Abstract
Background:
One of the most debilitating symptoms in breast cancer survivors is cancer-related fatigue (CRF). CRF weakens patients’ physical, cognitive, and occupational functions. It is associated with poorer quality of life and may reduce recurrence-free and overall survival. This study aimed to evaluate the efficacy of a group psychoeducational intervention in improving CRF in breast cancer patients.
Materials and Methods:
Fifty breast cancer patients who suffered from CRF were randomly assigned to receive a group psychoeducational intervention or control group. This study was designed as an eight weeks clinical trial. The psychoeducational intervention mainly consisted of concentrative movement therapy and energy conservation strategies. Primary outcome measures were the changes in the Fatigue Visual Analogue scale, Cancer Fatigue scale, and Piper Fatigue scale at the study endpoint. Measure assessments were made on four occasions: at baseline, after the intervention, one week, and four weeks post intervention. Statistical analysis was performed using SPSS26.
Results:
The intervention improved CRF significantly (P < 0.001). All subscales of the Cancer Fatigue scale and the sensory, affective, and cognitive subscales of the Piper Fatigue scale showed statistically significant effects (P < 0.001) at all time points. However, the behavioral subscale of the Piper Fatigue scale was different only at the end of the study (P < 0.001).
Conclusions:
The group psychoeducational intervention improved CRF significantly. All the sensory, behavioral, physical, affective, and cognitive subscales improved. Accessible and confirmatory treatment can help patients to cope with fatigue in communities.
Keywords: Breast cancer, energy resources conservation, fatigue, group psychotherapy, movement
INTRODUCTION
Breast cancer is the most common cancer in women worldwide, with a standardized incidence rate of about 28 per 100,000 people in Iran.[1,2] Every year, 1.7 million women are diagnosed with breast cancer.[3] And the incidence rates have risen by up to 5% per year in many populations in developing countries.[4] The number of breast cancer survivors has also increased because of advances in breast cancer diagnosis and treatment.[5]
One of the most debilitating symptoms in breast cancer survivors before, during, and after treatment is cancer-related fatigue (CRF).[6] The National Comprehensive Cancer Network (NCCN) is interpreted CRF as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.” CRF can be distinguished from fatigue experienced by healthy individuals as it is more severe and distressing and not being relieved by rest.[7] Depending on the patient population, type of treatment, and methodology employed, it is estimated that about 25-99% of oncology patients experience fatigue at different steps from detection to some point during the treatment course.[8,9,10] Nearly 25% of breast cancer patients suffer from severe fatigue.[11] Cancer-related fatigue weakens patients’ physical, cognitive, and occupational function.[12] Cancer-related fatigue is associated with poorer quality of life, and may reduce recurrence-free and overall survival.[13] Cancer-related fatigue has disabling effects on patients while causing distress and uncomforting in patients’ family members and caregivers.[14] The etiology of CRF is complex. However, tumor-related factors, comorbid conditions, psychological problems, and iatrogenic factors are believed to play a role.[9]
National Comprehensive Cancer Network guidelines recommend that all oncology patients should be screened for fatigue at their first clinical visit and then regularly during the treatment course and post-treatment. Both non-pharmacological and pharmacological interventions could be used for CRF management[7] Non-pharmacological treatments, including exercise and psychological interventions, are more effective and should be used as first-line therapies.[15] National Comprehensive Cancer Network recommended psychoeducation as a helpful strategy in CRF management.[7] Psychoeducation is a didactic intervention for a disorder and its treatment. Psychoeducation empowers patients to cope with the illness and improves treatment adherence and efficacy.[16] We designed a psychoeducational intervention consisting of concentrative body movement therapy (CMT) and energy conservation.
Concentrative body movement therapy is a body-oriented psychotherapy method consisting of practical sessions, which assure self-awareness during resting and moving.[17] We used CMT because it has both cognitive and mild motor components. To the best of our knowledge, CMT has not been used for CRF management.
Energy conservation is a programmed management of an individual's energy resources to prevent debilitation. It includes a practical activity that potentiates patients to set realistic priorities and balance, rest and activity during periods of high fatigue. Other strategies consist of pacing oneself, delegating less essential activities, post-pone all non-essential activities, and programming high-energy activities at times of peak energy.[18] Barsevick et al., in a randomized clinical trial RCT) demonstrated that energy conservation intervention had a modest but significant effect in reducing tiredness and recommended designing more studies using combination strategies for symptom management.[18]
Psychoeducational modalities have shown a beneficial effect on CRF outcomes in many trials,[19] while some studies reported controversial results.[20,21] Definite conclusions are not yet possible.[19]
But, despite the emphasis on before trials, a few published studies used combination therapy for CRF. Most of the previous treatments were individual rather than group based. We couldn’t find any study that assesses CMT for modulating CRF. Prior studies recommended evaluating treatments in other communities to localize treatment methods. Therefore, we designed a group psychoeducational intervention for energy/fatigue management in Iranian breast cancer patients.
MATERIALS AND METHODS
The study was designed as an eight-week randomized, single-blind clinical trial. The patients were recruited from Seyedoshohada hospital affiliated with Isfahan University of Medical Science, Ala medical center, a charity for oncology patients, and Kashani hospital affiliated with Shahrekord University of Medical Science, from January 2018 to May 2018.
Considering Pillai, V = 0.30, the number of groups = 2, response variables = 4, α error = 0.05, and power (1-β) 90%, the minimum sample size was calculated as 42 individuals with the actual power of 0.908. By a 10% dropout rate (DR), the total number of samples was considered 50 via N adjusted = n (here 42)/(1-DR).[22] Fifty-five patients with CRF who met the study inclusion criteria were selected and included by a convenience sampling method. Finally, fifty eligible patients were allocated to either the intervention (n = 25) or control group (n = 25) using a random number generator software.
All the patients provided written informed consent. The ethics committee of the Shahrekord University of Medical Science approved this study with a reference number of IR. SKUMS. REC. 1396. 247. The registration identifier in the Iranian Registry of Clinical Trials is IRCT 2018623040193N2. The study was designed concerning extended consolidated standards of reporting trials (consort) statements for non-pharmacologic treatments.[23]
Women aged 18–60 years old, with at least two months’ history of stage I or II breast cancer undergoing chemotherapy were recruited for this study. In routine visits of breast cancer patients from January 2018 to May 2018, the oncologist introduced those complaining about fatigue to the research coordinator. Patients interested in participating in the study were assessed for eligibility. The eligibility criteria were: a Fatigue Visual Analogue Scale score (F-VAS) ≥7, hemoglobin ≥11, ability to read and write, lack of metastasis, lack of any chronic cardiac or respiratory diseases, severe bone pain, and balance problems. Exclusion criteria were: acute respiratory, cardiac, or infectious diseases, hemoglobin <11, uncontrolled hypothyroidism, receiving any treatment (drug or psychotherapy) for fatigue in the past, and history of any major psychiatric illnesses for which the patient was hospitalized or medicated.
Intervention
Before the beginning of therapy sessions, both control and intervention groups were assessed for their fatigue characteristics [session 0, Table 1]. A trained psychiatrist provided education for all the patients weekly. Patients were divided into six–eight person groups.
Table 1.
Topics and content of the therapy sessions
| Session | Title | Content | Topic | Methods | Material | Time |
|---|---|---|---|---|---|---|
| 0 | Evaluation | Evaluation of fatigue characteristics in the interview | When did the first fatigue start? | Short speeches | Overhead projector and transparencies, | 60 min |
| When did you first discover this fatigue is different from the usual fatigue? | ||||||
| How was the severity of fatigue during the course of treatment or after the diagnosis? | ||||||
| What does alleviate your fatigue? | ||||||
| What will exacerbate your fatigue? | ||||||
| How does fatigue affect your daily activities or meaningful and enjoyable activities in your life? | ||||||
| Assessing the Fatigue Visual Analogue Scale (FVAS) | According to various conditions (At rest-post-exercise-post recovery) | |||||
| Evaluation of effective and treatable factors in fatigue | Pain, depression, emotional distress, and sleep disorders | |||||
| Nutrition status: Weight changes/calorie intake, water imbalance, and electrolyte | ||||||
| Activity level: Changes in exercise or activity patterns | ||||||
| Comorbidities | ||||||
| 1 | Introduction and Education | Introducing members, Expressing the biography and hearing the story of everyone | Short speeches Group discussion | Overhead projector and transparencies | 90 min | |
| Provide general education | Teaching well-known patterns of fatigue during and after treatment | |||||
| Ensure that fatigue is not a prominent indicator of disease progression | ||||||
| Expecting fatigue and accepting the problem | ||||||
| Identifying fatigue-inducing activities | ||||||
| Nutrition and proper fluid intake | ||||||
| Energy conservation methods and prioritization | ||||||
| Division of activities into smaller components | ||||||
| Balance between work/rest/recreation | ||||||
| Precise realistic and appropriate goals | ||||||
| Postponing unnecessary activities | ||||||
| Mental fatigue and mental-enhancing activities | ||||||
| Discussion about the meaning and effects of fatigue | What effect does cancer have on your definition (who am I?) | |||||
| Study psychological and social stressors and their negative impact | ||||||
| Explain the causes of avoiding activity | ||||||
| Teaching practical activities | Daily self-evaluation of fatigue and energy | |||||
| Environmental or operational changes | ||||||
| Planning important daily activities at minimum fatigue times | ||||||
| Remove unnecessary and stressful activities | ||||||
| Save energy by managing activity (divided into smaller steps) | ||||||
| Night sleep monitoring | ||||||
| Teaching the cycle of hyperactivity/rest | ||||||
| Set rest periods | ||||||
| List activities and prioritize them in terms of importance and urgency | ||||||
| 2 | Rope Practice | Evaluation of the effectiveness of the fatigue intervention program presented in the previous session | Short speeches Group discussion Practical exercises | Overhead projector and transparencies, Rope | 90 min | |
| A gradual increase in activity | Walking, gardening, watching birds | |||||
| Planned exercise in the power range | ||||||
| Planning for enjoyable activities | ||||||
| Implementing rope practice | Two people should hold a rope at a distance of 2.5 meter apart. Close your eyes. Do I feel the rope in my hands? Do I feel the person holding the rope on the other side? Do I feel the connection between me and the other one? How is this connection? Do I like to be stronger or not? Would I like to talk to the other one or make a message? Don’t I feel that standing with the rope in hand should be tedious? Maybe a better feeling can be made. Can I give a message to my opponent? | |||||
| Can I get this message by moving the rope gently? I’m looking at the distance with me and the other one. I try to make a more appropriate distance. Will I allow myself to be close to or far away, or do I want the other side to do this? Is there a movement in your hand? Is it in the rope? How is the connection between me and another one? Tight or loose? Do I like it to be tighter or not? How much would we like to get closer in this relationship? Not active or active? Open your eyes whenever you feel the right and proper distance. | ||||||
| 3 | Ball Practice | Review the effectiveness of the program to increase the activity provided in the previous meeting | Do I feel the rope in my hands? Do I feel the person holding the rope on the other side? | Overhead projector and transparencies, Ball | 90 mis | |
| Examining people’s emotions about rope practice | ||||||
| Implementing ball practice | Both of you lean toward the ball | |||||
| Close your eyes. | ||||||
| We start from the foot and go to the neck. | ||||||
| We breathe deeply with each other. | ||||||
| Focus on heart rate and number and depth of breathing | ||||||
| Put your hands on the ball. Does the ball move under my hand? | ||||||
| Does the other person move the ball? Would I like to give him a message through the ball? | ||||||
| I’m now trying to move with the ball. Without the ball falling. | ||||||
| Can I tell my friend which side to move with the ball? | ||||||
| What do I feel? Does he pay attention to my messages? | ||||||
| I now rely more on the ball. I leaned on the ball that someone else leaned on. | ||||||
| How do we feel? Is this backrest comfortable? Am I tired? | ||||||
| I want to send a message to my opponent through stretching and moving. | ||||||
| Now, slowly retract the nut into the nut and open the eyes. | ||||||
An outline of the proposed strategies used in each session in the intervention group is presented in Table 1. In the control group, patients received three 90 minutes weekly educational sessions about how to deal with lymphedema and hair loss, stress management, managing family matters, external prosthesis, and how to access self-help groups and support centers. At the end of each session, a booklet containing the provided education was given to patients. Participants were encouraged to implement lifestyle changes and register their fatigue daily in a notebook for self-monitoring.
A trained psychologist assessed the patients at baseline, after the intervention, one week, and four weeks after the end of the study, using FVAS, Cancer Fatigue scale (CFS), and revised-Piper Fatigue scale (PFS-R). The patient and the psychologist did not inform about the group type.
Tools
To fatigue evaluation, F-VAS, CFS, and PFS-R were used. The F-VAS is a valid, reliable, and sensitive self-report tool that could be used to assess the overall effectiveness of healthcare interventions. Patients rated their fatigue on a continuum anchored at each end by the following declarations: “not at all tired” or “extremely tired.”[24] The CFS is a questionnaire with a brief rating scale to evaluate the nature of fatigue. It consists of 15 items and 3 subscales (physical, affective, and cognitive) in which patients clarify their fatigue on a scale of 1(not at all) to 5 (very much).[25] The PFS-R is a multidimensional fatigue measure consisting of 22 questions, including subdomains of behavioral (6 items), sensory (5 items), affective (5 items), and cognitive/mood (6 items).[26] The reliability and validity of CFS and PFS have been confirmed in Persian.[27,28]
Statistical analysis
Initially, control and intervention groups were compared with respect to baseline demographic characteristics shown as mean ± standard deviation (SD) or number (percent). We used an independent sample t-test and Chi-square analysis to compare quantitative and qualitative variables between the two groups, respectively. The repeated variables (baseline, after the last session, one week, and four weeks after the end of the study) were regarded as dependent variables, groups as a factor, while we controlled age on multivariate analysis of covariance (MANCOVA). Multiple comparisons were done with Bonferroni. The assumptions of MANCOVA were checked. We have three continuous dependent variables and one continuous covariate (age). Observations are independent. There was a linear relationship between each pair of dependent variables within each group of the independent variable. Also, there was a linear relationship between the covariate and each dependent variable within each group of the independent variable. There was homogeneity of regression slopes. There was the normality distribution via the Shapiro–Wilk test. Also, no significant multivariate outliers. The multivariate outliers were checked by Mardia's test. We used SPSS26 for statistical analysis. We considered P value < 0.05 as statistically significant.
RESULTS
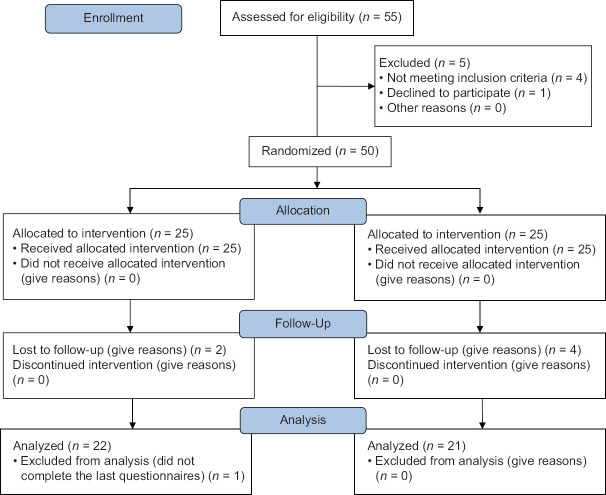
Fifty-five patients were evaluated for eligibility [Figure 1]. Five patients were excluded because of not meeting inclusion criteria (4) or unwillingness to participate (1), and ultimately, 25 patients were assigned to each group. Four patients from the control group and three from the intervention group refused to complete the study [Figure 1]. Table 2 displays the baseline demographics of the patients in each group. The age of the participants ranged from 32 to 60 years, with a mean of 46.27 ± 7.52 years. The groups were similar in respecting age, marital status, stage of cancer, and occupational and literacy status. All patients were under chemotherapy and recruited at either the third or fourth cycle of their treatment course, suffering from fatigue for more than six months. There are significant differences between intervention and control groups in all the time points (after the last session, one week, and four weeks) concerning F-VAS, CFS, and PFS (P < 0.001) [Table 3 and Figure 2]. All three subscales of CFS (physical, affective, and cognitive) significantly decreased in the intervention group comparing the control group (P < 0.001) [Table 3]. Multivariate analysis of covariance demonstrated significant differences in affective, sensory, and cognitive PFS subscales at all time points. However, the behavioral subscale was different between groups only at the end of the study (P < 0.001) [Table 3].
Figure 1.
Study profile
Table 2.
Comparison of baseline characteristics in intervention and control groups
| Characteristics | Intervention group | Control group | P |
|---|---|---|---|
| Age (year) | 45.91±7.57 | 46.67±1.67 | 0.74* |
| Marital status n (%) | |||
| Married | 16 (72.7) | 15 (71.4) | 0.92** |
| Single | 4 (18.2) | 5 (23.8) | |
| Divorced | 2 (9.1) | 1 (4.7) | |
| Employment status n (%) | |||
| Employed | 18 (81.8) | 17 (81) | 0.94** |
| Housewife | 4 (18.2) | 4 (19) | |
| Cancer stage n (%) | |||
| I | 7 (31.8) | 8 (38.1) | 0.66** |
| II | 15 (68.2) | 13 (61.9) | |
| Education status n (%) | |||
| No post-school qualification | 16 (72.7) | 14 (66.6) | 0.66** |
| College certificate | 6 (27.2) | 15 (33.3) |
*t-test, **χ2 test
Table 3.
Analysis of changes in fatigue experiences
| Intervention group | Control group | P | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | SD | Mean | SD | ||
| F-VAS | |||||
| Preintervention | 8.23 | 1.07 | 8.19 | 1.08 | 0.82 |
| Post-intervention | 3.95 | 1.09 | 5.29 | 0.78 | <0.001 |
| Follow-up at 1 wk | 4.18 | 1.14 | 5.48 | 0.87 | <0.001 |
| Follow-up at 4 wks | 4.23 | 1.34 | 6.38 | 1.28 | <0.001 |
| P CFS (Total score) | <0.001* | ||||
| Preintervention | 3.79 | 0.26 | 3.84 | 0.25 | 0.51 |
| Post-intervention | 2.28 | 0.23 | 2.83 | 0.28 | <0.001 |
| Follow-up at 1 wk | 2.47 | 0.21 | 3.06 | 0.02 | <0001 |
| Follow-up at 4 wks | 2.53 | 0.22 | 3.22 | 0.24 | <0.001 |
| P | <0.001* | ||||
| CFS (Physical subscale) | |||||
| Pre intervention | 3.8 | 0.44 | 3.84 | 0.46 | 0.71 |
| Post intervention | 2.35 | 0.39 | 2.76 | 0.49 | 0.003 |
| Follow up at 1 wk | 2.52 | 0.30 | 3.05 | 0.37 | <0.001 |
| Follow up at 4 wks | 2.55 | 0.31 | 3.13 | 0.44 | <0.001 |
| P CFS (Affective subscale) | <0.001* | ||||
| Preintervention | 3.82 | 0.36 | 3.87 | 0.52 | 0.68 |
| Post-intervention | 2.39 | 0.30 | 3.06 | 0.47 | <0.001 |
| Follow-up at 1 wk | 2.57 | 0.28 | 3.20 | 0.41 | <0.001 |
| Follow-up at 4 wks | 2.69 | 0.53 | 3.42 | 0.51 | <0.001 |
| P | <0.001* | ||||
| CFS (Cognitive subscale) | |||||
| Preintervention | 3.75 | 0.72 | 3.80 | 0.51 | 0.89 |
| Post-intervention | 2.05 | 0.47 | 2.71 | 0.48 | <0.001 |
| Follow up at 1 wk | 2.30 | 0.45 | 2.95 | 0.33 | <0.001 |
| Follow up at 4 wks | 2.38 | 0.51 | 3.18 | 0.42 | <0.001 |
| P PFS (Total score) | <0.001* | ||||
| Preintervention | 7.92 | 0.32 | 7.73 | 0.23 | 0.036 |
| Post-intervention | 3.81 | 0.18 | 4.26 | 0.21 | <0.001** |
| Follow-up at 1 wk | 4.08 | 0.34 | 4.63 | 0.45 | <0.001** |
| Follow-up at 4 wks | 4.21 | 0.21 | 4.98 | 0.28 | <0.001** |
| P | <0.001* | ||||
| PFS (Behavioral subscale) | |||||
| Preintervention | 7.87 | 0.63 | 7.79 | 0.43 | 0.60 |
| Post-intervention | 4.17 | 0.41 | 4.33 | 0.28 | 0.18 |
| Follow-up at 1 wk | 4.48 | 0.45 | 4.54 | 0.68 | 0.7 |
| Follow-up at 4 wks | 4.33 | 0.51 | 4.95 | 0.36 | <0.001 |
| P | <0.001* | ||||
| PFS (Affective subscale) | |||||
| Preintervention | 7.86 | 0.72 | 7.71 | 0.48 | 0.68 |
| Post-intervention | 3.67 | 0.45 | 4.20 | 0.44 | <0.001 |
| Follow-up at 1 wk | 3.68 | 1.04 | 4.80 | 1.00 | <0.001 |
| Follow-up at 4 wks | 4.22 | 0.42 | 4.89 | 0.50 | <0.001 |
| P | <0.001* | ||||
| PFS (Sensory subscale) | |||||
| Preintervention | 8.09 | 0.74 | 7.71 | 0.55 | 0.06 |
| Post-intervention | 3.73 | 0.53 | 4.20 | 0.43 | 0.003 |
| Follow-up at 1 wk | 4.54 | 0.62 | 4.97 | 0.72 | 0.044 |
| Follow-up at 4 wks | 4.23 | 0.49 | 4.95 | 0.51 | <0.001 |
| P | <0.001* | ||||
| PFS (Cognitive subscale) | |||||
| Preintervention | 7.86 | 0.67 | 7.69 | 0.61 | 0.39 |
| Post-intervention | 3.64 | 0.42 | 4.28 | 0.44 | <0.001 |
| Follow-up at 1 wk | 3.64 | 0.42 | 4.28 | 0.44 | <0.001 |
| Follow-up at 4 wks | 4.05 | 0.41 | 5.11 | 0.60 | <0.001 |
| P | <0.001* | ||||
PFS=Piper Fatigue scale, CFS=Cancer Fatigue scale, F-VAS=Fatigue Visual Analogue scale, SD=Standard deviation. *P of Wilks’ lambda, **P with controlling baseline variable
Figure 2.
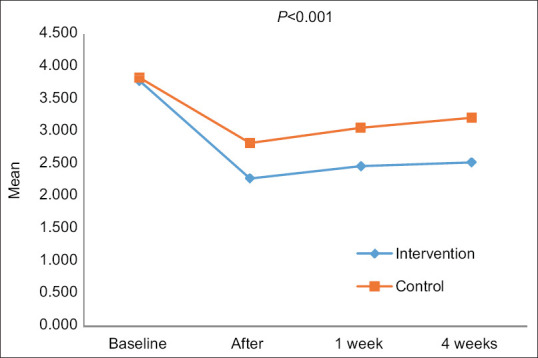
The trend of the changes in the Caner Fatigue scale (total score) over time in the intervention group comparing the control group
DISCUSSION
Findings of the current study indicated that our group's psychoeducational intervention improved CRF significantly. The pattern of scores demonstrated a decrease in all fatigue subscales at all time points except for the behavioral subscale. The behavioral subscale in patients only improved four weeks post-intervention. It is conceivable that it takes more time for patients to make behavioral changes in life.
In accordance with other studies,[19,29,30,31,32,33] we illustrated that psychoeducation is a helpful intervention for fatigue management. Psychosocial interventions are category 1 of recommendation in NCCN.[7] Two of the most effective treatments for CRF are exercise and psychological interventions that clinicians should prescribe as first-line modalities.[15] A systematic review of non-pharmacological therapies for cancer patients suggested multidisciplinary approach as the best way to manage CRF to address reducing fatigue and increasing activity together.[32] Our intervention consisted of concentrative movement therapy and energy conservation. The aim of our study was to apply the most evidence-based treatments in a group-based design for empowering therapeutic efficacy. A supportive group environment brings up a feeling of group unity and acceptance and strengthens health benefits. We used CMT because it has both cognitive and mild motor components. Our multidisciplinary intervention reduced fatigue. Our final goal in designing such a short-term intervention was to afford an applicable and easily used procedure that would become part of accessible and confirmed treatments in oncology centers.
Although the efficacy of this intervention was observed at all time points, the fatigue score increased from the first week onward. The increased fatigue over time is more prominent in the control group [Figure 2]. It is suggested that patients should be followed up for a more period to assess the efficacy of the intervention in future studies. Booster sessions may be required to preserve the therapeutic effectiveness.
Our study, using CMT in combination with psychoeducational modalities, is the first trial to use CMT as an intervention to reduce CRF. Concentrative body movement therapy is a helpful treatment for neurotic, stress-related, and somatoform disorders, some forms of personality disorders, crisis intervention, and preventing illnesses.[17] However, it is not known if CMT alone is beneficial for CRF. More investigation in this regard is suggested.
Only non-metastatic breast cancer patients were recruited for our study. It is unclear whether this intervention is useful for metastatic breast cancer patients or other cancer types. Another limitation of our study was the small number of participants and the short duration of follow-up.
CONCLUSIONS
In conclusion, our study showed that the group psychoeducational intervention improved CRF significantly. All the sensory, behavioral, physical, affective, and cognitive subscales improved. Accessible and confirmatory treatment can help patients to cope with fatigue in communities.
Ethical approval
This study has been approved by the ethics committee of Shahrekord University of medical science with a reference number of IR. SKUMS. REC. 1396. 247.
Informed consent
All the patients provided written informed consent.
Financial support and sponsorship
The study was funded from 2017 to 2018 by Shahrekord University of medical science. The sponsor was not involved in any level of the study process (from study design, collection, analysis, and interpretation of data and writing the report to the decision to submit and publication of the paper).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank all the staff of Kashani and Seyedoshohada hospitals, departments of oncology, and also, Ala institute for their contribution to this project. The authors would like to acknowledge the Clinical Research Development Unit, Hajar Hospital, Shahrekord University of Medical Sciences, Shahrekord, Iran, for supporting this study. The authors also would like to extend their thanks to Dr. Forough Jahandideh for her guidance.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Shamshirian A, Heydari K, Shams Z, Aref AR, Shamshirian D, Tamtaji OR, et al. Breast cancer risk factors in Iran: A systematic review and meta-analysis. Horm Mol Biol Clin Investig. 2020;41:20200021. doi: 10.1515/hmbci-2020-0021. [DOI] [PubMed] [Google Scholar]
- 3.Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women's cancers: A grand challenge in global health. Lancet. 2017;389:847–60. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–8. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Abrahams HJG, Gielissen MFM, Verhagen CAHHVM, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: A systematic review. Clin Psychol Rev. 2018;63:1–11. doi: 10.1016/j.cpr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 6.O’Higgins CM, Brady B, O’Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: Current controversies. Supportive Care Cancer. 2018;26:3353–64. doi: 10.1007/s00520-018-4318-7. [DOI] [PubMed] [Google Scholar]
- 7.NCCN, Clinical Practice Guidelines in Oncology. Cancer-Related Fatigue Version 1 2021. 2020. [Last accessed on 2021 May 01]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
- 8.Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koornstra RH, Peters M, Donofrio S, van den Borne B, de Jong FA. Management of fatigue in patients with cancer – A practical overview. Cancer Treat Rev. 2014;40:791–9. doi: 10.1016/j.ctrv.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–53. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 11.Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAHHVM, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12327 breast cancer survivors. Ann Oncol. 2016;27:965–74. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 12.Yennurajalingam S, Konopleva M, Carmack CL, Dinardo CD, Gaffney M, Michener HK, et al. Treatment of cancer-related-fatigue in acute hematological malignancies: Results of a feasibility study of using cognitive behavioral therapy. J Pain Symptom Manage. 2023;65:e189–97. doi: 10.1016/j.jpainsymman.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med. 2018;52:651–8. doi: 10.1136/bjsports-2016-096422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escalante CP, Manzullo EF. Cancer-related fatigue: The approach and treatment. J Gen Intern Med. 2009;24(Suppl 2):S412–6. doi: 10.1007/s11606-009-1056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 2017;3:961–8. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekhtiari H, Rezapour T, Aupperle RL, Paulus MP. Neuroscience-informed psychoeducation for addiction medicine: A neurocognitive perspective. Prog Brain Res. 2017;235:239–64. doi: 10.1016/bs.pbr.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamacher-Erbguth A. Concentrative Movement Therapy CMT (KBT): An evaluated Body Psychotherapy for psychosomatic and psychic disorders. European Psychotherapy 2012/2013 (ed) 11:1–98. [Google Scholar]
- 18.Barsevick AM, Dudley W, Beck S, Sweeney C, Whitmer K, Nail L. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100:1302–10. doi: 10.1002/cncr.20111. [DOI] [PubMed] [Google Scholar]
- 19.Corbett TK, Groarke A, Devane D, Carr E, Walsh JC, McGuire BE. The effectiveness of psychological interventions for fatigue in cancer survivors: Systematic review of randomised controlled trials. Syst Rev. 2019;8:324. doi: 10.1186/s13643-019-1230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purcell A, Fleming J, Burmeister B, Bennett S, Haines T. Is education an effective management strategy for reducing cancer-related fatigue? Support Care Cancer. 2011;19:1429–39. doi: 10.1007/s00520-010-0970-2. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, et al. The effect of group psychosocial support on survival in metastatic breast cancer. New Engl J Med. 2001;345:1719–26. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 22.Gupta KK, Attri JP, Singh A, Kaur H, Kaur G. Basic concepts for sample size calculation: Critical step for any clinical trials. Saudi J Anaesth. 2016;10:328–31. doi: 10.4103/1658-354X.174918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P. CONSORT statement for randomized trials of nonpharmacologic treatments: A 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med. 2017;167:40–7. doi: 10.7326/M17-0046. [DOI] [PubMed] [Google Scholar]
- 24.Armes J, Chalder T, Addington-Hall J, Richardson A, Hotopf M. A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer-related fatigue. Cancer. 2007;110:1385–95. doi: 10.1002/cncr.22923. [DOI] [PubMed] [Google Scholar]
- 25.Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, et al. Development and validation of the cancer fatigue scale: A brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5–14. doi: 10.1016/s0885-3924(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 26.Reeve BB, Stover AM, Alfano CM, Smith AW, Ballard-Barbash R, Bernstein L, et al. The Piper Fatigue Scale-12 (PFS-12): Psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2012;136:9–20. doi: 10.1007/s10549-012-2212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baresari ZB, Abbaszadeh A, Heydarirad G, Khabazkhoob M. The psychometrics of the Persian version of the “cancer fatigue scale” in Iran. Eurasia J Biosci. 2018;12:149–56. [Google Scholar]
- 28.Khoshakhlagh AH, Ghasemi M, Pourtaghi G. Association between fatigue and occupational physical trauma among male Iranian workers in the copper extraction industry. Trauma Mon. 2017:22. [Google Scholar]
- 29.Pearson EJM, Morris ME, di Stefano M, McKinstry CE. Interventions for cancer-related fatigue: A scoping review. Eur J Cancer Care (Engl) 2018:27. doi: 10.1111/ecc.12516. [DOI] [PubMed] [Google Scholar]
- 30.Wangnum K, Thanarojanawanich T, Chinwatanachai K, Jamprasert L, Maleehuan O, Janthakun V. Impact of the multidisciplinary education program in self-care on fatigue in lung cancer patients receiving chemotherapy. J Med Assoc Thai. 2013;96:1601–8. [PubMed] [Google Scholar]
- 31.Bennett S, Pigott A, Beller EM, Haines T, Meredith P, Delaney C. Educational interventions for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2016;11:CD008144. doi: 10.1002/14651858.CD008144.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–41. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol. 2007;26:660–7. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]