Abstract
Follicular lymphoma (FL) is an indolent B-cell lymphoma with a germinal center (GC) B cell phenotype that typically harbors t(14;18)(q32;q21). t(14;18) juxtaposes IGH on 14q32 and BCL2 on 18q21, resulting in overexpression of the anti-apoptotic BCL2 protein. However, t(14;18) is also found in the peripheral blood or lymphoid nodes (LNs) of otherwise healthy individuals. Moreover, overt FL has several additional gene alterations involved in epigenetic modification, JAK/STAT signaling, immune modulation, and NF-κB signaling, indicating multi-step lymphomagenesis in FL. There are two early or precursory lesions of FL: t(14;18)-positive cells in the peripheral blood of otherwise healthy individuals and in situ follicular B-cell neoplasm (ISFN). t(14;18)-positive cells are found in 10%–50% of healthy populations, and their incidence and frequency increase with age. The detection of t(14;18) in peripheral blood is a predictive factor for an increased risk of overt FL development. In contrast, ISFN is a histopathologically recognizable precursory lesion, in which t(14;18)-positive cells are confined to the GC of otherwise reactive LNs. ISFN is usually detected incidentally, with an incidence ranging from 2.0% to 3.2%. Occasional ISFN cases have concurrent or metachronous clonally related overt FL or aggressive B-cell lymphoma of a GC phenotype. t(14;18)-positive cells in peripheral blood and isolated ISFN, by themselves, are asymptomatic with limited clinical significance; however, investigations of t(14;18)-positive precursory or early lesions offer meaningful insights into the pathogenesis of FL. This review summarizes the epidemiology, clinical features, pathology, and genetics of precursory or early lesions of FL.
Keywords: t(14; 18), BCL2, follicular lymphoma, in situ follicular B-cell neoplasm
INTRODUCTION
Follicular lymphoma (FL) is a common indolent B-cell lymphoma that exhibits an immunophenotype of germinal center (GC) B cells. In Japan, FL accounts for 13.5% of newly diagnosed cases of lymphoma.1 Histopathologically, typical FL is characterized by predominantly nodular proliferation of centrocytic B cells.2 In addition to pan-B cell markers (including CD20, CD19, and CD79a), GC B cell markers (CD10 and BCL6) are typically expressed in tumor cells.2 A genetic hallmark of FL is a balanced translocation of chromosome 14q32 and 18q21, which is found in approximately 90% of FLs.2–4 This t(14;18)(q32;q21) juxtaposes immunoglobulin heavy chain (IGH) gene enhancer on 14q32 and BCL2 on 18q21, leading to the transcription upregulation of BCL2. As BCL2 is an anti-apoptotic protein, this IGH::BCL2 gene rearrangement enables FL cells to resist apoptosis in GCs.4 This crucial anti-apoptotic property facilitates the lymphomagenesis of FL. However, t(14;18) is not infrequently detected in lymphocytes in peripheral blood and reactive lymph nodes (LNs) of otherwise healthy individuals.5 In addition, recent molecular studies on FL identified a number of somatic mutations of genes involved in epigenetic modification (e.g., KMT2D, CREBBP, and EZH2), JAK/STAT signaling (SOCS1 and STAT6), immune modulation (B2M, CD58, and TNFRSF14), and B cell receptor (BCR)–NF-κB signaling (BCL10, CARD11, and CD79B).4 These findings suggest that lymphomagenesis of FL is a multi-step process.
Considering the multi-step pathogenesis of FL, some FL-like early or precursory lesions have been recognized. These FL-like lesions include t(14;18)-positive cells in peripheral blood and in situ follicular B-cell neoplasm (ISFN).6 In these conditions, FL-like cells harboring t(14;18) are, most often incidentally, detected by molecular and/or histopathological examination. These neoplastic processes are confined to a certain anatomical component without systemic distribution or symptoms such as overt FL. This review discusses the epidemiological, clinicopathological, pathological, and genetic features of precursory or early lesions of FL.
t(14;18)-POSITIVE CELLS IN PERIPHERAL BLOOD OF OTHERWISE HEALTHY INDIVIDUALS
t(14;18) translocation is due to erroneous VDJ recombination of the IGH gene in bone marrow at the pre-B cell stage,4,7 and t(14;18)-positive B lymphocytes circulate in the peripheral blood or reactive lymphoid tissues of individuals without FL. These t(14;18)-positive B cells exist at very low frequency in peripheral blood approximately ranging from 1 × 10-6 to 1 × 10-2, as quantified by quantitative PCR.5,8–15 Of note, a study investigated the distribution of t(14;18)-positive cells with a cell sorter and showed that the positive cells are more enriched in IgM memory (median: 380 × 10-6) than in antigen-naive (median: 16 × 10-6) or switched memory (median: 5 × 10-6) B cells.16 This finding demonstrated that t(14;18) is generated during early B cell development in bone marrow and that affected cells may mature and expand in GCs.
Similar to other subclinical monoclonal proliferations, such as monoclonal gammopathy of undetermined significance (MGUS) and monoclonal B-cell lymphocytosis (MBL), t(14;18)-positive cells are not uncommon and are found in 10%–50% of otherwise healthy individuals by highly sensitive quantitative or nested PCR (Table 1). The reported incidence varies considerably according to the age and ethnicity of the individuals. Dölken et al. reported an age-dependent increase in the incidence of t(14;18)-positive cells.9 According to their study, young individuals aged less than 10 years had no t(14;18)-positive cells in their peripheral blood. In contrast, the prevalence of circulating t(14;18)-positive cells increased from the second to fifth decades of life, from 20% to 66%, and remained stable thereafter. In addition, the median frequency of circulating t(14;18)-positive cells showed an increase with age.9 Hirt et al. also reported that t(14;18) frequency is positively associated with age but not with sex or smoking.12 These findings suggest that the cumulative chance of erroneous VDJ recombination increases and that B cells with t(14;18) clonally expand during aging.
Table 1. Incidences of t(14;18)-positive cells in peripheral blood and in situ follicular B-cell neoplasm (ISFN).
| Study, year (reference) | Screening method | Country of origin | Sample (n) | Prevalence (%) |
|---|---|---|---|---|
| t(14;18)-positive cells in peripheral blood | ||||
| Roulland, 2014 (5) | qPCR | Europe | Total peripheral blood leukocytes (n=218) | 29 |
| Summers, 2001 (8) | qPCR | United Kingdom | Total peripheral blood leukocytes (n=481) | 23 |
| Dolken, 2008 (9) | qPCR | Germany | Mononuclear peripheral blood leukocytes (n=644) | 45 |
| Nambiar, 2010 (10) | Nested PCR | India | Mononuclear peripheral blood leukocytes (n=253) | 34 |
| Yasukawa, 2001 (11) | Nested PCR | Germany | Mononuclear peripheral blood leukocytes (n=75) | 52 |
| Yasukawa, 2001 (11) | Nested PCR | Japan | Mononuclear peripheral blood leukocytes (n=241) | 16 |
| Hirt, 2013 (12) | qPCR | Germany | Total peripheral blood leukocytes (n=3966) | 39 |
| Schmitt, 2006 (13) | Nested PCR | Germany | Total peripheral blood leukocytes (n=204) | 24 |
| Schmitt, 2006 (13) | qPCR | Germany | Total peripheral blood leukocytes (n=204) | 19 |
| Colon-Otero, 2017 (14) | Semi-nested PCR | USA, African American | Total peripheral blood leukocytes (n=77) | 14 |
| Colon-Otero, 2017 (14) | Semi-nested PCR | USA, White | Total peripheral blood leukocytes (n=167) | 16 |
| Levy, 2017 (15) | Nested PCR | Brazil, White | Mononuclear peripheral blood leukocytes (n=85) | 53 |
| Levy, 2017 (15) | Nested PCR | Brazil, Black | Mononuclear peripheral blood leukocytes (n=72) | 79 |
| Levy, 2017 (15) | Nested PCR | Brazil, Japanese-descent | Mononuclear peripheral blood leukocytes (n=70) | 97 |
| in situ follicular B-cell neoplasm (ISFN) | ||||
| Henopp, 2011 (29) | IHC (BCL2) | Germany | Lymph nodes (n=132) | 2.3 |
| Bermudez, 2016 (25) | IHC (CD10 and BCL2) | Spain | Lymph nodes (n=341) | 3.2 |
| Carvajal-Cuenca, 2012 (30) | IHC (BCL2) | Spain | Lymph nodes (n=100) | 2.0 |
| Oishi, 2022 (24) | IHC (BCL2) | Japan | Lymph nodes (n=340) | 2.1 |
The incidence of overt FL may vary according to ethnicity. An earlier study by Chihara et al. showed that the population-based incidence of FL in Japan was significantly lower than that in the United States: the age-adapted FL incidence per 100 000 people in 2008 was 1.1 in Japan and 2.6 in the United States.1 On the other hand, it is controversial whether the incidence of t(14;18)-positive cells in healthy individuals differs by ethnicity (Table 1). Yasukawa et al. investigated the incidence of t(14;18)-positive cells in Japan and Germany.11 They reported that 16% (39/241) and 52% (39/75) of Japanese and German healthy individuals, respectively, harbor positive cells, suggesting a major reason for the difference in incidence of FL between Western countries and Japan.11 In contrast, Levy et al. compared the incidences in Brazil by ethnic background. Interestingly, t(14;18) translocation in the peripheral blood was found in 53% (45/85) of white individuals, 79% (57/72) of black individuals, and 97% (68/70) of Japanese-descent.15 Therefore, further validation is needed to determine whether the difference in the incidence of t(14;18)-positive cells in peripheral blood explains the different incidences of manifested FL across international populations.
Although t(14;18) constitutes the primary lymphomagenesis of FL, it is unclear whether healthy individuals harboring t(14;18)-positive cells have a higher risk of subsequent development of overt FL. Roulland et al. investigated t(14;18) in prediagnostic blood samples of healthy participants who developed FL after enrollment and those who did not (controls).5 Notably the incidence of t(14;18) was significantly higher in FL participants (61.8%, 102/165) than in controls (33.0%, 115/346). In addition, the t(14;18) frequency—that is, the relative number of t(14;18)-positive cells in each individual—was significantly higher in participants who developed FL (median, 1.8 × 10-5, range 0.1-2130 × 10-5) than in controls (median, 0.2 × 10-5, range 0.1-45.6 × 10-5). There was demonstrated to be a 23-fold higher risk of subsequent FL in blood samples associated with a t(14;18) frequency > 10-4 (odds ratio, 23.17; 95% confidence interval, 9.98 to 67.3).5 These risk estimates remained high and significant up to 15 years before diagnosis.5 Collectively, although t(14;18)-positive cells are uncommonly detected in otherwise healthy individuals, especially in older individuals, high t(14;18) frequency seems to be a predictive biomarker for subsequent FL, effective years before diagnosis.
It has not been fully understood how t(14;18)-positive cells develop into early or precursory FL lesions. A possible key factor determining the development of FL includes the affinity of t(14;18)-positive cells to GC, as an Eμ-BCL2 mice model indicated that deregulated BCL2-expressing B cells recirculate as memory B cells and accumulate genetic abnormalities while they repeatedly pass through GCs.17
in situ FOLLICULAR B-CELL NEOPLASM (ISFN)
Definition and terminology
The 5th World Health Organization (WHO) classification defines ISFN as partial or complete colonization of some GCs by follicular B cells with IGH::BCL2 and strong BCL2 expression in otherwise normal reactive LNs or lymphoid tissues at extranodal sites.6,18 ISFN was first described as follicular lymphoma in situ (FLIS) by Cong et al. in 2002 and was subsequently defined more strictly by Jegalian et al. in 2011.19,20 This entity was included as FLIS in the 4th WHO classification; however, given the limited clinical and prognostic impact, the term “neoplasia” rather than “lymphoma” was preferred.21 Therefore, the revised 4th WHO classification defined in situ follicular neoplasia. Now, the 5th WHO classification slightly modified the terminology to in situ follicular “B-cell neoplasm” (ISFN).22 The current WHO classification also notes that “in situ follicular neoplasia” is still acceptable, but that FLIS is not recommended.
Clinical features
Similar to overt FL, ISFN usually affects middle-aged and older individuals, with a mean age in the 40s.6,18 Patients younger than 40 years old are rare, although an exceptional ISFN case of a 24-year-old man with a history of Crohn’s disease and autoimmune hepatitis has been reported.23 Isolated ISFN (i.e., the pure form of ISFN without any overt lymphoma) is usually found incidentally in LNs resected for another reason or because of enlargement.18 In a small number of cases, ISFN may be seen in GCs of secondary lymphoid follicles of extranodal organs. ISFN can involve more than one lymph node without progression to overt lymphoma; therefore, multiple lesions do not exclude the diagnosis of ISFN.24,25 Individuals with isolated ISFN are usually asymptomatic and have excellent outcomes without developing overt FL, requiring no anti-lymphoma therapy.24–26 If the lesion is precisely differentiated from FL, the progression of isolated ISFN is very low and at most 5%.
In contrast, some ISFN lesions are associated with concurrent or subsequent overt FL.27,28 Cases of ISFN associated with non-FL lymphoma have also been reported.27 Clonally-related ISFN and diffuse large B-cell lymphoma (DLBCL) lacking clinically manifested FL as an intermediate step have also been described.27
Epidemiology
Because ISFN is most often an incidental finding in pathological examinations for other medical conditions, such as LN dissection for solid tumors, the exact incidence of ISFN is difficult to estimate. Four studies have investigated the incidence of isolated ISFN.24,25,29,30 These studies examined LNs from individuals who underwent surgery, including lymphadenectomy for solid tumors or inflammatory conditions, using immunohistochemistry (Table 1). The reported incidence of isolated ISFN ranged from 2.0% (2 of 100) to 3.2% (11 of 341).24,25,29,30 In Japan, a screening study using the BCL2 immunohistochemistry identified the incidence of ISFN to be 2.1% (7 of 340).24 One caveat is that these screening studies mostly investigated LNs of relatively older individuals who underwent surgical treatment for solid cancers. Because B cells with t(14;18) are more prevalent in older individuals, the reported incidence of ISFN in otherwise healthy individuals could be potentially biased and may be higher than the actual incidence.
Histopathology
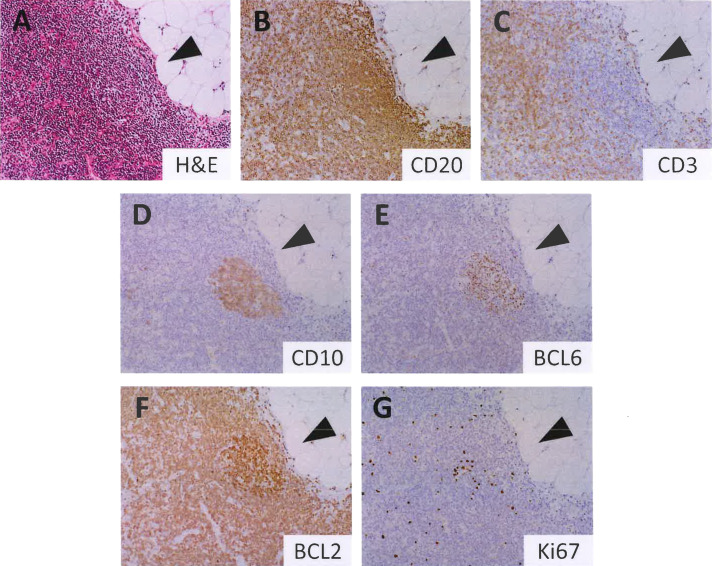
ISFN is usually not detected by routine hematoxylin and eosin staining, and immunohistochemistry is required for its detection. The LN or extranodal lymphoid tissue involved by ISFN appears otherwise normal or reactive, exhibiting lymphoid hyperplasia with secondary lymphoid follicles.6,18,31 The GCs with ISFN involvement may appear somewhat monotonous, lacking the tingible body macrophages and polarization seen in normal GCs (Figure 1); however, it is challenging to confirm the presence of ISFN without immunohistochemistry. Therefore, immunohistochemistry (especially for CD10 and BCL2) is essential for confirming ISFN.
Fig. 1.
Histopathology of isolated in situ follicular B-cell neoplasm (ISFN). Hematoxylin and eosin staining showing a lymphoid follicle with a monotonous germinal center (GC) (A). GC is involved by ISFN, in which neoplastic B cells are CD20+ (B), CD3- (C), CD10+ (D), BCL6+ (E), and BCL2+ (F). The ISFN lesion exhibits more intense BCL2 staining than the background B and T cells. The Ki67-labeling index is low (G).
The immunophenotype of ISFN is basically identical to that of classic FL (Figure 1). Neoplastic cells are positive for pan-B cell markers (i.e., CD19, CD20, CD79a, and PAX5) and GC B cell markers such as CD10 and BCL6. As t(14;18) generates IGH::BCL2 leading to the transcriptional upregulation of BCL2, neoplastic ISFN cells express the BCL2 protein.18,29 This gene rearrangement can be confirmed at the DNA level by fluorescence in situ hybridization (FISH). Immunohistochemical analysis reveals that ISFN cells exhibit more intense BCL2 positivity than other BCL2-positive non-GC cells, and even classic FL with t(14;18).24,29 This intense expression of BCL2 is a diagnostic hallmark of ISFN. The Ki67 labeling index is typically low. Neoplastic GCs contain a meshwork of follicular dendritic cells (FDCs) in the background, which is best highlighted by immunohistochemical staining for CD21 and/or CD23. Notably, the population of neoplastic ISFN cells varies by GCs: some GCs contain confluent BCL2-positive GC B cells, while others have scattered B cells strongly expressing BCL2 (Figure 2 and Figure 3).
Fig. 2.
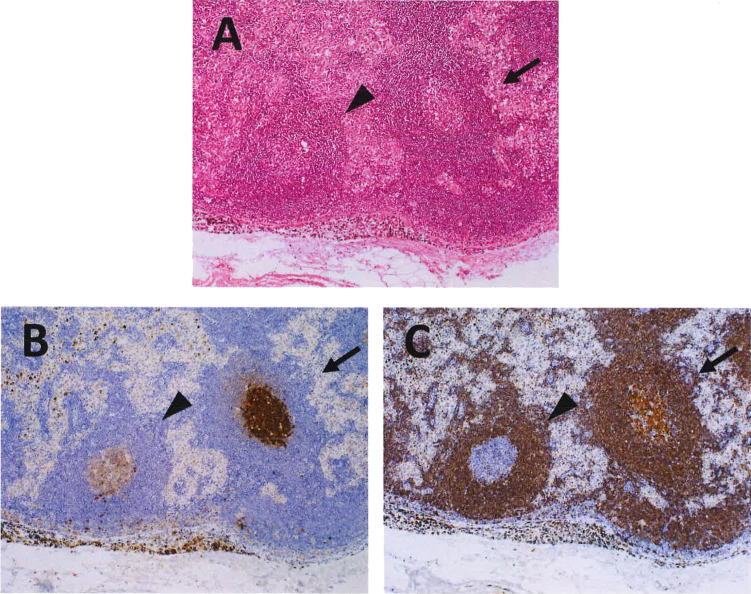
ISFN and a reactive GC. This example shows an ISFN (right, arrow) situated adjacent to a reactive GC (left, arrowhead) (A). ISFN exhibits co-expression of CD10 and BCL2.
Fig. 3.
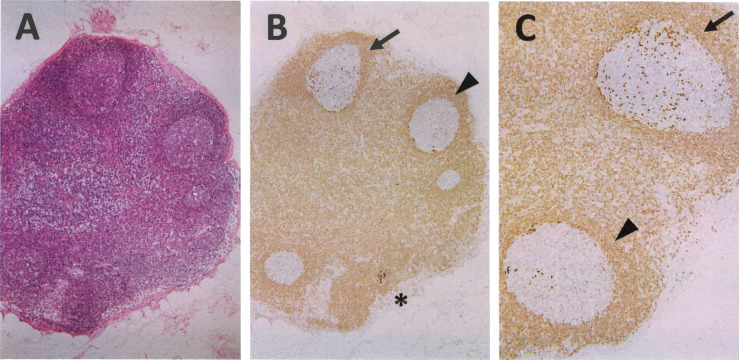
GCs variably involved by ISFN. (A) Hematoxylin and eosin staining demonstrates reactive-looking GCs. (B and C) Immunohistochemistry for BCL2 highlights a clear ISFN lesion (*) and GCs with scattered ISFN cells strongly expressing BCL2 (arrow and arrowhead). Note that the number of BCL2-positive cells in GCs are variable as indicated by the arrow and arrowhead.
An important difference in the immunophenotype of ISFN and classic FL is the expression of activation-induced cytidine deaminase (AID). AID expression is a hallmark of GC reactions and at least a subset of classic FLs express AID. In contrast, Goyal et al. demonstrated that ISFN is negative for AID.32 A Lack of AID expression was also noted in duodenal-type FL,33 an extremely indolent form of FL. As additional genetic abnormalities in FL cells are presumably triggered by AID, resulting in genomic instability, it is interesting that both low-risk FL variants, ISFN and duodenal-type FL, lack AID expression.
Differential diagnosis
Pure ISFN has limited clinical significance with very low risk to transform into overt FL. Therefore, in routine clinical practice, it is unnecessary to perform immunohistochemical screening for BCL2 (± CD10) in every reactive-looking LN for the purpose of excluding ISFN. Nonetheless, ISFN should be precisely differentiated from reactive follicular hyperplasia, FL, and partial involvement by FL (PFL). The distinction between reactive follicular hyperplasia and ISFN is usually straightforward, as GCs in reactive secondary lymphoid follicles are positive for CD10 and BCL6 but negative for BCL2. Primary lymphoid follicles consist of BCL2-positive B lymphocytes, potentially mimicking ISFN; however, B cells in primary lymphoid follicles are negative for GC markers such as CD10 and BCL6.
Differentiating FL from ISFN is clinically important. In classic FL, normal LN architecture is effaced by nodular/follicular or partially diffuse proliferation of neoplastic B cells co-expressing CD10 and BCL2. The neoplastic follicles in FL show so-called “back-to-back” arrangement, effacing normal structures of LNs. In contrast, ISFN preserved the follicular architecture of LNs with intact interfollicular areas and the medulla, mimicking reactive follicular hyperplasia.
The distinction between PFL and ISFN can be more challenging and is critically important because the former has a higher risk of progression to clinically significant FL.20 An earlier study reported that approximately half of all patients with untreated PFL developed overt FL in a 14-year follow-up period. PFL is histopathologically distinct from ISFN in that it exhibits focal architectural alterations in the lymphoid follicles. Neoplastic follicles of FL are often larger than those of ISFN, and their margins may be ill-defined with attenuated mantle zones. Neoplastic GCs in the PFL are typically monotonous and consist mostly of centrocytes, although centroblasts are occasionally present. In contrast to ISFN, in which CD10 and BCL2 are strongly expressed, PFL exhibits more variable and often less intense CD10 and BCL2 expression. In addition, neoplastic B cells may show interfollicular distribution in the PFL. Genetically, an array comparative genomic hybridization (aCGH) study identified significantly more genomic alterations per sample in PFL than in ISFN.34 Thus, given these clinical, pathological, and genetic data, partial involvement of FL is at a stage closer to overt FL than ISFN.
Molecular Pathogenesis
Since the pathogenesis of FL is a multi-step process with a number of genetic abnormalities other than t(14;18),4 it is crucial to understand the genetic features of ISFN, the histologically recognizable first step of FL lymphomagenesis. Genetic analysis of ISFN is technically challenging because neoplastic t(14;18)-positive cells are confined to GCs, requiring microdissection to obtain sufficient DNA from neoplastic cells.
Previous studies have investigated copy number alterations (CNAs) in ISFN using aCGH. According to Schmidt et al., CNAs were rare in ISFN and, when present, ISFNs had fewer CNAs per sample than overt FL.34 CNAs in paired samples of ISFN and overt FL were also studied. ISFN components showed no CNAs in five of the six cases. In contrast, Mamessier et al. examined CNAs in ISFNs (n=4) and found that all had chromosomal imbalances, although the number and length of CNAs in ISFN were smaller than those in overt FL.35 Hence, both studies indicated that ISFN shows less genomic complexity than overt FL and may represent an earlier lesion in the clonal evolution pathway of these lesions.
Some studies have investigated gene alterations in ISFN using next-generation sequencing (NGS). A Japanese study examined gene mutations in isolated ISFN cases (n=5) and found that ISFN had a few variants in FL-associated genes with varying but relatively low variant allele frequencies (VAFs). While variants in HIST1H1E (2/5), KMT2D (1/5), ARID1A (2/5), BCL7A (1/5), CREBBP (1/5), and TNFRSF14 (1/5) were identified, no isolated ISFNs harbored EZH2 Y646, one of the most frequently affected genes in overt FL.24 In contrast, Schmidt et al. performed targeted sequencing on ISFNs paired with overt FL (n=6) and isolated ISFNs (n=5). They found frequent CREBBP mutations in ISFNs paired with overt FL (83%, 5/6), but only in one of five cases with isolated ISFN (20%, 1/5).28 This finding suggests that CREBBP mutations are possibly early driver mutations that facilitate the acquisition of additional mutations and clonal evolution. These mutation profiling studies also imply that the molecular heterogeneity of isolated ISFNs, pure ISFNs, and ISFNs found in association with overt FL could be genetically distinct.
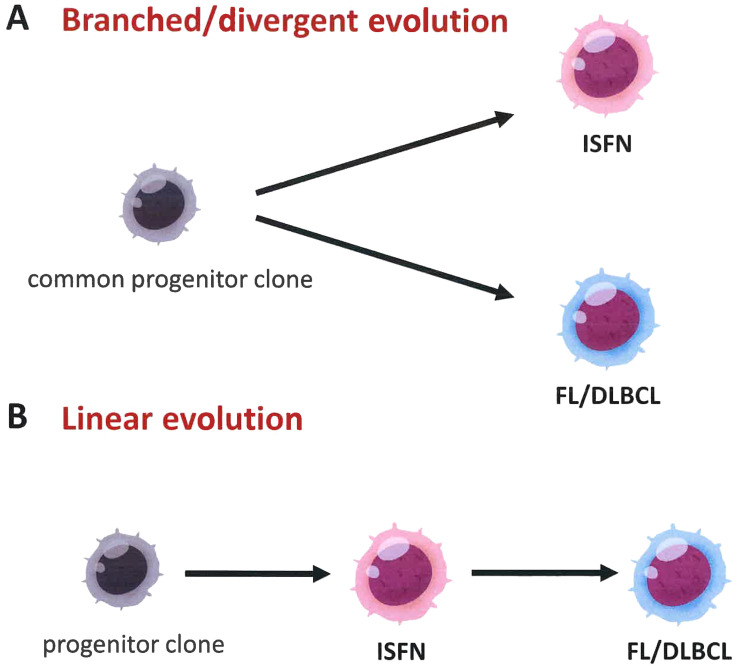
The clonal association between ISFN and associated aggressive B-cell lymphoma was investigated by Vogelsberg et al.27 They collected ten paired cases of ISFN and DLBCL or high-grade B-cell lymphoma (HGBCL), and investigated their clonal evolution using microdissection and NGS. As expected, all ten patients exhibited a clonal relationship between ISFN and aggressive B-cell lymphoma, which was confirmed by immunoglobulin and/or BCL2 rearrangements and/or shared somatic mutations. Consistent with the results of their previous study, CREBBP, KMT2D, EZH2, TNFRSF14, and BCL2 mutations were detected in ISFN. Notably, two clonal evolutionary patterns were observed (Figure 4). Eighty percent (8/10) of cases showed “branched” evolution, in which aggressive B-cell lymphoma, ISFN, and, when present, FL showed divergent evolution from a common progenitor. In contrast, a small number of cases (20%, 2/10) showed “linear” evolution with sequential stratification of mutations.
Fig. 4.
Two progression models for the generation of ISFN and overt B-cell lymphomas with a GC B cell phenotype. (A) In the branched or divergent evolution model, each ISFN and follicular lymphoma (FL) or DLBCL arises divergently from a shared progenitor clone harboring t(14;18). (B) In contrast, in the linear model, FL/DLBCL evolves directly from ISFN acquiring additional gene alterations.
CONCLUSIVE REMARKS
Although early or precursory lesions of FL, including t(14;18)-positive cells in peripheral blood and ISFNs, have limited clinical significance when identified, careful pathologic and molecular studies offer meaningful insights into the lymphomagenesis and progression of FL. In clinical practice, it is essential for both pathologists and clinicians to recognize the clinical relevance of these early lesions and share information concerning disease distribution in patients.
Footnotes
CONFLICT OF INTEREST
The author has no conflicts of interest directly relevant to the content of this article.
REFERENCES
- 1.Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in J apan and the United States. Br J Haematol. 2014; 164: 536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takata K, Miyata-Takata T, Sato Y, Yoshino T. Pathology of follicular lymphoma. J Clin Exp Hematop. 2014; 54: 3-9. [DOI] [PubMed] [Google Scholar]
- 3.Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest. 2012; 122: 3424-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huet S, Sujobert P, Salles G. From genetics to the clinic: a translational perspective on follicular lymphoma. Nat Rev Cancer. 2018; 18: 224-239. [DOI] [PubMed] [Google Scholar]
- 5.Roulland S, Kelly RS, Morgado E, et al. t(14;18) Translocation: A predictive blood biomarker for follicular lymphoma. J Clin Oncol. 2014; 32: 1347-1355. [DOI] [PubMed] [Google Scholar]
- 6.Ganapathi KA, Pittaluga S, Odejide OO, Freedman AS, Jaffe ES. Early lymphoid lesions: conceptual, diagnostic and clinical challenges. Haematologica. 2014; 99: 1421-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujimoto Y, Gorham J, Cossman J, Jaffe E, Croce CM. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985; 229: 1390-1393. [DOI] [PubMed] [Google Scholar]
- 8.Summers KE, Goff LK, Wilson AG, et al. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001; 19: 420-424. [DOI] [PubMed] [Google Scholar]
- 9.Dölken G, Dölken L, Hirt C, et al. Age-dependent prevalence and frequency of circulating t(14;18)-positive cells in the peripheral blood of healthy individuals. J Natl Cancer Inst Monogr. 2008; 2008: 44-47. [DOI] [PubMed] [Google Scholar]
- 10.Nambiar M, Raghavan SC. Prevalence and analysis of t(14;18) and t(11;14) chromosomal translocations in healthy Indian population. Ann Hematol. 2010; 89: 35-43. [DOI] [PubMed] [Google Scholar]
- 11.Yasukawa M, Bando S, Dölken G, et al. Low frequency of BCL-2/J(H) translocation in peripheral blood lymphocytes of healthy Japanese individuals. Blood. 2001; 98: 486-488. [DOI] [PubMed] [Google Scholar]
- 12.Hirt C, Weitmann K, Schüler F, et al. Circulating t(14;18)-positive cells in healthy individuals: association with age and sex but not with smoking. Leuk Lymphoma. 2013; 54: 2678-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitt C, Balogh B, Grundt A, et al. The bcl-2/IgH rearrangement in a population of 204 healthy individuals: Occurrence, age and gender distribution, breakpoints, and detection method validity. Leuk Res. 2006; 30: 745-750. [DOI] [PubMed] [Google Scholar]
- 14.Colon-Otero G, Van Wier SA, Ahmann GJ, et al. Prevalence of BCL-2/J(H) translocation in healthy African Americans. Ann Hematol. 2017; 96: 51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy D, Bertoldi ERM, Ruiz JLM, Pereira J, Bydlowski SP. Presence of t(14;18) translocation in healthy individuals varies according to ethnic background in the Brazilian population. Braz J Med Biol Res. 2017; 50: e6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirt C, Dölken G, Janz S, Rabkin CS. Distribution of t(14;18)-positive, putative lymphoma precursor cells among B-cell subsets in healthy individuals. Br J Haematol. 2007; 138: 349-353. [DOI] [PubMed] [Google Scholar]
- 17.Sakai T, Nishikori M, Tashima M, et al. Distinctive cell properties of B cells carrying the BCL2 translocation and their potential roles in the development of lymphoma of germinal center type. Cancer Sci. 2009; 100: 2361-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oishi N, Montes-Moreno S, Feldman AL. In situ neoplasia in lymph node pathology. Semin Diagn Pathol. 2018; 35: 76-83. [DOI] [PubMed] [Google Scholar]
- 19.Cong P, Raffeld M, Teruya-Feldstein J, et al. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002; 99: 3376-3382. [DOI] [PubMed] [Google Scholar]
- 20.Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011; 118: 2976-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022; 36: 1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobson R, Venkatraman L, Cucco F, et al. In situ follicular neoplasia in a young post‐liver transplant patient. Pathol Int. 2023; 73: 58-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oishi N, Segawa T, Miyake K, Mochizuki K, Kondo T. Incidence, clinicopathological features and genetics of in‐situ follicular neoplasia: a comprehensive screening study in a Japanese cohort. Histopathology. 2022; 80: 820-826. [DOI] [PubMed] [Google Scholar]
- 25.Bermudez G, González de Villambrosía S, Martínez-López A, et al. Incidental and isolated follicular lymphoma in situ and mantle cell lymphoma in situ lack clinical significance. Am J Surg Pathol. 2016; 40: 943-949. [DOI] [PubMed] [Google Scholar]
- 26.Pillai RK, Surti U, Swerdlow SH. Follicular lymphoma-like B cells of uncertain significance (in situ follicular lymphoma) may infrequently progress, but precedes follicular lymphoma, is associated with other overt lymphomas and mimics follicular lymphoma in flow cytometric studies. Haematologica. 2013; 98: 1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelsberg A, Steinhilber J, Mankel B, et al. Genetic evolution of in situ follicular neoplasia to aggressive B-cell lymphoma of germinal center subtype. Haematologica. 2021; 106: 2673-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt J, Ramis-Zaldivar JE, Bonzheim I, et al. CREBBP gene mutations are frequently detected in in situ follicular neoplasia. Blood. 2018; 132: 2687-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henopp T, Quintanilla-Martínez L, Fend F, Adam P. Prevalence of follicular lymphoma in situ in consecutively analysed reactive lymph nodes. Histopathology. 2011; 59: 139-142. [DOI] [PubMed] [Google Scholar]
- 30.Carvajal-Cuenca A, Sua LF, Silva NM, et al. In situ mantle cell lymphoma: clinical implications of an incidental finding with indolent clinical behavior. Haematologica. 2012; 97: 270-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mamessier E, Broussais-Guillaumot F, Chetaille B, et al. Nature and importance of follicular lymphoma precursors. Haematologica. 2014; 99: 802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal T, Ondrejka SL, Bodo J, Durkin L, Hsi ED. Lack of activation-induced cytidine deaminase expression in in situ follicular neoplasia. Haematologica. 2021; 106: 1212-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takata K, Sato Y, Nakamura N, et al. Duodenal follicular lymphoma lacks AID but expresses BACH2 and has memory B-cell characteristics. Mod Pathol. 2013; 26: 22-31. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt J, Salaverria I, Haake A, et al. Increasing genomic and epigenomic complexity in the clonal evolution from in situ to manifest t(14;18)-positive follicular lymphoma. Leukemia. 2014; 28: 1103-1112. [DOI] [PubMed] [Google Scholar]
- 35.Mamessier E, Song JY, Eberle FC, et al. Early lesions of follicular lymphoma: a genetic perspective. Haematologica. 2014; 99: 481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]