of health professionals play a role. Female and younger health professionals were reported to favor the prescription of digital therapeutics in Germany and the Netherlands [147,196], as did health professionals located in rural environments in Australia, the United States, and Taiwan [62,151,155,236]. The digital connectivity and literacy of health professionals were widely recognized as key components in determining their willingness toward and likelihood of prescribing digital therapeutics across countries [1,5,17,42-46,62,70,76,80,81,90,100,101,104,106,116,117,135, 145,146,162,174,183,184,187,189,191,192,194,196,197,199-201,203, 210,224,225,228,231], as were their personal attitudes toward, familiarity with, and trust in digital therapeutics [1,42,43,62,70,104,105,116,139,145-147,183,184, 187,189,192,196,198-200,203,209,210,230,231,236,248].
With respect to process measures, professionals reported rigidity in their work process [146,187,192,196], as well as a tendency to be risk averse and to resist change unless a digital therapeutic had been nationally accredited or endorsed [63,104,139,147,231,248], reemphasizing the need for policy mechanisms aimed at the accreditation of digital therapeutics. Approval from the institutional or social environment was also reported as an important indicator of the uptake and use of digital therapeutics by health professionals [19,42,62,104,112,115,139,156,159,174,200,202,203]. Finally, certain medical specialties (eg, psychology, psychiatry, and neurology) may be more amenable to adopting digital therapeutics than other specialties (eg, ophthalmology, dermatology, and surgery) [140,145,146, 162,167,183,196,213,235,236,247], which may be explained by the varying degrees of availability or applicability of digital therapeutics for different medical specialties [183,196].
Innovation Domain
Characteristics of Digital Therapeutics and Manufacturer Provisions
On the basis of the mapped literature, the nature, content, and structure of digital therapeutics are major determinants of their uptake and effectiveness across countries [1,4,13,19,40,63,100,102,189,191-194,200,205,230,246,265,266]. The design of digital therapeutics needs to be driven by the needs and expectations of patients [56,61,66, 68,84,100,102,117,118,122,132,136,140,141,168,177,189, 191-194,200,202,208,214-216,221,230,233,250,266,273,274] while acknowledging that patient needs may shift with time and disease progression [140]. They need to have clear aims and a position in the health care pathway that is understood by patients and professionals, with comprehensive and trusted information provided about the qualities and services of the devices and targeted health condition [56,57,93,118,142, 174,177,178,186,190,214,221,228,250,252,259, 265,266,273]. They should also be easy and straightforward to understand and use [1,4,13,19,40,42,117,135,141,150,193,194,203,208,217,250, 252,265,266,273], as well as have the ability to be personalized according to patient needs and preferences [4,13,62,117,208,216,217,220,246]. Finally, a key property and enabler of the reimbursement of digital therapeutics is their interaction with the target population: they should be co-designed and coimplemented with patients and health professionals to maximize the likelihood of their uptake by the relevant stakeholders, with the recognition of the fact that engagement might differ across population groups in different countries [4,13,19,40,47,56,57,89,129,166,174,189, 190,200,214,215,220,230,250].
With respect to structure measures, the interoperability of digital therapeutics (ie, the ability to communicate with local health informatics systems, such as electronic health records) was the most often emphasized requirement from a design viewpoint [1,4,13,15,17,19,44,45,54,57,61,66,80,82,103,106,107, 110,111,124,147,189-192,197,199,200,202,222,224, 230,231,246,264-266,270,273]. However, it is important that digital therapeutics remain compliant with local privacy and security legislation and prevent direct-to-consumer advertisements through the application [4,47-49,51,57,93,115,125,138,187,211,219,220,224], as well as identification through secondary data sharing [79,91,93,138,217]. The hardware and software requirements [63,91,163,194,233], implementation and upkeep costs [41,63,65,133,146,198,211,231,237,238,254], required input and oversight from health professionals [11,107,254,265], ability to work without a constant internet connection [91,135,217,233], price [165,222], and scalability were also deemed important factors, as they dictate the investment threshold for adopting digital therapeutics and how widely and cost-effectively digital therapeutics can be deployed, including the potential to realize economies of scale [164,199,231,265].
With respect to process measures, certain additional provisions from the developer broaden the potential uptake of digital therapeutics in addition to enhancing the digital therapeutics themselves. These additional provisions include dedicated training courses for the digital therapeutic aimed at patients and health professionals, and user support during and after implementation were noted as critical uptake factors [13,19,61-63,66,73,115,118,145,148,159,184,199,203,206,252].
Finally, a key property of digital therapeutics was the existence of a strong evidence base that matches the corresponding evidence tier of the national regulatory framework. This should contain data on the positive health effects of using the digital therapeutic compared with the status quo or preexisting treatment options (eg, pragmatic randomized controlled trials using a real-world data arm), the context in which the digital therapeutic is to be implemented, how it affects the clinical workflow in the target setting, economic evaluations (cost-effectiveness, cost utility, and cost benefit), economic risk to the payer, budget impact, qualitative evidence on patients’ and health professionals’ experiences with using the tool, health system needs assessment, the safety of the patient and their data (eg, reports of regular penetration testing), and transferability and generalizability across population groups [1,4, 13,15,44,46,49,54,61,65,85,86,91,98,105,106,108-112,117, 118,133,144,188,196,197,200,210,228,229,232,266]. In this context, positive health effects include not only medical benefits, such as improved health status, shortening of the duration of disease, prolongation of survival, and improvement of the quality of life, but also broader elements, such as treatment adherence, patient safety and sovereignty, coping capabilities, and the reduction of therapy-related efforts [108,109,229].
Relative Advantages of Digital Therapeutics
The mapped literature highlighted the multidimensionality and versatility of digital therapeutics by showcasing their relative advantages compared with their competitors. At the patient level, the most reported relative advantages were improvements in health outcomes and shortened disease duration through novel health care options, faster diagnoses, decrease in adverse medical events, and improved monitoring of clinical and patient-reported outcome measures [11,17,41,43, 45,47-49,53,60,61,69,71,74,80,81,88,92,94,95,100,102,105,124,128,130, 132,133,135,137,138,146,153,163,172,173,179,189,202,207,218,224,226,228, 229,231,232,234,239-242,244,246,248,257,260,262,263,265,266,270,275], as well as changes in the quality of life, well-being, and life expectancy [13,47,48,58,88,92,120,128,153,224,226, 229,244,246,257]. Digital therapeutics can also induce changes in the quality of care, patient satisfaction, and diagnostic accuracy [48,52,53,55,60,69,76,88,89,92,102,128,137, 147,165,168,179,189,203,219,242,243,270,273] while empowering the patient to have a more active role in their health care and further enabling shared decision-making [4,56,82,92,102,105,134,139,175,189,191,201, 216,225,226,229,231,241,246,248,258,266,273]. At the behavioral level, digital therapeutics have been shown to positively affect treatment adherence [41,48,69,137,196,226,229,231,246,266,273], patients’ coping and health management abilities [4,13,76,82,126,134,137,145,175,226,229,244,254,257,263,266,270,273], and general behavioral and lifestyle aspects [13,43,56,246,254]. Furthermore, digital therapeutics could improve access to health care, reduce waiting times, and lower thresholds for seeking out health services [1,41,43,45,48,49,53,55,61,62,67,70,71, 74,76,80,88,90,91,95,99,102,111,113,120,124,130-133,137-139,142,150, 151,162,171,173,181,183,185,193,196,203,216,217,224-226,229,232,234, 239,240,242,247,249,253,256,261,262,269,273,275]. They can also aid in the development of (digital) health literacy [4,13,41,43,47,76,82,83,89,92,139,147,165,196,202,208, 219,226,229,242,250,251,257,266,270,274].
At the health system level, digital therapeutics can influence health care costs and resource use [41,47,48, 53,69,71,72,80,81,94,95,105,107,111,113,121,124,128,131,133,147, 153,154,160,164,171,172,180,189,207,211,212,228,231,232,237,238,255-258, 261,262,265,269,273] and affect the workload and workflow of the health workforce, potentially bringing about significant improvements [19,47,60,62,78,80,90,105,116,124,126,128, 134,146,147,176,188,194,196,221,231]. They can also improve coordination among health professionals [60,90,92,225,226,229,232,273]. Furthermore, as they require less health professional input, digital therapeutics can help address clinical workforce shortages [47,72,80,124,128,173,176,228,243,270].
Digital therapeutics have secondary relative advantages at the public health and data science levels. The data generated through their use can allow for the tracking of disease burden [1,100,151,253], resulting in the ability to monitor and model the spread patterns of infectious diseases and inform national priorities for health care procurement and resource allocation [1,17,41,45,151]. As they reduce the need for patients to travel to health care facilities, they are both environmentally friendly and can reduce the risk of hospital-borne infections [120,139,165,171,180,185]. When designed and deployed adequately, digital therapeutics can also contribute to the reduction of health inequalities; however, when designed poorly or without the consideration of the potential for the digital exclusion of groups with lower levels of digital literacy, they have the potential to exacerbate health inequalities [47,53,68,70,81,95,99,100,102,107,120,127,132,139,140, 142,143,173,182,210,218,221,242]. Finally, digital therapeutics can aid in the generation and analysis of meaningful real-world (health) data through their integration with electronic health records [5,136,231,273], enable the use of cloud-based diagnostics and (big data) analyses [48,72,223], and be continuously developed and improved through iterative user feedback [201]. However, privacy-conscious data-sharing platforms (eg, PhysioNet and the US National Institute of Health All of Us research program) are vital for minimizing the risk of reidentification [79].
Comprehensive Map of Uptake Factors
From the 244 included documents, we extracted 85 factors that determine the uptake of digital therapeutics within a health system. At the policy level, the need to develop a legal framework for digital therapeutics was very frequently iterated (37/244, 15.2%). As for patients, their demographics (57/244, 23.4%), perceptions of digital therapeutics (39/244, 16%), and digital skills and risk perceptions (38/244, 15.6%) were very frequently reported to influence the potential uptake of digital therapeutics. The interoperability of digital therapeutics was also very frequently reported to influence their uptake (39/244, 16%). As for health professionals, their digital connectivity and literacy were the most stated factors affecting their uptake of digital therapeutics (43/244, 17.6%). In terms of the relative advantages of digital therapeutics, improved access to health services (65/244, 26.6%), improved health outcomes (63/244, 25.8%), and changes in health care costs and resource use (46/244, 18.9%) were very frequently reported. Further details on the frequency with which uptake factors were reported in the included studies are shown in Table 1. Finally, a visual map that outlines all the extracted factors color coded by frequency is shown in Figure 2.
Table 1.
Frequency table of the most reported uptake factors in the included documents (n=244).
| Domain and factors | Frequency, n (%) | |||
| Policy | ||||
|
|
Very frequently reported factors (≥15%) | |||
|
|
|
Developing a legal framework | 37 (15.2) | |
|
|
Frequently reported factors (5%-15%) | |||
|
|
|
Implementing a high-quality digital infrastructure | 28 (11.5) | |
|
|
|
Developing funding options | 27 (11.1) | |
|
|
|
Redesigning protocols and professional guidelines | 25 (10.2) | |
|
|
|
Inclusion of digital health modules in medical education | 18 (7.4) | |
|
|
|
Financing model for digital therapeutics | 17 (7) | |
| Patient characteristics | ||||
|
|
Very frequently reported factors (≥15%) | |||
|
|
|
Demographic characteristics | 57 (23.4) | |
|
|
|
Patient perceptions of digital therapeutics | 39 (16) | |
|
|
|
Digital skills and risk perceptions | 38 (15.6) | |
| Digital therapeutic | ||||
|
|
Very frequently reported factors (≥15%) | |||
|
|
|
Interoperability | 39 (16) | |
|
|
Frequently reported factors (5%-15%) | |||
|
|
|
Need-driven and patient-centered design | 34 (13.9) | |
|
|
|
Evidence base | 33 (13.5) | |
|
|
|
Simple and straightforward to use | 20 (8.2) | |
|
|
|
Clear aim that is understood by patients and professionals | 19 (7.8) | |
|
|
|
Accounting for different needs across populations and disease stages | 19 (7.8) | |
|
|
|
Provision of training courses for patients and professionals | 17 (7) | |
|
|
|
Remaining compliant with local privacy and security legislation | 15 (6.1) | |
| Health professional | ||||
|
|
Very frequently reported factors (≥15%) | |||
|
|
|
Digital literacy | 43 (17.6) | |
|
|
Frequently reported factors (5%-15%) | |||
|
|
|
Professional preference | 28 (11.5) | |
|
|
|
Effect on clinical workflow and professional autonomy | 16 (6.6) | |
|
|
|
Financial, human resource, or time- and workload-related barriers | 15 (6.1) | |
|
|
|
Approval from the institutional or social environment | 13 (5.3) | |
| Outcome | ||||
|
|
Very frequently reported factors (≥15%) | |||
|
|
|
Improved access to health services | 65 (26.6) | |
|
|
|
Improved health outcomes | 63 (25.8) | |
|
|
|
Change in health care costs and resource use | 46 (18.9) | |
|
|
Frequently reported factors (5%-15%) | |||
|
|
|
Improving digital literacy, health literacy, or digital health literacy in patients | 26 (10.7) | |
|
|
|
Improving the quality of care, patient satisfaction, and diagnostic accuracy | 24 (9.8) | |
|
|
|
Enabling shared decision-making | 23 (9.4) | |
|
|
|
Affecting health inequalities | 23 (9.4) | |
|
|
|
Affecting the workload and workflow of the health workforce | 21 (8.6) | |
Figure 2.
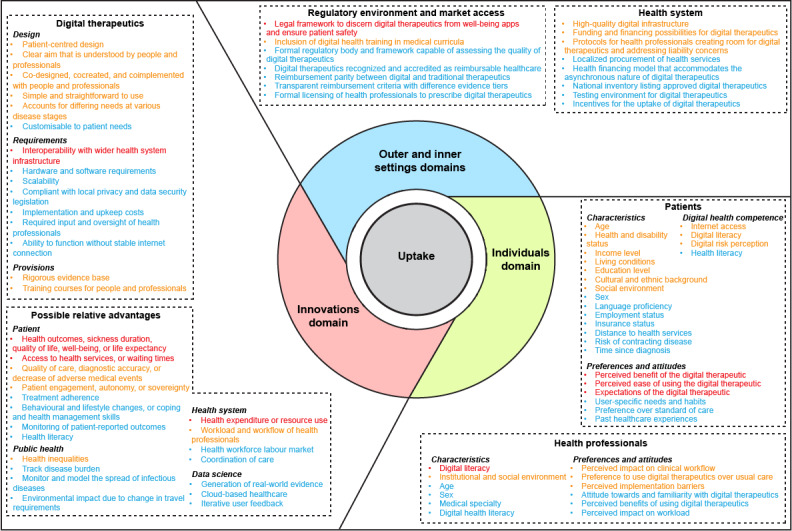
Map of the factors that can affect the uptake of digital therapeutics. The items in red are reported in 15% or more of the included articles. Items in yellow are reported in 5% to 15% of included articles. Items in blue are reported in less than 5% of the included articles.
Discussion
Principal Findings
This review aimed to create the first map of the most frequently discussed uptake factors of digital therapeutics that will aid their implementation across different health systems. Our map contains 85 factors that can affect the uptake of digital therapeutics within a health system. This map offers a novel and comprehensive overview of the known uptake factors from both technological and human perspectives. This bridges the dichotomy of the digital health literature in which articles either focus on the technological or patient and professional perspectives, which inadvertently highlights the novelty of this review. The map is intended to be scaled and adopted by countries looking to introduce digital therapeutics in their health service portfolio, expand the proportion of their population that benefits from digital therapeutics, or extend access to digital therapeutics to excluded communities. As this map was based on a standardized set of constructs in the CFIR, it can support future research on and implementation endeavors of digital therapeutics. It offers policy makers, health insurers, and other change agents specific digital health–related action points to focus on in their pursuit of implementing digital therapeutics in the health care system. In doing so, it can help address the existing barriers to the uptake of digital therapeutics, harmonize digital therapeutic infrastructure across health systems, and aid researchers in analyzing health system readiness for using digital therapeutics.
This map of factors aims to guide the integration of digital therapeutics into clinical practice by helping stakeholders identify, assess, and address key uptake barriers unique to digital therapeutics, including issues related to policy readiness, regulatory approval, reimbursement, engagement, data privacy and security, adoption, and clinical effectiveness. It highlights the finding that the innovation and individuals domains of the CFIR contain the most frequently cited uptake factors of digital therapeutics. The relative focus on these factors is unsurprising, seeing how digital therapeutics are unprecedented in terms of the required user input. It also signals these uptake factors as action points for policy makers, health insurers, and health promotion experts. Patient groups may need to be sensitized to the concept of digital therapeutics before they are ready and willing to use these novel tools in their health care pathways [22]. Simultaneously, it points to an opportunity for investors to update funding requirements to better enfranchise future users of digital therapeutics, especially if they are designed to combat the existing health inequalities.
Users of this map should consider the unique characteristics and needs of different health care settings and digital therapeutics on a per-case basis. Consequently, health care organizations can ensure that they are able to effectively leverage the potential of digital therapeutics to improve patient outcomes and address pressing health care challenges [1,71,128,194]. The map is not solely reliant on clinical health outcomes but allows room for a broader interpretation of positive health effects, such as treatment adherence, patient safety and sovereignty, coping capabilities, and reduction of therapy-related efforts [109,229]. This interpretation allows for a more versatile and holistic assessment of digital therapeutics, which could contribute to the value-based pricing of digital therapeutics [12,229].
The focus on the United States in the digital health literature included in this review is likely related to the current health system challenges worldwide. In particular, the United States is experiencing stark rises in health care costs, inequitable access to health care, and labor shortages in the health workforce [276], for which digital therapeutics can be seen as a potential (partial) solution. Although these issues exist worldwide, the United States has a favorable environment for developing and adopting innovations, as it hosts the largest technology companies and venture capital investors [277]. By contrast, the focus on Germany, for example, is attributable to the recent adoption of the Digital Healthcare Act, which only now formally enabled digital therapeutics to be prescribed by general practitioners [266].
In this review, we also found that countries with multipayer reimbursement systems (the United States, Germany, and the Netherlands) form the predominant countries of interest in the literature on reimbursing digital therapeutics. These health systems are built on the basis of (managed) competition and a mix of supplementary and substitutive private health insurance markets [278]. Stereotypically, these types of systems are more conducive to adopting health care innovations, as insurers are actively incentivized to look for a competitive advantage and potential cost-saving solutions [279]. This reasoning also suggests a partial explanation for why countries with a single-payer system (eg, Australia, Canada, or the European Nordic countries) are poorly represented in the current digital health literature. The outlier here is the United Kingdom, where there has been a broad policy discussion around this topic with a strong impetus from national bodies (eg, NHS England) [280]. A downside of introducing digital therapeutics in multipayer countries is the high investment cost of setting up the digital infrastructure in a health system. The Netherlands offers a partial solution to this problem by allowing health insurers to pool their resources and collectively buy the digital infrastructure required to run digital therapeutics [281].
Limitations and Future Research
Some limitations of this review need to be considered. First, the findings of this review should be interpreted as scoping, meaning it provides a high-level overview of the literature and may not capture more intricate and field-specific factors that can affect the uptake of digital therapeutics in health care. Second, the quality of the included sources was not assessed, which should be considered when interpreting the results. Third, we acknowledge the presence of evidence selection bias, as only 3 academic databases and Google Scholar were used, and the search strategy was not exhaustive. That said, an exhaustive search was not required to reach the aim of this review, namely the development of a map of uptake factors. In fact, we reached data saturation during the extraction of the current sample of articles. Fourth, we acknowledge that this review only captures the uptake factors that are considered important within the current digital health literature. Fifth, the high representation of the United States in the included articles might skew our results to be geared toward the US health ecosystem, although we believe that this is counterbalanced by the articles that focused on countries other than the United States. Sixth, these results cannot be directly transferred to the context of artificial intelligence, even though it overlaps to some degree with digital therapeutics, as the latter are often powered by some form of artificial intelligence. Finally, we acknowledge that this review makes broad conclusions about digital therapeutics holistically and may not be applicable to individual applications.
Various avenues for further research were also identified in this review. A comprehensive evidence synthesis for each domain within the map of factors could further improve its quality and robustness. An assessment of the relative importance of the uptake factors within each domain can provide more actionable guidance for policy makers, health professionals, and patients. Patient engagement and empowerment lie at the core of digital therapeutics, yet these concepts remain unquantified. Future research should investigate conceptualizing key performance indicators for patient activity and engagement with digital therapeutics. Even though qualitative studies comprised a decent proportion of the included studies (39/244, 16%), these studies focused predominantly on the experiences of implementing digital health applications in finalized or near-finalized development stages into practical situations. However, research on the process by which a digital therapeutic is developed and how patients can be enfranchised within that process is lacking. Future research should also investigate the possibility, risks, and benefits of migrating the necessary health data infrastructure for digital therapeutics to cloud-based environments compared with local storage. Finally, future research should explore how different financing models for digital therapeutics interact with different types of health systems to identify best practices and further streamline the integration of digital therapeutics in health care pathways.
Conclusions
Ultimately, digital therapeutics are set to disrupt the delivery of health care by challenging the fundamental assumption that health care needs to be location bound and episodic in nature [130]. Understanding the array of factors that determine the uptake of digital therapeutics in the health care landscape is a vital step in progressing the development of digitally augmented health systems. The map of factors developed in this review offers a multistakeholder approach to recognizing the uptake factors of digital therapeutics in the health care pathway and provides an analytical tool for policy makers to assess their health system’s readiness for digital therapeutics.
Acknowledgments
The authors would like to thank Ms Andra Fry at the London School of Economics and Political Science Library for her input on the methodology.
The authors declare that no funding was acquired for this research.
Abbreviations
- CFIR
Consolidated Framework for Implementation Research
- NHS
National Health Service
- NICE
National Institute of Health and Care Excellence
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews
Additional figures, tables, and the search strategy per database.
Footnotes
Authors' Contributions: RvK contributed to conceptualization, the methodology, visualization, software use, validation, formal analysis, writing (original draft, as well as review and editing), data curation, supervision, and project administration. AR-U contributed to conceptualization, the methodology, validation, visualization, formal analysis, and writing (original draft, as well as review and editing). MA contributed to the methodology, validation, and writing (review and editing). IK contributed to the methodology, validation, and writing (review and editing). SF contributed to validation and writing (review and editing). GM contributed to validation and writing (review and editing). SDR contributed to validation and writing (review and editing). MP contributed to validation and writing (review and editing). GW contributed to validation and writing (review and editing). EM contributed to conceptualization, the methodology, validation, writing (original draft, as well as review and editing), supervision, and project administration.
Conflicts of Interest: None declared.
References
- 1.Fahy N, Williams GA, Habicht T, Köhler K, Jormanainen V, Satokangas M, Tynkkynen LK, Lantzsch H, Winklemann J, Cascini F, de Belvis AG, Morsella A, Poscia A, Ricciardi W, Silenzi A, Farcasanu D, Scintee SG, Vladescu C, Delgado EB, Pueyo EA, Romero FE. Use of Digital Health Tools in Europe: Before, During and After COVID-19. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2021. [2022-11-01]. https://www.ncbi.nlm.nih.gov/books/NBK576970/ [PubMed] [Google Scholar]
- 2.Patel NA, Butte AJ. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit Med. 2020 Dec 11;3(1):159. doi: 10.1038/s41746-020-00370-8. doi: 10.1038/s41746-020-00370-8.10.1038/s41746-020-00370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowland SP, Fitzgerald JE, Holme T, Powell J, McGregor A. What is the clinical value of mHealth for patients? NPJ Digit Med. 2020 Jan 13;3:4. doi: 10.1038/s41746-019-0206-x. doi: 10.1038/s41746-019-0206-x.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essén A, Stern AD, Haase CB, Car J, Greaves F, Paparova D, Vandeput S, Wehrens R, Bates DW. Health app policy: international comparison of nine countries' approaches. NPJ Digit Med. 2022 Mar 18;5(1):31. doi: 10.1038/s41746-022-00573-1. doi: 10.1038/s41746-022-00573-1.10.1038/s41746-022-00573-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brassel S, Radu P, Bell E, Garau M. Navigating the landscape of digital health. Office of Health Economics. 2022. [2023-01-11]. https://www.ohe.org/publications/navigating-the-landscape-of-digital-health/
- 6.Kollins SH, DeLoss DJ, Cañadas E, Lutz J, Findling RL, Keefe RS, Epstein JN, Cutler AJ, Faraone SV. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial. Lancet Digit Health. 2020 Apr;2(4):e168–78. doi: 10.1016/S2589-7500(20)30017-0. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(20)30017-0 .S2589-7500(20)30017-0 [DOI] [PubMed] [Google Scholar]
- 7.Kollins SH, Childress A, Heusser AC, Lutz J. Effectiveness of a digital therapeutic as adjunct to treatment with medication in pediatric ADHD. NPJ Digit Med. 2021 Mar 26;4(1):58. doi: 10.1038/s41746-021-00429-0. doi: 10.1038/s41746-021-00429-0.10.1038/s41746-021-00429-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brezing CA, Brixner DI. The rise of prescription digital therapeutics in behavioral health. Adv Ther. 2022 Dec;39(12):5301–6. doi: 10.1007/s12325-022-02320-0. https://europepmc.org/abstract/MED/36242730 .10.1007/s12325-022-02320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fast N, van Kessel R, Humphreys K, Ward NF, Roman-Urrestarazu A. The evolution of telepsychiatry for substance use disorders during COVID-19: a narrative review. Curr Addict Rep. 2023;10(2):187–97. doi: 10.1007/s40429-023-00480-9. https://europepmc.org/abstract/MED/37266192 .480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah N, Velez FF, Colman S, Kauffman L, Ruetsch C, Anastassopoulos K, Maricich Y. Real-world reductions in healthcare resource utilization over 6 months in patients with substance use disorders treated with a prescription digital therapeutic. Adv Ther. 2022 Sep;39(9):4146–56. doi: 10.1007/s12325-022-02215-0. https://europepmc.org/abstract/MED/35819569 .10.1007/s12325-022-02215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lantzsch H, Panteli D, Martino F, Stephani V, Seißler D, Püschel C, Knöppler K, Busse R. Benefit assessment and reimbursement of digital health applications: concepts for setting up a new system for public coverage. Front Public Health. 2022 Apr 21;10:832870. doi: 10.3389/fpubh.2022.832870. https://europepmc.org/abstract/MED/35530738 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju JH, Sim B, Lee J, Lee JY. Reimbursement of digital therapeutics: future perspectives in Korea. Korean Circ J. 2022 Apr;52(4):265–79. doi: 10.4070/kcj.2022.0014. https://europepmc.org/abstract/MED/35388995 .52.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tromp J, Jindal D, Redfern J, Bhatt A, Séverin T, Banerjee A, Ge J, Itchhaporia D, Jaarsma T, Lanas F, Lopez-Jimenez F, Mohamed A, Perel P, Perez GE, Pinto F, Vedanthan R, Verstrael A, Yeo KK, Zulfiya K, Prabhakaran D, Lam CS, Cowie MR. World heart federation roadmap for digital health in cardiology. Glob Heart. 2022 Aug 26;17(1):61. doi: 10.5334/gh.1141. https://europepmc.org/abstract/MED/36051317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Huben A, Howell M, Howard K, Carrello J, Norris S. Health technology assessment for digital technologies that manage chronic disease: a systematic review. Int J Technol Assess Health Care. 2021 May 26;37(1):e66. doi: 10.1017/S0266462321000362.S0266462321000362 [DOI] [PubMed] [Google Scholar]
- 15.Prodan A, Deimel L, Ahlqvist J, Birov S, Thiel R, Toivanen M, Kolitsi Z, Kalra D. Success factors for scaling up the adoption of digital therapeutics towards the realization of P5 medicine. Front Med (Lausanne) 2022 Apr 12;9:854665. doi: 10.3389/fmed.2022.854665. https://europepmc.org/abstract/MED/35492346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Kessel R, Wong BL, Rubinić I, O'Nuallain E, Czabanowska K. Is Europe prepared to go digital? Making the case for developing digital capacity: an exploratory analysis of Eurostat survey data. PLOS Digit Health. 2022 Feb 17;1(2):e0000013. doi: 10.1371/journal.pdig.0000013. https://europepmc.org/abstract/MED/36812527 .PDIG-D-21-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong BL, Maaß L, Vodden A, van Kessel R, Sorbello S, Buttigieg S, Odone A, European Public Health Association (EUPHA) Digital Health Section The dawn of digital public health in Europe: implications for public health policy and practice. Lancet Reg Health Eur. 2022 Mar;14:100316. doi: 10.1016/j.lanepe.2022.100316. https://linkinghub.elsevier.com/retrieve/pii/S2666-7762(22)00009-6 .S2666-7762(22)00009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holly L, Wong BL, van Kessel R, Awah I, Agrawal A, Ndili N. Optimising adolescent wellbeing in a digital age. BMJ. 2023 Mar 20;380:e068279. doi: 10.1136/bmj-2021-068279. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=36940933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitelaw S, Pellegrini DM, Mamas MA, Cowie M, Van Spall HG. Barriers and facilitators of the uptake of digital health technology in cardiovascular care: a systematic scoping review. Eur Heart J Digit Health. 2021 Feb 04;2(1):62–74. doi: 10.1093/ehjdh/ztab005. https://europepmc.org/abstract/MED/34048508 .ztab005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Kessel R, Kyriopoulos I, Mastylak A, Mossialos E. Changes in digital healthcare search behavior during the early months of the COVID-19 pandemic: a study of six English-speaking countries. PLOS Digit Health. 2023 May 01;2(5):e0000241. doi: 10.1371/journal.pdig.0000241. https://europepmc.org/abstract/MED/37126489 .PDIG-D-23-00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Kessel R, Kyriopoulos I, Wong BL, Mossialos E. The effect of the COVID-19 pandemic on digital health-seeking behavior: big data interrupted time-series analysis of Google trends. J Med Internet Res. 2023 Jan 16;25:e42401. doi: 10.2196/42401. https://www.jmir.org/2023//e42401/ v25i1e42401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A'Court C, Hinder S, Fahy N, Procter R, Shaw S. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017 Nov 01;19(11):e367. doi: 10.2196/jmir.8775. https://www.jmir.org/2017/11/e367/ v19i11e367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon WJ, Landman A, Zhang H, Bates DW. Beyond validation: getting health apps into clinical practice. NPJ Digit Med. 2020 Feb 03;3:14. doi: 10.1038/s41746-019-0212-z. doi: 10.1038/s41746-019-0212-z.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chawla V. Where is the money in digital health? The roadmap to digital health app reimbursement in Europe. Research 2 Guidance. 2022. [2022-11-11]. https://research2guidance.com/where-is-the-money-in-digital-health-the-roadmap-to-digital-health-app-reimbursement-in-europe/
- 25.Wharton GA, Sood HS, Sissons A, Mossialos E. Virtual primary care: fragmentation or integration? Lancet Digit Health. 2019 Nov;1(7):e330–1. doi: 10.1016/S2589-7500(19)30152-9. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(19)30152-9 .S2589-7500(19)30152-9 [DOI] [PubMed] [Google Scholar]
- 26.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005 Feb;8(1):19–32. doi: 10.1080/1364557032000119616. https://www.tandfonline.com/doi/abs/10.1080/1364557032000119616 . [DOI] [Google Scholar]
- 27.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010 Sep 20;5:69. doi: 10.1186/1748-5908-5-69. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-5-69 .1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton A, Clowes M, Preston L, Booth A. Meeting the review family: exploring review types and associated information retrieval requirements. Health Info Libr J. 2019 Sep;36(3):202–22. doi: 10.1111/hir.12276. https://onlinelibrary.wiley.com/doi/10.1111/hir.12276 . [DOI] [PubMed] [Google Scholar]
- 29.Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Syst Rev. 2016 May 04;5:74. doi: 10.1186/s13643-016-0249-x. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0249-x .10.1186/s13643-016-0249-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MD, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018 Oct 02;169(7):467–73. doi: 10.7326/M18-0850. https://www.acpjournals.org/doi/abs/10.7326/M18-0850?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .2700389 [DOI] [PubMed] [Google Scholar]
- 31.Peters MD, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Scoping reviews. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Adelaide, Australia: JBI; 2020. [Google Scholar]
- 32.Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of Google scholar in evidence reviews and its applicability to grey literature searching. PLoS One. 2015 Sep 17;10(9):e0138237. doi: 10.1371/journal.pone.0138237. https://dx.plos.org/10.1371/journal.pone.0138237 .PONE-D-15-27398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960 Apr;20(1):37–46. https://journals.sagepub.com/doi/10.1177/001316446002000104 . [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–74. [PubMed] [Google Scholar]
- 35.Better systematic review management. Covidence. [2023-03-14]. https://www.covidence.org/
- 36.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi: 10.1007/s10597-020-00591-x. https://www.tandfonline.com/doi/abs/10.1191/1478088706qp063oa . [DOI] [Google Scholar]
- 37.Damschroder LJ, Reardon CM, Widerquist MA, Lowery J. The updated consolidated framework for implementation research based on user feedback. Implement Sci. 2022 Oct 29;17(1):75. doi: 10.1186/s13012-022-01245-0. https://implementationscience.biomedcentral.com/articles/10.1186/s13012-022-01245-0 .10.1186/s13012-022-01245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wienert J, Zeeb H. Implementing health apps for digital public health - an implementation science approach adopting the consolidated framework for implementation research. Front Public Health. 2021 May 07;9:610237. doi: 10.3389/fpubh.2021.610237. https://europepmc.org/abstract/MED/34026702 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donabedian A. Evaluating the quality of medical care. 1966. Milbank Q. 2005;83(4):691–729. doi: 10.1111/j.1468-0009.2005.00397.x. https://europepmc.org/abstract/MED/16279964 .MILQ397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honeyman M, Maguire D, Evans H, Davies A. Digital Technology and Health Inequalities: A Scoping Review. Cardiff, Wales: Public Health Wales NHS Trust; 2020. [Google Scholar]
- 41.Dávalos ME, French MT, Burdick AE, Simmons SC. Economic evaluation of telemedicine: review of the literature and research guidelines for benefit-cost analysis. Telemed J E Health. 2009 Dec;15(10):933–48. doi: 10.1089/tmj.2009.0067. [DOI] [PubMed] [Google Scholar]
- 42.Gagnon MP, Ngangue P, Payne-Gagnon J, Desmartis M. m-Health adoption by healthcare professionals: a systematic review. J Am Med Inform Assoc. 2016 Jan;23(1):212–20. doi: 10.1093/jamia/ocv052. http://hdl.handle.net/20.500.11794/353 .ocv052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Heuvel JF, Groenhof TK, Veerbeek JH, van Solinge WW, Lely AT, Franx A, Bekker MN. eHealth as the next-generation perinatal care: an overview of the literature. J Med Internet Res. 2018 Jun 05;20(6):e202. doi: 10.2196/jmir.9262. https://www.jmir.org/2018/6/e202/ v20i6e202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolasa K, Kozinski G. How to value digital health interventions? A systematic literature review. Int J Environ Res Public Health. 2020 Mar 23;17(6):2119. doi: 10.3390/ijerph17062119. https://www.mdpi.com/resolver?pii=ijerph17062119 .ijerph17062119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Omboni S, Padwal RS, Alessa T, Benczúr B, Green BB, Hubbard I, Kario K, Khan NA, Konradi A, Logan AG, Lu Y, Mars M, McManus RJ, Melville S, Neumann CL, Parati G, Renna NF, Ryvlin P, Saner H, Schutte AE, Wang J. The worldwide impact of telemedicine during COVID-19: current evidence and recommendations for the future. Connect Health. 2022 Jan 04;1:7–35. doi: 10.20517/ch.2021.03. https://boris.unibe.ch/id/eprint/166317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan K, Balijepalli C, Druyts E. The impact of digital therapeutics on current health technology assessment frameworks. Front Digit Health. 2021 Jun 09;3:667016. doi: 10.3389/fdgth.2021.667016. https://europepmc.org/abstract/MED/34713140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Y, Bernard L, Jorgensen C, Dusfour G, Pers YM. The challenges of telemedicine in rheumatology. Front Med (Lausanne) 2021 Oct 13;8:746219. doi: 10.3389/fmed.2021.746219. https://europepmc.org/abstract/MED/34722584 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilius G, Schroeder M, Schoebel C. Telehealth and its implementation in respiratory sleep medicine. Curr Opin Pulm Med. 2021 Nov 01;27(6):523–8. doi: 10.1097/MCP.0000000000000830.00063198-202111000-00006 [DOI] [PubMed] [Google Scholar]
- 49.Ambrosino N, Fracchia C. The role of tele-medicine in patients with respiratory diseases. Expert Rev Respir Med. 2017 Nov;11(11):893–900. doi: 10.1080/17476348.2017.1383898. [DOI] [PubMed] [Google Scholar]
- 50.Zaman SB, Khan RK, Evans RG, Thrift AG, Maddison R, Islam SM. Exploring barriers to and enablers of the adoption of information and communication technology for the care of older adults with chronic diseases: scoping review. JMIR Aging. 2022 Jan 07;5(1):e25251. doi: 10.2196/25251. https://aging.jmir.org/2022/1/e25251/ v5i1e25251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Briggs LG, Labban M, Alkhatib K, Nguyen DD, Cole AP, Trinh QD. Digital technologies in cancer care: a review from the clinician's perspective. J Comp Eff Res. 2022 May;11(7):533–44. doi: 10.2217/cer-2021-0263. https://www.becarispublishing.com/doi/10.2217/cer-2021-0263?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 52.Butz B, Kloep L, Kriegesmann B. User experience reevaluation and diffusion of technology in the context of compulsory usage illustrated by the example of telepsychotherapy-a literature review. Digit Health. 2022 Nov 13;8:20552076221134448. doi: 10.1177/20552076221134448. https://journals.sagepub.com/doi/10.1177/20552076221134448?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_20552076221134448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel P, Dhindsa D, Eapen DJ, Khera A, Gulati M, Stone NJ, Yancy CW, Rumsfeld JS, Sperling LS. Optimizing the potential for telehealth in cardiovascular care (in the era of COVID-19): time will tell. Am J Med. 2021 Aug;134(8):945–51. doi: 10.1016/j.amjmed.2021.03.007. https://europepmc.org/abstract/MED/33845033 .S0002-9343(21)00218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kozik M, Isakadze N, Martin SS. Mobile health in preventive cardiology: current status and future perspective. Curr Opin Cardiol. 2021 Sep 01;36(5):580–8. doi: 10.1097/HCO.0000000000000891. https://europepmc.org/abstract/MED/34224437 .00001573-202109000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kompala T, Neinstein AB. Telehealth in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2021 Feb 01;28(1):21–9. doi: 10.1097/MED.0000000000000600.01266029-202102000-00005 [DOI] [PubMed] [Google Scholar]
- 56.Almalki M, Giannicchi A. Health apps for combating COVID-19: descriptive review and taxonomy. JMIR Mhealth Uhealth. 2021 Mar 02;9(3):e24322. doi: 10.2196/24322. https://mhealth.jmir.org/2021/3/e24322/ v9i3e24322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koumpouros Y, Georgoulas A. A systematic review of mHealth funded R and D activities in EU: trends, technologies and obstacles. Inform Health Soc Care. 2020;45(2):168–87. doi: 10.1080/17538157.2019.1656208. [DOI] [PubMed] [Google Scholar]
- 58.Neubeck L, Hansen T, Jaarsma T, Klompstra L, Gallagher R. Delivering healthcare remotely to cardiovascular patients during COVID-19: a rapid review of the evidence. Eur J Cardiovasc Nurs. 2020 Aug;19(6):486–94. doi: 10.1177/1474515120924530. https://europepmc.org/abstract/MED/32380858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sauermann S, Herzberg J, Burkert S, Habetha S. DiGA - a chance for the German healthcare system. J Eur CME. 2022 Dec 23;11(1):2014047. doi: 10.1080/21614083.2021.2014047. https://europepmc.org/abstract/MED/34992948 .2014047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parikh RB, Basen-Enquist KM, Bradley C, Estrin D, Levy M, Lichtenfeld JL, Malin B, McGraw D, Meropol NJ, Oyer RA, Sheldon LK, Shulman LN. Digital health applications in oncology: an opportunity to seize. J Natl Cancer Inst. 2022 Oct 06;114(10):1338–9. doi: 10.1093/jnci/djac108. https://europepmc.org/abstract/MED/35640986 .6596055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raney L, Bergman D, Torous J, Hasselberg M. Digitally driven integrated primary care and behavioral health: how technology can expand access to effective treatment. Curr Psychiatry Rep. 2017 Sep 30;19(11):86. doi: 10.1007/s11920-017-0838-y.10.1007/s11920-017-0838-y [DOI] [PubMed] [Google Scholar]
- 62.Meurk C, Leung J, Hall W, Head BW, Whiteford H. Establishing and governing e-Mental health care in Australia: a systematic review of challenges and a call for policy-focussed research. J Med Internet Res. 2016 Jan 13;18(1):e10. doi: 10.2196/jmir.4827. https://www.jmir.org/2016/1/e10/ v18i1e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018 Jan;24(1):4–12. doi: 10.1177/1357633X16674087. https://journals.sagepub.com/doi/abs/10.1177/1357633X16674087?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodspeed TA, Page RE, Koman LE, Hollenbeck AT, Gilroy AS. Legal and regulatory issues with teledermatology. Curr Derm Rep. 2019 May 3;8(2):46–51. doi: 10.1007/s13671-019-0254-0. https://link.springer.com/article/10.1007/s13671-019-0254-0 . [DOI] [Google Scholar]
- 65.Boriani G, Vitolo M, Svennberg E, Casado-Arroyo R, Merino JL, Leclercq C. Performance-based risk-sharing arrangements for devices and procedures in cardiac electrophysiology: an innovative perspective. Europace. 2022 Oct 13;24(10):1541–7. doi: 10.1093/europace/euac045. https://hal.archives-ouvertes.fr/hal-03713140 .6582466 [DOI] [PubMed] [Google Scholar]
- 66.Jennett PA, Scott RE, Affleck Hall L, Hailey D, Ohinmaa A, Anderson C, Thomas R, Young B, Lorenzetti D. Policy implications associated with the socioeconomic and health system impact of telehealth: a case study from Canada. Telemed J E Health. 2004;10(1):77–83. doi: 10.1089/153056204773644616. [DOI] [PubMed] [Google Scholar]
- 67.Lopéz DM, Blobel B. mHealth in low- and middle-income countries: status, requirements and strategies. Stud Health Technol Inform. 2015;211:79–87. [PubMed] [Google Scholar]
- 68.Weinstein RS, Lopez AM, Joseph BA, Erps KA, Holcomb M, Barker GP, Krupinski EA. Telemedicine, telehealth, and mobile health applications that work: opportunities and barriers. Am J Med. 2014 Mar;127(3):183–7. doi: 10.1016/j.amjmed.2013.09.032.S0002-9343(13)00919-4 [DOI] [PubMed] [Google Scholar]
- 69.Kario K, Harada N, Okura A. The first software as medical device of evidence-based hypertension digital therapeutics for clinical practice. Hypertens Res. 2022 Dec;45(12):1899–905. doi: 10.1038/s41440-022-01016-w. https://europepmc.org/abstract/MED/36207530 .10.1038/s41440-022-01016-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasselberg MJ. The digital revolution in behavioral health. J Am Psychiatr Nurses Assoc. 2020 Jan;26(1):102–11. doi: 10.1177/1078390319879750. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez RC. The promise of e-Health - a Canadian perspective. eHealth Int. 2002 Sep 17;1(1):4. doi: 10.1186/1476-3591-1-4. https://europepmc.org/abstract/MED/12459044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meessen B. The role of digital strategies in financing health care for universal health coverage in low- and middle-income countries. Glob Health Sci Pract. 2018 Oct 10;6(Suppl 1):S29–40. doi: 10.9745/GHSP-D-18-00271. http://www.ghspjournal.org/lookup/pmidlookup?view=long&pmid=30305337 .GHSP-D-18-00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chua V, Koh JH, Koh CH, Tyagi S. The willingness to pay for telemedicine among patients with chronic diseases: systematic review. J Med Internet Res. 2022 Apr 13;24(4):e33372. doi: 10.2196/33372. https://www.jmir.org/2022/4/e33372/ v24i4e33372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leon N, Balakrishna Y, Hohlfeld A, Odendaal WA, Schmidt BM, Zweigenthal V, Anstey Watkins J, Daniels K. Routine Health Information System (RHIS) improvements for strengthened health system management. Cochrane Database Syst Rev. 2020 Aug 13;8(8):CD012012. doi: 10.1002/14651858.CD012012.pub2. https://europepmc.org/abstract/MED/32803893 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haverinen J, Keränen N, Falkenbach P, Maijala A, Kolehmainen T, Reponen J. Digi-HTA: health technology assessment framework for digital healthcare services. FinJeHeW. 2019 Nov 02;11(4):326–41. doi: 10.23996/fjhw.82538. https://journal.fi/finjehew/article/view/82538 . [DOI] [Google Scholar]
- 76.Nanda M, Sharma R. A review of patient satisfaction and experience with telemedicine: a virtual solution during and beyond COVID-19 pandemic. Telemed J E Health. 2021 Dec;27(12):1325–31. doi: 10.1089/tmj.2020.0570. [DOI] [PubMed] [Google Scholar]
- 77.Abbadessa G, Brigo F, Clerico M, De Mercanti S, Trojsi F, Tedeschi G, Bonavita S, Lavorgna L. Digital therapeutics in neurology. J Neurol. 2022 Mar;269(3):1209–24. doi: 10.1007/s00415-021-10608-4. http://hdl.handle.net/2318/1844671 .10.1007/s00415-021-10608-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ali S, Kleib M, Paul P, Petrovskaya O, Kennedy M. Compassionate nursing care and the use of digital health technologies: a scoping review. Int J Nurs Stud. 2022 Mar;127:104161. doi: 10.1016/j.ijnurstu.2021.104161.S0020-7489(21)00306-0 [DOI] [PubMed] [Google Scholar]
- 79.Chikwetu L, Miao Y, Woldetensae MK, Bell D, Goldenholz DM, Dunn J. Does deidentification of data from wearable devices give us a false sense of security? A systematic review. Lancet Digit Health. 2023 Apr;5(4):e239–47. doi: 10.1016/S2589-7500(22)00234-5. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(22)00234-5 .S2589-7500(22)00234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barrios V, Cinza-Sanjurjo S, García-Alegría J, Freixa-Pamias R, Llordachs-Marques F, Molina CA, Santamaría A, Vivas D, Suárez Fernandez C. Role of telemedicine in the management of oral anticoagulation in atrial fibrillation: a practical clinical approach. Future Cardiol. 2022 Sep;18(9):743–54. doi: 10.2217/fca-2022-0044. https://www.futuremedicine.com/doi/abs/10.2217/fca-2022-0044?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 81.Burg F, Pscherer A, Opitz OG. Digital communication in visceral medicine: regulatory framework for digital communication. Visc Med. 2021 Oct 20;96(6):1–7. doi: 10.1159/000519359. doi: 10.1159/000519359.vis-0096-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng L, Liu F, Mao X, Peng W, Wang Y, Huang H, Duan M, Wang Y, Yuan C. The pediatric cancer survivors' user experiences with digital health interventions: a systematic review of qualitative data. Cancer Nurs. 2022 Jan;45(1):E68–82. doi: 10.1097/NCC.0000000000000885.00002820-202201000-00018 [DOI] [PubMed] [Google Scholar]
- 83.Chuang TY, Cheng W, Chiu YC, Fan YH, Chi CC, Chang CC, Liao CH. Free interactive counselling program in a mobile communication application for improving health education on indwelling ureteric stents after ureterorenoscopic lithotripsy: an observational study. Digit Health. 2022 Aug 04;8:20552076221117754. doi: 10.1177/20552076221117754. https://journals.sagepub.com/doi/10.1177/20552076221117754?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_20552076221117754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwards KJ, Maslin K, Andrade J, Jones RB, Shawe J. Mobile health as a primary mode of intervention for women at risk of or diagnosed with gestational diabetes mellitus: a scoping review. JBI Evid Synth. 2022 Sep 01;20(9):2195–243. doi: 10.11124/JBIES-21-00294.02174543-202209000-00003 [DOI] [PubMed] [Google Scholar]
- 85.Feldman N, Back D, Boland R, Torous J. A systematic review of mHealth application interventions for peripartum mood disorders: trends and evidence in academia and industry. Arch Womens Ment Health. 2021 Dec;24(6):881–92. doi: 10.1007/s00737-021-01138-z. https://europepmc.org/abstract/MED/33929636 .10.1007/s00737-021-01138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hilty DM, Serhal E, Crawford A. A telehealth and telepsychiatry economic cost analysis framework: scoping review. Telemed J E Health. 2023 Jan;29(1):23–37. doi: 10.1089/tmj.2022.0016. [DOI] [PubMed] [Google Scholar]
- 87.Intan Sabrina M, Defi IR. Telemedicine guidelines in South East Asia-a scoping review. Front Neurol. 2020 Jan 13;11:581649. doi: 10.3389/fneur.2020.581649. https://europepmc.org/abstract/MED/33519669 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones C, Miguel-Cruz A, Smith-MacDonald L, Cruikshank E, Baghoori D, Kaur Chohan A, Laidlaw A, White A, Cao B, Agyapong V, Burback L, Winkler O, Sevigny PR, Dennett L, Ferguson-Pell M, Greenshaw A, Brémault-Phillips S. Virtual trauma-focused therapy for military members, veterans, and public safety personnel with posttraumatic stress injury: systematic scoping review. JMIR Mhealth Uhealth. 2020 Sep 21;8(9):e22079. doi: 10.2196/22079. https://mhealth.jmir.org/2020/9/e22079/ v8i9e22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kvedarienė V, Burzdikaitė P, Česnavičiūtė I. mHealth and telemedicine utility in the monitoring of allergic diseases. Front Allergy. 2022 Sep 02;3:919746. doi: 10.3389/falgy.2022.919746. https://europepmc.org/abstract/MED/36118170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Odendaal WA, Anstey Watkins J, Leon N, Goudge J, Griffiths F, Tomlinson M, Daniels K. Health workers' perceptions and experiences of using mHealth technologies to deliver primary healthcare services: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2020 Mar 26;3(3):CD011942. doi: 10.1002/14651858.CD011942.pub2. https://europepmc.org/abstract/MED/32216074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skeen SJ, Cain D, Gamarel KE, Hightow-Weidman L, Reback CJ. mHealth for transgender and gender-expansive youth: harnessing gender-affirmative cross-disciplinary innovations to advance HIV prevention and care interventions. Mhealth. 2021 Apr 20;7:37. doi: 10.21037/mhealth-20-60. https://europepmc.org/abstract/MED/33898606 .mh-07-20-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tripepi M, Pizzocaro E, Giardino A, Frigerio I, Guglielmi A, Butturini G. Telemedicine and pancreatic cancer: a systematic review. Telemed J E Health. 2023 Mar;29(3):352–60. doi: 10.1089/tmj.2022.0140. [DOI] [PubMed] [Google Scholar]
- 93.Wong CA, Madanay F, Ozer EM, Harris SK, Moore M, Master SO, Moreno M, Weitzman ER. Digital health technology to enhance adolescent and young adult clinical preventive services: affordances and challenges. J Adolesc Health. 2020 Aug;67(2S):S24–33. doi: 10.1016/j.jadohealth.2019.10.018. https://linkinghub.elsevier.com/retrieve/pii/S1054-139X(19)30867-5 .S1054-139X(19)30867-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y, Chen H, Qazi H, Morita PP. Intervention and evaluation of mobile health technologies in management of patients undergoing chronic dialysis: scoping review. JMIR Mhealth Uhealth. 2020 Apr 03;8(4):e15549. doi: 10.2196/15549. https://mhealth.jmir.org/2020/4/e15549/ v8i4e15549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grogan J, Simmons Z. Telemedicine for the care of neuromuscular disorders. Curr Treat Options Neurol. 2020 May 11;22(6):17. doi: 10.1007/s11940-020-00625-5. https://link.springer.com/article/10.1007/s11940-020-00625-5 . [DOI] [Google Scholar]
- 96.Mossialos E, Thomson S, Ter Linden A. Information technology law and health systems in the European Union. Int J Technol Assess Health Care. 2004;20(4):498–508. doi: 10.1017/s0266462304001424. [DOI] [PubMed] [Google Scholar]
- 97.Sheikh A, Anderson M, Albala S, Casadei B, Franklin BD, Richards M, Taylor D, Tibble H, Mossialos E. Health information technology and digital innovation for national learning health and care systems. Lancet Digit Health. 2021 Jun;3(6):e383–96. doi: 10.1016/S2589-7500(21)00005-4. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(21)00005-4 .S2589-7500(21)00005-4 [DOI] [PubMed] [Google Scholar]
- 98.Oliver A, Mossialos E, Robinson R. Health technology assessment and its influence on health-care priority setting. Int J Technol Assess Health Care. 2004;20(1):1–10. doi: 10.1017/s026646230400073x. [DOI] [PubMed] [Google Scholar]
- 99.Mehrotra A, Bhatia RS, Snoswell CL. Paying for telemedicine after the pandemic. JAMA. 2021 Feb 02;325(5):431–2. doi: 10.1001/jama.2020.25706. https://europepmc.org/abstract/MED/33528545 .2775723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Kessel R, Hrzic R, O'Nuallain E, Weir E, Wong BL, Anderson M, Baron-Cohen S, Mossialos E. Digital health paradox: international policy perspectives to address increased health inequalities for people living with disabilities. J Med Internet Res. 2022 Feb 22;24(2):e33819. doi: 10.2196/33819. https://www.jmir.org/2022/2/e33819/ v24i2e33819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Kessel R, Wong BL, Clemens T, Brand H. Digital health literacy as a super determinant of health: more than simply the sum of its parts. Internet Interv. 2022 Feb 07;27:100500. doi: 10.1016/j.invent.2022.100500. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(22)00007-0 .S2214-7829(22)00007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Kessel R, Wong BL, Forman R, Gabrani J, Mossialos E. The European health data space fails to bridge digital divides. BMJ. 2022 Jul 08;378:e071913. doi: 10.1136/bmj-2022-071913. [DOI] [PubMed] [Google Scholar]
- 103.Vallespin B, Cornet J, Kotzeva A. Ensuring evidence-based safe and effective mHealth applications. Stud Health Technol Inform. 2016;222:248–61. [PubMed] [Google Scholar]
- 104.Asthana S, Jones R, Sheaff R. Why does the NHS struggle to adopt eHealth innovations? A review of macro, meso and micro factors. BMC Health Serv Res. 2019 Dec 21;19(1):984. doi: 10.1186/s12913-019-4790-x. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-019-4790-x .10.1186/s12913-019-4790-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Hattem NE, Silven AV, Bonten TN, Chavannes NH. COVID-19's impact on the future of digital health technology in primary care. Fam Pract. 2021 Nov 24;38(6):845–7. doi: 10.1093/fampra/cmab081. https://europepmc.org/abstract/MED/34268563 .6322430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Williams G, Fahy N, Aissat D, Lenormand M, Stüwe L, Zablit-Schmidt I, Delafuys S, Le DY, Azzopardi-Muscat N. COVID-19 and the use of digital health tools: opportunity amid crisis that could transform health care delivery. Eurohealth. 2022;28(1):29–34. https://apps.who.int/iris/bitstream/handle/10665/351081/Eurohealth-28-1-29-34-eng.pdf?sequence=1 . [Google Scholar]
- 107.Podlewska AM, van Wamelen DJ. Parkinson's disease and COVID-19: the effect and use of telemedicine. Int Rev Neurobiol. 2022;165:263–81. doi: 10.1016/bs.irn.2022.04.002. https://europepmc.org/abstract/MED/36208904 .S0074-7742(22)00031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerke S, Stern AD, Minssen T. Germany's digital health reforms in the COVID-19 era: lessons and opportunities for other countries. NPJ Digit Med. 2020 Jul 10;3:94. doi: 10.1038/s41746-020-0306-7. doi: 10.1038/s41746-020-0306-7.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stern AD, Brönneke J, Debatin JF, Hagen J, Matthies H, Patel S, Clay I, Eskofier B, Herr A, Hoeller K, Jaksa A, Kramer DB, Kyhlstedt M, Lofgren KT, Mahendraratnam N, Muehlan H, Reif S, Riedemann L, Goldsack JC. Advancing digital health applications: priorities for innovation in real-world evidence generation. Lancet Digit Health. 2022 Mar;4(3):e200–6. doi: 10.1016/S2589-7500(21)00292-2. https://linkinghub.elsevier.com/retrieve/pii/S2589-7500(21)00292-2 .S2589-7500(21)00292-2 [DOI] [PubMed] [Google Scholar]
- 110.Genovese S, Bengoa R, Bowis J, Harney M, Hauck B, Pinget M, Leers M, Stenvall T, Guldemond N. The European health data space: a step towards digital and integrated care systems. J Integr Care. 2022 Oct 12;30(4):363–72. doi: 10.1108/jica-11-2021-0059. https://www.emerald.com/insight/content/doi/10.1108/JICA-11-2021-0059/full/pdf?title=the-european-health-data-space-a-step-towards-digital-and-integrated-care-systems . [DOI] [Google Scholar]
- 111.Peek N, Sujan M, Scott P. Digital health and care in pandemic times: impact of COVID-19. BMJ Health Care Inform. 2020 Jun;27(1):e100166. doi: 10.1136/bmjhci-2020-100166. https://informatics.bmj.com/lookup/pmidlookup?view=long&pmid=32565418 .bmjhci-2020-100166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ruwaard J, Kok R. View of wild west eHealth: time to hold our horses? Eur Health Psychol. 2015;17(1):45–9. https://www.ehps.net/ehp/index.php/contents/article/view/765/pdf_44 . [Google Scholar]
- 113.Aremu EO, Heffernan JL, Kvedar JC. The difference in practice expense costs between telehealth and in-office care could serve as the basis for differential reimbursement structures. Telemed J E Health. 2022 Jun;28(6):912–6. doi: 10.1089/tmj.2021.0229. [DOI] [PubMed] [Google Scholar]
- 114.Bashshur RL, Doarn CR, Frenk JM, Kvedar JC, Shannon GW, Woolliscroft JO. Beyond the COVID pandemic, telemedicine, and health care. Telemed J E Health. 2020 Nov;26(11):1310–3. doi: 10.1089/tmj.2020.0328. [DOI] [PubMed] [Google Scholar]
- 115.Bediang G, Perrin C, Ruiz de Castañeda R, Kamga Y, Sawadogo A, Bagayoko CO, Geissbuhler A. The RAFT telemedicine network: lessons learnt and perspectives from a decade of educational and clinical services in low- and middle-incomes countries. Front Public Health. 2014 Oct 07;2:180. doi: 10.3389/fpubh.2014.00180. https://europepmc.org/abstract/MED/25340048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brooks E, Turvey C, Augusterfer EF. Provider barriers to telemental health: obstacles overcome, obstacles remaining. Telemed J E Health. 2013 Jun;19(6):433–7. doi: 10.1089/tmj.2013.0068. [DOI] [PubMed] [Google Scholar]
- 117.Cohen AB, Stump L, Krumholz HM, Cartiera M, Jain S, Scott Sussman L, Hsiao A, Lindop W, Ying AK, Kaul RL, Balcezak TJ, Tereffe W, Comerford M, Jacoby D, Navai N. Aligning mission to digital health strategy in academic medical centers. NPJ Digit Med. 2022 Jun 02;5(1):67. doi: 10.1038/s41746-022-00608-7. doi: 10.1038/s41746-022-00608-7.10.1038/s41746-022-00608-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smuck M, Odonkor CA, Wilt JK, Schmidt N, Swiernik MA. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digit Med. 2021 Mar 10;4(1):45. doi: 10.1038/s41746-021-00418-3. doi: 10.1038/s41746-021-00418-3.10.1038/s41746-021-00418-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haque SN. Telehealth beyond COVID-19. Psychiatr Serv. 2021 Jan 01;72(1):100–3. doi: 10.1176/appi.ps.202000368. [DOI] [PubMed] [Google Scholar]
- 120.Tenderich A. Virtual nation: telemedicine's breakout moment. J Diabetes Sci Technol. 2020 Jul;14(4):799–800. doi: 10.1177/1932296820929359. https://journals.sagepub.com/doi/abs/10.1177/1932296820929359?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alami H, Lehoux P, Auclair Y, de Guise M, Gagnon MP, Shaw J, Roy D, Fleet R, Ag Ahmed MA, Fortin JP. Artificial intelligence and health technology assessment: anticipating a new level of complexity. J Med Internet Res. 2020 Jul 07;22(7):e17707. doi: 10.2196/17707. https://www.jmir.org/2020/7/e17707/ v22i7e17707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stroetmann KA. Achieving the integrated and smart health and wellbeing paradigm: a call for policy research and action on governance and business models. Int J Med Inform. 2013 Apr;82(4):e29–37. doi: 10.1016/j.ijmedinf.2012.05.008.S1386-5056(12)00094-9 [DOI] [PubMed] [Google Scholar]
- 123.Flannery D, Jarrin R. Building a regulatory and payment framework flexible enough to withstand technological progress. Health Aff (Millwood) 2018 Dec;37(12):2052–9. doi: 10.1377/hlthaff.2018.05151. [DOI] [PubMed] [Google Scholar]
- 124.Tracy J, Rheuban K, Waters RJ, DeVany M, Whitten P. Critical steps to scaling telehealth for national reform. Telemed J E Health. 2008 Nov;14(9):990–4. doi: 10.1089/tmj.2008.0125. [DOI] [PubMed] [Google Scholar]
- 125.Fried BM, Weinreich G, Cavalier GM, Lester KJ. e-Health: technologic revolution meets regulatory constraint. Health Aff (Millwood) 2000 Nov;19(6):124–31. doi: 10.1377/hlthaff.19.6.124. [DOI] [PubMed] [Google Scholar]
- 126.Jenkins M, Levy J, Cohen E, Yoon S, Singer J, Mostashari F. Integration of self-management tools in personal and provider e-health records. Stud Health Technol Inform. 2009;146:179–84. [PubMed] [Google Scholar]
- 127.Ben-Zeev D. Mobile health for all: public-private partnerships can create a new mental health landscape. JMIR Ment Health. 2016 Jun 06;3(2):e26. doi: 10.2196/mental.5843. https://mental.jmir.org/2016/2/e26/ v3i2e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Woods L, Eden R, Canfell OJ, Nguyen KH, Comans T, Sullivan C. Show me the money: how do we justify spending health care dollars on digital health? Med J Aust. 2023 Feb 06;218(2):53–7. doi: 10.5694/mja2.51799. https://europepmc.org/abstract/MED/36502453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alverson DC, Edison K, Flournoy L, Korte B, Magruder C, Miller C. Telehealth tools for public health, emergency, or disaster preparedness and response: a summary report. Telemed J E Health. 2010 Jan;16(1):112–4. doi: 10.1089/tmj.2009.0149. [DOI] [PubMed] [Google Scholar]
- 130.Speedie SM, Ferguson AS, Sanders J, Doarn CR. Telehealth: the promise of new care delivery models. Telemed J E Health. 2008 Nov;14(9):964–7. doi: 10.1089/tmj.2008.0114. [DOI] [PubMed] [Google Scholar]
- 131.North F, Crane SJ, Takahashi PY, Ward WJ, Tulledge-Scheitel SM, Ytterberg K, Tangalos EG, Stroebel RJ. Telemedicine barriers associated with regional quality measures. Telemed J E Health. 2014 Feb;20(2):179–81. doi: 10.1089/tmj.2013.0167. [DOI] [PubMed] [Google Scholar]
- 132.Myers KM, Lieberman D. Telemental health: responding to mandates for reform in primary healthcare. Telemed J E Health. 2013 Jun;19(6):438–43. doi: 10.1089/tmj.2013.0084. [DOI] [PubMed] [Google Scholar]
- 133.Rajda J, Paz HL. The future of virtual care services: a payor's perspective. Telemed J E Health. 2020 Mar;26(3):267–9. doi: 10.1089/tmj.2019.0020. [DOI] [PubMed] [Google Scholar]
- 134.Amorim P, Brito D, Castelo-Branco M, Fàbrega C, Gomes da Costa F, Martins H, Gonçalves L, Gonçalves LM, Martin V, Milner J, Nêveda R, Ferreira AN, Pardo R, Peralta-Santos A, Pessoa T, Silva J, Salvador Vergès À. Telehealth opportunities in the COVID-19 pandemic early days: what happened, did not happen, should have happened, and must happen in the near future? Telemed J E Health. 2021 Oct;27(10):1194–9. doi: 10.1089/tmj.2020.0386. [DOI] [PubMed] [Google Scholar]
- 135.Bonnechère B, Kossi O, Mapinduzi J, Panda J, Rintala A, Guidetti S, Spooren A, Feys P. Mobile health solutions: an opportunity for rehabilitation in low- and middle income countries? Front Public Health. 2022 Jan 24;10:1072322. doi: 10.3389/fpubh.2022.1072322. https://europepmc.org/abstract/MED/36761328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Canfell OJ, Littlewood R, Burton-Jones A, Sullivan C. Digital health and precision prevention: shifting from disease-centred care to consumer-centred health. Aust Health Rev. 2022 Jun;46(3):279–83. doi: 10.1071/AH21063.AH21063 [DOI] [PubMed] [Google Scholar]
- 137.D'Anza B, Pronovost PJ. Digital health: unlocking value in a post-pandemic world. Popul Health Manag. 2022 Feb;25(1):11–22. doi: 10.1089/pop.2021.0031. [DOI] [PubMed] [Google Scholar]
- 138.van Eijk RP, Beelen A, Kruitwagen ET, Murray D, Radakovic R, Hobson E, Knox L, Helleman J, Burke T, Rubio Pérez MÁ, Reviers E, Genge A, Steyn FJ, Ngo S, Eaglesham J, Roes KC, van den Berg LH, Hardiman O, McDermott CJ. A road map for remote digital health technology for motor neuron disease. J Med Internet Res. 2021 Sep 22;23(9):e28766. doi: 10.2196/28766. https://www.jmir.org/2021/9/e28766/ v23i9e28766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jagannath S, Mikhael J, Nadeem O, Raje N. Digital health for patients with multiple myeloma: an unmet need. JCO Clin Cancer Inform. 2021 Oct;5:1096–105. doi: 10.1200/CCI.20.00145. https://ascopubs.org/doi/10.1200/CCI.20.00145?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 140.Patel S, Goldsack JC, Cordovano G, Downing A, Fields KK, Geoghegan C, Grewal U, Nieva J, Patel N, Rollison DE, Sah A, Said M, Van De Keere I, Way A, Wolff-Hughes DL, Wood WA, Robinson EJ. Advancing digital health innovation in Oncology: priorities for high-value digital transformation in cancer care. J Med Internet Res. 2023 Jan 04;25:e43404. doi: 10.2196/43404. https://www.jmir.org/2023//e43404/ v25i1e43404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Strauss G, Flannery JE, Vierra E, Koepsell X, Berglund E, Miller I, Lake JI. Meaningful engagement: a crossfunctional framework for digital therapeutics. Front Digit Health. 2022 Aug 11;4:890081. doi: 10.3389/fdgth.2022.890081. https://europepmc.org/abstract/MED/36052316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Willis HA, Gonzalez JC, Call CC, Quezada D, Scholars For Elevating Equity And Diversity Seed. Galán CA. Scholars for Elevating Equity and Diversity (SEED), Galán CA. Culturally responsive telepsychology and mHealth interventions for racial-ethnic minoritized youth: research gaps and future directions. J Clin Child Adolesc Psychol. 2022 Nov;51(6):1053–69. doi: 10.1080/15374416.2022.2124516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Y, Chandra S, Peña MT, Lal L, Summers RL, Swint JM. Framework for evaluating and developing sustainable telehealth programs. Telemed J E Health (Forthcoming) 2023 Jan 30; doi: 10.1089/tmj.2022.0407. [DOI] [PubMed] [Google Scholar]
- 144.Jayawardana S, Mossialos E. How should economic evaluation be used to measure value and set priorities in health care? AMA J Ethics. 2021 Aug 01;23(8):E613–8. doi: 10.1001/amajethics.2021.613. https://journalofethics.ama-assn.org/article/how-should-economic-evaluation-be-used-measure-value-and-set-priorities-health-care/2021-08 .amajethics.2021.613 [DOI] [PubMed] [Google Scholar]
- 145.Della Vecchia C, Leroy T, Bauquier C, Pannard M, Sarradon-Eck A, Darmon D, Dufour JC, Preau M. Willingness of French general practitioners to prescribe mHealth apps and devices: quantitative study. JMIR Mhealth Uhealth. 2022 Feb 11;10(2):e28372. doi: 10.2196/28372. https://mhealth.jmir.org/2022/2/e28372/ v10i2e28372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muehlensiepen F, Knitza J, Marquardt W, Engler J, Hueber A, Welcker M. Acceptance of telerheumatology by rheumatologists and general practitioners in Germany: nationwide cross-sectional survey study. J Med Internet Res. 2021 Mar 29;23(3):e23742. doi: 10.2196/23742. https://www.jmir.org/2021/3/e23742/ v23i3e23742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Treskes RW, Wildbergh TX, Schalij MJ, Scherptong RW. Expectations and perceived barriers to widespread implementation of eHealth in cardiology practice: results from a national survey in the Netherlands. Neth Heart J. 2019 Jan;27(1):18–23. doi: 10.1007/s12471-018-1199-9. https://europepmc.org/abstract/MED/30488379 .10.1007/s12471-018-1199-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Antoniotti NM, Drude KP, Rowe N. Private payer telehealth reimbursement in the United States. Telemed J E Health. 2014 Jun;20(6):539–43. doi: 10.1089/tmj.2013.0256. [DOI] [PubMed] [Google Scholar]
- 149.Avanesova AA, Shamliyan TA. Worldwide implementation of telemedicine programs in association with research performance and health policy. Health Policy Technol. 2019 Jun;8(2):179–91. doi: 10.1016/j.hlpt.2019.04.001. https://www.sciencedirect.com/science/article/abs/pii/S2211883718302636 . [DOI] [Google Scholar]
- 150.Bos WH, van Tubergen A, Vonkeman HE. Telemedicine for patients with rheumatic and musculoskeletal diseases during the COVID-19 pandemic; a positive experience in the Netherlands. Rheumatol Int. 2021 Mar;41(3):565–73. doi: 10.1007/s00296-020-04771-6. https://europepmc.org/abstract/MED/33449162 .10.1007/s00296-020-04771-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brady CJ, D'Amico S, Withers N, Kim BY. Using public datasets to identify priority areas for ocular telehealth. Telemed J E Health. 2021 Nov;27(11):1293–8. doi: 10.1089/tmj.2020.0433. https://europepmc.org/abstract/MED/33600257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yu Q, Zhu C, Feng S, Xu L, Hu S, Chen H, Chen H, Yao S, Wang X, Chen Y. Economic burden and health care access for patients with inflammatory bowel diseases in China: web-based survey study. J Med Internet Res. 2021 Jan 05;23(1):e20629. doi: 10.2196/20629. https://www.jmir.org/2021/1/e20629/ v23i1e20629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harju A, Neufeld J. The impact of the Medicaid expansion on telemental health utilization in four midwestern states. Telemed J E Health. 2021 Nov;27(11):1260–7. doi: 10.1089/tmj.2020.0476. [DOI] [PubMed] [Google Scholar]
- 154.Zhao M, Hamadi H, Haley DR, Xu J, White-Williams C, Park S. Telehealth: advances in alternative payment models. Telemed J E Health. 2020 Dec;26(12):1492–9. doi: 10.1089/tmj.2019.0294. [DOI] [PubMed] [Google Scholar]
- 155.Ranganathan C, Balaji S. Key factors affecting the adoption of telemedicine by ambulatory clinics: insights from a statewide survey. Telemed J E Health. 2020 Feb;26(2):218–25. doi: 10.1089/tmj.2018.0114. [DOI] [PubMed] [Google Scholar]
- 156.Alishahi Tabriz A, Turner K, Williams Jr D, Babu N, North S, Shea CM. Association of financial factors and telemedicine adoption for heart attack and stroke care among rural and urban hospitals: a longitudinal study. Telemed J E Health. 2022 Jun;28(6):781–8. doi: 10.1089/tmj.2021.0341. https://europepmc.org/abstract/MED/34559014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ágh T, Hadžiabdić MO, Garuoliene K, Granas AG, Aarnio E, Menditto E, Gregório J, Barnestein-Fonseca P, Mevsim V, Kardas P, European Network to Advance Best Practices and Technology on Medication Adherence (ENABLE) Reimbursed medication adherence enhancing interventions in European countries: results of the EUREcA study. Front Pharmacol. 2022 Jun 17;13:892240. doi: 10.3389/fphar.2022.892240. https://europepmc.org/abstract/MED/35784711 .892240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith AC, Armfield NR, Croll J, Gray LC. A review of Medicare expenditure in Australia for psychiatric consultations delivered in person and via videoconference. J Telemed Telecare. 2012 Apr;18(3):169–71. doi: 10.1258/jtt.2012.SFT111.jtt.2012.SFT111 [DOI] [PubMed] [Google Scholar]
- 159.Philippi P, Baumeister H, Apolinário-Hagen J, Ebert DD, Hennemann S, Kott L, Lin J, Messner EM, Terhorst Y. Acceptance towards digital health interventions - model validation and further development of the unified theory of acceptance and use of technology. Internet Interv. 2021 Sep 20;26:100459. doi: 10.1016/j.invent.2021.100459. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(21)00099-3 .S2214-7829(21)00099-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Powers B, Bucher A. An economic impact model for estimating the value to health systems of a digital intervention for diabetes primary care: development and usefulness study. JMIR Form Res. 2022 Sep 26;6(9):e37745. doi: 10.2196/37745. https://formative.jmir.org/2022/9/e37745/ v6i9e37745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Borowiack E, Marzec M, Nowotarska A, Jarosz J, Orkisz A, Prząda-Machno P. Chance of reimbursement for ADD-ON therapies in Poland and in the world - review of the reimbursement recommendations. Przegl Epidemiol. 2018;72(1):99–109. http://www.przeglepidemiol.pzh.gov.pl/pobierz-artykul?id=2203 . [PubMed] [Google Scholar]
- 162.Ganapathy K, Das S, Reddy S, Thaploo V, Nazneen A, Kosuru A, Shankar Nag U. Digital health care in public private partnership mode. Telemed J E Health. 2021 Dec;27(12):1363–71. doi: 10.1089/tmj.2020.0499. [DOI] [PubMed] [Google Scholar]
- 163.Lewis T, Synowiec C, Lagomarsino G, Schweitzer J. E-health in low- and middle-income countries: findings from the center for health market innovations. Bull World Health Organ. 2012 May 01;90(5):332–40. doi: 10.2471/BLT.11.099820. https://europepmc.org/abstract/MED/22589566 .BLT.11.099820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moro Visconti R, Morea D. Healthcare digitalization and pay-for-performance incentives in smart hospital project financing. Int J Environ Res Public Health. 2020 Mar 30;17(7):2318. doi: 10.3390/ijerph17072318. https://www.mdpi.com/resolver?pii=ijerph17072318 .ijerph17072318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu H, Deng Z, Wang B, Gupta S. How does service price influence patients’ decisions? An examination of the free-market pricing mechanism in online health communities. Electron Mark. 2021 Mar 12;31:877–93. doi: 10.1007/s12525-020-00453-0. https://link.springer.com/article/10.1007/s12525-020-00453-0 . [DOI] [Google Scholar]
- 166.Jamaladin H, van de Belt TH, Luijpers LC, de Graaff FR, Bredie SJ, Roeleveld N, van Gelder MM. Mobile apps for blood pressure monitoring: systematic search in app stores and content analysis. JMIR Mhealth Uhealth. 2018 Nov 14;6(11):e187. doi: 10.2196/mhealth.9888. https://mhealth.jmir.org/2018/11/e187/ v6i11e187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Whitten P, Buis L. Private payer reimbursement for telemedicine services in the United States. Telemed J E Health. 2007 Feb;13(1):15–23. doi: 10.1089/tmj.2006.0028. [DOI] [PubMed] [Google Scholar]
- 168.Wu H, Lu N. Service provision, pricing, and patient satisfaction in online health communities. Int J Med Inform. 2018 Feb;110:77–89. doi: 10.1016/j.ijmedinf.2017.11.009.S1386-5056(17)30423-9 [DOI] [PubMed] [Google Scholar]
- 169.Neufeld JD, Doarn CR, Aly R. State policies influence Medicare telemedicine utilization. Telemed J E Health. 2016 Jan;22(1):70–4. doi: 10.1089/tmj.2015.0044. [DOI] [PubMed] [Google Scholar]
- 170.Neufeld JD, Doarn CR. Telemedicine spending by Medicare: a snapshot from 2012. Telemed J E Health. 2015 Aug;21(8):686–93. doi: 10.1089/tmj.2014.0185. [DOI] [PubMed] [Google Scholar]
- 171.Bynum AB, Irwin CA, Cranford CO, Denny GS. The impact of telemedicine on patients' cost savings: some preliminary findings. Telemed J E Health. 2003;9(4):361–7. doi: 10.1089/153056203772744680. [DOI] [PubMed] [Google Scholar]
- 172.Rajan B, Sainathan A, Agnihothri S, Cui L. The promise of mHealth for chronic disease management under different payment systems. Manuf Serv Oper Manag. 2022;24(6):3158–76. doi: 10.1287/msom.2022.1143. https://pubsonline.informs.org/doi/abs/10.1287/msom.2022.1143 . [DOI] [Google Scholar]
- 173.Qin X, Hsieh CR. Understanding and addressing the treatment gap in mental healthcare: economic perspectives and evidence from China. Inquiry. 2020 Jan;57:46958020950566. doi: 10.1177/0046958020950566. https://journals.sagepub.com/doi/10.1177/0046958020950566?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nuño-Solinís R, Urizar E, Merino M, Del Barrio J, Errea Rodríguez M. Validation study of a value-based digital health questionnaire. Int J Environ Res Public Health. 2022 Jun 08;19(12):7034. doi: 10.3390/ijerph19127034. https://www.mdpi.com/resolver?pii=ijerph19127034 .ijerph19127034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ajayi KV, Wachira E, Onyeaka HK, Montour T, Olowolaju S, Garney W. The use of digital health tools for health promotion among women with and without chronic diseases: insights from the 2017-2020 health information national trends survey. JMIR Mhealth Uhealth. 2022 Aug 19;10(8):e39520. doi: 10.2196/39520. https://mhealth.jmir.org/2022/8/e39520/ v10i8e39520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bärlund M, Takala L, Tianen L, Kellokumpu-Lehtinen PL. Real-world evidence of implementing eHealth enables fluent symptom-based follow-up of a growing number of patients with breast cancer with the same healthcare resources. Clin Breast Cancer. 2022 Apr;22(3):261–8. doi: 10.1016/j.clbc.2021.09.005. https://linkinghub.elsevier.com/retrieve/pii/S1526-8209(21)00263-9 .S1526-8209(21)00263-9 [DOI] [PubMed] [Google Scholar]
- 177.Ector GI, Verweij L, Hermens RP, Blijlevens NM. Filling the gaps of patient information needs and information perception in chronic myeloid leukemia with the patient-physician co-produced web-based platform CMyLife. Patient Educ Couns. 2022 Mar;105(3):686–94. doi: 10.1016/j.pec.2021.06.025. https://linkinghub.elsevier.com/retrieve/pii/S0738-3991(21)00424-9 .S0738-3991(21)00424-9 [DOI] [PubMed] [Google Scholar]
- 178.Khairat S, Wallace E, Bohlmann A, Zebrowski A, Stabile K, Yao Y, Lakdawala A, Edson B, Catlett T. Digital health experiences of incarcerated populations using telemedicine in North Carolina prisons. J Patient Exp. 2022 Apr 19;9:23743735221092611. doi: 10.1177/23743735221092611. https://journals.sagepub.com/doi/10.1177/23743735221092611?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_23743735221092611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liou H, Lane C, Huang C, Mookadam M, Joseph M, Hecker DuVal J. Eye movement desensitization and reprocessing in a primary care setting: assessing utility and comparing efficacy of virtual versus in-person methods. Telemed J E Health. 2022 Sep;28(9):1359–66. doi: 10.1089/tmj.2021.0454. [DOI] [PubMed] [Google Scholar]
- 180.Papavero S, Fracasso A, Ramaglia P, Cicchetti A, de Belvis AG, Ferrara FM. Telemedicine has a social impact: an Italian national study for the evaluation of the cost-opportunity for patients and caregivers and the measurement of carbon emission savings. Telemed J E Health (Forthcoming) 2023 Jan 13; doi: 10.1089/tmj.2022.0333. [DOI] [PubMed] [Google Scholar]
- 181.Radó N, Girasek E, Békási S, Győrffy Z. Digital technology access and health-related internet use among people experiencing homelessness in Hungary: quantitative survey. J Med Internet Res. 2022 Oct 19;24(10):e38729. doi: 10.2196/38729. https://www.jmir.org/2022/10/e38729/ v24i10e38729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ratcliff CL, Krakow M, Greenberg-Worisek A, Hesse BW. Digital health engagement in the us population: insights from the 2018 health information national trends survey. Am J Public Health. 2021 Jul;111(7):1348–51. doi: 10.2105/AJPH.2021.306282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rossano A, Crijns T, Ring D, Reichenberg J. Clinician preferences for current and planned future use of telemedicine. Telemed J E Health. 2022 Sep;28(9):1293–9. doi: 10.1089/tmj.2021.0486. [DOI] [PubMed] [Google Scholar]
- 184.Teferi GH, Tilahun BC, Guadie HA, Amare AT. Smartphone medical app use and associated factors among physicians at referral hospitals in Amhara region, North Ethiopia, in 2019: cross-sectional study. JMIR Mhealth Uhealth. 2021 Mar 26;9(3):e19310. doi: 10.2196/19310. https://mhealth.jmir.org/2021/3/e19310/ v9i3e19310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tilahun BB, Thompson NR, Bautista JF, Sankary LR, Stanton S, Punia V. Telepsychology may improve treatment adherence in patients with psychogenic nonepileptic seizures. Telemed J E Health. 2022 Aug;28(8):1159–65. doi: 10.1089/tmj.2021.0463. [DOI] [PubMed] [Google Scholar]
- 186.Uncovska M, Freitag B, Meister S, Fehring L. Patient acceptance of prescribed and fully reimbursed mHealth apps in Germany: an UTAUT2-based online survey study. J Med Syst. 2023 Jan 27;47(1):14. doi: 10.1007/s10916-023-01910-x. https://europepmc.org/abstract/MED/36705853 .10.1007/s10916-023-01910-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bouvet R, Desmarais P, Minvielle E. Legal and organizational barriers to the development of eHealth in France. Med Law. 2015 Sep;34(1):361–79. [PubMed] [Google Scholar]
- 188.Peeters JM, Krijgsman JW, Brabers AE, Jong JD, Friele RD. Use and uptake of eHealth in general practice: a cross-sectional survey and focus group study among health care users and general practitioners. JMIR Med Inform. 2016 Apr 06;4(2):e11. doi: 10.2196/medinform.4515. https://medinform.jmir.org/2016/2/e11/ v4i2e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Swinkels IC, Huygens MW, Schoenmakers TM, Oude Nijeweme-D'Hollosy W, van Velsen L, Vermeulen J, Schoone-Harmsen M, Jansen YJ, van Schayck OC, Friele R, de Witte L. Lessons learned from a living lab on the broad adoption of eHealth in primary health care. J Med Internet Res. 2018 Mar 29;20(3):e83. doi: 10.2196/jmir.9110. https://www.jmir.org/2018/3/e83/ v20i3e83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Silven AV, Petrus AH, Villalobos-Quesada M, Dirikgil E, Oerlemans CR, Landstra CP, Boosman H, van Os HJ, Blanker MH, Treskes RW, Bonten TN, Chavannes NH, Atsma DE, Teng YK. Telemonitoring for patients with COVID-19: recommendations for design and implementation. J Med Internet Res. 2020 Sep 02;22(9):e20953. doi: 10.2196/20953. https://www.jmir.org/2020/9/e20953/ v22i9e20953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dritsakis G, Trenkova L, Śliwińska-Kowalska M, Brdarić D, Pontoppidan NH, Katrakazas P, Bamiou DE. Public health policy-making for hearing loss: stakeholders' evaluation of a novel eHealth tool. Health Res Policy Syst. 2020 Oct 29;18(1):125. doi: 10.1186/s12961-020-00637-2. https://health-policy-systems.biomedcentral.com/articles/10.1186/s12961-020-00637-2 .10.1186/s12961-020-00637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brebner JA, Brebner EM, Ruddick-Bracken H. Experience-based guidelines for the implementation of telemedicine services. J Telemed Telecare. 2005;11 Suppl 1:3–5. doi: 10.1258/1357633054461778. [DOI] [PubMed] [Google Scholar]
- 193.Freund J, Buntrock C, Braun L, Thielecke J, Baumeister H, Berking M, Ebert DD, Titzler I. Digital prevention of depression for farmers? A qualitative study on participants' experiences regarding determinants of acceptance and satisfaction with a tailored guided internet intervention program. Internet Interv. 2022 Aug 09;29:100566. doi: 10.1016/j.invent.2022.100566. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(22)00073-2 .S2214-7829(22)00073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yagiz JI, Goderis G. The impact of the COVID-19 pandemic on eHealth use in the daily practice and life of Dutch-speaking general practitioners in Belgium: qualitative study with semistructured interviews. JMIR Form Res. 2022 Nov 28;6(11):e41847. doi: 10.2196/41847. https://formative.jmir.org/2022/11/e41847/ v6i11e41847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Spanhel K, Schweizer JS, Wirsching D, Lehr D, Baumeister H, Bengel J, Sander L. Cultural adaptation of internet interventions for refugees: results from a user experience study in Germany. Internet Interv. 2019 May 21;18:100252. doi: 10.1016/j.invent.2019.100252. https://linkinghub.elsevier.com/retrieve/pii/S2214-7829(19)30012-0 .S2214-7829(19)30012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dahlhausen F, Zinner M, Bieske L, Ehlers JP, Boehme P, Fehring L. Physicians' attitudes toward prescribable mHealth apps and implications for adoption in Germany: mixed methods study. JMIR Mhealth Uhealth. 2021 Nov 23;9(11):e33012. doi: 10.2196/33012. https://mhealth.jmir.org/2021/11/e33012/ v9i11e33012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dahlhausen F, Zinner M, Bieske L, Ehlers JP, Boehme P, Fehring L. There's an app for that, but nobody's using it: insights on improving patient access and adherence to digital therapeutics in Germany. Digit Health. 2022 Jul 03;8:20552076221104672. doi: 10.1177/20552076221104672. https://journals.sagepub.com/doi/10.1177/20552076221104672?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_20552076221104672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Voogt MP, Opmeer BC, Kastelein AW, Jaspers M, Peute L. Obstacles to Successful Implementation of eHealth Applications into Clinical Practice. Stud Health Technol Inform. 2018;247:521–5. [PubMed] [Google Scholar]
- 199.Cremers HP, Theunissen L, Hiddink J, Kemps H, Dekker L, van de Ven R, Monroy M, van Waes L, Scheele K, van Veghel D. Successful implementation of eHealth interventions in healthcare: development of an eHealth implementation guideline. Health Serv Manage Res. 2021 Nov;34(4):269–78. doi: 10.1177/0951484821994421. [DOI] [PubMed] [Google Scholar]
- 200.Bally EL, Cesuroglu T. Toward integration of mHealth in primary care in the Netherlands: a qualitative analysis of stakeholder perspectives. Front Public Health. 2020 Jan 15;7:407. doi: 10.3389/fpubh.2019.00407. https://europepmc.org/abstract/MED/32010660 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Agbakoba R, McGee-Lennon M, Bouamrane MM, Watson N, Mair F. Implementing a national Scottish digital health and wellbeing service at scale: a qualitative study of stakeholders' views. Stud Health Technol Inform. 2015;216:487–91. [PubMed] [Google Scholar]
- 202.Webers C, Beckers E, Boonen A, van Eijk-Hustings Y, Vonkeman H, van de Laar M, van Tubergen A. Development, usability and acceptability of an integrated eHealth system for spondyloarthritis in the Netherlands (SpA-Net) RMD Open. 2019 Apr 11;5(1):e000860. doi: 10.1136/rmdopen-2018-000860. https://rmdopen.bmj.com/lookup/pmidlookup?view=long&pmid=31168405 .rmdopen-2018-000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Christie HL, Boots LM, Peetoom K, Tange HJ, Verhey FR, de Vugt ME. Developing a plan for the sustainable implementation of an electronic health intervention (partner in balance) to support caregivers of people with dementia: case study. JMIR Aging. 2020 Jun 25;3(1):e18624. doi: 10.2196/18624. https://aging.jmir.org/2020/1/e18624/ v3i1e18624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bae YS, Kim KH, Choi SW, Ko T, Lim JS, Piao M. Satisfaction and usability of an information and communications technology-based system by clinically healthy patients with COVID-19 and medical professionals: cross-sectional survey and focus group interview study. JMIR Form Res. 2021 Aug 26;5(8):e26227. doi: 10.2196/26227. https://formative.jmir.org/2021/8/e26227/ v5i8e26227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Velayati F, Ayatollahi H, Hemmat M, Dehghan R. Key components and critical factors for developing a telehealth business framework: a qualitative study. BMC Med Inform Decis Mak. 2021 Dec 04;21(1):339. doi: 10.1186/s12911-021-01707-3. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-021-01707-3 .10.1186/s12911-021-01707-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schoebel V, Wayment C, Gaiser M, Page C, Buche J, Beck AJ. Telebehavioral health during the COVID-19 pandemic: a qualitative analysis of provider experiences and perspectives. Telemed J E Health. 2021 Aug;27(8):947–54. doi: 10.1089/tmj.2021.0121. [DOI] [PubMed] [Google Scholar]
- 207.Lin JC, Humphries MD, Shutze WP, Aalami OO, Fischer UM, Hodgson KJ. Telemedicine platforms and their use in the coronavirus disease-19 era to deliver comprehensive vascular care. J Vasc Surg. 2021 Feb;73(2):392–8. doi: 10.1016/j.jvs.2020.06.051. https://linkinghub.elsevier.com/retrieve/pii/S0741-5214(20)31473-7 .S0741-5214(20)31473-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hermaszewska S, Sin J. End-user perspectives on the development of an online intervention for parents of children on the autism spectrum. Autism. 2021 Jul;25(5):1234–45. doi: 10.1177/1362361320984895. https://journals.sagepub.com/doi/abs/10.1177/1362361320984895?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Faes L, Rosenblatt A, Schwartz R, Touhami S, Ventura CV, Chatziralli IP, Ruiz-Medrano J, Vogt D, Savastano A, Ruiz-Garcia H, Pohlmann D, Loewenstein A, International Retinal Collaborative Overcoming barriers of retinal care delivery during a pandemic-attitudes and drivers for the implementation of digital health: a global expert survey. Br J Ophthalmol. 2021 Dec;105(12):1738–43. doi: 10.1136/bjophthalmol-2020-316882.bjophthalmol-2020-316882 [DOI] [PubMed] [Google Scholar]
- 210.Moshi MR, Tooher R, Merlin T. Development of a health technology assessment module for evaluating mobile medical applications. Int J Technol Assess Health Care. 2020 Jun;36(3):252–61. doi: 10.1017/S0266462320000288.S0266462320000288 [DOI] [PubMed] [Google Scholar]
- 211.Thielscher C, Doarn CR. Long-term future of telemedicine in Germany: the patient's, physician's, and payer's perspective. Telemed J E Health. 2008 Sep;14(7):701–6. doi: 10.1089/tmj.2007.0104. [DOI] [PubMed] [Google Scholar]
- 212.Powell AC, Bowman MB, Harbin HT. Reimbursement of apps for mental health: findings from interviews. JMIR Ment Health. 2019 Aug 06;6(8):e14724. doi: 10.2196/14724. https://mental.jmir.org/2019/8/e14724/ v6i8e14724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Belghiti J, Oget-Gendre C, Berthon AF, Fagon JY. Supporting innovation in health care: a short experience in a dedicated unit of the French ministry of public health. J Visc Surg. 2021 Jun;158(3S):S6–11. doi: 10.1016/j.jviscsurg.2021.01.009.S1878-7886(21)00021-7 [DOI] [PubMed] [Google Scholar]
- 214.Al-Mondhiry J, D'Ambruoso S, Pietras C, Strouse T, Benzeevi D, Arevian AC, Wells KB. Co-created mobile apps for palliative care using community-partnered participatory research: development and usability study. JMIR Form Res. 2022 Jun 23;6(6):e33849. doi: 10.2196/33849. https://formative.jmir.org/2022/6/e33849/ v6i6e33849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Eaves ER, Doerry E, Lanzetta SA, Kruithoff KM, Negron K, Dykman K, Thoney O, Harper CC. Applying user-centered design in the development of a supportive mHealth app for women in substance use recovery. Am J Health Promot. 2023 Jan;37(1):56–64. doi: 10.1177/08901171221113834. https://europepmc.org/abstract/MED/35815770 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lackie ME, Parrilla JS, Lavery BM, Kennedy AL, Ryan D, Shulman B, Brotto LA. Digital health needs of women with postpartum depression: focus group study. J Med Internet Res. 2021 Jan 06;23(1):e18934. doi: 10.2196/18934. https://www.jmir.org/2021/1/e18934/ v23i1e18934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meyer CL, Surmeli A, Hoeflin Hana C, Narla NP. Perceptions on a mobile health intervention to improve maternal child health for Syrian refugees in Turkey: opportunities and challenges for end-user acceptability. Front Public Health. 2022 Nov 22;10:1025675. doi: 10.3389/fpubh.2022.1025675. https://europepmc.org/abstract/MED/36483243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Panda N, Sinyard R, Margo J, Henrich N, Cauley CE, Onnela JP, Haynes AB, Brindle ME. Perceptions of mobile health technology in elective surgery: a qualitative study of north American surgeons. Ann Surg. 2023 Mar 01;277(3):423–8. doi: 10.1097/SLA.0000000000005208.00000658-900000000-93274 [DOI] [PubMed] [Google Scholar]
- 219.Shih P, Prokopovich K, Degeling C, Street J, Carter SM. Direct-to-consumer detection of atrial fibrillation in a smartwatch electrocardiogram: medical overuse, medicalisation and the experience of consumers. Soc Sci Med. 2022 Jun;303:114954. doi: 10.1016/j.socscimed.2022.114954.S0277-9536(22)00260-X [DOI] [PubMed] [Google Scholar]
- 220.Smits M, Nacar M, D S Ludden G, van Goor H. Stepwise design and evaluation of a values-oriented ambient intelligence healthcare monitoring platform. Value Health. 2022 Jun;25(6):914–23. doi: 10.1016/j.jval.2021.11.1372. https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(21)03228-9 .S1098-3015(21)03228-9 [DOI] [PubMed] [Google Scholar]
- 221.Van Grootven B, Irusta LA, Christiaens W, Mistiaen P, De Meester C, Cornelis J, Dierckx de Casterlé B, Van Durme T, van Achterberg T. Experiences with the implementation of remote monitoring in patients with COVID-19: a qualitative study with patients and healthcare professionals. J Nurs Scholarsh. 2023 Jan;55(1):67–78. doi: 10.1111/jnu.12814. https://europepmc.org/abstract/MED/36165577 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van Velthoven MH, Cordon C, Challagalla G. Digitization of healthcare organizations: the digital health landscape and information theory. Int J Med Inform. 2019 Apr;124:49–57. doi: 10.1016/j.ijmedinf.2019.01.007.S1386-5056(18)30321-6 [DOI] [PubMed] [Google Scholar]
- 223.Salas-Vega S, Haimann A, Mossialos E. Big data and health care: challenges and opportunities for coordinated policy development in the EU. Health Syst Reform. 2015 May 19;1(4):285–300. doi: 10.1080/23288604.2015.1091538. [DOI] [PubMed] [Google Scholar]
- 224.Recognising the value of digital health apps: an assessment of five European healthcare systems. MedTech Europe. 2021. Nov 16, [2022-11-11]. https://www.medtecheurope.org/wp-content/uploads/2021/11/2111_v4.8_mte_dht_reimbursement16.11.2021-2.pdf .
- 225.From innovation to implementation: eHealth in the WHO European region. World Health Organization. 2016. [2022-12-15]. https://apps.who.int/iris/bitstream/handle/10665/326317/9789289051378-eng.pdf .
- 226.Schliess F, Affini Dicenzo T, Gaus N, Bourez JM, Stegbauer C, Szecsenyi J, Jacobsen M, Müller-Wieland D, Kulzer B, Heinemann L. The German fast track toward reimbursement of Digital Health Applications (DiGA): opportunities and challenges for manufacturers, healthcare providers, and people with diabetes. J Diabetes Sci Technol (Forthcoming) 2022 Sep 03;:19322968221121660. doi: 10.1177/19322968221121660. https://journals.sagepub.com/doi/abs/10.1177/19322968221121660?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lluch M. Incentives for telehealthcare deployment that support integrated care: a comparative analysis across eight European countries. Int J Integr Care. 2013 Nov 04;13:e042. doi: 10.5334/ijic.1062. https://europepmc.org/abstract/MED/24250282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Holterman S, Hettinga M, Buskens E, Lahr M. Factors influencing procurement of digital healthcare: a case study in Dutch district nursing. Int J Health Policy Manag. 2021 Aug 29;11(9):1883–93. doi: 10.34172/ijhpm.2021.115. https://europepmc.org/abstract/MED/34634888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gensorowsky D, Witte J, Batram M, Greiner W. Market access and value-based pricing of digital health applications in Germany. Cost Eff Resour Alloc. 2022 Jun 13;20(1):25. doi: 10.1186/s12962-022-00359-y. https://resource-allocation.biomedcentral.com/articles/10.1186/s12962-022-00359-y .10.1186/s12962-022-00359-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Christie HL, Boots LM, Hermans I, Govers M, Tange HJ, Verhey FR, de Vugt M. Business models of eHealth interventions to support informal caregivers of people with dementia in the Netherlands: analysis of case studies. JMIR Aging. 2021 Jun 03;4(2):e24724. doi: 10.2196/24724. https://aging.jmir.org/2021/2/e24724/ v4i2e24724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ahlqvist J, Kalliola M. How can digital therapeutics help Europe? Sitra. 2021. [2022-12-30]. https://www.sitra.fi/app/uploads/2021/11/sitra-how-can-digital-therapeutics-help-europe.pdf .
- 232.Powell AC, Torous JB, Firth J, Kaufman KR. Generating value with mental health apps. BJPsych Open. 2020 Feb 05;6(2):e16. doi: 10.1192/bjo.2019.98. https://europepmc.org/abstract/MED/32019619 .S205647241900098X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Reid HW, Proeschold-Bell RJ, Makarushka C, Melgar Vega KD, Huchko M, Jeronimo J, Vasudevan L. Using the consolidated framework for implementation research to inform the design of the mobile Inspección Visual con Ácido Acético system: mixed methods case study. JMIR Form Res. 2022 Jun 23;6(6):e32577. doi: 10.2196/32577. https://formative.jmir.org/2022/6/e32577/ v6i6e32577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bush J, Barlow DE, Echols J, Wilkerson J, Bellevin K. Impact of a mobile health application on user engagement and pregnancy outcomes among Wyoming Medicaid members. Telemed J E Health. 2017 Nov;23(11):891–8. doi: 10.1089/tmj.2016.0242. https://europepmc.org/abstract/MED/28481167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Drake C, Lian T, Cameron B, Medynskaya K, Bosworth HB, Shah K. Understanding telemedicine's "new normal": variations in telemedicine use by specialty line and patient demographics. Telemed J E Health. 2022 Jan;28(1):51–9. doi: 10.1089/tmj.2021.0041. https://europepmc.org/abstract/MED/33769092 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tzeng YH, Yin WH, Lin KC, Wei J, Liou HR, Sung HJ, Lang HC. Factors associated with the utilization of outpatient virtual clinics: retrospective observational study using multilevel analysis. J Med Internet Res. 2022 Aug 12;24(8):e40288. doi: 10.2196/40288. https://www.jmir.org/2022/8/e40288/ v24i8e40288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dansky KH, Palmer L, Shea D, Bowles KH. Cost analysis of telehomecare. Telemed J E Health. 2001;7(3):225–32. doi: 10.1089/153056201316970920. [DOI] [PubMed] [Google Scholar]
- 238.Armstrong AW, Dorer DJ, Lugn NE, Kvedar JC. Economic evaluation of interactive teledermatology compared with conventional care. Telemed J E Health. 2007 Apr;13(2):91–9. doi: 10.1089/tmj.2006.0035. [DOI] [PubMed] [Google Scholar]
- 239.Das S, Wang J, Chen SY, Chen CE. Telemental health collaborative care medication management: implementation and outcomes. Telemed J E Health. 2022 Jul;28(7):1035–43. doi: 10.1089/tmj.2021.0401. https://europepmc.org/abstract/MED/34939839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Owusu JT, Wang P, Wickham RE, Varra AA, Chen C, Lungu A. Real-world evaluation of a large-scale blended care-cognitive behavioral therapy program for symptoms of anxiety and depression. Telemed J E Health. 2022 Oct;28(10):1412–20. doi: 10.1089/tmj.2021.0590. https://europepmc.org/abstract/MED/35263185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ritterband LM, Thorndike FP, Morin CM, Gerwien R, Enman NM, Xiong R, Luderer HF, Edington S, Braun S, Maricich YA. Real-world evidence from users of a behavioral digital therapeutic for chronic insomnia. Behav Res Ther. 2022 Jun;153:104084. doi: 10.1016/j.brat.2022.104084. https://linkinghub.elsevier.com/retrieve/pii/S0005-7967(22)00055-9 .S0005-7967(22)00055-9 [DOI] [PubMed] [Google Scholar]
- 242.Scheer J, Areias AC, Molinos M, Janela D, Moulder R, Lains J, Bento V, Yanamadala V, Dias Correia F, Costa F. Engagement and utilization of a complete remote digital care program for musculoskeletal pain management in urban and rural areas across the United States: longitudinal cohort study. JMIR Mhealth Uhealth. 2023 Mar 16;11:e44316. doi: 10.2196/44316. https://mhealth.jmir.org/2023//e44316/ v11i1e44316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tan LF, Chan YH, Tay A, Jayasundram J, Low NA, Merchant RA. Practicality and reliability of self vs administered rapid geriatric assessment mobile app. J Nutr Health Aging. 2021;25(9):1064–9. doi: 10.1007/s12603-021-1672-9. https://europepmc.org/abstract/MED/34725662 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tumeh IB, Bergerot CD, Lee D, Philip EJ, Freitas-Júnior R. mHealth program for patients with advanced cancer receiving treatment in a public health hospital in Brazil. Psychooncology. 2023 Jan;32(1):125–32. doi: 10.1002/pon.6059. [DOI] [PubMed] [Google Scholar]
- 245.Melchiorre MG, Papa R, Quattrini S, Lamura G, Barbabella F, on behalf of ICARE4EU Consortium Integrated care programs for people with multimorbidity in European countries: eHealth adoption in health systems. Biomed Res Int. 2020 Apr 08;2020:9025326. doi: 10.1155/2020/9025326. doi: 10.1155/2020/9025326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Voncken-Brewster V, Tange H, Moser A, Nagykaldi Z, de Vries H, van der Weijden T. Integrating a tailored e-health self-management application for chronic obstructive pulmonary disease patients into primary care: a pilot study. BMC Fam Pract. 2014 Jan 08;15:4. doi: 10.1186/1471-2296-15-4. https://bmcfampract.biomedcentral.com/articles/10.1186/1471-2296-15-4 .1471-2296-15-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Balchander D, Cabrera CI, Zack B, Porter S, Sunshine J, D'Anza B. Assessing telehealth through the lens of the provider: considerations for the post-COVID-19 era. Telemed J E Health. 2022 Dec;28(12):1806–16. doi: 10.1089/tmj.2021.0508. [DOI] [PubMed] [Google Scholar]
- 248.Romm KL, Nilsen L, Gjermundsen K, Holter M, Fjell A, Melle I, Repål A, Lobban F. Remote care for caregivers of people with psychosis: mixed methods pilot study. JMIR Ment Health. 2020 Jul 28;7(7):e19497. doi: 10.2196/19497. https://mental.jmir.org/2020/7/e19497/ v7i7e19497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chukwu E, Garg L, Eze G. Mobile health insurance system and associated costs: a cross-sectional survey of primary health centers in Abuja, Nigeria. JMIR Mhealth Uhealth. 2016 May 17;4(2):e37. doi: 10.2196/mhealth.4342. https://mhealth.jmir.org/2016/2/e37/ v4i2e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ayyoubzadeh SM, Shirkhoda M, R Niakan Kalhori S, Mohammadzadeh N, Zakerabasali S. A smartphone remote monitoring app to follow up colorectal cancer survivors: requirement analysis. JMIR Cancer. 2022 Jan 05;8(1):e18083. doi: 10.2196/18083. https://cancer.jmir.org/2022/1/e18083/ v8i1e18083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Becker ER, Shegog R, Savas LS, Frost EL, Coan SP, Healy CM, Spinner SW, Vernon SW. Parents' experience with a mobile health intervention to influence human papillomavirus vaccination decision making: mixed methods study. JMIR Pediatr Parent. 2022 Feb 21;5(1):e30340. doi: 10.2196/30340. https://pediatrics.jmir.org/2022/1/e30340/ v5i1e30340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.LoBuono DL, Shea KS, Tovar A, Leedahl SN, Mahler L, Xu F, Lofgren IE. Acceptance and perception of digital health for managing nutrition in people with Parkinson's disease and their caregivers and their digital competence in the United States: a mixed-methods study. Health Sci Rep. 2021 Nov 11;4(4):e412. doi: 10.1002/hsr2.412. https://europepmc.org/abstract/MED/34796282 .HSR2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Saha S, Quazi ZS. Does digitally enabling frontline health workers improve coverage and quality of maternal and child health services? Findings from a mixed methods evaluation of TECHO+ in Gujarat. Front Public Health. 2022 Jul 22;10:856561. doi: 10.3389/fpubh.2022.856561. https://europepmc.org/abstract/MED/35958841 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lewkowicz D, Wohlbrandt AM, Bottinger E. Digital therapeutic care apps with decision-support interventions for people with low back pain in Germany: cost-effectiveness analysis. JMIR Mhealth Uhealth. 2022 Feb 07;10(2):e35042. doi: 10.2196/35042. https://mhealth.jmir.org/2022/2/e35042/ v10i2e35042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Aardoom JJ, Dingemans AE, van Ginkel JR, Spinhoven P, Van Furth EF, Van den Akker-van Marle ME. Cost-utility of an internet-based intervention with or without therapist support in comparison with a waiting list for individuals with eating disorder symptoms: a randomized controlled trial. Int J Eat Disord. 2016 Dec;49(12):1068–76. doi: 10.1002/eat.22587. [DOI] [PubMed] [Google Scholar]
- 256.Ambrens M, van Schooten KS, Lung T, Clemson L, Close JC, Howard K, Lord SR, Zijlstra GA, Tiedemann A, Valenzuela T, Vandelanotte C, Chow J, McInerney G, Miles L, Woodbury A, Delbaere K. Economic evaluation of the e-Health StandingTall balance exercise programme for fall prevention in people aged 70 years and over. Age Ageing. 2022 Jun 01;51(6):afac130. doi: 10.1093/ageing/afac130.6604744 [DOI] [PubMed] [Google Scholar]
- 257.Li J, Sun L, Hou Y, Chen L. Cost-effectiveness analysis of a mobile-based intervention for patients with type 2 diabetes mellitus. Int J Endocrinol. 2021 Jul 01;2021:8827629. doi: 10.1155/2021/8827629. doi: 10.1155/2021/8827629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Luo X, Xu W, Ming WK, Jiang X, Yuan Q, Lai H, Huang C, Zhong X. Cost-effectiveness of mobile health-based integrated care for atrial fibrillation: model development and data analysis. J Med Internet Res. 2022 Apr 19;24(4):e29408. doi: 10.2196/29408. https://www.jmir.org/2022/4/e29408/ v24i4e29408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McManus RJ, Little P, Stuart B, Morton K, Raftery J, Kelly J, Bradbury K, Zhang J, Zhu S, Murray E, May CR, Mair FS, Michie S, Smith P, Band R, Ogburn E, Allen J, Rice C, Nuttall J, Williams B, Yardley L, HOME BP investigators Home and Online Management and Evaluation of Blood Pressure (HOME BP) using a digital intervention in poorly controlled hypertension: randomised controlled trial. BMJ. 2021 Jan 19;372:m4858. doi: 10.1136/bmj.m4858. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=33468518 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Allen AR, Smith J, Hobbs MJ, Loughnan SA, Sharrock M, Newby JM, Andrews G, Mahoney AE. Internet-delivered cognitive behaviour therapy for post-traumatic stress disorder: a randomised controlled trial and outcomes in routine care. Behav Cogn Psychother. 2022 Nov;50(6):649–55. doi: 10.1017/S1352465822000285.S1352465822000285 [DOI] [PubMed] [Google Scholar]
- 261.Loohuis AM, Van Der Worp H, Wessels NJ, Dekker JH, Slieker-Ten Hove MC, Berger MY, Vermeulen KM, Blanker M. Cost-effectiveness of an app-based treatment for urinary incontinence in comparison with care-as-usual in Dutch general practice: a pragmatic randomised controlled trial over 12 months. BJOG. 2022 Aug;129(9):1538–45. doi: 10.1111/1471-0528.17191. https://europepmc.org/abstract/MED/35460163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Powell J, Williams V, Atherton H, Bennett K, Yang Y, Davoudianfar M, Hellsing A, Martin A, Mollison J, Shanyinde M, Yu LM, Griffiths KM. Effectiveness and cost-effectiveness of a self-guided internet intervention for social anxiety symptoms in a general population sample: randomized controlled trial. J Med Internet Res. 2020 Jan 10;22(1):e16804. doi: 10.2196/16804. https://www.jmir.org/2020/1/e16804/ v22i1e16804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Stuhlreyer J, Roder C, Krug F, Zöllner C, Flor H, Klinger R. A digital application and augmented physician rounds reduce postoperative pain and opioid consumption after primary total knee replacement (TKR): a randomized clinical trial. BMC Med. 2022 Dec 05;20(1):469. doi: 10.1186/s12916-022-02638-0. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-022-02638-0 .10.1186/s12916-022-02638-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Open API architecture policy. National Health Service, England. 2014. [2022-11-30]. https://www.england.nhs.uk/wp-content/uploads/2018/09/open-api-policy.pdf .
- 265.Evidence standards framework (ESF) for digital health technologies. National Institute for Health and Care Excellence. 2018. [2022-11-11]. https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies .
- 266.The Fast-Track process for digital health applications (DiGA) according to section 139e SGB V. Federal Institute for Drugs and Medical Devices. 2022. [2022-10-15]. https://www.bfarm.de/SharedDocs/Downloads/EN/MedicalDevices/DiGA_Guide.pdf?__blob=publicationFile&v=2 .
- 267.Cowey A. Establishing the NHS digital academy: future vision and implementation areas for expansion. National Health Service Health Education, England. 2021. [2023-01-15]. https://digital-transformation.hee.nhs.uk/binaries/content/assets/digital-transformation/nhs-digital-academy/digital-academy-future-vision-report---mar-2021.pdf .
- 268.Bradford WD, Kleit A, Krousel-Wood MA, Re RM. Comparing willingness to pay for telemedicine across a chronic heart failure and hypertension population. Telemed J E Health. 2005 Aug;11(4):430–8. doi: 10.1089/tmj.2005.11.430. [DOI] [PubMed] [Google Scholar]
- 269.Chang J, Savage SJ, Waldman DM. Estimating willingness to pay for online health services with discrete-choice experiments. Appl Health Econ Health Policy. 2017 Aug;15(4):491–500. doi: 10.1007/s40258-017-0316-z.10.1007/s40258-017-0316-z [DOI] [PubMed] [Google Scholar]
- 270.Chen N, Liu P. Assessing elderly user preference for telehealth solutions in China: exploratory quantitative study. JMIR Mhealth Uhealth. 2022 Jan 12;10(1):e27272. doi: 10.2196/27272. https://mhealth.jmir.org/2022/1/e27272/ v10i1e27272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.NHS apps library. National Health Service Digit. 2021. [2023-01-31]. https://digital.nhs.uk/services/nhs-apps-library .
- 272.Early Value Assessment (EVA) for medtech. National Institute for Health and Care Excellence. 2023. [2023-02-20]. https://www.nice.org.uk/about/what-we-do/eva-for-medtech .
- 273.Aanestad M, Grisot M, Hanseth O, Vassilakopoulou P. Information Infrastructures within European Health Care: Working with the Installed Base. Cham, Switzerland: Springer; 2017. Strategies for building eHealth infrastructures. [PubMed] [Google Scholar]
- 274.Ramirez M, Duran MC, Pabiniak CJ, Hansen KE, Kelley A, Ralston JD, McCurry SM, Teri L, Penfold RB. Family caregiver needs and preferences for virtual training to manage behavioral and psychological symptoms of dementia: interview study. JMIR Aging. 2021 Feb 10;4(1):e24965. doi: 10.2196/24965. https://aging.jmir.org/2021/1/e24965/ v4i1e24965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cox NS, Dal Corso S, Hansen H, McDonald CF, Hill CJ, Zanaboni P, Alison JA, O'Halloran P, Macdonald H, Holland AE. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021 Jan 29;1(1):CD013040. doi: 10.1002/14651858.CD013040.pub2. https://europepmc.org/abstract/MED/33511633 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Buntin MB. Confronting challenges in the US health care system: potential opportunity in a time of crisis. JAMA. 2021 Apr 13;325(14):1399–400. doi: 10.1001/jama.2021.1471.2778509 [DOI] [PubMed] [Google Scholar]
- 277.VC digital health deal count by country worldwide HY1 2020. Statista. 2022. [2023-01-27]. https://www.statista.com/statistics/1063913/vc-backed-digital-health-deals-in-select-countries/
- 278.Tikkanen R, Osborn R, Mossialos E, Djordjevic A, Wharton G. International profiles of health care systems. The Commonwealth Fund. 2020. [2022-11-26]. https://www.commonwealthfund.org/sites/default/files/2020-12/International_Profiles_of_Health_Care_Systems_Dec2020.pdf .
- 279.Folland S, Goodman A, Stano M. The economics of health and health care. 8th edition. London, United Kingdom: Routledge, Taylor & Francis Group; 2017. [Google Scholar]
- 280.Anderson M, Pitchforth E, Asaria M, Brayne C, Casadei B, Charlesworth A, Coulter A, Franklin BD, Donaldson C, Drummond M, Dunnell K, Foster M, Hussey R, Johnson P, Johnston-Webber C, Knapp M, Lavery G, Longley M, Clark JM, Majeed A, McKee M, Newton JN, O'Neill C, Raine R, Richards M, Sheikh A, Smith P, Street A, Taylor D, Watt RG, Whyte M, Woods M, McGuire A, Mossialos E. LSE-Lancet Commission on the future of the NHS: re-laying the foundations for an equitable and efficient health and care service after COVID-19. Lancet. 2021 May 22;397(10288):1915–78. doi: 10.1016/S0140-6736(21)00232-4. https://eprints.whiterose.ac.uk/174078/ S0140-6736(21)00232-4 [DOI] [PubMed] [Google Scholar]
- 281.Wegwijzer bekostiging digitale zorg 2023. Dutch Healthcare Authority. 2022. [2022-11-11]. https://puc.overheid.nl/nza/doc/PUC_655318_22/1/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional figures, tables, and the search strategy per database.


