Compared with other embolic agents, particles offer distinct attributes for a broad range of clinical applications, including treatment of vascular tumors to achieving hemostasis in life-threatening emergencies. 1 Microparticle embolization is often the agent of choice when smaller, more distal vessels are targeted for occlusion. Due to their varied attributes, including size, shapes (i.e., microspheres), and material composition, particles can provide excellent versatility in embolization procedures. The aim of this article is to provide a back-to-the-basics overview of particle embolization. Here, we discuss the various microparticle types, their respective advantages and disadvantages, as well as technical considerations when using microparticles for embolization.
History of Microparticles
The first-generation of microparticles, polyvinyl alcohol (PVA), were invented by Chuck Kerber in the 1970s as embolic agents with thrombogenic properties. 2 The second generation involved more spherical PVA with compressible properties. 3 The following generation focused on microspheres composed of a hydrogel core, allowing more consistent size calibration when immersed in saline or contrast. Over the 21st century, there have been considerable efforts in the development of degradable microspheres for embolization. 4
General Particle Technology
Microparticles are flow-directed agents delivered via microcatheters to provide temporary or permanent embolization through targeted occlusion of blood vessels ( Fig. 1 ). This is achieved by (1) creating a mechanical occlusion of the vessels, (2) providing a framework for thrombus formation, and (3) inciting a foreign body and inflammatory response. The timeframe for vascular occlusion is dependent on the type of embolic agent, size of target vessel, and flow dynamics. 1 5
Fig. 1.
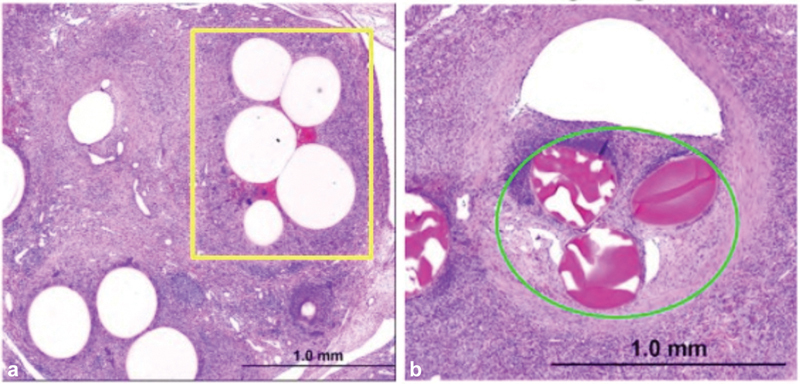
Microparticle histology within porcine interlobular arteries of the lower poles of the left and right kidneys. ( a ) Polyethylene glycol microspheres (yellow box) show dense luminal packing due to tighter size distribution (Terumo Interventional Systems, California). ( b ) Trisacryl gelatin microspheres (green circle) with diffuse packing. (Images used with permission from Terumo Interventional Systems.)
Patient Selection
Microparticles aim to provide complete mechanical occlusion of vessels while working complementary to a patient's innate coagulation cascade. As a result, microparticles, like other embolic devices, are most effective in patients with intact coagulation cascades. Careful consideration and planning must be met when utilizing microparticles in patients with coagulopathic concerns, such as those with platelet dysfunction, severe thrombocytopenia, or clotting cascade impairments. 6 For these coagulopathic patients, tightly calibrated microparticles, with minimal reliance on clotting cascade, are a better choice. When determining which embolic agent to select for the patient, it is important to consider target vessel size and tissue viability. Smaller particles are more appropriate when the goal is tumor embolization or tissue death. 7
Materials
Commercially available microparticles have various designs, each possessing distinctive advantages for peripheral embolization. One of the first non-absorbable microparticles was composed of PVA. 8 9 PVA is derived from a vacuum-dried sheet of its own synthetic polymer subsequently filtered through sieves based on size. 9 PVA's high compressibility and irregular composition ultimately leads to vessel adherence and particle aggregation ( Table 1 ). These properties allow for more efficient occlusion of blood vessels with a smaller volume of material when compared with microspheres. 10
Table 1. Advantages, disadvantages, and cost of main microparticle components.
| Advantages | Disadvantages | Cost | Market examples | |
|---|---|---|---|---|
| Permanent particles | ||||
| Polyvinyl alcohol | Dry form can be mixed with 100% contrast. Less embolic needed. Inexpensive. | Size variability could impact the level of vessel occlusion, microcatheter occlusion, or particle aggregation. | $ | Contour 1 , Bearing 2 , Beadblock 1 |
| Trisacryl with gelatin | Ease of injection. Reduced aggregation. More predictable level of occlusion. Less clogging of microcatheters | Need intermittent stirring to prevent sedimentation. Porcine gelatin allergic potential. | $$ | Embosphere 2 , EmboGold 2 |
| Polyethylene glycol | Greater compressibility for more distal occlusion. Tightly calibrated size distribution | Risk of clogging microcatheter with larger size | $$ | HydroPearl 3 |
| Resorbable particles | ||||
| Gelatin sponge | Complete resorption of embolic while maintaining vessel integrity. | Inflammation that leads to long-term occlusion of target vessels. | $ | Gelfoam 4 , Torpedo Gelatin Foam 2 , Embocube 2 |
| Drug-eluting particles | ||||
| Sulfonate-modified acrylamido-polyvinyl alcohol hydrogel | Compatible with chemotherapy (Doxorubicin, Irinotecan). Real-time feedback both during and after embolization. | Shorter suspension time due to increased density and stiffness. Potential for microcatheter blockage | $$$ | DC Bead 1 |
| Sodium acrylate alcohol copolymer | Expand four times original size | Prone to fragmentation after drug-loading | $$ | Quadrasphere 2 |
| Hydrogel core with Polyzene-F coating | Precise calibration of particles and narrow size distributions allows for deeper penetration | Variable deformation may result in more distal embolization | $$ | Embozene 5 , Oncozene 5 |
**Manufacturer details:
Boston Scientific; Washington, D.C., US
MeritMedical; Utah, US
Terumo Interventional Systems; California, US
Pfizer: New York; US
Varian; California, US
Tri-acryl gelatin microspheres are another commonly used particle material. These are precisely calibrated with cationic charge on the particle surface to create hydrophilic and non-aggregating properties. Owing to their biochemical properties, tris-acryl gelatin microspheres are easier to inject and possess a reduced risk of microcatheter clogging compared with PVA particles. However, a few downsides of tris-acryl gelatin microspheres include the continual need for agitation to prevent sedimentation, and the allergic potential it has for susceptible patients due to its porcine gelatin composition. 8
Gelatin sponge is an absorbable embolic agent derived from purified pork skin gelatin. It contains a highly porous structure which permits the absorption of surrounding fluid. Platelets are trapped within the porous structure of a gelatin sponge which in turn induces the clotting cascade. 8 Gelatin sponge can be cut into pledgets or made into a slurry for temporary embolization. 11 However, due to the heterogenicity in shape and size of gelatin sponge, calibrated gelatin-based microspheres were developed for more predictable embolization. 11 A similar temporary embolic agent composed of microfibrillar collagen works by activating platelet aggregation and the formation of fibrin to enhance hemostasis. 12
Drug-eluting beads (DEBs) are a newer generation of microspheres that have the capability to be loaded with chemotherapeutic agents to provide localized tumor treatment. The drug-loading capacity of these particles utilizes a negatively charged ionic group to bind to positively charged chemotherapy drugs ( Fig. 2 ). For example, acrylamide-PVA hydrogel microspheres utilize sulfonate binding groups on their surface for loading doxorubicin and irinotecan: two notable drugs frequently utilized in transarterial chemoembolization.
Fig. 2.
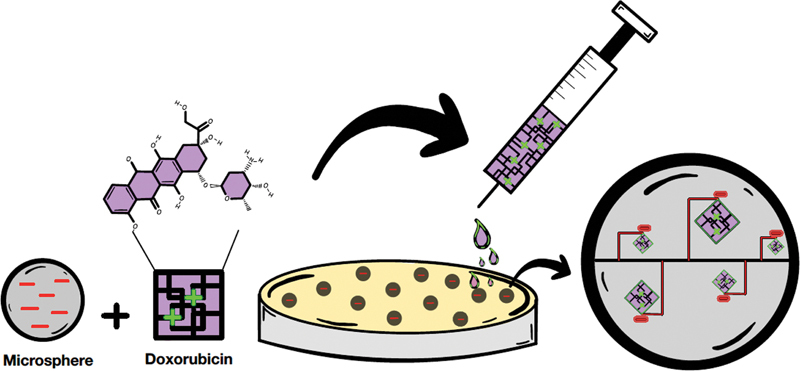
Loading mechanism of drug-eluting particles. Chemotherapy agents (i.e., doxorubicin) are loaded and eluted onto microspheres via a reversible ionic exchange mechanism. Here, the positively charged doxorubicin hydrochloride interacts with the negatively charge acrylate of a microsphere to achieve proper drug loading in a petri dish.
Shapes and Sizes
Microparticles are available in various shapes and sizes ( Fig. 3 ). Currently, microparticle diameters range from 50 to 1,200 μm; device packaging typically lists a defined minimum and maximum within this range. Microspheres on the higher end of the size spectrum typically necessitate higher compressibility to be deliverable through narrow lumen microcatheters. Common particle shapes include spherical, nonspherical, cuboidal, and torpedo designs. 5
Fig. 3.
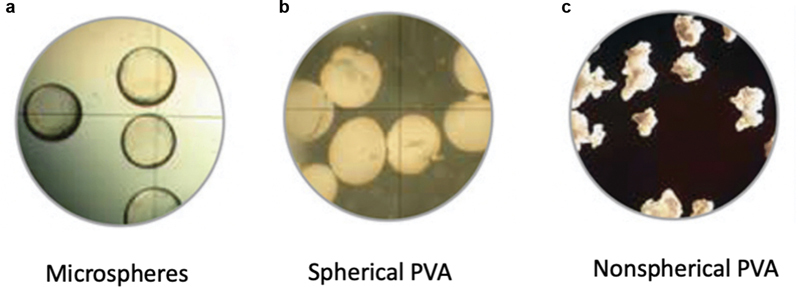
Common particle shapes. Microscopic representation of the shapes of traditional ( a ) microspheres, ( b ) spherical PVA particles, and ( c ) nonspherical PVA particles. (Images used; © Merit Medical, reprinted by permission.)
Modern microspheres, which have more controlled shape and size distributions, have fewer episodes of catheter occlusion and more predictable embolization. 7 Tightly calibrated microspheres of the same size, such as polyethylene glycol, allow for more homogeneous distal distribution of the embolic. 11 Narrow size distribution also allows for tighter luminal packing due to the higher surface contact between microspheres and the surrounding vessel walls. 11 As a result, these precisely calibrated spherical microparticles are associated with better-targeted devascularization and tissue necrosis. 5 In fact, increased calibration among microspheres has been shown to effectively embolize target vasculature without the need for additional thrombus formation. 13 This is potentially beneficial for patients with coagulopathic concerns due to decreased reliance on innate thrombolytic processes.
Microparticle Performance
The performance of microparticles is determined by their size range, tendency to aggregate, compressibility, and elasticity. These biomechanical properties dictate how microparticles will behave as they traverse through a microcatheter and within a blood vessel. Most microspheres are elastic particles that can undergo temporary compression.
Microparticle Aggregation
Traditional PVA particles occlude vasculature by adhering to vessel walls and clumping together to provide mechanical occlusion. Thrombogenic factors are released from this vascular adhesion, ultimately leading to fibrosis of the underlying vessel. The notable irregularity in the shape of PVA particles helps reduce the amount of material needed to achieve the desired vascular endpoint ( Fig. 4 ). However, this dramatic size variability has also been classically associated with increased risk for catheter clogging and more proximal vessel occlusion. 7 11
Fig. 4.

Common particle compositions. ( a ) Small and irregular flakes of Contour (Boston Scientific, Washington, DC) PVA particles (yellow arrow) are seen upon deployment through a microcatheter. ( b ) More uniform sizing and distribution is available in a permanent particle model, such as the EmboGold (Merit Medical, Utah) particles (green arrow). ( c ) Similarly, homogenous particle composition is also available in a resorbable model, such as the EmboCube (Merit Medical) particles (red circle). (Images used with permissions from Boston Scientific [Image provided courtesy of Boston Scientific.© 2022 Boston Scientific Corporation or its affiliates. All rights reserved.] and © Merit Medical, reprinted by permission.)
The development of precisely calibrated spherical particles helped remedy the above concerns with PVA by providing more homogeneity in particle embolization. This uniformity in shape contributed to microspheres demonstrating a decreased inflammatory response compared with regular PVA particles by relying more heavily on a sphere's space-occupying properties to achieve compact embolization. 14
Compressibility and Elasticity
A microsphere's compressibility and elasticity (or ability to recover to original size) determine how far distally it can travel. 11 Among particles, polyethylene glycol and hydrogel core microspheres exhibit high compressibility, which allows for more distal embolization at the arteriolar level. 9 13 Additionally, these two microsphere subtypes demonstrate high elastic recovery which permits shape retention following microcatheter exit. 9 Regarding drug-eluting particles, microspheres demonstrate decreased diameters upon loading with medication but a concurrent increase in elastic rebound to account for size differences. 11 15 16 Drug-eluting microspheres, along with PVA hydrogels with covalently bound radiopaque moieties, demonstrate significantly higher rigidity compared with other particles due to the drug or contrast agent attached to their scaffold. 5
Gelatin Sponge Reabsorption
Gelatin sponge is a widely used, inexpensive, resorbable (or temporary) embolic agent. Vessel occlusion typically lasts 3 to 6 weeks with preservation of normal vascular function. 8 Temporary embolization using a gelatin sponge is appropriate in time-sensitive scenarios such as posttraumatic bleeds or patients at high risk of bleeding. 9 17 Gelatin sponge come in a variety of shapes and sizes to fit clinical needs. For instance, the torpedo shape can be used for large vessel embolization or for scenarios necessitating more proximal occlusion. Often, a three-way stopcock will be utilized to generate a gelatin slurry with specific size range and density based on operator's discretion. 18 One notable drawback of gelatin sponges is their makeup of small fragments, which can contribute to increased inflammation and permanent occlusion through dense packing. 8 19
Drug-Eluting Capability
Drug-eluting beads provide a new avenue by which chemotherapy agents can be delivered to tumor cells with broad treatment applications from hepatocellular carcinoma to metastatic colon cancer. Upon occlusion of the target vessel, the microparticles provide sustained and local chemotherapy to tumor cells while mitigating the adverse systemic effects associated with traditional chemotherapy. 20
The sulfonate-modified acrylamide-PVA hydrogel and sodium acrylate alcohol copolymer have proven to be effective in providing a combined ischemic and local cytotoxic effect in transarterial embolization procedures. 20 21 Of note, sodium acrylate microspheres are super absorbent micropolymers that can absorb up to 64 times its dry weight volume. 19 22 Compared with other microparticles, the hydrogel core with Polyzene-F coating demonstrates the tightest size distribution among DEBs, with minimal size change upon drug-loading. 8 23 An added benefit of the hydrogel is that the small size distribution allows for deeper penetration with increased density of beads for improved tumor coverage. 24 Currently, some drawbacks of DEBs include potential for permanent occlusion, nontarget distal embolization upon drug loading, limited selection of loadable Food and Drug Administration (FDA)-approved drugs, and microcatheter occlusion. 20 22
General Technical Considerations
Preprocedural Planning, Catheter Selection, and Microparticle Release Strategies
As with any other intervention, achieving technical success with vascular embolization requires meticulous preprocedural planning. The interventional radiologist must have a thorough understanding of the biomechanical properties of each embolic microsphere, target vessel size, duration of occlusion (permanent or temporary), vascular shunts, and collateral circulation.
Pertinent information related to microsphere size range, minimum introducer sheath size, and guide catheter/microcatheter compatibility is provided by the manufacturer. The interventionalist must be cognizant of microparticle size and catheter compatibility to prevent procedural complications. For example, a 600-μm microsphere would be compatible with a 2.4- or 2.7-Fr microcatheter; however, catheter occlusion may occur if deployed through a 2.0-Fr microcatheter.
The selection of microparticle size should be based on target vessel diameter and end-goal of the procedure, whether that be bleeding control or tissue necrosis. Larger microparticles may be used to prevent acute bleeds in cases of bronchial artery embolization for recurrent hemoptysis. 7 Smaller microparticles (< 300 μm) may be preferred for more distal penetration of malignant tumors and for minimizing the risk of embolization of normal tissue. 7 25
Appropriate suspension of the microparticles is necessary to avoid aggregation and microcatheter occlusion. Certain microparticles, such as PVA, require agitation with a three-way stopcock to promote homogeneity and prevent catheter clogging. The suspension time is impacted by size, density, and viscosity of the microparticle in the suspended medium. Caution should also be exercised with the relative force of suspension, as fragmentation creates smaller microparticles, potentially leading to more distal embolization.
Flow Stability and Microparticle Reflux
An interventional radiologist must be keenly aware of vessel flow dynamics as microparticle deployment is dependent on blood flow and the relative resistance of vascular beds. Initially, injection should be constant as blood flow is stable. 1 3 Microparticles should be introduced into the delivery catheter under fluoroscopic visualization while observing the contrast flow rate. 1 Selective catheterization should be achieved by placing the microcatheter tip as close as possible to the target, through the specific branch/branches supplying it. 20 The operator must then ensure that the catheter position is stable, as deployment can lead to microcatheter recoil, particle reflux, and nontarget embolization. Novel tools such as antireflux microcatheters utilize a balloon positioned near the tip to ensure precise deployment. 26 If flow rate does not decrease after the initial delivery of particles, a common practice is to increase the size of the particulates. 13 As embolization proceeds, originally fast-flowing vessels will become increasingly stagnant; operators must adjust accordingly to avoid reflux of embolization agents into nontarget vessels. 1 20
The interventionalist should change to a slower and more careful injection if resistance in the vasculature is observed. Forceful injection to overcome vascular resistance may result in vessel damage or alter the distribution of particles. 3 In the event of vascular resistance, the system is cleaned out with saline and observed for several minutes prior to readmission of embolic agents. 11
The Microparticle Advantage and the Combination of Embolic Agents
The selection of an embolic agent (microparticles, liquid embolic, coils, and plugs) is based on size of target vessel, timing (temporary or permanent), and tissue viability. Under certain clinical scenarios, microparticles hold several advantages over other available embolic agents. As previously stated, flow dynamics influence microparticle delivery, which poses a unique advantage in tumor devascularization. For example, due to the hypervascularization in uterine fibroids, microparticles preferentially flow to the fibroids while relatively sparing normal myometrium thus allowing for preservation of fertility. 27 28 29 Smaller microparticles (< 300 μm) are indicated for more distal occlusion where tissue necrosis is desired. 7 The operator must be knowledgeable of the fact that the usage of smaller microparticles increases ischemic risk and injury to healthy tissue. 30 When larger vessels require occlusion, such as organs with collateral blood supply or pulmonary arteriovenous malformations, coils or Amplatzer vascular plugs may be a more appropriate choice. 31 32
In many scenarios, it is beneficial for a combination of embolic agents to be used. For instance, in the pelvis and liver where persistent bleeding may occur despite initial embolization due to extensive collateral circulation, a “sandwich technique” could be employed in which microparticles are used for distal embolization, followed by coils and plugs for more proximal occlusion. 7 17 33 For coagulopathic patients, coils could be used as scaffold followed by gelatin sponge for mechanical occlusion. 31
Complications of Microparticle Occlusion
The most common complications of microparticle embolization include target vessel recanalization, nontarget embolization, and hypersensitivity reactions ( Figs. 5 and 6 ). Inflammation can progress to vessel remodeling, ultimately resulting in thrombosis owing to the ingrowth of connective tissue between the particles. 3 Vessel perforation is not common, and the risk can be mitigated with controlled injections. When microparticles are injected too rapidly, they may aggregate leading to a false endpoint. 28 Nontarget embolization is often due to substandard angiographic mapping, deployment technique, or patient anatomy. These complications may lead to a variety of clinical sequelae, including organ ischemia, stroke, pulmonary embolism, and even death.
Fig. 5.
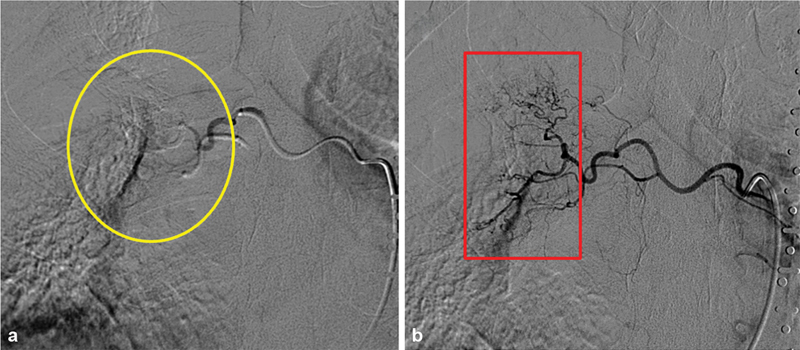
Recanalization in bronchial artery embolization with microspheres. ( a ) Immediate postprocedural angiogram demonstrates relatively proximal vessel occlusion (yellow circle). Distal irregular vasculature is no longer identified. ( b ) Repeat bronchial angiogram a few days later demonstrates recanalization (red box) and recurrent hemorrhage, likely secondary to proximal particle clumping on the initial intervention leading to a false endpoint.
Fig. 6.
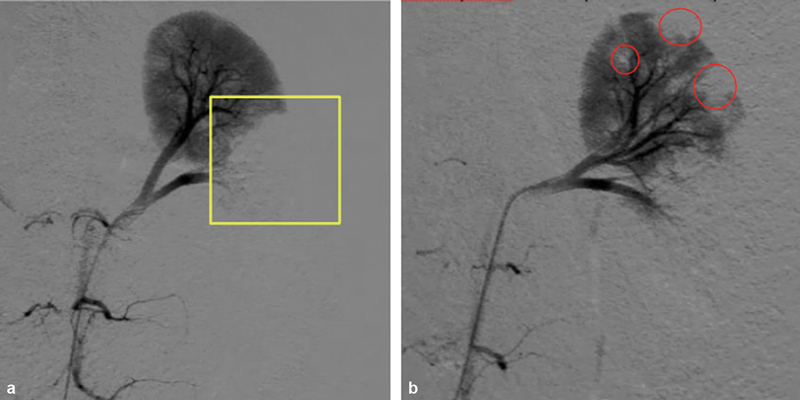
Target versus nontarget embolization of porcine kidney. ( a ) Postprocedural angiogram with polyethylene glycol microspheres (Terumo Interventional Systems, California) demonstrates target occlusion of the lower pole of kidney (yellow box). ( b ) In comparison, trisacryl gelatin microspheres (TAGM) displayed more nontarget embolization (red circles) into the upper pole of kidney. Here, this is most attributable to the tighter spectrum of luminal packing density and size distribution exhibited by polyethylene microspheres compared with TAGM microspheres. (Images used with permission from Terumo Interventional Systems.)
The “Perfect” Microparticle and Future Applications
The ideal microparticle should be cost efficient, require minimal preparation, and provide precise occlusion of the target vessel. Moreover, this microparticle should lead to complete occlusion of the target vessel without requiring an additional embolization agent. Biodegradability might also be a useful feature to minimize inflammation and maximize particle implant tolerability within vasculature. Additionally, if novel particles could be easily identified on imaging (fluoroscopy, follow-up cross-sectional imaging), this would offer a distinct advantage in assessing successful embolization as well as any nontarget embolization. 13 One modern microparticle that confers this advantage is the LC Bead LUMI, which is composed of PVA hydrogel that is covalently bound to a radiopaque moiety. It can be visualized using conventional imaging techniques to provide physicians with intra-procedural and postprocedural microsphere location ( Fig. 7 ). However, additional studies are being conducted to design a particle that is intrinsically radiopaque. 34 35 It is worth mentioning that a potential drawback of imageable particles is obscuration of future imaging findings, unless the imageability is reversible.
Fig. 7.
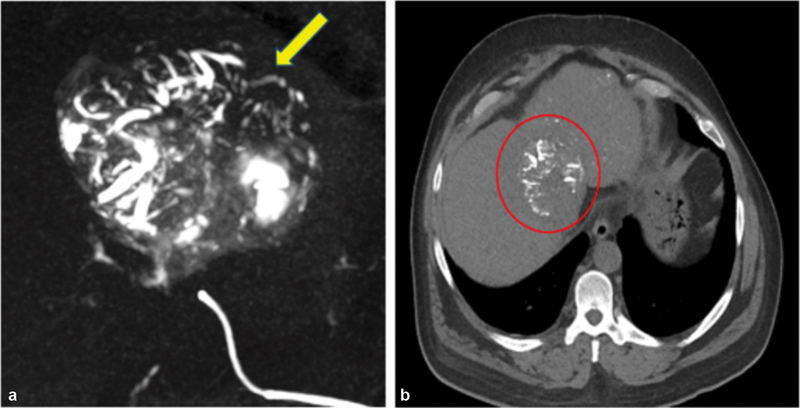
Radiopaque feature of particles. These newer age particles provide operators with real-time feedback both during and after procedures. ( a ) The above scan can showcase vessels being filled with LC Bead LUMI (Boston Scientific, Washington, DC) particles (yellow arrow) downstream of catheter placement during an HCC embolization procedure. ( b ) These same particles (red circle) are still able to be later visualized on a 48-hour postoperative scan. (Images used with permission from Boston Scientific. Image provided courtesy of Boston Scientific.© 2022 Boston Scientific Corporation or its affiliates. All rights reserved.)
Conclusion
In practice, microparticles have broad procedural application in a variety of clinical situations and are an important tool in the interventionalist's toolbox. Having a thorough understanding of the advantages and disadvantages of each microparticle is essential for optimizing clinical outcomes.
Acknowledgments
None.
Footnotes
Conflicts of Interest B.F.: Consultant, Okami Medical.
R.J.L: Consultant, BD, Boston Scientific, Varian.
References
- 1.Karim V. Saunders/Elsevier: Philadelphia; 2006. Vascular and Interventional Radiology; pp. 40–46. [Google Scholar]
- 2.Sheth R A, Sabir S, Krishnamurthy S et al. Endovascular embolization by transcatheter delivery of particles: past, present, and future. J Funct Biomater. 2017;8(02):12. doi: 10.3390/jfb8020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guimaraes M.Does Material Matter? Particulate EmbolicsEndovascular Today2013. 072013:4
- 4.Doucet J, Kiri L, O'Connell K et al. Advances in degradable embolic microspheres: a state of the art review. J Funct Biomater. 2018;9(01):14. doi: 10.3390/jfb9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilbao J I, de Luis E, García de Jalón J A et al. Comparative study of four different spherical embolic particles in an animal model: a morphologic and histologic evaluation. J Vasc Interv Radiol. 2008;19(11):1625–1638. doi: 10.1016/j.jvir.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Xiao N, Lewandowski R J. Embolic agents: coils. Semin Intervent Radiol. 2022;39(01):113–118. doi: 10.1055/s-0041-1740939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medsinge A, Zajko A, Orons P, Amesur N, Santos E. A case-based approach to common embolization agents used in vascular interventional radiology. AJR Am J Roentgenol. 2014;203(04):699–708. doi: 10.2214/AJR.14.12480. [DOI] [PubMed] [Google Scholar]
- 8.Abada H T, Golzarian J. Gelatine sponge particles: handling characteristics for endovascular use. Tech Vasc Interv Radiol. 2007;10(04):257–260. doi: 10.1053/j.tvir.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Poursaid A, Jensen M M, Huo E, Ghandehari H. Polymeric materials for embolic and chemoembolic applications. J Control Release. 2016;240:414–433. doi: 10.1016/j.jconrel.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spies J B, Allison S, Flick P et al. Polyvinyl alcohol particles and tris-acryl gelatin microspheres for uterine artery embolization for leiomyomas: results of a randomized comparative study. J Vasc Interv Radiol. 2004;15(08):793–800. doi: 10.1097/01.RVI.0000136982.42548.5D. [DOI] [PubMed] [Google Scholar]
- 11.Caine M, Carugo D, Zhang X, Hill M, Dreher M R, Lewis A L.Review of the development of methods for characterization of microspheres for use in embolotherapy: translating bench to CathlabAdv Healthc Mater 2017;6(09): [DOI] [PubMed]
- 12.Cziperle D J. Avitene™ microfibrillar collagen hemostat for adjunctive hemostasis in surgical procedures: a systematic literature review. Med Devices (Auckl) 2021;14:155–163. doi: 10.2147/MDER.S298207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patetta M A, Isaacson A J, Stewart J K. Initial experience with HydroPearl microspheres for uterine artery embolization for the treatment of symptomatic uterine fibroids. CVIR Endovasc. 2021;4(01):32. doi: 10.1186/s42155-021-00223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siskin G P, Dowling K, Virmani R, Jones R, Todd D. Pathologic evaluation of a spherical polyvinyl alcohol embolic agent in a porcine renal model. J Vasc Interv Radiol. 2003;14(01):89–98. doi: 10.1097/01.rvi.0000052296.26939.4c. [DOI] [PubMed] [Google Scholar]
- 15.Lewis A L, Dreher M R, O'Byrne V et al. DC BeadM1™: towards an optimal transcatheter hepatic tumour therapy. J Mater Sci Mater Med. 2016;27(01):13. doi: 10.1007/s10856-015-5629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis A L, Gonzalez M V, Leppard S W et al. Doxorubicin eluting beads - 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18(09):1691–1699. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- 17.Lopera J E. Embolization in trauma: principles and techniques. Semin Intervent Radiol. 2010;27(01):14–28. doi: 10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsumori T, Kasahara T. The size of gelatin sponge particles: differences with preparation method. Cardiovasc Intervent Radiol. 2006;29(06):1077–1083. doi: 10.1007/s00270-006-0059-y. [DOI] [PubMed] [Google Scholar]
- 19.Wang C Y, Hu J, Sheth R A, Oklu R. Emerging embolic agents in endovascular embolization: an overview. Prog Biomed Eng (Bristol) 2020;2(01):12003. doi: 10.1088/2516-1091/ab6c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencioni R, de Baere T, Burrel M et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35(05):980–985. doi: 10.1007/s00270-011-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan O, Denys A, De Baere T, Boulens N, Doelker E. Comparative study of chemoembolization loadable beads: in vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J Vasc Interv Radiol. 2010;21(07):1084–1090. doi: 10.1016/j.jvir.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 22.Liapi E, Lee K H, Georgiades C C, Hong K, Geschwind J F. Drug-eluting particles for interventional pharmacology. Tech Vasc Interv Radiol. 2007;10(04):261–269. doi: 10.1053/j.tvir.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Anderson M E, Kalva S P. Wolters Kluwer; 2014. Drug-eluting agents; p. 816. [Google Scholar]
- 24.Delicque J, Guiu B, Boulin M, Schwanz H, Piron L, Cassinotto C. Liver chemoembolization of hepatocellular carcinoma using TANDEM ® microspheres . Future Oncol. 2018;14(26):2761–2772. doi: 10.2217/fon-2018-0237. [DOI] [PubMed] [Google Scholar]
- 25.Lazzaro M A, Badruddin A, Zaidat O O, Darkhabani Z, Pandya D J, Lynch J R. Endovascular embolization of head and neck tumors. Front Neurol. 2011;2:64. doi: 10.3389/fneur.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borota L, Mahmoud E, Nyberg C, Lewén A, Enblad P, Ronne-Engström E. Dual lumen balloon catheter - an effective substitute for two single lumen catheters in treatment of vascular targets with challenging anatomy. J Clin Neurosci. 2018;51:91–99. doi: 10.1016/j.jocn.2018.01.070. [DOI] [PubMed] [Google Scholar]
- 27.Smith S J.Uterine fibroid embolization Am Fam Physician 200061123601–3607., 3611–3612 [PubMed] [Google Scholar]
- 28.Vo N J, Andrews R T. Uterine artery embolization: a safe and effective, minimally invasive, uterine-sparing treatment option for symptomatic fibroids. Semin Intervent Radiol. 2008;25(03):252–260. doi: 10.1055/s-0028-1085923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malone C D, Banerjee A, Alley M T, Vasanawala S S, Roberts A C, Hsiao A. Pelvic blood flow predicts fibroid volume and embolic required for uterine fibroid embolization: a pilot study with 4D flow MR angiography. AJR Am J Roentgenol. 2018;210(01):189–200. doi: 10.2214/AJR.17.18127. [DOI] [PubMed] [Google Scholar]
- 30.Lubarsky M, Ray C, Funaki B. Embolization agents-which one should be used when? Part 2: small-vessel embolization. Semin Intervent Radiol. 2010;27(01):99–104. doi: 10.1055/s-0030-1247891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubarsky M, Ray C E, Funaki B. Embolization agents-which one should be used when? Part 1: large-vessel embolization. Semin Intervent Radiol. 2009;26(04):352–357. doi: 10.1055/s-0029-1242206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A, Xiao N, Gordon A C, Funaki B, Lewandowski R J. Embolic agents: vascular plugs. Semin Intervent Radiol. 2022;39(05):526–532. doi: 10.1055/s-0042-1758112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz J, Sheth D, Patel J. Bronchial artery embolization. Semin Intervent Radiol. 2012;29(03):155–160. doi: 10.1055/s-0032-1326923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson C G, Tang Y, Beck A et al. Preparation of radiopaque drug-eluting beads for transcatheter chemoembolization. J Vasc Interv Radiol. 2016;27(01):117–126000. doi: 10.1016/j.jvir.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dreher M R, Sharma K V, Woods D L et al. Radiopaque drug-eluting beads for transcatheter embolotherapy: experimental study of drug penetration and coverage in swine. J Vasc Interv Radiol. 2012;23(02):257–640000. doi: 10.1016/j.jvir.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


