Key Points
Question
Is greater intake of sugar-sweetened beverages associated with greater risk of liver cancer or chronic liver disease mortality?
Findings
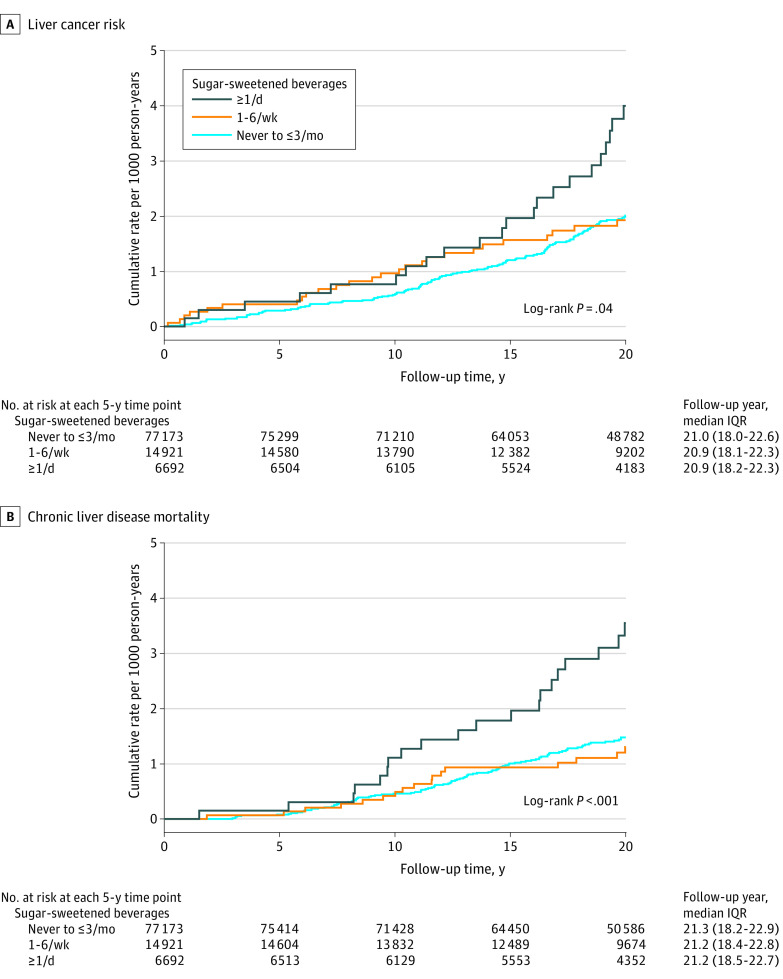
In 98 786 postmenopausal women followed up for a median of 20.9 years, compared with consuming 3 servings or less of sugar-sweetened beverages per month, women consuming 1 or more servings per day had significantly higher rates of liver cancer (18.0 vs 10.3 per 100 000 person-years; adjusted hazard ratio [HR], 1.85) and chronic liver disease mortality (17.7 vs 7.1 per 100 000 person-years; adjusted HR, 1.68).
Meaning
Compared with 3 or fewer sugar-sweetened beverages per month, consuming 1 or more sugar-sweetened beverages per day was associated with a significantly higher incidence of liver cancer and death from chronic liver diseases.
Abstract
Importance
Approximately 65% of adults in the US consume sugar-sweetened beverages daily.
Objective
To study the associations between intake of sugar-sweetened beverages, artificially sweetened beverages, and incidence of liver cancer and chronic liver disease mortality.
Design, Setting, and Participants
A prospective cohort with 98 786 postmenopausal women aged 50 to 79 years enrolled in the Women’s Health Initiative from 1993 to 1998 at 40 clinical centers in the US and were followed up to March 1, 2020.
Exposures
Sugar-sweetened beverage intake was assessed based on a food frequency questionnaire administered at baseline and defined as the sum of regular soft drinks and fruit drinks (not including fruit juice); artificially sweetened beverage intake was measured at 3-year follow-up.
Main Outcomes and Measures
The primary outcomes were (1) liver cancer incidence, and (2) mortality due to chronic liver disease, defined as death from nonalcoholic fatty liver disease, liver fibrosis, cirrhosis, alcoholic liver diseases, and chronic hepatitis. Cox proportional hazards regression models were used to estimate multivariable hazard ratios (HRs) and 95% CIs for liver cancer incidence and for chronic liver disease mortality, adjusting for potential confounders including demographics and lifestyle factors.
Results
During a median follow-up of 20.9 years, 207 women developed liver cancer and 148 died from chronic liver disease. At baseline, 6.8% of women consumed 1 or more sugar-sweetened beverage servings per day, and 13.1% consumed 1 or more artificially sweetened beverage servings per day at 3-year follow-up. Compared with intake of 3 or fewer servings of sugar-sweetened beverages per month, those who consumed 1 or more servings per day had a significantly higher risk of liver cancer (18.0 vs 10.3 per 100 000 person-years [P value for trend = .02]; adjusted HR, 1.85 [95% CI, 1.16-2.96]; P = .01) and chronic liver disease mortality (17.7 vs 7.1 per 100 000 person-years [P value for trend <.001]; adjusted HR, 1.68 [95% CI, 1.03-2.75]; P = .04). Compared with intake of 3 or fewer artificially sweetened beverages per month, individuals who consumed 1 or more artificially sweetened beverages per day did not have significantly increased incidence of liver cancer (11.8 vs 10.2 per 100 000 person-years [P value for trend = .70]; adjusted HR, 1.17 [95% CI, 0.70-1.94]; P = .55) or chronic liver disease mortality (7.1 vs 5.3 per 100 000 person-years [P value for trend = .32]; adjusted HR, 0.95 [95% CI, 0.49-1.84]; P = .88).
Conclusions and Relevance
In postmenopausal women, compared with consuming 3 or fewer servings of sugar-sweetened beverages per month, those who consumed 1 or more sugar-sweetened beverages per day had a higher incidence of liver cancer and death from chronic liver disease. Future studies should confirm these findings and identify the biological pathways of these associations.
This observational study evaluated incidence of liver cancer and death from chronic liver disease among postmenopausal women by comparing intake of 3 or fewer servings of sugar-sweetened beverages per month vs intake of 1 or more sugar-sweetened beverages per day.
Introduction
Chronic liver disease is a major cause of mortality and liver cancer.1 Between 1985 and 2015, the annual incidence of liver cancer increased from 3.0 to 9.4 per 100 000 in the US. In 2023, it was anticipated that there would be 41 210 new cases of liver cancer.2 Risk factors for liver cancer include chronic hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, metabolic disorders (type 2 diabetes, obesity, and metabolic syndrome), excessive alcohol consumption, and aflatoxin-contaminated foods such as peanuts and corn.3 However, approximately 40% of patients with liver cancer do not have these risk factors.3 Epidemiological studies on dietary factors and liver cancer and chronic liver disease mortality are limited. Therefore, it is important to identify dietary risk factors for liver cancer and chronic liver disease mortality.
Between 2017 and 2018, more than 65% of US adults consumed sugar-sweetened beverages daily.4 Epidemiologic studies have reported positive associations of sugar-sweetened beverages with risk of breast, colorectal, and prostate cancers.5 Two cohort studies including 553 874 and 477 206 participants reported a potential association between sugar-sweetened beverage intake and risk of liver cancer.6,7 However, neither study reported rates of liver cancer among women. Additionally, one prospective study reported that consumption of sugar-sweetened beverages was associated with higher risk of nonalcoholic fatty liver diseases.8 However, whether higher sugar-sweetened beverage intake is associated with chronic liver disease mortality remains unknown. Artificially sweetened beverages are healthier alternatives of sugar-sweetened beverages,9 but animal-based studies reported that artificially sweetened beverage intake was associated with nonalcoholic fatty liver diseases.10
This study evaluated whether intake of sugar-sweetened beverages or artificially sweetened beverages was associated with higher rates of liver cancer and chronic liver disease mortality in the Women’s Health Initiative (WHI).
Methods
Study Population
The WHI is a large prospective study composed of 161 808 postmenopausal women aged 50 to 79 years, enrolled at 40 clinical centers in the US between 1993 and 1998.11 The study was approved by the institutional review board of the Fred Hutchinson Cancer Center (IR# 3467-EXT).12 Participants gave written consent for participation and medical records review. The study included 68 132 women who participated in 4 overlapping clinical trials, and 93 676 women participated in the observational study. This study excluded participants with implausible total energy intake (<600 kcal/day or >5000 kcal/day), history of cancer at baseline (except for nonmelanoma skin cancer), and those missing sugar-sweetened beverage intake or covariate data (Figure 1).
Figure 1. Potential Participants, Exclusions, and Cohort Development for the Women’s Health Initiative.
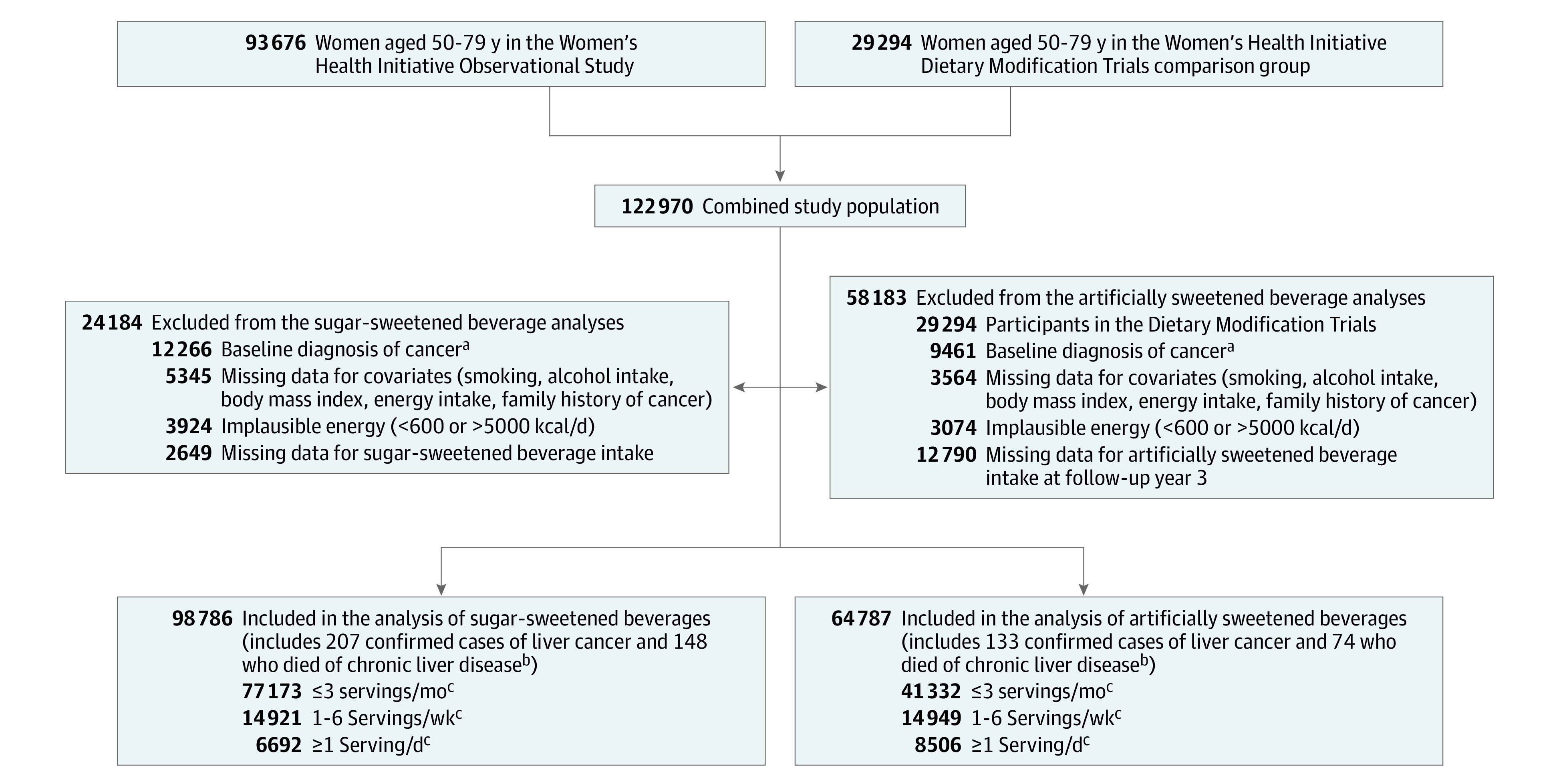
aThis exclusion indicates all cancers except nonmelanoma skin cancer (ie, patients with nonmelanoma skin cancer were not excluded).
bIndicates new cases of liver cancer and confirmed deaths over a median 20.9 years of follow-up.
cOne serving was defined as 12 fl oz or 355 mL.
Assessment of Beverage Intake
Participants in the WHI were asked to report their usual intake of regular soft drinks (not diet) or fruit drinks (Tang, Kool-Aid, Hi-C, and other fruit drinks not including fruit juice) from “never or less than once per month” to “6 or more per day” and serving sizes from small (6 fl oz) to large (18 fl oz) during the past 3 months on a baseline food frequency questionnaire.13 On the annual follow-up questionnaire at year 3, the WHI Observational Study participants were asked about intake of diet drinks or diet fruit drinks in the past 3 months with a median size of 12 fl oz. Total sugar-sweetened beverage intake was defined as the sum of regular soft drinks and fruit drinks. The original categories were collapsed into 3 groups for sugar-sweetened beverages or artificially sweetened beverages: never or 3 or fewer servings per month; 1 to 6 servings per week; and 1 or more servings per day. The median intake of each category was assigned to create continuous variables.
Liver Cancer and Chronic Liver Disease Mortality Ascertainment
The 2 primary outcomes were incident liver cancer and chronic liver disease mortality. These outcomes were assessed through March 1, 2020. People who developed liver cancer were excluded from the outcome of chronic liver disease mortality. Incident liver cancers were assessed using self-administered questionnaires (every 6 months for the clinical trial and annually for the observational study). The diagnosis was verified and adjudicated through centralized review of pathology reports, discharge or consultant summaries, operative and radiology reports, or tumor registry abstracts.14 Cause of death was determined using medical record or death certificate review using International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes through March 1, 2020. Chronic liver disease death (excluding liver cancer) was defined as death from nonalcoholic fatty liver diseases, liver fibrosis, cirrhosis, alcoholic liver diseases, and chronic hepatitis (ICD-9: 571; ICD-10: K70, K73, and K74).15 Overall, 95.6% of causes of death were determined either by medical records or the National Death Index. The specificity and sensitivity of the algorithm via the National Death Index in the test and training sets were 98%.16
Covariates Assessment
Potential confounders and effect modifiers were identified based on published literature,17 which were then integrated into a directed acyclic graph to inform the modeling approach (eFigure 1 in Supplement 1). Self-administered questionnaires were used to collect baseline information on demographic variables (age at entry, self-reported race and ethnicity, education), hormone or oral contraceptive use, family history of cancer, use of nonsteroidal anti-inflammatory drugs, self-reported diabetes, total energy intake per day (calculated from the food frequency questionnaire), modified alternate healthy eating index (AHEI), smoking, alcohol intake, body mass index (BMI), waist-hip ratio, and physical activity (eAppendix in Supplement 1).
Statistical Analysis
Descriptive statistics were calculated according to categories of sugar-sweetened beverage or artificially sweetened beverage intake, including medians for continuous variables and percentages for categorical variables.
For liver cancer, person-years were calculated from the date of baseline enrollment (sugar-sweetened beverage analyses) or the date for questionnaire return at 3-year follow-up (artificially sweetened beverage analyses) until the date of diagnosis of liver cancer, date of death, or the end of follow-up (March 1, 2020), whichever came first. For chronic liver disease mortality, person-years were calculated from the date at baseline (sugar-sweetened beverage analyses) or the date at 3-year follow-up (artificially sweetened beverage analyses) until the date of death due to chronic liver disease or the end of follow-up. Deaths or liver cancers occurring before the year 3 visit were excluded for artificially sweetened beverage analyses. Time to first liver cancer or to chronic liver disease death was analyzed using Kaplan-Meier methods to calculate cumulative probabilities by categories of beverage intake. Cox proportional hazards regression models were used to estimate age- and energy-adjusted and multivariable-adjusted hazard ratios (HRs) and 95% CIs. Potential confounding factors were selected a priori including age at entry, race and ethnicity, education, smoking status, alcohol intake, BMI, physical activity, total energy intake, nonsteroidal anti-inflammatory drugs use, family history of cancer, oral contraceptive use, postmenopausal hormone therapy, and self-reported diabetes.18 The median intake of each category was modeled as a continuous variable to calculate the P value for trend. The proportionality assumptions of the hazard models across beverage categories were visually examined by using the Schoenfeld residual plot with no violations observed. Several sensitivity analyses and substitution analyses by replacing beverages with coffee or tea were performed (eAppendix in Supplement 1).
To address the potential influence of HBV or HCV infection status on the associations between beverages and the primary outcomes, the Spearman correlations between HBV and HCV infection status and sugar-sweetened beverage or artificially sweetened beverage intake were calculated using data from the National Health and Nutrition Examination Survey (NHANES 2007-2018 [n = 23 520]), a nationally representative survey,19 and a subsample of the WHI (n = 214) with the available HBV and HCV data. Additional information about the HBV and HCV measurement can be found in eAppendix in Supplement 1.
Two-sided P values less than .05 were considered statistically significant. All statistical analyses were performed in SAS version 9.4 (SAS Institute).
Results
A total of 98 786 postmenopausal women were included in analyses for sugar-sweetened beverage intake (Figure 1). After excluding participants missing artificially sweetened beverage intake data, the sample size was 64 787 for artificially sweetened beverage analyses (eTable 1 in Supplement).
Over a median 20.9 years of follow-up, 207 new cases of liver cancer and 148 chronic liver disease deaths were confirmed. Approximately 6.8% (6692) of women consumed 1 or more servings of sugar-sweetened beverages per day, and 13.1% (8506) consumed 1 or more artificially sweetened beverages per day (Table 1). At enrollment, women who consumed more sugar-sweetened beverages were younger, less physically active; higher proportions were non-Hispanic Black, never drank alcohol; and they had a lower education level, higher BMI, a lower modified AHEI score, and higher total energy intake. Women who consumed more artificially sweetened beverages were younger, had a higher BMI, and a lower modified AHEI score (Table 1).
Table 1. Characteristics of Participants in the Women’s Health Initiative According to Sugar-Sweetened Beverage and Artificially Sweetened Beverage Intake.
| Variables | Sugar-sweetened beverage intake (n = 98 786)a | Artificially sweetened beverage intake (n = 64 787)a | ||||
|---|---|---|---|---|---|---|
| Never to ≤3/mo | 1-6/wk | ≥1/d | Never to ≤3/mo | 1-6/wk | ≥1/d | |
| No. | 77 173 | 14 921 | 6692 | 41 332 | 14 949 | 8506 |
| Age, median (IQR), y | 63.0 (58.0-69.0) | 62.0 (56.0-68.0) | 60.0 (55.0-66.0) | 64.0 (58.0-70.0) | 63.0 (57.0-68.0) | 60.0 (55.0-66.0) |
| Race and ethnicityb | ||||||
| Hispanic | 2.8 | 6.0 | 6.8 | 3.1 | 3.1 | 3.0 |
| Non-Hispanic Black | 5.1 | 16.0 | 22.1 | 6.4 | 6.4 | 6.0 |
| Non-Hispanic White | 88.0 | 72.7 | 66.4 | 85.4 | 87.1 | 87.8 |
| Other | 4.2 | 5.2 | 4.6 | 5.0 | 3.4 | 3.2 |
| Education | ||||||
| <High school | 3.7 | 7.2 | 9.2 | 3.7 | 4.0 | 4.5 |
| High school or some college | 52.0 | 58.5 | 59.4 | 50.9 | 53.4 | 54.2 |
| ≥4 y of college | 44.3 | 34.3 | 31.4 | 45.3 | 42.6 | 41.3 |
| Never use of alcohol | 9.5 | 14.0 | 15.9 | 10.6 | 9.4 | 11.2 |
| Alcohol intake, median (IQR), drinks/wk | 0.4 (0-3.2) | 0.2 (0-1.4) | 0 (0-0.8) | 0.4 (0-3.2) | 0.4 (0-3.2) | 0.4 (0-2.6) |
| Smoking status | ||||||
| Never | 50.2 | 57.1 | 52.9 | 52.9 | 50.3 | 46.3 |
| Past | 44.4 | 35.4 | 35.6 | 41.4 | 44.8 | 46.7 |
| Current | 5.5 | 7.5 | 11.5 | 5.7 | 4.8 | 7.0 |
| Medication use | ||||||
| Nonsteroidal anti-inflammatory drug | 54.1 | 53.5 | 52.3 | 49.9 | 54.9 | 56.5 |
| Oral contraceptive | 41.6 | 42.3 | 45.6 | 39.6 | 44.6 | 48.5 |
| Menopausal hormone therapy | 61.6 | 58.6 | 54.1 | 61.0 | 64.6 | 63.9 |
| Family history of cancer | 64.2 | 61.9 | 60.7 | 63.8 | 64.3 | 64.3 |
| Self-reported diabetes | 5.5 | 4.1 | 5.5 | 3.0 | 6.6 | 9.2 |
| History of liver disease | 2.3 | 2.5 | 2.4 | 2.3 | 2.1 | 2.4 |
| Body mass index, median (IQR)c | 26.2 (23.3-30.1) | 27.3 (24-31.4) | 28.6 (24.9-33.4) | 25.2 (22.6-28.7) | 26.8 (23.9-30.7) | 27.8 (24.6-32.2) |
| Waist-hip ratio, median (IQR)d | 0.80 (0.75-0.85) | 0.81 (0.76-0.86) | 0.82 (0.77-0.87) | 0.79 (0.75-0.84) | 0.80 (0.75-0.85) | 0.81 (0.76-0.86) |
| Recreational physical activity, median (IQR), metabolic equivalent h/wk | 10.5 (3.8-19.5) | 7.3 (1.7-15) | 5.0 (0.5-13) | 10.5 (3.8-21) | 10.5 (3.8-20) | 9.1 (2.5-18.8) |
| Total energy intake, median (IQR), kcal/d | 1451 (1131-1830) | 1583 (1217-2025) | 1849 (1426-2385) | 1409 (1094-1786) | 1442 (1119-1821) | 1472 (1136-1887) |
| Modified alternate healthy eating index, median (IQR)e | 48.9 (42.4-55.4) | 44.7 (39.1-51.1) | 42.2 (36.6-48.4) | 50.5 (43.6-57.7) | 48.9 (42.6-55.6) | 46.9 (40.4-53.6) |
Percent values are reported unless otherwise indicated.
Race and ethnicity data were collected because the association between beverage intake and liver outcomes could differ by participants’ race and ethnicity. The information was collected by asking the question: “How would you describe your racial or ethnic group?” Six response options were given: American Indian or Alaska Native, Asian or Pacific Islander, Black or African American (not of Hispanic origin), Hispanic/Latino, White (not of Hispanic origin), or other.
Calculated as weight in kilograms divided by height in meters squared.
In the sugar-sweetened beverage intake group, 356 had missing values for waist-hip ratio, and 243 in the artificially sweetened beverage intake group had missing values.
Values were calculated by excluding the sugar-sweetened beverage component from the alternate healthy eating index. The alternate healthy eating index grades diet quality based on 11 foods and nutrients that prevent chronic diseases (range, 0 to 110 [with higher scores indicating healthier diets]).
Association Between Sugar-Sweetened Beverages, Artificially Sweetened Beverages, and Rates of New Liver Cancer Diagnoses
Rates of liver cancer were 18.0 per 100 000 person-years in women who consumed 1 or more sugar-sweetened beverages per day and 10.3 per 100 000 person-years in women who consumed 3 or fewer per month (P value for trend = .02; multivariable-adjusted HR, 1.85 [95% CI, 1.16-2.96]; P = .01) (Figure 2 and Table 2). There were no statistically significant associations with intake of regular soft drinks (≥1/day vs ≤3/month, 16.9 vs 10.7 per 100 000 person-years [P value for trend = .18]; HR, 1.66 [95% CI, 0.97-2.85]; P = .06) or fruit drinks (≥1/day vs ≤3/month, 19.3 vs 10.5 per 100 000 person-years [P value for trend = .06]; HR, 1.71 [95% CI, 0.75-3.92]; P = .20) with liver cancer. Replacing 1 serving per day of sugar-sweetened beverages with 1 serving per day of coffee or 1 serving of tea was associated with nonsignificant lower liver cancer incidence (eFigure 2 in Supplement 1).
Figure 2. Cumulative Probability of Liver Cancer and Chronic Liver Disease Mortality, According to Categories of Sugar-Sweetened Beverage Intake.
Table 2. Associations Between Sugar-Sweetened and Artificially Sweetened Beverage Intake and Liver Cancer Risk in the Women’s Health Initiative, 1993-2020.
| Beverages | Consumption categoriesa | P value for trendb | ||
|---|---|---|---|---|
| Never to ≤3 servings/mo | 1-6 servings/wk | ≥1 serving/d | ||
| Sugar-sweetened beverages | ||||
| Cases of liver cancer, No. | 153 | 31 | 23 | |
| No. of participants | 77 173 | 14 921 | 6692 | |
| Unadjusted rate/100 000 person-years | 10.3 | 10.8 | 18.0 | .02 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 1.12 (0.76-1.65) | 2.03 (1.30-3.18) | .003 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 1.10 (0.74-1.64) | 1.85 (1.16-2.96) | .01 |
| Soft drinks | ||||
| Cases of liver cancer, No. | 169 | 22 | 16 | |
| Unadjusted rate/100 000 person-years | 10.7 | 9.7 | 16.9 | .18 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 0.96 (0.61-1.50) | 1.86 (1.10-3.14) | .06 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 0.94 (0.60-1.48) | 1.66 (0.97-2.85) | .14 |
| Fruit drinks | ||||
| Cases of liver cancer, No. | 189 | 12 | 6 | |
| Unadjusted rate/100 000 person-years | 10.5 | 15.0 | 19.3 | .06 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 1.51 (0.84-2.72) | 1.93 (0.85-4.36) | .04 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 1.38 (0.76-2.51) | 1.71 (0.75-3.92) | .11 |
| Artificially sweetened beveragese | ||||
| Cases of liver cancer, No. | 83 | 30 | 20 | |
| No. of participants | 41 332 | 14 949 | 8506 | |
| Unadjusted rate/100 000 person-years | 10.2 | 10.1 | 11.8 | .70 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 1.06 (0.70-1.61) | 1.37 (0.83-2.24) | .29 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 0.99 (0.65-1.51) | 1.17 (0.70-1.94) | .66 |
Abbreviation: HR, hazard ratio.
One serving defined as 12 fl oz or 355 mL.
The median intake of each category was modeled as a continuous variable to calculate the P value for trend.
Results were from the Cox proportional hazards model adjusting for age at entry (continuous) and total energy intake (quartile).
Results were from the Cox proportional hazards model additionally adjusting for race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, other), education (<high school, high school or some college, >college), smoking status (non-smoker, past smoker, current smoker), alcohol consumption (nondrinker, past drinker, <1 drink/month, <1 drink/week, 1-<7 drinks/week, ≥7 drinks/week), body mass index (<18.5, 18.5-<25, 25-<30, 30-<35, 35-<40, ≥40 [calculated as weight in kilograms divided by height in meters squared]), physical activity (quartile), nonsteroidal anti-inflammatory drugs use (yes, no), family history of cancer (yes, no), prior oral contraceptive use (yes, no), postmenopausal hormone therapy (yes, no), and self-reported diabetes (yes, no).
For artificially sweetened beverage analyses, we used the 3-year follow-up as the baseline in the observational study (64 787 participants).
Higher intake of artificially sweetened beverages was not associated significantly with liver cancer risk (Table 2).
Association Between Sugar-Sweetened Beverages, Artificially Sweetened Beverages, and Chronic Liver Disease Mortality
Rates of chronic liver disease mortality were 17.7 per 100 000 person-years in women who consumed 1 or more sugar-sweetened beverages per day and 7.1 per 100 000 person-years in women who consumed 3 or fewer per month (P value for trend <.001; multivariable-adjusted HR, 1.68 [95% CI, 1.03-2.75]; P = .04) (Table 3 and Figure 2). Rates of chronic liver disease mortality were 19.8 per 100 000 person-years in women who consumed 1 or more soft drinks per day compared with 7.2 per 100 000 person-years in women who consumed 3 or fewer regular soft drinks per month (P value for trend <.001; multivariable-adjusted HR, 1.80 [95% CI, 1.07-3.03]; P = .03). Fruit drinks were not associated significantly with chronic liver disease mortality (1 or more per day vs 3 or fewer per month, 9.6 vs 7.5 per 100 000 person-years [P value for trend = .33]; HR, 0.95 [95% CI, 0.30-3.02]; P = .93). Replacing sugar-sweetened beverages with coffee or tea was not significantly associated with chronic liver disease mortality (eFigure 2 in Supplement 1).
Table 3. Associations Between Sugar-Sweetened and Artificially Sweetened Beverage Intake and Chronic Liver Disease Mortality in the Women’s Health Initiative, 1993-2020.
| Beverages | Consumption categoriesa | P value for trendb | ||
|---|---|---|---|---|
| Never to ≤3 servings/mo | 1-6 servings/wk | ≥1 serving/d | ||
| Sugar-sweetened beverages | ||||
| Cases of chronic liver disease mortality, No. | 107 | 18 | 23 | |
| No. of participants | 77 173 | 14 921 | 6692 | |
| Unadjusted rate/100 000 person-years | 7.1 | 6.2 | 17.7 | <.001 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 0.87 (0.53-1.44) | 2.50 (1.57-3.99) | <.001 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 0.71 (0.42-1.18) | 1.68 (1.03-2.75) | .08 |
| Soft drinks | ||||
| Cases of chronic liver disease mortality, No. | 115 | 14 | 19 | |
| Unadjusted rate/100 000 person-years | 7.2 | 6.1 | 19.8 | <.001 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 0.84 (0.48-1.47) | 2.76 (1.67-4.56) | <.001 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 0.68 (0.39-1.20) | 1.80 (1.07-3.03) | .11 |
| Fruit drinks | ||||
| Cases of chronic liver disease mortality, No. | 136 | 9 | 3 | |
| Unadjusted rate/100 000 person-years | 7.5 | 11.1 | 9.6 | .33 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 1.46 (0.74-2.87) | 1.22 (0.39-3.83) | .38 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 1.10 (0.55-2.20) | 0.95 (0.30-3.02) | .93 |
| Artificially sweetened beveragese | ||||
| Cases of chronic liver disease mortality, No. | 43 | 19 | 12 | |
| No. of participants | 41 332 | 14 949 | 8506 | |
| Unadjusted rate/100 000 person-years | 5.3 | 6.4 | 7.1 | .32 |
| Age and energy-adjusted HR (95% CI)c | 1 [Reference] | 1.24 (0.72-2.12) | 1.41 (0.74-2.70) | .47 |
| Multivariable-adjusted HR (95% CI)d | 1 [Reference] | 1.01 (0.59-1.76) | 0.95 (0.49-1.84) | .92 |
Abbreviation: HR, hazard ratio.
One serving defined as 12 fl oz or 355 mL.
The median intake of each category was modeled as a continuous variable to calculate the P value for trend.
Results were from the Cox proportional hazards model adjusting for age at entry (continuous) and total energy intake (quartile).
Results were from the Cox proportional hazards model additionally adjusting for race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, other), education (< high school, high school or some college, >college), smoking status (non-smoker, past smoker, current smoker), alcohol consumption (non-drinker, past drinker, <1 drink/mo, <1 drink/week, 1-<7 drinks/week, ≥7 drinks/week), body mass index (<18.5, 18.5-<25, 25-<30, 30-<35, 35-<40, ≥40 [calculated as weight in kilograms divided by height in meters squared]), physical activity (quartile), nonsteroidal anti-inflammatory drugs use (yes, no), family history of cancer (yes, no), prior oral contraceptive use (yes, no), postmenopausal hormone therapy (yes, no), and self-reported diabetes (yes, no).
For artificially sweetened beverage analyses, we used the 3-year follow-up as the baseline in the observational study (64 787 participants).
Artificially sweetened beverage intake was not associated significantly with chronic liver disease mortality (Table 3).
Sensitivity Analyses
Results were similar after additional adjustment for coffee or tea intake, history of liver diseases, modified AHEI, added sugar, waist-hip ratio, or without adjusting for BMI and self-reported diabetes (eTables 2 and 3 in Supplement 1). Similar results were observed across BMI or waist-hip ratio groups. However, the association between sugar-sweetened beverages and liver cancer was significantly greater among women without obesity (P for interaction = .01) (eTable 4 in Supplement 1). The positive association between sugar-sweetened beverage intake and liver cancer persisted when excluding first 2-year cases, liver diseases at baseline, diabetes at baseline, participants in the clinical trials, or participants with alcohol intake of 1 or more drinks per day (eTable 5 and 6 in Supplement 1).
Correlations Between Sugar-Sweetened Beverages, Artificially Sweetened Beverages, and HBV or HCV Infection Status
Sugar-sweetened or artificially sweetened beverage intake was not associated with prevalent HBV (sugar-sweetened beverage ρ = 0.002 [P = .74]; artificially sweetened beverage ρ = −0.003 [P = .69]) and HCV infection status (sugar-sweetened beverage ρ = −0.001 [P = .87]; artificially sweetened beverage ρ = −0.003 [P = .67]) in the NHANES. In a study nested within the WHI with available HBV/HCV infection status, no correlation of sugar-sweetened beverage intake with HBV (ρ = −0.001 [P = .94]) or HCV infection status (ρ = 0.002 [P = .74]) was observed. Similarly, artificially sweetened beverage intake was not associated with HBV (ρ = 0.066 [P = .44]) or HCV in the WHI (ρ = −0.015 [P = .86]).
Discussion
Results reported in this study demonstrated a statistically significant association of greater sugar-sweetened beverage intake and increased risk of liver cancer and chronic liver disease mortality. There were no statistically significant associations of artificially sweetened beverage intake with liver cancer or chronic liver disease mortality.
The World Cancer Research Fund/American Institute for Cancer Research concluded that sugar-sweetened beverages increased the risk of overweight and obesity but due to limited evidence, did not describe an association of sugar-sweetened beverages with cancer risk.20 A meta-analyses including 27 cohort and 37 case-control studies reported positive associations between sugar-sweetened beverage intake and cancers of the breast and prostate.21 To our knowledge, only 2 prior studies evaluated the association between sugar-sweetened beverages and liver cancer. The European Prospective Investigation into Cancer and Nutrition (EPIC) cohort reported an increased hepatocellular carcinoma risk between participants who consumed more than 6 soft drink cans per week and those consuming less than 1 can per week (6.4 vs 2.8 per 100 000 person-years; P value for trend = .01).6 This positive association was mainly due to artificially sweetened soft drinks rather than sugar-sweetened soft drinks. In data reported here, there was a positive association between sugar-sweetened beverages and liver cancer (≥1 beverages/day vs ≤3 beverages/month, HR = 1.85) without significant difference between regular soft drinks and fruit drinks. The difference in findings may be due to different consumption levels among people living in European countries compared with those living in the US. The mean regular soft drink intake was less than 100 g per day in European countries22 but more than 200 g per day in the US.23 Another US study pooling the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial and the NIH-AARP (National Institutes of Health—American Association of Retired Persons) Diet and Health Study reported each additional serving of regular soda consumption per day was associated with an 18% greater risk of liver cancer among persons without diabetes.7 In our study, similar results were observed among women without diabetes.
Chronic liver disease was the fourth leading cause of death for women aged 45 to 54 years and the fifth leading cause of death for men aged 45 to 64 years in 2019 in the US.24 However, evidence for the associations between diet and chronic liver disease mortality is limited. To our knowledge, this is the first study to report a positive association between sugar-sweetened beverage intake and chronic liver disease mortality. This finding is consistent with a meta-analysis of 6 studies, which reported that higher intake of sugar-sweetened beverages was associated with a 40% increased odds of nonalcoholic fatty liver diseases (OR, 1.40 [95% CI, 1.07-1.82]).25 The Framingham Heart Study reported that 1 or more servings per week of sugar-sweetened beverages was associated with a 77% increased incident odds of nonalcoholic fatty liver diseases compared with people who did not drink sugar-sweetened beverages (OR, 1.77 [95% CI, 1.11-2.83]).8
Replacing sugars with artificial sweeteners may reduce caloric intake.26 However, data regarding artificial sweeteners and risk of cancer have been mixed. In this study, a statistically significant association between artificially sweetened beverages and liver cancer was not observed. Discrepancies between results reported here and in prior literature may be due to the types or doses of sweeteners commonly used in the US and in European countries. The European Food Safety Authority have approved 2 additional sweeteners (cyclamate and neohesperidine DC) than the US.27 Although these artificial sweeteners are considered to have no carcinogenic risk, based on a 2004 literature review,28 further studies are needed to evaluate associations between different artificial sweeteners and liver cancer risk, considering its increasing consumption.29
This study was not able to discern the biologic pathways by which sugar-sweetened beverage consumption was associated with adverse liver outcomes. Potential pathways include the following considerations. First, sugar sweetened beverages are associated with obesity,30 and obesity is a risk factor for liver diseases. However, adjustments for BMI or waist-hip ratio did not alter the estimates meaningfully. Second, high intake of sweetened beverages may lead to rapid and dramatic increases in blood glucose, further resulting in insulin resistance, a risk factor of liver cancer and liver diseases.31 Third, sugar-sweetened beverages are associated with liver fat accumulation.32 Fourth, adverse changes in the gut microbiome33 can influence liver health. Fifth, sugar-sweetened beverage–associated metabolites such as taurine and phenylalanine were associated with hepatocellular carcinoma.34,35 Sixth, added sugar from liquid sources, such as soft soda and fruit drinks, is rapidly absorbed, which might lead to metabolic conditions and liver problems.36 Seventh, chemicals contained in these sugar-sweetened beverages might contribute to detrimental effects, such as caramel color, citric acid, natural flavors, organic diol, and others.37
This study had several strengths including its large and geographically dispersed cohort and the 20.9-year length of follow-up. Liver cancer cases were verified with medical record review, and data on death due to chronic liver disease was confirmed with medical records or the National Death Index.
Limitations
This study had several limitations. First, only 2 questions on sugar-sweetened beverage intake (regular soft drinks and fruit drinks) and 1 question about artificially sweetened beverage intake were administered to participants. Second, detailed information about sugar content or sweetener type was unavailable, preventing quantification of subtypes of sugar-sweetened beverages or artificially sweetened beverages such as sports drinks. Third, the study was observational and causality cannot be inferred. Fourth, the effect of changes in beverage intake over time could not be assessed. Fifth, the study may have been confounded by unmeasured factors, such as HBV or HCV infections. However, given no corrections between HBV or HCV infection status and beverage consumption in this study, the observed associations are unlikely to be substantially confounded by HBV or HCV infection. Sixth, chronic liver disease mortality rather than incidence data were used because chronic liver disease registries are not available yet in the US. Seventh, only 207 liver cancer cases and 148 chronic liver disease deaths were included in this study, which limited statistical power.
Conclusions
In postmenopausal women, compared with consuming 3 or fewer servings of sugar-sweetened beverages per month, people who consumed 1 or more sugar-sweetened beverages per day had higher rates of liver cancer and higher rates of death due to chronic liver disease. Future studies should confirm these findings and identify the biological pathways of these associations.
eAppendix
eTable 1. Participants in the Women's Health Initiative According to Beverage Consumption
eTable 2. Sensitivity Analyses on Associations Between Beverages and Liver Cancer Risk With Additional Adjustments
eTable 3. Sensitivity Analyses on Associations Between Beverages and Chronic Liver Disease Mortality With Additional Adjustments
eTable 4. Stratified Analyses by Body Mass Index and Waist-Hip Ratio for the Associations Between Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality
eTable 5. Sensitivity Analyses on Associations Between Beverages and Liver Cancer Risk With Different Exclusion Criteria
eTable 6. Sensitivity Analyses on Associations Between Beverages and Chronic Liver Disease Mortality With Different Exclusion Criteria
eFigure 1. Hypothesized Directed Acyclic Graphs (DAGS) on Associations Between Sugar-Sweetened and Artificially Sweetened Beverage Intake and Liver Cancer Risk and Chronic Liver Disease Mortality in the Women's Health Initiative
eFigure 2. Hazard Ratios (95% CIs) for Liver Cancer Risk and Chronic Liver Disease Mortality Associated With the Replacement of 1 Serving Per Day of Sugar-Sweetened or Artificially Sweetened Beverages With 1 Serving Per Day of Coffee or Tea at Follow-Up Year 3
Data Sharing Statement
References
- 1.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151-171. doi: 10.1016/j.jhep.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi: 10.3322/caac.21763 [DOI] [PubMed] [Google Scholar]
- 3.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122(11):1757-1765. doi: 10.1002/cncr.29971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J, Soto MJ, Dunn CG, Bleich SN. Trends and patterns in sugar-sweetened beverage consumption among children and adults by race and/or ethnicity, 2003-2018. Public Health Nutr. 2021;24(9):2405-2410. doi: 10.1017/S1368980021001580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Guo L, He K, Huang C, Tang S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: a systematic review and dose-response meta-analysis of observational studies. J Cancer. 2021;12(10):3077-3088. doi: 10.7150/jca.51322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepien M, Duarte-Salles T, Fedirko V, et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur J Nutr. 2016;55(1):7-20. doi: 10.1007/s00394-014-0818-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones GS, Graubard BI, Ramirez Y, et al. Sweetened beverage consumption and risk of liver cancer by diabetes status: a pooled analysis. Cancer Epidemiol. 2022;79:102201. doi: 10.1016/j.canep.2022.102201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park WY, Yiannakou I, Petersen JM, Hoffmann U, Ma J, Long MT. Sugar-sweetened beverage, diet soda, and nonalcoholic fatty liver disease over 6 years: the Framingham Heart Study. Clin Gastroenterol Hepatol. 2022;20(11):2524-2532e2. doi: 10.1016/j.cgh.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. doi: 10.1136/bmj.k4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emamat H, Ghalandari H, Tangestani H, Abdollahi A, Hekmatdoost A. Artificial sweeteners are related to non-alcoholic fatty liver disease: microbiota dysbiosis as a novel potential mechanism. EXCLI J. 2020;19:620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Women’s Health Initiative Study Group . Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61-109. doi: 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services . Office for Human Research Protections, 25 CFR 46. Accessed June 15, 2023. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html
- 13.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178-187. doi: 10.1016/S1047-2797(98)00055-6 [DOI] [PubMed] [Google Scholar]
- 14.Curb JD, McTiernan A, Heckbert SR, et al. ; WHI Morbidity and Mortality Committee . Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9)(suppl):S122-S128. doi: 10.1016/S1047-2797(03)00048-6 [DOI] [PubMed] [Google Scholar]
- 15.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145(2):375-382.e1-2. doi: 10.1053/j.gastro.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Women’s Health Initiative . Summary of WHI National Death Index (NDI) data. Published 2022. Accessed March 22, 2023. https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/Summary-of-National-Death-Index-Data.pdf
- 17.Yang W, Ma Y, Liu Y, et al. Association of intake of whole grains and dietary fiber with risk of hepatocellular carcinoma in US adults. JAMA Oncol. 2019;5(6):879-886. doi: 10.1001/jamaoncol.2018.7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Sui J, Zhao L, et al. Association of inflammatory and insulinemic potential of diet and lifestyle with risk of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(4):789-796. doi: 10.1158/1055-9965.EPI-20-1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention; National Center for Health Statistics . National Health and Nutrition Examination Survey data. Published 2022. Accessed March 22, 2023. https://www.cdc.gov/nchs/nhanes/index.htm
- 20.World Cancer Research Fund; American Institute for Cancer Research . Diet, nutrition, physical activity and cancer: a global perspective. Continuous update project expert report. Accessed June 15, 2023. https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf
- 21.Llaha F, Gil-Lespinard M, Unal P, de Villasante I, Castañeda J, Zamora-Ros R. Consumption of sweet beverages and cancer risk: a systematic review and meta-analysis of observational studies. Nutrients. 2021;13(2):516. doi: 10.3390/nu13020516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nissensohn M, Sánchez-Villegas A, Galan P, et al. Beverage consumption habits among the European population: association with total water and energy intakes. Nutrients. 2017;9(4):383. doi: 10.3390/nu9040383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106(12):1992-2000. doi: 10.1016/j.jada.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021;70(9):1-114. [PubMed] [Google Scholar]
- 25.Asgari-Taee F, Zerafati-Shoae N, Dehghani M, Sadeghi M, Baradaran HR, Jazayeri S. Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Nutr. 2019;58(5):1759-1769. doi: 10.1007/s00394-018-1711-4 [DOI] [PubMed] [Google Scholar]
- 26.McGlynn ND, Khan TA, Wang L, et al. Association of low- and no-calorie sweetened beverages as a replacement for sugar-sweetened beverages with body weight and cardiometabolic risk: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(3):e222092. doi: 10.1001/jamanetworkopen.2022.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiano C, Grimaldi V, Scognamiglio M, et al. Soft drinks and sweeteners intake: possible contribution to the development of metabolic syndrome and cardiovascular diseases. Food Res Int. 2021;142:110220. doi: 10.1016/j.foodres.2021.110220 [DOI] [PubMed] [Google Scholar]
- 28.Weihrauch MR, Diehl V. Artificial sweeteners–do they bear a carcinogenic risk? Ann Oncol. 2004;15(10):1460-1465. doi: 10.1093/annonc/mdh256 [DOI] [PubMed] [Google Scholar]
- 29.Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet. 2017;117(3):441-448.e2. doi: 10.1016/j.jand.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik VS, Hu FB. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat Rev Endocrinol. 2022;18(4):205-218. doi: 10.1038/s41574-021-00627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laguna JC, Alegret M, Roglans N. Simple sugar intake and hepatocellular carcinoma: epidemiological and mechanistic insight. Nutrients. 2014;6(12):5933-5954. doi: 10.3390/nu6125933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Softic S, Stanhope KL, Boucher J, et al. Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. 2020;57(5):308-322. doi: 10.1080/10408363.2019.1711360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063-1075. doi: 10.1016/j.jhep.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibbons H, McNulty BA, Nugent AP, et al. A metabolomics approach to the identification of biomarkers of sugar-sweetened beverage intake. Am J Clin Nutr. 2015;101(3):471-477. doi: 10.3945/ajcn.114.095604 [DOI] [PubMed] [Google Scholar]
- 35.Kimhofer T, Fye H, Taylor-Robinson S, Thursz M, Holmes E. Proteomic and metabonomic biomarkers for hepatocellular carcinoma: a comprehensive review. Br J Cancer. 2015;112(7):1141-1156. doi: 10.1038/bjc.2015.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundborn G, Thornley S, Merriman TR, et al. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity (Silver Spring). 2019;27(6):879-887. doi: 10.1002/oby.22472 [DOI] [PubMed] [Google Scholar]
- 37.Leis-Keeling K. Comprehensive evaluation of soft drinks, effects on health, and nutritional strategies to reverse damage. Nutr Perspect. 2010;33(1):15-23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix
eTable 1. Participants in the Women's Health Initiative According to Beverage Consumption
eTable 2. Sensitivity Analyses on Associations Between Beverages and Liver Cancer Risk With Additional Adjustments
eTable 3. Sensitivity Analyses on Associations Between Beverages and Chronic Liver Disease Mortality With Additional Adjustments
eTable 4. Stratified Analyses by Body Mass Index and Waist-Hip Ratio for the Associations Between Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality
eTable 5. Sensitivity Analyses on Associations Between Beverages and Liver Cancer Risk With Different Exclusion Criteria
eTable 6. Sensitivity Analyses on Associations Between Beverages and Chronic Liver Disease Mortality With Different Exclusion Criteria
eFigure 1. Hypothesized Directed Acyclic Graphs (DAGS) on Associations Between Sugar-Sweetened and Artificially Sweetened Beverage Intake and Liver Cancer Risk and Chronic Liver Disease Mortality in the Women's Health Initiative
eFigure 2. Hazard Ratios (95% CIs) for Liver Cancer Risk and Chronic Liver Disease Mortality Associated With the Replacement of 1 Serving Per Day of Sugar-Sweetened or Artificially Sweetened Beverages With 1 Serving Per Day of Coffee or Tea at Follow-Up Year 3
Data Sharing Statement