Abstract
Transfusion of red blood cells (RBCs) is one of the most valuable and widespread treatments in modern medicine. Lifesaving RBC transfusions are facilitated by the cold storage of RBC units in blood banks worldwide. Currently, RBC storage and subsequent transfusion practices are performed using simplistic workflows. More specifically, most blood banks follow the “first-in-first-out” principle to avoid wastage, whereas most healthcare providers prefer the “last-in-first-out” approach simply favoring chronologically younger RBCs. Neither approach addresses recent advances through -omics showing that stored RBC quality is highly variable depending on donor-, time-, and processing-specific factors. Thus, it is time to rethink our workflows in transfusion medicine taking advantage of novel technologies to perform RBC quality assessment. We imagine a future where lab-on-a-chip technologies utilize novel predictive markers of RBC quality identified by -omics and machine learning to usher in a new era of safer and precise transfusion medicine.
Keywords: red blood cells, transfusion, preservation
Red blood cells (RBCs) constitute more than 90% of the cellular blood content in humans and perform one of the most essential roles for life, oxygen transport. RBC transfusion is a common preventive and therapeutic procedure with 36,000 units transfused daily in the US alone (1–3). Here, we review the decades-old quality control practices for stored RBCs and contend that advances are warranted using novel lab-on-a-chip (LOC) technologies to enable precision transfusion medicine.
Blood transfusion therapy has evolved into today's practice over the last four centuries (4). Consequently, many patients and practitioners mistakenly assume that current transfusion workflows have reached their final form. Historically, the first convincing demonstrations of blood transfusion and its utility occurred during the First World War. These demonstrations were facilitated by advances in blood storage practices (5) including additive solutions-enabled extended storage (6, 7), and later plastic blood bags (8) that permitted lighter weight and reduced contamination.
The World Health Organization reports that whole blood donation exceeds 120 million units annually, in response to a massive global need (3). The logistics of the RBC supply-and-demand chain are sustained through commonly agreed-upon storage practices in blood banks. The US Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) are involved in keeping the blood supply safe by mandating quality control and assurance parameters, and monitoring incidents during manufacturing and adverse posttransfusion reactions (9). These US agencies, along with their international counterparts, have been instrumental in establishing quality and product clearance regulations for RBC storage and transfusion. Specifically, the current practice for RBC banking is storage at 4 °C for 42 d (10–12). This practice is based primarily on the FDA-mandated requirements that RBCs undergo less than 1% hemolysis during storage and that at least 75% of transfused RBCs survive at 24 h after transfusion (13, 14). The slow pace of change and improvements to these common practices, however, should not imply that RBC storage and transfusion strategies are optimal. It rather signals a lack of low-cost and translatable tools to assess quantitatively, (i.e., objectively via unbiased measurements), the quality of RBC units.
Stored RBCs exhibit unique donor-, time-, and processing-dependent metabolic and morphological changes collectively termed storage lesions (15, 16) (Fig. 1). For example, increased cellular acidity inhibits glycolytic pathway enzymes, depleting adenosine triphosphate (ATP) reserves. A decline in 2,3-diphosphoglycerate (2,3-DPG) changes oxygen kinetics (17, 18). Morphological changes and oxidative injuries jointly cause RBC breakdown (i.e., intravascular hemolysis) or increased sequestration in the spleen (i.e., extravascular hemolysis) (19, 20). Overall, these lesions imply reduced viability and impaired functionality of stored RBCs, negatively impacting their efficacy and safety in transfusion.
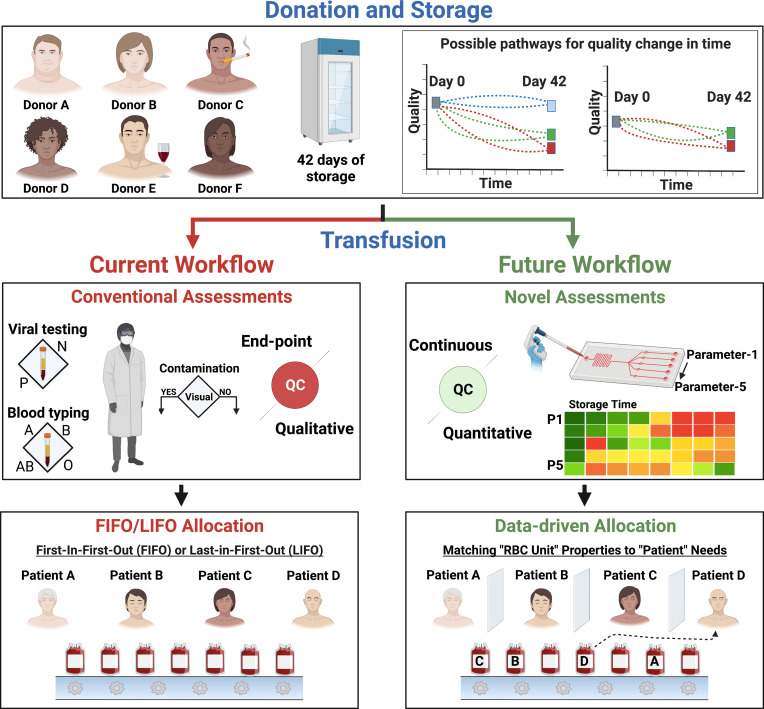
Fig. 1.
The current and proposed future workflow for RBC storage and transfusion medicine. Donors vary in sex, age, ethnicity, genetic disposition, body mass index, metabolism, and habits. These variations can lead to different initial quality levels for donated RBCs and to different quality changes during storage. Currently, blood banks store donated RBCs at 4 °C for 42 d. This storage can induce injuries collectively termed the “storage lesions” in a donor-, time-, and processing-dependent manner. Thus, the injury to each stored RBC unit can be unique. The current quality assessments performed on individual RBC units before transfusion are infectious disease marker testing and donor screening, blood typing, and visual inspection for bacterial contamination. These assessments are agreed upon and mandated by health agencies worldwide—including the FDA and CDC. RBC units are generally allocated following the “first-in-first-out” protocol. However, this workflow may result in posttransfusion complications and significantly reduced efficacy. The advances in LOC technologies armed with information from -omics and ML studies offer a better alternative. We envision a future where each RBC unit is assessed continuously during storage using multiple quality metrics based on objective unbiased measurements via LOC technologies. Patients with different medical conditions will then be administered the right RBC unit via data-driven allocation. Such an approach will maximize the efficacy of transfusion therapies and reduce complications.
Several studies in the early 2000s have highlighted risks associated with the use of older stored RBCs versus fresh units (21–26). Others have challenged such claims using large-scale randomized clinical trials (RCTs) (27–31). However, these trials have also drawn criticism due to their study design and intrinsic limitations (poor delineation of storage time, mortality as the primary outcome, etc.) (27, 28, 32). Thus, a consensus on the clinical impact of the time-dependent RBC storage lesion has not yet been reached. This stems from a lack of data on the actual quality [real age (33)] of RBC units beyond their storage time (chronological age) (Fig. 1). Following initial pathogen screening and blood typing, only some arbitrary RBC units—if any—go through destructive (sacrificing the tested RBC units) quality control assays, and only visual inspections are conducted as end-point quality control release tests (Fig. 1). However, recent studies indicate that the temporal evolution of storage lesions is dependent on donor variability factors such as sex, age, ethnicity, genetic disposition, body mass index, metabolism, and lifestyle (34–38). The severity of adverse effects from RBC transfusion also depends on recipient factors (e.g., health state, transfusion volume, and frequency). Objective assessment of storage lesions can lead to RBC quality and “real age” determination and rational decision-making for donor-to-recipient selection. Doing so will improve transfusion safety and efficacy, and potentially lead to better storage practices.
Two emerging approaches have been used to quantitatively assess the RBC storage lesions: -omics and machine learning (ML). Specifically, -omics methods (proteomics, lipidomics, metabolomics) that provide comprehensive characterization and quantification of biomolecules via mass spectrometry have been used to study RBC changes upon storage. These studies have led to identification of potential biomarkers to assess RBC storage injuries (39). Similarly, ML combined with imaging flow cytometry has captured the progression of storage-induced changes in RBC morphology and deformability (40, 41). Together, -omics and ML technologies have the potential to identify key quality parameters that are predictive of clinical transfusion success for stored RBCs. Yet, the adoption of -omics on a large scale for routine use is hindered by their cost and analysis time, though these hurdles are overcome with the introduction of high-throughput -omics.
LOC technologies offer an alternative for routinely measuring RBC unit quality. These LOC technologies allow miniaturization of analytical assays with the benefits of sensitivity, specificity, ease of use, portability, rapid turnaround, and high-throughput (42–45). As such, LOCs can become a new frontier for assessing stored RBC products using quality parameters that result from combined -omics and ML studies. More specifically, LOCs can measure and immediately report these key quality parameters for every stored RBC unit to enable safer transfusion (without any unintended adverse consequences) by matching the properties of the RBC unit to the needs of the patient (right unit to right patient). Nevertheless, despite their increasing adoption and commercial successes in other areas (pregnancy, glucose, and rapid COVID-19 tests), LOCs are scarcely used to assess stored RBCs. Yet, recent research is demonstrating the potential of LOCs to improve RBC safety assessment (46–53).
This Perspective introduces the unexplored intersection of i) RBC storage lesions, ii) the need for RBC quality assessment, and iii) the potential for integrated LOC technologies to provide for safer transfusion therapy. First, we highlight the issues regarding the quality of stored RBCs. Specifically, we and others posit that stored RBCs, under current practices, are not always safe to transfuse due to donor, time, and processing factors. This is especially problematic for critically ill patients, those on chronic transfusion regimens, and surgeries where large volumes of RBCs are transfused. The lack of assessment of stored RBCs before transfusion on a unit-by-unit basis represents a failure, especially in this era of precision medicine. We then discuss solutions to this critical issue using accessible and robust LOC platforms and their convergence with other state-of-the-art approaches (-omics and ML).
Current Practices in RBC Storage and Quality Assessment
Storage of RBCs.
RBCs undergo donor-, time-, and processing-dependent metabolic, biochemical, and morphological injuries during their extended storage, collectively termed storage lesions (54–56). These include progressive increases in hemolysis (57); intracellular and extracellular acidification (58); depletion of glucose (15); accumulation of extracellular potassium and intracellular calcium ions (54); alteration in osmotic fragility; depletion of intracellular energy and redox pools (reduced and oxidized glutathione) (55); and decrease in deformability (59) (Fig. 2). The identification of storage lesions and efforts to mitigate them have led to the development of improved storage solutions to extend storage time for banking purposes. As a result, the current widely used storage method for RBCs is cold storage at 4 °C for up to 42 d of storage in various additive solutions (SAG-M, AS-1, AS-3, etc.) (54).
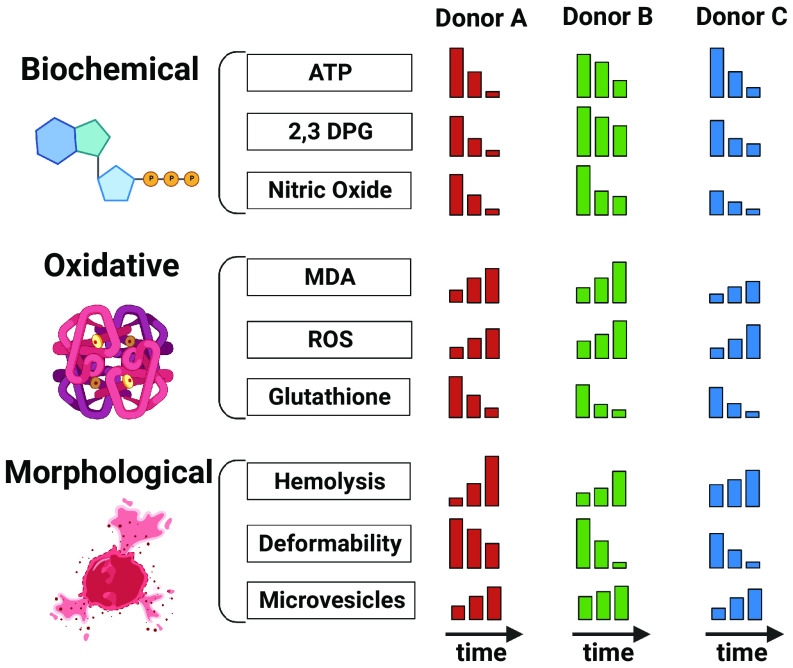
Fig. 2.
Donor-, time-, and processing-dependent RBC storage lesions. RBCs undergo various injuries during storage in their biochemical content, oxidative state, and morphology. Major biochemical changes include the depletion of ATP and 2,3-DPG. This is accompanied by an increase in reactive oxygen species damaging proteins and lipids (through increased malondialdehyde—MDA), among other injuries affecting the cellular integrity. These injuries are often observed as irreversible RBC morphological changes resulting in loss of integrity (hemolysis), deformability, and discoid morphology. While these alterations have a general time-dependent trend, they also depend on the donor and the initial blood processing, which varies among blood collection centers. These donor-, time-, and processing-dependent changes are not monitored in today’s practice of blood banking.
Quality Assessment of Stored RBCs.
There are only two criteria mandated by the FDA and international counterparts for the quality and acceptable storage of RBCs: i) at least 75% of autologous RBCs must survive at 24 h in vivo after transfusion, and ii) there must be less than 1% hemolysis in the storage container (13, 14). Novel RBC products—featuring new storage solutions, methods, or other improvements—must meet these FDA criteria for clinical use. The standard practice of up to 42 d of cold storage for RBCs is primarily driven by the need to meet these two criteria. In 2008, several clinical retrospective studies presented data that suggested chronologically old RBCs (stored for more than 2 wk) were associated with worse outcomes upon transfusion in different patient groups. In contrast, subsequent RCTs indicated that there are no significant differences between transfusing chronologically fresh versus older stored RBCs. In the following paragraphs, we summarize the findings of these different studies and the ongoing discussion on stored RBC quality and their chronological age. Nevertheless, the different views on this question stem from a lack of data on the real age—i.e., quantitative and objective metrics of quality—of RBC units beyond their chronological age. In this Perspective, our goal is to urge that the field adopt robust and routine quality assessment of stored RBCs.
According to the 2008 retrospective clinical study, transfusion of RBCs cold-stored for more than 2 wk was associated with increased risks of postoperative complications and reduced survival after cardiac surgery (21). A similar result was found for trauma patients and corroborated by further studies (22, 23). Specifically, trauma patients administered three or more RBC units within 24 h of their hospitalization had a higher mortality risk when relatively old (>14 d) RBCs were used. A follow-up study indicated inhibition of regional microvascular perfusion in anemic (but otherwise stable) patients when older (>21 d) RBCs were used (24). However, these studies were retrospective and hence lacked proper randomization. Nevertheless, their findings prompted agencies such as the US National Heart, Lung, and Blood Institute (NHLBI) to reexamine the suitability of the current practices (25, 60).
Subsequent RCTs on a subset of patient groups indicated no inferiority of selective transfusion of freshest available RBC products versus standard of care for various patient groups (27–31). These studies contrast with the previous retrospective and observational clinical studies and with recent animal studies that predict high mortality in disease models of animals when older (42-d-old) RBCs are transfused (61, 62). While RCTs are regarded as the gold standard for clinical studies, several issues were raised regarding these RCTs (27, 28). These RCTs were conducted in specific patient groups, and their results may not be generalizable to all patient populations. Also, none of the RCTs evaluated the effect of transfusing RBCs stored between 35 and 42 d, which is the period other clinical trials have questioned (63). In fact, Pereira et al. suggested that the RCTs lack enough power to resolve the issue of the “old” RBCs (32) and recommended reducing the maximum storage time to 35 d.
The quality of stored RBCs may not necessarily be dictated by their chronological age. As we already indicated, the RBC quality varies based on not only storage time but also donor, processing, storage solution, and environmental factors. Indeed, within individual RBC units, there are heterogenous subpopulations with unique structural, functional, and metabolic dissimilarities due to donor-dependent differences in hematopoiesis and in vivo eryptosis (64). Similarly, the needs of each patient for transfusion vary depending on several factors, including their own disposition, condition, and the anticipated operation. Therefore, the focus when using stored RBCs should be on assessing the quality and thus the transfusibility of each stored unit for the particular patient in need, whether the storage lesions be due to donor, time, or processing factors. These considerations have fueled the debate on personalized transfusion medicine approaches, a key goal in the agenda of the NHLBI, as discussed in the State of the Science in Transfusion Medicine Workshop (60). Thus, rather than an overly simplistic “first-in-first-out” or “last-in-first-out” approach, a data-driven selection of stored RBC units for each patient will prove superior (Fig. 1). The routine use of such a data-driven approach will reduce transfusion complications, improve outcomes, and enable better allocation of RBC units for every patient.
State-of-the-Art Assessment Approaches via -Omics and Machine Learning.
-Omics for transfusion medicine.
There has been progress in recent years in our understanding of storage lesions using -omics technologies (55)—especially metabolomics (65). This has been primarily driven by advancements in high-throughput mass spectrometry (66), a technology allowing the analysis of large populations. A paradigmatic example is the Recipient Epidemiology and Donor Evaluation Study III—RBC Omics (REDS). This study enrolled ~14,000 healthy donors across four US blood centers, and performed both donor genomics and metabolomic analyses of stored RBC units (67). These data were gathered in parallel to laboratory measurements of hemolysis (35, 68), clinical measurements of iron metabolism (69), and recipient databases for functional readouts [e.g., posttransfusion hemoglobin increments (70)], ultimately resulting in what is defined as a “vein-to-vein” database (71).
The opportunity to generate a wealth of data through metabolomics testing of thousands of RBC units in the REDS study has allowed the field to appreciate the impact of donor biological variables [sex (34), age (35), ethnicity (72), body mass index (36), testosterone levels (37)] on RBC storage quality. Interestingly, the genetic background of the donor significantly impacts the heterogeneity observed in storage profiles. Such is the case for common polymorphisms—e.g., glucose 6-phosphate dehydrogenase deficiency that affects ~7% of the world population (73). Similar considerations apply to other common polymorphisms, such as those found in beta-thalassemia minor (74), band 3 (75), and glutathione peroxidase 4 (76). Metabolomics has also demonstrated the impact of processing [e.g., leukoreduction/leukodepletion (77)], and the blood donor “exposome” on the onset, progression, and ultimate storage lesion severity (78). Specifically, based on -omics studies, we now appreciate the impact of factors such as donor exposure to smoking (38), alcohol (79), and caffeine (80) on RBC storage quality. Furthermore, -omics has shown that certain drugs—that are not grounds for donor deferral—are routinely identifiable in stored blood products, with the potential to negatively impact storage quality and promote untoward consequences in transfusion recipients (78).
Overall, large-scale -omics studies have paved the way for personalized medicine, revealing that storage duration (chronological age) might be less relevant than the real age (metabolic age) of the unit (81). Applications of -omics to transfusion medicine have recently yielded potential new metrics of the storage lesion and posttransfusion performances. These include posttransfusion recovery (82), storage or oxidative hemolysis (34, 73), or oxygen kinetics (17). These parameters can now drive the development of cost-effective, targeted strategies to monitor theoretically all the >100 million blood units donated yearly worldwide.
Machine Learning for transfusion medicine.
Advanced technologies to assess RBC quality and state—such as -omics—generate complex multivariate data (83). This data-intensive (i.e., big data) approach to assessing stored RBC quality necessitates using computational statistical techniques to objectively extract meaningful patterns from these datasets (84). Such combination of big data and computational statistical tools is critical to biological interpretation and aiding the data-driven selection of stored RBCs for precision transfusion medicine (85).
Among computational approaches, ML has shown promise in accurately classifying complex multivariate data and aiding critical decision-making in biomedicine (86–88). Of interest to transfusion medicine, ML was recently leveraged to predict preoperative RBC demand and the objective assessment of stored RBC quality (40, 89). One study involved a retrospective analysis of ~130,000 surgery patients using seven ML algorithms (81). This led to a more objective approach for predicting the quantity of RBCs needed by each patient, compared to clinicians making the same decisions without ML. In another study, brightfield images were used to distinguish between RBC morphologies and predict stored RBC quality using ML algorithms (40). ML results correlated better with physiological tests of RBC quality, compared to subjective expert annotation. Further examples at the intersection of transfusion medicine, big data, and ML include the improved quality monitoring of stored RBC units via the identification of new injury markers (40) and the development of new products (90); novel storage additives (91) and strategies (92); rejuvenated units (93).
These advanced technologies, especially -omics, have enriched our understanding of storage lesions and led to the development of potential new metrics for RBC quality assessment. Currently, these technologies are limited to research, predominantly due to the relatively low accessibility of the instruments and the complexity of the data. Although the ML toolbox has demonstrated success in tackling such data, implementation of ML-based solutions in healthcare will require expertise and installation of software infrastructure. This necessitates an alternative approach that can harness the results of -omics and ML and provide quick, noninvasive, economically feasible, and user-friendly quality measurements on stored RBC units.
LOC Technologies for the Assessment of Stored RBCs
Overview of LOC Technologies.
LOC platforms are miniaturized fluidic systems that facilitate chemical and biological analyses with sample volume requirements as low as pico-to-nanoliters (Fig. 3A) (42–45). Many materials (polycarbonate, paper, polydimethylsiloxane) are available for applications with different requirements. Fabrication methods are dictated by the material choice and include lithography, hot embossing, and 3D printing. Chemical and biological analyses require the manipulation of fluids facilitated by components including i) pumps to drive, ii) valves to selectively direct, and iii) mixers to combine fluids (94, 95). Optical, ultrasonic, electrochemical, electrical, and other analytical components can also be integrated (96–102), further expanding the LOC toolbox for sensing biomarkers (proteins, metabolites, etc.).
Fig. 3.
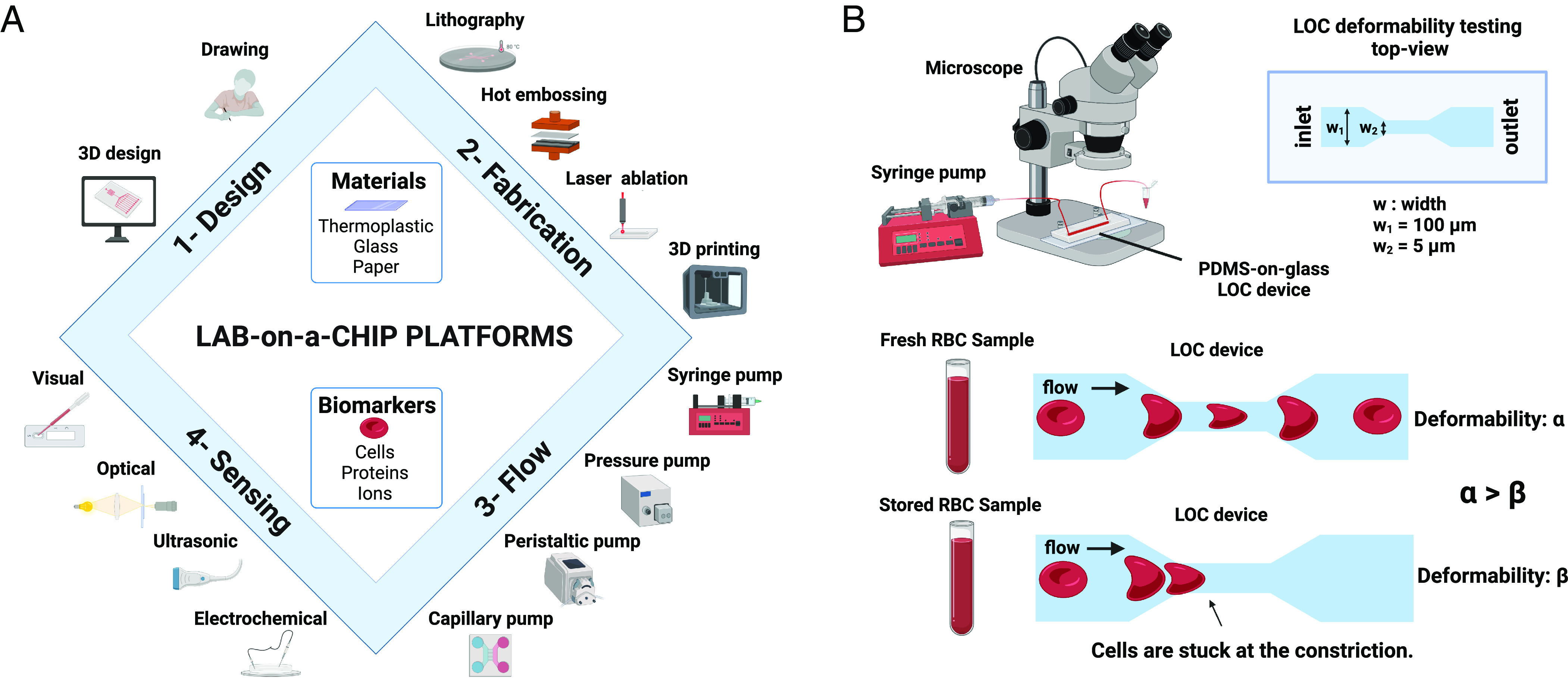
LOC platforms for RBC quality assessment. Figure (A) shows different aspects, components, and materials of LOC platforms from design and fabrication to operation (flow) and sensing. Such platforms enable detection of various biomarkers ranging from cells and proteins to ions. Figure (B) shows a schematic representation of one of the most commonly used RBC deformability measurements using LOC platforms. Such platforms use microfluidic constrictions to quantify the degree of deformability.
During recent decades, LOCs have enabled several transformative technologies that have improved public health and become commercial successes (45). These range from nucleic acid sequencing (103) to rapid diagnostics (103), biochemical analyses (104), and antibody selection (105). Today, LOCs are also indispensable in single-cell -omics (106), synthetic biology (107), liquid biopsy (108), organs-on-chips (109), and wearable sensors (110). Despite their increasing utility, LOCs have not yet made a translational impact in transfusion medicine. However, the promising examples described below demonstrate the utility of LOCs to quantify stored RBC quality.
LOC Platforms for RBC Quality Assessment.
Microfluidic platforms have been utilized to characterize storage-induced alterations in RBCs, with a predominant focus on the stiffness analysis of stored RBCs (Fig. 3B). The simplest approach allows RBCs to adhere to glass in a microchannel (46). A pressure-driven flow is then used to deform the adherent RBCs and measure their shear modulus via image analysis. Similar shear modulus measurements are also possible by deforming RBCs flowing through a constriction and quantifying their subsequent recovery in a wider channel through image analysis (47, 48). These suspension-based approaches enable a higher throughput than the adherent cell approach. Together, these studies have confirmed storage-induced increases in RBC stiffness.
An alternative approach to direct measurement of shear modulus is the margination and subsequent fractionation of RBCs of different deformability. Several groups have developed microfluidic cell sorters where deformable RBCs migrate toward the channel center, and stiffened ones toward the sidewalls in laminar flow (49, 50). These studies show significant correlations between storage-induced osmotic fragility and storage-induced stiffness in RBCs (49), and deduce storage time from the fraction of stiff RBCs (50).
Such deformability-based fractionation of stored RBCs can also be achieved with higher fidelity using a microfluidic ratchet device (51). This device features an array of constrictions (tapered micropillars) and 12 outlets. The size of constrictions varies gradually perpendicular to the sample flow direction. Using an oscillatory crossflow, cells can be filtered through constrictions until they reach a limiting constriction size. The device can fractionate cells into 12 stiffness levels to study donor and storage-induced variability in RBC stiffness. A micropillar array of reducing constriction size along the flow direction can also be used to measure RBC deformation (52). This device (OcclusionChip) mimics a transition from capillaries to microcapillaries and reports the number of occluded constrictions in each array zone. This allows the quantification of deformability associated with microcapillary occlusion (53). This device has been integrated with microelectrode arrays to enable real-time, electronic, portable readout of RBC microcapillary occlusion via the Microfluidic Impedance Red Cell Assay (111). The American Medical Association has issued the first and only CPT® Proprietary Laboratory Analyses Codes (CPT® 0303U and 0304U) for measuring RBC health using this LOC technology.
An active research area using LOCs is measuring RBC metabolite release. Two examples feature measurements of the ability of stored RBCs to i) release oxygen (112) and ii) release ATP and nitric oxide (NO) in circulation rather than measuring the intracellular levels (113, 114). Measurement of oxygen release rate is possible using precisely controlled microenvironments at the single-cell level in LOCs via luminescent probes (112). Similarly, ATP and NO can be facilitated by the chemiluminescent detection of the luciferin/luciferase reaction for ATP and amperometric (113) or fluorogenic detection for NO (114). Indeed, LOCs can measure ATP and NO release from RBCs (113) and quantify the downstream effects of ATP release—stimulation of NO release by endothelial cells—by integrated microfluidic components (114). These studies, together, have assessed the quality of stored RBCs with different glucose levels and compared normoxic and hypoxic conditions for storage.
An inevitable and detrimental effect of RBC storage is oxidative stress, which usually correlates well with hemolysis and, thus, the real age of stored RBCs. A study shows that oxidative stresses of stored RBCs can be predicted from the dielectrophoretic mobility of the cells. Specifically, an optical trap was used to immobilize RBCs in a microfluidic chip, and then a dielectrophoretic trap was activated to measure the dielectrophoretic force on each RBC (115). This study indicates that longer stored RBCs and RBCs subjected to oxidative stress have significantly lower dielectrophoretic mobilities compared to fresh and shorter-stored RBCs.
A Convergent Future of RBC Quality Assessment for Precision Transfusion Medicine
How best to assess the freshness, quality, and transfusion efficacy of unique RBC units is still unresolved. The current RBC transfusion workflow fails to assess the donor-, time-, and processing-dependent quality (real age) of RBCs prior to transfusion. The inability to use real age dismisses variables that affect RBC quality and the suitability of the unit for the particular patient. Consequently, a long list of complications, ranging from sepsis and multiorgan failure to other morbidities, and ultimately mortality can occur following RBC transfusion. This is especially true for critically and chronically ill patients who require multiple transfusions.
We have highlighted the lack of quantitative assessment of stored RBCs before transfusion on a unit-by-unit basis, a notable failure in this era of personalized medicine. We foresee that, over the next decade, state-of-the-art technologies such as -omics and ML can be combined to first identify a set of key quantitative quality metrics (i.e., a quality index) for stored RBCs. This work is already underway, led by the pioneers in the field (116). Subsequent translation of such quality metrics to a noninvasive and continuous assessment of RBC quality during storage could enable better and more appropriate allocation, less waste, and, most importantly, safer transfusion with better efficacy for all patients.
LOC platforms are now mature enough to be integrated with advanced biosensors featuring flexible electronics and wireless transmitters. We anticipate that such integrated LOCs can be installed on every RBC storage bag and enable measurements of multiple biochemical and morphological parameters. Accordingly, we envision a future where LOCs leverage quantitative quality indices—identified by -omics and ML– to monitor stored RBC quality continuously and affordably (Fig. 4).
Fig. 4.
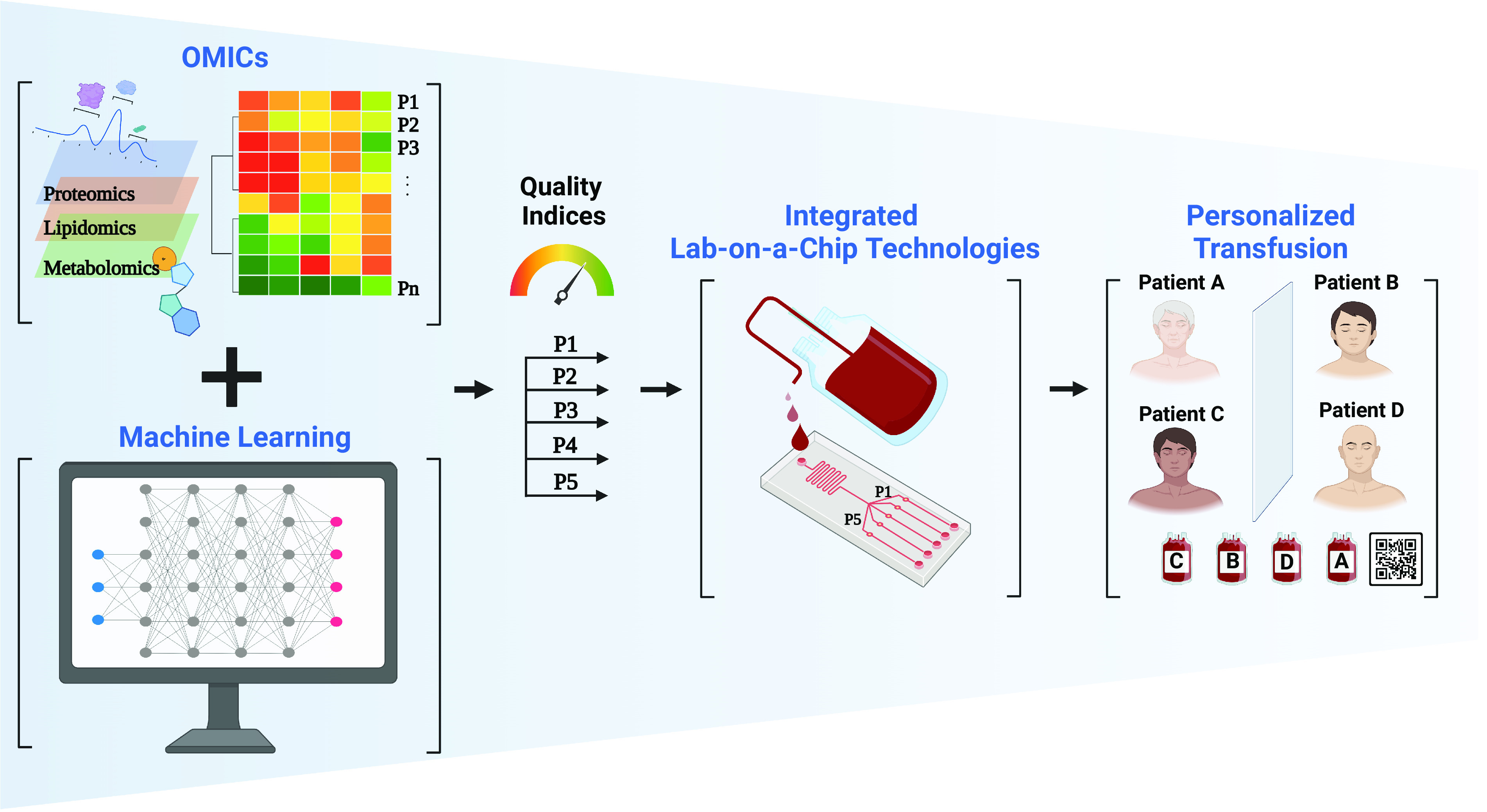
Convergent future of stored RBC assessment toward precision transfusion. Novel -omics and ML technologies have recently been used to assess RBC storage lesions. We envision that the integration of these technologies will identify key quality metrics (quality indices) for stored RBCs in the near future. These quality indices can then guide LOC platforms armed with novel biosensors to continuously monitor stored RBC quality and then match the properties of the RBC unit to the needs of the patient.
Limitations and Challenges for Precision Transfusion Medicine.
The path to our envisioned convergent future (Fig. 4), where integrated LOCs on each RBC unit provide quality assessment prior to transfusion, is not without its hurdles and challenges. The ASSURED criteria for LOC platforms stand for “affordable, sensitive, specific, user‐friendly, rapid and robust, equipment free, and deliverable to end‐user” (117). As summarized in this Perspective, the current LOC platforms developed for stored RBC assessment present a promising foundation. For successful translation to blood banks, we envision that these platforms must address three main technical challenges in terms of sampling, detection and detection miniaturization, and integration with blood units.
First, the sampling of RBC units should be performed without any contamination and any changes to the quality of the unit, i.e., in a noninvasive manner. Further, the sampling should be representative of the entire unit and bias-free. Given that an advantage of LOCs is the use of low volumes compared to traditional assays, this implies the use of repeated or continuous sampling to ensure robust and representative measurements. While current sampling approaches such as sampling site couples or sterile connected containers can be used in early nonintegrated versions of LOC safety assessments, they are cumbersome and impractical for the long-term integrated and streamlined vision for RBC safety assessment. In this regard, engineering solutions providing small volume, and one-way/contamination-free sampling from inside the RBC units will be necessary.
Second, the detection of biomarkers for quality assessments should be performed in reasonably miniaturized platforms suitable for integration with 180- to 500-mL storage bags. Further, the ideal set of quality indices for RBC efficacy might necessitate multiple different detection modalities, which may pose additional challenges for achieving a reasonably small footprint for the integrated LOC platforms. Currently, most of the research LOC platforms for RBC analyses feature cumbersome detection techniques such as benchtop microscopic imaging. Miniaturization and integration of detection units on LOCs requires advanced engineering approaches. Reassuringly, many examples of commercially available LOCs that are stand-alone and portable demonstrate feasibility. Furthermore, continuous advances in microelectronics and microelectromechanical system devices and micro/nano fabrication are expected to enable manufacturing of miniaturized detection units. A common limitation for LOC platforms used in research for RBC assessment thus far has been their inability to work with the high hematocrit samples that are the norm for packed RBC units. This detection-related limitation can likely be overcome with on-chip manipulation, dilution, and multiplexing approaches.
Once the best strategy to perform aseptic and noninvasive sampling is established and the LOC platforms are sufficiently miniaturized, the next step will be integration of these platforms with the storage bags. We envision two routes for this integration. The first features a fully closed system where sampled volumes are routed to an LOC platform that is integrally manufactured with the blood bags. The second approach features a modular cartridge-based system that can accommodate different LOC platform designs through a universal fluid coupling port. The latter approach might allow rapid iteration of the platform technologies without affecting the design of the rest of the blood bag unit. The final engineering step is to integrate user-friendly reporting of the generated data through wireless technologies and cloud-based data management approaches.
Beyond the technical and practical challenges, the economics of integrating LOC platforms into the transfusion medicine workflow needs to be addressed. This challenge can be overcome by economies of scale and rapid advances in fabrication of microsystems technologies, which are expected to bring the unit costs down. The rapid cost and size reduction that we have witnessed during the microprocessor revolution is a prime example of how similar economic challenges have been overcome. User-friendly implementation of LOC platforms should impose few new skillset requirements, which will ease the adoption process.
Concluding Remarks.
LOC technologies can ensure a more successful transfusion workflow by enabling objective assessment of stored RBC units using quality metrics identified by -omics and ML, thus ushering in a new era of precision transfusion medicine. Addressing the potential and challenges of precision transfusion medicine will facilitate the proposed convergent future, as depicted in Fig. 4. This will require all stakeholders (including experts in blood banking, biopreservation, transfusion medicine, -omics, ML, bioengineering, ethics, regulation, and ultimately patients) to participate in multidisciplinary discussions and collaborations rather than their current isolated efforts. This Perspective—where we have a diverse team of such stakeholders—is a step toward this envisioned era of precision in transfusion medicine.
Acknowledgments
This work was supported partially by grants from the National Institutes of Health (R21GM136002, R21GM141683, and R21GM10656 for O.B.U., A.A.G., and M.L.Y., R01AR081529 for O.B.U. and A.A.G., and R01HL145031 for O.B.U., J.P.A., Z.I., and M.L.Y.; R00HL143149, R01HL157803, and R01DK134590 for S.N.T.; and R01HL146442, R01HL149714, R01HL148151, and R21HL150032 for A.D.) and National Science Foundation (EEC-1941543 for O.B.U., Z.I., A.A.G., M.L.Y., M.T., J.B., S.N.T., and S.M.W.). C.E. was supported by the DigiHealth strategic profiling project (Academy of Finland no. 326291) and by the European Union (European Research Council, BiNET, 101043314). The content of this article is the responsibility of the authors and does not necessarily represent the official views of the NIH or the NSF. Figures were created with BioRender.com.
Author contributions
Z.I., A.D., J.P.A., and O.B.U. designed research; Z.I., A.D., J.P.A., and O.B.U. performed research; Z.I., A.D., S.M.W., D.H.M., S.N.T., E.K., A.A.G., N.W., R.D.S., J.B., N.M., M.P.B., C.E., U.A.G., M.T., J.P.A., M.L.Y., and O.B.U. reviewed and revised; and Z.I., A.D., S.M.W., D.H.M., S.N.T., E.K., A.A.G., N.W., R.D.S., J.B., N.M., M.P.B., C.E., U.A.G., M.T., J.P.A., M.L.Y., and O.B.U. wrote the paper.
Competing interests
U.A.G. and Case Western Reserve University have financial interests in Hemex Health Inc. and Xatek Inc. U.A.G., E.K., and Case Western Reserve University have financial interests in BioChip Labs Inc. U.A.G. has financial interests in DxNow Inc. Financial interests include licensed intellectual property, stock ownership, research funding, employment, and consulting. Hemex Health Inc. offers point-of-care diagnostics for hemoglobin disorders, anemia, and malaria. BioChip Labs Inc. offers commercial clinical microfluidic biomarker assays for inherited or acquired blood disorders. Xatek Inc. offers point-of-care global assays to evaluate the hemostatic process. DxNow Inc. offers microfluidic and bio-imaging technologies for in vitro fertilization, forensics, and diagnostics. Competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. The study was conducted prior to E.K. employment at IDEXX Laboratories, and the ideas expressed are not in his capacity as an IDEXX employee. A.D. serves on the Scientific Advisory Board of Hemanext Inc. and Macopharma Inc.; his competing interests are managed by the University of Colorado in accordance with their conflict-of-interest policies. A.D. owns stock in Omix Technologies Inc. A.D. has multiple patents on novel blood storage strategies. S.N.T., M.T., R.D.S., and M.L.Y. have multiple patent applications that relate blood storage and storage in general. The remaining authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Angelo D’Alessandro, Email: angelo.dalessandro@cuanschutz.edu.
O. Berk Usta, Email: ousta@mgh.harvard.edu.
Data, Materials, and Software Availability
There are no data underlying this work.
References
- 1.Jones J. M., et al. , Has the trend of declining blood transfusions in the United States ended? Findings of the 2019 National Blood Collection and Utilization Survey. Transfusion 61, S1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein H. G., Spahn D. R., Carson J. L., Red blood cell transfusion in clinical practice. Lancet 370, 415–426 (2007). [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, “Global status report on blood safety and availability 2021” (2022). https://www.who.int/publications/i/item/9789240051683.
- 4.Moore S. B., A brief history of the early years of blood transfusion at the Mayo Clinic: The first blood bank in the United States (1935). Transfus. Med. Rev. 19, 241–245 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Stansbury L. G., Hess J. R., Blood transfusion in World War I: The roles of Lawrence Bruce Robertson and Oswald Hope Robertson in the “most important medical advance of the war”. Transfus. Med. Rev. 23, 232–236 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Mollison P. L., The introduction of citrate as an anticoagulant for transfusion and of glucose as a red cell preservative. Br. J. Haematol. 108, 13–18 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Loutit J. F., Mollison P. L., Disodium-Citrate—Glucose Mixture as a Blood Preservative. Br Med J 2, 744–745 (1943). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prowse C. V., de Korte D., Hess J. R., van der Meer P. F., Commercially available blood storage containers. Vox Sang. 106, 1–13 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention, “Monitoring Blood Safety” (2022). https://www.cdc.gov/bloodsafety/monitoring/blood_safety.html.
- 10.Sut C., et al. , Duration of red blood cell storage and inflammatory marker generation. Blood Transfus. 15, 145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel R., et al. , Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion 56, 1690–1698 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Sparrow R. L., Red blood cell storage and transfusion-related immunomodulation. Blood Transfus. 8, s26 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont L. J., Aubuchon J. P., Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion 48, 1053–1060 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Hess J. R., Scientific problems in the regulation of red blood cell products. Transfusion 52, 1827–1835 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Yoshida T., Prudent M., D’Alessandro A., Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 17, 27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J. S., Gladwin M. T., Bad Blood: The risks of red cell storage. Nat. Med. 16, 381–382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan K., et al. , Stored blood has compromised oxygen unloading kinetics that can be normalized with rejuvenation and predicted from corpuscular side-scatter. Haematologica 107, 298–302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabcuka J., et al. , Metabolic reprogramming under hypoxic storage preserves faster oxygen unloading from stored red blood cells. Blood Adv. 6, 5415–5428 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Alessandro A., D’Amici G. M., Vaglio S., Zolla L., Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica 97, 107–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roussel C., et al. , Rapid clearance of storage-induced microerythrocytes alters transfusion recovery. Blood 137, 2285–2298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch C. G., et al. , Duration of red-cell storage and complications after cardiac surgery. N. Engl. J. Med. 358, 1229–1239 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Weinberg J. A., et al. , Duration of red cell storage influences mortality after trauma. J. Trauma 69, 1427–1432 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparrow R. L., Red blood cell storage duration and trauma. Transfus. Med. Rev. 29, 120–126 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Weinberg J. A., et al. , The deleterious effect of red blood cell storage on microvascular response to transfusion. J. Trauma Acute Care Surg. 75, 807 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn S. A., The red blood cell storage lesion: A method to the madness. Transfusion 50, 1164–1169 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Bennett-Guerrero E., et al. , Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. U.S.A. 104, 17063–17068 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glynn S. A., Klein H. G., Ness P. M., The red blood cell storage lesion: The end of the beginning. Transfusion 56, 1462–1468 (2016). [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A., Liumbruno G. M., Red blood cell storage and clinical outcomes: New insights. Blood Transfus. 15, 101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimring J. C., Spitalnik S. L., Large retrospective effects, clear differences in animals, and multiple negative randomised controlled trials: This is exactly how it is supposed to work. Blood Transfus. 15, 104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai-Adisaksopha C., et al. , Mortality outcomes in patients transfused with fresher versus older red blood cells: A meta-analysis. Vox Sang. 112, 268–278 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Steiner M. E., et al. , Effects of red-cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med. 372, 1419–1429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira A., Will clinical studies elucidate the connection between the length of storage of transfused red blood cells and clinical outcomes? An analysis based on the simulation of randomized controlled trials Transfusion 53, 34–40 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Koch C. G., et al. , Real Age: Red blood cell aging during storage. Ann. Thorac. Surg. 107, 973–980 (2019). [DOI] [PubMed] [Google Scholar]
- 34.D’Alessandro A., et al. , Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica 106, 1290–1302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanias T., et al. , Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: Results of the REDS-III RBC-Omics study. Blood Adv. 1, 1132–1141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazegh K., et al. , Blood donor obesity is associated with changes in red blood cell metabolism and susceptibility to hemolysis in cold storage and in response to osmotic and oxidative stress. Transfusion 61, 435–448 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander K., et al. , Testosterone replacement therapy in blood donors modulates erythrocyte metabolism and susceptibility to hemolysis in cold storage. Transfusion 61, 108–123 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefanoni D., et al. , Nicotine exposure increases markers of oxidant stress in stored red blood cells from healthy donor volunteers. Transfusion 60, 1160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paglia G., et al. , Biomarkers defining the metabolic age of red blood cells during cold storage. Blood 128, e43–e50 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Doan M., et al. , Objective assessment of stored blood quality by deep learning. Proc. Natl. Acad. Sci. U.S.A. 117, 21381–21390 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamoureux E. S., et al. , Assessing red blood cell deformability from microscopy images using deep learning. Lab Chip 22, 26–39 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Manz A., Graber N., Widmer H. M., Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuat. B Chem. 1, 244–248 (1990). [Google Scholar]
- 43.Whitesides G. M., The origins and the future of microfluidics. Nat. 4427101 442, 368–373 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Isiksacan Z., Guler M. T., Kalantarifard A., Asghari M., Elbuken C., "Lab-on-a-Chip platforms for disease detection and diagnosis" in Biosensors and Nanotechnology: Applications in Health Care Diagnostics, Altintas Z., Ed. (John Wiley & Sons, Hoboken, NJ, USA, 2018) pp. 155–181. [Google Scholar]
- 45.Iyer V., Yang Z., Ko J., Weissleder R., Issadore D., Advancing microfluidic diagnostic chips into clinical use: A review of current challenges and opportunities. Lab Chip 22, 3110–3121 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z., et al. , Stiffness increase of red blood cells during storage. Microsystems Nanoeng. 4, 1–6 (2018). [Google Scholar]
- 47.Zheng Y., et al. , Characterization of red blood cell deformability change during blood storage. Lab Chip 14, 577–583 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Saadat A., et al. , A system for the high-throughput measurement of the shear modulus distribution of human red blood cells. Lab Chip 20, 2927–2936 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Huang S., et al. , Towards microfluidic-based depletion of stiff and fragile human red cells that accumulate during blood storage. Lab Chip 15, 448–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y., Feng Y., Wan J., Chen H., Enhanced separation of aged RBCs by designing channel cross section. Biomicrofluidics 12, 024106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Islamzada E., et al. , Deformability based sorting of stored red blood cells reveals donor-dependent aging curves. Lab Chip 20, 226–235 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Man Y., et al. , Microfluidic assessment of red blood cell mediated microvascular occlusion. Lab Chip 20, 2086–2099 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurkan U. A., Biophysical and rheological biomarkers of red blood cell physiology and pathophysiology. Curr. Opin. Hematol. 28, 138–149 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Alessandro A., Liumbruno G., Grazzini G., Zolla L., Red blood cell storage: The story so far. Blood Transfus. 8, 82–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Alessandro A., et al. , An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 55, 205–219 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Hess J. R., Measures of stored red blood cell quality. Vox Sang. 107, 1–9 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Kim-Shapiro D. B., Lee J., Gladwin M. T., Storage lesion: Role of red blood cell breakdown. Transfusion 51, 844–851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagerberg J. W., Korsten H., Van Der Meer P. F., De Korte D., Prevention of red cell storage lesion: A comparison of five different additive solutions. Blood Transfus. 15, 456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Relevy H., Koshkaryev A., Manny N., Yedgar S., Barshtein G., Blood banking-induced alteration of red blood cell flow properties. Transfusion 48, 136–146 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Custer B., et al. , Proceedings of the 2022 NHLBI and OASH state of the science in transfusion medicine symposium. Transfusion 63, 1074–1091 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Solomon S. B., et al. , Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood 121, 1663–1672 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortés-Puch I., et al. , In a canine pneumonia model of exchange transfusion, altering the age but not the volume of older red blood cells markedly alters outcome. Transfusion 55, 2564–2575 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rapido F., et al. , Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Invest. 127, 375–382 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mykhailova O., Olafson C., Turner T. R., D'Alessandro A., Acker J. P., Donor-dependent aging of young and old red blood cell subpopulations: Metabolic and functional heterogeneity. Transfusion 60, 2633–2646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nemkov T., Hansen K. C., Dumont L. J., D’Alessandro A., Metabolomics in transfusion medicine. Transfusion 56, 980–993 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nemkov T., Yoshida T., Nikulina M., D’Alessandro A., High-throughput metabolomics platform for the rapid data-driven development of novel additive solutions for blood storage. Front. Physiol. 13, 466 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Alessandro A., et al. , Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: Lessons from REDS-III-Omics. Transfusion 59, 89–100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanias T., Acker J. P., Biopreservation of red blood cells–the struggle with hemoglobin oxidation. FEBS J. 277, 343–356 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Mast A. E., et al. , The benefits of iron supplementation following blood donation vary with baseline iron status. Am. J. Hematol. 95, 784–791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roubinian N. H., et al. , Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood 134, 1003–1013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Josephson C. D., et al. , The recipient epidemiology and donor evaluation study-IV-pediatric (REDS-IV-P): A research program striving to improve blood donor safety and optimize transfusion outcomes across the lifespan. Transfusion 62, 982–999 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Page G. P., et al. , Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J. Clin. Invest. 131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Francis R. O., et al. , Donor glucose-6-phosphate dehydrogenase deficiency decreases blood quality for transfusion. J. Clin. Invest. 130, 2270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tzounakas V. L., et al. , Beta thalassemia minor is a beneficial determinant of red blood cell storage lesion. Haematologica 107, 112–125 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Issaian A., et al. , The interactome of the N-terminus of band 3 regulates red blood cell metabolism and storage quality. Haematologica 106, 2971–2985 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stolwijk J. M., et al. , Red blood cells contain enzymatically active GPx4 whose abundance anticorrelates with hemolysis during blood bank storage. Redox Biol. 46, 102073 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pertinhez T. A., et al. , A comparative study of the effect of leukoreduction and pre-storage leukodepletion on red blood cells during storage. Front. Mol. Biosci. 3, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nemkov T., et al. , Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight 6, e146175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Alessandro A., et al. , Ethyl glucuronide, a marker of alcohol consumption, correlates with metabolic markers of oxidant stress but not with hemolysis in stored red blood cells from healthy blood donors. Transfusion 60, 1183–1196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.D’Alessandro A., et al. , Stored RBC metabolism as a function of caffeine levels. Transfusion 60, 1197 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D’Alessandro A., Zimring J. C., Busch M., Chronological storage age and metabolic age of stored red blood cells: Are they the same? Transfusion 59, 1620–1623 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Nemkov T., et al. , Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 103, 361–372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sebastian J. A., Kolios M. C., Acker J. P., Emerging use of machine learning and advanced technologies to assess red cell quality. Transfus. Apher. Sci. 59, 103020 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Ching T., et al. , Opportunities and obstacles for deep learning in biology and medicine. J. R. Soc. Interface 15, 20170387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ning S., Li N., Barty R., Arnold D., Heddle N. M., Database-driven research and big data analytic approaches in transfusion medicine. Transfusion 62, 1427–1434 (2022). [DOI] [PubMed] [Google Scholar]
- 86.Gunčar G., et al. , An application of machine learning to haematological diagnosis. Sci. Reports 8, 1–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doan M., et al. , Label-free leukemia monitoring by computer vision. Cytom. Part A 97, 407–414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blasi T., et al. , Label-free cell cycle analysis for high-throughput imaging flow cytometry. Nat. Commun. 7, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng Y., Xu Z., Sun X., Wang D., Yu Y., Machine learning for predicting preoperative red blood cell demand. Transfus. Med. 31, 262–270 (2021). [DOI] [PubMed] [Google Scholar]
- 90.Patel R. M., et al. , Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion 55, 544–552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rolfsson O., et al. , Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 112, 326–335 (2017). [DOI] [PubMed] [Google Scholar]
- 92.D'Alessandro A., et al. , Hypoxic storage of red blood cells improves metabolism and post-transfusion recovery. Transfusion 60, 786–798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gehrke S., et al. , Metabolic impact of red blood cell exchange with rejuvenated redblood cells in sickle cell patients. Transfusion 59, 3102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guler M. T., Isiksacan Z., Serhatlioglu M., Elbuken C., Self-powered disposable prothrombin time measurement device with an integrated effervescent pump. Sensors Actuators, B Chem. 273, 350–357 (2018). [Google Scholar]
- 95.Melin J., Quake S. R., Microfluidic large-scale integration: The evolution of design rules for biological automation. Annu. Rev. Biophys. Biomol. Struct. 36, 213–231 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Isiksacan Z., Erel O., Elbuken C., A portable microfluidic system for rapid measurement of the erythrocyte sedimentation rate. Lab Chip 16, 4682–4690 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Isiksacan Z., Serhatlioglu M., Elbuken C., In vitro analysis of multiple blood flow determinants using red blood cell dynamics under oscillatory flow. Analyst 145, 5996–6005 (2020). [DOI] [PubMed] [Google Scholar]
- 98.Isiksacan Z., Hastar N., Erel O., Elbuken C., An optofluidic point-of-care device for quantitative investigation of erythrocyte aggregation during coagulation. Sensors Actuators, A Phys. 281, 24–30 (2018). [Google Scholar]
- 99.Sun L., Lehnert T., Li S., Gijs M. A. M., Bubble-enhanced ultrasonic microfluidic chip for rapid DNA fragmentation. Lab Chip 22, 560–572 (2022). [DOI] [PubMed] [Google Scholar]
- 100.Kuan D. H., Huang N. T., Recent advancements in microfluidics that integrate electrical sensors for whole blood analysis. Anal. Methods 12, 3318–3332 (2020). [DOI] [PubMed] [Google Scholar]
- 101.Martín A., et al. , Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sensors 2, 1860–1868 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Mishra A., et al. , Ultrahigh-throughput magnetic sorting of large blood volumes for epitope-agnostic isolation of circulating tumor cells. Proc. Natl. Acad. Sci. U.S.A. 117, 16839–16847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hindson B. J., et al. , High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang L., Chen Q., Ma Y., Sun J., Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl. Bio Mater. 3, 107–120 (2020). [DOI] [PubMed] [Google Scholar]
- 105.Sinton D., Kelley S. O., AbCellera’s success is unprecedented: What have we learned? Lab Chip 21, 2330–2332 (2021). [DOI] [PubMed] [Google Scholar]
- 106.Duncombe T. A., Tentori A. M., Herr A. E., Microfluidics: Reframing biological enquiry. Nat. Rev. Mol. Cell Biol. 16, 554–567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gach P. C., Iwai K., Kim P. W., Hillson N. J., Singh A. K., Droplet microfluidics for synthetic biology. Lab Chip 17, 3388–3400 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Jeffrey S. S., Toner M., Liquid biopsy: A perspective for probing blood for cancer. Lab Chip 19, 548–549 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Bhatia S. N., Ingber D. E., Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Vinoth R., Nakagawa T., Mathiyarasu J., Mohan A. M. V., Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sensors 6, 1174–1186 (2021). [DOI] [PubMed] [Google Scholar]
- 111.Man Y., et al. , Microfluidic electrical impedance assessment of red blood cell-mediated microvascular occlusion. Lab Chip 21, 1036–1048 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chng K. Z., et al. , Assessment of transient changes in oxygen diffusion of single red blood cells using a microfluidic analytical platform. Commun. Biol. 4, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hayter E. A., et al. , A 3D-printed, multi-modal microfluidic device for measuring nitric oxide and ATP release from flowing red blood cells. Anal. Methods 14, 3171–3179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y., Giebink A., Spence D. M., Microfluidic evaluation of red cells collected and stored in modified processing solutions used in blood banking. Integr. Biol. 6, 65–75 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Jeon H. J., Lee H., Yoon D. S., Kim B. M., Dielectrophoretic force measurement of red blood cells exposed to oxidative stress using optical tweezers and a microfluidic chip. Biomed. Eng. Lett. 7, 317–323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D'Alessandro A., et al. , Red blood cell omics and machine learning in transfusion medicine: Singularity is near. Transfus. Med. Hemother. 50, 174–183 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Land K. J., Boeras D. I., Chen X. S., Ramsay A. R., Peeling R. W., REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.