Abstract
Objective
To describe the incidence of adverse events following immunisation (AEFI) and determine the factors that affect the onset and duration of AEFI after COVISHIELD vaccination among healthcare workers.
Design
Prospective cohort study.
Setting
Tertiary healthcare, Korle-Bu, Ghana.
Participant
Three thousand and twenty-two healthcare workers at least 18 years of age were followed up for 2 months after receiving two doses of the COVISHIELD vaccine.
Primary outcome
The occurrence of the AEFI was identified by self-reporting to the AEFI team members.
Results
A total of 3022 healthcare workers had at least one AEFI (incidence rate of 706.0 (95% CI 676.8 to 736.1) per 1000 doses) with an incidence rate of 703.0 (95% CI 673.0 to 732.0) per 1000 doses for non-serious AEFI and an incidence rate of 3.3 (95% CI 1.6 to 6.1) per 1000 doses for serious AEFI. The most commonly reported systemic adverse events were headache (48.6%), fever (28.5%), weakness (18.4%) and body pains (17.9%). The estimated median time to onset of the AEFI following the first-dose vaccination was 19 hours and the median AEFI duration was 40 hours or 2 days. Delayed-onset AEFI occurred in 0.3% after first dose and 0.1% after second dose. Age, sex, previous SARS-CoV-2 infection, history of allergies and comorbidity were not significantly associated with the onset and duration of AEFI. However, participants who used paracetamol seemed to be significantly protected (HR 0.15; 95% CI 0.14, 0.17) from having a long duration of AEFI.
Conclusion
The results of our study indicate a high incidence of non-serious AEFI and the rare occurrence of serious AEFI after COVISHIELD vaccination in healthcare workers. The rate of AEFI was higher after the first dose than after the second dose. Sex, age, previous SARS-CoV-2 infection, allergies and comorbidity were not significantly associated with the onset and duration of AEFI.
Keywords: COVID-19, Immunology, Public health
STRENGTHS AND LIMITATIONS OF THIS STUDY
Our study is a large-scale prospective cohort with comprehensive monitoring of adverse events following immunisation (AEFI) among healthcare workers.
The study had the advantage of collecting information on risk factors before the occurrence of AEFI.
The method of self-reporting AEFI symptoms by healthcare workers is inherently associated with reporting bias and residual confounding.
Our study is composed of health workers, who may have different sociodemographic characteristics compared with the general population, which may limit the generalisability of the study.
A prospective cohort with a good response of 75.5% of the original participants for 2 months.
Introduction
COVID-19 caused by SARS-CoV-2 is an unprecedented public health emergency affecting people’s lives and the world’s social fabric. This infection was first reported in Wuhan, China, in December 2019 and has rapidly spread across the globe. As of January 2022, COVID-19 has infected over 200 million people and has caused over 4 million deaths.1 2 To overcome this pandemic, the vaccination strategy to prevent COVID-19 infection remains an essential tool as the world searches for an effective treatment. There are many myths surrounding the use of vaccines, especially in Africa, and any false report on its safety may increase vaccine hesitancy.
COVISHIELD, a DNA vaccine developed by Oxford University and AstraZeneca, consists of a non-replicating chimpanzee adenovirus that carries DNA encoding (instructions) for the spike protein of SARS-CoV-2 to the human cells.3 The DNA is released into the cytoplasm and then to the nucleus, where it is transcribed into the messenger RNA (mRNA). The mRNA is then translated by ribosome into spike protein, eliciting the immunogenic response.
As of January 2022, about 53.0% of the world population has been vaccinated with at least one dose of the COVID-19 vaccine.4 In Ghana, the expanded immunisation programme has administered over 4 million doses, with the majority being AstraZeneca (COVISHIELD). Only 8.4% of Ghana’s population have been vaccinated—2.6% fully vaccinated and 5.7% partially vaccinated.4 However, this vaccine is not without adverse events. To our knowledge, there have been no prospective studies of adverse events following immunisation (AEFI) of the COVISHIELD vaccine. Most of these studies found in the literature were cross-sectional or retrospective cohort studies that could not estimate the duration of the adverse events after a COVISHIELD vaccination. Thus, we conducted a prospective study that aims to describe the incidence of AEFI and determine factors that affect the time to onset and duration of AEFI after COVISHIELD vaccination among a cohort of healthcare workers in a teaching hospital.
Materials and methods
Study design and setting
This is a prospective cohort study involving healthcare workers to determine the incidence of adverse events following COVISHIELD vaccination. The study was conducted at Korle-Bu Teaching Hospital in Ghana. With a 2000-bed capacity and over 7000 healthcare workers, the hospital is one of Ghana’s treatment centres for SARS-CoV-2-infected patients.
Participants and recruitment
The study recruited 4000 healthcare workers across the various departments of Korle-Bu Teaching Hospital, Korle-Bu, Accra. Recruitment took place between 24 February and 2 March 2021. After the ethical approval, the study was publicised on all hospital’s noticeboards and social media platforms. All healthcare workers in the hospital were sensitised about the new COVISHIELD vaccination.
All eligible healthcare workers were invited to participate in the study after indicating their willingness to be vaccinated by pre-registration at the vaccination centres. A healthcare worker was defined as a person employed by the Ghana’s Ministry of Health to work in Korle-Bu Teaching Hospital. To be eligible, a healthcare worker had to be at least 18 years of age, an employee of Korle-Bu Teaching Hospital and had completed the consent form. Healthcare workers were excluded if they had been partially vaccinated (with any vaccine), tested positive for SARS-CoV-2 infection at the time of recruitment, very ill, refused to be vaccinated or had medical contraindications or allergies to the vaccine (COVISHIELD). Details of the study, PCR COVID-19 test and vaccination procedure were explained to each healthcare worker after written informed consent was obtained. COVISHIELD vaccines were provided to all eligible healthcare workers because it was the only COVID-19 vaccine approved by Ghana’s Ministry of Health.
Data collection and follow-up
All eligible healthcare workers were tested for SARS-CoV-2 infection using a GeneXpert PCR machine. Those that were positive for SARS-COV-2 infection were excluded from the study. At the baseline, prevaccination online questionnaire was to obtain information about employee identification number, age, sex, occupation or profession, department, history of COVID-19 infection, pre-existing medical condition (comorbidity) and history of allergy. The baseline data were collected and managed using REDCap (electronic data capturing system).5 6 Among the 3237 healthcare workers who completed the prevaccination baseline questionnaire, we restricted the analysis to 3022 healthcare workers who took both the first and second doses of the COVISHIELD vaccine. The 3022 healthcare workers were followed up for 2 months—1 month after receiving the first dose and another month after receiving the second dose. Figure 1 shows participant flow in the study.
Figure 1.
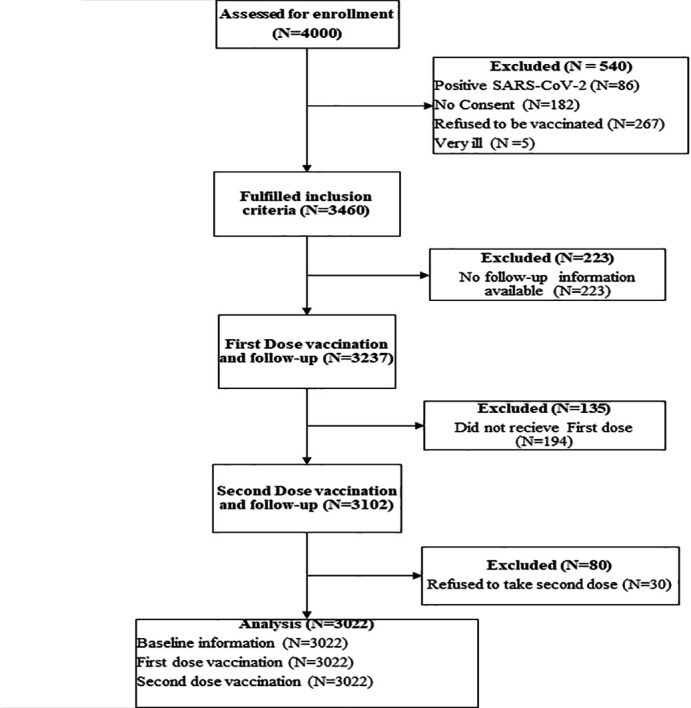
Participant flow in the study. Four thousand healthcare workers were invited to participate in the adverse events following immunisation (AEFI) COVISHIELD vaccination study. Three thousand and twenty-two out of the original participants received the first and second doses of the COVISHIELD vaccine after a 2-month follow-up. About 978 healthcare workers were excluded from the study: 80 were partially vaccinated, 135 refused vaccinations, 223 had missing information and 540 did not meet eligibility criteria.
Vaccination (first or second dose) of the healthcare workers was conducted by the nurses from the Public Health Unit. All the healthcare workers received two separate doses of 0.5 mL of COVISHIELD vaccine intramuscularly. The interval between the first and second doses was 6–8 weeks. To verify healthcare workers who received the first dose of the COVISHIELD vaccine, their employee identification number provided at the baseline was used to retrieve their vaccination information from the national vaccination registry. Any unmatched employee identification number indicated that the healthcare worker was not available for the first-dose vaccination and was excluded from the study. The second-dose vaccination intake was verified by entering the vaccination card number into the national vaccination registry before follow-up. All healthcare workers whose vaccination status indicated the first dose only from the national vaccination registry were excluded from the study.
During the follow-up, participants were asked to report any symptoms after the COVISHIELD vaccination to the hospital’s AEFI team members, and record the time of onset and end of AEFI. Participants were called daily during the week after the first dose of vaccination, followed by a weekly call for 3 weeks. The daily and weekly calls were repeated after the second dose of vaccination.
Outcomes
Study participants were asked to record the occurrence of the AEFI to the AEFI team, which was made up of four physicians, a pharmacist with experience in pharmacovigilance and four nurses with experience in AEFI. Participants were contacted periodically via phone calls about the occurrence of AEFI. All self-reported AEFIs were confirmed based on WHO’s operational definition. The AEFI is defined as any untoward medical occurrence that follows immunisation and does not necessarily have a causal relationship with the usage of the vaccine.7 The AEFIs were classified into non-serious and serious AEFIs based on WHO’s definition. Non-serious adverse events were defined as any event that is not serious and does not pose a potential risk to the health of the recipient. Serious adverse events were defined as events involving hospitalisation, prolongation of existing hospitalisation, life-threatening illness or permanent disability or death.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Science (SPSS) version 26 software. Kaplan-Meier curves were used to plot the survival function between the group. Survival functions were compared using the log-rank test. Cox proportional models were used to determine the effect of covariates on the onset and duration AEFI.
Patient and public involvement
Patients or the public were not involved in our research’s design, conduct, reporting or dissemination plans.
Results
A total of 3022 healthcare workers who received the first and second doses of COVISHIELD vaccine were followed up for 2 months. The average follow-up time was 56 days (median: 58) after baseline. Table 1 shows the descriptive characteristics of the study participants. The age of the 3022 healthcare workers ranged from 30 to 39 with a mean of 36.2 (SD=9.7) years. Nurse and doctor were the most predominant professions. Almost 15.1% reported having previous SARS-CoV-2 infection, 10.0% had a history of allergies and 8.0% had pre-existing medical conditions.
Table 1.
Study participant characteristics
| Variables | (n=3022) |
| Age group (%) | |
| <30.0 | 814 (26.9) |
| 30.0–39 | 1335 (44.2) |
| 40.0–49 | 528 (17.5) |
| 50.0–59 | 276 (9.1) |
| 60.0 and over | 69 (2.3) |
| Sex (%) | |
| Female | 1751 (57.9) |
| Male | 1271 (42.1) |
| Profession (%) | |
| Doctor | 916 (30.2) |
| Nurse | 1053 (34.8) |
| Pharmacist | 102 (3.4) |
| Medical laboratory professional | 93 (3.1) |
| Physiotherapist | 36 (1.2) |
| Dietician | 19 (0.6) |
| Administrator | 116 (3.8) |
| Accountant | 73 (2.4) |
| Healthcare assistant | 41 (1.4) |
| Orderly | 48 (1.6) |
| Security | 38 (1.3) |
| Clinical psychologist | 48 (1.6) |
| Medical student/national service person | 96 (3.2) |
| Others | 345 (11.4) |
| Previous COVID-19 infection (%) | |
| No | 2559 (84.6) |
| Yes | 466 (15.4) |
| AEFI first dose (%) | |
| No | 1125 (37.2) |
| Non-serious | 1887 (62.4) |
| Serious | 10 (0.33) |
| AEFI second dose (%) | |
| No | 2406 (76.6) |
| Non-serious | 617 (20.4) |
| Serious | 0 (0.0) |
| History of allergies (%) | |
| Yes | 304 (10.0) |
| No | 2718 (90.0) |
| Comorbidity (%) | |
| Yes | 242 (8.0) |
| No | 2780 (92.0) |
AEFI, adverse events following immunisation.
Adverse events following immunisation
The incidence of AEFI was 706.0 (95% CI 676.8 to 736.1) per 1000 doses, with a higher incidence of non-serious AEFI (incidence rate of 703.0 (95% CI 673.0 to 732.0) per 1000 doses) compared with serious AEFI (incidence rate of 3.3 (95% CI 1.6 to 6.1) per 1000 doses). The occurrence of AEFI was lower in the second dose (incidence rate of 204.1 (95% CI 188.4 to 220.9) per 1000 doses) compared with the first dose (incidence rate of 627.3 (95% CI 599.8 to 656.7) per 1000 doses) regardless of the interval between the two doses. 62.8% (1897/3022) of participants had adverse events after the first dose. Among the participants who experienced adverse events after the first dose, 19.6% (372/1897) developed adverse events after second-dose vaccination. Of these participants, most (68.7%, 256/372) developed second-dose adverse events early than their first-dose adverse events. 8.1% (245/3022) had adverse events after the second dose but had no adverse events after the first dose. The most common AEFI symptom combination was the influenza-like or COVID-like symptoms experienced by healthcare workers after the first and second doses. The occurrence of AEFI increased with age in the first dose; however, no adverse events were reported among age groups greater than 50 years during the second-dose vaccination.
Types of AEFI
The most common local AEFI was pain at the injection site. No other local AEFI such as swelling was reported. In the case of systemic adverse events, headache (48.6%), fever (28.5%), weakness (18.4%) and feeling of body pains (17.9%) were the most commonly reported adverse events (figure 2). The type of AEFI observed after the first-dose vaccination was clinically similar to that observed after the second-dose vaccination (figure 2).
Figure 2.
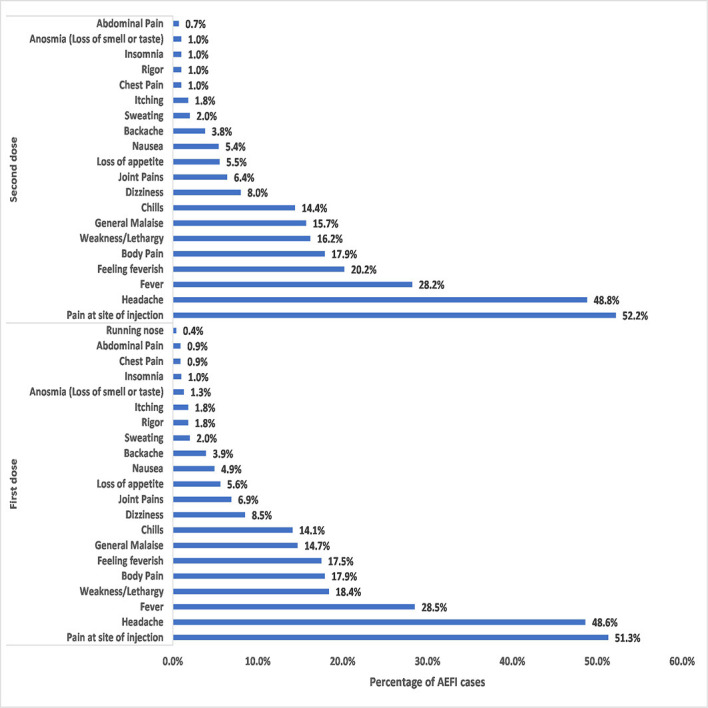
Various types of adverse events following immunisation (AEFI) after first and second doses of COVISHIELD vaccination. Study participants presented with various types of adverse events after vaccination. The adverse events observed after first-dose vaccination were similar to those observed after second-dose vaccination. The only local adverse event reported by half (52%, 51%) of the participants was pain at the injection site. The six most commonly reported systematic adverse events in descending order of percentage were headache, fever, weakness, body pains, feeling feverish and generalised malaise.
Onset and duration of AEFI
The estimated median time to onset of the AEFI following the first-dose vaccination was 19 hours, indicating that 50% of the AEFI occurs within a day (figure 3). The median time to developing AEFI after second-dose vaccination could not be estimated from the Kaplan-Meier curve since less than 50% of the study participants experienced AEFI. In figure 4, most participants developed AEFI within 24 hours (91.1% for the first dose and 92.1% for the second dose), while the delayed-onset AEFI that started after 7 days occurred in 0.3% and 0.1% of the participants after administration of first and second doses, respectively.
Figure 3.
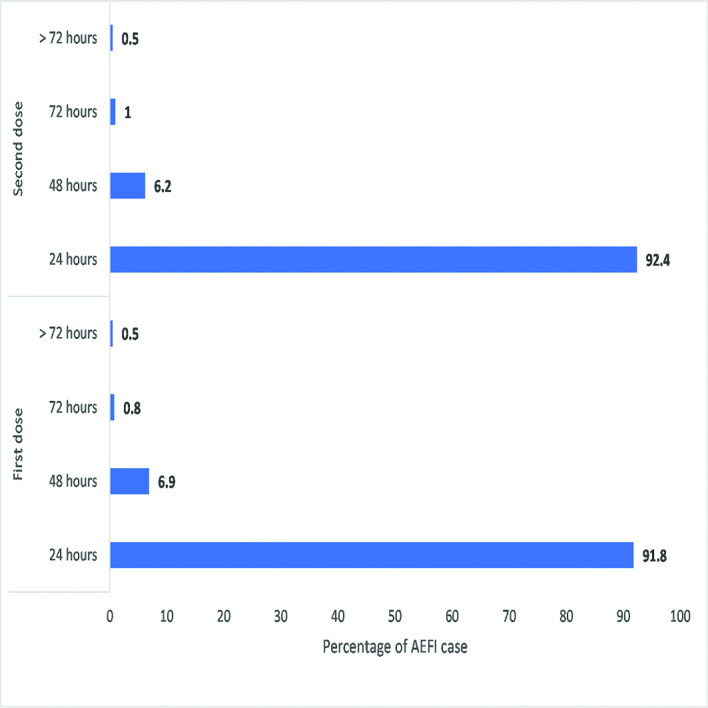
Onset of adverse events following immunisation (AEFIs) by time interval after inoculation. The onset of AEFIs after inoculation was similar in both vaccinations (first and second doses). The majority of adverse events occurred within 24 hours after vaccination. The delayed onset of adverse events was rare (0.1%, 0.3%) among the participants.
Figure 4.
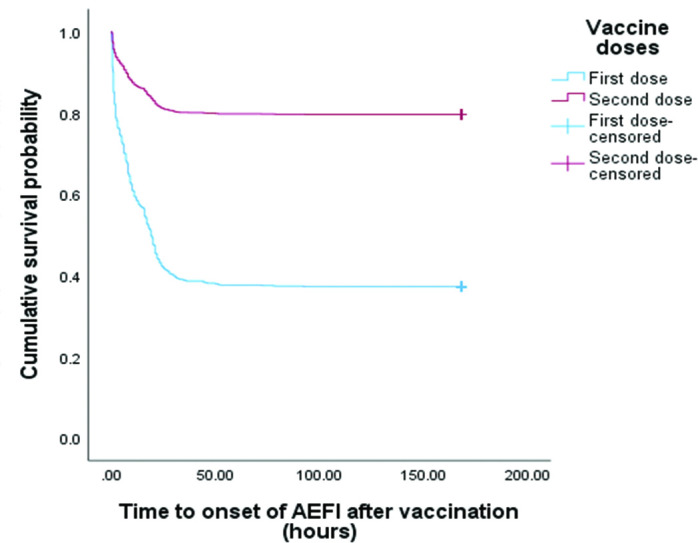
Comparison of the Kaplan-Meier survival curves of adverse events following immunisation (AEFI) onset between first and second doses of COVISHIELD vaccination. A Kaplan-Meier curve that demonstrates the onset of AEFI after COVISHIELD vaccination. After first-dose vaccination, the median time to onset of AEFI was 19.0 (95% CI 18.0 to 20.0) hours. The median time to onset after the second-dose vaccination could not be estimated. There was a significantly higher incidence of adverse events after the first-dose vaccination than after the second-dose vaccination (p<0.00).
The median duration after the onset of AEFI was 40 hours or approximately 2 days (figure 5). First-dose adverse events had a mean duration of 55.5 hours and were similar to the second-dose adverse events (54.9 hours). Regarding the association between the duration of AEFI and vaccine doses, the log-rank test revealed that vaccine doses had no association with the duration of adverse events. This indicates that AEFIs after the first and second doses were similar in the adverse event duration. Although there was a high number of recoveries after first-dose vaccination, this was proportional to the number of adverse events reported.
Figure 5.
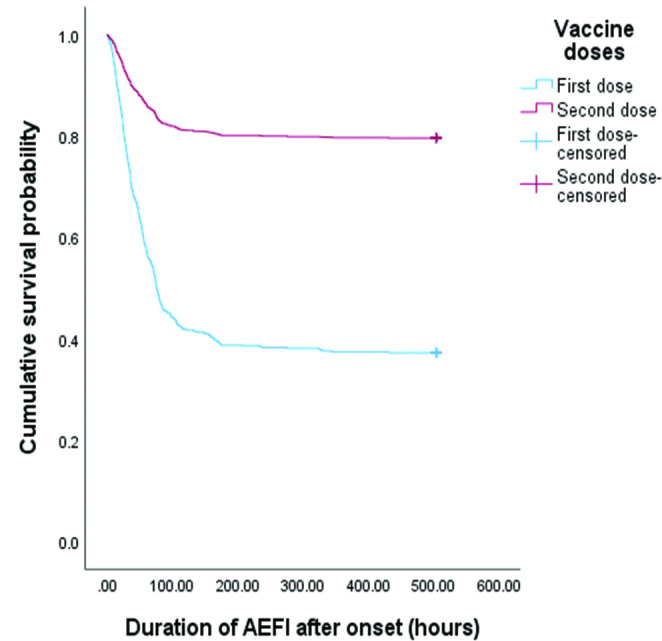
Comparison of the Kaplan-Meier survival curve of adverse events following immunisation (AEFI) duration between first and second doses of COVISHIELD vaccination. Kaplan-Meier curves show the duration of AEFI after the onset. The estimated median duration of AEFI was 76 hours (95% CI 70.8 to 81.1) (approximately 3 days). There was no difference in duration between the first and second doses.
Healthcare workers who reportedly took paracetamol (56 hours) had a significantly shorter adverse event duration than those who did not take paracetamol (349 hours, approximately 14 days) (figure 6). This indicates that paracetamol shortens the duration of adverse events by 294 hours (12 days).
Figure 6.
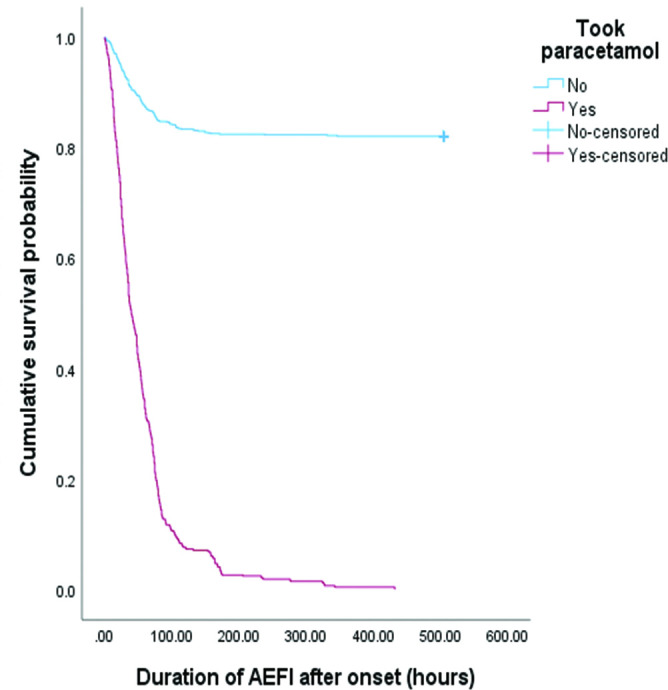
Kaplan-Meier survival curve shows the effect of paracetamol on the adverse events following immunisation (AEFI) duration. The participants who took paracetamol had significantly shorter recovery times than those who did not. Statistical significance between this group was evaluated using the log-rank test.
Intervention used in managing AEFI
Various interventions were administered to help participants who were experiencing AEFI. About 66.4% (1935) of AEFI participants reported the use of paracetamol (antipyretic) for treatment. However, 26% reported not taking any treatment or intervention. Only 2% of the participants reported to the hospital.
Factors affecting onset and duration of AEFI
A multivariate Cox regression model was used to identify factors that predicted the onset of AEFI independently of the other variables under consideration. Our analysis shows that age, sex, previous SARS-CoV-2 infection, history of allergies and comorbidity did not reach statistical significance (table 2). Table 3 shows the HR for several factors associated with the duration of AEFI. In the multivariate analysis (table 3), participants who used paracetamol were significantly protected (HR 0.15; 95% CI 0.14, 0.17) from having long adverse event duration. However, other variables like age, sex, history of allergies and comorbidity were not significantly associated with the duration of AEFI recovery.
Table 2.
Multivariate adjusted HR (95% CI) for associations between selected characteristics and the onset of AEFI
| HR (95% CI) | P value | |
| Age (years) | 1.00 (0.99 to 1.00) | 0.265 |
| Sex | ||
| Female | 1 | |
| Male | 0.95 (0.87 to 1.05) | 0.324 |
| Previous infection | ||
| No | 1 | |
| Yes | 1.11 (0.93 to 1.19) | 0.562 |
| History of allergies | ||
| No | 1 | |
| Yes | 0.99 (0.86 to 1.15) | 0.912 |
| Comorbidity | ||
| No | 1 | |
| Yes | 1.02 (0.87 to 1.21) | 0.775 |
AEFI, adverse events following immunisation.
Table 3.
Multivariate adjusted HR (95% CI) for associations between selected characteristics and the duration of AEFI
| HR (95% CI) | P value | |
| Age (years) | 1.00 (0.99 to 1. 00) | 0.421 |
| Sex | ||
| Female | 1 | |
| Male | 1.03 (0.94 to 1.13) | 0.507 |
| Previous infection | ||
| No | 1 | |
| Yes | 1.05 (0.93 to 1.19) | 0.432 |
| Antipyretic (paracetamol) | ||
| No | 1 | |
| Yes | 0.15 (0.14 to 0.17) | 0.00 |
| History of allergies | ||
| No | 1 | |
| Yes | 0.99 (0.86 to 1.16) | 0.984 |
| Comorbidity | ||
| No | 1 | |
| Yes | 1.01 (0.85 to 1.18) | 0.929 |
AEFI, adverse events following immunisation.
Discussion
We found that the incidence rate of AEFI was 706 per 1000 doses after the COVISHIELD vaccination. Non-serious AEFI was 703 per 1000 doses, and serious or life-threatening AEFI was 3.3 per 1000 doses. However, the incidence of AEFI decreases from 627.3 per 1000 doses after first-dose vaccination to 204.1 per 1000 doses after the second-dose vaccination. Our results confirmed the previous reports8–10 on the rare occurrence of serious or life-threatening AEFI and the high incidence of non-serious AEFI associated with COVISHIELD vaccine. No serious adverse events were reported after the second dose of COVISHIELD vaccination. The rate of AEFI varies from study to study due to differences in study methodologies, population and context. The studies from the UK (75%11), India (57.0%12), Togo (75%13) and Ethiopia (43.4%14) reported findings that were consistent with our results.
Previous studies in adverse events following the COVID-19 vaccination have reported that reactogenicity increases with pre-existing natural immunity leading to an increased risk of adverse events among those with previous SARS-CoV-2 infection.15 16 Although not significant, our HR of 1.11 (0.93–1.19) is consistent with these studies. On the other hand, a survey study conducted by Bardenheier et al8 found that pre-existing natural immunity from SARS-CoV-2 infection did not increase the risk of AEFI. This study did not find this association due to the relatively small number of participants with previous SARS-CoV-2 infection.
Contrary to a previous study, we found that the second-dose vaccination of the COVISHIELD vaccine was associated with a lower number of adverse events (200 per 1000 doses) than the first-dose vaccination. Menni et al reported that high reactogenicity following the second-dose vaccination is related to post first-dose vaccination immunogenicity.11
Our results found that the median time to onset of AEFI was 19 hours after vaccination. Most AEFIs started within 24 hours (92.1% for the first dose and 91.8% for the second dose) after inoculation. The rate of AEFI tends to decline with time, with no incidence being recorded after a week. Our results differ from two survey studies that reported that most adverse events occur within 48 hours.9 10 The delayed onset of adverse events (after 7 days) was 0.3% for the first-dose vaccination and 0.1% for the second-dose vaccination.
Analysing the effect of demographic characteristics on adverse events following COVISHIELD vaccination, we found no effect of sex and age on adverse events. Our results indicate that the rate of adverse events was similar for males and females. This is consistent with the findings from previous studies.8 9
The most common systematic adverse event was headache (48.6% in the first dose and 48.8% in the second dose). Our results indicate that the occurrence of headaches was higher than studies reporting 27%10 and 21.0%14 but lower than studies reporting 58.5%9 and 59.0%.17 The differences in these results are due to different populations and research methods. Studies reporting lower values than our results were survey or cross-sectional studies which are usually subject to recall bias. In contrast, studies reporting higher values than our results were cohort studies or studies that used both passive and active surveillance.
The most common local AEFI was pain at the injection site (51.0% in the first dose and 52.2% in the second dose) and this is consistent with the studies found in literature.8–10 17 Pain perception is subjective and depends on personal exposures like psychological, sociocultural and genetic factors, which may be explained by the slight differences in our rate of pain at the injection site compared with other studies.8–10 17 Among those who developed AEFI, most healthcare workers had two or more adverse event symptoms. Although the COVISHIELD vaccine is known to increase the risk of thrombosis,10 our study did not find any coagulopathy like deep vein thrombosis, cardiovascular accident (stroke), neurological deficits, etc among healthcare workers. The use of antipyretics such as paracetamol to ameliorate adverse events following vaccination was pervasive among healthcare workers. Sixty-nine per cent of those who experienced adverse events took paracetamol. Paracetamol was effective in reducing the recovery time. However, paracetamol is known to suppress vaccine effectiveness through various mechanisms,18 and unnecessary use of it should be avoided, especially during vaccination.
This prospective study had the advantage of collecting information on possible risk factors before the occurrence of AEFI. Therefore, it prevents recall bias once adverse events have occurred. All participants were from the hospital, eliminating the hospital as a variation. One strength of this study is the large sample size with AEFI. Because of limited resources, we did not collect information on all possible risk factors. We found that those who took the first dose only relatively experienced severe symptoms that may deter them from receiving the second dose. The method of self-reporting AEFI symptoms by healthcare workers is inherently associated with reporting bias and residual confounding. In addition, it should be emphasised that we did focus on healthcare workers, not the general population.
In conclusion, the results of our study indicate that the rate of non-serious AEFI is higher and serious AEFI is low with COVISHIELD vaccination in healthcare workers. The rate of AEFI was higher in the first-dose vaccination compared with the second-dose vaccination. Sex, age, previous SARS-CoV-2 infection, allergies and comorbidity were not significantly associated with the onset and duration of AEFI. However, antipyretic (paracetamol) was able to speed up AEFI recovery, and its use is encouraged in the event of unbearable symptoms.
Supplementary Material
Footnotes
Contributors: KM, AS, FA and EOk conceived and designed the original protocol. All authors were involved in amending the protocol. KM, YM, EOw and DNAD conducted the study, including staff recruitment and data collection. Data entry was carried out by JZ, EOw, HM and EA. KM, DNAD and HM carried out the data cleaning and preliminary analysis. KM analysed the data. KM wrote the first draft of the manuscript with EOk, AS and YM. All authors contributed to subsequent and final drafts. KM and AS are guarantors for the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The data for this article are available upon reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
This study involves human participants and was approved by the Korle-Bu Teaching Hospital Institutional Review Board (IRD: KBTH-ADM/00014/2022) in Ghana. Participants gave informed consent to participate in the study before taking part.
References
- 1.World Health Organization . Coronavirus (COVID-19) dashboard 2021; Available: https://covid19.who.int/ [Accessed 15 Jan 2022].
- 2.Worldometer . Coronavirus deaths worldwide 2021. 2022. Available: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1?%22%20%5Cl%22 [Accessed 9 Jan 2022].
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.University of Oxford, Our World in Data . Coronavirus (COVID-19) Vaccinations (statistics and research); 2021.
- 5.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Global manual on surveillance of adverse events following immunization. 2021. Available: https://apps.who.int/iris/rest/bitstreams/914830/retrieve [Accessed 09 Oct 2021].
- 8.Bardenheier BH, Gravenstein S, Blackman C, et al. Adverse events following one dose of mRNA COVID-19 vaccination among US nursing home residents with and without a previous SARS-CoV-2 infection. J Am Med Dir Assoc 2021;22:2228–32. 10.1016/j.jamda.2021.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran VN, Nguyen HA, Le TTA, et al. Factors influencing adverse events following immunization with AZD1222 in Vietnamese adults during first half of 2021. Vaccine 2021;39:6485–91. 10.1016/j.vaccine.2021.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almohaya AM, Qari F, Zubaidi GA, et al. Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia. Prev Med Rep 2021;24:101595. 10.1016/j.pmedr.2021.101595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis 2021;21:939–49. 10.1016/S1473-3099(21)00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamal D, Thakur V, Nath N, et al. Adverse events following ChAdOx1 nCoV-19 vaccine (COVISHIELD) amongst health care workers: a prospective observational study. Med J Armed Forces India 2021;77:S283–8. 10.1016/j.mjafi.2021.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konu YR, Gbeasor-Komlanvi FA, Yerima M, et al. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (COVISHIELD) in Togo, March 2021. Arch Public Health 2021;79:207. 10.1186/s13690-021-00741-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tequare MH, Abraha HE, Adhana MT, et al. Adverse events of Oxford/Astrazeneca’s COVID-19 vaccine among health care workers of ayder comprehensive specialized hospital, Tigray, Ethiopia. IJID Reg 2021;1:124–9. 10.1016/j.ijregi.2021.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ossato A, Tessari R, Trabucchi C, et al. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (comirnaty, Pfizer-Biontech) vaccine: a post-marketing Italian study conducted between 1 January and 28 February 2021. Eur J Hosp Pharm 2021:ejhpharm-2021-002933. 10.1136/ejhpharm-2021-002933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F, Srivastava K, Alshammary H, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021;384:1372–4. 10.1056/NEJMc2101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh E, Moody MA, Walter EB. Effect of antipyretic analgesics on immune responses to vaccination. Hum Vaccin Immunother 2016;12:2391–402. 10.1080/21645515.2016.1183077 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The data for this article are available upon reasonable request from the corresponding author.


