Abstract
Background
This study aimed to report clinical practice patterns of postoperative radiotherapy for stage I to II endometrial carcinoma (EC) patients treated in 13 Chinese medical centers.
Methods
We included early stage EC patients treated by hysterectomy and adjuvant RT between 2003 and 2017 from 13 institutions. Patients were classified into 4 risk groups based on ESMO-ESGO-ESTRO recommendations (2014).
Results
A total of 1,227 cases were analyzed. Along the 15 years of the study, an increasing tendency was found towards administration for vaginal brachytherapy (VBT) alone, while the proportion of external beam pelvic radiotherapy (EBRT) alone remained stable in the corresponding period. When radiation modalities were stratified by risk groups, proportion of VBT alone significantly increased in all risk groups. The higher the risk, the later VBT became the main adjuvant treatment modality. However, EBRT alone or with VBT remained the main adjuvant method for high-risk patients.
There were 13 dose-fractionation schemes for VBT alone with the scheme of 30 Gy in 6 fractions prescribed at 0.5cm under the vaginal mucosa accounting for most. There were 17 schemes for VBT boost and the most common schedule was 10 Gy in 2 fractions. The upper 3–5cm part of vagina was the most frequent target. 89.6% of the practitioners performed two-dimensional VBT technique. The median dose for EBRT was 50 Gy. From 2003 to 2017, conventional radiotherapy was gradually replaced by three-dimensional conformal radiotherapy modality and intensity modulated radiotherapy.
Conclusion
We report a significant shift from EBRT to VBT alone for high-intermediate-risk, intermediate-risk and low-risk EC patients from 2003 to 2017 while EBRT remained the main radiation modality for high-risk early stage patients. There has been remarkable heterogeneity among VBT dose fractionation schedules across China.
Trial registration
The clinical trial ID was ChiCTR-PRC-17010712. It was authorized by the Institutional Review Board of Peking Union Medical College Hospital (N0. S-K139).
Keywords: External beam pelvic radiotherapy, Endometrial neoplasms, Vaginal brachytherapy, Practice patterns
Introduction
Tumor of the uterus is the secondly diagnosed gynecological tumor in China, with endometrial cancer (EC) accouting for most of it [1]. Due to the obvious clinical manifestations, majority of EC patients are diagnosed early. Surgery is the main procedure for EC confined to the uterus. For patients with risk factors, such as lympho-vascular space invasion, high grade and deep myometrial invasion, postoperative radiotherapy including external beam pelvic radiotherapy (EBRT) and/or vaginal brachytherapy (VBT) is recommended [2–4].
As many randomized researches have been performed [5–10] along with increasing institutional data, the clinical practice patterns of VBT and/or EBRT in the postoperative management of EC continues to change. In 2005, the American Brachytherapy Society (ABS) published data on clinical utilization of postoperative RT and in 2016, the data was updated [11, 12]. They demonstrated an increasing trend towards administration for VBT alone. Modh et al. [13] published data on trends in the real practice on utilization of VBT alone vs. EBRT or EBRT with VBT for stage I-II (FIGO 1988) EC by Surveillance, Epidemiology, and End Results database. He reported a significant increase for the use of VBT alone from 1995 to 2012, which was not limited to histological type, age, stage et al. Meanwhile, the proportion of EBRT with or without VBT decreased during the same period. However, there was limited data on clinical practice patterns of VBT and EBRT in China.
In this research, we would demonstrate radiotherapy pattern evolvement, treatment planning and dose fractionation schedules for early stage EC patients treated between 2003 to 2017 from 13 institutions in China.
Methods and materials
Data of EC patients treated between 2003.1 and 2017.12 from 13 institutions in China was retrospectively reviewed. Patients of the following clinical characteristics were included: hysterectomy followed by postoperative RT, stage I to II, and complete clinical data. International Federation of Gynecology and Obstetrics (FIGO) (2009) staging system was used for all patients. Patients were stratified into high-risk (HR), high-intermediate-risk (HIR), intermediate-risk (IR) and low-risk (LR) groups based on ESMO-ESGO-ESTRO consensus (2014). This trial was retrospectively registered at 2017.02.23 and the clinical trial number is ChiCTR-PRC-17010712.
SPSS software was used for data analysis. The Kaplan–Meier method was performed to estimate survival outcomes. We considered a p-value < 0.05 statistically significant.
Results
Patients and treatments
One thousand two hundred twenty-seven early stage EC cases were included (Table 1). The pathology of most patients was endometrioid adenocarcinoma (92.7%, n = 1138). Percentage of HR, HIR, IR and LR groups were: 25.9% (n = 318), 19.2% (n = 235), 27.2% (n = 334) and 27.7% (n = 340), respectively.
Table 1.
Baseline clinical characteristics for all patients treated from 2003 to 2017
| Patients(N = 1,227) | ||
|---|---|---|
| Clinical Characteristic | No | % |
| Age, years | ||
| Mean | 56.2 | |
| Range | 23–86 | |
| Lymphadenectomy | ||
| No | 361 | 29.4 |
| Yes | 866 | 70.6 |
| Mean Number | 23.7 | |
| Range | 1–99 | |
| Pathology type | ||
| Endometrioid Adenocarcinoma | 1137 | 92.7 |
| Nonendometrioid Carcinoma | ||
| Mixed cell carcinoma | 43 | 3.5 |
| Serous carcinoma | 22 | 1.8 |
| Clear cell carcinoma | 16 | 1.3 |
| Undifferentiated carcinoma | 6 | 0.5 |
| Others | 3 | 0.2 |
| Stage(FIGO 2009) | ||
| IA | 566 | 46.1 |
| IB | 500 | 40.7 |
| II | 161 | 13.1 |
| Diameter | ||
| < 2 cm | 141 | 11.5 |
| ≥ 2 cm | 685 | 55.8 |
| Missing | 401 | 32.7 |
| Gradea | ||
| G1 | 395 | 34.7 |
| G2 | 518 | 45.5 |
| G3 | 219 | 19.2 |
| Missing | 6 | 0.5 |
| Myometrial invasion | ||
| < 1/2 | 638 | 52.0 |
| ≥ 1/2 | 581 | 47.4 |
| Missing | 8 | 0.7 |
| Invasion of lower uterine segment | ||
| No | 885 | 72.1 |
| Yes | 342 | 27.9 |
| Involvement of cervix | ||
| No | 975 | 79.5 |
| Yes | ||
| Cervical mucosa | 91 | 7.4 |
| Cervical stromal | 161 | 13.1 |
| Lympho-vascular Space Invasion | ||
| Present | 222 | 18.1 |
| Absent | 1005 | 81.9 |
| Chemotherapy | ||
| No | 997 | 81.3 |
| Yes | 230 | 18.7 |
Abbreviation: FIGO International Federation of Gynecology and Obstetrics
aonly for endometrioid adenocarcinoma
A hysterectomy and bilateral salpingo-oophorectomy surgery was performed for all cases. A total of 70.6% of all patients underwent lymphadenectomy. All patients underwent adjuvant RT, which included EBRT alone (n = 122), or with VBT (n = 491) and VBT alone (n = 614). EBRT was delivered to the pelvic lymphatic drainage regions and upper part of vagina. A total of 230 patients received chemotherapy. There were 154 patients in the HR group, 43 patients in the HIR group, 29 patients in the IR group, and 4 patients in the LR group, respectively. When used on the concurrent setting, cisplatin was administrated. When used as sequential chemotherapy, regimens such as paclitaxel, carboplatin/paclitaxel, cisplatin/doxorubicin and cisplatin/doxorubicin/paclitaxel were administrated. The number of adjuvant chemotherapy cycles ranged from 1 to 6.
RT Pattern evolvement
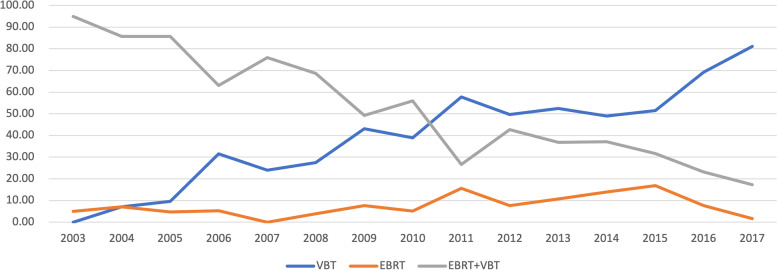
An increasing tendency was found towards utilization for VBT alone overall. EBRT with VBT was the main treatment modality from 2003 to 2010 and VBT dominated from 2011. In 2003, there was no record of VBT alone, and the proportion rose to 81.1% in 2017. Meanwhile, the proportion of EBRT with VBT decreased. In 2003, 95.0% of patients received combined EBRT and VBT, and the proportion decreased to 17.3% in 2017. The proportion of EBRT alone remained stable in the corresponding period (Fig. 1).
Fig. 1.
Radiotherapy pattern evolvement: percentage of different modalities from 2003 to 2017
In this research, trends of VBT alone, and EBRT with or without VBT were stratified by 4 risk groups (Table 2). Data demonstrated that the utilization of VBT alone increased remarkably among all groups from 2003 to 2017 while the proportion of EBRT with or without VBT decreased correspondingly in the period. In the LR group, VBT became the main adjuvant treatment early in the second five years (2008–2012). In the IR and HIR groups, VBT dominated in the third five years (2013–2017). In the HR group, EBRT with or without VBT remained the main adjuvant treatment modality from 2003 to 2017.
Table 2.
Clinical practice of VBT alone and EBRT (with or without VBT) among different risk groups from 2003 to 2017
| Patients(N = 1,227) | |||||
|---|---|---|---|---|---|
| 2003–2007 (%) | 2008–2012 (%) | 2013–2017 (%) | p | ||
| Low-risk (N = 340) | VBT alone (N = 243) | 36.1 a | 61.7 a | 85.8 a | 0.000 |
| EBRT with or without VBT (N = 97) | 63.9 a | 38.3 a | 14.2 a | ||
| Intermediate -risk (N = 334) | VBT alone (N = 184) | 6.1 a | 48.6 | 67.0 a | 0.000 |
| EBRT with or without VBT (N = 150) | 93.9 a | 51.4 | 33.0 a | ||
| High-intermediate-risk (N = 235) | VBT alone (N = 139) | 0.0 a | 42.0 | 66.3 a | 0.000 |
| EBRT with or without VBT (N = 96) | 100.0 a | 58.0 | 33.7 a | ||
| High-risk (N = 318) | VBT alone (N = 48) | 0.0 | 13.0 | 17.4 | 0.013 |
| EBRT with or without VBT (N = 270) | 100.0 | 87.0 | 82.6 | ||
Abbreviation: EBRT External Beam Radiation, VBT Vaginal Brachytherapy
aadjusted residuals, only values greater than ± 3 were marked
Treatment planning and dose fractionation
A total of 1105 patients received VBT, including 614 cases of VBT alone and 491 cases of VBT as a boost to EBRT. For patients receiving VBT, the vaginal irradiated target was mostly the proximal 3 to 5 cm (93.5%) of the vagina. The proximal 2 cm accounted for 6.3% and proximal 6 to 7 cm for 0.2% of the patients. The median irradiated vaginal length was 3 cm. Cylinders were the most commonly used applicators, followed by ovoids. A total of 1056 (95.6%) patients were treated with vaginal cylinders, including 154 (14.6%) single, central channel applicators and 902 (85.4%) multichannel vaginal applicators. For treatment of the vagina, the choice of applicator was both institution and doctor-dependent.
In terms of VBT planning pattern, 990 (89.6%) practitioners performed two-dimensional VBT technique, and others (115, 10.4%) used a three-dimensional VBT technique. For the former, all practitioners specified the dose to a 0.5 cm depth from the vaginal surface, while for the latter, clinical target volume was formed by expanding a 0.5 cm margin around the vaginal cylinder applicator excluding surrounding organs at risk. High-dose-rate appeared to be the only approach, accounting for 100% of all patients with diverse dose fractionation regimens. As to VBT alone, a total of 13 dose-fractionation schemes were performed, and for VBT boost, 17 dose-fractionation schemes were used. Overall, for VBT alone, the most common schedule was six fractions 5 Gy each, accounting for 87.8% (539/614) of all patients, followed by eight fractions 5 Gy each (2.6%, 16/614) (Fig. 2). For VBT boost, the most common schedule was two fractions 5 Gy each (42.4%, 208/491), followed by four fractions 5 Gy each (24.0%, 118/491) (Fig. 3).
Fig. 2.
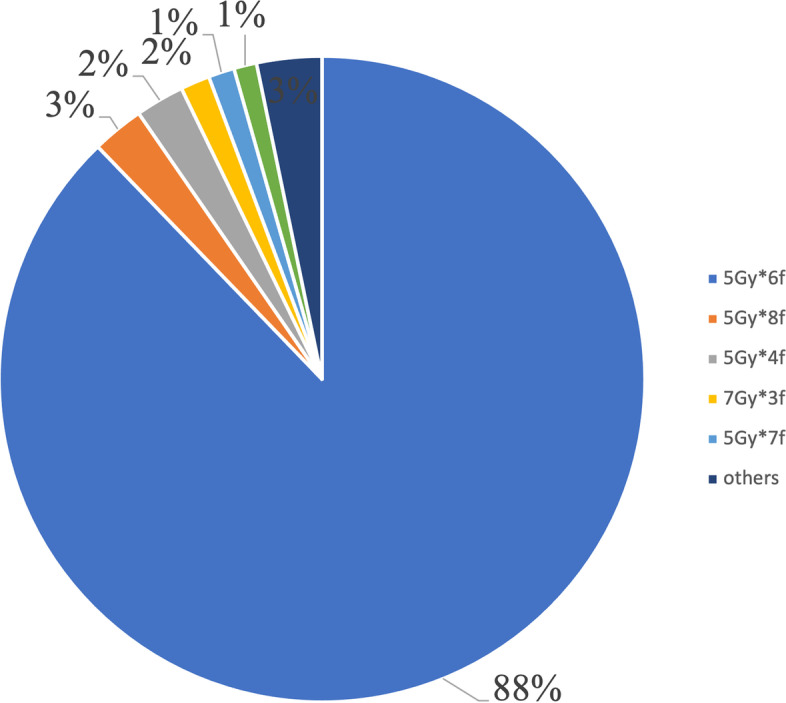
Dose-fractionation schemes for VBT alone
Fig. 3.
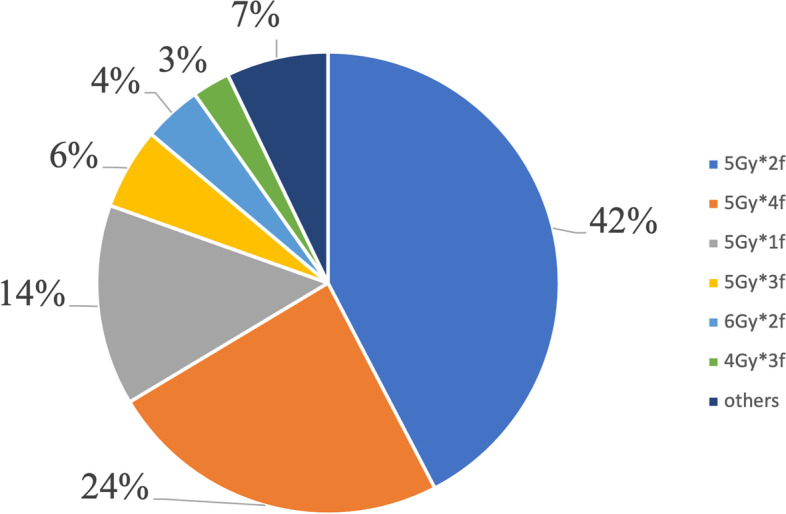
Dose-fractionation schemes for VBT boost
In all, 613 patients received EBRT. The median dose and fractions for EBRT was 50 Gy (ranging from 39.6 to 54.0 Gy), and 25 fractions (ranging from 20 to 30 fx) respectively. For treatment planning, the computer tomography-based intensity modulated radiotherapy technique (IMRT) (n = 302, 49.3%) accounted for most, followed by conventional technique (n = 166, 27.0%) and three-dimensional conformal radiotherapy modality (3D-CRT) (n = 145, 23.7%). In 2003, all patients received conventional RT. Along the 15 years of the study, conventional RT has been gradually replaced by 3D-CRT and IMRT. 3D-CRT peaked in 2011 (73.7%) and then gradually replaced by IMRT.
As to timing of adjuvant radiotherapy, when VBT alone was used, most patients initiated radiotherapy at 4–6 (45.0%, n = 276) weeks postoperatively. 25.6% (n = 157), 20.8% (n = 128) and 8.6% (n = 53) of patients initiated VBT alone at 6–8, > 8 and 2–4 weeks postoperatively, respectively. When EBRT was used, timing of radiotherapy was equally distributed. Patients initiated EBRT at 4–6 (32.0%, n = 196), > 8 (25.3%, n = 155), 2–4 (24.0%, n = 147) and 6–8 (18.7%, n = 115) weeks, respectively. As to the median overall treatment time, it was 38 days (range, 26 to 83 days) for patients receiving EBRT with VBT and it was 13 days (range, 4 to 33 days) for patients receiving VBT alone.
Survival
For all the patients, the overall follow-up time was 52 months (range, 2 to 204 months). The 5-year overall survival (OS), disease-free survival (DFS), local recurrence-free survival and distant metastasis-free survival rates were: 94.7%, 90.5%, 92.7% and 91.7%, respectively. When stratified by risk groups, 5-year OS rates were similar among HIR, IR and LR groups (95.9%, 95.5%, and 96.4%, respectively), while 5-year OS rate was lower for HR group (91.1%). The 5-year DFS rates were 84.8%, 90.2%, 92.2% and 94.1% for HR, HIR, IR and LR patients, respectively.
Discussion
In this study, we reported the clinical practice patterns, treatment planning and dose prescription of different radiation modalities for early stage EC patients treated in 13 Chinese institutions. These results suggested a significant shift from EBRT to VBT for LR, IR and HIR stage I to II EC over the study period. Adjuvant EBRT with or without VBT remained the main radiation modality for HR stage I to II patients. There was great heterogeneity on dose-fractionation schedules which was slightly different from what was performed in the USA.
RT Pattern evolvement
Radiotherapy patterns have evolved over the last two decades. From 2000 to 2010, prospective trials were conducted [5, 6, 8, 14] to compare postoperative EBRT with observation. The results demonstrated an increased local–regional control rate for patients receiving postoperative EBRT. With the promising data of the PORTEC-2 trial and other institutional researches [7, 9, 10], VBT alone was highly recommended for IR or HIR patients, as VBT was almost equally effective to EBRT in terms of disease control while women receiving VBT alone had a better life quality. The ABS performed a series of surveys on practice patterns of postoperative RT [11, 12] and reported an increasing trend towards referrals for VBT alone. Modh et al. [13] evaluated radiation patterns by Surveillance, Epidemiology, and End Results database for early stage patients treated from 1995 to 2012. He demonstrated the proportion of patients receiving VBT increased yearly while utilization of EBRT with VBT deceased significantly, which was consistent to our findings. However, a decreasing trend was shown on the use of EBRT alone from 1995 to 2012. That was different to our results that EBRT kept stable from 2003 to 2017. Besides, Modh et al. [13] reported that from 1995 to 2006, EBRT alone was the main adjuvant radiotherapy approach for all the EC patients and VBT dominated after 2007. This was different from our results that combined EBRT with VBT was the main treatment modality from 2003 to 2010. In the early years, some single-center experience on VBT as a boost have been reported [15–18]. Most data demonstrated EBRT with VBT and EBRT alone had equivalent local and regional control rates as well as OS [19, 20]. Therefore, combined EBRT with VBT was a choice in early years. The current view is, VBT as a boost to EBRT may be a choice for stage II (FIGO 2009) or grade 3 disease or lympho-vascular space invasion positive patients on an individual basis. As far as we know, most LR, IR and HIR patients in some centers in China received VBT alone since 2017. We look forward to our subsequent updated results.
As to trends in different risk groups, Modh et al. [13] demonstrated, the proportion of VBT alone increased significantly even for HR patients like stage II (FIGO 1988) disease or clear-cell/serous histology, which was consistent to our findings. However, in Modh’s research, VBT alone (67.4%) was the main RT modality for clear-cell/serous histology in 2012. In our research, EBRT with or without VBT was always preferred as the main RT modality in the HR group. Researches have been conducted for the proper treatment approach for high-risk early stage EC patients [21–24]. GOG249 [22] was designed to replace EBRT with combined chemotherapy and VBT. However, it failed to report a superiority of chemotherapy and VBT compared to EBRT on survival while the former had greater acute AE. In our study, the HR group included patients with stage IB grade 3 disease, stage II disease and serous/ clear-cell histology. Due to the small amount of HR patients, analysis stratified by various factors was not carried out which might interfere comparation to Modh’s research.
Treatment planning and dose fractionation
There was significant variety among VBT dose-fractionation schemes, as there were 13 different schedules as monotherapy and 17 as a boost, not to mention the diversity of dose-fractionation schemes of combined EBRT with VBT.
When VBT alone was used as adjuvant monotherapy, as Harkenrider et al. [2] summarized, in the US, the most commonly performed regimen was 3 fractions 7 Gy each prescribed to a 0.5-cm depth, followed by 5 fractions 6 Gy each prescribed to the vaginal surface. Since different doses per fraction would result in different biological effects, we used EQD210 (Equivalent Dose in 2 fractions, α/β = 10) to compare different dose-fractionation effects. The total 5 mm depth EQD210 (Gy) for 3 fractions 7 Gy each was 29.75 Gy and for 5 fractions 6 Gy each was 22.59. Different from that in the USA, the most commonly used scheme of VBT alone in China was 30 Gy in 6 fractions prescribed to 5 mm below the vaginal mucosa (total 5-mm depth EQD210 (Gy): 37.5 Gy), followed by 8 fractions 5 Gy each prescribed to 5 mm below the vaginal mucosa (total 5-mm depth EQD210 (Gy): 50 Gy). Therefore, the prescription dose of VBT alone in China has been higher than that in the United States. However, vaginal recurrence rates after surgery and adjuvant VBT were low in stage I to II EC patients and were not significantly different by VBT dose fractionation schedules [25].
When VBT was used as an EBRT boost, the dose fractionation schedules were variable, as there were diverse EBRT and VBT dose prescriptions. In this research, the median dose for EBRT was 50 Gy. Meanwhile, the commonly used scheme for VBT boost was 2 fractions 5 Gy each followed by 4 fractions 5 Gy each prescribed to a 0.5 cm depth. The recommended fractionation schedules by ABS included 45 Gy EBRT + 15–18 Gy in 3 fractions VBT to the vaginal surface or 50.4 Gy EBRT + 12 Gy in 2 fractions VBT to the vaginal surface [26]. The dose prescriptions were slightly different from those in the US.
The technical details and prescription doses for the administration of postoperative RT have been described in different guidelines. There were various dose fractionation prescriptions practically performed. There was no common view on the superiority of one scheme over others. Therefore, the ABS did not propose the best regimen [2, 26]. The prescription dose was the result of the balance between efficacy and toxicity, and was adjusted by different radiation oncologists in different regions. We do not aim to seek for the best treatment options but rather simply describe how we are actually performing evidenced-based care.
Limitations
The following were the limitations of this research. As a retrospective research, there might be selection bias and some pathology and chemotherapy details were not complete. As a multicenter study, the number of centers included in this research was too small to summarize the current situation of EC treatment in China. Besides, in-depth group analysis could not be carried out due to the sample size. Despite these limitations, this was the first and largest sample sized study that has investigated the real practice patterns of adjuvant VBT and EBRT with or without VBT from 2003 to 2017 for early stage patients in China.
Conclusions
The utilization of postoperative VBT alone has increased remarkably in all risk groups and EBRT with or without VBT remained stable as the main treatment modality in the HR groups along the 15 years of the study. There has been significant heterogeneity among VBT dose fractionation schemes which was different from that in the US.
Acknowledgements
None.
Abbreviations
- EC
Endometrial cancer
- EBRT
External beam radiation
- VBT
Vaginal brachytherapy
- RT
Radiotherapy
- ESMO
European Society for Medical Oncology
- ESTRO
European Society for Radiotherapy & Oncology
- ESGO
European Society of Gynaecological Oncology
- FIGO
International Federation of Gynecology and Obstetrics
- IMRT
Intensity modulated radiation therapy
- 3D-CRT
Three-dimensional conformal radiotherapy
- OS
Overall survival
- DFS
Disease-free survival
Authors’ contributions
WW was a major contributor in writing the manuscript. TW and ZL analyzed and interpreted the patient data. TW, ZL, JH, XS, WZ, FZ, XL, SL, HZ and ZM contributed to resources collection, data curation, and supervision.FZ performed the project administration. XH and KH reviewed the manuscript. LW and LZ performed conceptualization and supervision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-036), National High Level Hospital Clinical Research Funding (No.2022-PUMCH-B-052), Ministry of Science and Technology of the People’s Republic of China (grant number 2016YFC0105207), Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant number 2019XK320046) and Peking Union Medical College Postgraduate Education Reform Program (No. 10023201900104). The funds had no role in study design, subject enrollment, or data analysis.
Availability of data and materials
The datasets used and analyzed during the current research are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by Institutional Review Board (IRB) of Peking Union Medical College Hospital (N0. S-K139) at Oct. 24, 2016. As it was a retrospective study, the IRB waived the need for obtaining the informed consent. We confirm that the methods were performed under the relevant regulations and guidelines.
Consent for publication
Not applicable.
Competing interests
Declarations of interest: none.
Trial registration number
The trial was retrospectively registered at 2017.02.23 and the registration number is ChiCTR-PRC-17010712.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenhui Wang, Tiejun Wang and Zi Liu contributed equally to this work.
Contributor Information
Xiaorong Hou, Email: hxr_pumch@163.com.
Lichun Wei, Email: weilichun@fmmu.edu.cn.
Lijuan Zou, Email: suxindai@163.com.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Harkenrider MM, Block AM, Alektiar KM, Gaffney DK, Jones E, Klopp A, et al. American Brachytherapy Task Group Report: adjuvant vaginal brachytherapy for early-stage endometrial cancer: a comprehensive review. Brachytherapy. 2017;16(1):95–108. doi: 10.1016/j.brachy.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27(1):16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- 4.Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother Oncol . 2021;154:327–53. doi: 10.1016/j.radonc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet (London England) 2009;373(9658):137–46. doi: 10.1016/s0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic Oncology Group study. Gynecol Oncol. 2004;92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 7.Nout RA, Smit VT, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet (London England) 2010;375(9717):816–23. doi: 10.1016/s0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 8.Scholten AN, van Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. Postoperative radiotherapy for stage 1 endometrial carcinoma: long-term outcome of the randomized PORTEC trial with central pathology review. Int J Radiat Oncol Biol Phys. 2005;63(3):834–8. doi: 10.1016/j.ijrobp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Wortman BG, Creutzberg CL, Putter H, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens L, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer. 2018;119(9):1067–74. doi: 10.1038/s41416-018-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer SM, Nout RA, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, et al. Long-term impact of Endometrial Cancer diagnosis and treatment on health-related quality of Life and Cancer survivorship: results from the Randomized PORTEC-2 Trial. Int J Radiat Oncol Biol Phys. 2015;93(4):797–809. doi: 10.1016/j.ijrobp.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Small W, Jr, Erickson B, Kwakwa F. American Brachytherapy Society survey regarding practice patterns of postoperative irradiation for endometrial cancer: current status of vaginal brachytherapy. Int J Radiat Oncol Biol Phys. 2005;63(5):1502–7. doi: 10.1016/j.ijrobp.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Harkenrider MM, Grover S, Erickson BA, Viswanathan AN, Small C, Kliethermes S, et al. Vaginal brachytherapy for postoperative endometrial cancer: 2014 survey of the american Brachytherapy Society. Brachytherapy. 2016;15(1):23–9. doi: 10.1016/j.brachy.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Modh A, Ghanem AI, Burmeister C, Rasool N, Elshaikh MA. Trends in the utilization of adjuvant vaginal brachytherapy in women with early-stage endometrial carcinoma: results of an updated period analysis of SEER data. Brachytherapy. 2016;15(5):554–61. doi: 10.1016/j.brachy.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet (London England) 2000;355(9213):1404–11. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 15.Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56(4):419–27. [PubMed] [Google Scholar]
- 16.Sorbe B, Horvath G, Andersson H, Boman K, Lundgren C, Pettersson B. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma–a prospective randomized study. Int J Radiat Oncol Biol Phys. 2012;82(3):1249–55. doi: 10.1016/j.ijrobp.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Fayed A, Mutch DG, Rader JS, Gibb RK, Powell MA, Wright JD, et al. Comparison of high-dose-rate and low-dose-rate brachytherapy in the treatment of endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2007;67(2):480–4. doi: 10.1016/j.ijrobp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Cannon GM, Geye H, Terakedis BE, Kushner DM, Connor JP, Hartenbach EM, et al. Outcomes following surgery and adjuvant radiation in stage II endometrial adenocarcinoma. Gynecol Oncol. 2009;113(2):176–80. doi: 10.1016/j.ygyno.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Randall ME, Wilder J, Greven K, Raben M. Role of intracavitary cuff boost after adjuvant external irradiation in early endometrial carcinoma. Int J Radiat Oncol Biol Phys. 1990;19(1):49–54. doi: 10.1016/0360-3016(90)90133-5. [DOI] [PubMed] [Google Scholar]
- 20.Greven KM, D’Agostino RB, Jr, Lanciano RM, Corn BW. Is there a role for a brachytherapy vaginal cuff boost in the adjuvant management of patients with uterine-confined endometrial cancer? Int J Radiat Oncol Biol Phys. 1998;42(1):101–4. doi: 10.1016/s0360-3016(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 21.de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(3):295–309. doi: 10.1016/s1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. Phase III trial: Adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage Endometrial Cancer. J Clin Oncol . 2019;37(21):1810–8. doi: 10.1200/jco.18.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynaers EA, Jutzi L, Ezendam NP, Kwon JS, Pijnenborg JM. Improved outcome of High-Grade, early 1-Stage Endometrioid Endometrial Carcinoma with Adjuvant Chemotherapy and Radiotherapy: comparison of 2 treatment strategies. Int J Gynecol Cancer. 2017;27(3):467–72. doi: 10.1097/igc.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 24.Smogeli E, Cvancarova M, Wang Y, Davidson B, Kristensen G, Lindemann K. Clinical outcome of patients with high-risk Endometrial Carcinoma after treatment with Chemotherapy only. Int J Gynecol Cancer . 2018;28(9):1789–95. doi: 10.1097/igc.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 25.Ager BJ, Francis SR, Do OA, Huang YJ, Soisson AP, Dodson MK, et al. Do vaginal recurrence rates differ among adjuvant vaginal brachytherapy regimens in early-stage endometrial cancer? Brachytherapy. 2019;18(4):453–61. doi: 10.1016/j.brachy.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Small W, Jr, Beriwal S, Demanes DJ, Dusenbery KE, Eifel P, Erickson B, et al. American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy. Brachytherapy. 2012;11(1):58–67. doi: 10.1016/j.brachy.2011.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current research are available from the corresponding author upon reasonable request.