Abstract
The T cell lineage–restricted protein THEMIS plays a critical role in T cell development at the positive selection stage. In the SHP1 activation model, THEMIS is proposed to enhance the activity of the tyrosine phosphatase SHP1 (encoded by Ptpn6), thereby dampening T cell antigen receptor (TCR) signaling and preventing the inappropriate negative selection of CD4+CD8+ thymocytes by positively selecting ligands. In contrast, in the SHP1 inhibition model, THEMIS is proposed to suppress SHP1 activity, rendering CD4+CD8+ thymocytes more sensitive to TCR signaling initiated by low-affinity ligands to promote positive selection. We sought to resolve the controversy regarding the molecular function of THEMIS. We found that the defect in positive selection in Themis−/− thymocytes was ameliorated by pharmacologic inhibition of SHP1 or by deletion of Ptpn6 and was exacerbated by SHP1 overexpression. Moreover, overexpression of SHP1 phenocopied the Themis−/− developmental defect, whereas deletion of Ptpn6, Ptpn11 (encoding SHP2), or both did not result in a phenotype resembling that of Themis deficiency. Finally, we found that thymocyte negative selection was not enhanced but was instead impaired in the absence of THEMIS. Together, these results provide evidence favoring the SHP1 inhibition model, supporting a mechanism whereby THEMIS functions to enhance the sensitivity of CD4+CD8+ thymocytes to TCR signaling, enabling positive selection by low-affinity, self-ligand–TCR interactions.
Introduction
A distinguishing feature of T cell development is the stage referred to as thymocyte selection, at which immature CD4+CD8+ (double-positive, DP) thymocytes receive signals transmitted by the T cell antigen receptor (TCR) that determine cell fate (survival or death) and lineage choice, resulting in the generation of either mature CD4+CD8- (CD4 single-positive, CD4 SP) or mature CD4-CD8+ (CD8 single-positive, CD8 SP) thymocytes (1). As a consequence of the semi-stochastic process of V-(D)-J recombination at the Tcrα and Tcrβ loci and the activity of terminal deoxynucleotidyl transferase (TdT), each DP thymocyte expresses a unique, clonotype-specific TCR with the potential to recognize peptides in the thymus (predominantly derived from self-proteins) presented by self major histocompatibility complexes (MHCs) (2). DP thymocytes that express a TCR that does not bind to self-peptide bound self-MHC (self-pMHC) in the thymus fail to receive survival and differentiation signals from the TCR and die by a process called nonselection or neglect. DP thymocytes that express a TCR that binds with low affinity to self-pMHC receive “low-moderate” TCR signals that promote their survival and transition to the SP stage through a process called positive selection. Finally, TCRs that bind to self-pMHC with high affinity transmit “strong,” high-intensity TCR signals that trigger apoptotic cell death by a process called negative selection, preventing the further development of overtly self-reactive thymocytes with the potential to cause autoimmune disease (1).
Experimental data indicate that DP thymocytes are more sensitive to stimulation by self-ligands than are mature T cells (3). Several proteins that have activating roles in the TCR signaling response [for example, protein kinase D2 (PKD2), PKD3, thymocyte-expressed, positive selection associated–1 (TESPA), and sodium voltage-gated channel alpha subunit 5a and 4b (SCN5a/4b)] are more highly abundant in DP thymocytes than in SP thymocytes and peripheral T cells (4). On the other hand, several molecules that inhibit TCR signaling [for example, the protein tyrosine phosphatases PTPN6 (SHP1), PTPN11 (SHP2), and PTPN22] are less abundant in DP thymocytes than in mature SP thymocytes and T cells (4). Together, these findings suggest that DP thymocytes are “primed” to be especially sensitive to TCR engagement, and this enables the transmission of signals sufficient to promote positive selection in response to very low-affinity TCR–self-pMHC interactions (4).
THEMIS was identified as a T cell lineage–specific protein that, similar to PKD2, PKD3, TESPA, and SCN5a/4b, is highly abundant in DP thymocytes but is subsequently reduced in abundance in mature SP thymocytes and T cells (5–7). THEMIS contains a proline-rich region that binds to the ubiquitous cytosolic adaptor protein, GRB2, and tandem copies of a globular sequence designated the CABIT (cysteine-containing-all-beta-in-THEMIS) domain (5, 8). In the absence of THEMIS, thymocyte development is strongly impaired at the positive selection stage, resulting in a marked decrease in CD4 SP and, to a lesser extent, CD8 SP thymocytes and a reduction in CD4+ and CD8+ T cells (5–9). Although the phenotype of Themis−/− mice suggests that it plays a role in the TCR signaling response in DP thymocytes, and therefore in thymocyte selection, a clearly defined signaling defect has not been consistently demonstrated in Themis−/− thymocytes (5–12). The only consistent finding is that Themis−/− DP thymocytes contain reduced amounts of tyrosine-phosphorylated SHP1, a tyrosine phosphatase that also binds to GRB2 and that inhibits TCR signaling through its dephosphorylation of several activated (phosphorylated) effector molecules, including LCK, ZAP-70, and LAT (13, 14).
Independent studies from several groups, including our own, have led to the proposal of distinct and directly contrasting models for THEMIS function in TCR signaling (10, 11, 15–18). The first model (herein designated the SHP1 activation model) maintains that THEMIS, through its interaction with SHP1 facilitated by GRB2, positively regulates SHP1 phosphatase activity, thereby dampening or inhibiting TCR signaling and preventing inappropriate negative selection in response to otherwise positively selecting TCR–self-pMHC interactions (10, 17, 18). We proposed a second model (herein designated the SHP1 inhibition model) that maintains that THEMIS inhibits the tyrosine phosphatase activity of SHP1, enhancing the TCR signaling sensitivity of DP thymocytes (15, 16). We previously reported that the THEMIS CABIT domains inhibit SHP1 by binding to and promoting or sustaining the oxidation of the SHP1 catalytic cysteine by cellular reactive oxygen species (ROS), thereby inhibiting SHP1 tyrosine phosphatase activity (16). This function, which is predicted to have an activating effect on the TCR signaling response, is postulated to facilitate positive selection by low-affinity self-ligands (15, 16). These two models for THEMIS function also offer contrasting explanations for the reduced abundance of tyrosine-phosphorylated SHP1 (pSHP1) in Themis−/− DP thymocytes. The SHP1 activation model maintains that the reduced pSHP1 abundance reflects reduced SHP1 tyrosine phosphatase activity (17, 18) based on findings from previous studies indicating that phosphorylation of two C-terminal SHP1 tyrosines (Tyr536 and Tyr564) increases SHP1 phosphatase activity, although this causal relationship remains controversial (13, 19). The SHP1 inhibition model on the other hand maintains that the reduced pSHP1 abundance in Themis−/− DP thymocytes reflects increased SHP1 phosphatase activity (16) and is due to increased auto- or trans- dephosphorylation of SHP1 by SHP1 (14). We previously reported that extinguishing SHP1 expression in DP thymocytes by CD4-Cre-mediated deletion of Ptpn6 substantially alleviated the developmental block in Themis−/− thymocytes, providing support for the SHP1 inhibition model (16). However, this result was not reproduced by the proponents of the SHP1 activation model (17); thus, the mechanism of THEMIS function in DP thymocytes remains unresolved. Given the key role played by THEMIS in T cell development, and the widespread expression of CABIT domain–containing proteins in metazoans (8), resolution of the mechanism of THEMIS function remains an important priority.
Here, we designed and performed experiments to test the two models of THEMIS function with the goal of resolving the issue of mechanism. We showed that deletion of the gene encoding SHP1 (Ptpn6) by two different, early expressed T cell lineage–specific Cre transgenic lines or pharmacologic inhibition of SHP1 tyrosine phosphatase activity substantially alleviated the developmental block in Themis−/− thymocytes. We also showed that transgenic overexpression of SHP1 phenocopied Themis−/− mice and did not rescue, but instead exacerbated the developmental defects in Themis−/− mice, whereas deletion of Ptpn6, Ptpn11 (encoding SHP2), or both Ptpn6 and Ptpn11 did not result in a phenotype resembling that of Themis−/− mice. Lastly, we demonstrated that negative selection was not enhanced in Themis−/− thymocytes as predicted by the SHP1 activation model (10), but instead was impaired, reflecting reduced TCR signal intensity. Together, these findings validate the SHP1 inhibition model of THEMIS function and support the idea that the high abundance of THEMIS in DP thymocytes contributes to their enhanced sensitivity to self-ligands, enabling positive selection.
Results
Deletion of Ptpn6 alleviates the developmental block in Themis−/− thymocytes
We previously reported that the developmental block at the positive selection stage in Themis−/− thymocytes is substantially alleviated by CD4-Cre–mediated deletion of Ptpn6, the gene encoding SHP1 (16). CD4-Cre; Ptpn6fl/fl; Themis−/− mice have significantly more mature CD4 SP and CD8 SP thymocytes and peripheral CD4+ and CD8+ T cells compared to Themis−/− mice, reflecting the substantial rescue of the DP to SP developmental block. However, alleviation of the developmental block was not observed in CD4-Cre; Ptpn6fl/fl; Themis−/− mice generated and analyzed in another study, although a substantial restoration of peripheral T cell numbers was reported (17). Because CD4-Cre begins to be expressed at the DP stage when positive selection occurs, we reasoned that variations in the timing of Themis deletion in DP thymocytes might explain the incongruous results obtained in the two studies. To circumvent this potential issue, we used two different early expressed Cre transgenes to direct the T cell–lineage specific deletion of Themis: CD2-iCre (20) and Lck-Cre (21) (Fig. 1A). Both Cre transgenes begin to be expressed at the immature CD4-CD8- (double-negative, DN) stage and continue to be expressed at all subsequent stages of T cell development (20, 21). The developmental defect in Themis−/− thymocytes was substantially alleviated in both CD2-iCre; Ptpn6fl/fl; Themis−/− mice (Fig. 1B) and Lck-Cre; Ptpn6fl/fl; Themis−/− mice (Fig. 1C and fig. S1) as demonstrated by restored generation of mature CD24lo CD4 SP and CD8 SP thymocytes, and the near-normal numbers of CD4 SP and CD8 SP thymocytes and peripheral CD4+ and CD8+ T cells (Fig. 2). We also confirmed that this rescue effect was specific to Ptpn6 deletion because CD2-iCre– or Lck-Cre–mediated deletion of Ptpn11 (Shp2) failed to rescue the developmental block in Themis−/− cells (Fig. 2 and fig. S1), consistent with our previous data obtained with CD4-Cre;Ptpn11fl/fl;Themis−/− mice (16).
Fig. 1. CD2-iCre– or Lck-Cre–mediated deletion of Ptpn6 restores T cell development in Themis−/− mice.
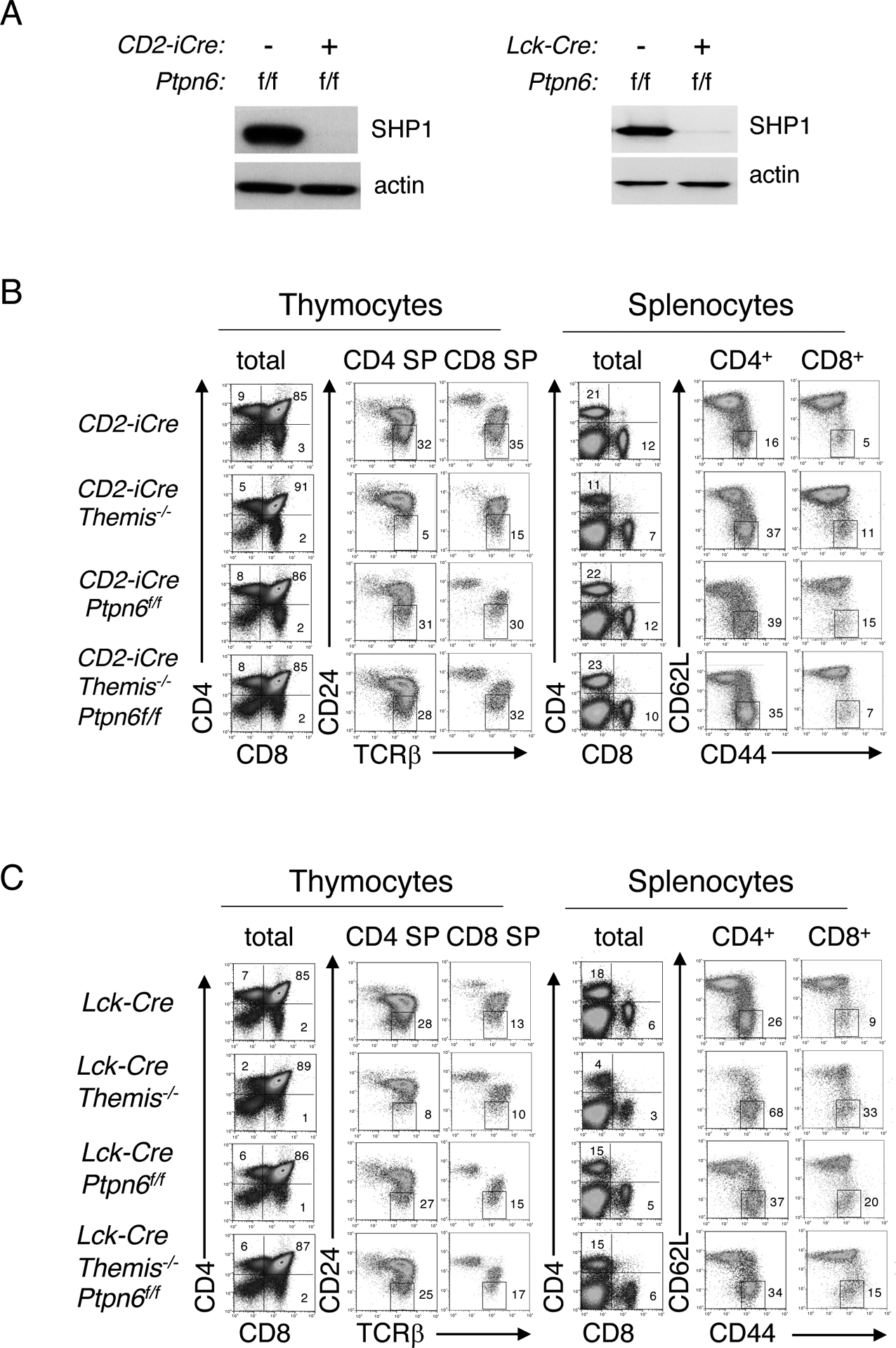
(A) Western blotting analysis of SHP1 in total thymocytes from CD2-iCre or CD2-iCre;Ptpn6f/f mice (left) or in total thymocytes from Lck-Cre or Lck-Cre;Ptpn6f/f mice (right). Actin was used as a loading control. For all subsequent experiments, efficient Cre-mediated deletion was also confirmed, where appropriate, by Western blotting analysis. (B and C) Phenotypic comparison of thymocytes (left) and splenocytes (right) from representative CD2-iCre (B) or Lck-Cre (C) mice of the indicated genotype. Numbers indicate the percentage of cells within each gate. Data are representative of at least ten mice for each genotype.
Fig. 2. Evaluation of thymocyte and T cell subset numbers in mice.
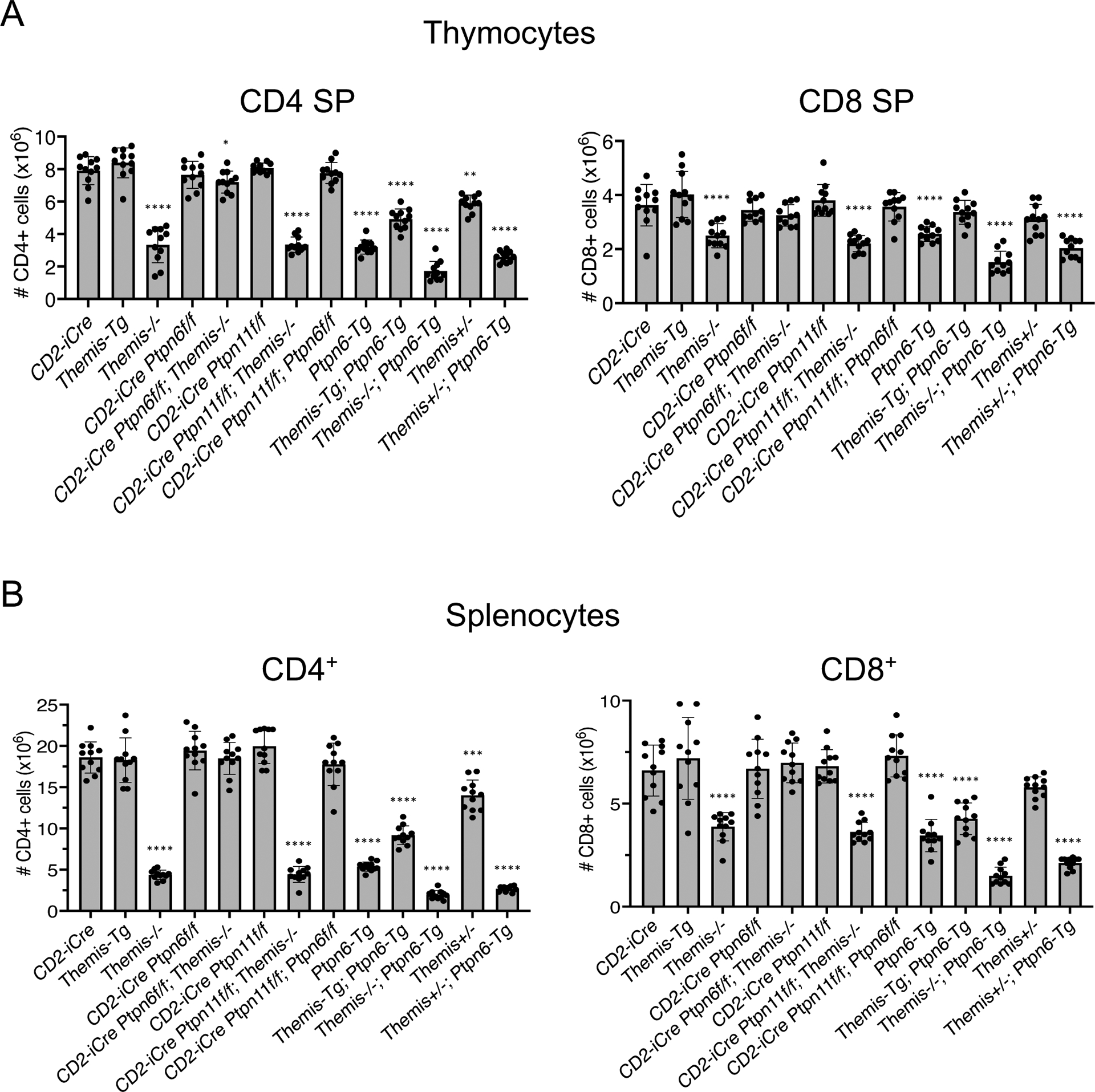
(A and B) Numbers of CD4 SP (left) or CD8 SP (right) thymocytes (A) and CD4+ (left) or CD8+ (right) splenocytes (B) from mice of the indicated genotype. Data are means ± SD of thymocyte or T cell subset numbers and were analyzed by two-tailed, type 2, t test. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001. All comparisons are to CD2-iCre controls.
In Themis−/− mice, a high percentage of CD4+ and CD8+ T cells exhibited a “memory-like” phenotype (CD62L-CD44+) as a result of lymphopenia-induced expansion (Fig. 1, B and C) (5, 22). We also noted a high percentage of CD62L-CD44+ SP T cells in both CD2-iCre; Ptpn6fl/fl; Themis−/− and Lck-Cre; Ptpn6fl/fl; Themis−/− mice (Fig. 1, B and C). However, this phenotype, which was previously reported in mice with a T cell–specific deletion of Ptpn6 (23–25) and which we also observed in CD2-iCre; Ptpn6fl/fl and Lck-Cre; Ptpn6fl/fl mice (Fig. 1, B and C), was attributed to an effect caused by deletion of Ptpn6 rather than lymphopenia-induced expansion.
A sensitive method of evaluating the effect of signaling molecules on positive selection is the use of Tcrα and Tcrβ transgenes to fix the ligand-specificity of the TCR so that all DP thymocytes are subjected to the same TCR signaling responses by engagement of the same positively selecting ligands. We used the MHC II–restricted ovalbumin peptide–specific TCR, OT2 (26) to test the effect of Ptpn6 deletion on a cohort of developing Themis−/− thymocytes that expressed the same TCR, because the main defect resulting from Themis deletion is on CD4 SP thymocyte development (5). OT2; Themis−/− thymocytes exhibited a substantial block in T cell development at the DP to CD4 SP transition stage as evinced by a reduction in the numbers of total and mature CD24lo CD4 SP thymocytes and CD4+ peripheral T cells and the high percentage of lymphopenia-induced, “memory phenotype” (CD62L-CD44+) CD4+ T cells (Fig. 3A). Consistent with the results obtained with polyclonal thymocytes, the percentages and numbers of CD4 SP thymocytes and CD4+ T cells in OT2; CD2-Cre; Themis−/−; Ptpn6fl/fl mice were similar to those in OT2 Themis+/+ mice, demonstrating that deletion of Ptpn6 rescued the development of OT2 TCR–expressing thymocytes in the absence of THEMIS (Fig. 3, A and B and data not shown). Positive selection efficiency was proportional to THEMIS abundance because the numbers of CD4 SP thymocytes and CD4+ T cells were reduced in OT2; Themis+/− mice, which have approximately half as much THEMIS as that of wild-type, Themis+/+ mice, and were increased in OT2; Themis-Tg mice (Fig. 3, A and B), which have approximately 2 to 2.5 times the normal amount of THEMIS protein (Fig. 4A) (27). Together, these results demonstrate that deletion of Ptpn6 corrects the developmental block in Themis−/− thymocytes, supporting the SHP1 inhibition model of THEMIS function.
Fig. 3. . Deletion of Ptpn6 restores T cell development in OT2 TCR transgenic Themis−/− mice.
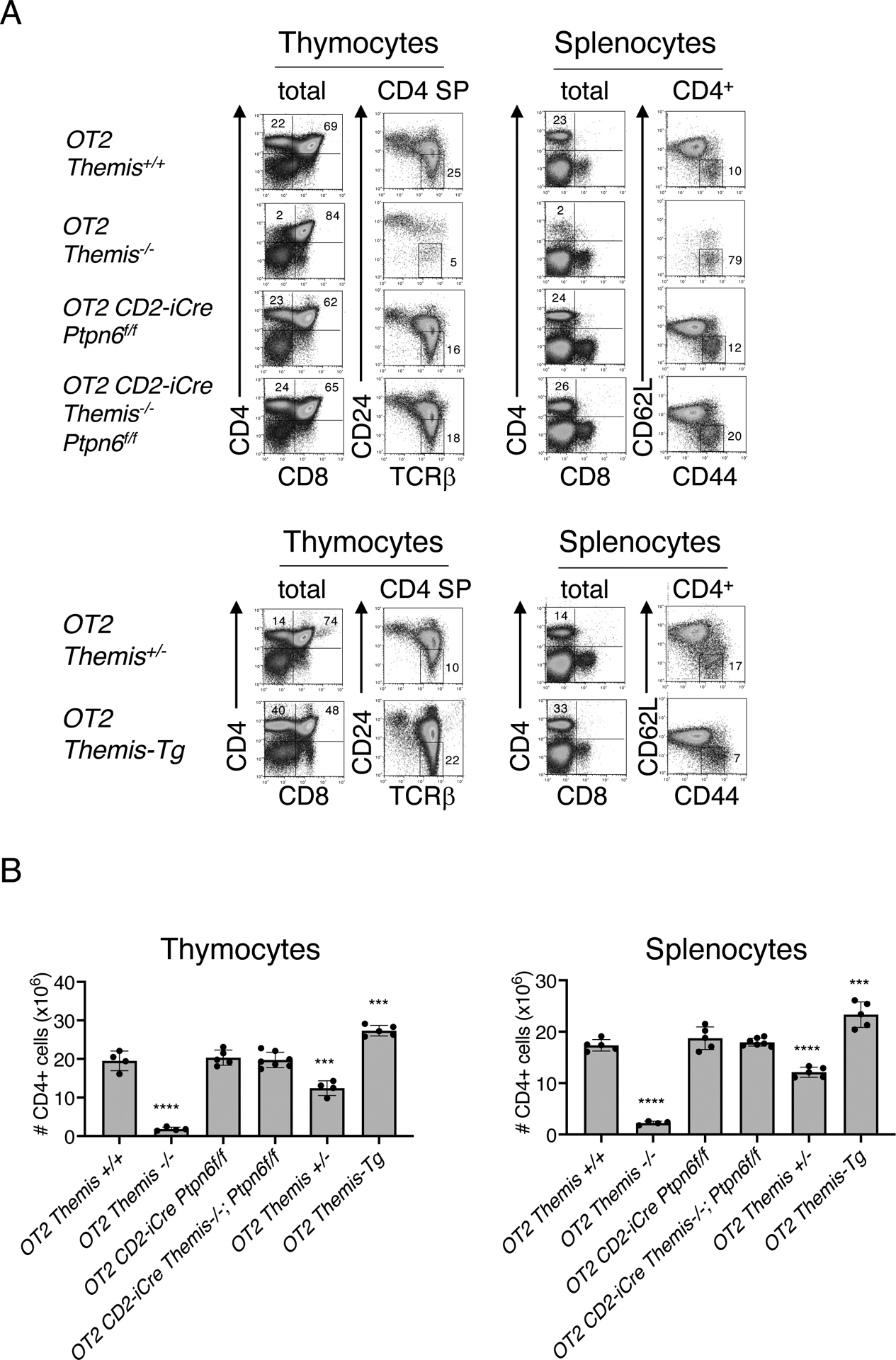
(A) Phenotypic comparison of thymocytes (left) and splenocytes (right) from representative mice of the indicated genotype. Numbers indicate the percentage of cells within each gate. Plots are representative of four or more mice analyzed for each genotype. (B) Numbers of CD4 SP thymocytes (left) and CD4+ splenocytes (right) from mice of the indicated genotype. Data are means ± SD of thymocyte or splenocyte subset numbers and were analyzed by two-tailed, type 2, t test. ***P < 0.005, ****P < 0.001. All comparisons are to OT2+/+ controls.
Fig. 4. Overexpression of SHP1 results in a phenotype that closely resembles that of Themis−/− mice.
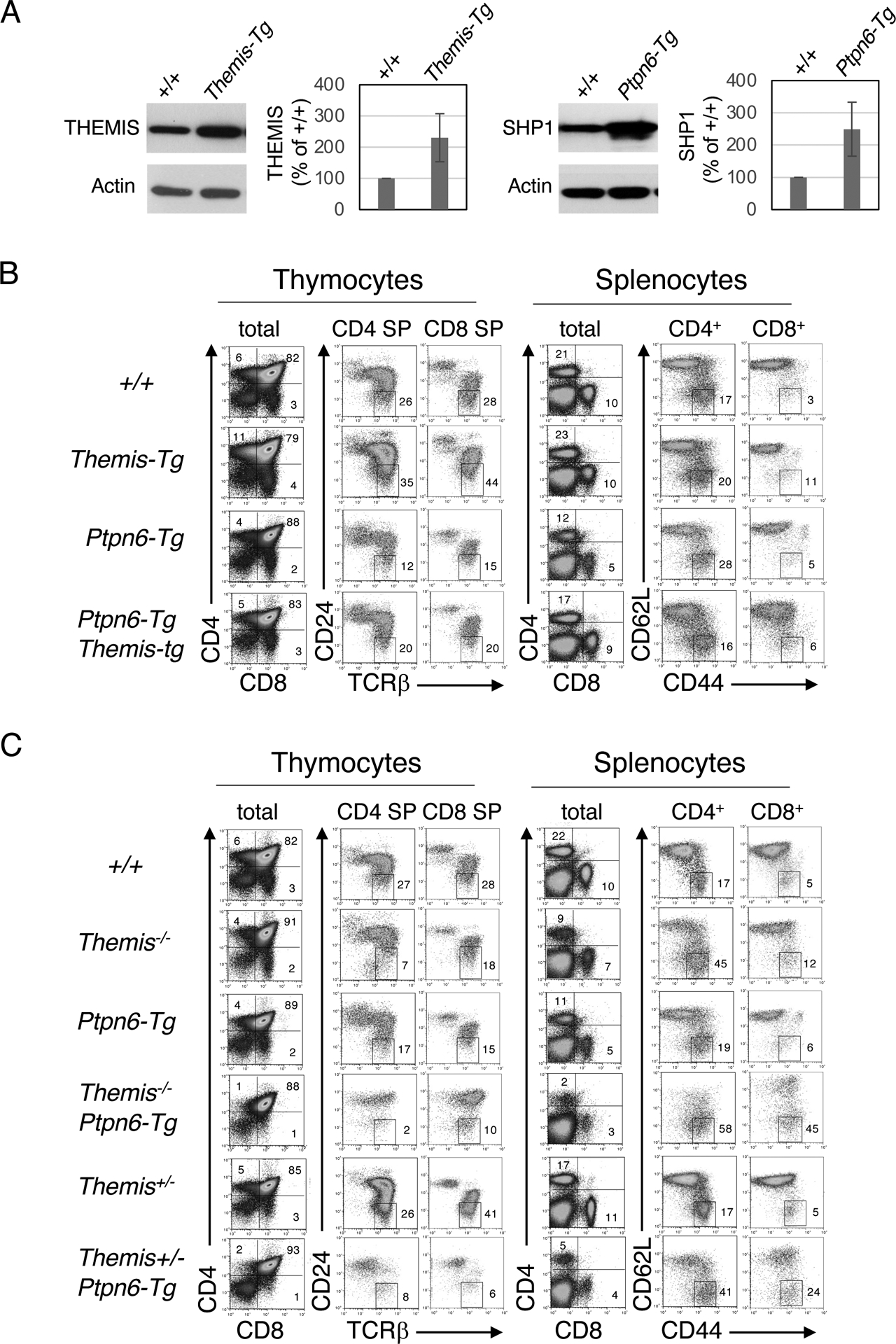
(A) Left: Western blotting analysis of the relative abundance of THEMIS in total thymocytes from Themis+/+ and Themis-Tg littermates. Right: Western blotting analysis of the relative abundance of SHP1 in total thymocytes from Ptpn6+/+ and Ptpn6-Tg littermates. The intensities of THEMIS and SHP1 bands from three experiments were quantitated by densitometry relative to those for actin bands. The value for WT (Themis+/+) cells was arbitrarily set at 100% and all results are shown relative to that. (B) Phenotypic comparison of thymocytes (left) and splenocytes (right) from representative mice of the indicated genotype. Numbers indicate the percentage of cells within each gate. (C) Effect of SHP1 overexpression on the developmental block in Themis+/− and Themis−/− thymocytes. Phenotypic comparison of thymocytes (left) and splenocytes (right) from representative mice of the indicated genotype. Numbers indicate the percentage of cells within each gate. Results are representative of at least ten mice for each genotype.
Overexpression of SHP1 phenocopies Themis deletion
A prediction of the SHP1 inhibition model is that transgenic overexpression of SHP1 should phenocopy Themis−/− mice and should exacerbate the developmental defect in Themis−/− thymocytes. Alternatively, the SHP1 activation model predicts that overexpression of SHP1 should alleviate (or possibly have no effect on, but not exacerbate) the Themis−/− phenotype because the developmental defect is presumed to be due to reduced SHP1 tyrosine phosphatase activity (10). To test these predictions, we made use of a Ptpn6 BAC transgenic mouse line (Ptpn6-Tg) that expresses approximately a two- to three-fold greater amount of SHP1 in thymocytes compared to that in thymocytes from nontransgenic mice (Fig. 4A). Ptpn6-Tg mice exhibited a partial block in T cell development at the positive selection stage that closely resembled the phenotype of Themis−/− mice (Fig. 4B). Overexpression of THEMIS (Themis-Tg) partially alleviated the inhibitory effect of SHP1 overexpression on T cell development (Ptpn6-tg; Themis-tg mice, Fig. 2 and Fig. 4B), whereas transgenic overexpression of SHP1 failed to ameliorate and instead markedly worsened the developmental defect in Themis−/− thymocytes (Fig. 2 and Fig. 4C). Moreover, the effect of SHP1 overexpression was sensitive to the amount of cellular THEMIS, because the developmental block in Themis+/−; Ptpn6-Tg mice was noticeably greater than that in Themis+/+; Ptpn6-Tg mice (Fig. 2 and Fig. 4C). Similar phenotypes were observed in the corresponding OT2 TCR-tg lines (fig. S2, A and B). The effects of Themis heterozygosity (Themis+/−) and THEMIS overexpression (Themis-Tg) and the effects of SHP1 overexpression (Ptpn6-Tg) on positive selection were more pronounced in the TCR-Tg background (Figs. 2 to 4, fig. S2) (12), reminiscent of the effect of Cd5 deletion on positive selection (28, 29).
Deletion of Ptpn6 and Ptpn11 does not phenocopy Themis−/− mice
In contrast to the similar effects of Themis deletion and SHP1 overexpression on positive selection and DP-to-SP thymocyte development, deletion of Ptpn6 had no discernable effect on positive selection (Figs. 1 to 3 and fig. S1), as reported previously (23–25). The proponents of the SHP1 activation model of THEMIS function have suggested that functional redundancy of SHP1 and SHP2 might explain the failure of SHP1-deficient thymocytes to phenocopy Themis−/− thymocytes as predicted by their model (30). We tested this hypothesis by generating CD2-iCre or Lck-Cre Ptpn6fl/fl; Ptpn11fl/fl mice. We found that deletion of Ptpn6, Ptpn11, or both Ptpn6 and Ptpn11 failed to result in a phenotype resembling that of Themis−/− mice (Figs. 1 to 3 and figs. S1 and S3,), suggesting that the Themis−/− phenotype cannot be attributed to the reduced tyrosine phosphatase activities of SHP1, SHP2, or both.
Inhibition of the tyrosine phosphatase activity of SHP1 alleviates the developmental block in Themis−/− thymocytes
The rescue or exacerbation of the Themis−/− developmental defect by deletion or overexpression of SHP1, respectively, is consistent with our previous data demonstrating that THEMIS directly inhibits the tyrosine phosphatase activity of SHP1 (16). To test this further, we performed fetal thymic organ culture (FTOC) of day 16 Themis−/− thymocytes for three days in the presence of the SHP1 phosphatase inhibitor TPI-1, the pan-phosphatase inhibitor pervanadate, or Buthionine sulfoximine (BSO), which reduces the amount of cellular glutathione, thereby stabilizing oxidized (inactivated) SHP1. Each of these treatments partially alleviated the DP-to-SP developmental block in Themis−/− thymocytes as reflected by the increased percentage of CD4 SP thymocytes and the increased ratio of CD4 SP:DP cells (Fig. 5). On the other hand, culture of Themis−/− fetal thymi in the presence of N-Acetyl-L-cysteine (NAC), a thiol-reducing agent and ROS scavenger, aggravated the Themis−/− phenotype as evinced by the reduced generation of CD4 SP thymocytes (Fig. 5). Effects similar to those observed with Themis−/− FTOC were also observed with wild-type B6 (Themis+/+) FTOC (fig. S4). However, none of these reagents significantly affected CD4 SP thymocyte development in Ptpn6-deficient fetal thymocytes, indicating that their effect on CD4 SP thymocyte development was due to inhibition or enhancement of SHP1 tyrosine phosphatase activity (fig. S5).
Fig. 5. Inhibition of the tyrosine phosphatase activity of SHP1 alleviates the block in positive selection in Themis−/− mice.
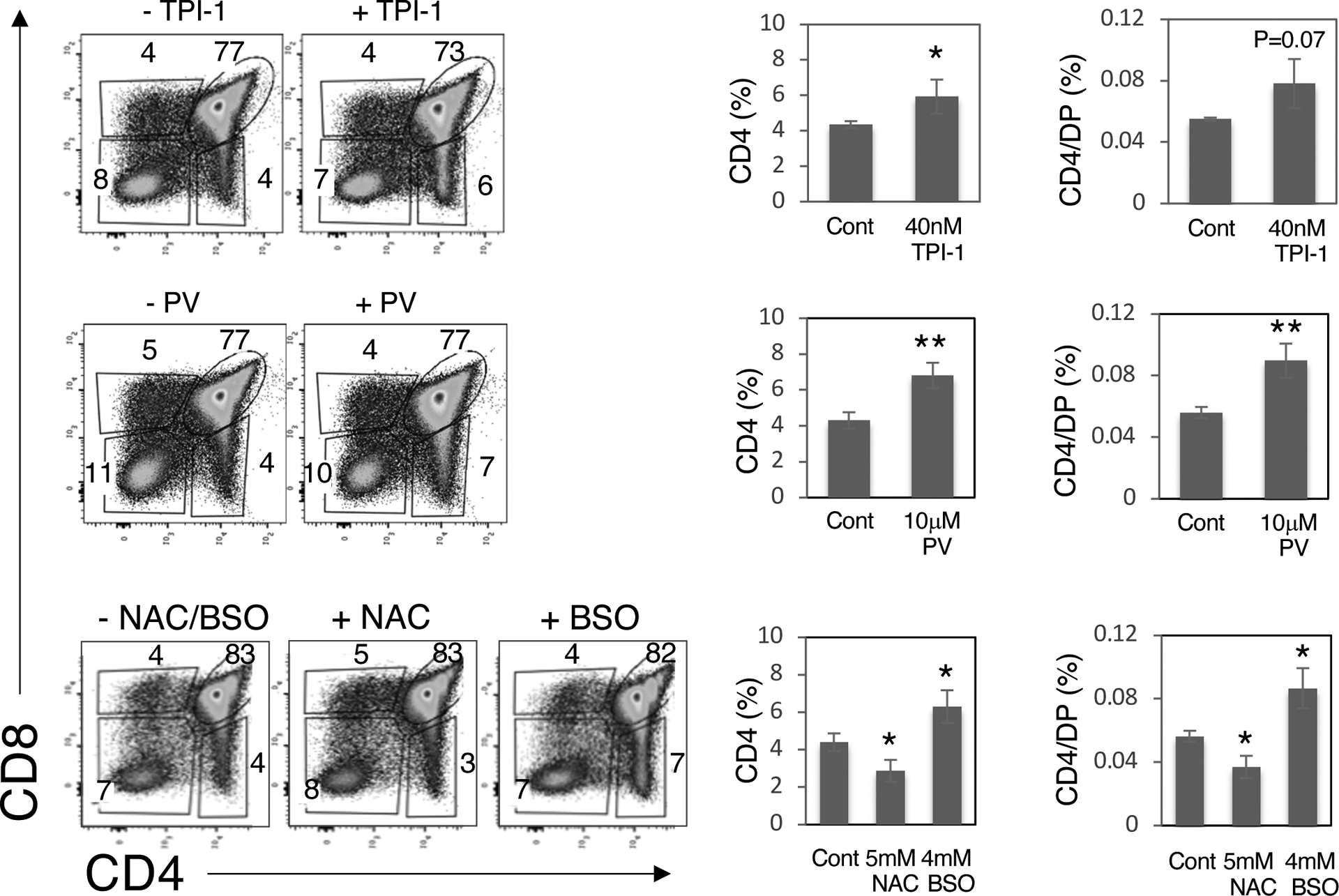
Gestation day 16 fetal thymus lobes were cultured in medium for 2 days and then for a further 3 days with medium alone or medium containing 40 nM TPI-1, 10 μM pervanadate (PV),, 5 mM N-acetyl-L-cysteine (NAC), or 4 mM L-buthionine-sulfoximine (BSO). Left: The thymocytes were then harvested and analyzed by flow cytometry. Numbers indicate the percentage of cells within each gate. Right: The percentages of CD4 SP thymocytes and the percentage of CD4 SP thymocytes divided by the percentage of DP thymocytes for the indicated treatments. Data are means ± SD of the indicated thymocyte subset percentages and were analyzed by two-tailed, type 2, t test. All comparisons are to untreated controls. *P < 0.05, **P < 0.01. Combined results are from four to six thymus lobes for each treatment. The genotype of fetal thymi was confirmed by PCR.
To confirm the proposed mechanism for THEMIS function, namely, that THEMIS facilitates the oxidation of SHP1, we evaluated the effect of THEMIS deletion (Themis−/−) or THEMIS overexpression (Themis-Tg) on SHP1 oxidation by H2O2, which can reversibly or irreversibly oxidize SHP1 depending upon the concentration of H2O2 and treatment time (31). We found that irreversible oxidation of SHP1 was augmented in Themis-Tg thymocytes and decreased in Themis−/− thymocytes (16) relative to that in Themis+/+ thymocytes (Fig. 6A and fig. S6). Similar results were observed with thymocytes treated with pervanadate, which irreversibly oxidizes SHP1 (Fig. 6B and fig. S7). Together, these results demonstrate that THEMIS quantitatively influences SHP1 oxidation by ROS.
Fig. 6. Oxidation (inactivation) of SHP1 in thymocytes is enhanced by overexpression of THEMIS (Themis-Tg) and is diminished by deletion of Themis (Themis−/−).
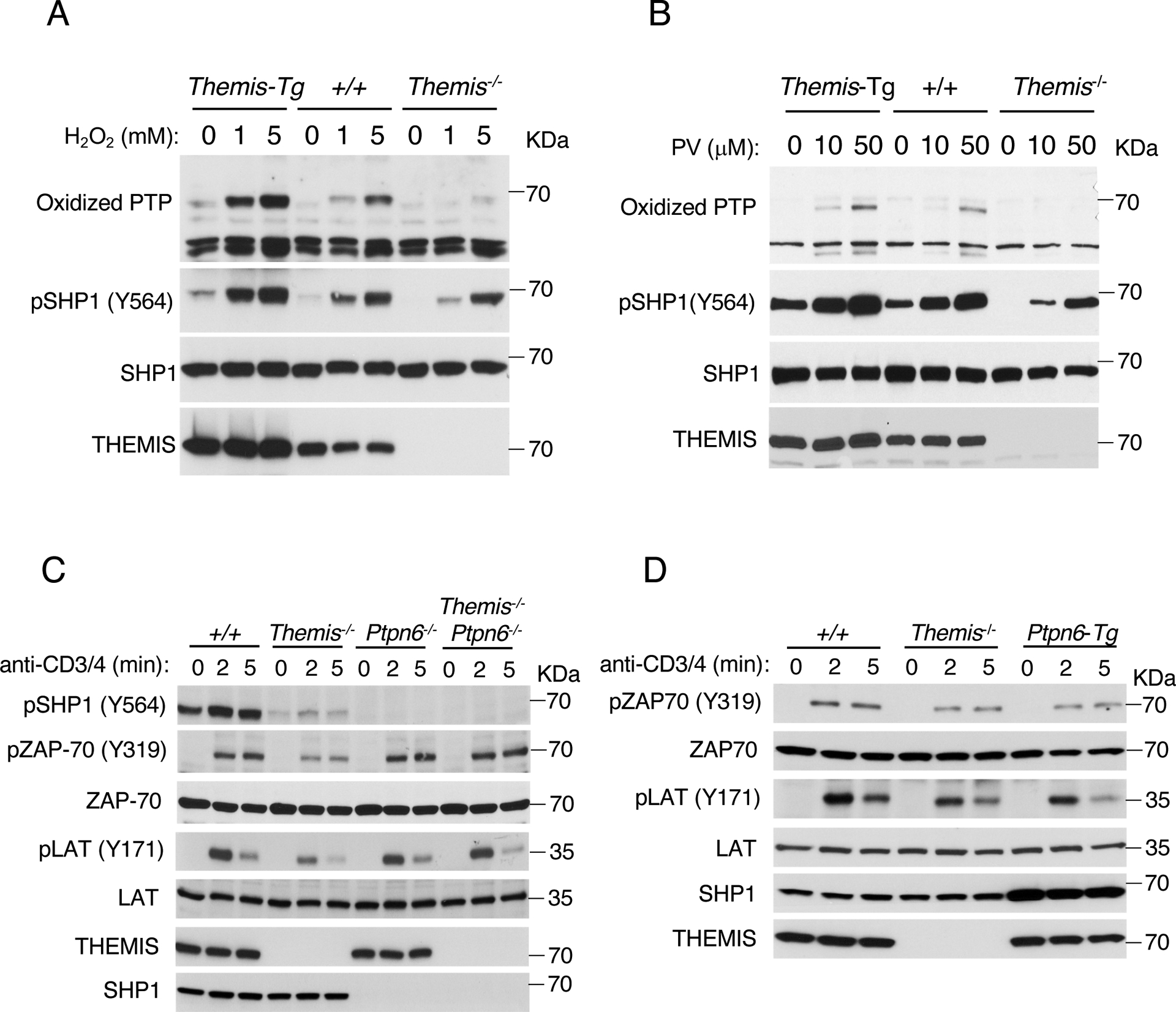
(A and B) Effect of THEMIS overexpression (Themis-tg) or deletion (Themis−/−) on the oxidation of SHP1 by H2O2 (A) or pervanadate (B). Western blots show the analysis of cell lysates with antibodies against the indicated proteins. The antibody against oxidized PTP detects irreversibly oxidized (SO3H) phosphatases. Data are representative of three independent experiments (see figs. S6 and S7 for quantification data). (C and D) Effect of deletion of SHP1 (Ptpn6) (C) or overexpression of SHP1 (Ptpn6-Tg) (D) on TCR signaling. Total thymocytes from the indicated mice were incubated in complete medium for 6 hours at 37°C and then were stimulated with anti-CD3 and anti-CD4 antibodies for 0, 2, or 5 min. Western blotting analysis of cell lysates with antibodies against the indicated proteins. Data are representative of four (C) or three (D) independent experiments (see figs. S8 and S9 for data quantification).
The TCR signaling defect in Themis−/− thymocytes is alleviated by deletion of Ptpn6
We previously reported that although freshly isolated Themis−/− thymocytes do not show an obvious TCR signaling defect, impaired TCR-mediated signaling responses were observed after 6 hours of culture (16). This effect is presumably due to the cumulative effects of ROS, which are increased in cell culture (32), on the tyrosine phosphatase activity of SHP1 through increased oxidation of its catalytic cysteine in the absence of THEMIS (16). Here, we found that thymocytes from Themis−/− mice that were rested in complete medium for 6 hours exhibited mild but discernible defects in ZAP-70 and LAT phosphorylation after stimulation with anti-CD3 and anti-CD4 antibodies (Fig. 6C and fig. S8). Consistent with the phenotypic rescue of the positive selection of Themis−/− thymocytes by deletion of Ptpn6, the TCR signaling defect in Themis−/− thymocytes was substantially rescued by deletion of Ptpn6 (Fig. 6C and fig. S8). A mild but reproducible defect in TCR signaling responses was observed in Ptpn6-Tg thymocytes (Fig. 6D and fig. S9), whereas it was previously shown that TCR signaling responses are mildly enhanced in Themis-Tg thymocytes (16).
Tyrosine-phosphorylation of SHP1 is not essential for its phosphatase activity
A major point of contention between the two models of THEMIS function is the cause of the reduced amount of tyrosine-phosphorylated SHP1 (pSHP1) in both ex vivo and TCR-stimulated Themis−/− thymocytes and its effect on SHP1 activity. As mentioned earlier, the proponents of the SHP1 activation model postulate that phosphorylation of SHP1 (which occurs at the C-terminal residues Tyr536 and Tyr564) is required for or enhances the tyrosine phosphatase activity of SHP1 and that, consequently, the reduction in pSHP1 abundance in Themis−/− thymocytes reflects reduced SHP1 tyrosine phosphatase activity (17). On the other hand, the SHP1 inhibition model posits that SHP1 phosphorylation is not critical for SHP1 phosphatase activity and that the reduction in pSHP1 abundance in Themis−/− thymocytes reflects increased SHP1 trans- or auto-dephosphorylation due to its enhanced tyrosine phosphatase activity.
We previously demonstrated that the effect of SHP1 tyrosine phosphorylation on its phosphatase activity cannot be accurately quantified by immunoprecipitating SHP1 protein under the usual cell lysis conditions, which include phosphatase inhibitors, because, in the absence of phosphatase inhibitors, SHP1 is rapidly dephosphorylated in cell lysates (Fig. 7A) (16). SHP1 phosphorylation can be preserved by the addition of sodium orthovanadate (Na3VO4) to cell lysates, however, SHP1 phosphatase activity is inhibited by Na3VO4 (Fig. 7A). When immunoprecipitated in the presence of the ROS scavenger NAC, SHP1 was completely dephosphorylated but SHP1 phosphatase activity was greater in both Themis+/+ and Themis−/− thymocytes than in the corresponding immunoprecipitates performed in the absence of NAC (Fig. 7A). Moreover, we consistently observed increased tyrosine phosphorylation of SHP1 in thymocytes treated with H2O2 or pervanadate, which resulted in the oxidation and inactivation of SHP1 (Fig. 6, A and B). Together, these results suggest that phosphatase activity of SHP1 is not dependent upon its tyrosine phosphorylation.
Fig. 7. Tyrosine phosphorylation of SHP1 is not essential for its catalytic activity.
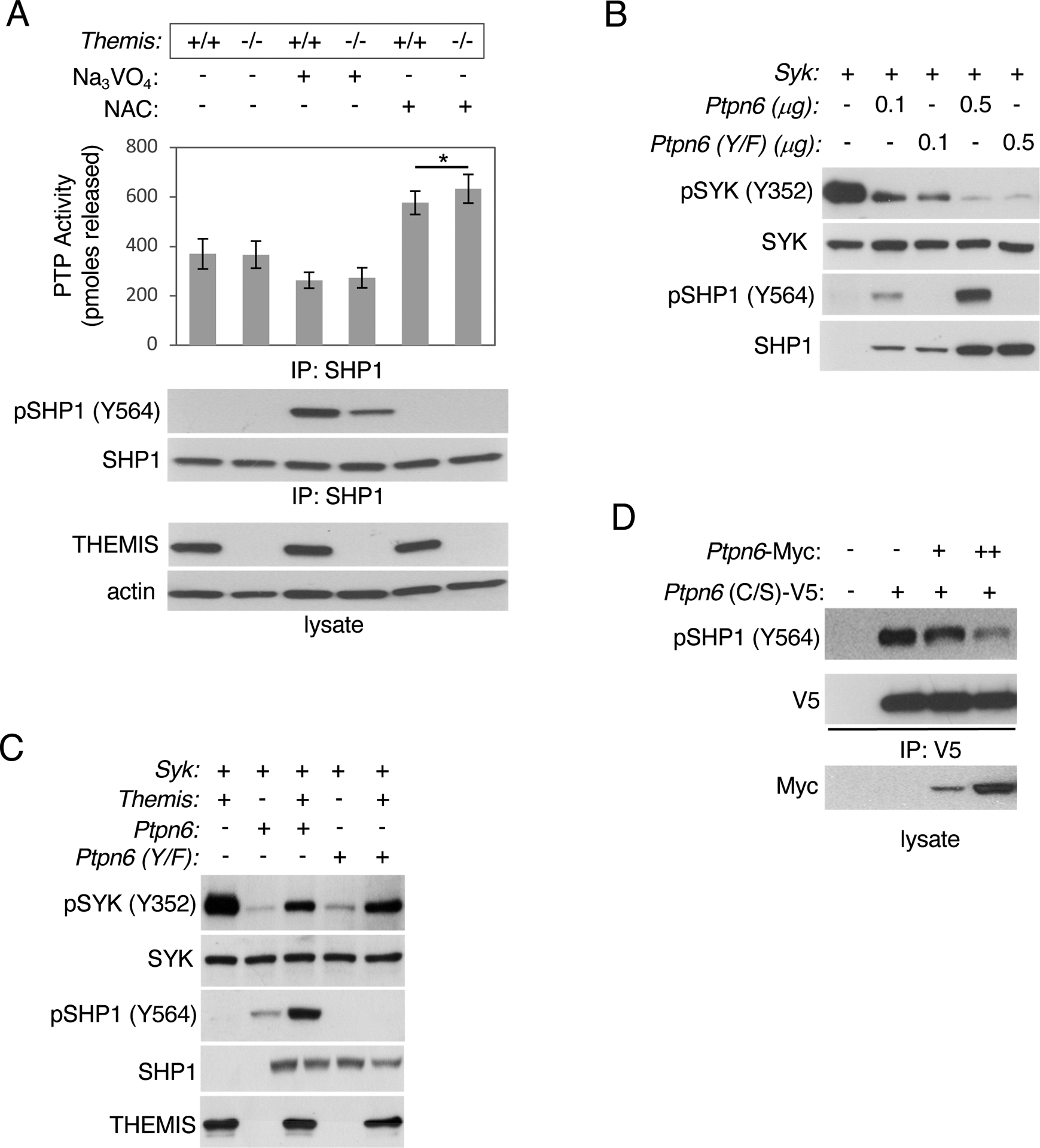
(A) Total thymocytes from the indicated mice were lysed in the presence of the phosphatase inhibitor Na3VO4 or the ROS scavenger NAC, incubated with anti-SHP1 antibody at 4°C for 4 hours, and immunoprecipitated proteins were evaluated for phosphatase activity as described in Materials and Methods or evaluated by SDS-PAGE and Western blotting with the indicated antibodies. Data are means ± SD from three experiments and were analyzed by two-tailed, type 2, t test. *P < 0.05. (B) SHP1 (Ptpn6) and SHP1 Y536F/Y564F [Ptpn6(Y/F)] were evaluated for tyrosine phosphatase activity against pSYK by Western blotting analysis of lysates from transfected HEK293 cells. (C) Evaluation of the ability of THEMIS to inhibit SHP1(Y/F) Western blotting analysis of lysates of transfected HEK293 cells. (D) Evaluation of the ability of SHP1 to de-phosphorylate SHP1 in trans. HEK293 cells were transfected with plasmids encoding V5- tagged, catalytically inactive SHP1(C/S) and Myc-tagged WT SHP1. Twenty-four hours later, the cells were lysed and SHP1(C/S) was immunoprecipitated and phosphorylation at Tyr564 (Y564) was evaluated by SDS-PAGE and Western blotting. Blots are representative of at least three experiments.
To test further whether tyrosine-phosphorylation of SHP1 is required for or affects its phosphatase activity, we mutated both Tyr536 and Tyr564 of SHP1 to phenylalanine (F) and compared the activity of wild-type (WT) SHP1 and SHP1 with the Y536F and Y564F mutations (SHP1 Y/F) in an in vitro transfection assay using phosphorylation of a known SHP1 target, the tyrosine kinase SYK, as a readout (33). SHP1(Y/F) was approximately equivalent to WT SHP1 in its ability to dephosphorylate SYK, despite its inability to be tyrosine-phosphorylated (Fig. 7B and fig. S10). SHP1(Y/F) and WT SHP1 were also approximately equivalent in their ability to dephosphorylate another known SHP1 target, the tyrosine kinase ZAP-70, which requires LCK for its activation (fig. S11A). SHP1(Y/F) bound to THEMIS and Grb2 (fig. S11B) and was inhibited by THEMIS similarly to WT SHP1 (Fig. 7C and fig. S12). Together, these results indicate that the tyrosine-phosphorylation of SHP1 is not critical for its phosphatase activity. Finally, we demonstrated that SHP1 dephosphorylated a catalytically inactive SHP1 mutant [SHP1 (C/S)], confirming that SHP1 was capable of trans-dephosphorylation of SHP1 (Fig. 7D and fig. S13).
Negative selection is impaired in Themis−/− thymocytes
The SHP1 activation model for THEMIS function postulates that the marked reduction in the number of SP thymocytes in Themis−/− mice is caused by increased negative selection (clonal deletion) as a result of reduced SHP1 activity in DP and immature SP thymocytes (10). Demonstrating alterations in negative selection (enhanced or reduced) is made difficult by the fact that the cell population of interest is apoptotic and is rapidly and efficiently removed by scavenger cells in the thymus (34). However, in the absence of the co-stimulatory molecule CD28, autoreactive thymocytes that would otherwise be clonally deleted by negative selection do not undergo apoptosis but instead differentiate into DN TCR+ PD-1hi cells that eventually exit the thymus and populate the intestine (35). Compared to those in CD28−/− control mice, the percentages and numbers of DN TCR+ PD-1hi thymocytes were significantly reduced in CD28−/− Themis−/− mice (Fig. 8, A and B). Transgenic overexpression of THEMIS increased the percentages and numbers of DN TCR+ PD-1hi thymocytes, whereas, similar to CD28−/−Themis−/− thymocytes, transgenic overexpression of SHP1 (CD28−/−Ptpn6-Tg) reduced the percentages and numbers of DN TCR+ PD-1hi thymocytes (Fig. 8, A and B). Together, these results demonstrate that negative selection is not enhanced but is instead impaired in the absence of THEMIS supporting the SHP1 inhibition model of THEMIS function.
Fig. 8. Defective negative selection in the absence of THEMIS.
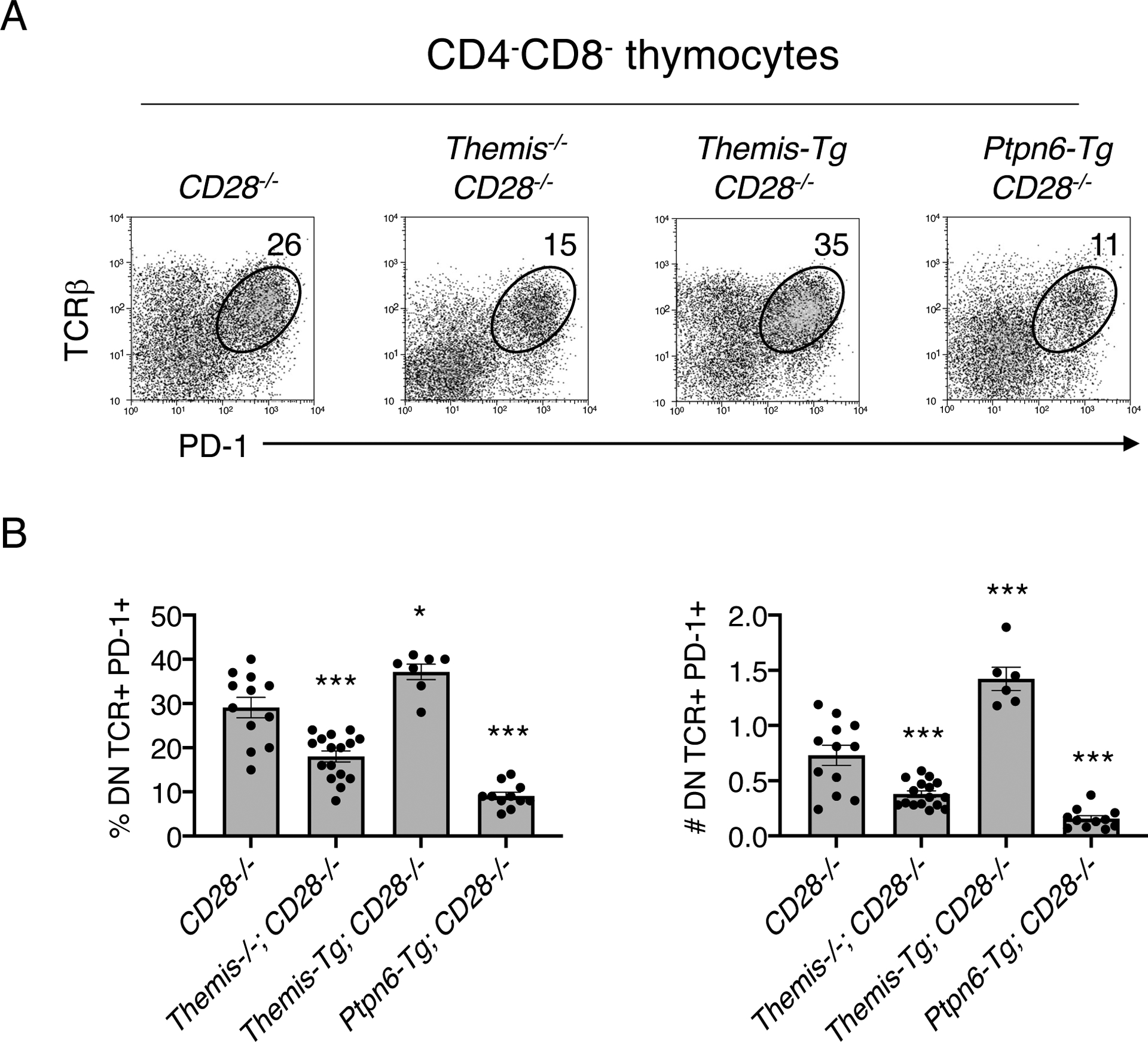
(A and B) Thymocytes from the indicated mice were stained with anti-CD4, anti-CD8, anti-TCRβ, and anti-PD-1 antibodies. (A) Flow cytometry analysis of the TCRβ vs. PD-1 profile of gated CD4-CD8- (DN) thymocytes. (B) Percentages (left) and numbers (right) of DN TCRβ+PD-1+ thymocytes in the indicated mice. Data are means + SD and were analyzed by two-tailed, type 2, t test. All comparisons are to CD28−/− controls. *P < 0.05, ***P < 0.005.
Discussion
In this study, we sought to resolve an outstanding controversy regarding the proposed function of the protein THEMIS during T cell development, in particular, its role at the thymocyte selection stage where the developmental defect caused by THEMIS deficiency is most severe. Using two different early-acting, T cell lineage–specific Cre transgenes, we showed that deletion of Ptpn6 alleviated the developmental block at the DP-to-SP transition stage in Themis−/− thymocytes. Furthermore, Ptpn6 overexpression phenocopied Themis−/− mice and worsened rather than rescued the developmental block in Themis−/− thymocytes, and deletion of Ptpn6, Ptpn11, or both failed to phenocopy Themis−/− thymocytes contrary to the prediction of the SHP1 activation model of THEMIS function (30). Pharmacologic inhibition of SHP1 tyrosine phosphatase activity alleviated, and prevention of SHP1 oxidation worsened the Themis−/− developmental defect in FTOC. Finally, we demonstrated that negative selection was impaired in Themis−/− thymocytes, consistent with the SHP1 inhibition model, rather than enhanced as would be predicted by the SHP1 activation model (10). Although we do not claim that our results are unequivocal, as that term has no proper place in experimental biology, we believe that they provide strong, multifaceted support for the SHP1 inhibition model of THEMIS function.
THEMIS is the prototype of a family of proteins that contain one or two copies of a sequence termed the CABIT domain (8). Three THEMIS family members (defined by the presence of two tandem CABIT domains) are expressed in mice: THEMIS (or THEMIS1) which is restricted to T cells, THEMIS2, which is restricted to B cells and myeloid cells, and THEMIS3, which is restricted to the intestine, but unlike THEMIS and THEMIS2, is not expressed in humans. CABIT domains are present in many, mostly uncharacterized, proteins in all metazoans, with the exception of nematodes from cnidarians onwards (8); thus, they likely serve important functions in many cell types, underscoring the need to elucidate the function of THEMIS and the CABIT domain.
Initial studies of THEMIS-deficient or THEMIS-inactivating mutant mice published in 2009 by five independent groups reported either no TCR signaling defect or a modest reduction in TCR signaling in thymocytes and the reduced efficiency of positive selection or both positive and negative selection (5–9). Subsequent work by two groups suggested that THEMIS plays a predominantly inhibitory role in the TCR signaling response (10, 18), whereas studies performed by our group suggested an activating role for THEMIS in TCR signaling (12, 16) and for THEMIS2 in BCR signaling (36). We also proposed, based on biochemical studies, that the THEMIS CABIT domains functioned to promote or stabilize oxidation of the catalytic cysteine residue of SHP1 by ROS, thereby providing a potential mechanism for the THEMIS-mediated inhibition of SHP1 (16). To our knowledge, no molecular mechanism has yet been offered for the proposed THEMIS-mediated activation of SHP1. In other studies performed by the group proposing that THEMIS activates SHP1 in thymocytes (10, 17), the results indicate that THEMIS inhibits SHP1 activity in peripheral T cells to regulate T cell homeostasis (37) and cytokine signaling (38). Because it is reasonable to assume that THEMIS performs similar rather than opposing biological functions with respect to the identical target (SHP1) in thymocytes and mature T cells, these findings appear incongruous.
As mentioned previously, we speculate that the inability of others to recapitulate rescue of the developmental block in CD4-Cre;Themis−/−;Ptpnf/f mice may be due to differences in the timing of Cre expression driven by the CD4 promoter, because we have shown here that near complete phenotypic rescue was observed in Themis−/− mice with Lck-Cre– or CD2-iCre–mediated deletion of Ptpn6 at the DN stage. A consensus finding from both groups is that ERK1/2 activation is enhanced in the absence of THEMIS (12, 18), a result that has been cited as support for the SHP1 activation model (30). However, THEMIS inhibits the activities of both SHP1 and SHP2 (15, 16). Whereas SHP1 appears to have exclusively inhibitory effects on TCR signaling, SHP2 has mixed effects, including a proposed role in ERK1/2 activation (39). These and other findings (40, 41) demonstrate that the effect of Themis deletion on TCR signaling may be more complex than can be explained by an entirely activating or inhibitory model. Notwithstanding, the results reported here demonstrate that Themis deletion has an overall dampening or attenuating effect on the integrated TCR signaling response in DP thymocytes undergoing thymocyte selection as a result of the increased tyrosine phosphatase activity of SHP1.
The relatively mild effects of Ptpn6 deletion on thymocyte selection, also noted in previous reports (23–25), has led to the conclusion that SHP1 does not have a critical function during T cell development (24). However, our results demonstrate that both positive selection and late T cell development are very sensitive to SHP1 abundance, as was revealed by the phenotype of Ptpn6-Tg mice. The absence of a similarly profound phenotype in Ptpn6−/− mice could reflect that THEMIS effectively inactivates the critical pool of SHP1 in DP thymocytes at the stage of positive or negative selection. Consistent with this interpretation, we found that positive selection was extremely sensitive to THEMIS and SHP1 amounts, as revealed by the phenotypes of Themis+/−, Themis-Tg, and Ptpn6-Tg mice. Our results demonstrate that the relatively high abundance of THEMIS in DP thymocytes provides a mechanism for the transient (stage-specific) inactivation of SHP1 at the critical positive selection stage, enhancing TCR signaling initiated by the engagement of low-affinity self-ligands to enable positive selection.
Materials and Methods
Mice
Themis−/− mice were described previously (5). Shp1f/f [Ptpn6f/f] mice (42) were provided by Markus Muschen, UCSF. CD2-iCre mice [Strain# 008520], OT2 TCR transgenic mice [Strain# 004194]m CD28−/− mice [Strain# 002666], and Shp2f/f [Ptpn11f/f] mice [Strain# 025758], were obtained from Jackson Labs. Shp1(Ptpn6) transgenic [Shp1-tg] mice (43) and Themis transgenic (27) mice were generated as described previously. Lck-Cre transgenic mice (21) were provided by Remy Bosselut (NCI).
FTOC
Thymic lobes were excised from fetuses at a gestational age of day 16 (the detection of the vaginal plug is considered as day 1). The lobes were put on polycarbonate membranes in a 12-well transwell plate (Costar, cat. 3401) in DMEM supplemented with 10% fetal bovine serum (FBS), penicillin, streptomycin, 2 mM glutamine, and 10 mM β-mercaptoethanol. After 2 days, the medium was exchanged with medium alone or including 40 nM TPI-1, 5 mM NAC, 10 μM pervanadate (PV), or 4 mM BSO (4mM). After 3 more days in culture, thymocytes were released from the lobes by pressing the tissue through nylon mesh. The cells were then analyzed by flow cytometry.
Antibodies and reagents
The following antibodies were used for flow cytometry: anti-CD4-Qdot 605 (Q10092) and anti-CD8-APC eFluor 780 (47–0081-82) from Invitrogen; anti-CD5-PerCP (100616) from Biolegend; anti-CD24-FITC (553261), anti-CD44-APC (559250), anti-CD62L-PerCP Cy5.5 (560513), and anti-PD1-PE (551892) from BD Biosciences; and anti-TCRβ-eFluor 450 (48–5961-82) was from eBioscience. For cell stimulations, biotin-conjugated anti-CD3 (553060) and biotin-conjugated anti-CD4 (553728), used at 1 μg each per 1 × 107 thymocytes, were obtained from BD Biosciences. For immunoprecipitations, anti-V5 agarose (A7345) was from Sigma-Aldrich, and anti-SHP1 (04–742), with 1 μg of antibody used for 1 × 107 cells, were from EMD Millipore. For Western blotting analysis, anti-HA (11583816001) was from Roche; anti-oxidized PTP active site monoclonal antibody (MAB2844) was from R&D Systems; anti-V5 (A190–120A) was from Bethyl; anti-pTyr (05321) and anti-LAT (06807) were from EMD Millipore; anti-SHP1 (MA5–111669) was from Invitrogen; anti-pTyr564-SHP1 (8849), anti-pTyr171-LAT (3581), and anti-Myc tag (2276) were from Cell Signaling Technology; anti-pTyr319-ZAP-70 (612574), anti-ZAP-70 (610240), and anti-GRB2 (610111) were from BD Biosciences; anti-LCK (SC433), anti-SYK (SC1077), anti-actin (SC58673), anti-SHP2 (SC7384), anti-CD5 (SC6986), and anti-CD28 (SC1624) were from Santa Cruz Biotechnology. The antibodies for Western blotting analysis were diluted 1:1000. The rabbit polyclonal antiserum to THEMIS was described previously (5). Streptavidin was purchased from Southern Biotechnology. Mouse IgGκBP-HRP (SC516102) and anti-rabbit IgG-HRP (SC2357) were purchased from Santa Cruz Biotechnology. NAC(N-Acetyl-L-cysteine) (A9165) and BSO (L-Buthionine-sulfoximine) (B2515) were purchased from Sigma-Aldrich. TPI-1 (22480) was purchased from Cayman Chemical. A solution of 1 mM Pervanadate was made with 10 μl of 100 mM Na3VO4 and 5 μl of 3% H2O2 (Sigma Aldrich, cat. 88597) in 1 ml of H2O.
Plasmids and constructs
The cDNA encoding Flag-tagged THEMIS was subcloned into pFLAG-CMV2 vector by PCR from the THEMIS-eGFP plasmid. DDK-SYK, DDK-ZAP70, and DDK-LCK plasmids were purchased from OriGene. HA- and Myc-tagged SHP1 were subcloned into the pCDNA3-HA and pCDNA3-Myc vectors by PCR from human cDNA for SHP1 from Addgene. V5-tagged SHP1(C/S) was subcloned into pCDNA3-V5 vector by PCR from human cDNA for SHP1(C/S) from Addgene. SHP1 (Y536F and Y564F) was generated by site-directed mutagenesis with the Quik Change Kit (Stratagene). Mutations at Tyr536 and Tyr564 were confirmed by DNA sequencing.
Flow cytometry
Cells were treated with the Fc-blocking antibody 2.4G2 [BD Biosciences] at 1/1000 dilution of stock. Antibody cocktails were prepared in Hanks’ Balanced Salt Solution (HBSS) containing 1% BSA Fraction V (MP Biomedicals), 0.1% NaN3 (Sigma), and cells were labelled for 30 min at 4°C in the dark. Samples were washed and resuspended in the described buffer and acquired on an LSRFortessa X-20 cell analyzer (BD Biosciences). Analysis was conducted with FlowJo software (v10.6.1, TreeStar Inc.).
Immunoprecipitation and Western blotting analysis
Thymocytes were stimulated with biotin-conjugated anti-CD3 and anti-CD4 antibodies, followed by cross-linking with streptavidin. Cells were then washed in ice-cold PBS and lysed in standard lysis buffer [1% Nonidet P-40, 10 mM Tris (pH 7.5), 150 mM NaCl, 2 mM EGTA, 50 mM β-glycerophosphate, 2 mM Na3VO4, 10 mM NaF, and protease inhibitors]. Immunoprecipitations and Western blotting analysis were performed as described previously (16).
Transient transfections
HEK-293 cells were cultured in DMEM supplemented with 10% (vol/vol) FBS and 2 mM glutamine, and penicillin and streptomycin (100 U/ml each). Cells (1 × 106) were co-transfected with the appropriate plasmid with Lipofectamine 2000 (Thermo Scientific). The cells were incubated for 24 hours, washed in ice-cold PBS, and lysed in standard lysis buffer.
In vitro PTP assay
Thymocytes from WT and Themis KO mice were lysed in degassed PTP lysis buffer [1% Nonidet P-40, 25 mM HEPES (pH 7.0), 150 mM NaCl] in the presence or absence of 2 mM Na3VO4 or 1 mM NAC. Cell lysates were subjected to immunoprecipitation with anti-SHP1 antibody for 4 hours at 4°C. Gamma-bind G-Sepharose (GE Healthcare) was added, and the lysates were rotated for 1 hour at 4°C. The beads were washed twice with degassed PTP lysis buffer. Immunoprecipitated SHP1 protein was incubated with 0.2 mM PTP substrate peptide (RRLIEDAEpYAARG) in phosphatase assay buffer (20–180, EMD Millipore) in the presence or absence of 1 mM NAC and incubated for 30 min at room temperature. Released phosphate was detected by the addition of malachite green (17–125, EMD Millipore) and quantitated from a standard curve.
Statistical analysis
For the detection of SHP1 PTP activity, FTOC and cell (thymocyte and lymphocyte) counts, statistical significance was calculated by a two-tailed, type 2 t test (unpaired equal variance).
Data and materials availability:
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
Supplementary Material
Acknowledgments:
The authors thank A. Grinberg for generating the Themis-/- mice and M. Muschen (UCSF) and R. Bosselut (NCI) for providing mouse lines for this study. This paper is dedicated to the memory of our colleague Tibor Glant.
Funding:
This work was supported by intramural NIH funding (to P.E.L.): Project number 1 ZIAHD001803-28. All animal experiments were approved by the NIH Animal Car and Use Committee.
Footnotes
References and Notes
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA, Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nature reviews 14, 377–391 (2014); published online EpubJun ( 10.1038/nri3667). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth DB, V(D)J Recombination: Mechanism, Errors, and Fidelity. Microbiol Spectr 2, (2014); published online EpubDec ( 10.1128/microbiolspec.MDNA3-0041-2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA, Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. The Journal of experimental medicine 188, 1867–1874 (1998); published online EpubNov 16 ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaud G, Lesourne R, Love PE, Regulatory mechanisms in T cell receptor signalling. Nature reviews 18, 485–497 (2018); published online EpubAug ( 10.1038/s41577-018-0020-8). [DOI] [PubMed] [Google Scholar]
- 5.Lesourne R, Uehara S, Lee J, Song KD, Li L, Pinkhasov J, Zhang Y, Weng NP, Wildt KF, Wang L, Bosselut R, Love PE, Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol 10, 840–847 (2009); published online EpubAug (ni.1768 [pii] 10.1038/ni.1768). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, Salek M, Fallen PR, Hoerter JA, Munshi A, Huang YH, Hu J, Fox HS, Sauer K, Acuto O, Gascoigne NR, Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol 10, 848–856 (2009); published online EpubAug ( 10.1038/ni.1766). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrick MS, Oda H, Hayakawa K, Sato Y, Eshima K, Kirikae T, Iemura S, Shirai M, Abe T, Natsume T, Sasazuki T, Suzuki H, Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proceedings of the National Academy of Sciences of the United States of America 106, 16345–16350 (2009); published online EpubSep 22 ( 10.1073/pnas.0908593106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson AL, Aravind L, Shulzhenko N, Morgun A, Choi SY, Crockford TL, Lambe T, Domaschenz H, Kucharska EM, Zheng L, Vinuesa CG, Lenardo MJ, Goodnow CC, Cornall RJ, Schwartz RH, Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol 10, 831–839 (2009); published online EpubAug ( 10.1038/ni.1769). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakugawa K, Yasuda T, Miura I, Kobayashi A, Fukiage H, Satoh R, Matsuda M, Koseki H, Wakana S, Kawamoto H, Yoshida H, A novel gene essential for the development of single positive thymocytes. Molecular and cellular biology 29, 5128–5135 (2009); published online EpubSep ( 10.1128/MCB.00793-09). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu G, Casas J, Rigaud S, Rybakin V, Lambolez F, Brzostek J, Hoerter JA, Paster W, Acuto O, Cheroutre H, Sauer K, Gascoigne NR, Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 504, 441–445 (2013); published online EpubDec 19 ( 10.1038/nature12718). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paster W, Brockmeyer C, Fu G, Simister PC, de Wet B, Martinez-Riano A, Hoerter JA, Feller SM, Wulfing C, Gascoigne NR, Acuto O, GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J Immunol 190, 3749–3756 (2013); published online EpubApr 1 ( 10.4049/jimmunol.1203389). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zvezdova E, Mikolajczak J, Garreau A, Marcellin M, Rigal L, Lee J, Choi S, Blaize G, Argenty J, Familiades J, Li L, Gonzalez de Peredo A, Burlet-Schiltz O, Love PE, Lesourne R, Themis1 enhances T cell receptor signaling during thymocyte development by promoting Vav1 activity and Grb2 stability. Sci Signal 9, ra51 (2016) 10.1126/scisignal.aad1576). [DOI] [PubMed] [Google Scholar]
- 13.Neel BG, Gu H, Pao L, The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci 28, 284–293 (2003); published online EpubJun ( 10.1016/S0968-0004(03)00091-4). [DOI] [PubMed] [Google Scholar]
- 14.Pao LI, Badour K, Siminovitch KA, Neel BG, Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annual review of immunology 25, 473–523 (2007) 10.1146/annurev.immunol.23.021704.115647). [DOI] [PubMed] [Google Scholar]
- 15.Choi S, Cornall R, Lesourne R, Love PE, THEMIS: Two Models, Different Thresholds. Trends in immunology 38, 622–632 (2017); published online EpubSep ( 10.1016/j.it.2017.06.006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Warzecha C, Zvezdova E, Lee J, Argenty J, Lesourne R, Aravind L, Love PE, THEMIS enhances TCR signaling and enables positive selection by selective inhibition of the phosphatase SHP-1. Nat Immunol 18, 433–441 (2017); published online EpubApr ( 10.1038/ni.3692). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta M, Brzostek J, Chen EW, Tung DWH, Chen S, Sankaran S, Yap J, Rybakin V, Gascoigne NRJ, Themis-associated phosphatase activity controls signaling in T cell development. Proceedings of the National Academy of Sciences of the United States of America 115, E11331–E11340 (2018); published online EpubNov 27 ( 10.1073/pnas.1720209115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paster W, Bruger AM, Katsch K, Gregoire C, Roncagalli R, Fu G, Gascoigne NR, Nika K, Cohnen A, Feller SM, Simister PC, Molder KC, Cordoba SP, Dushek O, Malissen B, Acuto O, A THEMIS:SHP1 complex promotes T-cell survival. The EMBO journal 34, 393–409 (2015); published online EpubFeb 3 ( 10.15252/embj.201387725). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poole AW, Jones ML, A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal 17, 1323–1332 (2005); published online EpubNov ( 10.1016/j.cellsig.2005.05.016). [DOI] [PubMed] [Google Scholar]
- 20.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D, Transgenic mice with hematopoietic and lymphoid specific expression of Cre. European journal of immunology 33, 314–325 (2003); published online EpubFeb ( 10.1002/immu.200310005). [DOI] [PubMed] [Google Scholar]
- 21.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB, A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001); published online EpubNov (S1074–7613(01)00227–8 [pii]). [DOI] [PubMed] [Google Scholar]
- 22.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM, The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. The Journal of experimental medicine 206, 435–448 (2009); published online EpubFeb 16 ( 10.1084/jem.20081829). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez RJ, Morris AB, Neeld DK, Evavold BD, Targeted loss of SHP1 in murine thymocytes dampens TCR signaling late in selection. European journal of immunology 46, 2103–2110 (2016); published online EpubSep ( 10.1002/eji.201646475). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson DJ, Pao LI, Dhanji S, Murakami K, Ohashi PS, Neel BG, Shp1 regulates T cell homeostasis by limiting IL-4 signals. The Journal of experimental medicine 210, 1419–1431 (2013); published online EpubJul 01 ( 10.1084/jem.20122239). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler CC, Pao LI, Blattman JN, Greenberg PD, SHP-1 in T cells limits the production of CD8 effector cells without impacting the formation of long-lived central memory cells. J Immunol 185, 3256–3267 (2010); published online EpubSep 15 ( 10.4049/jimmunol.1001362). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnden MJ, Allison J, Heath WR, Carbone FR, Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76, 34–40 (1998); published online EpubFeb ( 10.1046/j.1440-1711.1998.00709.x). [DOI] [PubMed] [Google Scholar]
- 27.Lesourne R, Zvezdova E, Song KD, El-Khoury D, Uehara S, Barr VA, Samelson LE, Love PE, Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J Immunol 189, 1154–1161 (2012); published online EpubAug 1 ( 10.4049/jimmunol.1200123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tarakhovsky A, Muller W, Rajewsky K, Lymphocyte populations and immune responses in CD5-deficient mice. European journal of immunology 24, 1678–1684 (1994); published online EpubJul ( [DOI] [PubMed] [Google Scholar]
- 29.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K, A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 269, 535–537 (1995); published online EpubJul 28 ( [DOI] [PubMed] [Google Scholar]
- 30.Gascoigne NR, Acuto O, THEMIS: a critical TCR signal regulator for ligand discrimination. Curr Opin Immunol 33, 86–92 (2015); published online EpubApr ( 10.1016/j.coi.2015.01.020). [DOI] [PubMed] [Google Scholar]
- 31.Choi S, Love PE, Detection of Intracellular Reduced (Catalytically Active) SHP-1 and Analyses of Catalytically Inactive SHP-1 after Oxidation by Pervanadate or H2O2. Bio Protoc 8, (2018); published online EpubJan 5 ( 10.21769/BioProtoc.2684). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halliwell B, Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J 37, 99–105 (2014); published online EpubMay-Jun ( 10.4103/2319-4170.128725). [DOI] [PubMed] [Google Scholar]
- 33.Dustin LB, Plas DR, Wong J, Hu YT, Soto C, Chan AC, Thomas ML, Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol 162, 2717–2724 (1999); published online EpubMar 1 ( [PubMed] [Google Scholar]
- 34.Dzhagalov IL, Chen KG, Herzmark P, Robey EA, Elimination of self-reactive T cells in the thymus: a timeline for negative selection. PLoS Biol 11, e1001566 (2013) 10.1371/journal.pbio.1001566). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A, Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol 13, 569–578 (2012); published online EpubApr 29 ( 10.1038/ni.2292). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng D, Deobagkar-Lele M, Zvezdova E, Choi S, Uehara S, Baup D, Bennett SC, Bull KR, Crockford TL, Ferry H, Warzecha C, Marcellin M, de Peredo AG, Lesourne R, Anzilotti C, Love PE, Cornall RJ, Themis2 lowers the threshold for B cell activation during positive selection. Nat Immunol 18, 205–213 (2017); published online EpubFeb ( 10.1038/ni.3642). [DOI] [PubMed] [Google Scholar]
- 37.Brzostek J, Gautam N, Zhao X, Chen EW, Mehta M, Tung DWH, Chua YL, Yap J, Cho SH, Sankaran S, Rybakin V, Fu G, Gascoigne NRJ, T cell receptor and cytokine signal integration in CD8(+) T cells is mediated by the protein Themis. Nat Immunol 21, 186–198 (2020); published online EpubFeb ( 10.1038/s41590-019-0570-3). [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Cong Y, Niu Y, Yuan Y, Tan F, Lai Q, Hu Y, Hou B, Li J, Lin C, Zheng H, Dong J, Tang J, Chen Q, Brzostek J, Zhang X, Chen XL, Wang HR, Gascoigne NRJ, Xu B, Lin SH, Fu G, Themis is indispensable for IL-2 and IL-15 signaling in T cells. Sci Signal 15, eabi9983 (2022); published online EpubFeb 15 ( 10.1126/scisignal.abi9983). [DOI] [PubMed] [Google Scholar]
- 39.Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA, Schraven BL, Philips MR, Neel BG, Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell 13, 341–355 (2004); published online EpubFeb 13 ( [DOI] [PubMed] [Google Scholar]
- 40.Kinosada H, Yasunaga JI, Shimura K, Miyazato P, Onishi C, Iyoda T, Inaba K, Matsuoka M, HTLV-1 bZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-inhibitory Receptors. PLoS Pathog 13, e1006120 (2017); published online EpubJan ( 10.1371/journal.ppat.1006120). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun C, Shou P, Du H, Hirabayashi K, Chen Y, Herring LE, Ahn S, Xu Y, Suzuki K, Li G, Tsahouridis O, Su L, Savoldo B, Dotti G, THEMIS-SHP1 Recruitment by 4–1BB Tunes LCK-Mediated Priming of Chimeric Antigen Receptor-Redirected T Cells. Cancer Cell 37, 216–225 e216 (2020); published online EpubFeb 10 ( 10.1016/j.ccell.2019.12.014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K, B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 27, 35–48 (2007); published online EpubJul ( 10.1016/j.immuni.2007.04.016). [DOI] [PubMed] [Google Scholar]
- 43.Markovics A, Toth DM, Glant TT, Mikecz K, Regulation of autoimmune arthritis by the SHP-1 tyrosine phosphatase. Arthritis Res Ther 22, 160 (2020); published online EpubJun 26 ( 10.1186/s13075-020-02250-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


