Abstract
High-grade B-cell lymphoma with 11q aberrations (HGBL-11q) has been classified for the first time as a high-grade mature B-cell neoplasm according to the 5th edition of the World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissues. HGBL-11q is morphologically and immunohistochemically similar to Burkitt lymphoma (BL) or HGBL; it is characterized by gain in the 11q23.2-11q23.3 region and loss in the 11q24.1-qter region but it lacks MYC translocation. HGBL-11q is a rare tumor, and its exact frequency in Japan remains unclear. In this study, we classified 113 Germinal center B-cell (GCB) type aggressive B-cell lymphomas (BCLs), which were divided into BL, high-grade (HG), and large cell (LC) morphologies. We performed fluorescence in situ hybridization (FISH) to identify 11q aberrations. Nine patients had 11q aberrations (7.96%, 9/113), including six HGBL-11q. The age range was from 8 to 87 years, and all were male. Six out of 14 patients with HG morphology were diagnosed with HGBL-11q (6/14, 42.9%). HGBL-11q has been found to occur primarily in children and young adults but also in middle-aged and older adults. Patients with HG morphology without MYC translocation should undergo FISH for 11q aberrations regardless of age. However, the pathogenesis, clinical findings, and prognosis of HGBL-11q remain unclear. The accumulation of cases with an accurate HGBL-11q diagnosis in daily practice and accurate and detailed data on HGBL-11q will contribute to further understanding of 11q aberrations.
Keywords: high-grade B-cell lymphoma, 11q aberrations, fluorescence in situ hybridization
INTRODUCTION
High-grade B-cell lymphoma with 11q aberrations (HGBL-11q) is a new mature high-grade B-cell lymphoma (HGBL) to be described in the World Health Organization Classification 5th edition (WHO-5).1 HGBL-11q is a relatively rare B-cell lymphoma that exhibits a Burkitt-like or an intermediate to blastoid appearance but has 11q aberrations without MYC translocation. The concept of HGBL originated from B-cell lymphoma (BCL), unclassifiable, with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) (BCL-U), which was proposed as a provisional entity in the WHO 4th edition (WHO-4).2 This category was thought to apply to aggressive lymphomas with inferior prognosis, such as BL without MYC translocation, atypical BL with marked nuclear atypia, BL with BCL2 expression, and double-hit lymphoma, which had been discussed previously. In the revised 4th edition of WHO (WHO-4R), BCL-U was reclassified as HGBL with MYC and BCL2 and/or BCL6 rearrangements, and HGBL, not otherwise specified (NOS).3 HGBL, NOS included cases that could not be morphologically, immunohistochemically, or genetically classified as BL, Burkitt-like lymphoma with 11q aberration (BLL-11q), or other aggressive BCLs. HGBL without MYC translocation with BLL-11q was tentatively designated as a separate disease as BLL-11q in WHO-4R; BLL-11q will be changed to HGBL-11q in WHO-5.
BL is an aggressive lymphoma of well-known origin in the germinal center dark zone, characterized by marked cell proliferation due to MYC translocation. The dense, cohesive growth pattern of medium-sized tumor cells and the “starry sky” appearance produced by scattered macrophages are characteristic of BL. BLL-11q was established because 11q aberrations were found in MYC-negative BL and post-transplant-associated BL.4-6 Several studies of HIV-associated MYC-negative BL with 11q aberrations have been reported.7,8
Recent studies have reported that 11q aberrations were not limited to the Burkitt-like morphology cases, but were also found in BL, HGBL, and DLBCL.7,9,10 Regarding genetic variation, HGBL-11q was more similar to germinal center B-cell (GCB) type DLBCL than to BL,10 and it has also been reported to have the same properties of the dark zone in the germinal center as those of BL.11
Based on these studies, the International Consensus Classification (ICC) proposed a provisional entity named “Large B-cell lymphoma with 11q aberration”, composed mainly of centroblasts-like cells.12 This name emphasizes the fact that large B-cell lymphoma with 11q aberration is composed of larger cells than those of BL without MYC translocation, and its gene expression is similar to that of GCB type DLBCL. On the contrary, the description of HGBL-11q in WHO-5 includes high-grade (HG) morphology.1 HG morphology is defined as intermediate morphology between DLBCL and BL, or blastoid morphology.1
There are no large cohorts to investigate the frequency of HGBL with 11q aberrations in Japan. HGBL-11q is treated with aggressive chemotherapy as in BL. This disease is different from BL and may have different therapeutic targets, but detailed studies have yet to be reported. Accurate diagnosis is essential to understand the biology of HGBL-11q. In this study, we used fluorescence in situ hybridization (FISH) to select cases with 11q aberrations from aggressive BCLs showing GCB type and performed histological and immunohistochemical analyses. This study will facilitate HGBL-11q extraction, accumulate data on HGBL-11q, and contribute to a better understanding of 11q aberrations and to a more compatible treatment.
MATERIAL AND METHOD
Case selection
The Ethics Committee approved this study of Fukushima Medical University (No. 30257). From the archive of 274 aggressive BCL cases stored in formalin-fixed paraffin-embedded (FFPE) sections between 2005 and 2020 at Fukushima Medical University Hospital, 113 cases of GCB type with appropriate immunohistochemistry or FISH were selected for this study. According to Hans’s algorithm using immunohistochemistry, whether the disease was GCB or non-GCB type was determined. Cases classified as non-GCB type by immunohistochemistry were excluded. The 113 cases selected were 70 males and 43 females with a median age of 68 years (range 4-94 years). Prior to FISH analysis, the diagnoses performed in 113 cases were BL in 8 cases, BCL-U, intermediate DLBCL and BL, or HGBL in 11 cases, and DLBCL in 94 cases. DLBCL included DLBCL, NOS, and primary large B-cell lymphoma of the central nervous system (CNS).
Morphological classification
The 113 cases were reclassified into BL morphology, HG morphology, and large cell (LC) morphology in this study. These morphological classifications were made according to the description in WHO-5. BL morphology case was defined as medium-sized, round or square uniform nuclei, small and multiple nucleoli, coarsely granular or finely clumped chromatin, cohesive growth patterns, and diffuse starry-sky appearance.13 HG morphology was further classified into two categories: HG-intermediate (HG-int) morphology and HG-blastoid morphology.14 HG-int morphology was defined as cases with medium to large sized, irregular round or square nuclei, small and multiple nucleoli or single prominent nucleoli, coarsely granular or slightly vesicular chromatin, and cohesive growth patterns. HG-int was applicable when cases were not classified as BL morphology or LC morphology. Cases with nuclei slightly larger than those of BL and a well-defined nucleolus were classified as HG-int in this study, whereas in the past, they were referred to as atypical BL. HG-blastoid morphology was defined as medium-sized nuclei resembling lymphoblastic lymphomas with fine chromatin and no or inconspicuous nucleoli. LC morphology showed large-sized, irregular round nuclei, multiple or single prominent nucleoli, and coarsely granular or vesicular chromatin.15
Some studies have demonstrated that increased apoptotic bodies and coarse apoptotic debris within macrophages were characteristic features of 11q aberrations.16,17 In this study, a starry sky appearance was classified into three levels according to the extent to which macrophages were scattered: diffuse (>50%), partial (10–49%), and none (<10%). A field of view with many macrophages was selected and evaluated by counting 50 macrophages and the number of apoptotic bodies within the macrophages. We defined increased apoptotic bodies as an average of five or more apoptotic bodies within macrophages. These were reviewed by three pathologists (S.Y., Y.O., and Y.H.).
Immunohistochemistry
Thinly sliced FFPE sections of 4 μm thickness were deparaffinized, and immunohistochemistry was performed using a fully automated immunohistochemistry system Leica BOND-III (Leica Biosystems, Australia). The antibodies used in this study were: CD20 (FB1, in-house produced18), CD10 (ready-to-use product/RTU, 56C6, Leica Biosystems, UK), BCL6 (RTU, LN22, Leica Biosystems), MUM1 (RTU, EAU32, Leica Biosystems), BCL2 (RTU, BCL-2/100/D5, Leica Biosystems), Ki-67 (RTU, MM1, Leica Biosystems), c-myc (dilution: 1: 50, Y69, Abcam, UK), TdT (RTU, SEN28, Leica Biosystems), CD5 (dilution: 1: 50, SP19, Thermo Fisher Scientific, UK), and Cyclin D1(RTU, EP12, Leica Biosystems). Expression of c-myc was evaluated in 10% increments, and 40% or more was considered positive. CD10, BCL6, and MUM1 were deemed positive if they stained at least 30% of tumor cells according to the Hans algorithm.19 BCL2 was considered positive if it stained more than 50% of tumor cells.
In situ hybridization (ISH) of Epstein-Barr Virus encoded small RNA (EBER)
ISH was performed using Leica BOND III to detect EBV infected cells. The BOND EBER probe (RTU, PB0589, Leica Biosystems) was used in ISH.
Fluorescence in situ hybridization (FISH)
Pretreatment and hybridization were performed using Leica Elite (Leica Biosystems, USA). FISH analysis was performed on interphase nuclei of FFPE samples (4 μm thick sections) using DNA probes. For MYC translocation, BCL2 translocation, and BCL6 translocation, ZytoLight SPEC MYC Dual Color Break Apart Probe (Zytovision GmbH, Germany), ZytoLight SPEC BCL2 Dual Color Break Apart Probe (Zytovision GmbH, Germany) and ZytoLight SPEC BCL6 Dual Color Break Apart Probe (Zytovision GmbH, Germany) were used. We confirmed translocations of BCL2 and BCL6 by FISH only in cases with positive for MYC translocations or 11q aberrations. For copy number analysis of 11q23.3 and 11q24.3, Zytolight SPEC 11q gain/loss Triple Color Probe (Zytovision GmbH, Germany) was used. To analyze the gain or loss of 11q, we used probes that were labeled with different fluorescent dyes. Specifically, we used a green fluorescent dye probe that was directly labeled with the minimal region of gain at 11q23.3, an orange fluorescent dye probe that was directly labeled with the minimal region of loss at 11q24.3, and a blue fluorescent dye probe that was directly labeled with the centromere of chromosome 11. The FISH signal for the MYC, BCL2, and BCL6 Break Apart probe counted 200 cells with a positive cutoff of 10%. The 11q gain/loss probe FISH signal counted 200 cells. As for the 11q aberrations, 11q24.3 loss has been shown to be highly specific compared to 11q23.3 gain.9,16,20,21 200 cells were counted, and 11q aberrations were defined as those with one or more copy deletions in more than 20% of the cells. Only the gain or loss of 11q23.3 and gain of both 11q23.3 and 11q24.3 were excluded from 11q aberrations.
Final Diagnosis
BL morphology or HG morphology case with MYC translocation was comprehensively diagnosed as BL. BL morphology or HG morphology case with 11q aberrations was diagnosed as HGBL-11q in the absence of MYC translocation. This study interpreted the HG morphology case with both MYC translocation and 11q aberrations as BL (described as BL-11q). LC morphology cases with 11q aberrations were described as DLBCL-11q.
RESULTS
Morphological reclassification and final diagnosis
The 113 eligible GCB type aggressive BCLs were reclassified into BL morphology cases (n=6), HG morphology cases (n=14), and LC morphology cases (n=93). Based on the FISH result, these cases were finally classified as BL (n=7), BL-11q (n=1), HGBL-11q (n=6), DLBCL/HGBL with MYC and BCL2 rearrangements (DLBCL/HGBL-MYC/BCL2) (n=8), HGBL, NOS (n=5), DLBCL-11q (n=2) and GCB type DLBCL (n=84) (Figure 1 and Table 1). FISH examination revealed 11q aberrations in nine cases (9/113, 7.96%). 11q aberrations were found in seven HG morphology cases (five HG-int and two HG-blastoid cases) and two LC morphology cases. No 11q aberrations were observed in the BL morphology cases. HGBL-11q was found in six cases of HG morphology (42.9%, 6/14) and BL-11q in one HG morphology (7.14%, 1/14). BCL2 and BCL6 translocations were not observed in cases with 11q aberrations. No 11q aberrations were identified in the six cases of BL morphology. DLBCL-11q was found in two cases of LC morphology (2/93, 2.15%). There were no cases of DLBCL/HGBL-MYC/BCL2 with 11q aberrations.
Fig. 1.
Morphological classification and final diagnosis
Numbers in parentheses indicate the number of cases.
*DLBCL included DLBCL, NOS, and primary large B-cell lymphoma of the CNS.
BCLs, B-cell lymphomas; GCB, germinal center B-cell; BL, Burkitt lymphoma; HG, High-grade; LC, Large cell; BL-11q, BL with 11q aberrations; HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; HGBL, High-grade B-cell lymphoma; NOS, not otherwise specified; DLBCL, Diffuse large B-cell lymphoma; DLBCL-11q, DLBCL with 11q aberrations; FISH, Fluorescence in situ hybridization
Table 1. Clinical findings, morphological classification, and chromosomal abnormality of GCB type aggressive BCLs.
| BL (n=7) | BL-11q (n=1) | HGBL-11q (n=6) | HGBL, NOS (n=5) | DLBCL/HGBL-MYC/BCL2 (n=8) | DLBCL (n=84) | DLBCL-11q (n=2) | ||
|---|---|---|---|---|---|---|---|---|
| Clinical findings | Age (years) (median) | 8-52 (13.0) | 83 | 8-87 (68.5) | 13-90 (66.0) | 46-78 (71.0) | 19-94 (68.5) | 68-75 (71.5) |
| Sex | ||||||||
| Male | 7 (100%) | 1 (100%) | 6 (100%) | 4 (80.0%) | 5 (62.5%) | 46 (54.8%) | 2 (100%) | |
| Female | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20.0%) | 3 (37.5%) | 38 (45.2%) | 0 (0%) | |
| CNS primary | 0 (0%) | 0 (0%) | 2 (33.3%) | 0 (0%) | 0 (0%) | 6 (7.14%) | 0 (0%) | |
| Morphological classification | BL morphology | 6 (85.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HG morphology | 1 (14.3%) | 1 (100%) | 6 (100%) | 5 (100%) | 1 (12.5%) | 0 (0%) | 0 (0%) | |
| LC morphology | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (87.5%) | 84 (100%) | 2 (100%) | |
| Chromosomal abnormality | MYC-R | 7 (100%) | 1 (100%) | 0 (0%) | 2 (40.0%) | 8 (100%) | 9 (10.7%) | 0 (0%) |
| 11q aberrations | 0 (0%) | 1 (100%) | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (100%) |
BL, Burkitt lymphoma; BL-11q, Burkitt lymphoma with 11q aberrations; HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; HGBL, High-grade B-cell lymphoma;. NOS, not otherwise specified; DLBCL, Diffuse large B-cell lymphoma; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations; CNS, Central nervous system; HG, High-grade; LC, Large cell; MYC-R, MYC rearrangement
Histological features in aggressive BCLs with 11q aberrations
Histological characteristics are described in Table 2. In HGBL-11q (n=6), four cases showed HG-int morphology, and two showed HG-blastoid morphology (Figure 2). HG-int morphology cases in HGBL-11q showed a cohesive growth pattern with medium to large nuclei, coarsely granular or dense nuclei, and inconspicuous nucleoli. Of the six HGBL-11q cases, only two pediatric cases had a diffuse starry sky appearance (2/6, 33.3%), and the average number of apoptotic bodies in macrophages was more than five (Figure 3). Although HG-int morphology in HGBL-11q has more prominent nuclear shape irregularity than typical BL, it was challenging to accurately distinguish BL or HGBL-11q based on the histology alone. One HG-blastoid morphology case (Case 5) showed cohesive growth, but this pattern was not observed in the other case (Case 3). HG-blastoid morphology cases did not show a starry-sky appearance and increased apoptotic bodies. HG-blastoid morphology cases had medium-sized round nuclei with somewhat irregular nuclei size and fine chromatin.
Table 2. Histological findings of aggressive BCLs with 11q aberrations.
| Case | Final diagnosis | Morphological classification | Nuclear size | Irregularity of nuclear shape | Chromatin pattern | Nucleoli | Starry-sky appearance | Increased apoptotic bodies (Average numbers) | Cohesive growth pattern |
|---|---|---|---|---|---|---|---|---|---|
| BL-1 | BL | BL | Medium | - | Finely clumped | inconspicuous | Diffuse | 2.3 | + |
| BL-2 | BL | BL | Medium | - | Finely clumped | inconspicuous | Diffuse | 2.1 | + |
| BL-3 | BL | BL | Medium | - | Finely clumped | inconspicuous | Diffuse | 2.9 | + |
| BL-4 | BL | HG-int | Medium to Large | + | Finely clumped | conspicuous | Diffuse | 1.8 | + |
| BL-5 | BL | BL | Medium | - | Finely clumped | conspicuous | Diffuse | 1.7 | + |
| BL-6 | BL | BL | Medium | - | Finely clumped | inconspicuous | Diffuse | 1.3 | + |
| BL-7 | BL | BL | Medium | - | coarsely granular | inconspicuous | Partial | 2.2 | + |
| Case 1 | HGBL-11q | HG-int | Medium to Large | + | Finely clumped | inconspicuous | Diffuse | 5.7 | + |
| Case 2 | HGBL-11q | HG-int | Medium to Large | + | Finely clumped | inconspicuous | Diffuse | 5.5 | + |
| Case 3 | HGBL-11q | HG-blastoid | Medium to Large | + | Fine | inconspicuous | None | NA | - |
| Case 4 | HGBL-11q | HG-int | Medium to Large | + | coarsely granular | inconspicuous | None | NA | + |
| Case 5 | HGBL-11q | HG-blastoid | Medium | + | Fine | inconspicuous | None | NA | + |
| Case 6 | HGBL-11q | HG-int | Medium to Large | + | coarsely granular | inconspicuous | None | NA | + |
| Case 7 | BL-11q | HG-int | Medium to Large | + | Finely clumped | conspicuous | None | NA | + |
| Case 8 | DLBCL-11q | LC | Large | + | Vesicular | inconspicuous | Partial | 5.3 | +/- |
| Case 9 | DLBCL-11q | LC | Large | + | Vesicular | inconspicuous | Partial | 6.1 | +/- |
BL, Burkitt lymphoma; HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; BL-11q, Burkitt lymphoma with 11q aberrations; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations; HG-int, High-grade-intermediate; HG-blastoid, High-grade-blastoid; LC, Large cell; NA, not available; BCLs, B-cell lymphomas
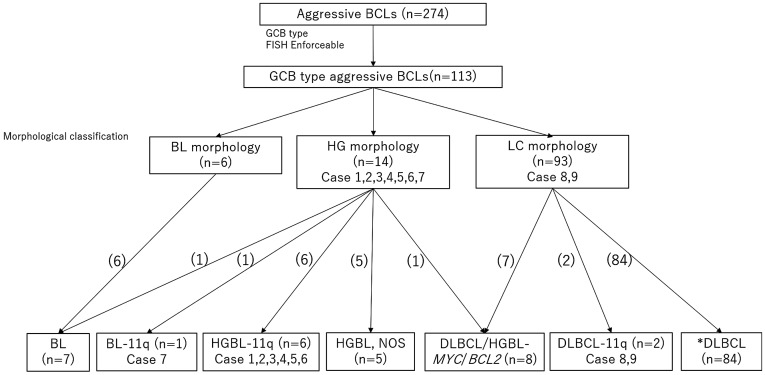
Fig. 2.
Histological findings of HGBL-11q, BL-11q, DLBCL-11q and BL
(A) Case 1 HGBL-11q (HG-int), (B) Case 2 HGBL-11q (HG-int), (C) Case 3 HGBL-11q (HG-blastoid), (D) Case 4 HGBL-11q (HG-int), (E) Case 5 HGBL-11q (HG-blastoid), (F) Case 6 HGBL-11q (HG-int), (G) Case 7 BL-11q (HG-int), (H) Case 8 DLBCL-11q (LC), (I) Case 9 DLBCL-11q (LC), (J) BL-6 (BL)
HGBL-11q was characterized by irregular nuclei and variations in size when compared to typical BL. BL-11q also showed HG-int morphology, not BL. DLBCL-11q is identified as the centroblastic type of DLBCL.
HG-int, High-grade intermediate morphology; HG-blastoid, High-grade blastoid morphology; HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; BL-11q, Burkitt lymphoma with 11q aberrations; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations; HG, High-grade; LC, Large cell, BL: Burkitt lymphoma
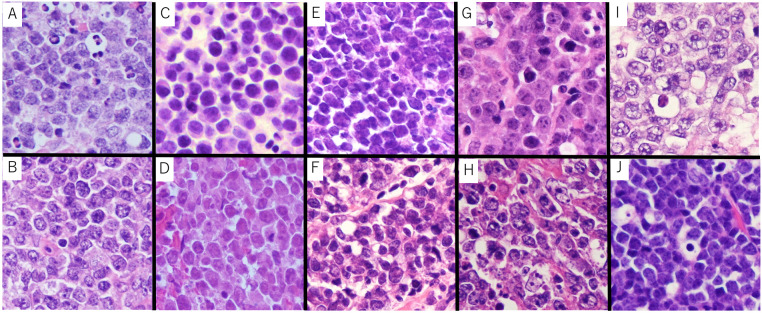
Fig. 3.
Increased apoptotic bodies in macrophages
(A) Case 1 HGBL-11q, (B) Case 2 HGBL-11q, (C) Case 8 DLBCL-11q, (D) Case 9 DLBCL-11q, (E) BL-6, (F) BL-4
In HGBL-11q and DLBCL-11q, increased apoptotic bodies within macrophages were observed (yellow arrowhead). While partial increased apoptotic bodies within macrophages were often observed in BL, they typically did not exceed an average of five.
HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations, BL, Burkitt lymphoma
BL-11q (n=1, Case 7) showed HG-int morphology, which differed from typical BL morphology. BL-11q showed a cohesive growth pattern with medium to large nuclei, coarsely granular nuclei, and conspicuous nucleoli. No starry sky appearance and no increase in apoptotic bodies were observed.
DLBCL-11q (n=2, Case 8 and Case 9) showed LC morphology. These cases showed diffuse proliferation of centroblastic tumor cells with vesicular and large nuclei and multiple nucleoli. There was little uniformity in the morphology of the tumor cells. The morphology of DLBCL-11q was identical to that of centroblastic type DLBCL. DLBCL-11q showed a partial starry-sky appearance and numerous phagocytosed apoptotic bodies in its macrophages (Figure 3), and the average number of apoptotic bodies was more than five.
Immunohistochemical and EBER-ISH findings in aggressive BCLs with 11q aberrations
Immunohistochemistry results are shown in Table 3.
Table 3. Immunohistochemical findings and FISH analysis of BL, HGBL-11q, BL-11q, and DLBCL-11q.
| Case | Final diagnosis | Immunohistochemistry | FISH | Diagnosis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hans’s classifer | CD10 | BCL6 | MUM1 | CD5 | CyclinD1 | TdT | BCL2 | c-myc % | Ki-67% | *BL phenotype | MYC-R | 11q aberration (copy number) | Initial diagnosis | WHO-4R | ||||
| BL-1 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | BL | BL | ||
| BL-2 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | BL | BL | ||
| BL-3 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | BL | BL | ||
| BL-4 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | BL | BL | ||
| BL-5 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | BL | BL | ||
| BL-6 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | BL | BL | ||
| BL-7 | BL | GCB | + | + | - | - | - | - | - | >70 | >95 | + | + | - | Atypical BL | BL | ||
| Case 1 | HGBL-11q | GCB | + | + | - | - | - | - | - | >70 | > 95 | + | - | 11q23.3 gain (3) 11q24.3 loss (1) | Atypical BL | BLL-11q | ||
| Case 2 | HGBL-11q | GCB | + | + | - | - | - | - | - | >70 | > 95 | + | - | 11q23.3 gain (3) 11q24.3 loss (1) | HGBL | BLL-11q | ||
| Case 3 | HGBL-11q | GCB | - | + | - | - | - | - | + | 50 | > 95 | - | - | 11q24.3 loss (1) | DLBCL | BLL-11q | ||
| Case 4 | HGBL-11q | GCB | + | + | - | - | - | - | + | 60 | 80 | - | - | 11q23.3 gain (3) 11q24.3 loss (1) | DLBCL | BLL-11q | ||
| Case 5 | HGBL-11q | GCB | + | + | + | - | - | - | - | > 70 | 90 | - | - | 11q23.3 gain (3) 11q24.3 loss (1) | BCLU | BLL-11q | ||
| Case 6 | HGBL-11q | GCB | + | + | - | - | - | - | - | > 70 | 90 | - | - | 11q23.3 gain (3) 11q24.3 loss (1) | DLBCL or HGBL | BLL-11q | ||
| Case 7 | BL-11q | GCB | + | + | + | - | - | - | - | > 70 | > 95 | - | + | 11q23.3 gain (4) 11q24.3 loss (1) | DLBCL or HGBL | BL | ||
| Case 8 | DLBCL-11q | GCB | + | + | - | - | - | - | - | 50 | 80 | - | - | 11q23.3 gain (3) 11q24.3 loss (1) | DLBCL, NOS | DLBCL, NOS | ||
| Case 9 | DLBCL-11q | GCB | + | + | - | - | - | - | - | 40 | 80 | - | - | 11q23.3 gain (3) 11q24.3 loss (1) | DLBCL, NOS | DLBCL, NOS | ||
FISH, Fluorescence in situ hybridization; BL, Burkitt lymphoma; HGBL-11q, High grade B-cell lymphoma with 11q aberrations; BL-11q, Burkitt lymphoma with 11q aberrations; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations; GCB, Germinal center B-cell; MYC-R, MYC rearrangement; DLBCL, Diffuse large B-cell lymphoma; HGBL, High grade B-cell lymphoma; BCL-U, B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL; BLL-11q, Burkitt-like lymphoma with 11q aberration; NOS, not otherwise specified; NA, Not available; *BL phenotype: CD10, BCL6 and c-myc were positive and Ki-67 positivity was >95%
HGBL-11q all showed c-myc >40%. Two pediatric HGBL-11q and two adults HGBL-11q showed c-myc >70%. MUM1 was positive only in one case in HGBL-11q. The Ki-67 positivity rate was >95% in three cases of HGBL-11q. The primary brain HGBL-11q was positive for BCL2, but the other HGBL-11q was negative for BCL2. Thus, HGBL-11q included complicated cases that were not immunohistochemically distinguishable from BL and DLBCL, NOS. In particular, two pediatric cases of HGBL-11q showed typical BL traits and did not differ from BL immunohistochemically. Indeed, HGBL-11q had previously been diagnosed with atypical BL, HGBL, BCLU, or DLBCL (Table 3). DLBCL-11q showed slightly fewer Ki-67 positive cells, expression of c-myc >40% and was negative for BCL2. DLBCL-11q was similar to the BL phenotype except for Ki-67 positivity. In all cases with 11q aberrations, EBER-ISH was negative.
Patterns of 11q aberrations in the FISH
The patterns of 11q aberrations are shown in Table 3 and Figure 4. The 11q aberrations included two patterns: a combination of 11q23.3 gain and 11q24.3 loss and 11q24.3 loss only. A variety of 11q23.3 gain and 11q24.3 loss was seen in eight cases, and 11q24.3 loss only in one case. Most of the 11q23.3 gain patterns included three copies, an increase by one copy, and only BL-11q had four copies of 11q23.3. The pattern of 11q24.3 loss was one copy deletion in all cases. No clustered amplification of 11q23.3 was observed. In this study, heterozygous deletion of 11q24.3 was common in all 11q aberrations, and homozygous deletion was not found. Five cases with LC morphology showed increased copy numbers of both 11q23.3 and 11q24.3, but this pattern is not characteristic of HGBL-11q and was not classified as 11q aberrations.
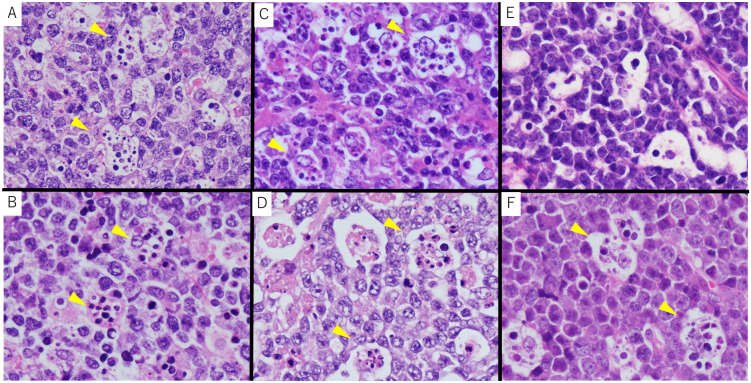
Fig. 4.
FISH analysis
(A) Case 1 HGBL-11: 11q23.3 gain (three green signals) and 11q24.3 loss (one orange signal), (B) Case 8 DLBCL-11q: 11q23.3 gain (three green signals) and 11q24.3 loss (one orange signal), (C) Case 7 BL-11q: 11q23.3 gain (four green signals) and 11q24.3 loss (one orange signal), (D) BL-11q: MYC split signal positive
FISH, Fluorescence in situ hybridization; HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations; BL-11q, Burkitt lymphoma with 11q aberrations
Clinical data of aggressive BCLs with 11q aberrations
Clinical data is provided in Table 4. HGBL-11q was found in two children and four adults. BL-11q and DLBCL-11q were adult cases. All Aggressive BCLs with 11q aberrations were male. HGBL-11q was also found in both nodal and extra-nodal sites. There were two cases of primary HGBL-11q in the central nervous system. Pediatric cases (Case 1 and 2) achieved remission with intense chemotherapy and have not experienced a relapse. Case 6 and Case 7 had a history of DLBCL and had been treated with R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone) therapy before developing HGBL-11q, therefore these two patients were treated with RB (Rituximab and Bendamustine) therapy.
Table 4. Clinical data of aggressive BCLs with 11q aberrations.
| Age | Sex | Site | Stage | BM involvement | Treatment | Response | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 8 | M | Intra-abdominal tumor(bulky), systemic LNs | IV | + | B-NHL-14 Group B | CR | ANED |
| Case 2 | 13 | M | Parotid gland | I | - | B-NHL03 Group 2 | CR | ANED |
| Case 3 | 63 | M | Brain | - | - | R-MPV→WBRT→HD-AraC | CR | Alive, relapse |
| Case 4 | 74 | M | Brain | - | NT | No treatment | - | DOD |
| Case 5 | 74 | M | Ileocecum-ascending colon, intra-abdominal LNs | II | - | EPOCH→R-CHOP→R-MA→R-CHOEP | CR | TUM |
| Case 6 | 87 | M | Cervical LNs | I | - | RB | ND | ND |
| Case 7 | 83 | M | Systemic LNs, retroperitoneum | III | - | RB→GDP | PD | DOD |
| Case 8 | 68 | M | Para-aortic LNs | II | - | R-CHOP | CR | TUM |
| Case 9 | 75 | M | Spleen | ND | NT | Splenectomy | ND | TUM |
HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; BL-11q, Burkitt lymphoma with 11q aberrations; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations; ND, No data, BM, Bone marrow; NT, No test; B-NHL-14 Group B, R-COPADM (Rituximab, Cyclophosphamide, Vincristine, Prednisolone, Doxorubicin, High-dose methotrexate); B-NHL03 Group 2 (P [Prednisolone, Vincristine, Cyclophosphamide, Methotrexate-IT, Hydrocortisone-IT, Cytarabine-IT], 2A [Prednisolone, Methotrexate, Vincristine, Cyclophosphamide, THP-adriamycin, Dexamethasone, Methotrexate-IT, Hydrocortisone-IT], 2B [Methotrexate, Cytarabine, Methotrexate-IT, Hydrocortisone-IT]); R-MPV, Rituximab, methotrexate, procarbazine, vincristine; WBRT, whole brain radiotherapy; HD-AraC, High-dose cytarabine; EPOCH, Etoposide, Prednisolone, Vincristine, Cyclophosphamide, Doxorubicin; R-CHOP, Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone; R-MA, Rituximab, Methotrexate, Cytarabine; R-CHOEP, Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Etoposide, Prednisolone; CR, Complete remission; PD, Progressive disease; ANED, Alive, no evidence of disease; DOD, Died of disease progression; TUM, Treatment or disease unrelated mortality
Stage was classified using the St.Jude/Murphy classification for pediatric cases and the Ann Arbor classification for adult cases.
DISCUSSION
This study selected 113 cases of aggressive GCB type BCLs, and FISH was performed to determine the frequency and characteristics of HGBL-11q and other lymphomas with 11q aberrations. As a result, nine cases with 11q aberration were extracted (9/113, 7.96%), including BL-11q (n=1), HGBL-11q (n=6), and DLBCL-11q (n=2). Although the HG morphology itself was infrequent, 6 of the 14 HG morphology were diagnosed with HGBL-11q (6/14, 42.9%). As in previous reports, cases that resemble BL morphology but could not be confirmed as BL or were suspected to be HGBL had a high probability of containing the 11q aberrations. In a previous study, 52% of MYC translocation-negative BL-like lymphoma or HGBL, NOS had 11q aberrations.16 These results suggest that 11q aberrations are frequently seen in cases of HG morphology and that it is essential to actively search for 11q aberrations.
Some cases of HGBL-11q resembled BL in exterior appearance, especially in the HG-int morphology of pediatric cases. These cases could not be strictly classified as BL morphology because the nuclei of the tumor cells were slightly larger than typical BL, and the nuclear irregularities and size discrepancies were somewhat more pronounced. However, these pediatric cases would have been diagnosed as BL or atypical BL if FISH had not been performed, as they showed immunohistochemically typical features of BL. Cohesive growth and a starry sky appearance were common findings in some of BL and HGBL-11q.
The histological finding of many apoptotic bodies and coarse apoptotic debris in macrophages is a characteristic of 11q aberrations cases.16,17 Yu et al. reported that these findings in more than 50% of macrophages are predictive of the presence of 11q aberrations.17 In this study, increased apoptotic bodies in a starry-sky appearance were observed in two pediatric HGBL-11q and two DLBCL-11q. The increased apoptotic bodies in macrophages were rarely seen in BL, and an average increase of more than five bodies was not recognized in BL. In DLBCL without 11q aberrations, the local increase in apoptotic bodies was seen in only five cases (5/84, 5.95%) and was rare. These results suggest that the 11q aberrations may induce marked apoptosis and phagocytosis. The finding of increased apoptotic bodies can be a distinguishing point between BL and HGBL-11q harboring immunohistochemical BL phenotype and between usual DLBCL and DLBCL-11q. However, it is important to note that increased apoptotic bodies are not always present in HGBL-11q.
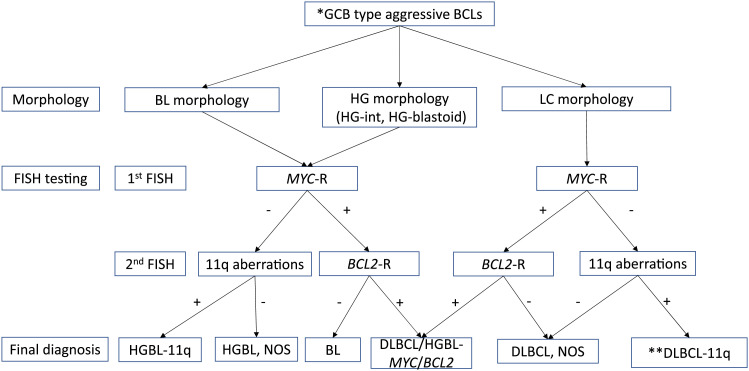
As mentioned above, morphological and immunohistochemical differentiation between BL and HGBL-11q can often be difficult, and FISH screening for chromosomal abnormality is ultimately essential for an accurate pathological diagnosis. However, it is not practical to perform FISH on 11q in all cases of aggressive BCLs. Therefore, we have developed a flowchart to distinguish HGBL-11q from other aggressive BCLs (Figure 5). In cases that show BL or HG morphology and no MYC translocation, FISH of 11q can be used to differentiate HGBL-11q from HGBL, NOS. In this study, HGBL-11q and HGBL, NOS were found in approximately equal numbers. It is possible that HGBL-11q was included among the previously diagnosed HGBL, NOS, and it is expected that the frequency of HGBL-11q will increase by performing FISH on 11q.
Fig. 5.
Diagnostic flowchart of HGBL-11q
Classification should take into account the patient’s immune status.
HG-int, High-grade intermediate morphology; HG-blastoid, High-grade blastoid morphology; GCB, Germinal center B-cell; BL, Burkitt lymphoma; HG, High-grade; LC, Large cell; MYC-R, MYC rearrangement; FISH, Fluorescence in situ hybridization; HGBL-11q, High-grade B-cell lymphoma with 11q aberrations; HGBL, High-grade B-cell lymphoma; NOS, not otherwise specified; DLBCL, Diffuse large B-cell lymphoma; DLBCL-11q, Diffuse large B-cell lymphoma with 11q aberrations
*Age or tumor location is not a consideration.
** DLBCL-11q is currently included in DLBCL, NOS, but its classification may change in the future.
It has also been reported that 11q aberrations are rarely found in DLBCL.10,16 In this study, DLBCL-11q was seen in two LC morphology cases (2/93, 2.15%), although the number was small. It is also described as “Large B-cell lymphoma with 11q aberration”12 in the ICC, and the morphology is centroblast-like cells, which may include LC morphology. However, the number of DLBCL-11q is very small, and the differences in clinical behavior and pathological findings between DLBCL, NOS are currently unclear. At this time, it is not realistic to perform FISH for 11q in all cases that could be diagnosed as DLBCL, NOS. Increased apoptotic bodies may be a clue to diagnosing DLBCL-11q, but this matter needs to be investigated in future case series.
Furthermore, BL with MYC translocations has been reported to also have 11q aberrations.9 There are also case reports of BL and HGBL-11q composite lymphoma.22 One case of BL-11q was present in this study. It is unclear whether BL-11q is clinically or molecular pathologically different from pure BL, but for the time being, it should be diagnosed and treated as BL. Importantly, since 11q aberrations have been observed in aggressive BCLs other than HGBL-11q, it is suggested that the 11q aberrations may be secondary copy number abnormalities in addition to other chromosomal abnormalities. However, chromosomal aberrations are not as complex in pediatric BL as they are in adult BL,23 and pediatric HGBL-11q may be significantly affected by the 11q aberrations themselves. This study did not examine whole chromosome aberrations by G-banding, nor did it examine copy number aberrations in regions other than 11q23.3 and 11q24.3; therefore, the exact chromosome aberrations could not be ascertained. We would expect less complex additional chromosomal abnormalities in pediatric cases than in adult cases, and it is possible that 11q aberrations may directly affect tumorigenesis.
Although genetic variation was not examined in this study, HGBL-11q and BL also differ in terms of genetic mutations. BL shows MYC translocation and mutations in TCF3, ID3, and CCND3, with an exceptionally high frequency of mutations in ID3.24-26 HGBL-11q was reported to have no mutations in ID3, TCF3, CCND3, and SMARCA4, suggesting it may be genetically different from BL.10,20,27 Recurrent genetic mutations found in HGBL-11q include BTG2, DDX3X, ETS1, EP300, GNA13, and epigenetic modifier genes; HGBL-11q is genetically more similar to GCB type DLBCL than it is to BL.10 Candidate genes for HGBL-11q include GNA13, ETS1, and NFRKB,27 but a more definitive one is yet to be found.
HGBL-11q overwhelmingly affects males more than females,9,10 and all patients with 11q aberrations were male in this study (Table 4). The reasons why aggressive BCLs with 11q aberrations are more common in males are still unknown. The small number of cases with 11q aberrations in this study may have led to a bias, and more cases need to be analyzed to determine the exact sex ratio. Most case reports of HGBL-11q have been conducted in children and young adults,8,21,28-33 but some cases in middle and old age have been reported.7,22,34-37 In this study, two HGBL-11q cases were pediatric patients, while five HGBL-11q and two DLBCL-11q cases were middle-aged and elderly patients. Although HGBL-11q tends to be more frequent among younger patients, it is important to recognize that the tumor can occur in middle-aged and older patients. Exclusion of HGBL-11q on the basis of age is not recommended. However, it is unclear whether the 11q aberrations have the same significance in younger and older patients. The differences in HGBL-11q according to age and site of origin need to be studied in detail in the future. HGBL-11q also occurs in post-organ transplant and immunocompromised patients associated with HIV infection.8,38,39 Immunocompromised and post-organ transplant patients were excluded from this study, and EBV infection was not implicated in any cases. In Japan, the association of 11q aberrations with HIV infection and medically-induced immunodeficiency has yet to be clarified. In this study, two cases of primary CNS HGBL-11q were found. A case report of orbital primary HGBL-11q showed direct infiltration of the optic nerve,40 but no HGBL-11q of central nervous system origin was found in the literature.
To conclude, we performed 11q gain/loss FISH on 113 GCB type aggressive BCLs and found six cases of HGBL-11q, one case of BL-11q, and two cases of DLBCL-11q. The spectrum of 11q aberration is not limited to those with HG morphology. However, DLBCL-11q requires clinical and molecular pathological investigation. In addition, HGBL-11q is not limited to children and young adults but also occurs in middle-aged and older adults. Therefore, morphologically suspected 11q aberration should be aggressively searched for 11q aberration by FISH, regardless of age.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022; 36: 1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SHC, Campo E, Jaffe ES, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue [OP] (Medicine). 4th ed, World Health Organization. 2008. [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pienkowska-Grela B, Rymkiewicz G, Grygalewicz B, et al. Partial trisomy 11, dup(11)(q23q13), as a defect characterizing lymphomas with Burkitt pathomorphology without MYC gene rearrangement. Med Oncol. 2011; 28: 1589-1595. [DOI] [PubMed] [Google Scholar]
- 5.Salaverria I, Martin-Guerrero I, Wagener R, et al. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014; 123: 1187-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreiro JF, Morscio J, Dierickx D, et al. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/loss pattern. Haematologica. 2015; 100: e275-e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebauer N, Witte HM, Merz H, et al. Aggressive B-cell lymphoma cases with 11q aberration patterns indicate a spectrum beyond Burkitt-like lymphoma. Blood Adv. 2021; 5: 5220-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JA, Kim HY, Kim SJ, Kim HJ, Kim SH. A case of Burkitt-like lymphoma with 11q aberration with HIV infection in East Asia and literature review. Ann Lab Med. 2021; 41: 593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grygalewicz B, Woroniecka R, Rymkiewicz G, et al. The 11q-gain/loss aberration occurs recurrently in MYC-negative Burkitt-like lymphoma with 11q aberration, as well as MYC-positive Burkitt lymphoma and MYC-positive high-grade B-cell lymphoma, NOS. Am J Clin Pathol. 2017; 149: 17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Farre B, Ramis-Zaldivar JE, Salmeron-Villalobos J, et al. Burkitt-like lymphoma with 11q aberration: a germinal center-derived lymphoma genetically unrelated to Burkitt lymphoma. Haematologica. 2019; 104: 1822-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeffler-Wirth H, Kreuz M, Hopp L, et al. A modular transcriptome map of mature B cell lymphomas. Genome Med. 2019; 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood. 2022; 140: 1229-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leoncini L, Siebert R, Ferry JA, et al. Burkitt lymphoma. In: WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet; beta version ahead of print]. Lyon (France): International Agency for Research on Cancer; 2022 [cited 2023/03/01]. (WHO classification of tumours series, 5th ed.; vol. 11). Available from: https: //tumourclassification.iarc.who.int/chapters/63.
- 14.Rosenwald A, Leoncini L, Macon WR, et al. Diffuse large B-cell lymphoma / high grade B-cell lymphoma with MYC and BCL2 rearrangements. In: WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet; beta version ahead of print]. Lyon (France): International Agency for Research on Cancer; 2022 [cited 2023/03/01]. (WHO classification of tumours series, 5th ed.; vol. 11). Available from: https: //tumourclassification.iarc.who.int/chapters/63.
- 15.Delabie J, Medeiros LJ, Klapper W, et al. Diffuse large B-cell lymphoma, NOS. In: WHO Classification of Tumours Editorial Board. Haematolymphoid tumours [Internet; beta version ahead of print]. Lyon (France): International Agency for Research on Cancer; 2022 [cited 2023/03/01]. (WHO classification of tumours series, 5th ed.; vol. 11). Available from: https: //tumourclassification.iarc.who.int/chapters/63.
- 16.Horn H, Kalmbach S, Wagener R, et al. A diagnostic approach to the identification of Burkitt-like lymphoma with 11q aberration in aggressive B-cell lmphomas. Am J Surg Pathol. 2021; 45: 356-364. [DOI] [PubMed] [Google Scholar]
- 17.Yu YT, Takeuchi K, Baba S, Chang KC. Morphologically suspected Burkitt-like lymphoma with 11q aberrations confirmed by fluorescence in situ hybridization. Am J Surg Pathol. 2022; 46: 576-577. [DOI] [PubMed] [Google Scholar]
- 18.Nozawa Y, Abe M, Ohno H, Fukuhara S, Wakasa H. Production of two monoclonal antibodies (FB1 and FB21) useful for the identification of human B lymphocytes in formalin-fixed, paraffin-embedded tissues. J Pathol. 1994; 173: 347-354. [DOI] [PubMed] [Google Scholar]
- 19.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282. [DOI] [PubMed] [Google Scholar]
- 20.Havelange V, Ameye G, Théate I, et al. The peculiar 11q-gain/loss aberration reported in a subset of MYC-negative high-grade B-cell lymphomas can also occur in a MYC-rearranged lymphoma. Cancer Genet. 2016; 209: 117-118. [DOI] [PubMed] [Google Scholar]
- 21.Au-Yeung RKH, Arias Padilla L, Zimmermann M, et al. Experience with provisional WHO‐entities large B‐cell lymphoma with IRF4 ‐rearrangement and Burkitt‐like lymphoma with 11q aberration in paediatric patients of the NHL‐BFM group. Br J Haematol. 2020; 190: 753-763. [DOI] [PubMed] [Google Scholar]
- 22.Kim M, Hwang HS, Yoon DH, Chun SM, Go H. Distinct genetic alterations in Burkitt-like lymphoma with 11q aberration and Burkitt lymphoma: a novel case report of composite lymphoma. Haematologica. 2022; 107: 1999-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salaverria I, Zettl A, Beà S, et al. Chromosomal alterations detected by comparative genomic hybridization in subgroups of gene expression-defined Burkitt’s lymphoma. Haematologica. 2008; 93: 1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rohde M, Bonn BR, Zimmermann M, et al. Relevance of ID3-TCF3-CCND3 pathway mutations in pediatric aggressive B-cell lymphoma treated according to the non-Hodgkin Lymphoma Berlin-Frankfurt-Münster protocols. Haematologica. 2017; 102: 1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012; 44: 1321-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012; 490: 116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagener R, Seufert J, Raimondi F, et al. The mutational landscape of Burkitt-like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood. 2019; 133: 962-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel HA, Cairo MS, Heerema NA, et al. Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Leukemia. 2009; 23: 323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westwood AC, Ali H. Burkitt-like lymphoma with 11q aberration. Diagn Histopathol. 2020; 26: 440-443. [Google Scholar]
- 30.Aypar U, Ewalt MD. A very Burkitt-like case of Burkitt-like lymphoma with 11q aberration. Blood. 2022; 139: 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asadbeigi SN, Deel CD. Burkitt-like lymphoma with 11q aberration: a case report and review of a rare entity. Case Rep Hematol. 2020; 2020: 8896322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimsza L, Pittaluga S, Dirnhofer S, et al. The clinicopathologic spectrum of mature aggressive B cell lymphomas. Virchows Arch. 2017; 471: 453-466. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Brown LE, Bowen LM, et al. Application of 2016 WHO classification in the diagnosis of paediatric high-grade MYC -negative mature B-cell lymphoma with Burkitt-like morphological features. J Clin Pathol. 2020; 73: 563-570. [DOI] [PubMed] [Google Scholar]
- 34.Collins K, Mnayer L, Shen P. Burkitt‐like lymphoma with 11q aberration. Clin Case Rep. 2019; 7: 1823-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moshref Razavi H, Hrynchak M. Unusual presentation of Burkitt-like lymphoma with 11q aberration in an elderly patient. Blood. 2019; 133: 381. [DOI] [PubMed] [Google Scholar]
- 36.Okwan-Duodu D, Huang Q. Primary splenic Burkitt-like lymphoma with 11q aberration. Blood. 2021; 138: 1642. [DOI] [PubMed] [Google Scholar]
- 37.Rymkiewicz G, Grygalewicz B, Chechlinska M, et al. A comprehensive flow-cytometry-based immunophenotypic characterization of Burkitt-like lymphoma with 11q aberration. Mod Pathol. 2018; 31: 732-743. [DOI] [PubMed] [Google Scholar]
- 38.Grygalewicz B, Woroniecka R, Rymkiewicz G, et al. Genetic progression of post-transplant Burkitt-like lymphoma case with 11q-Gain/Loss and MYC amplification. Cancer Genet. 2020; 245: 1-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Ma L, Guo J, Xi Y, Xu E. Burkitt-like lymphoma with 11q aberration in a patient with AIDS and a patient without AIDS: two cases reports and literature review. Open Med (Wars). 2021; 16: 428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azad F, Miranda CJ, Zhang J, Gravina M. Early orbital involvement in a rare diagnosis of Burkitt-like lymphoma with 11q aberration. Proc Bayl Univ Med Cent. 2023; 36: 240-242. [DOI] [PMC free article] [PubMed] [Google Scholar]