Abstract
Background
As an emerging treatment strategy for triple-negative breast cancer (TNBC), immunotherapy acts in part by inducing ferroptosis. Recent studies have shown that protein arginine methyltransferase 5 (PRMT5) has distinct roles in immunotherapy among multiple cancers by modulating the tumor microenvironment. However, the role of PRMT5 during ferroptosis, especially for TNBC immunotherapy, is unclear.
Methods
PRMT5 expression in TNBC was measured by IHC (immunohistochemistry) staining. To explore the function of PRMT5 in ferroptosis inducers and immunotherapy, functional experiments were conducted. A panel of biochemical assays was used to discover potential mechanisms.
Results
PRMT5 promoted ferroptosis resistance in TNBC but impaired ferroptosis resistance in non-TNBC. Mechanistically, PRMT5 selectively methylated KEAP1 and thereby downregulated NRF2 and its downstream targets which can be divided into two groups: pro-ferroptosis and anti-ferroptosis. We found that the cellular ferrous level might be a critical factor in determining cell fate as NRF2 changes. In the context of higher ferrous concentrations in TNBC cells, PRMT5 inhibited the NRF2/HMOX1 pathway and slowed the import of ferrous. In addition, a high PRMT5 protein level indicated strong resistance of TNBC to immunotherapy, and PRMT5 inhibitors potentiated the therapeutic efficacy of immunotherapy.
Conclusions
Our results reveal that the activation of PRMT5 can modulate iron metabolism and drive resistance to ferroptosis inducers and immunotherapy. Accordingly, PRMT5 can be used as a target to change the immune resistance of TNBC.
Keywords: Immunotherapy, Breast Neoplasms
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Triple-negative breast cancer (TNBC) is the worst subtype of breast carcinoma which has lacked effective targets in past years. Immunotherapy is an emerging strategy which acts in part by inducing ferroptosis. However, immune-resistance is inevitable during the treatment and combined therapy might be a strategy to solve the problem. Recent studies have shown protein arginine methyltransferase 5 (PRMT5), a promising druggable target, has distinct roles in immunotherapy among various cancers by modulating tumor microenvironment. But the role of PRMT5 during ferroptosis, especially for TNBC immunotherapy, is unclear.
WHAT THIS STUDY ADDS
PRMT5 downregulated NRF2/HMOX1 pathway and slowed down the import of ferrous by methylating and stabilizing KEAP1. In the context of higher ferrous concentration in TNBC cells, PRMT5 promoted the resistance of ferroptosis and immunotherapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our study demonstrated that the activation of PRMT5 can modulate iron metabolism and drive resistance to ferroptosis inducers and immunotherapy. Accordingly, PRMT5 can be used as a marker to screen candidates and as a promising target to change the immune resistance of TNBC.
Introduction
As the most frequently diagnosed carcinoma, breast cancer is considered the leading cause of cancer-associated mortality in women.1 2 Triple-negative breast cancer (TNBC), a subtype that lacks human epidermal growth factor 2, progesterone receptor, and estrogen receptor expression, accounts for 16% of all patients with breast carcinoma. However, more than 30% of breast cancer-associated deaths are attributed to TNBC.3
In recent years, chemotherapy has been the main choice for the treatment of patients with TNBC due to the deficiency of therapeutic targets.4 With an in-depth understanding of the immune microenvironment of breast cancer,5 6 the advent of immunotherapy, which can reinvigorate inactivated T cells, has improved the prognosis of patients with TNBC.7–9 However, only a few patients with TNBC respond to immunotherapy, and it is still difficult to accurately distinguish patients with early TNBC with different responses.10 Consequently, novel markers for patient stratification and therapeutic targets to improve efficacy are urgently needed.
Regulated cell death (RCD), which includes necrosis, apoptosis, pyroptosis, etc, has become increasingly important in tumor therapy in recent years, as it can target specific tumor cells and increase the efficacy of drug killing while reducing side effects in normal tissues. Ferroptosis has been found to be an RCD in recent studies. Ferroptosis is generated by excessive iron and is characterized by the peroxidation of polyunsaturated fatty acids.11 12 It is believed that ferroptosis mainly results from ferrous ion accumulation, antioxidant system malfunction, free radical production, and lipid peroxidation.13 However, the precise mechanisms underlying ferroptosis have not been fully elucidated. A variety of studies have shown that ferroptosis plays an important role in breast cancer treatment; ferroptosis activators such as RSL3 and erastin induce breast cancer cell death, and a variety of clinically used drugs, such as sorafenib, sulfasalazine, and statins, can also induce ferroptosis in cancer cells.14–18 Recent studies have demonstrated that ferroptosis induced by interferon (IFN)-γ plays a major role in the immunotherapy of TNBC19 20; thus, targeting ferroptosis could furnish new therapeutic opportunities for augmenting immunotherapy efficacy.
Protein arginine methyltransferase 5 (PRMT5) is an important arginine methyltransferase.21 By transferring methyl groups to targeted proteins, PRMT5 can regulate gene transcription and participate in various signaling networks associated with tumor initiation and progression.22 Due to the materiality of PRMT5 in tumor progression, the usage of PRMT5 inhibitors, such as GSK3326595, in the treatment of solid tumors, as well as its activity and safety, were investigated by a phase I clinical trial, and obvious remission was observed in a proportion of patients who received PRMT5 inhibitors orally, suggesting the great potential of PRMT5 inhibitors for therapeutic applications.23
Recent findings have reported that PRMT5 exerts an effect in a cancer-specific way in the immunotherapy of diverse cancers. The inhibition of PRMT5 can augment the efficacy of immunotherapy against melanoma and cervical cancer.24 25 However, attenuation of immunotherapeutic efficiency and induction of immune resistance were also observed in lung cancer after treatment with PRMT5 inhibitors.26 However, the role of PRMT5 in the resistance of TNBC to immunotherapy and its association with ferroptosis are completely unknown.
Herein, we aimed to discover the function of PRMT5 in the immunotherapy of breast cancer, and explore potential therapeutic targets for overcoming immune resistance in TNBC. We found that PRMT5 can regulate the NRF2/HMOX1 axis by methylating KEAP1 to influence ferroptosis in breast cancer and that targeting PRMT5 is a promising strategy to enhance sensitivity to immunotherapy by regulating the intracellular iron concentration to facilitate ferroptosis in TNBC.
Methods
Cells and cell culture
The cells used in this study were supplied by Pricella (Wuhan, China) and authenticated by the supplier. All cells were cultivated in a specialized medium purchased from Pricella and maintained at 37°C in a 5% CO2 incubator.
Antibodies and reagents
MG132 (HY-13259), Z-VAD-FMK (HY-16658B), erastin (HY-15763), 3-MA (HY-19312), Fer-1 (HY-100579), necrostatin-1 (HY-15760), cycloheximide (HY-12320) and RSL3 (HY-100218A) were obtained from MedChemExpress. JNJ-64619178 (S8624) and GSK3326595 (S8664) were purchased from Selleck Chemicals. Mouse anti-PD-1 antibody (BE0146) and IgG control (BE0089) were purchased from Bio X Cell. Recombinant murine IFN-γ (P6137) was purchased from Beyotime. PRMT5 (D5P2T), actin (8H10D10), and symmetric di-Methyl arginine motif (13222) were purchased from Cell Signaling Technology (Massachusetts, USA). KEAP1 antibody (10 503–2-AP), NRF2 antibody (16 396–1-AP), HMOX1 antibody (10 701–1-AP), V5 antibody (14 440–1-AP), HA antibody (51 064–2-AP), TRIM25 antibody (12 573–1-AP), FLAG antibody (20 543–1-AP), SLC7A11 antibody (26 864–1-AP), and GPX4 antibody (67 763–1-Ig) were purchased from Proteintech.
MTT assay
Cell viability was assessed using MTT assays as directed by the manufacturer. Cells were cultivated at the appropriate density in 96-well plates. Thiazolyl blue was dissolved in sterile PBS (phosphate buffered saline) and added to each well. The supernatant was removed after 3 hours of incubation, and 150 µl of DMSO (dimethyl sulfoxide) was used to dissolve the formazan. The absorbance at 570 nm was determined using a plate reader.
Flow cytometry
The viability of cells was estimated by PI and Annexin V-FITC staining coupled with flow cytometry. PI-negative and Annexin V-FITC-negative cells were considered viable cells. Each cell line was analyzed in three independent experiments.
BODIPY 581/591 C11 assay
C11-BODIPY 581/591 (D3861, Thermo Fisher Scientific, Massachusetts, USA) was used to determine the level of lipid ROS (reactive oxygen species). The cells were cultured in confocal dishes, and 1 µM RSL3 was added for 12 hours. Fluorescence was detected by confocal microscopy at excitation wavelengths of 565 nm and 488 nm after 1 hour of staining with 5 µM BODIPY 581/591 C11 at 37°C and two washes with PBS. Fluorescence at an emission wavelength of 590 nm represented normal cells, while fluorescence at 510 nm represented oxidized cell membranes.
Malondialdehyde assay
A Lipid Peroxidation MDA Assay Kit (ab118970, Abcam, UK) was employed to assess the level of malondialdehyde (MDA). After homogenization of the collected cells or tissues in 400 µl specialized lysis buffer, the supernatant was collected by sonication and centrifugation. After a 1-hour incubation at 95°C, a mixture of 600 µl of TBA (thiobarbituric acid) solution and 200 µl of supernatant was applied to determine the absorbance at 532 nm.
Quantitative RT-PCR
For RNA extraction and cDNA synthesis, TRIzol reagent (TaKaRa, China) and a reverse transcription kit (TaKaRa) were employed. Then, RT-PCR detection was performed. β-actin expression was applied for the normalization of target gene expression. The sequences of the primers are shown in online supplemental table S1.
jitc-2023-006890supp001.pdf (3.2MB, pdf)
Coimmunoprecipitation and western blotting
Protease inhibitor cocktail containing RIPA (radioimmunoprecipitation assay buffer) buffer was employed to lyse the collected cells or tissues. Then, the lysates were incubated with the indicated antibodies for 12 hours at 4°C and mixed with protein A/G magnetic beads for 4 hours. After three washes using PBST (phosphate buffered saline with Tween-20) and 8 min of boiling, immunoblotting was performed to determine the eluents. Briefly, after separation using SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), a nitrocellulose membrane was used to combine with the protein samples. Next, after 1 hour of blocking using 5% fat-free milk, overnight incubation of the membranes with particular antibodies was performed at 4°C. Then, after three washes using PBST and a 1-hour incubation with the corresponding HRP (horseradish peroxidase)-conjugated secondary antibody, enhanced chemiluminescence was applied to examine the protein bands.
Intracellular iron assay
An iron assay kit from Abcam (ab83366) was used to determine the intracellular ferrous levels.
FerroOrange assay
To determine the concentrations of iron in cells, FerroOrange (Dojindo, Japan) was employed. Briefly, after a 30-min incubation with 1 mM FerroOrange in confocal dishes at 37°C, the iron concentration in the cells was examined under a confocal microscope at 561 nm to examine the fluorescence.
In vivo tumorigenesis assay
To explore the function of PRMT5 inhibitors on the immune resistance of tumors in vivo, a 4T1 cell-bearing murine model was employed. Briefly, 1×105 4T1 cells in 100 µl PBS and Matrigel (47 743–720, Corning) mixture (1:1) were injected into the mammary fat pads. Then 3 days later, five intraperitoneal injections (two times a week) with 100 µg mouse anti-PD-1 antibody (BE0146, Bio X Cell) were performed. Mouse IgG was also injected as a negative control. Meanwhile, treatment with the PRMT5 inhibitor GSK3326595 (40 mg/kg, oral gavage, daily) or vehicle control was also performed for 15 days. The volume of the tumor was measured as 0.5 × width2 × length, and the tumor was collected after 20 days of treatment. The study was approved by the Air Force Medical University Experimental Animal Ethics Committee.
Patient samples
Eight patients who received immunotherapy were retrospectively screened for PRMT5, NRF2, and HMOX1 protein expression. Four patients had a better clinical response, while the other four patients had a poor clinical response. PRMT5 protein expression was determined by immunohistochemistry (IHC) staining.
IHC staining
The breast cancer tissues embedded in paraffin were provided by The First Affiliated Hospital, Fourth Military Medical University, Xi’an, China. All enrolled subjects signed the informed consent form before the initiation of this study. Then tissue sections (5 µm) were prepared. The slides were incubated with anti-PRMT5 (1:100) antibody, anti-KEAP1 (1:100) antibody, anti-NRF2 (1:100) antibody and anti-HMOX1 (1:100) antibody.
Statistics
Student’s t-tests were conducted to compare two treatment groups. Except tumor volume which is expressed as the mean±SEM, all results are presented as the mean±SD. The raw data were analyzed by SPSS V.22.0, and a p value less than 0.05 was set as a significant difference. A minimum of three independent replications were performed for all experiments.
Results
PRMT5 prevents ferroptosis of TNBC cells
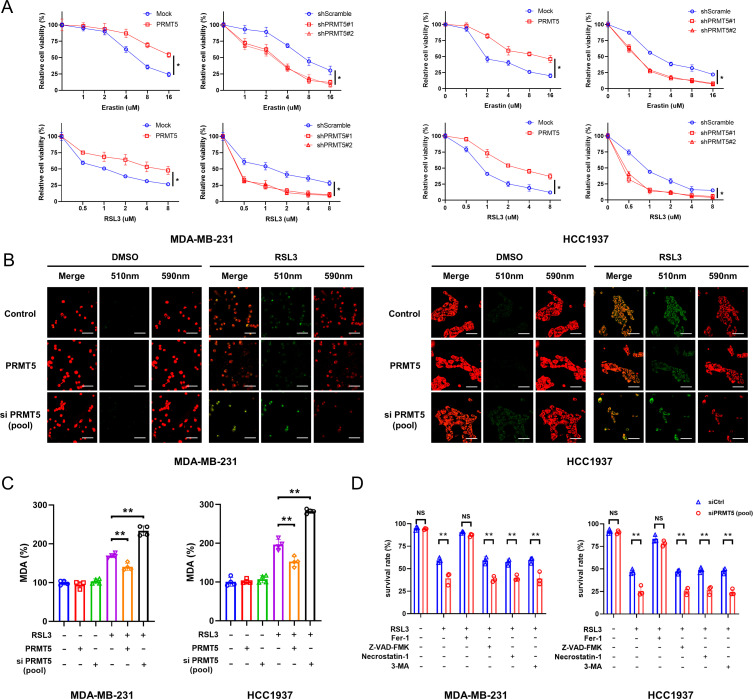
First, we investigated whether PRMT5 could regulate the ferroptosis induced by RSL3 and erastin in TNBC cell lines. Due to the comparable expression of PRMT5 among different TNBC cells (online supplemental figure S1A,B), MDA-MB-231 and HCC1937, two classical TNBC cell lines, were selected for subsequent studies. PRMT5-overexpressing or PRMT5-silenced cells were treated with RSL3 or erastin for 24 hours. We found that PRMT5 overexpression significantly promoted cell viability in the presence of ferroptosis inducers, while silencing PRMT5 obviously increased the ferroptosis caused by ferroptosis inducers (figure 1A).
Figure 1.
PRMT5 overexpression prevents the ferroptosis of TNBC cells. (A) After knockdown or overexpression of PRMT5, HCC1937 and MDA-MB-231 cells were treated with 0, 1, 2, 4, 8, and 16 μM erastin or 0, 0.5, 1, 2, 4, and 8 μM RSL3 for 24 hours. The killing efficiency was examined using the MTT assay. (B,C) PRMT5 overexpressing or PRMT5-deficient HCC1937 and MDA-MB-231 cells were treated with 3 μM RSL3 (3 μM) for 12 hours, and then BODIPY 581/591 C11 staining (B) and MDA assays (C) were conducted to detect the content of cellular lipid ROS (B) and MDA (C), respectively. (D) After 24 hours of treatment with 1 μM RSL3 in combination with 60 μM 3-MA, 10 μM Z-VAD-FMK, 10 μM necrostatin-1, or 1 μM Fer-1, the death of PRMT5-deficient HCC1937 and MDA-MB-231 cells was examined by flow cytometry. Average of three experiments. *p<0.05; **p<0.01; NS, no significance. PRMT5, protein arginine methyltransferase 5; TNBC, triple-negative breast cancer; DMSO, dimethyl sulfoxide; ROS, reactive oxygen species.
It has been widely used to detect ferroptosis by measuring lipid peroxidation. To evaluate the effect of PRMT5 on lipid peroxidation in the presence of RSL3, BODIPY-581/591 staining and MDA detection were performed. As expected, lipid ROS accumulation and MDA levels were markedly increased in the PRMT5-silenced cells and decreased in the PRMT5-overexpressing cells (figure 1B,C).
Moreover, the increased ferroptosis caused by PRMT5 silencing (siRNA sequences are shown in online supplemental table S1) in the presence of RSL3 was reversed by Fer-1 but not agents for autophagy (3-MA), necroptosis (necrostatin-1), or apoptosis (Z-VAD-FMK) (figure 1D). This suggests that PRMT5 overexpression can inhibit TNBC ferroptosis.
PRMT5 induces the resistance of TNBC cells to ferroptosis by downregulating HMOX1
To discover potential mechanisms of PRMT5-regulated ferroptosis, the GSE132407 and GSE65965 databases were employed. The analysis showed differential gene expression in the case of PRMT5 knockdown or inhibition (online supplemental figure S2A). Then, we intersected the differentially expressed genes and ferroptosis-associated genes from the FerrDb data set and identified five ferroptosis-associated genes that might be regulated by PRMT5 (online supplemental figure S2B).27 Among the genes, HMOX1 was selected because of its inducible effect on ferroptosis in a previous study,28 as well as the changes in its expression after PRMT5 knockdown. Hence, we focused on HMOX1 in the following studies.
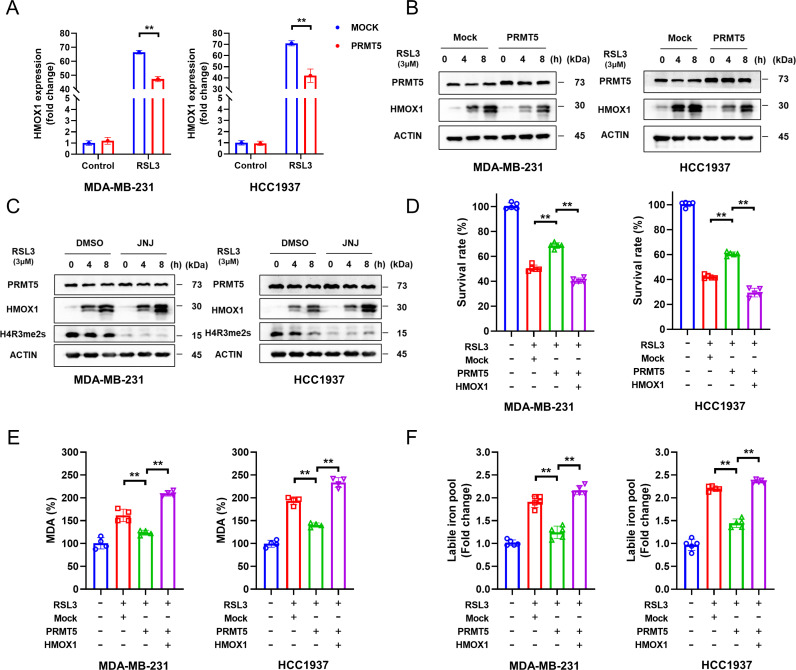
We first measured the mRNA levels of HMOX1 on PRMT5 overexpression. We found that overexpression of PRMT5 dramatically reduced the transcription of HMOX1 in the presence of RSL3 but not in the control group without RSL3 (figure 2A). Next, HMOX1 protein expression was determined by western blotting. As expected, PRMT5 overexpression greatly decreased the protein expression of HMOX1 in the presence of RSL3, while JNJ-64619178, a potent PRMT5 inhibitor, markedly increased HMOX1 protein expression (figure 2B,C). To further explore the function of HMOX1 during PRMT5-regulated ferroptosis, PRMT5-overexpressing HCC1937 and MDA-MB-231 cells were transfected with HMOX1 and then treated with RSL3. MTT and MDA assays demonstrated that HMOX1 overexpression enormously impaired the protective function of PRMT5 overexpression (figure 2D,E). Recent studies have revealed that HMOX1 can increase the concentration of ferrous ions in tumor cells.28 29 Thus, we next investigated whether the intracellular ferrous ion concentration could be regulated by PRMT5 overexpression. Strikingly, the ferrous ion concentration in both cell lines was obviously decreased by PRMT5 overexpression in the presence of RSL3, but this effect was reversed by HMOX1 overexpression (figure 2F). Taken together, these findings indicated that PRMT5 renders TNBC cells resistant to ferroptosis by suppressing HMOX1.
Figure 2.
PRMT5 suppresses the ferroptosis of TNBC cells by downregulating HMOX1. (A,B) After 6 hours of treatment with 3 μM RSL3, the expression of HMOX1 in differentially treated MDA-MB-231 and HCC1937 cells at the mRNA (B) and protein (C) levels was examined by RT-qPCR (B) and western blotting (C), respectively. (D–F) HCC1937 and MDA-MB-231 cells overexpressing or not overexpressing HMOX1 were transfected with PRMT5 and then treated with 1 μM RSL3. After 12-hour and 24-hour treatments, MTT and MDA assays were performed to determine cell viability and intracellular MDA, respectively. Intracellular ferrous content was assessed by an Iron Assay Kit after 6 hours. Average of three experiments. **p<0.01. PRMT5, protein arginine methyltransferase 5; TNBC, triple-negative breast cancer; DMSO, dimethyl sulfoxide; JNJ, PRMT5 inhibitor, Onametostat (JNJ-64619178).
PRMT5 inhibits NRF2/HMOX1 by methylating and stabilizing KEAP1
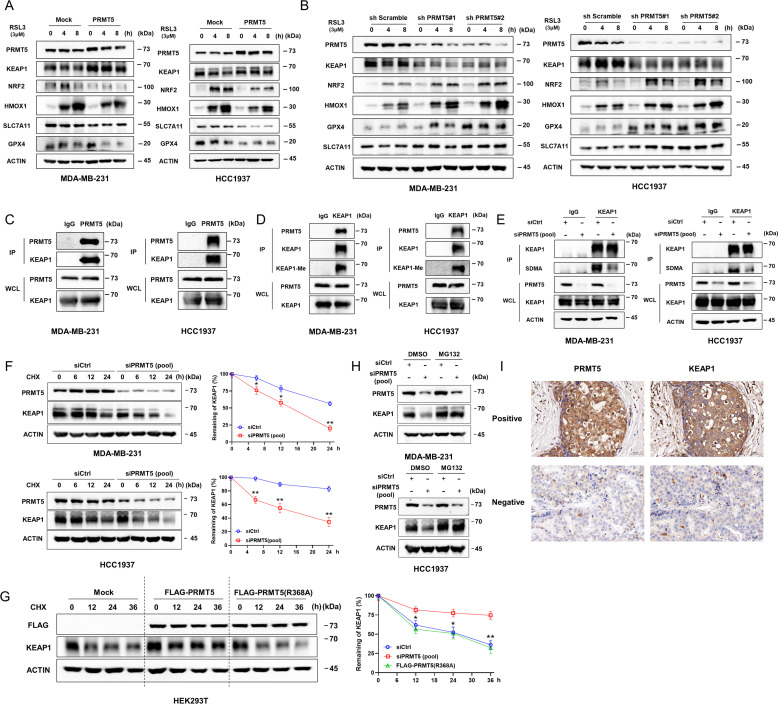
To gain insight into the molecular mechanisms for the downregulation of HMOX1 by PRMT5, the upstream molecules of HMOX1 were measured. In the presence of RSL3, PRMT5 overexpression significantly decreased the protein expression of NRF2, a classical transcription factor of HMOX1, and further increased the protein expression of KEAP1, which constitutively ubiquitinated NRF2 and vice versa (figure 3A,B).30 Additionally, obviously decreased expression of HMOX1 and other target genes of NRF2 was also observed after PRMT5 overexpression. However, no obvious changes in KEAP1 and NRF2 transcription were noted between cells with or without PRMT5 overexpression (online supplemental figure S3A).
Figure 3.
PRMT5 inhibits NRF2/HMOX1 by methylating and stabilizing KEAP1. (A,B) PRMT5 overexpression promotes the translation of KEAP1 and suppresses the expression of NRF2 and its targets (HMOX1, SLC7A11, and GPX4) in the presence of RSL3 and vice versa. (C,D) Representative blots showing the interaction of PRMT5 and KEAP1, as well as the methylation of KEAP1. (E) Deficiency of PRMT5 decreases the methylation of KEAP1 in MDA-MB-231 and HCC1937 cells. (F) Deficiency of PRMT5 significantly accelerated KEAP1 degradation in both cell lines. (G) Overexpression of PRMT5 increased the half-life of KEAP1. Representative graph showing the summarized results of the left panel. (H) The decreased expression of KEAP1 in HCC1937 and MDA-MB-231 cells caused by PRMT5 deficiency was blocked by MG132. (I) The positive correlation of PRMT5 expression with KEAP1 expression in human breast cancer specimens was determined by immunohistochemistry. Representative staining results are shown. Average of three experiments. *p<0.05. PRMT5, protein arginine methyltransferase 5.
PRMT5 is a methyltransferase that can modify histones and other proteins to regulate their function and stability.31 Therefore, the interaction of PRMT5 with KEAP1 or NRF2 was speculated by coimmunoprecipitation (co-IP) and mass spectrometry. As expected, a potential interaction between KEAP1 and PRMT5 was observed (online supplemental figure S3B). Additionally, we observed an obvious interaction between PRMT5 and KEAP1. Meanwhile, we also observed symmetrical methylation of KEAP1 in vivo, and this methylation was significantly impaired by PRMT5 knockdown (figure 3C,E).
Next, the stability of KEAP1 after di-methylation at the arginine was investigated. To this end, the half-life of KEAP1 in PRMT5-deficient cells was determined using cycloheximide. We found that PRMT5 knockdown accelerated KEAP1 protein turnover in both cell lines and that the suppression of KEAP1 expression caused by PRMT5 deficiency could be reversed by MG132. (figure 3F,H). Furthermore, PRMT5 and mutant PRMT5-R368A plasmids were transfected into HEK293T cells. The half-life of the protein was increased in the former but not in the latter (figure 3G; Online supplemental figure S3C). Additionally, IHC was used to assess the protein expression of PRMT5 and KEAP1 in breast carcinoma tissues and explore their clinical relevance in breast cancer. A significantly positive association was observed between PRMT5 and KEAP1 (figure 3I; Online supplemental table S3). These data suggested that PRMT5 could inhibit NRF2/HMOX1 by methylating and stabilizing KEAP1.
PRMT5 inhibits TRIM25-mediated KEAP1 ubiquitination by inducing KEAP1R596me2
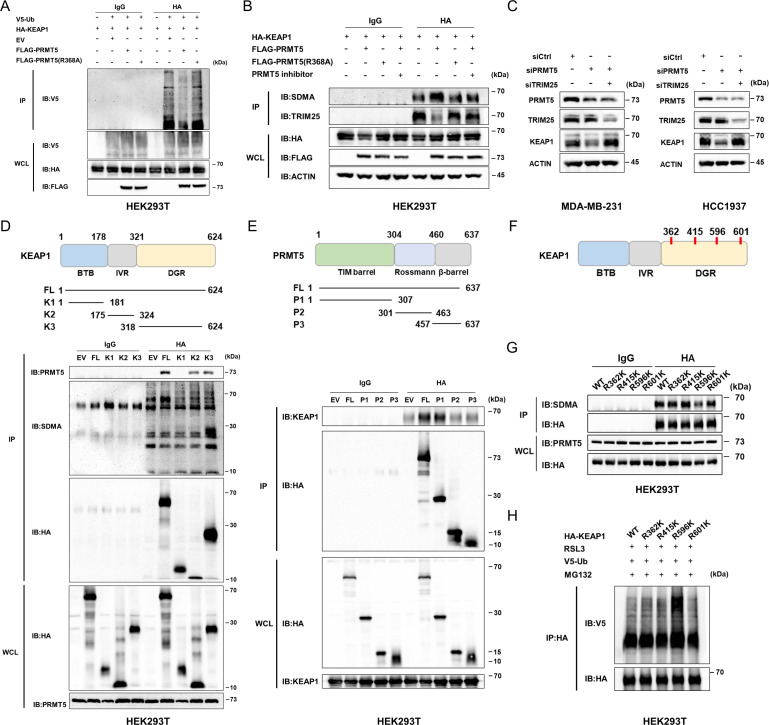
To discover the potential mechanism for the regulation of KEAP1 stability by methylation, the ubiquitination levels of KEAP1 were determined. Significantly decreased ubiquitination of KEAP1 was observed in the PRMT5-overexpressing HEK293T cells but not in the PRMT5-R368A cells(figure 4A). A previous study showed that KEAP1 is directly ubiquitinated by TRIM25 in its lysine at position 615,32 so we speculate that the methylation of KEAP1 could perturb the ubiquitination conducted by TRIM25. As expected, the interaction of KEAP1 and TRIM25 was significantly disrupted as PRMT5 was overexpressed but not its inactivated mutants, and this effect can be effectively reversed by the inhibition of PRMT5 (figure 4B). Moreover, the expression of KEAP1 was obviously rescued by the double knockdown of PRMT5 and TRIM25 in both cell lines (figure 4C) (siRNA sequences are shown in online supplemental table S1).
Figure 4.
PRMT5 inhibits TRIM25-mediated KEAP1 ubiquitination by inducing KEAP1R596me2. (A) Overexpression of PRMT5 suppresses the ubiquitination of KEAP1 in an arginine methyltransferase activity-dependent manner. (B) PRMT5 overexpression interfered with TRIM25 binding to KEAP1 in an arginine methyltransferase activity-dependent manner. The cells transfected with the indicated plasmids were extracted following IP and IB analysis. (C) The level of KEAP1 protein was restored by the double silencing of PRMT5 and TRIM25. (D) Representative graph showing the regions in KEAP1 that interact with PRMT5. KEAP1 179-321 aa and 322-624 aa could bind to PRMT5 while KEAP1 322-624 aa can be methylated. (E) Identification of the domain in PRMT5 involved in the interaction with KEAP1. The 1-324 aa region participates in the interaction of PRMT5 with KEAP1. (F) Representative graph showing the putative methylated residues of KEAP1. (G) R596 of KEAP1 can be methylated by PRMT5. After transfection with different plasmids, HEK293T cells were collected, and co-IP was conducted to determine methylation at the indicated site. (H) The level of ubiquitination of KEAP1-R596K was significantly lower than that of the other mutants. Average of three experiments. IB, immunoblotting; IP, immunoprecipitation; PRMT5, protein arginine methyltransferase 5; HA, anti-HA tag.
Next, to further clarify the domains involved in the binding of PRMT5 with KEAP1, different truncated mutants of PRMT5 and KEAP1 were constructed. For KEAP1, we generated three mutants composed of the BTB, IVR, or DGR domain. The co-IP assay suggested that both the IVR and DGR domains interact with PRMT5; however, only KEAP1 mutants containing the DGR domain could be methylated by PRMT5 (figure 4D). In addition, the interaction between KEAP1 and the TIM barrel domain of PRMT5 was also discovered (figure 4E).
Then, predictive tools for methylation, such as GPS-MSP and PRmePred, were applied to screen and identify the potential residue that can be methylated PRMT5 (online supplemental table S2). Four arginine residues in the DGR domain were selected for further analysis (Figure 4F). Furthermore, we generated four KEAP1 mutants with Arg362, Arg415, Arg596 and Arg601 replaced by lysine (termed R362K, R415K, R596K and R601K). These findings suggested that the replacement of Arg596 by Lys596 dramatically suppressed the methylation and enhanced the ubiquitination of KEAP1 (figure 4G,H), suggesting the binding of PRMT5 to KEAP1 at R596 and suppression of ubiquitination and degradation. Collectively, these results demonstrate that PRMT5 inhibits TRIM25-mediated KEAP1 ubiquitination by inducing KEAP1R596me2.
Cellular ferrous levels might be the key to determining cell fate in the case of alterations in PRMT5 or NRF2 protein levels
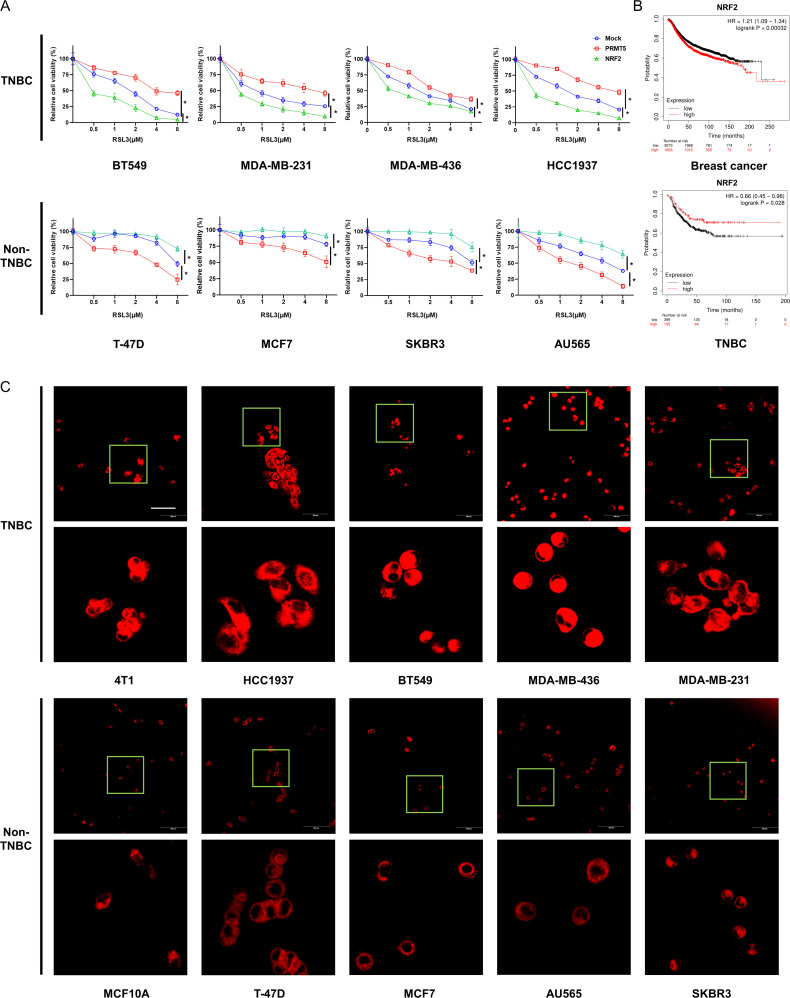
NRF2 is a critical regulatory protein in ferroptosis, but the role of NRF2 is controversial in different cellular and biological contexts.30 We found that the overexpression of PRMT5 or NRF2 resulted in the opposite outcome in different cell lines. In TNBC cells, such as BT549, HCC1937, MDA-MB-436, and MDA-MB-231 cells, the overexpression of PRMT5 and NRF2 significantly increased cell viability and death in the presence of RSL3. However, elevated cell sensitivity to RSL3 and enhanced cell survival were observed in non-TNBC cell lines (such as T-47D, MCF7, SKBR3, and AU565) after PRMT5 and NRF2 overexpression, respectively (figure 5A). Moreover, we also observed significantly negative correlations between the expression of NRF2 and the prognosis of all patients with breast cancer, as well as a positive correlation with the prognosis of patients with TNBC (figure 5B).33
Figure 5.
Cellular ferrous levels might be the key to influencing cell fate in the case of alterations in PRMT5 or NRF2 protein levels. (A) Different breast cancer cell lines were transfected with PRMT5 or NRF2 plasmids and then treated with RSL3 for 24 hours. Then, an MTT assay was conducted to determine cell viability. (B) NRF2 expression-related survival curves of all patients with breast cancer or patients with triple negative breast cancer. (C) Cellular ferrous levels of a panel of breast cancer cell lines were determined using FerroOrange. Scale bar, 100 μm. Average of three experiments. *p<0.05. PRMT5, protein arginine methyltransferase 5; TNBC, triple-negative breast cancer.
A previous study showed that compared with non-TNBC cells, TNBC cells exhibited high sensitivity to ferroptosis and increased iron importer expression.34 Thus, the ferrous ion concentration between TNBC and non-TNBC cells, such as 4T1, HCC1937, BT549, MDA-MB-231, MDA-MB-436, T-47D, MCF7, AU565, SKBR3 and MCF10A, was investigated using an Fe2+ iron probe known as FerroOrange. We observed that compared with the non-TNBC cells, the TNBC cells emitted much stronger orange fluorescence (figure 5C). These results indicated that the cellular ferrous ion levels might be the key to influencing cell fate in the case of alterations in PRMT5 or NRF2 protein levels.
PRMT5 inhibitors potentiated the therapeutic efficacy of immunotherapy
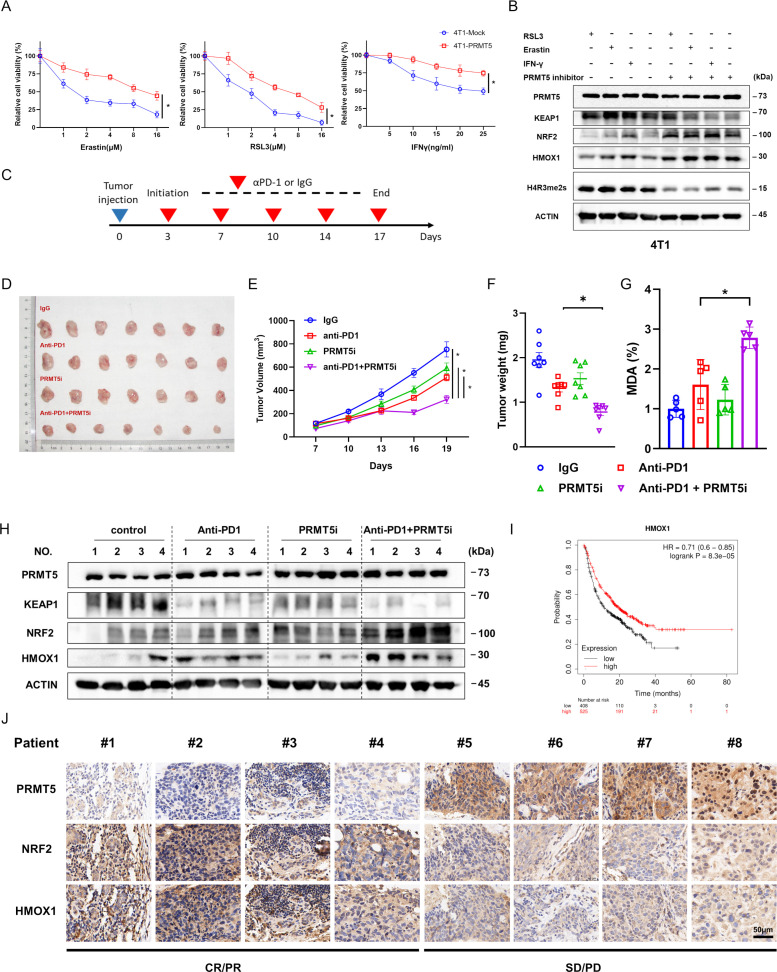
Recent studies have revealed that by promoting ferroptosis, IFN-γ can eliminate tumor cells in vivo. Thus, 4T1 murine mammary cancer cells were transfected with mock and PRMT5 plasmids following RSL3, erastin, and IFN-γ treatment. Markedly increased cell viability was found after PRMT5 overexpression (figure 6A). Furthermore, we found that the inhibition of PRMT5 markedly reduced the KEAP1 protein expression and upregulated the protein expression of NRF2 under treatment with RSL3, erastin, and IFN-γ (figure 6B).
Figure 6.
PRMT5 inhibitors potentiated the therapeutic efficacy of immunotherapy. (A) PRMT5 overexpression prevents the cytotoxic effect of IFN-γ, RSL3 and erastin on murine 4T1 mammary carcinoma cells. (B) Representative blots showing the expression of NRF2, HMOX1, and KEAP1 in 4T1 cells treated with RSL3 (3 µM), erastin (8 µM) or IFN-γ (20 ng/mL) in combination with or without PRMT5 inhibitors. (C) Schematic showing the animal anti-PD1 administration scheme. Tumor-bearing mice were given anti-PD1 antibody or IgG as a control on the indicated days for a total of five treatments. (D) Excised tumors collected on day 19. (E) Representative graph showing the calculated tumor volume at the indicated time points. (F) The tumor weights of excised tumors were measured at the end of the experiment. (G) Representative graph showing the content of MDA in the tumors collected at day 19. (H) PRMT5 inhibitors in combination with anti-PD1 significantly decreased the translation of KEAP1 and increased the production of NRF2 and HMOX1 at the protein level in the indicated tumor tissues. (I) The HMOX1 expression-related survival curves of patients with cancer who received immunotherapy. (J) Expression of PRMT5, NRF2 and HMOX1 protein in human specimens derived from eight patients with TNBC who received immunotherapy. *p<0.05. CR, complete response; IFN, interferon; PD, progressive disease; PR, partial response; PRMT5, protein arginine methyltransferase 5; SD, stable disease.
Given that immunotherapy with an anti-PD-1 antibody was largely dependent on the activity of CD8+ T cells, we further explored whether PRMT5 inhibitors promote immunotherapy-induced ferroptosis in vivo. To this end, 4T1 cells were injected to generate a syngenetic BALB/c mouse model. Mice were subdivided into four groups and administered IgG antibody, anti-PD-1 antibody, GSK3326595 (a PRMT5 inhibitor), and anti-PD-1 antibody combined with GSK3326595. The co-treatment dramatically inhibited tumor growth in comparison to anti-PD-1 antibody alone (figure 6D–F). However, no obvious influence was observed in body weight among the groups (data not shown). Meanwhile, we also observed markedly enhanced MDA in the mice from the co-treatment group in comparison to the mice from other groups (figure 6G).
Next, the protein levels of KEAP1, HMOX1, and NRF2 were measured. Western blotting results suggested that compared with the mice from other groups, the mice of the co-treatment groups showed significantly decreased KEAP1 production and increased NRF2 and HMOX1 production (figure 6H). Moreover, survival data suggested that the prognosis of patients who had been treated with immunotherapy was significantly associated with HMOX1 expression (figure 6I). Furthermore, we obtained eight human specimens derived from patients with TNBC who received immunotherapy and then measured the protein expression of PRMT5, NRF2 and HMOX1. Strikingly, the levels of these proteins were closely associated with the tumor response to immunotherapy (figure 6J; online supplemental figure S4). Taken together, these findings indicated that PRMT5 inhibitors can be applied as drug targets for tumor immunotherapy in the clinic due to their promotive effect on antitumor immune responses.
Discussion
Due to having the worst prognosis, TNBC is perceived as the most challenging subtype of breast carcinoma. Although emerging immunotherapy has brought promise for TNBC, resistance to immunotherapy also exists.35 Therefore, identifying novel molecular targets combined with immunotherapy is undoubtedly a promising strategy to overcome immune resistance.36 37
Previously, we demonstrated the elevated expression of PRMT5 in breast carcinoma and its regulatory function in the resistance of breast cancer cells to chemotherapies by regulating RNA m6A modification and governing stemness.38 39 In the current research, we explored the importance of PRMT5 in the regulation of ferroptosis in the immunotherapy response of TNBC. We found that PRMT5 can methylate and stabilize KEAP1, a ubiquitinated enzyme, thereby inhibiting the downstream NRF2/HMOX1 pathway to promote resistance to ferroptosis and immunotherapy in breast cancer.
As mentioned in the introduction, PRMT5 has been implicated in the communication of tumor and immune cells in the process of immunotherapy.24 However, less attention has been focused on the function of PRMT5 in the inherent immune tolerance of cancer cells. Here, our results demonstrated that PRMT5 overexpression could downregulate the expression of NRF2 and its downstream genes to promote inherent resistance to immunotherapy. The regulatory effect of PRMT5 on NRF2 and its target genes is consistent with the findings obtained by Chang et al but incongruent with those studies showing that PRMT5 promotes an increase in SLC7A11 mRNA levels.40 41 The reason for the discrepancy may be attributed to differences in the tumor context.
Various types of tumors have been linked to ferroptosis since the term ferroptosis was proposed in the last decade. However, some contradictions remain to be explored, perhaps the most notable example involves NRF2, a critical ferroptosis regulator. While some NRF2 target genes (ie, GPX4, SLC7A11, and GCLC) serve anti-ferroptotic functions, some evidence has revealed that NRF2/HMOX1 promotes the ferroptosis-associated cascade in some specific contexts42 43; however the underlying mechanisms are completely unknown. Previous studies have suggested that TNBC is more susceptible to ferroptosis than non-TNBC.34 44 Moreover, the amounts of iron import-associated proteins are higher in TNBC, while iron export-associated proteins are lower. Our study has shown that the levels of ferrous ion (Fe2+) in TNBC are significantly higher than those in non-TNBC, which may induce different cell fate transitions in the case of NRF2 overexpression.
In the context of oxidative stress, NRF2 expression is dramatically increased, which in turn activates downstream targets, including HMOX1 and GPX4.45 HMOX1 catalyzes heme breakdown and releases biliverdin, Fe2+, and monoxide. In TNBC, which exhibits a high ferrous ion concentration, the rapid upregulation of HMOX1 leads to a rapid rise in ferrous ion concentration and further promotes oxygen radical storms. However, the increase in Fe2+ levels may be slight and modest for non-TNBC with a low intracellular ferrous ion concentration. Moreover, the accumulation of anti-ferroptosis regulators, such as GPX4, can reduce lipid peroxidation products and prevent ferroptosis effects. This is compatible with previous findings from Fang et al.29 Moreover, the survival analysis also suggested the importance of HMOX1 in immunotherapy. Collectively, our results further illustrated the importance of iron metabolism for ferroptosis and may provide a reasonable basis for recommending a high-iron diet for patients treated with immunotherapies.
Recently, PRMT5 has been considered a promising treatment target, and multiple PRMT5-targeted agents are currently in clinical trials for a wide variety of tumors.46 47 Regrettably, it is difficult to obtain TNBC specimens treated by immunotherapy and validate the therapeutic efficacy of PRMT5 inhibitors in patient-derived tumor xenograft models or clinical trials. However, our study demonstrated that combining PRMT5 inhibitors with immunotherapy might hold immense promise for TNBC treatment.
The current study revealed that PRMT5 induces KEAP1/R596me2 to prevent KEAP1 ubiquitination mediated by TRIM25, subsequently suppressing NRF2/HMOX1 expression, and thereby preventing the ferroptosis of TNBC cells during immunotherapy. Thus, PRMT5-targeted drugs combined with immunotherapy may be a potential treatment strategy for TNBC.
Footnotes
ZW, RL and NH contributed equally.
Contributors: The experiments were conducted by ZW, RLi, NH, PF, CJ and BZ. The collection of the samples from patients with breast cancer and analysis were conducted by Juliang Zhang, TW, GZ and QY. The manuscript was written by ZW and JD, YW, LL and JK supervised the project and discussed the data. RLing and Jian Zhang conceived, designed and supervised the project and analyzed the data. RLing was the guarantor of this work.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81902677, 82073202), the Natural Science Basic Research Program of Shaanxi (2020JM-340), the Scientific and technological innovation team of Shaanxi Innovation Capability Support Plan (S2023-ZC-TD-0132).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information Additional supporting data are available from the corresponding authors upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee of The First Affiliated Hospital of the Fourth Military Medical University, KY20223273-1. Participants gave informed consent to participate in the study before taking part.
References
- 1. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer Statistics. CA Cancer J Clin 2019;69:438–51. 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3. Yin L, Duan J-J, Bian X-W, et al. Triple-negative breast cancer molecular Subtyping and treatment progress. Breast Cancer Res 2020;22:61. 10.1186/s13058-020-01296-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vagia E, Mahalingam D, Cristofanilli M. The landscape of targeted therapies in TNBC. Cancers (Basel) 2020;12:916. 10.3390/cancers12040916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zou Y, Ye F, Kong Y, et al. The Single‐Cell landscape of Intratumoral heterogeneity and the immunosuppressive Microenvironment in liver and brain metastases of breast cancer. Adv Sci (Weinh) 2023;10:2203699. 10.1002/advs.202203699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keren L, Bosse M, Marquez D, et al. A structured tumor-immune Microenvironment in triple negative breast cancer revealed by Multiplexed ion beam imaging. Cell 2018;174:1373–87. 10.1016/j.cell.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus NAB-paclitaxel as first-line treatment for Unresectable, locally advanced or metastatic triple-negative breast cancer (Impassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020;21:44–59. 10.1016/S1470-2045(19)30689-8 [DOI] [PubMed] [Google Scholar]
- 8. Schmid P, Cortés J, Dent R, et al. KEYNOTE-522: phase III study of Pembrolizumab (Pembro)+ chemotherapy (Chemo) vs placebo (Pbo)+ Chemo as Neoadjuvant treatment, followed by Pembro vs PBO as adjuvant treatment for early triple-negative breast cancer (TNBC). Annals of Oncology 2019;30:v853–4. 10.1093/annonc/mdz394.003 [DOI] [Google Scholar]
- 9. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet 2020;396:1817–28. 10.1016/S0140-6736(20)32531-9 [DOI] [PubMed] [Google Scholar]
- 10. Mediratta K, El-Sahli S, D’Costa V, et al. Current progresses and challenges of Immunotherapy in triple-negative breast cancer. Cancers (Basel) 2020;12:3529. 10.3390/cancers12123529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021;22:266–82. 10.1038/s41580-020-00324-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Cao F, Yin H, et al. Ferroptosis: past, present and future. Cell Death Dis 2020;11:1–13. 10.1038/s41419-020-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Yu C, Kang R, et al. Iron metabolism in Ferroptosis. Front Cell Dev Biol 2020;8:590226. 10.3389/fcell.2020.590226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, Nonapoptotic cell death in Oncogenic-RAS-harboring cancer cells. Chemistry & Biology 2008;15:234–45. 10.1016/j.chembiol.2008.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dolma S, Lessnick SL, Hahn WC, et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003;3:285–96. 10.1016/s1535-6108(03)00050-3 [DOI] [PubMed] [Google Scholar]
- 16. Su Y, Zhao B, Zhou L, et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett 2020;483:127–36. 10.1016/j.canlet.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 17. Yu H, Yang C, Jian L, et al. Sulfasalazine-induced Ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the Transferrin receptor. Oncol Rep 2019;42:826–38. 10.3892/or.2019.7189 [DOI] [PubMed] [Google Scholar]
- 18. Garwood ER, Kumar AS, Baehner FL, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat 2010;119:137–44. 10.1007/s10549-009-0507-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Green M, Choi JE, et al. Cd8+ T cells regulate tumour Ferroptosis during cancer Immunotherapy. Nature 2019;569:270–4. 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Z-H, Tang Y, Yu H, et al. The role of Ferroptosis in breast cancer patients: a comprehensive analysis. Cell Death Discov 2021;7:93. 10.1038/s41420-021-00473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stopa N, Krebs JE, Shechter D. The Prmt5 arginine Methyltransferase: many roles in development, cancer and beyond. Cell Mol Life Sci 2015;72:2041–59. 10.1007/s00018-015-1847-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H, Ronai ZA. Prmt5 function and targeting in cancer. Cell Stress 2020;4:199–215. 10.15698/cst2020.08.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siu LL, Rasco DW, Vinay SP, et al. METEOR-1: A phase I study of Gsk3326595, a first-in-class protein arginine Methyltransferase 5 (Prmt5) inhibitor, in advanced solid tumours. Annals of Oncology 2019;30:v159. 10.1093/annonc/mdz244 [DOI] [Google Scholar]
- 24. Kim H, Kim H, Feng Y, et al. Prmt5 control of cGAS/STING and Nlrc5 pathways defines Melanoma response to antitumor immunity. Sci Transl Med 2020;12:551. 10.1126/scitranslmed.aaz5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang Y, Yuan Y, Chen M, et al. Prmt5 disruption drives antitumor immunity in Cervical cancer by Reprogramming T cell-mediated response and regulating PD-L1 expression. Theranostics 2021;11:9162–76. 10.7150/thno.59605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ZHENG Y, Shen L, Xiao H, et al. Prmt5 inhibition promotes PD-L1 expression and Immuno-resistance in lung cancer. Front Immunol 2022;2021:5877. 10.3389/fimmu.2021.722188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou N, Bao J. Ferrdb: a manually Curated resource for regulators and markers of Ferroptosis and Ferroptosis-disease associations. Database (Oxford) 2020;2020:baaa021. 10.1093/database/baaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang L-C, Chiang S-K, Chen S-E, et al. Heme Oxygenase-1 mediates BAY 11–7085 induced Ferroptosis. Cancer Lett 2018;416:124–37. 10.1016/j.canlet.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 29. Fang X, Wang H, Han D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA 2019;116:2672–80. 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anandhan A, Dodson M, Schmidlin CJ, et al. Breakdown of an ironclad defense system: the critical role of Nrf2 in mediating Ferroptosis. Cell Chem Biol 2020;27:436–47. 10.1016/j.chembiol.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Motolani A, Martin M, Sun M, et al. The structure and functions of Prmt5 in human diseases. Life (Basel) 2021;11:1074. 10.3390/life11101074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, Tao S, Liao L, et al. Trim25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun 2020;11:1–13. 10.1038/s41467-019-14190-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Győrffy B. Survival analysis across the entire Transcriptome identifies biomarkers with the highest Prognostic power in breast cancer. Computational and Structural Biotechnology Journal 2021;19:4101–9. 10.1016/j.csbj.2021.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verma N, Vinik Y, Saroha A, et al. Synthetic lethal combination targeting BET uncovered intrinsic susceptibility of TNBC to Ferroptosis. Sci Adv 2020;6:eaba8968. 10.1126/sciadv.aba8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu Y, Zhu X, Tang C, et al. Progress and challenges of Immunotherapy in triple-negative breast cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2021;1876:188593. 10.1016/j.bbcan.2021.188593 [DOI] [PubMed] [Google Scholar]
- 36. Jiang Z, Lim S-O, Yan M, et al. Tyro3 induces anti–PD-1/PD-L1 therapy resistance by limiting innate immunity and Tumoral Ferroptosis. J Clin Invest 2021;131. 10.1172/JCI139434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tarantino P, Corti C, Schmid P, et al. Immunotherapy for early triple negative breast cancer: research agenda for the next decade. NPJ Breast Cancer 2022;8. 10.1038/s41523-022-00386-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Z, Kong J, Wu Y, et al. Prmt5 determines the sensitivity to chemotherapeutics by governing Stemness in breast cancer. Breast Cancer Res Treat 2018;168:531–42. 10.1007/s10549-017-4597-6 [DOI] [PubMed] [Google Scholar]
- 39. Wu Y, Wang Z, Han L, et al. Prmt5 regulates RNA M6A Demethylation for doxorubicin sensitivity in breast cancer. Molecular Therapy 2022;30:2603–17. 10.1016/j.ymthe.2022.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diao C, Chen Z, Qiu T, et al. Inhibition of Prmt5 attenuates oxidative stress-induced Pyroptosis via activation of the Nrf2/HO-1 signal pathway in a mouse model of renal ischemia-reperfusion injury. Oxid Med Cell Longev 2019;2019:2345658. 10.1155/2019/2345658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao L, Wu G, Zhu J, et al. Genotoxic stress-triggered Β-Catenin/Jdp2/Prmt5 complex facilitates reestablishing glutathione homeostasis. Nat Commun 2019;10:1–17. 10.1038/s41467-019-11696-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwon M-Y, Park E, Lee S-J, et al. Heme Oxygenase-1 accelerates Erastin-induced Ferroptotic cell death. Oncotarget 2015;6:24393–403.:27. 10.18632/oncotarget.5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hassannia B, Wiernicki B, Ingold I, et al. Nano-targeted induction of dual Ferroptotic mechanisms Eradicates high-risk neuroblastoma. J Clin Invest 2018;128:3341–55. 10.1172/JCI99032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun L-L, Linghu D-L, Hung M-C. Ferroptosis: a promising target for cancer Immunotherapy. Am J Cancer Res 2021;11:5856–63.:12. [PMC free article] [PubMed] [Google Scholar]
- 45. He F, Ru X, Wen T. Nrf2, a transcription factor for stress response and beyond. Int J Mol Sci 2020;21:4777. 10.3390/ijms21134777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jin Y, Zhou J, Xu F, et al. Targeting Methyltransferase Prmt5 eliminates leukemia stem cells in chronic Myelogenous leukemia. J Clin Invest 2016;126:3961–80. 10.1172/JCI85239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang S, Ma Y, Hu X, et al. Targeting Prmt5/AKT signalling axis prevents human lung cancer cell growth. J Cell Mol Med 2019;23:1333–42. 10.1111/jcmm.14036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-006890supp001.pdf (3.2MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information Additional supporting data are available from the corresponding authors upon reasonable request.