Abstract
Observation of chronic inflammatory cells and associated high-level gamma interferon (IFN-γ) production in ganglia during herpes simplex type 1 (HSV-1) latent infection in mice (E. M. Cantin, D. R. Hinton, J. Chen, and H. Openshaw, J. Virol. 69:4898–4905, 1995) prompted studies to determine a role of IFN-γ in maintaining latency. Mice lacking IFN-γ (GKO mice) or the IFN-γ receptor (RGKO mice) were inoculated with HSV-1, and the course of the infection was compared with that in IFN-γ-competent mice with the same genetic background (129/Sv//Ev mice). A time course study showed no significant difference in trigeminal ganglionic viral titers or the timing of establishment of latency. Spontaneous reactivation resulting in infectious virus in the ganglion did not occur during latency in any of the mice. However, 24 h after the application of hyperthermic stress to mice, HSV-1 antigens were detected in multiple neurons in the null mutant mice but in only a single neuron in the 129/Sv//Ev control mice. Mononuclear inflammatory cells clustered tightly around these reactivating neurons, and by 48 h, immunostaining was present in satellite cells as well. The incidence of hyperthermia-induced reactivation as determined by recovery of infectious virus from ganglia was significantly higher in the null mutant than in control mice: 11% in 129/Sv//Ev controls, 50% in GKO mice (P = 0.0002), and 33% in RGKO mice (P = 0.03). We concluded that IFN-γ is not involved in the induction of reactivation but rather contributes to rapid suppression of HSV once it is reactivated.
Recurrent disease caused by herpes simplex virus type 1 (HSV-1) results from reactivation of latent virus in ganglia, centripetal spread of the virus in axons, and viral replication at mucosal sites. Experimentally, spontaneous reactivation of HSV-1 occurs in some species, e.g., producing recurrent skin lesions in the guinea pig model (32) or recurrent shedding without clinical manifestations in the rabbit model (22). In mouse models of HSV-1 infection, spontaneous reactivation occurs rarely or not at all (33). However, diverse stimuli have been shown experimentally to induce reactivation in the mouse. These stimuli can be grouped under four headings: (i) epithelial irritants such as UV light or physical skin damage from dry ice burn, scarification, or other noxious stimuli (2, 3, 33); (ii) direct action on the ganglion itself in the form of neurectomy or the use of neurotoxic agents such as cadmium (10, 42); (iii) immunosuppressant or cytotoxic agents such as cyclophosphamide or X-irradiation (24); and (iv) general systemic insults stressing the whole organism, such as pneumococcal pneumonia, hyperthermia, or cytokines such as interleukin-6 and tumor necrosis factor alpha (16, 30, 38, 41).
The host mechanisms underlying spontaneous and induced HSV-1 reactivation are not well understood but are probably multiple, involving neural, endocrine, and immunological mediators, including a possible role of gamma interferon (IFN-γ). In addition to the production of an antiviral state, IFN-γ has a spectrum of immunoregulatory effects. These include its abilities to activate macrophages, induce expression of major histocompatibility complex (MHC) class II antigens, regulate the proliferation and function of activated T cells, enhance NK cell activity, and influence immunoglobulin isotype switching (1, 9). IFN-γ is produced by activated NK cells and certain T-cell subsets, but its effects are ubiquitous, mediated via the IFN-γ receptor, with resultant induction of over 200 IFN-γ responsive genes. The IFN-γ effect, then, varies depending on the target cell and the microenvironment of other immunoregulatory mediators present. IFN-γ secretion by T cells has been shown to be critical in clearing HSV-1 skin infection (37), and in nervous system tissue, it is likely that T cells limit HSV-1 infection by noncytolytic mechanisms, focusing IFN-γ and other antiviral cytokines at sites of viral replication (36). However, there are limited and conflicting studies concerning IFN-γ in recurrent HSV-1 infection. In support of a protective effect is the detection of higher levels of IFN-γ in stimulated peripheral blood mononuclear cells and in recurrent vesicles of patients with a greater interval between attacks (6, 40).
We and others have reported inflammatory cells, both CD4+ and CD8+ lymphocytes and macrophages, persisting in latently infected ganglia of mice along with high levels of IFN-γ immunostaining surrounding some neurons in these ganglia (5, 12, 34). On this basis, we have speculated that IFN-γ may act as a modulator of HSV-1 latency. One way of assessing the importance of IFN-γ or other specific biological mediators in HSV-1 infection is to exploit knockout or null mutant mice. The present study used null mutant mice having the same genetic background (129/Sv//Ev) but lacking IFN-γ (GKO mice) or lacking the IFN-γ receptor (RGKO mice). We found that induced HSV-1 reactivation occurred more frequently in IFN-γ incompetent mutant mice than in the isogenic controls.
Evaluation of spontaneous reactivation in IFN-γ incompetent and control mice.
It is well known that susceptibility to HSV-1 varies according to mouse strain (19). The GKO mice previously were available only in the C57BL/6 background (7), whereas RGKO mice were available in the more susceptible 129/Sv//Ev background (13). In a prior study comparing HSV-1 infection in RGKO and GKO mice, we derived the IFN-γ null mutation in the 129/Sv//Ev background by using the AB-1 ES cell clone 97E (7), obtained from Tim Stuart (Genentech, San Francisco, Calif.) (4a). Thus, the IFN-γ mutant mouse strains used in our prior study and those used in this study differ genetically only at the mutant locus.
To determine whether GKO and RGKO mice differ from 129/Sv//Ev controls in terms of infectious HSV-1 present in ganglia during latency, anesthetized 6- to 8-week-old male mice were inoculated with HSV-1 strain F (American Type Culture Collection, Rockville, Md.) by placing 106 PFU contained in 4 μl on the right cornea and scarifying the cornea with a 27-gauge needle. Mice were euthanatized 30 to 60 days after viral inoculation, the trigeminal ganglia corresponding to the inoculated eye were removed, and cell homogenates prepared from these ganglia were individually assayed for infectious virus. In results from over 100 GKO, RGKO, and 129/Sv//Ev mice, infectious HSV-1 was never isolated from these ganglionic homogenates at the latent stage of infection (results not shown). Infection in these mice was confirmed by (i) HSV-1 shedding in the tear film at the acute stage of infection, with a greater duration of shedding in the mutant mice than in the controls (data not shown); (ii) development of periocular hair loss and corneal clouding (also more pronounced in the knockout mice than in the control mice); and (iii) demonstration of latent infection in 100% of the HSV-1-inoculated littermates of these mice as shown by explanation of trigeminal ganglia in culture for 72 h and detection of infectious virus in cell homogenates prepared from these explants. (Unexpectedly, both left and right ganglia were found to harbor HSV-1, despite inoculation of only the right eye.) Therefore, mice lacking IFN-γ or the IFN-γ receptor do not have a persistent infection after HSV-1 inoculation but develop latency just like fully immunocompetent control mice. Moreover, once at the latent stage, the IFN-γ incompetent mice do not undergo spontaneous reactivation resulting in detectable infectious HSV-1 in the ganglia, although abortive spontaneous reactivation (e.g., Fig. 1F) may occur.
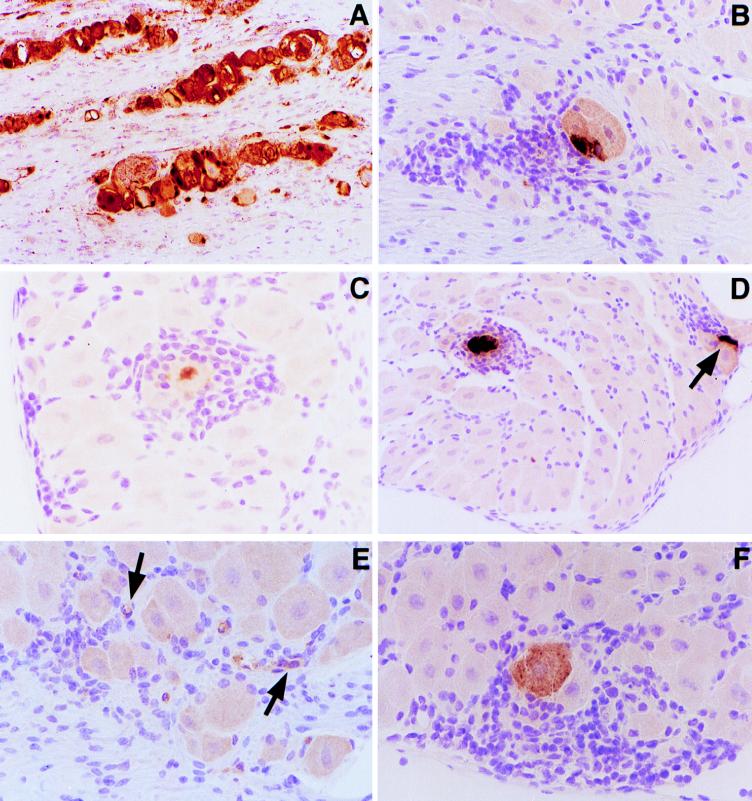
FIG. 1.
Immunoperoxidase staining of HSV-1 antigen in trigeminal ganglion sections. (A) Acute HSV-1 trigeminal ganglion section 4 days after viral inoculation. (B) RGKO trigeminal ganglion section and (C and D) GKO trigeminal ganglion sections 24 h after hyperthermia-induced HSV-1 reactivation. (E) GKO trigeminal ganglion section 48 h after hyperthermia-induced reactivation. (F) Latent infection of a GKO mouse showing spontaneous HSV-1 reactivation. Magnifications, ×10 (A), ×20 (B and D), and ×40 (C, E, and F). Images were further enlarged in PhotoShop for presentation. The arrows in panel E indicate HSV-1 antigen in satellite and/or infiltrating cells.
To evaluate whether reactivation occurs but is abortive (i.e., expression of viral antigens without infectious particles) or below the sensitivity of detection of HSV-1 in ganglionic homogenates, paraffin-embedded trigeminal ganglia were sectioned at 6 μm and HSV-1 antigens assayed by immunoperoxidase staining 30 to 60 days after HSV-1 inoculation. For immunostaining, the standard streptavidin-biotin immunoperoxidase technique was used with the kit from Vector Labs, Inc., Burlington, Calif. (5). Sections were hydrated with phosphate-buffered saline (pH 7.4), and anti-HSV-1 rabbit serum was added for a 1-h incubation at room temperature. After amplification (as described in the Vector Labs kit), the antibody-antigen complex was detected by using 3-amino-9-ethylcarbazole as the red chromogen, and sections were counterstained with hematoxylin.
In assays of over 20 sections of trigeminal ganglia from GKO, RGKO, and control 129/Sv//Ev mice, immunoperoxidase staining was detected in only one cell, a neuron, present in a section from a GKO ganglion. This neuron was surrounded by tightly clustered inflammatory cells, as shown in Fig. 1F. A positive control ganglionic section from a ganglion taken 4 days after inoculation of an RGKO mouse showed HSV-1 antigens confined primarily in neurons (Fig. 1A). No immunostaining was present in ganglionic sections from uninfected mice or from latently infected 129/Sv//Ev or RGKO mice. It is likely that the chronic inflammatory cells reported in prior studies of ganglionic sections at the latent stage (5, 12, 34) occur because of abortive spontaneous reactivation producing rare, sporadic HSV-1 antigen expression, as detected in the single latently infected neuron in a ganglionic section processed from a GKO mouse (Fig. 1F).
Evaluation of hyperthermia-induced HSV-1 reactivation in IFN-γ incompetent and control mice.
To determine if the absence of IFN-γ or the IFN-γ receptor influences the frequency of induced HSV-1 reactivation, GKO, RGKO, and control 129/Sv//Ev mice were treated with hyperthermia 30 to 60 days after inoculation by the method of Sawtell and Thompson (30) modified to maximize reactivation (38a). In brief, mice were treated with 10 min of hyperthermia in a 43°C water bath for three consecutive treatments with a 3-h recovery period between treatments. Mice were sacrificed 24 h later, and trigeminal ganglia were removed and assayed for infectious virus in ganglionic cell homogenates.
The results in Table 1, combining four separate trials, revealed a background rate of hyperthermia-induced reactivation of 11% in 129/Sv//Ev controls. The chi-square test showed a statistically significant 50% higher reactivation in GKO mice (P = 0.0002 compared to 129/Sv//Ev controls) and 33% higher reactivation in RGKO mice (P = 0.03 compared to 129/Sv//Ev controls). There was no statistically significant difference between the induced reactivation rates in the GKO and RGKO mice.
TABLE 1.
Hyperthermia-induced HSV-1 reactivation in male 129/Sv//Ev, GKO, and RGKO mice
| Expt no. | No. of mice with reactivated virus/no. tested (% with reactivated virus)a
|
||
|---|---|---|---|
| 129/Sv//Ev | GKO | RGKO | |
| 1 | 1/8 (13) | 5/12 (42) | 4/10 (40) |
| 2 | 1/7 (14) | 5/12 (42) | 1/10 (10) |
| 6 | 2/10 (20) | 5/10 (50) | 4/10 (40) |
| 7 | 0/13 (0) | 5/6 (83) | 4/10 (40) |
| Total | 4/38 (11) | 20/40 (50) | 13/40 (33) |
By chi-square test, P = 0.0002 for GKO mice and 0.03 for RGKO mice compared to 129/Sv//Ev mice.
Induced reactivation was also apparent in assays of ganglionic sections for HSV-1 antigen by immunoperoxidase staining. Two to four mice in each group (GKO, RGKO, and control 129/Sv//Ev mice) were euthanatized at three time points: just prior to hyperthermia, 24 h after hyperthermia, and 48 h after hyperthermia. The right trigeminal ganglion from each of the mice was dissected and snap frozen in liquid nitrogen-cooled isopentane. Frozen sections cut at 5 μm were used for detection of T cells (data not shown), but for in situ hybridization (ISH) and detection of HSV antigens, some of the ganglia were thawed and embedded in paraffin wax and then sectioned. Blocks of paired ganglia from the three groups were prepared, and 30 serial sections were obtained. Every fifth serial section was stained by the immunoperoxidase technique for HSV-1 antigens, and every sixth serial section was used for ISH for latency-associated transcripts (LAT). Figure 1 shows antigen-positive neurons in RGKO (Fig. 1B) and GKO (Fig. 1C and D) trigeminal ganglion sections prepared 24 h after hyperthermia treatment. In Fig. 1D, an antigen-positive satellite cell juxtaposed to a neuron is present (arrow). Interestingly, HSV antigen expression appeared to be confined to the nucleus in some neurons (Fig. 1C). In general, antigen-positive neurons were detected in at least two sections from GKO and RGKO mice, but only a single positive cell was detected in 129/Sv//Ev mice after hyperthermia treatment. As with the spontaneous reactivating neuron in Fig. 1F, chronic inflammatory cells clustered tightly around HSV-1 antigen-positive neurons (Fig. 1B, C, and D). Interestingly, in some GKO and RGKO ganglia assayed 48 h after induced reactivation, HSV-1 antigen tended to be found in surrounding satellite and or infiltrating cells rather than exclusively in neurons (Fig. 1E, arrows), suggesting intraganglionic spread of the reactivated HSV-1 or HSV-1 antigens. Again, only a single positive neuron was seen in ganglia from 129/Sv//Ev mice assayed 48 h after hyperthermic stress. HSV-1 replication was confined primarily to neurons at the acute stage (day 4) of infection (Fig. 1A), and no immunoreactivity was seen with ganglionic sections from uninfected mice (data not shown).
Assay of IFN-γ incompetent and control mice for degree of HSV-1 infection.
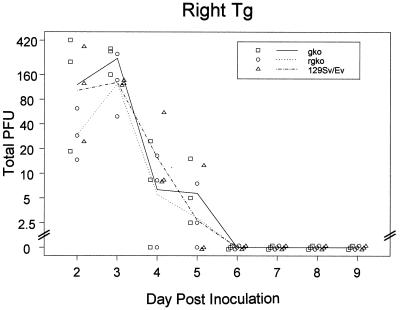
For a given HSV-1 strain, it has been shown that inoculum size and, to a lesser extent, virus titers during the acute ganglionic infection predict the latent viral genome burden (18, 28). This latency burden, estimated variously as the number of LAT-positive neurons, the number of neurons containing HSV DNA, or the average HSV genome copy number per neuron, is positively correlated with the frequency and efficiency of reactivation (18, 28). There is a formal possibility that induced reactivation occurs more often in GKO and RGKO mice (Table 1) because acute HSV-1 replication was enhanced and/or persisted in the ganglion, resulting in a greater burden of latent HSV-1 than in control mice. To evaluate this possibility, the time course of acute ganglionic infection was determined in these three strains of mice. The results in Fig. 2 show no difference in daily ganglionic HSV-1 titers from day 2 to day 5 post-HSV-1 inoculation and no difference in the time of latency entry (day 6). HSV titers in the three mouse groups were compared statistically by fitting the total plaque counts before day 6 to an overdispersed Poisson model. The total homogenate volume was used as denominator in a generalized linear model with log link and log volume as an offset (21). Comparison of the right trigeminal ganglion fit with and without the effect of strain using an F test produced a result of F = 3, P = 0.07, which is not significant at conventional levels. Furthermore, when a Kruskal-Wallis test (two degrees of freedom) was used, neither day 2 nor day 3 titers provided significant evidence of systematic variation among the three strains. This result is consistent with our earlier study showing very little difference in HSV-1 titer between RGKO and control ganglia (5) and with the results of Geiger et al. (11) that showed equivalent HSV-1 titers in the nervous systems of C57BL/6 control and GKO mice inoculated with HSV-1, even though the GKO mice developed encephalitis. Prolonged corneal virus shedding at the acute stage of infection has been reported in GKO mice (4), and we too have noted this in GKO and RGKO mice compared to 129/Sv//Ev controls, even though peak titers were not different in the three groups of mice (data not shown). However, this persistence at the cornea is not mirrored in ganglion or brain stem HSV-1 titers.
FIG. 2.
Time course of trigeminal ganglion (Tg) HSV-1 titers after inoculation of 129/Sv//Ev, GKO, and RGKO mice by the corneal route. Line segments show mean numbers of plaque-forming units (PFU) for each day. Symbols show individual mice with a horizontal offset to distinguish strains and a diagonal offset to distinguish identical points.
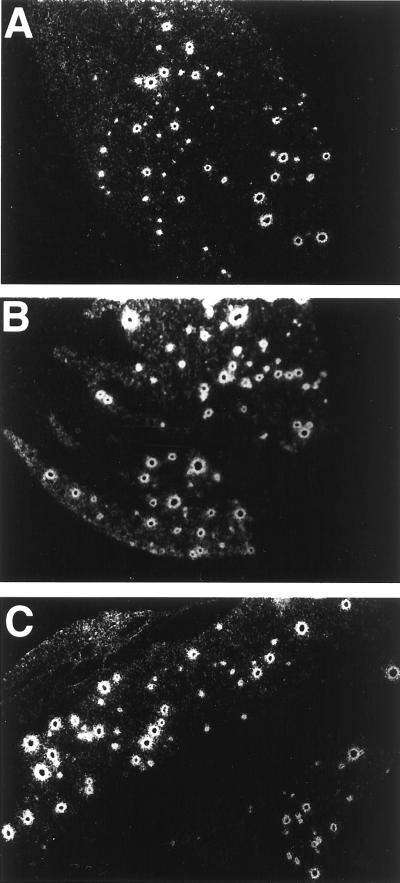
Evidence of latent HSV-1 ganglionic burden equivalency comes from ISH studies of LAT during latency. Although LAT-positive neurons are only a subset of the neurons that harbor latent HSV genomes (20, 28), it has been shown in the mouse ocular model that the number of LAT-positive neurons correlates positively with the efficiency of reactivation (20). Also, in a separate study, increasing the inoculum size resulted in an increased latency burden and a higher rate of reactivation (18). Although for distinct HSV-1 strains, the efficiency of reactivation correlated with the HSV-1 genome copy number per latently infected neuron (29), this was not the case when LAT+ and LAT− variants of the same strain were compared (39). In this instance, reactivation efficiency correlated with the number of latently infected neurons and there was no difference in latency burden per neuron. In the present study, LAT foci were readily identified in the three groups of mice (Fig. 3). With a sampling of five unselected sections more than 6 μm apart, the number of LAT foci in all three groups ranged from 145 to 229, with greater variability recorded within a group than between groups. Similar results suggesting equivalency of LAT foci were obtained in a screening study of latently infected trigeminal ganglia of GKO mice in the C57/BL6 background compared to control C57/BL6 mice (20a). To strictly exclude small quantitative differences in the number of LAT foci would require sectioning of the entire ganglion and, because of variability of infection, use of multiple animals in the three groups.
FIG. 3.
ISH for HSV-1 LAT in representative ganglionic sections from 129/Sv//Ev (A), GKO (B), and RGKO (C) mice photographed under dark-field illumination. Magnification was done with a 10× objective, and images were further enlarged in PhotoShop for presentation.
Conclusions.
Spontaneous HSV-1 reactivation leading to detectable virus in ganglia was not seen in GKO and RGKO mice. Therefore, it is unlikely that IFN-γ controls the reactivation process itself. However, after hyperthermia, there is a higher frequency of reactivation in the IFN-γ-incompetent mice, as detected by assay of viral antigens or infectious virus. We attribute this higher frequency to a controlling effect of IFN-γ on the virus immediately after reactivation. Similar results were obtained in a recent study of murine cytomegalovirus showing that IFN-γ controls reactivation by blocking the growth of low levels of reactivated virus rather than influencing the reactivation process itself or the burden of latent DNA (26). Another explanation for our results is the possibility of a greater burden of latent DNA in the ganglia of null mutant mice, leading to a higher frequency of induced reactivation (18, 20). Since the difference in detectable reactivation is quite large (fivefold greater in GKO mice than in controls), one would anticipate that the difference in latency burden also would be large, in analogy to published studies comparing the latency burdens in HSV-1 and HSV-2 strains which differ in reactivation efficiency (18). However, we found equivalent number of LAT foci in GKO, RGKO, and 129/Sv//Ev mice. In our study using a fixed inoculum size, equivalent acute-phase titers were obtained in the eyes and ganglia of the three groups of mice. Hence, the source of virus that could result in the hypothetical increased latency burden is not clear (14). To rigorously evaluate the possibility of an increased latency burden, a separate study would be required to compare the HSV genome copy number per latently infected neuron for the three mouse strains (GKO, RGKO, and 129/Sv//Ev) (28, 29).
In summary, from these results, we infer that IFN-γ suppresses replication of HSV-1 soon after reactivation. Because cytokine effects are short range (17, 27), we would predict that effector cells secreting IFN-γ and other cytokines should be focused at sites of reactivation, and indeed, we observed a characteristic halo of mononuclear inflammatory cells surrounding reactivating HSV-1 antigen-positive neurons (Fig. 1B to F). Prior studies have shown that CD8+ T cells control HSV-1 during acute infection through a nonlytic mechanism(s) most likely involving secretion of cytokines (35, 36). Although we did not immunophenotype the infiltrating cells around reactivating neurons (Fig. 1), it is reasonable to speculate that, analogous to the acute HSV-1 infection, T cells may control reactivated HSV-1 infection by secretion of IFN-γ and other soluble effectors. Spontaneous abortive reactivation events, as noted here (Fig. 1F) and in earlier reports of PCR and immunohistochemical studies, presumably drive the chronic inflammatory response, including CD4+ and CD8+ T cells in the ganglion during latency (5, 12, 34). It is these resident inflammatory cells, dispersed throughout the ganglion during latency, which rapidly home to reactivating neurons and control reactivated HSV-1.
Important questions to be addressed in future studies include identification of other cytokines and or chemokines involved and how they act to suppress HSV, the T cell, or other effector cell types involved and elucidation of how HSV-1 antigens expressed in reactivating neurons are recognized by inflammatory T cells, given that neurons are incapable of antigen expression because they are deficient in MHC class I or II antigens (15). Speculative mechanisms that might account for HSV-1 antigen presentation include expression of MHC antigens by neurons in vivo under special circumstances (23, 25) or recognition of HSV-1 antigens independent of MHC restriction, as shown recently for γδ T cells (31). Alternatively, HSV-1 antigens such as VP22, which has the unique ability to spread from the cell of synthesis (8), may be actively exported or traffic naturally to adjacent MHC-expressing satellite cells capable of antigen presentation. This is the first report to show that IFN-γ is important for controlling in vivo-reactivated HSV-1 and thereby contributes to the maintenance of virological latency, meaning the absence of infectious HSV-1 in the ganglion as opposed to molecular latency, which is manifested as repression of viral gene expression at the cellular level.
Acknowledgments
We thank Richard Thompson for advice about the in vivo HSV-1 reactivation procedure.
This work was supported by Public Health Service grant MH-55784 from the National Institute of Mental Health.
REFERENCES
- 1.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 2.Blatt A N, Laycock K A, Brady R H, Traynor P, Krogstad D J, Pepose J S. Prophylactic acyclovir effectively reduces herpes simplex virus type 1 reactivation after exposure of latently infected mice to ultraviolet B. Investig Ophthalmol Vis Sci. 1993;34:3459–3465. [PubMed] [Google Scholar]
- 3.Blyth W A, Hill T J, Field H J, Harbour D A. Reactivation of herpes simplex virus infection by ultraviolet light and possible involvement of prostaglandins. J Gen Virol. 1976;33:547–550. doi: 10.1099/0022-1317-33-3-547. [DOI] [PubMed] [Google Scholar]
- 4.Bouley D M, Kanangat S, Wire W, Rouse B T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 4a.Cantin, E. M. Unpublished data.
- 5.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham A L, Merigan T C. Gamma-interferon production appears to predict time of recurrence of herpes labialis. J Immunol. 1983;130:2397–2400. [PubMed] [Google Scholar]
- 7.Dalton D K, Pitts-Meek S, Keshev S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.Elliott G, O’Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 9.Farrar M A, Schreiber R D. The cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 10.Fawl R L, Roizman B. Induction of reactivation of herpes simplex virus in murine sensory ganglia in vivo by cadmium. J Virol. 1993;67:7025–7031. doi: 10.1128/jvi.67.12.7025-7031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiger K D, Nash T C, Sawyer S, Krahl T, Patstone G, Reed J C, Krajewski S, Dalton D, Buchmeier M J, Sarvetnick N. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 12.Halford W P, Gebhardt B M, Carr D J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 13.Haung S, Hendricks W, Althage A, Hemmi S, Bleuthmann H, Kamijo R, Vileck J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 14.Hill T J. Herpes simplex virus latency. In: Roizman B, editor. The herpesviruses. New York, N.Y: Plenum Press; 1985. pp. 175–240. [Google Scholar]
- 15.Joly E, Oldstone M B. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- 16.Kreisel J D, Ricigliano J, Spruance S L, Garza J H H, Hill J M. Neuronal reactivation of herpes simplex virus may involve interleukin-6. J Neurovirol. 1997;3:441–448. doi: 10.3109/13550289709031190. [DOI] [PubMed] [Google Scholar]
- 17.Kundig T M, Hengartner H, Zinkernagel R M. T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J Immunol. 1993;150:2316–2321. [PubMed] [Google Scholar]
- 18.Lekstrom-Himes J A, Pesnicak L, Straus S E. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J Virol. 1998;72:2760–2764. doi: 10.1128/jvi.72.4.2760-2764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez C. Immunological nature of genetic resistance of mice to herpes simplex virus type 1 infection. IARC Sci Publ. 1978;24:775–781. [PubMed] [Google Scholar]
- 20.Maggioncalda J, Mehta A, Su Y H, Fraser N W, Block T M. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology. 1996;225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 20a.Margolis, T. Personal communication.
- 21.McCullagh P, Nelder J A. Generalized linear models. 2nd ed. New York, N.Y: Chapman & Hall; 1989. [Google Scholar]
- 22.Nesburn A B, Elliott J H, Leibowitz H M. Spontaneous reactivation of experimental herpes simplex keratitis in rabbits. Arch Ophthalmol. 1967;78:523–529. doi: 10.1001/archopht.1967.00980030525021. [DOI] [PubMed] [Google Scholar]
- 23.Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Openshaw H, Asher L V, Wohlenberg C, Sekizawa T, Notkins A L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979;44:205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- 25.Pereira R A, Tscharke D C, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Presti R M, Pollock J L, Dal Canto A J, O’Guin A K, Virgin H W., IV Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J Exp Med. 1998;188:577–588. doi: 10.1084/jem.188.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 28.Sawtell N M. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawtell N M, Poon D K, Tansky C S, Thompson R L. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawtell N M, Thompson R L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciammas R, Johnson R M, Sperling A I, Brady W, Linsley P S, Spear P G, Fitch F W, Bluestone J A. Unique antigen recognition by a herpesvirus-specific TCR-gamma delta cell. J Immunol. 1994;152:5392–5397. [PubMed] [Google Scholar]
- 32.Scriba M. Herpes simplex virus infection in guinea pigs: an animal model for studying latent and recurrent herpes simplex virus infection. Infect Immun. 1975;12:162–165. doi: 10.1128/iai.12.1.162-165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekizawa T, Openshaw H, Wohlenberg C, Notkins A L. Latency of herpes simplex virus in absence of neutralizing antibody: model for reactivation. Science. 1980;210:1026–1028. doi: 10.1126/science.6254149. [DOI] [PubMed] [Google Scholar]
- 34.Shimeld C, Whiteland J L, Nicholls S M, Grinfeld E, Easty D L, Gao H, Hill T J. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 35.Simmons A, Tscharke D, Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 36.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex infection: T-cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 38.Stevens J G, Cook M L, Jordan M C. Reactivation of latent herpes simplex virus after pneumoccal pneumonia in mice. Infect Immun. 1975;11:635–639. doi: 10.1128/iai.11.4.635-639.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Thompson, R. L. Personal communication.
- 39.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torseth J W, Merigan C T. Significance of local γ-interferon production in recurrent herpes simplex infection. J Infect Dis. 1986;153:979–984. doi: 10.1093/infdis/153.5.979. [DOI] [PubMed] [Google Scholar]
- 41.Walev I, Podlech J, Falke D. Enhancement by TNF-alpha of reactivation and replication of latent herpes simplex virus from trigeminal ganglia of mice. Arch Virol. 1995;140:987–992. doi: 10.1007/BF01315409. [DOI] [PubMed] [Google Scholar]
- 42.Walz M A, Price R W, Notkins A L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974;184:1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]