Transporters of the mitochondrial carrier superfamily (SLC25), with 53 members, play a crucial role in maintaining metabolic homeostasis (1). Expression of the SLC25 family varies greatly among tissues, and SLC25A47 is a liver-specific mitochondrial carrier (1–3). Recently, Yook et al. identify that 9 single-nucleotide polymorphisms (SNPs) in SLC25A47 are significantly associated with glucose and lipid homeostasis, such as fasting glucose, random glucose, HbA1c, and high-density lipoprotein cholesterol levels in humans (1). However, whether and how these SNPs affect SLC25A47 gene expression remains unknown, which has prompted us to explore the findings further.
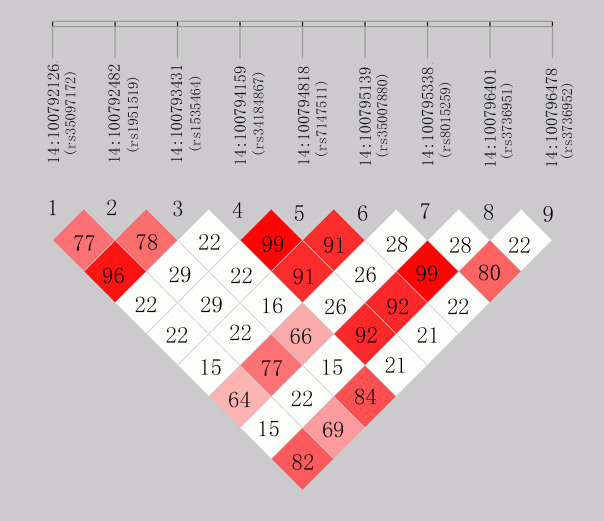
First, linkage disequilibrium (LD) among these 9 SNPs is calculated on the basis of the 1000 Genomes European panel (4–7). Interestingly, similar to our previous study (8), the LD analysis indicates that rs1535464, rs35097172, rs1951519, rs3736952, and rs8015259 are in high LD with each other (r2 range = 0.64 to 0.96), while rs34184867, rs35007880, rs3736951, and rs7147511 are in strong LD with each other (r2 range = 0.91 to 0.99) (Fig. 1). In addition, we compare allele frequencies of the 9 SNPs in Africans (AFR), Americans (AMR), East Asians (EAS), Europeans (EUR), and South Asians (SAS) descent from the 1000 genomes project. The results show that frequencies of rs1535464, rs35097172, rs1951519, rs3736952, and rs8015259 are identical or nearly identical in the same ethnicity, and frequencies of rs34184867, rs35007880, rs3736951, and rs7147511 are identical or nearly identical in the same ethnicity (Table 1). These results indicate that the 9 SNPs are not independent of each other.
Fig. 1.
The LD analysis for the nine SLC25A47 SNPs r2-value (×100).
Table 1.
The metabolic phenotypes-associated SNPs and SLC25A47 gene expression in the liver of human
| EA | NEA | 1000G Allele Frequencies | GTEx Association | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNPs | AFR (%) | AMR (%) | EAS (%) | EUR (%) | SAS (%) | β* | SE | P-Value† | ||
| rs1535464 | A | G | 7 | 12 | 12 | 21 | 16 | −0.013 | 0.057 | 0.82 |
| rs35097172 | T | C | 7 | 12 | 12 | 21 | 16 | −0.012 | 0.060 | 0.84 |
| rs1951519 | A | C | 48 | 16 | 24 | 25 | 19 | 0.004 | 0.054 | 0.95 |
| rs3736952 | T | C | 7 | 12 | 13 | 19 | 17 | −0.012 | 0.057 | 0.83 |
| rs8015259 | C | G | 50 | 16 | 25 | 23 | 20 | −0.038 | 0.055 | 0.49 |
| rs34184867 | G | C | 6 | 31 | 23 | 47 | 66 | −0.025 | 0.048 | 0.60 |
| rs35007880 | T | G | 4 | 31 | 23 | 49 | 66 | −0.030 | 0.048 | 0.53 |
| rs3736951 | T | C | 4 | 31 | 23 | 49 | 66 | −0.030 | 0.048 | 0.53 |
| rs7147511 | T | C | 4 | 31 | 23 | 47 | 66 | −0.032 | 0.048 | 0.51 |
EA, effect allele; NEA, nonEA. AFR, AMR, EAS, EUR, and SAS. SE standard error.
*β is the regression coefficient based on the EA. β > 0 and β < 0 indicate that the effect/mutant allele regulates increased and reduced gene expression, respectively.
†The threshold of statistical significance for eQTLs analysis was P < 0.05.
Because SLC25A47 is selectively expressed in the liver of humans, then we evaluate the association of these 9 SNPs with SLC25A47 expression using expression quantitative trait loci (eQTL) datasets from the Genotype-Tissue Expression (GTEx) project in the liver (9, 10). The eQTL analysis is performed by applying the linear regression based on an additive model. The results show that all effect alleles of the 9 SNPs could regulate a reduced SLC25A47 expression in the liver (β < 0), except for rs1951519 (β = 0.004). However, the association between the 9 SNPs and SLC25A47 expression does not pass the significance level of 0.05. More detailed results are presented in Table 1.
In summary, Yook et al. independently found that SLC25A47 is selectively expressed in the liver of humans and significant associations of SLC25A47 genetic variants with glycemic and lipid homeostasis. Here, we show that all the associated SNPs could not significantly regulate reduced SLC25A47 gene expression in the liver. We believe that our findings provide important supplementary information about the role of SLC25A47 genetic variants in gluconeogenesis and energy expenditure.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81703623), the Scientific Foundation of Hunan (No. 2023JJ30772), and the Wu Jieping Medical Foundation (320.6750.2020-04-14). We gratefully acknowledge GTEx project1000 genomes project for providing the data in this study, and LD analysis by SHEsis.
Author contributions
H.R. and J.-Q.L. designed research; H.R., C.-L.X., W.-R.L., M.-Z.L., and J.-Q.L. performed research; C.-L.X., M.-Z.L., and J.-Q.L. contributed new reagents/analytic tools; H.R., C.-L.X., W.-R.L., M.-Z.L., and J.-Q.L. analyzed data; and H.R., C.-L.X., and J.-Q.L. wrote the paper.
Competing interests
The authors declare no competing interest.
References
- 1.Yook J. S., et al. , The SLC25A47 locus controls gluconeogenesis and energy expenditure. Proc. Natl. Acad. Sci. U.S.A. 120, e2216810120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bresciani N., et al. , The Slc25a47 locus is a novel determinant of hepatic mitochondrial function implicated in liver fibrosis. J. Hepatol. 77, 1071–1082 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Cheng L., et al. , Hepatic mitochondrial NAD+ transporter SLC25A47 activates AMPKalpha mediating lipid metabolism and tumorigenesis. Hepatology, 10.1097/HEP.0000000000000314 (2023). [DOI] [PMC free article] [PubMed]
- 4.Li Z., et al. , A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: Update of the SHEsis (http://analysis.bio-x.cn). Cell Res. 19, 519–523 (2009). [DOI] [PubMed] [Google Scholar]
- 5.C. Genomes Project et al. , A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.C. Genomes Project et al. , An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y. Y., He L., SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 15, 97–98 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Luo J. Q., et al. , Rs495828 polymorphism of the ABO gene is a predictor of enalapril-induced cough in Chinese patients with essential hypertension. Pharmacogenet. Genomics 24, 306–313 (2014). [DOI] [PubMed] [Google Scholar]
- 9.G. T. Consortium et al. , Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.G. T. Consortium, The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]