Abstract
Myeloid sarcoma (MS) is a condition characterized by a tumor mass of myeloid blasts in any site of the body other than the bone marrow, with or without acute myeloid leukemia. A 93-year-old man underwent laparoscopy-assisted distal gastrectomy with D1 lymphadenectomy for advanced gastric cancer. Other than metastatic foci of gastric cancer cells, some dissected lymph nodes showed destructive architecture with proliferation of small- to medium-sized atypical hematopoietic cells. Those cells were focally positive for naphthol AS-D chloroacetate esterase. Immunohistochemically, positive results were obtained for CD4, CD33, CD68 (KP1), Iba-1, lysozyme, myeloperoxidase, and PU.1, with focally positive results for CD13, CD14, CD68 (PGM1), CD163, and CD204, and negative results for AE1/AE3, CD1a, CD3, CD20, and S-100 protein. These results suggested MS with phenotypically myelomonocytic differentiation. We report a rare case of MS incidentally found in specimens resected for other purposes. Careful diagnosis and consideration of differential diagnoses including MS using an adequate panel of antibody markers for dissected lymph nodes is warranted.
Keywords: myeloid sarcoma, gastric cancer, lymph node, stomach
INTRODUCTION
Myeloid sarcoma (MS) is a condition characterized by a tumor mass of myeloid blasts at extramedullary sites. MS can be concurrent with acute myeloid leukemia (AML) or other myeloid neoplasms, including myelodysplastic syndrome, myeloproliferative neoplasm, or myelodysplastic/myeloproliferative neoplasms, can precede AML, or can represent the initial manifestation of relapsing AML.1 However, MS arises in the absence of underlying AML or other myeloid neoplasms in about one-quarter of cases, as so-called de novo MS.1 Here, we report a rare case of MS found incidentally in lymph nodes dissected for advanced gastric cancer.
CASE REPORT
A 93-year-old man was referred to our hospital for the treatment of gastric cancer. He did not have any past medical history. Computed tomography suggested lymph node metastasis around the stomach, but no distant metastases were identified. Laparoscopy-assisted distal gastrectomy with D1 lymphadenectomy was performed. The pathologic stage of gastric cancer was determined as pT3, pN1, cM0, stage IIB (Union for International Cancer Control / American Joint Committee on Cancer 8th ed.).
Microscopic examination revealed poorly differentiated adenocarcinoma in the stomach with two lymph node metastases. A notable finding in the present case was that other than metastatic foci of adenocarcinoma, some lymph nodes showed destructive architecture and proliferation of hematopoietic cells with small- to medium-sized, round or cleaved nuclei, inconspicuous nucleoli, and scant eosinophilic cytoplasm. These cells were distributed mainly in the lymph node sinus and paracortex with perinodal fat infiltration (Figure 1A, B). Scattered hemophagocytosis by mature macrophages was found. Cytochemical staining for naphthol AS-D chloroacetate esterase (NASDCAE) yielded focally positive results (Figure 2A). Immunohistochemically, positive results were obtained for CD4, CD33, CD68 (KP1), Iba-1, lysozyme, myeloperoxidase, and PU.1 (Figure 2B–G), with focally positive results for CD11c, CD13, CD14, CD45, CD68 (PGM1), CD163, and CD204 (Figure 2H, I), and negative results for AE1/AE3, CD1a, CD3, CD5, CD8, CD20, CD21, CD34, CD123, CD169, CD206, and S-100 protein (Figure 2J, K). Ki-67 labeling index was 40–70% (Figure 2L). Confocal fluorescence microscopy using Ki-67 and Iba-1 or PU.1 antibodies revealed coexpression of Iba-1 or PU.1 and Ki-67 (Figure 3). The differential diagnoses based on morphological features were malignant lymphoma, MS, histiocytic sarcoma, Langerhans cell histiocytosis, and metastatic gastric cancer. Considering the positive results for NASDCAE, expression of myeloid and histiocytic markers, and the lack of pan B- and T-cell markers, Langerhans cell markers, and cytokeratins, we diagnosed MS showing myelomonocytic differentiation. Blood cell counts after surgery were as follows: white blood cell count, 28.5 ×102/μL; hemoglobin, 9.7 g/dL; and platelet count, 7.5 ×104/μL. No bone marrow biopsy was ever performed and the patient has been followed-up without chemotherapy for MS, because of old age. No evidence of recurrence or progression to AML was detected as of 3 months after the surgery.
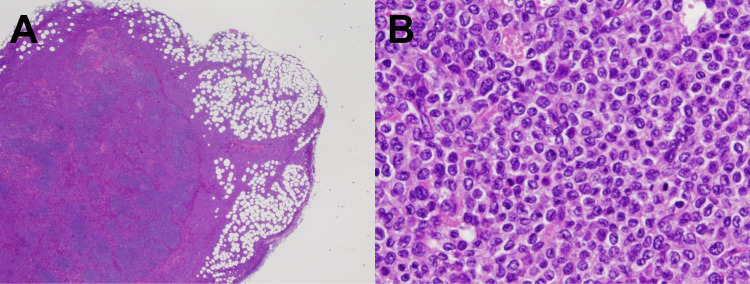
Fig. 1.
Histological findings. A) Hematoxylin and eosin staining shows infiltration of neoplastic cells into the paracortex and sinus area of a lymph node and perinodal fat. B) Neoplastic cells are small to medium in size, with round or cleaved nuclei, inconspicuous nucleoli, and scant eosinophilic cytoplasm.
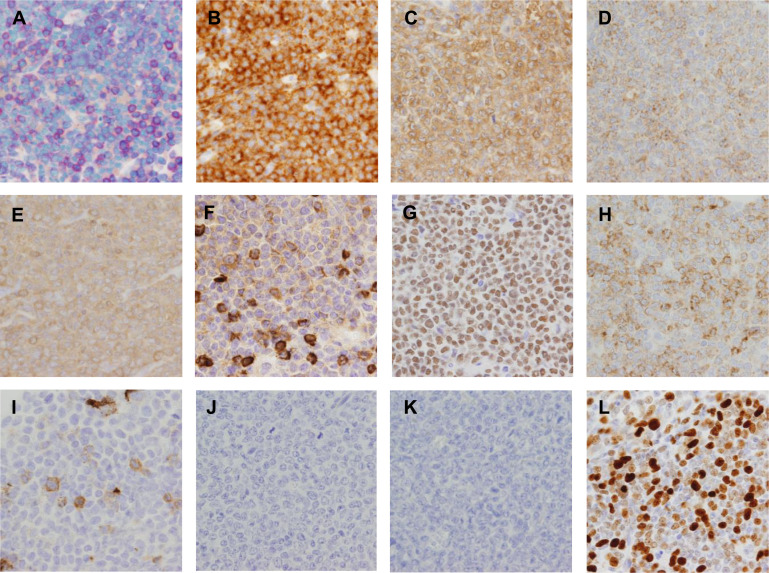
Fig. 2.
Cytochemical and representative immunohistochemical findings. A) Naphthol AS-D chloroacetate esterase (NASDCAE) is focally positive. B–G) Positive results are obtained for CD33 (B), CD68 (KP1) (C), Iba-1 (D), lysozyme (E), myeloperoxidase (F), and PU.1 (G). H, I) Focal positivity is seen for CD68 (PGM1) (H) and CD163 (I). J, K) CD3 (J) and CD20 (K) are negative. L) Ki-67 labeling index was about 70% in the highest area.
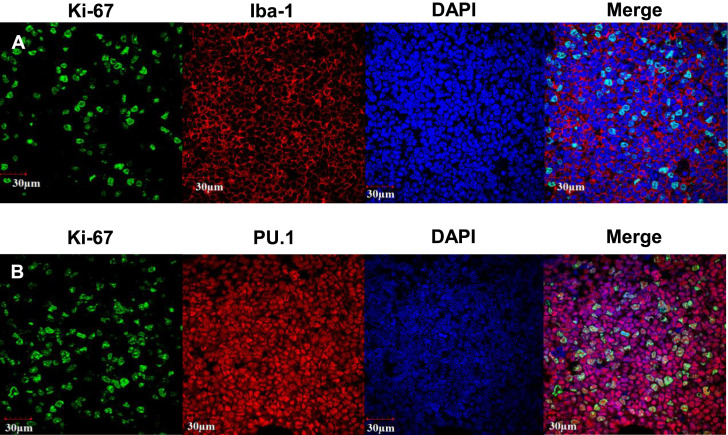
Fig. 3.
Confocal fluorescence microscopy images. Neoplastic cells coexpress Iba-1 or PU.1 and Ki-67. A) Ki-67 (green), Iba-1 (red), and DAPI (blue). B) Ki-67 (green), PU.1 (red), and DAPI (blue).
DISCUSSION
MS can involve any site of the body and is most frequently identified in lymph nodes. In the largest study of MS, 72 of 131 patients (55.0%) showed MS in lymph nodes.2 In another large study of MS, 15 of 92 patients (16.3%) had MS in lymph nodes.3 MS usually demonstrates a recognizable tumor mass, but cases of MS have been incidentally found in specimens resected for other purposes, as in the present case.4-7 In lymph nodes, MS grows diffusely or infiltrates the paracortex in particular, along the lymph node sinus.3,8 The morphology of MS is variable, ranging from blastic cells to large cells.9 In the present case, neoplastic cells were easily recognized in perinodal fat, but were easily overlooked in some lymph nodes that showed few neoplastic cells distributed only in the lymph node sinus, because of their morphological similarity to sinus macrophages. The immunophenotype of MS is most commonly myeloblastic, with or without features of promyelocytic or neutrophilic maturation.1 Some cases are myelomonocytic or monoblastic, and erythroblastic or megakaryoblastic differentiation is rare.1 Cytochemically, positivity for myeloperoxidase or NASDCAE indicates myeloid differentiation, whereas positivity for non-specific esterase indicates monocytic differentiation.1 The results of immunohistochemical staining in MS are summarized in Table 1. Myeloid markers include CD13, CD15, CD33, and myeloperoxidase, and monocytic markers include CD4, CD11c, CD14, CD68 (PGM1), and CD163.1,10 Lysosome and CD68 (KP1) are expressed in a wide spectrum, from myeloblastic to monoblastic tumors.8 A population of immature blasts often expresses CD34, CD117, and HLA-DR.10 Erythroblastic tumors are positive for glycophorin A and C, hemoglobin, and CD71, and megakaryoblastic tumors are positive for Factor VIII, CD31, and CD61.1,8 Since a certain proportion of MS cases lack some of these markers, use of several antibodies is necessary.2 MS can show varying degrees of TdT and CD56 expression, and aberrant antigenic expressions (such as B- or T-cell markers including CD2, CD3, CD4, CD7, CD20, CD30, and CD79a) can be observed.1-3,8 When adequate immunohistochemical analysis is not performed, cases may be misdiagnosed as malignant lymphoma, including diffuse large B-cell lymphoma, small lymphocytic lymphoma, peripheral T-cell lymphoma, lymphoblastic lymphoma/leukemia, or anaplastic large-cell lymphoma.3 The correct diagnosis is often missed, especially in cases of de novo MS.3,8 Another pitfall is that cytokeratin expression can be exceptional, leading to misdiagnosis as carcinoma.11
Table 1. Immunohistochemical staining in MS.
| Myeloid markers | CD13, CD15, CD33, myeloperoxidase |
| Monocytic markers | CD4, CD11c, CD14, CD68 (PGM1), CD163 |
| Erythroblastic markers | Glycophorin A and C, hemoglobin, CD71 |
| Megakaryoblastic markers | Factor VIII, CD31, CD61 |
The present case showed expression of some macrophage markers, including CD204, Iba-1, and PU.1. PU.1 is a critical transcriptional factor for macrophage and B-cell differentiation.12 PU.1 has been suggested as a marker for macrophage differentiation in Rosai–Dorfman disease and Erdheim–Chester disease.13,14 PU.1 may be an additional marker for MS showing monocytic and monoblastic differentiation.
To summarize, we have reported a rare case of MS incidentally found in lymph nodes dissected during surgery for advanced gastric cancer. The present case highlights that MS can be found incidentally in specimens resected for other purposes. To reach a correct diagnosis and prompt treatment, pathologists should consider MS in addition to malignant lymphoma and use an adequate panel of antibody markers when hematopoietic neoplasm is histologically suspected.
Footnotes
FUNDING INFORMATION
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 20H03459).
CONFLICT OF INTEREST
The authors have no conflict of interest. Y.K. is an editorial board member of JCEH.
REFERENCES
- 1.Pileri SA, Orazi A, Falomo B. Myeloid sarcoma. In: Swerdlow SH, Campo E, Harris NL, et al. (eds): WHO Classification of tumours of haematopoietic and lymphoid tissues. 4th ed, Lyon, IARC Press. 2017; pp. 167-168. [Google Scholar]
- 2.Kawamoto K, Miyoshi H, Yoshida N, et al. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg Pathol. 2016; 40: 1473-1483. [DOI] [PubMed] [Google Scholar]
- 3.Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007; 21: 340-350. [DOI] [PubMed] [Google Scholar]
- 4.Mullen C, Beverstock S, Roddie H, Campbell VL, Al-Qsous W. Myeloid sarcoma of uterine cervix: A case report with review of the literature. Gynecol Oncol Rep. 2022; 39: 100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro E, Morales L, Zreik R, Donner LR. A focus of differentiated myeloid sarcoma in a ligation specimen of the fallopian tube: no evidence of hematologic abnormality in 18 years of follow-up despite absence of treatment. Int J Surg Pathol. 2020; 28: 99-101. [DOI] [PubMed] [Google Scholar]
- 6.Rocca BJ, Ambrosio MR, Gozzetti A, et al. Myeloid sarcoma and adenocarcinoma of the large bowel as collision tumors: a case report. Histol Histopathol. 2012; 27: 941-947. [DOI] [PubMed] [Google Scholar]
- 7.Lazaris AC, Papanikolaou IS, Xirou PA. Coexistence of a granulocytic sarcoma and adenocarcinoma of the rectum. Am J Gastroenterol. 2001; 96: 615-616. [DOI] [PubMed] [Google Scholar]
- 8.Audouin J, Comperat E, Tourneau AL, et al. Myeloid sarcoma: clinical and morphologic criteria useful for diagnosis. Int J Surg Pathol. 2003; 11: 271-282. [DOI] [PubMed] [Google Scholar]
- 9.Dayton VD, Williams SJ, McKenna RW, Linden MA. Unusual extramedullary hematopoietic neoplasms in lymph nodes. Hum Pathol. 2017; 62: 13-22. [DOI] [PubMed] [Google Scholar]
- 10.Arber DA, Brunning RD, Orazi A, et al. Acute myeloid leukaemia, NOS. In: Swerdlow SH, Campo E, Harris NL, et al. (eds): WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed, Lyon, IARC Press. 2017; pp. 156-166. [Google Scholar]
- 11.Dayton VJ, Beckman A, Linden M. Myeloid sarcoma expressing keratins and mimicking carcinoma-case report and literature review. Lab Med. 2022; 53: 100-106. [DOI] [PubMed] [Google Scholar]
- 12.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994; 265: 1573-1577. [DOI] [PubMed] [Google Scholar]
- 13.Kiruthiga KG, Younes S, Natkunam Y. Strong Coexpression of transcription factors PU.1 and Oct-2 in Rosai-Dorfman disease. Am J Clin Pathol. 2022; 158: 672-677. [DOI] [PubMed] [Google Scholar]
- 14.Kai K, Komohara Y, Shinojima N, et al. A case of suprasellar Erdheim-Chester disease and characterization of macrophage phenotype. J Clin Exp Hematop. 2020; 60: 179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]