Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease affecting 1% of the world’s population, with significant morbidity and mortality. In this study, we investigated a recombinant adeno-associated virus (rAAV) vector for its potential application in RA gene therapy. rAAV encoding Escherichia coli β-galactosidase was injected into rat joints which had already been induced into acute arthritis after local lipopolysaccharide (LPS) administration, and the efficiency of in vivo transduction was evaluated. We observed a striking correlation between vector transgene expression and disease severity in arthritic joints. The inflammatory reaction peaked at 3 to 7 days after LPS treatment, and, at the same time, 95% of the synoviocytes had high-level transgene expression. Gene expression diminished to the basal level (5%) when the inflammation subsided at 30 days after LPS treatment. More importantly, the diminished transgene expression could be efficiently reactivated by a repeated insult. The transgene expression in normal joints transduced with rAAV remained low for a long period of time (30 days) but could still be induced to high levels (95%) at 3 to 7 days after LPS treatment. This is the first demonstration of disease state-regulated transgene expression. These findings strongly support the feasibility of therapeutic as well as preventative gene transfer approaches for RA with rAAV vectors containing therapeutic genes, which are expected to respond primarily to the disease state of the target tissue.
Rheumatoid arthritis (RA) and animal models of arthritis are inflammations of joints leading to the destruction of joint cartilage and eventually to destruction of joint function (24). In general, these diseases are characterized by abnormal proliferation of the specialized epithelial cells known as synoviocytes that form the lining tissue of the intra-articular space of diathodial joints (34). Although the causes of RA are not fully understood, laboratory and clinical evidence suggests that proinflammatory cytokines, particularly tumor necrosis factor (TNF) and interleukin-1 (IL-1), have an important role in its pathogenesis (2, 9). Soluble TNF receptor (sTNFR) and IL-1 receptor antagonist (IL-1Ra) are molecules that can prevent the binding of TNF and IL-1 to their respective cell surface receptors (20, 27, 38) and improve the inflammatory symptoms of arthritis (23, 24, 31). With the recent advance of gene therapy, sTNFR and IL-1Ra genes have been delivered by retrovirus-based and adenovirus-based vectors into synoviocytes to achieve anti-inflammatory functions both in vivo and in vitro, with variable success (13). Ex vivo transfer of the IL-1Ra gene to the synovium has lead to a suppression of the intra-articular response to IL-1 (23, 31). While effective, ex vivo gene delivery by transplantation of retroviral vector-transduced synoviocytes is laborious and expensive and thus is difficult to apply on a widespread scale (17). On the other hand, adenovirus vectors delivering IL-1Ra and sTNFR have also been reported to suppress collagen-induced arthritis in rats (3, 25). However, inflammation has been noted when adenovirus vectors themselves are injected into knee joints of rabbits and mice (30, 37). The elimination of transduced cells by the host immune system and the episomal nature of this vector cause a short-lived expression of adenovirus-transduced genes (45, 46).
Adeno-associated virus (AAV) is a single stranded, nonpathogenic virus. AAV vectors represent a promising alternative to current viral delivery systems (42, 43). Removal of all viral coding sequences (96% of the genome) eliminates the possibility of an immune response to residual viral gene expression (1, 15, 42). The recombinant AAV (rAAV) genome can integrate into the host chromosome, facilitating long-term transduction (29, 43). Recent studies with rAAV in vivo have resulted in efficient, long-term gene transfer in a variety of tissues (1, 15, 42). In addition, rAAV preparations are stable and can be produced at high titers of more than 1012 particles per ml (16). Recent research with tissue cultures indicated that cell proliferation can enhance rAAV transduction significantly (36). These findings make arthritis a candidate disease for AAV gene therapy, since arthritis is accompanied by synovial membrane cell proliferation.
In this study, gene delivery into arthritic joints by rAAV carrying the Escherichia coli β-galactosidase gene regulated by the cytomegalovirus (CMV) promoter was studied in an animal model of acute arthritis. The animal arthritis was established by intra-articular injection of lipopolysaccharide (LPS), which induces transient synoviocyte hyperplasia and polymorphonuclear cell infiltration (8, 11, 12, 18, 22, 39). We find that joint inflammation could be induced by LPS treatment effectively and that the expression of the transduced gene decreased significantly when the transient inflammation subsided, about 30 days after LPS treatment. Moreover, the reduced transgene expression could still be efficiently reactivated by a second LPS treatment.
MATERIALS AND METHODS
rAAV.
The rAAV-lacZ construct used in this work contains a lacZ reporter gene that harbors a nuclear localization signal under the regulation of the CMV immediate-early promoter (42). Preparations of the rAAV-lacZ viral vector were made by cotransfection methods according to published protocols with modifications (44). Briefly, at 1 to 2 h before transfection, 80 15-cm-diameter dishes of human 293 cells at 80% confluence were fed with 25 ml of fresh Iscove modified Eagle medium (Gibco) containing 10% fetal calf serum (Gibco) without antibiotics. A total of 49 μg of plasmid DNA (16 μg of rAAV-lacZ plasmid plus 8 μg of pXX2, which encodes Rep and Cap proteins, and 25 μg of pXX6, which encodes adenovirus gene products) was used to transfect 293 cells in each 15-cm-diameter dish by using a modified calcium phosphate precipitation method as described previously (44). Cells from these 80 dishes were harvested 48 h after transfection, resuspended in 40 ml of OptiMEM medium (Gibco), and frozen and thawed four times. Cell lysates were digested with 4,000 U of DNase (Sigma) and 1 mg of RNase (Sigma) at 37°C for 30 min, and deoxycholate (Sigma) was added to a final concentration of 1%. The mixture was then homogenized, and CsCl was added to a final density of 1.37 g/ml. CsCl density gradient purification was then carried out as previously described (42). Titers of rAAV-lacZ were determined by coinfection of 293 cells with various dilutions of rAAV-lacZ and adenovirus type 5 dl309 (multiplicity of infection of 1). The cells were fixed 24 h later and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (42). Each blue cell was considered to be transduced by one infectious rAAV-lacZ particle.
Animals and experimental arthritis.
Sprague-Dawley rats weighing 250 to 300 g were handled in accordance with government guidelines. Experiments were done with female rats 8 weeks of age. Experimental arthritis was achieved by the following protocol. LPS (Sigma) was dissolved in distilled H2O with gentle sonication and diluted to 1 mg/ml in phosphate-buffered saline (PBS). Rats were anesthetized with 30 mg of tribromoethanol (Avertin; Aldrich Chemical Co., Milwaukee, Wis.) per kg intraperitoneally. Intra-articular injections of LPS (10 μg) were then given through the patella tendon, using a 30-gauge needle adapted to a Hamilton syringe.
In situ staining for β-galactosidase transgene expression.
Rats were anesthetized with 30 mg of tribromoethanol (Avertin) per kg intraperitoneally. One hundred microliters of rAAV-lacZ (107 infectious units) or an equivalent volume of PBS was given through the patella tendon, using a 30-gauge needle adapted to a Hamilton syringe, slowly over 1 min. We made sure that there was no back flow of virus fluid after the removal of the needle. The sizes of the knees did not change significantly after injection, and the viral solution did not withdraw after injection. In situ staining for β-galactosidase activity was performed by a published procedure with modifications (40). Briefly, at various times after rAAV-lacZ infection, animals were euthanized with a intravenous overdose of pentobarbital. Knee joints were dissected, and synovial tissue together with the patella tendon were removed and washed extensively with 1× PBS. The synovial tissues were fixed in a solution freshly prepared by mixing equal volumes of 4% paraformaldehyde in PBS (pH 7.4) and 1.25% glutaraldehyde in PBS (pH 7.4) with gentle shaking for 2 h. After fixation, samples were rinsed with PBS (pH 7.4) three times and soaked in PBS for 1 h. Immediately after the fixation and rinsing, synovial tissues were placed in staining solution containing 5 mM K3Fe(CN)6, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40, and 1 mg of X-Gal per ml in PBS (pH 7.4) for 4 h at 37°C with gentle shaking. Samples were freshly frozen in OCT (Miles, Inc., Elkhart, Ind.). Sections (5 μm) were prepared on a CM1900 cryostat (Leica) and placed on microscope slides. For the estimation of percentages of lacZ-positive cells, X-Gal-stained sections were counterstained with hematoxylin and eosin. The numbers of X-Gal-stained cells and synoviocytes without X-Gal staining were determined by cell counting under a BX50 microscope (Olympus, Tokyo, Japan) from five randomly selected high-power fields (magnification, ×400). Percentages were derived by dividing the numbers of X-Gal-stained cells by the sums of the numbers of X-Gal-stained cells and the numbers of synoviocytes without X-Gal staining. The five results were then averaged.
Histology.
For histological study of synovial tissues, rats were euthanized with pentothal, and synovial membranes were surgically removed, fixed with 4% paraformaldehyde, sectioned with a CM1900 cryostat (Leica), and stained with hematoxylin and eosin.
RESULTS
Establishment of an LPS-induced arthritis model.
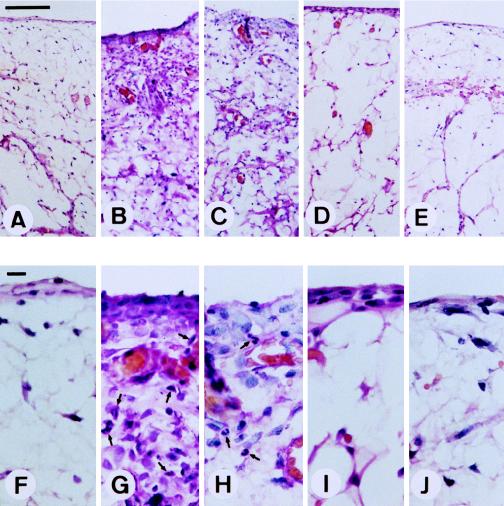
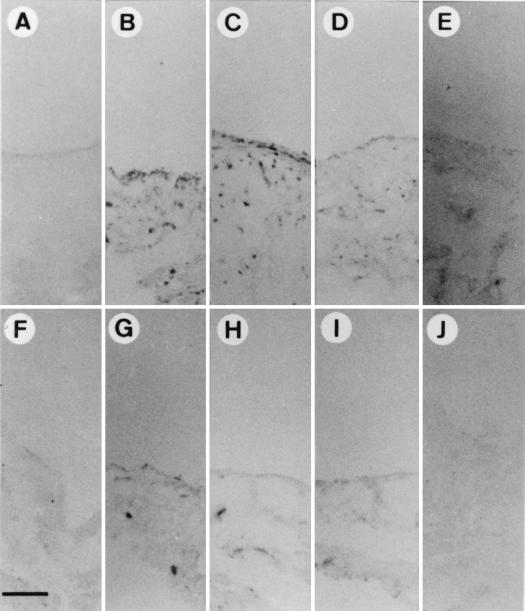
LPS induces primarily an acute arthritis of relatively short duration after intra-articular injection (8, 11, 12, 18, 22, 39). To study the effect of inflammation on gene delivery to synovial tissue, LPS was injected into the knee joints of Sprague-Dauley rats. Within 12 h after injection, redness and swelling of all of the injected joints could be observed (data not shown). As shown in Fig. 1A and F, before the injection of LPS, there were two or three layers of normal synovial lining cells and no polymorphonuclear cell infiltration of subsynovial adipose tissue. Synovial membrane hyperplasia, due to synovial fibroblast proliferation, was observed 3 days after LPS injection (Fig. 1B and G). We also observed polymorphonuclear cell infiltration in the synovial lining layer and subsynovial adipose tissue (arrows in Fig. 1G). These changes lasted for 7 days (Fig. 1C and H) and gradually subsided during the next 7 days (Fig. 1D and I). At 30 days after LPS injection, no signs of inflammation could be identified (Fig. 1E and J). These morphological and histological findings indicate that acute arthritis can be induced by LPS treatment and subsides within 30 days.
FIG. 1.
Induction of joint inflammation by LPS treatment. Ten micrograms of LPS was injected into individual joints. Three (B and G), 7 (C and H), 14 (D and I), and 30 (E and J) days later, synovial tissues were surgically removed, fixed, and stained with hematoxylin-eosin as described in Materials and Methods. (A and F) Tissues without LPS treatment. Arrows, polymorphonuclear cells. Magnifications, ×100 (A to E) and ×400 (F to J). Bar in panel A, 100 μm; bar in panel F, 10 μm.
Enhancement of rAAV gene delivery by the inflammatory process.
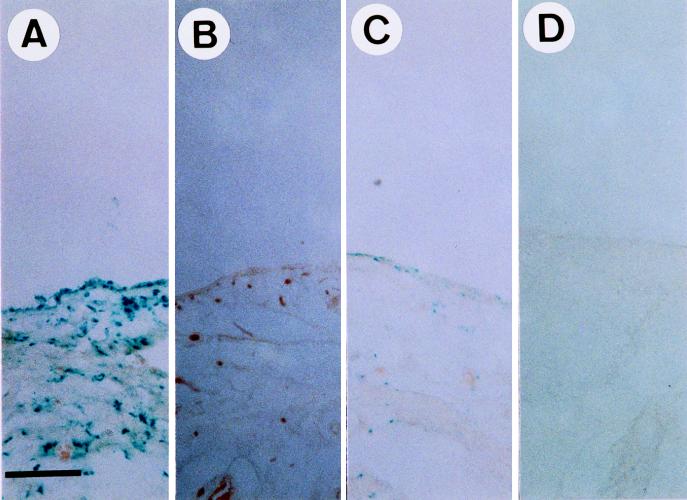
After the establishment of the animal model of arthritis, we intended to investigate rAAV-mediated gene delivery in inflammatory joint tissues and used the lacZ gene as a reporter. However, it has been reported that synoviocytes may produce significant endogenous levels of lysosomal galactosidase, an enzyme that may react with X-Gal and produce false-positive signals (35). To rule out the possibility that lacZ-positive signals were from lysosomal galactosidase in synoviocytes, LPS was injected into both knees of the same Sprague-Dauley rats. After 12 h, rAAV-lacZ was injected into the right knee joints and PBS (as a negative control) was injected into the left knee joints of the LPS-injected rats. Three days after LPS injection, synovial tissues were isolated, fixed, and stained with X-Gal. The lacZ-positive cells had blue staining in their nuclei because the rAAV used in this study carries a lacZ reporter gene that harbors a nuclear localization signal (42). The results indicate that LPS treatment resulted in accumulation of lacZ-positive cells in rAAV-lacZ-injected knee joints (Fig. 2A) but not in control knee joints (Fig. 2B). Thus, the lacZ-positive signals in LPS-treated joints indeed resulted from the expression of the E. coli β-galactosidase gene delivered by rAAV. In the control group of rats, we injected rAAV-lacZ into normal joints, and only a small number of lacZ-positive cells were observed (Fig. 2C). This was drastically different from the findings for knee joints that received both rAAV-lacZ injection and LPS treatment (Fig. 2A) and suggests that LPS-induced inflammation can enhance the gene delivery by rAAV. Again, no lacZ-positive cells were observed in the normal knees without rAAV-lacZ injection (Fig. 2D).
FIG. 2.
rAAV-mediated lacZ gene delivery into synovial tissues. In an experimental group of rats, both knee joints were injected with 10 μg of LPS. Twelve hours later, 107 infectious units of rAAV-lacZ virus in 100 μl of PBS was injected into the right knee joints (A), and 100 μl of PBS was injected into the left knee joints (B). In a control group of rats, 107 infectious units of rAAV-lacZ virus in 100 μl was injected into the right knee joints (C), and 100 μl of PBS was injected into the left knee joints (D). Three days after the first injection, synovial membranes were surgically removed, fixed, and stained for β-galactosidase (lacZ) activity. Bar, 100 μm.
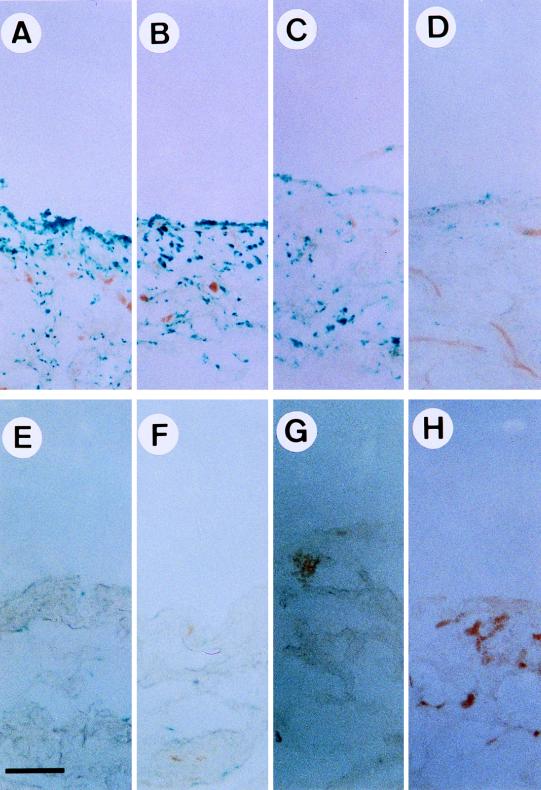
To further characterize the enhancement of gene delivery by LPS treatment, a time course analysis was performed. LPS was injected into the right knee and PBS was injected into the left knee of the same Sprague-Dauley rats. Twelve hours later, rAAV-lacZ was injected into these joints. At 3, 7, 14, and 30 days after rAAV injection, synovial tissues were isolated, fixed, and stained for β-galactosidase activity. The percentages of lacZ-positive cells were determined by counting blue cells from five randomly selected high-power fields under a microscope. At 3 days after rAAV-lacZ injection, about 95% of synovial lining cells were stained positive and lacZ-positive cells were distributed both in the synovial lining layer and the adipose tissue layer underneath (Fig. 3A). At 7 days after rAAV-lacZ injection, about 89% of synovial lining cells remained positive (Fig. 3B). At 14 days after rAAV-lacZ injection, there was a moderate reduction of lacZ-positive cells, to 70% (Fig. 3C). However, at 30 days after rAAV-lacZ injection, the lacZ-positive cells dropped to 8% (Fig. 3D). In synovial tissues from joints with PBS treatment, lacZ-positive cells were always fewer than 5% at 3 (Fig. 3E), 7 (Fig. 3F), 14 (Fig. 3G), and 30 (Fig. 3H) days after the injection of rAAV-lacZ. These observations further confirm that expression of the AAV-transduced gene can be stimulated by LPS pretreatment, as shown in Fig. 2. Moreover, it seems that the expression of the AAV-transduced gene is transient and correlates strongly with the degree of joint inflammation.
FIG. 3.
Correlation between inflammation status and lacZ gene expression. Normal rat knee joints were injected with 10 μg of LPS (A to D) or PBS (E to H). Twelve hours later, 107 infectious units of rAAV-lacZ was injected into knee joints. At 3 (A and E), 7 (B and F), 14 (C and G), and 30 (D and H) days after rAAV-lacZ injection, synovial membranes were surgically removed, fixed, and stained for β-galactosidase (lacZ) activity. Bar, 100 μm.
CMV promoter-controlled gene expression can be reinduced by LPS treatment.
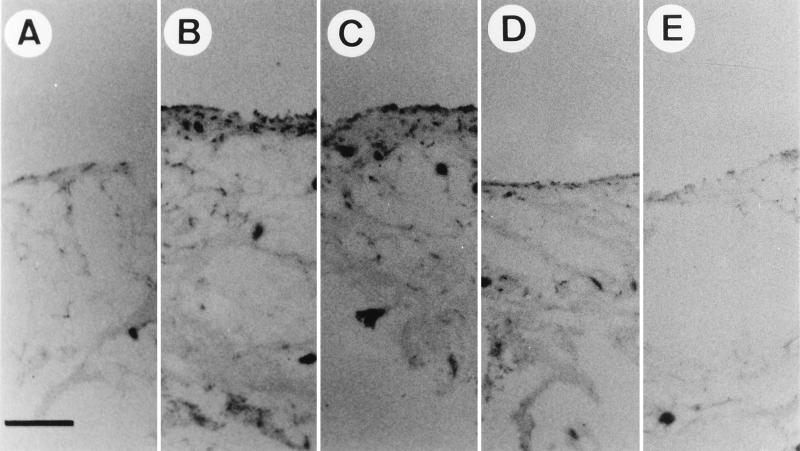
From the results shown in Fig. 3, there are several possible explanations for the gradual loss of lacZ-positive cells during the subsidence of LPS-induced arthritis. One is that the transduced cells may go into apoptotic or nonapoptotic cell death. If this is the case, the reexposure of the synovium to LPS must not increase lacZ-positive cells, since transduced cells are permanently lost. Another possibility is the suppression in normal synovial tissue of the CMV promoter, which was used in this study to drive lacZ gene expression. To test these two possibilities, we first pretreated rat knees with LPS. Twelve hours later, rAAV-lacZ was injected into the same joints. Thirty days later, X-Gal staining revealed that the expression of the lacZ gene was suppressed to less than 8% (Fig. 3D and 4A). A second dose (10 μg) of LPS was then injected into the same joints. The expression of the lacZ gene was monitored over time. At 3 days after LPS treatment, about 93% of the cells in the synovium were stained positive (Fig. 4B), and about 90% of the cells remained positive 7 days after treatment (Fig. 4C). Fourteen days later, there was a moderate reduction of lacZ-positive cells, to 70% (Fig. 4D), and 30 days later, the lacZ-positive cells dropped to 8% (Fig. 4E). The lacZ-positive cells were of the same percentage and were distributed in the same histology pattern as shown in Fig. 3. Taken together, these results demonstrate that the CMV promoter of the rAAV transgene may be induced by LPS and suggest that the decrease of cells expressing the transgene is from suppression of the CMV promoter rather than from loss of transduced cells.
FIG. 4.
Reinduction of the rAAV-delivered gene by LPS treatment. Normal rat knee joints were injected with 10 μg of LPS. Twelve hours later, 107 infectious units of rAAV-lacZ was injected into the same knee joints. Thirty days later, a second dose of 10 μg of LPS was injected into the same knees, and then 3 (B), 7 (C), 14 (D), and 30 (E) days after the second LPS treatment, synovial membranes were surgically removed, fixed, and stained for β-galactosidase (lacZ) activity. (A) Tissue from knees without the second LPS injection. Bar, 100 μm.
LPS can induce lacZ gene expression 30 days after rAAV-lacZ gene delivery.
As demonstrated above, the gene delivery by rAAV-lacZ was poor in the absence of LPS stimulation or an inflammatory process. This predicts that gene therapy may not be effective if rAAV is delivered when joint tissues are not in the condition of inflammation, such as in the early stage of arthritis, during remission, or with anti-inflammatory therapy. This may limit the application of rAAV-mediated gene therapy in arthritis. Hence, in this study, we investigated whether the inactive rAAV-delivered gene could be stimulated by a later inflammatory process. rAAV-lacZ was first injected into bilateral knee joints, and 30 days later, LPS was injected into the knee joints. Only 5% of the cells were lacZ positive before LPS injection (Fig. 5A), and about 92% of synoviocytes became lacZ positive at 3 (Fig. 5B) and 7 (Fig. 5C) days after LPS treatment. The percentage of lacZ-positive cells decreased to 65% at 14 days (Fig. 5D) and to 5% at 30 days (Fig. 5E) after LPS injection. Figure 5F, G, H, I, and J show fewer than 5% lacZ-positive cells at 0, 3, 7, 14, and 30 days, respectively, after control PBS treatment. These results in Fig. 5 are similar to those in Fig. 2, suggesting that the lacZ gene can be delivered and remain inactive in joint tissues in the absence of inflammation for at least 30 days and that this gene can then be induced by the occurrence of inflammation.
FIG. 5.
Induction of the rAAV-delivered gene by delayed LPS-induced arthritis. rAAV-lacZ virus (107 infectious units) was injected into both knee joints. Thirty days later, the right knees (B to E) were injected with 10 μg of LPS, and the left knees (G to J) were injected with PBS. Before treatment (A and F) or at 3 (B and G), 7 (C and H), 14 (D and I), and 30 (E and J) days after treatment, synovial membranes were surgically removed, fixed, and stained for β-galactosidase (lacZ) activity. Bar, 100 μm.
DISCUSSION
This is the first demonstration of disease state-regulated transgene expression. In this study, when the lacZ gene was delivered by rAAV through direct joint injection, we found that the transduction efficiency was low (Fig. 3E to H). However, after joint inflammation was induced by LPS treatment, most cells (95%) in the synovium became lacZ positive (Fig. 3A and B) during the peak of inflammation (3 to 7 days after LPS treatment), and the number of positive cells decreased with the subsidence of inflammation (14 to 30 days after LPS treatment) (Fig. 3C and D). The transient induction of gene expression by rAAV does not seem to be a specific response to LPS, since we have performed the same experiments by intra-articular injection of recombinant IL-1β, which was reported to induce acute arthritis in mice and rabbits (5), and observed a transient increase of lacZ-positive cells similar to that induced by LPS injection (data not shown). The mechanism of the enhancement of gene transduction in joint tissues by inflammation is unclear. LPS-induced cell proliferation may be one of the mechanisms that can activate the expression of the rAAV-transduced gene. Proliferating cells are transduced by rAAV about 10- to 1,000-fold more efficiently than quiescent cells (14). Thus, the synoviocyte proliferation induced by the inflammatory process (Fig. 1) may promote rAAV-mediated transduction.
More likely, the enhancement of gene expression by inflammation may be through the activation of the CMV promoter by LPS and proinflammatory cytokines. Recent findings indicated that the CMV promoter can be induced by LPS (26). The CMV promoter is active in many cell culture systems and is considered to be one of the strongest promoters in vitro. However, when CMV promoter is used in in vivo approaches to gene therapy, it becomes silent within a few weeks in several organs (19). Recent reports showed that the silenced CMV promoter can be reactivated by LPS treatment in liver, and the LPS-induced NF-κB signaling pathway has been proposed to be the mechanism of CMV promoter activation (26). LPS is a potent stimulator of macrophages and monocytes, which respond by producing TNF and IL-1, etc. (7, 10, 28). Other recent reports demonstrated that LPS, IL-1, and TNF can cause phosphorylation and degradation of IκB, an inhibitor of NF-κB (6, 33). In general, NF-κB is constitutively expressed in the cytoplasm, is bound to the inhibitor IκB, and remains inactive. Only when IκB is degraded can NF-κB be released, be translocated to the nucleus, and become functional for transcription activation (21, 32, 41). There are four NF-κB consensus binding sites in the CMV promoter, and efficient transcription from the CMV promoter is dependent on these sites (41). Hence, an explanation of the enhancement of gene expression could be that NF-κB is inactivated by its binding to IκB in normal synoviocytes, and the lack of binding between NF-κB and the CMV promoter renders the CMV promoter inactive. In the presence of LPS, TNF, or IL-1, the degradation of IκB leads to NF-κB translocation into the nucleus and activates the CMV promoter.
In this study, we observed a striking correlation between transgene expression and the severity of the arthritis. At the peak of the disease insult (3 to 7 days), 95% of the synoviocytes (Fig. 3A and B) had high-level transgene expression, which diminished to a basal level of 5% synoviocytes when the joint inflammation subsided at 30 days after LPS treatment (Fig. 3D). We exposed the joints which recovered from LPS-induced inflammation to a second injection of LPS and observed a dramatic reactivation of transgene expression, in which the gene expression pattern was similar to that induced by the first LPS treatment (Fig. 3 and 4). Regarding the mechanisms responsible for these observations, the reinduction of lacZ-positive cells may be explained by inflammation reactivating the suppressed CMV promoter. Since a single LPS treatment induces only transient inflammation of synovial tissues (Fig. 1), the CMV promoter is transiently activated. Interestingly, the transduced gene remains stable in synoviocytes and becomes readily induced by the second LPS treatment. This model strongly suggests the persistence of the rAAV-delivered gene in the synovium for at least 30 days. Indeed, the fact that the percentage of lacZ-positive cells (93%) induced by reexposure of synoviocytes was similar to that induced by primary LPS treatment indicates that there was no detectable reduction of transduced cells over 30 days.
Here, we present evidence that the lacZ gene could be predelivered by rAAV, remain inactive in synoviocytes for at least 30 days, and then still be efficiently induced by LPS treatment (Fig. 5). Since arthritis can be diagnosed at an early stage when joint inflammation is not severe, the application of rAAV gene delivery for the prevention of arthritis may be restricted if inflammation is necessary to facilitate gene delivery. It is comforting that the results shown in Fig. 5 indicate that the prevention of arthritis by gene delivery is feasible.
Among the challenges of developing gene therapy for arthritis is the achievement of efficient, prolonged, and yet regulated gene expression in vivo. Ex vivo gene transfer to cells derived from rabbit synoviocytes by using recombinant retrovirus has been reported; however, the efficiency of gene transfer is relative low, and the technique is laborious (4, 5). On the other hand, the use of adenovirus vectors for intra-articular infection is relatively simple and results in the efficient genetic transduction of synovial lining cells, but the gene expression is transient (19). From our observations, rAAV transduction to synoviocytes can be highly efficient (Fig. 2 and 3). The reinduction of lacZ gene expression in a majority of the cells (Fig. 4) indicates that the rAAV-transduced gene can be stably maintained in synoviocytes for at least 30 days. This stability can be attributed to the integration capability of rAAV and to the fact that rAAV-transduced cells do not elicit a cytotoxic-T-lymphocyte response; both are major differences between rAAV vectors and the currently used recombinant adenovirus vectors (1, 15, 42, 45, 46). In this study, we demonstrate efficient and stable gene delivery by rAAV, suggesting that rAAV is a superior tool for arthritis gene therapy. Moreover, this is the first demonstration of disease state-regulated transgene expression. These findings strongly support the feasibility of therapeutic as well as preventative gene transfer approaches to RA with rAAV vectors containing therapeutic genes, which respond primarily to the disease state of the target tissue.
ACKNOWLEDGMENTS
The first two authors contributed equally to this report.
We greatly appreciate the technical assistance of Junn-Liang Chang and Dai-Wei Liu. We thank John Wu for editing the manuscript.
This study was supported by National Health Research Institute grant DD01-86IX-MG609P and National Science Council grant NSC 88-2314-b-016-011-M20.
REFERENCES
- 1.Afione S A, Conrad C K, Kearns W G, Chunduru S, Adams R, Reynolds T, Guggino W B, Cutting G R, Carter B J, Flotte T R. In vivo model of adeno-associated virus vector persistence and rescue. J Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend W P, Dayer J M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 3.Bakker A C, Joosten L A, Arntz O J, Helsen M M, Bendele A M, van de Loo F A, van den Berg W B. Prevention of murine collagen-induced arthritis in the knee and ipsilateral paw by local expression of human interleukin-1 receptor antagonist protein in the knee. Arthritis Rheum. 1997;40:893–900. doi: 10.1002/art.1780400517. [DOI] [PubMed] [Google Scholar]
- 4.Bandara G, Robbins P D, Georgescu H I, Mueller G M, Glorioso J C, Evans C H. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:27–231. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- 5.Bandara G, Mueller G M, Galea-Laure M H, Tindal M H, Georgescu H I, Suchanek M K, Hung G L, Glorioso J C, Robbins P D, Evans C H. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci USA. 1993;90:10764–10768. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beg A A, Finco T S, Natermet P V, Baldwin A S. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 8.Braude A I, Jones J L, Douglas H. The behavior of Escherichia coli endotoxin (Somatic antigen) during infectious arthritis. J Immunol. 1963;90:297–311. [PubMed] [Google Scholar]
- 9.Dayer J M, Fenner H. The role of cytokines and their inhibitors in arthritis. Ballieres Clin Rheumatol. 1992;6:485–516. doi: 10.1016/s0950-3579(05)80186-4. [DOI] [PubMed] [Google Scholar]
- 10.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 11.Esser R E, Stimpson S A, Cromartie W J, Schwab J H. Reactivation of streptococcal cell wall-induced arthritis by homologous and heterologous cell wall polymers. Arthritis Rheum. 1985;28:1402–1411. doi: 10.1002/art.1780281213. [DOI] [PubMed] [Google Scholar]
- 12.Esser R E, Anderle S K, Chetty C, Stimpson S A, Cromartie W J, Schwab J H. Comparison of inflammatory reactions induced by intraarticular injections of bacterial cell wall polymers. Am J Pathol. 1986;122:323–334. [PMC free article] [PubMed] [Google Scholar]
- 13.Evans C H, Robbins P D. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77:1103–1114. doi: 10.2106/00004623-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P L, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flotte T R, Barraza-Ortiz X, Solow R, Afinoe S A, Carter B J, Guggino W B. An improved system for packaging recombinant adeno-associated virus vectors capable of in vivo transduction. Gene Ther. 1995;2:29–37. [PubMed] [Google Scholar]
- 17.Ghivizzani S C, Lechman E R, Kang R, Tio C, Kolls J, Evans C H, Robbins P D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor α soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci USA. 1998;95:4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenburg D L, Reed J I, Rice P A. Arthritis in rabbits induced by killed Neisseria gonorrhoeae and gonococcal lipopolysaccharide. J Rheumatol. 1984;11:3–8. [PubMed] [Google Scholar]
- 19.Guo Z S, Wang L H, Eisensmith R C, Woo C L. Evaluation of promoter strength for hepatic gene expression in vivo following adenovirus-mediated gene transfer. Gene Ther. 1996;3:802–810. [PubMed] [Google Scholar]
- 20.Henderson B, Thompson R C, Hardingham W, Lewthwaite J. Inhibition of interleukin-1 induced synovitis and articular cartilage proteoglycan loss in the rabbit knee by recombinant human interleukin-1-receptor antagonist. Cytokine. 1991;3:246–249. doi: 10.1016/1043-4666(91)90023-7. [DOI] [PubMed] [Google Scholar]
- 21.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle P A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth J W, Atkins E. Synovial inflammatory response to bacterial endotoxin. Yale J Biol Med. 1965;38:241–256. [PMC free article] [PubMed] [Google Scholar]
- 23.Hung G L, Galea-lauri J, Mueller G M, Georgescu H I, Larkin L A, Suchanek M K, Tindal M H, Robbins P D, Evans C H. Suppression of intraarticular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1:64–69. [PubMed] [Google Scholar]
- 24.Kaklamanis P M. Experimental animal models resembling rheumatoid arthritis. Clin Rheumatol. 1992;11:41–47. doi: 10.1007/BF02207082. [DOI] [PubMed] [Google Scholar]
- 25.Le C H, Nicolson A G, Morales A, Sewell K L. Suppression of collagen-induced arthritis through adenovirus-mediated transfer of a modified tumor necrosis factor alpha receptor gene. Arthritis Rheum. 1997;40:1662–1669. doi: 10.1002/art.1780400916. [DOI] [PubMed] [Google Scholar]
- 26.Loser P, Jennings G S, Strauss M, Sandig V. Reactivation of the previously silenced cytomegalovirus major immediate-early promoter in the mouse liver: involvement of NFκB. J Virol. 1998;72:180–190. doi: 10.1128/jvi.72.1.180-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohler K M, Torranceet D S, Smith C A, Goodwin R G, Stremler K E, Fung V P, Madani H, Widmer M B. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 28.Morrison D C, Ryan J. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 29.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 30.Nita I, Ghivizzani S C, Galea-Lauri J, Bandara G, Georgescu H I, Robbins P D, Evans C H. Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum. 1996;39:820–828. doi: 10.1002/art.1780390515. [DOI] [PubMed] [Google Scholar]
- 31.Otani K, Nita I, Macaulay W, Georgescu H I, Robbins P D, Evans C H. Suppression of antigen-induced arthritis in rabbits by ex vivo gene therapy. J Immunol. 1996;156:3558–3562. [PubMed] [Google Scholar]
- 32.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 33.Prosch S, Staak K, Stein J, Liebenthal C, Stamminger T, Volk H-D, Kruger D H. Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNFα is mediated via induction of NF-κB. Virology. 1995;208:197–206. doi: 10.1006/viro.1995.1143. [DOI] [PubMed] [Google Scholar]
- 34.Remmers E F, Lafyatis R, Kumkumaian G K, Case J P, Roberts A B, Sporn M B, Wilder R L. Cytokines and growth regulation of synoviocytes from patients with rheumatoid arthritis and rats with streptococcal cell wall arthritis. Growth Factors. 1990;2:179–188. [PubMed] [Google Scholar]
- 35.Roessler B J, Allen E D, Wilson J M, Hartman J W, Davidson B L. Adenoviral-mediated gene transfer to rabbit synovium in vivo. J Clin Invest. 1993;92:1085–1092. doi: 10.1172/JCI116614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell D W, Miller A D, Alexander I E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawchuk S J, Boivin G, Duwel L, Ball W, Bove K, Trapnell B, Hirsch R. Anti-T cell receptor monoclonal antibody prolongs transgene expression on following adenovirus-mediated in vivo gene transfer. Hum Gene Ther. 1996;7:499–506. doi: 10.1089/hum.1996.7.4-499. [DOI] [PubMed] [Google Scholar]
- 38.Smith R J, Chin J E, Sam L M, Justen J M. Biological effects of an interleukin-1 receptor antagonist protein on interleukin-1 stimulated cartilage erosion and chondrocyte responsiveness. Arthritis Rheum. 1991;34:78–83. doi: 10.1002/art.1780340112. [DOI] [PubMed] [Google Scholar]
- 39.Stimpson S A, Esser R E, Carter P B, Sartor R B, Cromartie W J, Schwab J H. Lipopolysaccharide induces recurrence of arthritis in rat joints previously injured by peptidoglycan-polysaccharide. J Exp Med. 1988;165:1688–1702. doi: 10.1084/jem.165.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan R, Lo C W. Histochemical and fluorochrome-based detection of β-galactosidase. In: Tuan R S, editor. Recombinant protein protocols: detection and isolation. Totowa, N.J: Humana Press; 1997. pp. 229–246. [DOI] [PubMed] [Google Scholar]
- 41.Thompson J E, Philips R J, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 42.Xiao X, Li J, Samulski R J. Efficient long-term transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao X, Li J, McCown T J, Samulski R J. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- 44.Xiao X, Li J, Samulski R J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y, Ertl H C, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenovirus. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]