Abstract
Materials & methods:
We recently reported the largest trial of breast cancer patients with HER2 positive leptomeningeal metastases (LM) treated with trastuzumab. An additional treatment indication was explored as part of a single institution retrospective case series of HER2 positive esophageal adenocarcinoma LM (n = 2).
Results:
One patient received intrathecal trastuzumab (80 mg twice weekly) as part of their treatment regimen with durable long-term response and clearance of circulating tumor cells in the cerebral spinal fluid. The other patient demonstrated rapid progression and death as previously described in the literature.
Conclusion:
Intrathecal trastuzumab is a well-tolerated and reasonable therapeutic option worthy of further exploration for patients with HER2 positive esophageal carcinoma LM. An associative, but not a causal relationship, can be made regarding therapeutic intervention.
Keywords: circulating tumor cells, esophageal cancer, HER2, intrathecal, leptomeningeal metastases, trastuzumab
Plain language summary
Cancer of the esophagus, the tube that connects the mouth to the stomach, tends to be aggressive. Very rarely, this cancer can spread to the lining that surrounds your brain, called the leptomeninges. Previous reports of patients who have experienced this specific spreading pattern of esophageal cancer to the leptomeninges are quite grim, with patients experiencing rapid decline and death within weeks to months. However, we write with two cases of esophageal cancer with this leptomeningeal spreading pattern, one of which involves a patient treated with a medication known as trastuzumab. As part of his long and complex course of treatment, this patient was given trastuzumab through a tube traveling directly to the area of the leptomeninges. This patient, now almost 2 years out from his initial diagnosis, has responded well to the treatment. As such, we believe that this specific treatment regimen as well as the ways in which our clinical team tracked this patient’s response to medications are worth exploring further.
Tweetable abstract
This new case series explores the well-tolerated use of intrathecal trastuzumab in the treatment of HER2 positive esophageal adenocarcinoma with leptomeningeal metastases.
Esophageal cancer represents approximately 1% of all cancers diagnosed in the USA, with an estimated 20,640 new cases in the USA in 2022 [1]. Leptomeningeal metastases (LM) due to esophageal carcinoma remain extremely rare, with no epidemiological data and only case reports/series documenting its natural history, prognostic factors and treatment [2–22]. Overall, the prognosis of esophageal cancer is poor, with an overall 5-year survival rate of approximately 10–20% [23]. American Joint Committee on Cancer Stage IV esophageal cancers, which includes Stage IVB cancers that have spread to distant lymph nodes or organs, have a 5-year survival rate of just 5–10% [24,25]. The prognosis for LM due to esophageal carcinoma is exceptionally grim, with the only known systematic review of 30 patients with an accompanying case series exhibiting a maximum survival of just 7 months from time of diagnosis [22]. Given the low incidence of esophageal carcinoma LM and its associated rapid progression, no clear standard of care has been established and there are no recommendations for the therapeutic management of HER2+ esophageal carcinoma LM. This paper describes the presentation, progression and treatment courses of two patients with HER2+ esophageal carcinoma LM.
Case 1
A 60-year-old man initially presented with a 2 month history of dysphagia, weight loss, headaches and progressive cognitive decline marked by confusion and memory deficits. The patient underwent extensive infectious, inflammatory and neoplastic workup including cerebrospinal fluid (CSF) analysis (Table 1) and was found to have diffuse intracranial leptomeningeal enhancement on MRI (case 1 in Figure 1), as well as a thickened distal esophagus detected by computed tomography. Biopsy of the distal esophagus demonstrated poorly differentiated invasive adenocarcinoma with signet-ring cell features and diffuse membranous HER2 expression by immunohistochemistry (IHC) (Score 3+, Figure 2A & B). Biopsy of the leptomeninges showed poorly differentiated adenocarcinoma morphologically compatible with metastasis from the biopsied esophageal lesion and non specific cytoplasmic HER2 expression by IHC (Figure 2C & D).
Table 1. . Cerebrospinal fluid studies upon admission.
| CSF studies | ||
|---|---|---|
| Case 1 | Case 2 | |
| Color | Colorless | Colorless |
| Clarity | Clear | Clear |
| Cell count | ||
| Red blood cells | 18 | 429 |
| Nucleated cells | 25 | <1 |
| Differential | ||
| Neutrophils | – | 30% |
| Monocytes | 10% | 0% |
| Lymphocytes | 89% | 70% |
| Cytology | Negative for malignancy | Negative for malignancy |
| Flow cytometry | T cells and monocytes, small polytypic B-cell population | – |
Figure 1. . MRI brain and spine images.
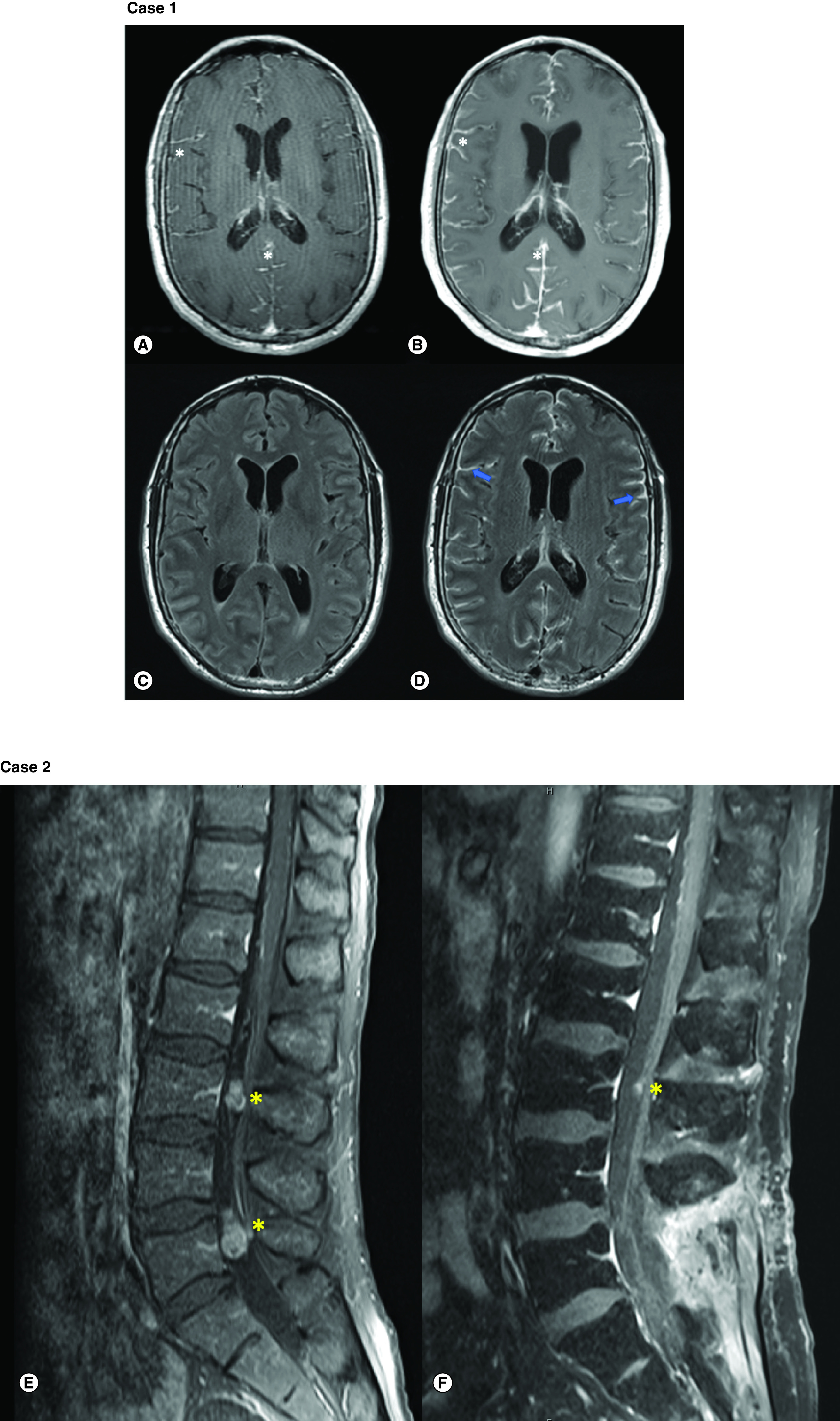
Case 1 MRI: MRI brain with and without contrast for patient 1. T1-CE sequences demonstrated rapid progression of leptomeningeal enhancement (white star) from symptom onset (A) to 11 days follow-up; (B) FLAIR sequence; (C) without sulcal hyperintensity but FLAIR-CE sequence; and (D) demonstrates significant enhancement in leptomeninges (blue arrows). Case 2 MRI: MRI spine with and without contrast for patient 2. Lumbar T1-CE sequence (E) demonstrates two enhancing metastases (yellow stars) in the cauda equina at L3 and L5 vertebrae levels. (F) Remarkable improvement in size of both lesions after intrathecal trastuzumab therapy.
CE: Contrast enhanced; FLAIR: Fluid-attenuated inversion recovery; T1: T1 weighted.
Figure 2. . Esophageal and CNS histology.
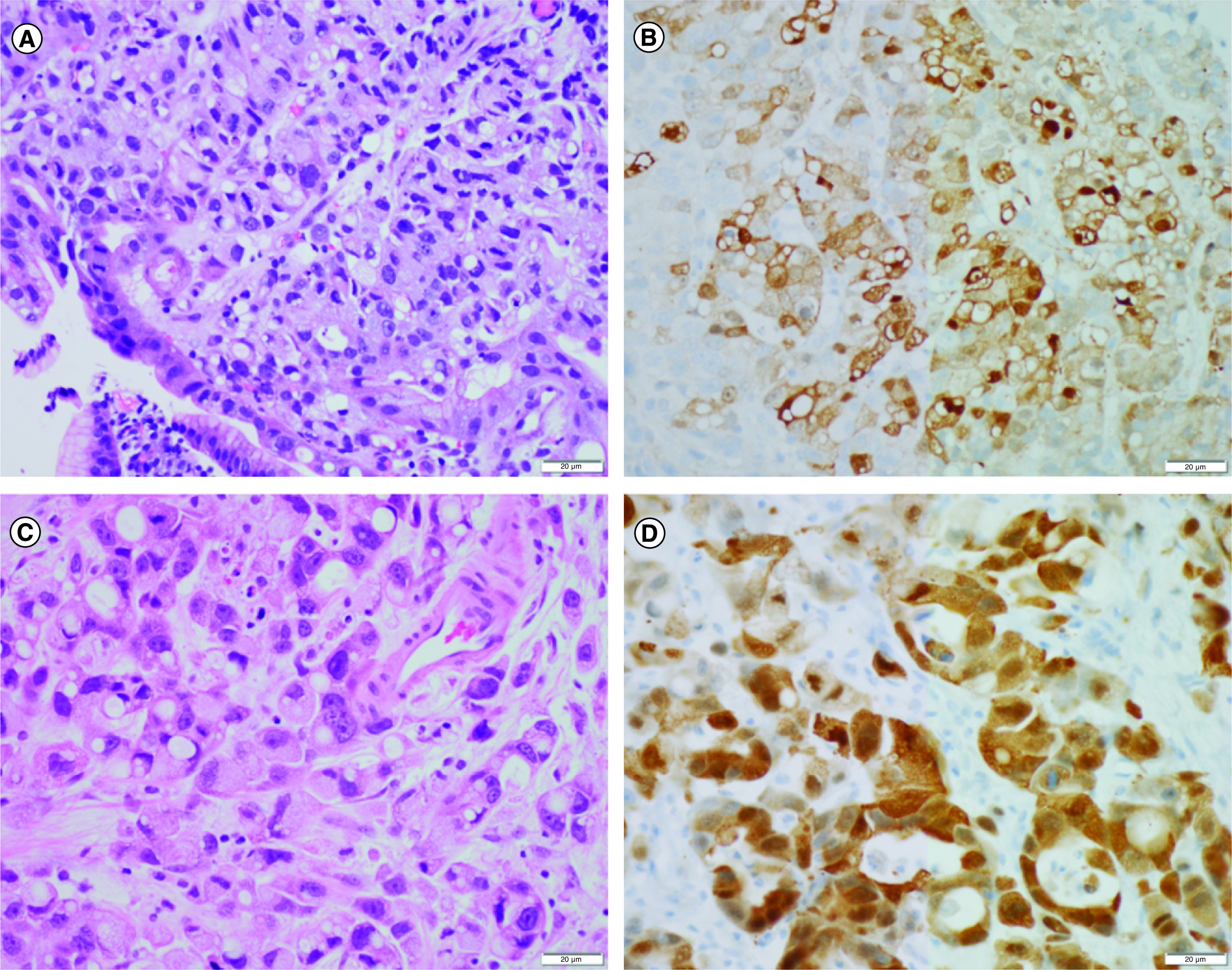
Case 1 pathology: endoscopic biopsy of the esophageal lesion demonstrated a poorly differentiated adenocarcinoma with signet ring features (A) and strong membranous Her2/Neu expression by immunohistochemistry (3+) (B). Biopsy of the right frontal leptomeninges demonstrated a morphologically similar metastatic adenocarcinoma (C) with nonspecific diffuse cytoplasmic staining for Her2/Neu (D).
Scale bars = 20 μm.
The patient received (whole-brain radiation therapy: 30 Gy in 10 planned fractions, six completed) with subsequent placement of Ommaya reservoir for anticipated intrathecal trastuzumab and topotecan chemotherapy. However, the patient passed away 22 days after initial presentation.
Case 2
A 56-year-old man initially presented with a history of back pain, weight loss and dysphagia. He was found to have abnormal thickening of the gastroesophageal junction and regionally enlarged lymph nodes concerning for metastatic spread. Initial CSF cytology was negative for malignancy (Table 1). Biopsy of the gastroesophageal junction demonstrated moderately differentiated invasive adenocarcinoma. MRI of the spine revealed intradural lesions at C2, L3 and L5 (case 2 in Figure 1). The patient underwent L4/5 partial laminectomy and biopsy of intradural tissue, which revealed metastatic adenocarcinoma. Molecular studies revealed amplified ERBB2 (HER2) and CCNE-1, deletion of SMAD4 and mutations in TP53, ARID1A, ERBB4, MYC and POLE. The patient was treated with palliative radiotherapy to the C1-C3 and T12-S1 levels (20 Gy in 5 fractions) followed by stereotactic radiosurgery to the right parietal brain metastasis (27 Gy in 3 fractions).
About 2 months after initial presentation, the patient began systemic therapy with intravenous (iv.) fluorouracil, oxaliplatin and leucovorin (FOLFOX) plus trastuzumab. An Ommaya reservoir was placed 2 months after initiating systemic chemotherapy and intrathecal trastuzumab was started at 80 mg twice weekly. Upon systemic non-CNS progression, the patient was switched to iv. fam-trastuzumab deruxtecan-nxki in combination with intrathecal trastuzumab. When he developed two new parenchymal brain metastases in the setting of stable LM, the patient received stereotactic radiosurgery (20 Gy) to the new lesions. With further systemic non-CNS progression, his systemic therapy was switched to iv. paclitaxel and pembrolizumab with continued intrathecal trastuzumab. He also received palliative radiotherapy to symptomatic bony metastases in the ribs and cervical spine.
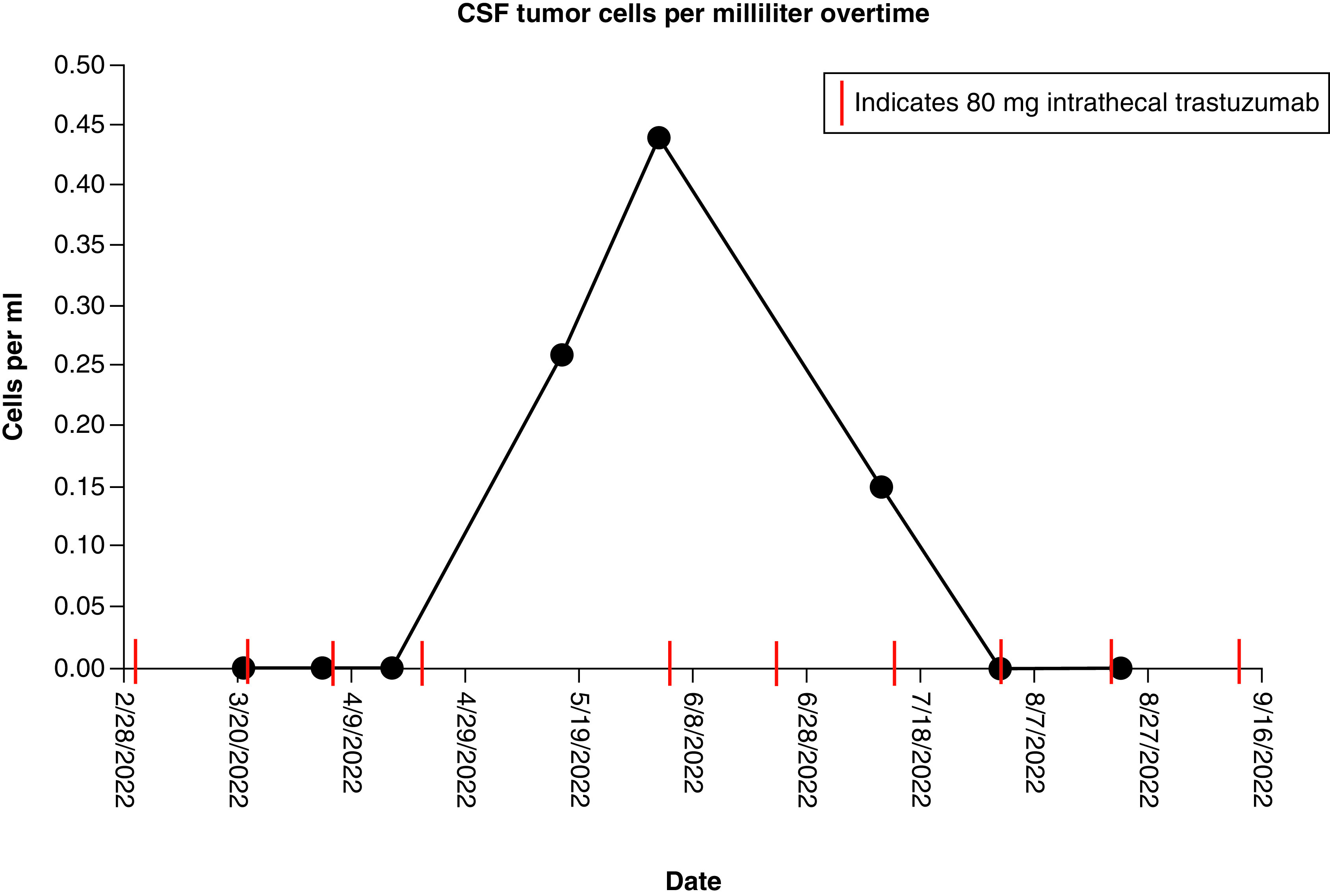
The patient continues to undergo treatment for 19 months after initial diagnosis without onset of new focal neurologic deficits. Quantifiable evaluation of circulating tumor cells (CTC) in the CSF demonstrates excellent control in the CSF with treatment. This remains durable, despite active disease outside the CNS (Figure 3).
Figure 3. . Circulating tumor cells cerebrospinal fluid trend for case 2.
Discussion
LM due to esophageal carcinoma is a rare occurrence with an aggressive but otherwise poorly understood disease course [2,22]. The cases here help to clarify the clinical presentation and broaden the understanding of the natural history of the disease. Among our two cases, males aged 56 and 60 at the time of presentation, both presented with dysphagia and weight loss, with the 60-year-old patient also presenting with headaches and cognitive decline. The 60-year-old patient declined rapidly and died just 22 days after initial presentation despite rapid initiation of whole-brain radiation therapy. This is in line with what has previously been reported [22]. The 56-year-old patient underwent extensive systemic and intrathecal trastuzumab therapy (80 mg twice weekly) with palliative radiation, resulting in non detectable to low CTC with treatment. This patient continues to undergo treatment with minimal side effects 18 months after initial presentation. The contrasting outcomes raise the potential of yet undescribed prognostic factors which may influence outcome in esophageal carcinoma LM. It is possible that this may be secondary to different states of the LM cells–with Case 1 representing predominantly free-floating neoplastic cells and Case 2 representing more adherent cells, both ultimately yielding dichotomous phenotypes previously described with divergent prognoses [26].
This paper demonstrates that intrathecal trastuzumab may be a reasonable treatment approach for patients with HER2+ esophageal carcinoma LM. Previous investigation has shown poor CSF penetration and bioavailability of systematically administered trastuzumab [27]. However, more recently published studies have begun to explore the utility of intrathecal trastuzumab for HER2+ cancers with LM [28,29]. Kumthekar et al. performed a Phase I/II study of intrathecal trastuzumab in HER2 positive cancer with LM that demonstrated favorable median OS of 10.5 months (n = 26) [28]. The nonrandomized nature of the trial precludes differentiating between a favorable natural history of HER2+ LM patients and a benefit from intrathecal HER2 directed therapy. Oberkampf et al. conducted a Phase II study of intrathecal trastuzumab in patients with HER2+ breast cancer with LM and noted that at 8 weeks, overall global health related quality-of-life status was preserved with 14 of their 19 patients free of neurological disease progression [29]. Neither of these trials included patients with esophageal carcinoma LM. The experience of our Case 2 patient aligns with these studies, their safety and efficacy, and reinforces the potential utility of intrathecal trastuzumab in patients with HER2+ carcinoma LM. Additionally, given its success in treating trastuzumab-refractory breast and gastric HER2+ malignancies [30,31], there may be a role for trastuzumab deruxtecan combination chemotherapy in the treatment of HER2+ esophageal carcinoma LM.
This paper also demonstrates the practicality and potential usefulness of CTC monitoring to track disease course. The quantification of CTC in the CSF at various points throughout treatment with patient 2 showed a marked decline with near complete resolution of these cells in response to treatment. Previous research highlights the utility of quantifiable CTC assays to diagnose and follow LM in the CSF [32,33]. A similar study probing LM survival in the context of CSF CTC studies and proton craniospinal irradiation timing by Wijetunga et al. supports early CTC quantification to assess LM disease burden [34].
Conclusion
Metastatic esophageal adenocarcinoma has a poor prognosis, with management of LM complicated by the rarity and rapid progression of the disease [1,22,24,25]. Despite trends of improved overall survival seen in patients with resectable HER2+ esophageal adenocarcinoma [35], our case series shows that HER2+ LM due to esophageal carcinoma can be associated with exceptionally rapid mortality. However, intrathecal trastuzumab-based treatment presents a new horizon which may prolong overall survival in patients with HER2+ LM, including potentially those with esophageal carcinoma, while being well-tolerated and without severe toxicity.
Summary points.
The prognosis for leptomeningeal metastases (LM) from esophageal carcinoma is exceptionally grim, with the only known systematic review of 30 patients with an accompanying case series exhibiting a maximum survival of just 7 months from time of diagnosis.
This article describes the presentation, progression and treatment courses of two patients with HER2+ esophageal carcinoma LM.
The cases presented help to clarify the clinical presentation and broaden the understanding of the natural history of the disease.
This paper demonstrates that intrathecal trastuzumab may be a reasonable treatment approach for patients with HER2+ esophageal carcinoma LM, while also being well-tolerated and without severe toxicity.
This paper also demonstrates the practicality and potential usefulness of circulating tumor cell monitoring to track disease course.
Footnotes
All authors contributed to the study conception, design, editing and review process. Material collection and analysis were performed by all authors. The first draft of the manuscript was written by SA Wu and RV Lukas and all authors commented on multiple previous versions of the manuscript. All authors read and approved the final manuscript.
Financial & competing interests disclosure
Authors SA Wu, DT Jia, M Mulcahy, K Guo, MC Tate, S Sachdev, N Kostelecky, DJ Escobar and DJ Brat declare that they have no financial interests. Author M Schwartz has received compensation for serving as a speaker for Novocure Inc. Author RV Lukas has received compensation for serving as a speaker for Novocure Inc. and Merck and consulting fees from Novocure Inc. and Merck as well as research support (drug only) from BMS. AB Heimberger has received consulting fees from WCG Oncology and Caris Life Science and research support from Alnylam and AbbVie. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.American Cancer Society. Key statistics for esophageal cancer. https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html
- 2.Lukas RV, Thakkar JP, Cristofanilli M et al. Leptomeningeal metastases: the future is now. J. Neurooncol. 156(3), 443–452 (2022). [DOI] [PubMed] [Google Scholar]; •• Discusses contemporary diagnostic and therapeutic leptomeningeal metastases (LM) from solid tumors.
- 3.Akhavan A, Navabii H. Leptomeningeal metastasis from squamous cell carcinoma of oesophagus with unusual presentation. BMJ Case Rep. 2012, bcr0220125846 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aulakh AS, Buttar A, Piperdi B. Leptomeningeal carcinomatosis in esophageal cancer: case report and review of literature. J. Gastrointest. Cancer 43(Suppl. 1), S84–S88 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Henninger N, Lin E, Fischer A, Kheradmand A. Leptomeningeal carcinomatosis: a rare complication in esophageal cancer. Acta Neurol. Belg. 109(2), 162 (2009). [PubMed] [Google Scholar]
- 6.Astner ST, Nieder C, Stock K, Gaa J, Grosu AL. Carcinomatous meningitis appearing as acoustic neuromas: two cases. Strahlenther. Onkol. 183(5), 279–283 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Goddeeris K, Verslype C, Wilms G, Van Cutsem E. Leptomeningeal carcinomatosis associated with oesophageal adenocarcinoma: two case reports and review of the literature. Acta Gastroenterol. Belg. 69(4), 377–380 (2006). [PubMed] [Google Scholar]
- 8.Wagemakers M, Verhagen W, Borne B, Venderink D, Wauters C, Strobbe L. Bilateral profound hearing loss due to meningeal carcinomatosis. J. Clin. Neurosci. 12(3), 315–318 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Okumura H, Natsugoe S, Yokomakura N et al. A case of leptomeningeal carcinomatosis from esophageal basaloid carcinoma diagnosed by quantitative reverse transcription-polymerase chain reaction for carcinoembryonic antigen. J. Gastroenterol. 40(1), 87–93 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Abdo AA, Coderre S, Bridges RJ. Leptomeningeal carcinomatosis secondary to gastroesophageal adenocarcinoma: a case report and review of the literature. Can. J. Gastroenterol. 16(11), 807–811 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Girola SS, Celano M, Arrigoni E, Di Giulio G, Di Maggio EM, Mazzone A. Meningeal carcinomatosis in patient with esophagus adenocarcinoma. Minerva Med. 88(3), 105–107 (1997). [PubMed] [Google Scholar]
- 12.Watanabe M, Tanaka R, Takeda N. Correlation of MRI and clinical features in meningeal carcinomatosis. Neuroradiology 35(7), 512–515 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Civantos F, Choi YS, Applebaum EL. Meningeal carcinomatosis producing bilateral sudden hearing loss: a case report. Am. J. Otol. 13(4), 369–371 (1992). [PubMed] [Google Scholar]
- 14.Teare JP, Whitehead M, Rake MO, Coker RJ. Rapid onset of blindness due to meningeal carcinomatosis from an oesophageal adenocarcinoma. Postgrad. Med. J. 67(792), 909–911 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coman I, Barroso B, Sawan B, Brochet B. Signet-ring cell carcinomatous meningitis complicating esophageal neoplasia. Rev. Neurol. (Paris) 157(12), 1539–1541 (2001). [PubMed] [Google Scholar]
- 16.Giglio P, Weinberg JS, Forman AD, Wolff R, Groves MD. Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract. Cancer 103(11), 2355–2362 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Ikuta Tanaka TY, Shugo Akazawa, Yasuki Nishibori, Tetsuya Sumiyoshi, Yojiro Niitsu. A case of recurrent esophageal cancer with meningeal carcinomatosis. Digestive Endoscopy 42(6), 1053–1057 (2000). [Google Scholar]
- 18.Jagtap SV, Khoja S, Jagtap SS, Gudur R, Janugade H. Leptomeningeal carcinomatosis secondary to esophageal cancer diagnosed on cytology. J. Neurosci. Rural Pract. 11(3), 495–497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinugasa S, Kobayashi I, Horikawa M et al. Brain metastasis and leptomeningeal carcinomatosis after surgery for esophageal cancer-a case report. Gan To Kagaku Ryoho 47(11), 1593–1595 (2020). [PubMed] [Google Scholar]
- 20.Lu Y, Wang L, Ajani JA. Rare esophageal leptomeningeal metastases detected on 18F-FDG PET/CT. Clin. Nucl. Med. 45(4), 334–335 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Dumbill R, Thompson S, Peschl H, Turner G, Woodrow C. Isolated leptomeningeal carcinomatosis and possible fungal meningitis as late sequelae of oesophageal adenocarcinoma. BMJ Case Rep. 12(11), e230117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukas RV, Mata-Machado NA, Nicholas MK, Salgia R, Antic T, Villaflor VM. Leptomeningeal carcinomatosis in esophageal cancer: a case series and systematic review of the literature. Dis. Esophagus 28(8), 772–781 (2015). [DOI] [PubMed] [Google Scholar]; •• This referenced work is the largest and most complete review of LM due to esophageal cancer.
- 23.Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J. Surg. 41(3), 210–215 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Short MW, Burgers KG, Fry VT. Esophageal cancer. Am. Fam. Physician 95(1), 22–28 (2017). [PubMed] [Google Scholar]
- 25.National Cancer Institute. Cancer of the esophagus. https://seer.cancer.gov/archive/csr/1975_2013/results_merged/sect_08_esophagus.pdf
- 26.Remsik J, Chi Y, Tong X et al. Leptomeningeal metastatic cells adopt two phenotypic states. Cancer Rep. (Hoboken) 5(4), e1236 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs 18(1), 23–28 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Kumthekar PU, Avram MJ, Lassman AB et al. A Phase I/II study of intrathecal trastuzumab in HER-2 positive cancer with leptomeningeal metastases: safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro.Oncol. 25(3), 557–565 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This referenced study completed by our authors and colleagues is the foundation for the LM due to esophageal carcinoma cases and treatment discussed.
- 29.Oberkampf F, Gutierrez M, Trabelsi Grati O et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro. Oncol. 25(2), 365–374 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modi S, Saura C, Yamashita T et al. DESTINY-Breast01 investigators. trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382(7), 610–621 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shitara K, Bang YJ, Iwasa S et al. DESTINY-Gastric01 investigators. trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382 (25), 2419–2430 2020). [DOI] [PubMed] [Google Scholar]
- 32.Van Bussel MTJ, Pluim D, Milojkovic Kerklaan B et al. Circulating epithelial tumor cell analysis in CSF in patients with leptomeningeal metastases. Neurology 94(5), e521–e528 (2020). [DOI] [PubMed] [Google Scholar]; • This referenced study reinforces the potential role of quantifiable circulating tumor cells (CTC) assays to diagnose and monitor LM in the cerebrospinal fluid (CSF).
- 33.Malani R, Fleisher M, Kumthekar P et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J. Neurooncol. 148(3), 599–606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reinforces the potential role of quantifiable CTC assays to diagnose and monitor LM in the CSF.
- 34.Wijetunga NA, Boire A, Young RJ et al. Quantitative cerebrospinal fluid circulating tumor cells are a potential biomarker of response for proton craniospinal irradiation for leptomeningeal metastasis. Neurooncol. Adv. 3(1), vdab181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reinforces the potential role of quantifiable CTC assays to diagnose and monitor LM in the CSF.
- 35.Creemers A, Ebbing EA, Hooijer GKJ et al. The dynamics of HER2 status in esophageal adenocarcinoma. Oncotarget 9(42), 26787–26799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


