Abstract
Objectives
The COVID-19 pandemic has required significant modifications of hospital care. The objective of this study was to examine the operational approaches taken by US hospitals over time in response to the COVID-19 pandemic.
Design, setting and participants
This was a prospective observational study of 17 geographically diverse US hospitals from February 2020 to February 2021.
Outcomes and analysis
We identified 42 potential pandemic-related strategies and obtained week-to-week data about their use. We calculated descriptive statistics for use of each strategy and plotted percent uptake and weeks used. We assessed the relationship between strategy use and hospital type, geographic region and phase of the pandemic using generalised estimating equations (GEEs), adjusting for weekly county case counts.
Results
We found heterogeneity in strategy uptake over time, some of which was associated with geographic region and phase of pandemic. We identified a body of strategies that were both commonly used and sustained over time, for example, limiting staff in COVID-19 rooms and increasing telehealth capacity, as well as those that were rarely used and/or not sustained, for example, increasing hospital bed capacity.
Conclusions
Hospital strategies during the COVID-19 pandemic varied in resource intensity, uptake and duration of use. Such information may be valuable to health systems during the ongoing pandemic and future ones.
Keywords: COVID-19, HEALTH SERVICES ADMINISTRATION & MANAGEMENT, Organisation of health services, Protocols & guidelines, PUBLIC HEALTH
Strengths and limitations of this study.
Strengths include the novelty, breadth of hospital measures examined and inclusion of multiple geographically diverse health centres.
Limitations include the relatively small sample and that the participating hospitals were mostly large teaching hospitals, which limits generalisability to other hospital types.
The study may have failed to capture some important hospital strategies as well as external influences affecting strategy uptake and sustainability.
Introduction
Background
The COVID-19 pandemic has required tremendous creativity from healthcare institutions. Hospitals have needed to deliver care to patients with a highly contagious disease, triage and manage them efficiently and protect staff and patients from contracting and transmitting the disease, all in the context of a rapidly changing knowledge base about necessary containment approaches and diagnosis and treatment strategies. While disaster management plans and knowledge base from prior pandemics exist,1 the exponential spread, severity and sustained nature of this crisis has required responsiveness and adaptability on an unprecedented scale.
Early in 2020, healthcare organisations did not know which responses and resources to COVID-19 were both feasible and valuable. There was a wide variety of potential responses,2 3 including changes to physical space, adapted triage and patient flow processes and clinical protocols, rules for use of limited availability supplies and equipment, withdrawal of non-essential staff from hospital campuses, limitation of visitors to the hospital and adaptations to increase the workforce.2 3 Detailed information from hospitals has been extremely limited and consisting of single-hospital or practice setting, cross-sectional or general, descriptive data only. Patterns and heterogeneity of practices as the pandemic progressed across the USA has not been well described.
The objective of this study was to examine the range of approaches taken by a sample of geographically diverse US healthcare organisations in response to the COVID-19 pandemic. We examined which strategies were most used, their duration of uptake and their association with hospital and pandemic factors (such as geographic location, hospital type and local COVID-19 case volume). As health systems across the country continued to grapple with successive waves of COVID-19, increased hospitalisations and staffing shortages, we sought to understand the range and prioritisation of institution-level actions to help determine optimal strategies for future pandemics. Our hypothesis was that hospital type would be associated with the types of strategy used, given the material and human resources needed for many of the strategies.
Methods and analysis
This was an observational study of 17 US hospitals over the first 12 months of the pandemic. Hospitals were invited from an existing consortium of US hospitals4 collecting data on COVID-19 cases and clinical information. Those who agreed to participate and provided at least 70% of data during the period of interest were included. From 3 February 2020—defined as the week the USA declared COVID-19 a public health emergency5—to 28 February 2021, research team members tracked active hospital strategies related to COVID-19
. This is a study of institutional characteristics and practices and does not involve human participants.
Patient and public involvement
As a study of institutional practices, patients and the public were not involved.
Hospital strategies of interest
In February and March 2020, we identified a body of potential hospital strategies. We used as a starting point a hospital disaster resilience framework,6 which was designed around the elements that allow a hospital to withstand a discrete ‘shock’ to the system: hospital safety and vulnerability (eg, disease testing and surveillance, workforce protections); disaster preparedness and resources (eg, new administrative or planning structures, supply); continuity of services (eg, modified patient care practices, workforce capacity); and recovery and adaptation (eg, timing of return of routine functions, workforce mental health). We conducted a web search of English language news and journal reports from major outlets using terms (hospital or health system), (strategies), (management or response) and (COVID-19 or pandemic); review of existing guidelines for hospitals from early COVID-19-affected areas7 8; solicitation of multidisciplinary experts in emergency care, public health, and hospital administration; social media sites related to COVID-19 healthcare response (eg, COVID-19 dedicated Facebook groups) and iterative review and input by the study team.
The final list included 42 strategies that we grouped into six general categories: (1) modifying patient volume or care practices; (2) increasing hospital and workforce capacity; (3) new administrative structures and resources; (4) means of protecting the healthcare workforce; (5) personal protective equipment (PPE) supply, training and use; and (6) COVID-19 testing availability and operations.
Data collection
We developed survey questions (online supplemental file 1) that captured each hospital strategy of interest. These were piloted with the study sites and iteratively refined for clarity and completeness and appropriateness of answer options. They were then entered into the REDCap electronic data capture programme hosted at Oregon Health & Science University.9 10
bmjopen-2022-067986supp001.pdf (6.8MB, pdf)
Participating institutions received an online training session, a study manual and codebook and a site liaison to answer specific questions. Sites were sent a form link weekly to the REDCap9 10 data entry form, within which sites reported the initiation, continuation, modification and discontinuation of strategies related to COVID-19. Information within each site was obtained according to the best practice identified by the site investigator and included review of internal emails, published policies, meeting minutes and direct conversation with hospital administrators involved in the COVID-19 response.
Data collection started in April 2020, with data from January to early April 2020 entered retrospectively and data from mid-April to February 2021 entered prospectively. The coordinating site performed line-by-line data review for incomplete entries or values inconsistent with codebook definitions and flagged these items on a data review sheet that was sent to each site for review, correction or completion prior to final analysis.
Strategy use might vary depending on local or regional case counts. Daily COVID-19 case data for the county in which a hospital is located was obtained from the New York Times, based on reports from state and local health agencies.11 Weekly COVID-19 cases were calculated from the daily data.
Hospital characteristics
To characterise study hospitals, we collected information on 2019 annual emergency department (ED) patient volume; number of inpatient beds in the facility12; Disproportionate Share Hospital percent13; trauma level; hospital type, based on IBM Watson Hospital categorisation14; the social vulnerability index (SVI) of the hospital’s county15; and the census region in which the hospital resides.16 ED volume information was reported by participating hospitals. Disproportionate Share Hospital percent is a measure of the amount of uncompensated care provided by each hospital. It was available from the Centers for Medicare and Medicaid Services for 15 hospitals in our study sample.
IBM Watson Hospital methodology uses bed count, resident-to-bed ratio and teaching status to place hospitals in one of five categories: major teaching hospital, teaching hospital, large community hospital, medium community hospital or small community hospitals. These categories were applied in order to capture the general context of care, patient volumes, resources and populations cared for, all of which may impact the use of COVID-19 strategies. Veterans Affairs (VA) hospitals, children’s hospitals and critical access hospitals are not included in this categorisation system, and these were classified as ‘Other’.
The SVI is a measure designed to help public agencies identify populations who are particularly at risk during public health emergencies. It ranks each census tract on a number of social factors, including socioeconomic status, household composition and disability, minority status and language, housing type and transportation.
Data analysis
All statistical analyses were carried out in SAS V.9.4 statistical software (SAS Institute Inc). We calculated descriptive statistics (counts, percentages, means, medians, IQRs) for the implementation of each strategy.
In addition to presenting specific use and duration of use data, we presented data in categories in order to provide a more condensed overview. We defined ‘uptake’ as when a hospital implemented a strategy and ‘duration’ as the number of study weeks they reported using a strategy. We plotted strategies based on percent uptake and percent weeks used, reasoning that strategies identified by hospitals that were felt to be potentially helpful and feasible would be frequently implemented and that strategies identified by hospitals as valuable and sustainable following implementation would be retained over time.
We did not find established cut-offs for use of novel hospital strategies so de novo defined asimple threshold of 50% uptake or more as ‘commonly used’ and an average of 50% of study weeks or more as ‘sustained’. This created four categories: (1) common and sustained; (2) common but not sustained; (3) uncommon but when used, sustained; and (4) uncommon and when used, not sustained.
We assessed the crude relationships between implementing a strategy and possible factors we thought were likely to influence strategy uptake: hospital type (major teaching hospital or other), US regions where the hospitals are located (northeast, south, west or midwest) and phase of the pandemic (first wave, defined as January through May 2020, or postfirst wave, defined as the subsequent period) separately, using generalised estimating equations (GEE). All these crude models were adjusted for weekly cases. After calculating crude odds of implementation of each strategy, we developed multivariable longitudinal models examining the associations between hospital type and strategy uptake, adjusting for weekly case counts, US region and phase of the pandemic via GEE. Given the numerous comparisons, we applied a Bonferroni adjustment to the threshold for statistical significance, establishing an alpha value of 0.0012.
Results
Characteristics of study hospitals
We invited 45 investigators to participate. Twenty-four accepted the initial invitation and 23 initiated data collection. Seventeen facilities, representing nine health systems across 11 cities and 7 states, completed at least 70% of data entry over the study period and were included in this report. There were few strategies used in late January, so the analysis was conducted on data from February 2020 to the end of February 2021 (56 weeks). Characteristics of participating hospitals are summarised in table 1.
Table 1.
Characteristics of included hospitals (n=17)
| Characteristics of included hospitals (n=17) | |
| Annual ED volume in 2019 – mean (median) | 63 287 (56 637) |
| Inpatient beds – count mean (median) | 598 (627) |
| Level 1 trauma centres – count (per cent) | 14 (82%) |
| Hospital type* – count (per cent) | |
| Major teaching hospital | 13 (76) |
| Medium community hospital | 1 (6) |
| Children’s hospital | 1 (6) |
| Critical access hospital | 1 (6) |
| Other | 1 (6) |
| DSH Index – mean (median)† | 0.40 (0.39) |
| County SVI index‡ – mean (median) | 0.58 (0.56) |
| Regions§ | |
| Midwest | 4 (24) |
| Northeast | 3 (18) |
| South | 4 (24) |
| West | 6 (35) |
| Uptake of strategies per site out of 42 possible strategies– median (range) | 32 (20 – 40) |
*IBM Watson Hospital categories or non-categorised hospitals (eg, children’s hospital, critical access hospital).
†Disproportionate share hospital percent – measure of amount of uncompensated care; available for 15 hospitals.
‡SVI: 0 (lowest vulnerability) to 1 (highest vulnerability).
§Percentages exceed 100% due to rounding.
ED, emergency department; SVI, social vulnerability index.
Main results
Table 2 provides a summary of strategy uptake and duration of use in average weeks over the study period. The final column of table 2 and figure 1 illustrate how common and sustained individual strategies were. Common and sustained strategies (category 1) include limiting staff in COVID-19 rooms and increasing telehealth capacity for routine or urgent-care visits, work-from-home policies for non-essential employees and wellness resources for employees. Commonly tried but not highly sustained strategies (category 2) included cancelling non-emergency surgeries, removing medical students from clinical rotations and halting research operations. Using separate waiting rooms for patients under investigation of COVID-19 was an uncommon strategy that was sustained by organisations using it (category 3). A number of strategies were both uncommon and even when used, not sustained for long (category 4), including converting operating rooms into intensive care units, increasing hospital bed capacity, waiving usual credentialing requirements for physicians and nurses and allowing medical students to graduate early to begin residency.
Table 2.
Strategies employed by study hospitals (n=17)
| Strategy | Uptake % of sites that used the strategy: count (%) |
Sustainability % of study weeks used, (among sites that used the strategy at least once) mean (SD) Median (IQR) |
How common/how sustained**
|
|
| Patient volume and flow, care practices | ||||
| Use separate waiting rooms for patients under investigation of COVID-19 | 8 (47%) | 74% (32) | 89% (26) | 3 |
| Use dedicated equipment for potential or positive COVID-19 patients (eg, ultrasound, EKG) | 9 (53%) | 72% (26) | 88% (38) | 1 |
| Have triage area outside of hospital | 11 (65%) | 47% (37) | 32% (80) | 2 |
| Send messages to the public to reduce visits to the emergency department | 12 (71%) | 46% (37) | 42 (79) | 2 |
| Use a phone or other video device (eg, iPad) for initial evaluation of stable patients | 12 (71%) | 72% (30) | 86% (15) | 1 |
| Create PPE donning/doffing areas outside clinical care rooms | 12 (71%) | 82% (21) | 89% (9) | 1 |
| Use a phone or other video device (eg, iPad) to register patients | 13 (76%) | 81% (23) | 87% (4) | 1 |
| Create dedicated COVID-19 care areas/zones | 14 (82%) | 76% (20) | 89% (40) | 1 |
| Cancel non-emergency dental visits* | 13 (93%) | 47% (31) | 35% (66) | 2 |
| Decrease use of positive pressure ventilation (CPAP, BiPAP) due to COVID-19 | 16 (94%) | 79% (21) | 88% (15) | 1 |
| Use video laryngoscopy instead of direct laryngoscopy | 16 (94%) | 87% (13) | 91% (4) | 1 |
| Cancel non-emergency outpatient visits | 17 (100%) | 41% (29) | 29% (38) | 2 |
| Cancel non-emergency surgeries | 17 (100%) | 34% (25) | 32% (30) | 2 |
| Limit staff in COVID-19 rooms | 17 (100%) | 86% (10) | 89% (6) | 1 |
| Use telehealth to screen potential COVID-19 cases | 17 (100%) | 89% (4) | 90% (5) | 1 |
| Hospital and workforce capacity | ||||
| Convert surgical operating rooms into intensive care units | 3 (18%) | 13% (19) | 3% (34) | 4 |
| Waive usual credentialing requirements for physicians and nurses | 5 (29%) | 34% (31) | 38% (32) | 4 |
| Significantly increase bed capacity in the ED (by >25%) | 6 (35%) | 28% (22) | 26% (29) | 4 |
| Significantly increase inpatient bed capacity on the floors (by >25%) | 7 (41%) | 37% (24) | 44% (46) | 4 |
| Allow medical students to graduate early to begin residency† | 6 (46%) | 6% (5) | 4% (11) | 4 |
| Significantly increase inpatient bed capacity in the intensive care unit (by >25%) | 11 (65%) | 37% (22) | 41% (36) | 2 |
| Construct or modify additional facilities to expand care capacity | 14 (82%) | 55% (34) | 56% (61) | 1 |
| Increase telehealth capacity for routine or urgent care visits | 17 (100%) | 87% (7) | 88% (7) | 1 |
| Administrative structures/resources | ||||
| Set up new committees, task forces for COVID-19 response | 17 (100%) | 7% (8) | 4% (7) | 2 |
| Posting wellness resources for healthcare workers | 17 (100%) | 86% (8) | 89% (4) | 1 |
| Minimise viral transmission across personnel | ||||
| Isolate healthcare workers with high risk exposures | 15 (88%) | 80% (24) | 89% (12) | 1 |
| Remove medical student clinical rotations | 17 (100%) | 34% (17) | 29% (10) | 2 |
| Encourage or implement voluntary work from home policies for non-essential employees | 17 (100%) | 67% (30) | 85% (39) | 1 |
| Halt (partial or full) basic science research operations* | 14 (100%) | 69% (26) | 84% (52) | 1 |
| Halt (partial or full) clinical science research operations‡ | 15 (100%) | 71% (26) | 84% (49) | 1 |
| Mandate work from home policies for non-essential employees | 17 (100%) | 71% (25) | 88% (38) | 1 |
| Implement a work-related travel ban | 17 (100%) | 86% (14) | 91% (6) | 1 |
| Implement (partial or full) visitor ban | 17 (100%) | 89% (3) | 89% (2) | 1 |
| PPE use and supply | ||||
| Redistribute PPE from non-clinical/non-essential facilities | 11 (65%) | 46% (35) | 40% (74) | 2 |
| Solicit PPE from the community | 12 (71%) | 45% (37) | 42% (82) | 2 |
| Restrict access to PPE | 15 (88%) | 77% (21) | 89% (16) | 1 |
| Conduct formal PPE donning/doffing training | 16 (94%) | 68% (33) | 87 (59) | 1 |
| Testing | ||||
| In-house COVID-19 testing | 12 (71%) | 39% (32) | 45% (64) | 2 |
| Conduct drive-through (or walk-through) COVID-19 testing for hospital employees | 12 (71%) | 68% (33) | 85% (30) | 1 |
| Conduct drive-through (or walk-through) COVID-19 testing for the public | 12 (71%) | 74% (22) | 82% (26) | 1 |
| Turnaround time for COVID-19 test <1 day | 13 (76%) | 75% (17) | 82% (30) | 1 |
| Conduct drive-through (or walk-through) COVID-19 testing for patients | 13 (76%) | 82% (12) | 86% (6) | 1 |
*n=14.
†n=13.
‡n=15.
BiPAP, Bilevel Positive Airway Pressure; CPAP, Continuous Positive Airway Pressure; ED, emergency department; PPE, personal protective equipment.
Figure 1.
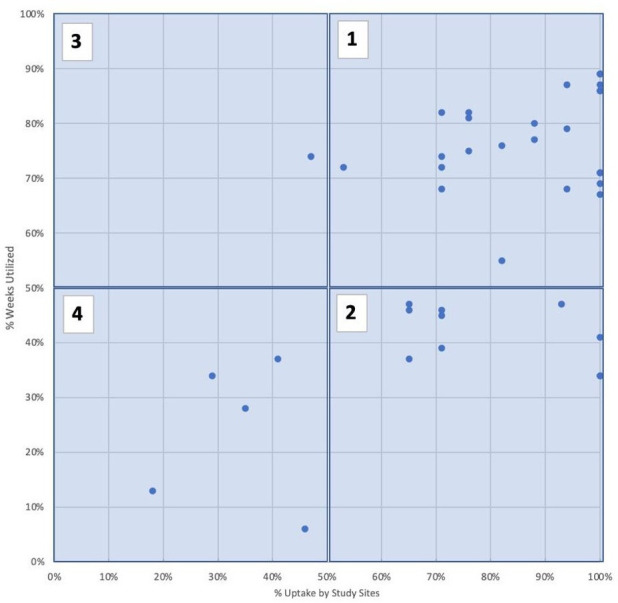
Uptake (x-axis) versus duration of use (y-axis). (1) Common (≥50% organisations used at some point during the study) + sustained (≥50% mean study weeks, among hospitals that used the strategy). (2) Common (≥50% organisations used at some point during the study)/not sustained (<50% mean study weeks, among hospitals that used the strategy). (3) Uncommon (≤50% organisations used at some point during the study)/sustained (≥50% mean study weeks, among hospitals that used the strategy). (4) Uncommon (<50% organisations used at some point during the study)/not sustained (<50% mean study weeks, among hospitals that used the strategy).
Results of the multivariable analysis are in table 3. Hospital type (being a major teaching hospital) was associated with increased odds of only one type of strategy: in-house COVID-19 testing capacity. Regions had several differences. Compared with the midwest (the referent category), the northeast was more likely to use a phone or other device to register patients, create dedicated COVID-19 care areas/zones and have rapid (<1 day) turnaround time for COVID-19 tests; all other regions were more likely to mandate work from home policies for non-essential employees; the south was more likely to restrict access to PPE; and the West was more likely to conduct drive-through (or walkthrough) COVID-19 testing for the public.
Table 3.
Multivariable models for odds of uptake of strategies, adjusted for weekly county-level COVID-19 case counts*
| Strategy (n=17) | OR, p value | ||||
| Hospital type† | Region‡:NE | Region‡:S | Region‡:W | Phase§ | |
| Patient volume and flow, modified clinical practices | |||||
| Use a phone or other video device (eg, iPad) to register patients | 0.29, 0.30 | 217.08,<0.0001 | 79.40, 0.03 | 25.09, 0.07 | 1.65, 0.19 |
| Create dedicated COVID-19 care areas/zones | 0.83, 0.87 | 37.66,<0.0001 | 9.82, 0.07 | 2.06, 0.49 | 1.70, 0.17 |
| Use video laryngoscopy instead of direct laryngoscopy | 2.43, 0.42 | 1.63, 0.62 | 0.85, 0.92 | 0.60, 0.59 | 0.20, 0.0003 |
| Cancel non-emergency outpatient visits | 2.64, 0.33 | 6.39, 0.09 | 1.47, 0.51 | 5.16, 0.04 | 6.15, 0.0007 |
| Cancel non-emergency surgeries | 5.40, 0.01 | 17.33, 0.003 | 1.91, 0.36 | 1.45, 0.68 | 11.39,<0.0001 |
| Hospital and workforce capacity | |||||
| Significantly increase inpatient bed capacity on the floors (by >25%) | 0.59, 0.68 | 1.09, 0.94 | 0.46, 0.56 | 0.20, 0.09 | 2.65,<0.0001 |
| Minimise viral transmission across personnel | |||||
| Encourage or implement voluntary work from home policies for non-essential employees | 0.89, 0.92 | 0.62, 0.70 | 0.97, 0.98 | 0.08, 0.04 | 0.19,<0.0001 |
| Mandate work from home policies for non-essential employees | 6.50, 0.03 | 26.79, 0.0002 | 142.23,<0.0001 | 13.25,<0.0001 | 0.90 0.86 |
| Remove medical student clinical rotations | 0.92, 0.87 | 5.89, 0.09 | 5.91, 0.02 | 0.44, 0.24 | 8.54,<0.0001 |
| PPE use and supply | |||||
| Restrict access to PPE | 1.16, 0.92 | 11.40, 0.002 | 91.11,<0.0001 | 0.78, 0.83 | 0.92, 0.85 |
| Testing | |||||
| Conduct drive-through (or walk-through) COVID-19 testing for the public | 10.42, 0.01 | 7.11, 0.19 | 10.33, 0.01 | 59.18, 0.0003 | 0.38, 0.008 |
| Turnaround time for COVID-19-test<1 day | 0.79, 0.80 | 68.06,<0.0001 | 3.28, 0.41 | 0.51, 0.56 | 0.15, 0.0003 |
| In-house COVID-19 testing | 21.38,<0.0001 | 4.12, 0.10 | 0.13, 0.09 | 7.02, 0.049 | 0.36, 0.07 |
Only strategies with significant relationships are shown here (the remainder are included in a table in online supplemental file 2).
*Alpha value of <0.0012 is significant.
†Hospital type baseline category=non-major teaching hospital (vs major teaching hospital).
‡Regions: NE=northeast; S=south; W=west; Midwest=referent category.
§Phase baseline (referent) category=post-June 2020.
PPE, personal protective equipment.
bmjopen-2022-067986supp002.pdf (108KB, pdf)
We detected several statistically significant differences between the first wave (the referent category) and post-first wave period. Postfirst wave, hospitals were more likely to report using video laryngoscopy instead of direct laryngoscopy, encourage or implement voluntary work from home policies for non-essential employees and have access to shorter turnaround times for COVID-19 tests. They were less likely to cancel non-emergency outpatient visits and non-emergency surgeries, significantly increase inpatient bed capacity on the floors (by >25%) and to remove medical student clinical rotations.
Limitations
We designed the study early in the pandemic and included strategies that appeared to be plausible and important at that time. However, strategies not identified at study outset or introduced later may have been missed. The study hospitals were all within the USA. While this allowed regional and institutional variability, a single-country sample meant there was relative uniformity in terms of stage of pandemic and outside influences, including national pandemic responses.
Furthermore, the study sites were primarily urban academic hospitals and minimally represented or did not represent smaller or mid-sized community hospitals, VA hospitals, children’s hospitals or rural settings; therefore, although we did capture some in-country heterogeneity, its generalisability to all settings across the USA is limited.
We cannot discern the exact influences determining strategy uptake, which likely included a complex interaction of feasibility, inertia, acceptability, community risk tolerance, cost and caution. We did not capture strategies that may have been desired but minimally possible. For example, while increasing ED, inpatient or ICU capacity did not rise to the surface as high uptake-measures, it is important to consider this in the context of the fact that many US hospitals operate at near full capacity at baseline.17
Discussion
The COVID-19 pandemic precipitated a series of institutional interventions that had broad and lasting impact across healthcare systems in the USA. Hospital operational decisions during the pandemic have been complex, likely influenced by numerous factors, including hospital case load, local or regional transmission rates, available resources, regional practice, real-time or projected organisational capacity, leadership types, workforce culture and risk tolerance. Some actions—such as halting elective surgeries—had tremendous implications for an institution’s financial survival and ability to sustain its workforce; in the first 4 months of the pandemic alone, hospitals lost an estimated $202.6 billion in US dollars.18
This study was designed in early 2020. At that time, there were no studies describing hospital responses to COVID-19 and few describing responses to public health threats similar to COVID-19. Hospital preparedness literature is often focused around self-limited events, like weather-related disasters or mass casualty incidents, and not the kind of sustained, high intensity stressor that the pandemic placed on health facilities.
In the intervening 3 years, numerous studies have been published related to various specific aspects of the hospital COVID-19 response, including visitation policies,19–23 practice adaptations in specific settings or types of care (eg, ICU, paediatric and cancer care, aerosol generating procedures),24–28 infection control procedures,29 use of technology to assist care and prevent disease transmission.30–33 Many of these manuscripts propose guidelines on deploying a strategy, explore experiences or outcomes of staff and patients or describe evolution of a specific guideline.
Our study is the only investigation we are aware of that captures a wide range of potential strategies over time through different phases of the pandemic. There are a number of important topics we did not capture, including specific changes in shiftwork models,34 changes in communication with staff,35 creation of specific roles like ‘command centre’ personnel35 and coordination with public agencies.36 Nevertheless, cataloguing the strategies and quantifying their sustained use informs future pandemic planning as organisations both reflect on their own responses and seek to learn lessons from the responses of other health systems.
Commonly employed strategies that endured were of great interest to us, as these strategies were likely to have been associated with perceptions or experience of effective resource utilisation, cost and fit within existing systems and structure across a variety of institution types and locations. Streamlining staff in COVID-19 rooms, boosting telehealth capacity, work from home policies—all high uptake and sustained use strategies—have obvious and multiple drivers, including reduction of transmission, efficiency and potential cost savings. Those that were high cost and disrupted standard operations and core missions of these organisations (eg, cancelling surgeries, keeping medical students from clinical rotations) were also those that in our data appear to have been minimised quickly, after the first wave.
We suspected that strategy utilisation would be driven in large part by hospital type, as all had a component of cost and resources. However, hospital type demonstrated little association with utilisation across strategy type. It may be that the small number of hospitals in our sample that were not major teaching hospitals limited our ability to examine this variable robustly or that cost and resources drove decision making across all sites in similar manner.
Regional variability was observed and validated our sense that geographic location impacted strategy uptake (eg, high odds of using video devices in the northeast, high odds of drive through testing in the west). There are many mechanisms for this kind of variation, including regional communication and coordination of response, supply chains (eg, PPE and testing), geographically driven organisational similarities and the timing of COVID-19, with areas affected early and/or more severely responding more rapidly and assertively with strategies and resources. The difference in use of some strategies likely reflect societal and economic conditions. For example, work from home policies, which were more used in all regions compared with the NE, may have been a factor of different regional availability of at-home work spaces, public transit, driving distances or differing regional social norms related to workplace culture.
Interestingly, many measures were initiated over a short period of time across geographically diverse sites, even those with low local case volumes. While judicious in such a rapidly evolving situation, hospitals likely did not need uniform implementation of many strategies, especially within adaptive and coordinated statewide systems.37 38
Conclusion
This study describes a broad collection of hospital operational modifications used in response to a pandemic and identify those that may have been seen as most effective, feasible and/or sustainable by the organisations implementing them. This may provide an expanded reference for institutions facing pandemics in the future. More data related to how organisations initiate, continue or reinstitute strategies during successive waves of infection, and how these relate to successful clinical operations, may be valuable to health systems during the ongoing crisis and into the future.
Supplementary Material
Acknowledgments
We acknowledge the support of the Oregon Clinical & Translational Research Institute, which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR002369.
Footnotes
Twitter: @StrehlowMatthew, @kcmadhav16
Correction notice: This article has been corrected since it was first published. Minor textual changes have been done.
Contributors: EC contributed to study conception, study design, data validation, data interpretation, drafting and revising the manuscript, final approval of the version to be published, agreeing to be accountable for all aspects of the work, and is responsible for the overall content as guarantor. MS contributed to study design, data collection, data interpretation, revising the manuscript, and final approval of the version to be published, agreeing to be accountable for all aspects of the work. MDR contributed to study design, data interpretation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. EO contributed to data analysis and validation, data interpretation, drafting and revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. RP, AN and SL contributed to study design, data collection and validation, data interpretation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. CH contributed to study conception, study design, data collection and validation, data interpretation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. SN and DK contributed to study conception, study design, data collection and validation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. SVC contributed to study design, data collection and validation, data interpretation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. AW contributed to study design, data collection, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. JJB contributed to data collection and validation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. DWC, ZG, CJG and NH contributed to data collection, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. MKD, AMa, MM and AMe contributed to data collection and validation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. AJ contributed to study design, data collection, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. MKC contributed to data analysis, drafting and revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. DP contributed to study design, data analysis, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. DR-A and LS contributed to data collection and validation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. CS contributed to data collection and validation, data interpretation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. SS contributed to study design, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. DDP contributed to data collection and validation, data interpretation,revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work. DMM contributed to study conception, study design, data collection and validation, data interpretation, revising the manuscript and final approval of the version to be published, agreeing to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Study code and study data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This is a study of institutional characteristics and practices and does not involve human participants.
References
- 1.EMS and disaster preparedness. American College of Emergency Physicians, [Google Scholar]
- 2.Cao Y, Li Q, Chen J, et al. Hospital emergency management plan during the COVID-19 epidemic. Acad Emerg Med 2020;27:309–11. 10.1111/acem.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GA, Buck CR, Kang CS, et al. COVID-19 in seattle-early lessons learned. J Am Coll Emerg Physicians Open 2020;1:85–91. 10.1002/emp2.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kline JA, Pettit KL, Kabrhel C, et al. Multicenter registry of United States emergency department patients tested for SARS-cov-2. J Am Coll Emerg Physicians Open 2020;1:1341–8. 10.1002/emp2.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Determination that a public health emergency exists. US Department of Health & Human Services, 2020. [Google Scholar]
- 6.Zhong S, Clark M, Hou XY, et al. Development of hospital disaster resilience: conceptual framework and potential measurement. Emerg Med J 2014;31:930–8. 10.1136/emermed-2012-202282 [DOI] [PubMed] [Google Scholar]
- 7.UW department of emergency medicine ED COVID-19 guide. n.d.
- 8.Global medxchange for combatting covid-19. n.d.
- 9.Harris PA, Taylor R, Minor BL, et al. The redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (redcap) -- a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The New York Times . Coronavirus (covid-19) data in the united states. 2021. Available: https://github.com/nytimes/covid-19-data [Accessed Nov-Dec 2021].
- 12.FY 2020 Final Rule and Correction Notice Data Files . n.d. Centers for medicare and medicaid services (CMS).
- 13.Disproprotionate Share Hospital (DSH) . n.d. Centers for medicare and medicaid services (CMS).
- 14.Watson health top 100 hospitals study. 2020. 10.1093/oso/9780198847533.001.0001 [DOI]
- 15.CDC social vulnerability index. n.d.
- 16.2010 census regions and divisions of the united states. United States Census Bureau, [Google Scholar]
- 17.Moskop JC, Geiderman JM, Marshall KD, et al. Another look at the persistent moral problem of emergency department crowding. Ann Emerg Med 2019;74:357–64. 10.1016/j.annemergmed.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 18.Hospitals and health systems face unprecedented financial pressures due to COVID-19. American Hospital Association, [Google Scholar]
- 19.Jaswaney R, Davis A, Cadigan RJ, et al. Hospital policies during COVID-19: an analysis of visitor restrictions. J Public Health Manag Pract 2022;28:E299–306. 10.1097/PHH.0000000000001320 [DOI] [PubMed] [Google Scholar]
- 20.Lanphier E, Mosley L, Antommaria AHM. Assessing visitor policy exemption requests during the COVID-19 pandemic. Pediatrics 2021;148:e2021051254. 10.1542/peds.2021-051254 [DOI] [PubMed] [Google Scholar]
- 21.Iness AN, Abaricia JO, Sawadogo W, et al. The effect of hospital visitor policies on patients, their visitors, and health care providers during the COVID-19 pandemic: a systematic review. Am J Med 2022;135:1158–67. 10.1016/j.amjmed.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeh RD, Santry HP, Monsour C, et al. Impact of visitor restriction rules on the postoperative experience of COVID-19 negative patients undergoing surgery. Surgery 2020;168:770–6. 10.1016/j.surg.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiest KM, Krewulak KD, Hiploylee C, et al. An environmental scan of visitation policies in Canadian intensive care units during the first wave of the COVID-19 pandemic. Can J Anaesth 2021;68:1474–84. 10.1007/s12630-021-02049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vranas KC, Golden SE, Mathews KS, et al. The influence of the COVID-19 pandemic on ICU organization, care processes, and frontline clinician experiences: a qualitative study. Chest 2021;160:1714–28. 10.1016/j.chest.2021.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international Collaborative group. Oncologist 2020;25:e936–45. 10.1634/theoncologist.2020-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Martínez CE, Sinha IP, Whittaker E, et al. Nebulization procedures for children with unknown viral status during the COVID-19 pandemic. J Asthma 2021;58:1597–8. 10.1080/02770903.2020.1827418 [DOI] [PubMed] [Google Scholar]
- 27.Abola RE, Schwartz JA, Forrester JD, et al. A practical guide for anesthesia providers on the management of coronavirus disease 2019 patients in the acute care hospital. Anesth Analg 2021;132:594–604. 10.1213/ANE.0000000000005295 [DOI] [PubMed] [Google Scholar]
- 28.Efendi D, Hasan F, Natalia R, et al. Nursing care recommendation for pediatric COVID-19 patients in the hospital setting: a brief scoping review. PLoS One 2022;17:e0263267. 10.1371/journal.pone.0263267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee C, Baker MA, Klompas M. Survey of coronavirus disease 2019 (COVID-19) infection control policies at leading US academic hospitals in the context of the initial pandemic surge of the severe acute respiratory coronavirus virus 2 (SARS-cov-2) omicron variant. Infect Control Hosp Epidemiol 2022:1–7. 10.1017/ice.2022.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilendrer S, Patel B, Chadwick W, et al. Rapid deployment of inpatient telemedicine in response to COVID-19 across three health systems. J Am Med Inform Assoc 2020;27:1102–9. 10.1093/jamia/ocaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ming LC, Untong N, Aliudin NA, et al. Mobile health apps on COVID-19 launched in the early days of the pandemic: content analysis and review. JMIR Mhealth Uhealth 2020;8:e19796. 10.2196/19796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anyanwu EC, Ward RP, Shah A, et al. A mobile APP to facilitate socially distanced Hospital communication during COVID-19: implementation experience. JMIR Mhealth Uhealth 2021;9:e24452. 10.2196/24452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaspar K. Motivations for social distancing and APP use as complementary measures to combat the COVID-19 pandemic: quantitative survey study. J Med Internet Res 2020;22:e21613. 10.2196/21613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X, Jiang L, Hu Y, et al. Nurses’ experiences regarding shift patterns in isolation wards during the COVID-19 pandemic in China: a qualitative study. J Clin Nurs 2020;29:4270–80. 10.1111/jocn.15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penwill NY, Roessler De Angulo N, Pathak PR, et al. Changes in pediatric hospital care during the COVID-19 pandemic: a national qualitative study. BMC Health Serv Res 2021;21:953. 10.1186/s12913-021-06947-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cha KS, Kim EM. A topic modeling analysis of the crisis response stage during the COVID-19 pandemic. Int J Environ Res Public Health 2022;19:14. 10.3390/ijerph19148331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.COVID-19: oregon hospitals share data, create real-time bed capacity system. n.d.
- 38.Schorsch K, Woelfel M. When some small hospitals in chicago were full, bigger ones had beds to spare. WBEZ 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067986supp001.pdf (6.8MB, pdf)
bmjopen-2022-067986supp002.pdf (108KB, pdf)
Data Availability Statement
Data are available on reasonable request. Study code and study data are available on reasonable request.


