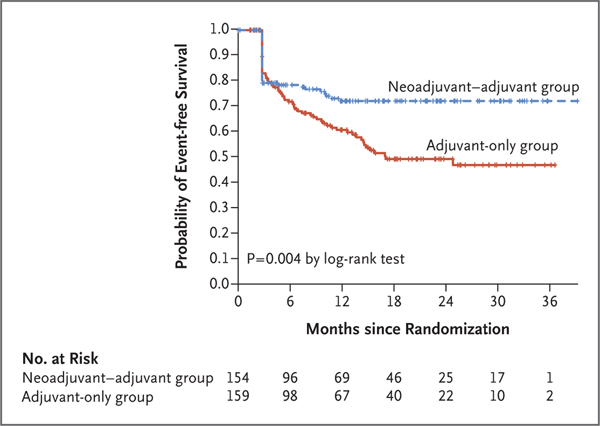
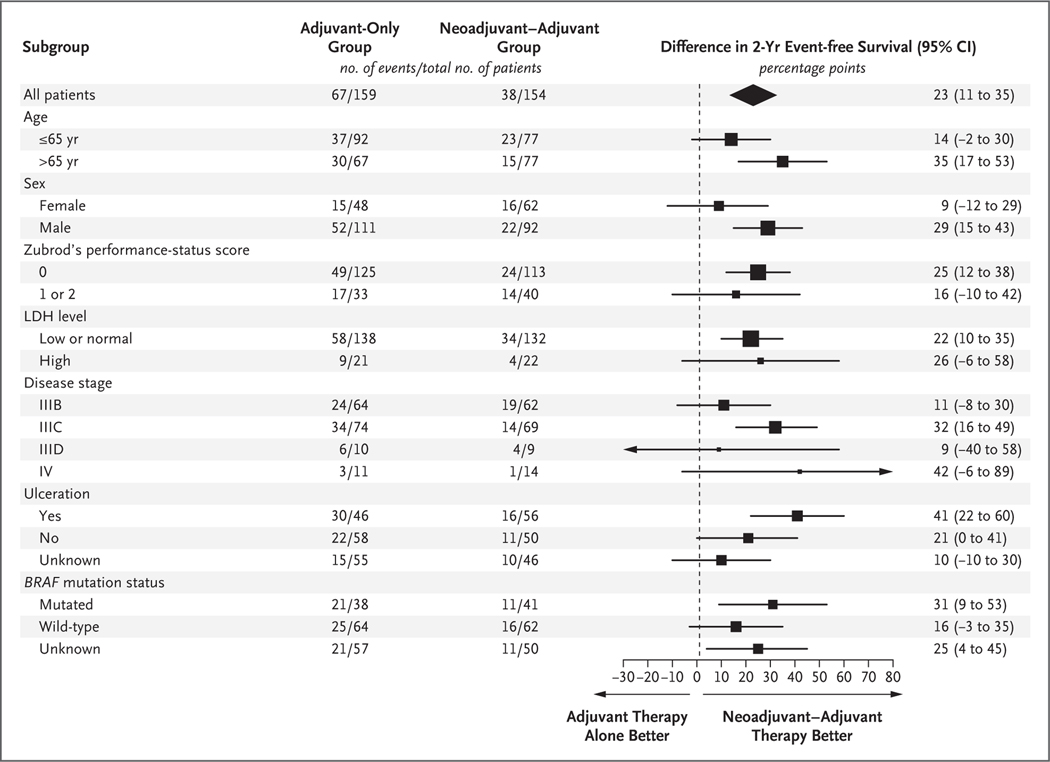
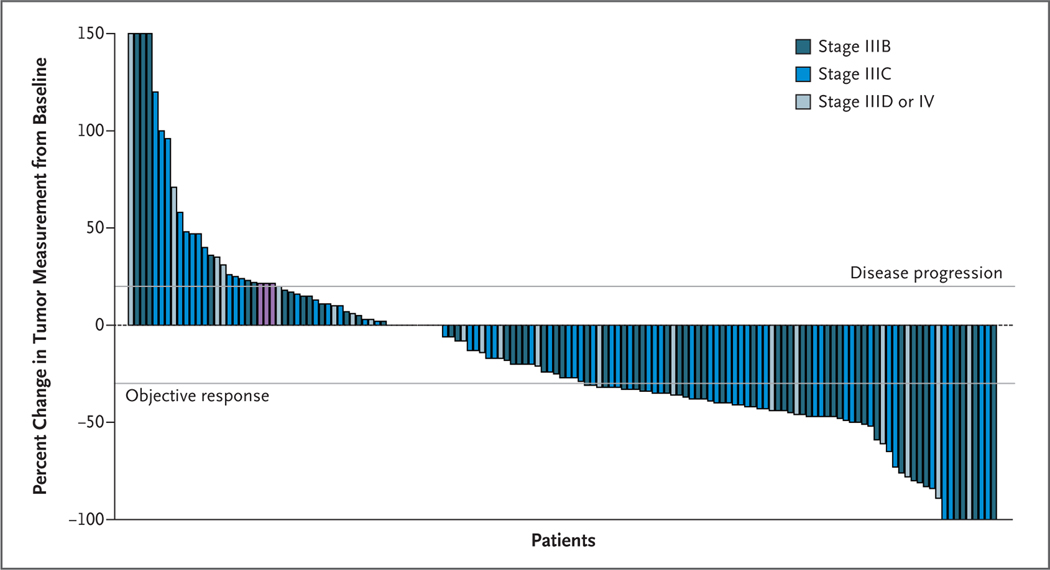
Sapna P Patel
Sapna P Patel, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Megan Othus
Megan Othus, Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Yuanbin Chen
Yuanbin Chen, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
G Paul Wright Jr
G Paul Wright Jr, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Kathleen J Yost
Kathleen J Yost, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
John R Hyngstrom
John R Hyngstrom, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Siwen Hu-Lieskovan
Siwen Hu-Lieskovan, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Christopher D Lao
Christopher D Lao, M.D., M.P.H.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Leslie A Fecher
Leslie A Fecher, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Thach-Giao Truong
Thach-Giao Truong, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Jennifer L Eisenstein
Jennifer L Eisenstein, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Sunandana Chandra
Sunandana Chandra, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Jeffrey A Sosman
Jeffrey A Sosman, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Kari L Kendra
Kari L Kendra, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Richard C Wu
Richard C Wu, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Craig E Devoe
Craig E Devoe, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Gary B Deutsch
Gary B Deutsch, M.D., M.P.H.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Aparna Hegde
Aparna Hegde, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Maya Khalil
Maya Khalil, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Ankit Mangla
Ankit Mangla, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Amy M Reese
Amy M Reese, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Merrick I Ross
Merrick I Ross, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Andrew S Poklepovic
Andrew S Poklepovic, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Giao Q Phan
Giao Q Phan, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Adedayo A Onitilo
Adedayo A Onitilo, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Demet G Yasar
Demet G Yasar, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Benjamin C Powers
Benjamin C Powers, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Gary C Doolittle
Gary C Doolittle, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Gino K In
Gino K In, M.D., M.P.H.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Niels Kokot
Niels Kokot, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Geoffrey T Gibney
Geoffrey T Gibney, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Michael B Atkins
Michael B Atkins, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Montaser Shaheen
Montaser Shaheen, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
James A Warneke
James A Warneke, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Alexandra Ikeguchi
Alexandra Ikeguchi, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Jose E Najera
Jose E Najera, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Bartosz Chmielowski
Bartosz Chmielowski, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Joseph G Crompton
Joseph G Crompton, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Justin D Floyd
Justin D Floyd, D.O.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Eddy Hsueh
Eddy Hsueh, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Kim A Margolin
Kim A Margolin, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Warren A Chow
Warren A Chow, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Kenneth F Grossmann
Kenneth F Grossmann, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Eliana Dietrich
Eliana Dietrich
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Victor G Prieto
Victor G Prieto, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Michael C Lowe
Michael C Lowe, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Elizabeth I Buchbinder
Elizabeth I Buchbinder, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
John M Kirkwood
John M Kirkwood, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Larissa Korde
Larissa Korde, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
James Moon
James Moon, M.S.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Elad Sharon
Elad Sharon, M.D., M.P.H.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Vernon K Sondak
Vernon K Sondak, M.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1,
Antoni Ribas
Antoni Ribas, M.D., Ph.D.
1University of Texas M.D. Anderson Cancer Center, Houston (S.P.P., M.I.R., V.G.P.), and the University of Texas Health Science Center at San Antonio, San Antonio (M.S.); Southwest Oncology Group Statistics and Data Management Center (M.O., J.M.) and the Fred Hutchinson Cancer Center (M.O., E.D., J.M.) — both in Seattle; the Cancer Research Consortium of West Michigan National Cancer Institute Community Oncology Research Program (NCORP)–Cancer and Hematology Centers of Western Michigan (Y.C.), the Cancer Research Consortium of West Michigan NCORP–Spectrum Health (G.P.W.), and the Cancer Research Consortium of West Michigan NCORP (K.J.Y.), Grand Rapids, and the University of Michigan Rogel Cancer Center, Ann Arbor (C.D.L., L.A.F.); the University of Utah Huntsman Cancer Institute, Salt Lake City (J.R.H., S.H.-L.); Kaiser Permanente Northern California, Vallejo (T.-G.T.), University of Southern California Norris Comprehensive Cancer Center (G.K.I., N.K.) and University of California Los Angeles Jonsson Comprehensive Cancer Center (B.C., J.G.C., A.R.), Los Angeles, City of Hope Comprehensive Cancer Center–Saint John’s Cancer Institute, Santa Monica (K.A.M.), and City of Hope Comprehensive Cancer Center–University of California, Irvine, Irvine (W.A.C.) — all in California; Kaiser Permanente Colorado, Lafayette (J.L.E.); Northwestern University, Chicago (S.C., J.A.S.), and the Cancer Care Specialists of Illinois–Heartland NCORP, O’Fallon (J.D.F.) — both in Illinois; Ohio State University Wexner Medical Center, Columbus (K.L.K., R.C.W.), and University Hospitals Seidman Cancer Center–Case Western Reserve University Case Comprehensive Cancer Center, Cleveland (A.M., A.M.R.); Northwell Health Cancer Institute, Lake Success, NY (C.E.D., G.B.D.); the University of Alabama at Birmingham, Birmingham (A.H., M.K.); Virginia Commonwealth University–Massey Cancer Center–VCU Massey Cancer Center Minority Underserved NCORP, Richmond (A.S.P., G.Q.P.); Marshfield Medical Center Wisconsin NCORP, Weston (A.A.O.), and Marshfield Medical Center Wisconsin NCORP, Minocqua (D.G.Y.); the University of Kansas Cancer Center, Overland Park (B.C.P.), and the University of Kansas Hospital–Westwood Cancer Center, Westwood (G.C.D.); MedStar Georgetown University Hospital, Washington, DC (G.T.G., M.B.A.); Banner University Medical Center–University of Arizona Cancer Center, Tucson (M.S., J.A.W.); the University of Oklahoma, Oklahoma City (A.I.), and the University of Oklahoma–Cancer Centers of Southwest Oklahoma, Lawton (J.E.N.); Saint Louis University School of Medicine, St. Louis (E.H.); Merck, Rahway, NJ (K.F.G.); Emory University, Atlanta (M.C.L.); Dana–Farber Cancer Institute–Harvard Cancer Center, Boston (E.I.B.); the University of Pittsburgh Medical Center Hillman Cancer Center, Pittsburgh (J.M.K.); the National Cancer Institute Cancer Therapy Evaluation Program, Bethesda, MD (L.K., E.S.); and Moffitt Cancer Center, Tampa, FL (V.K.S.).
1