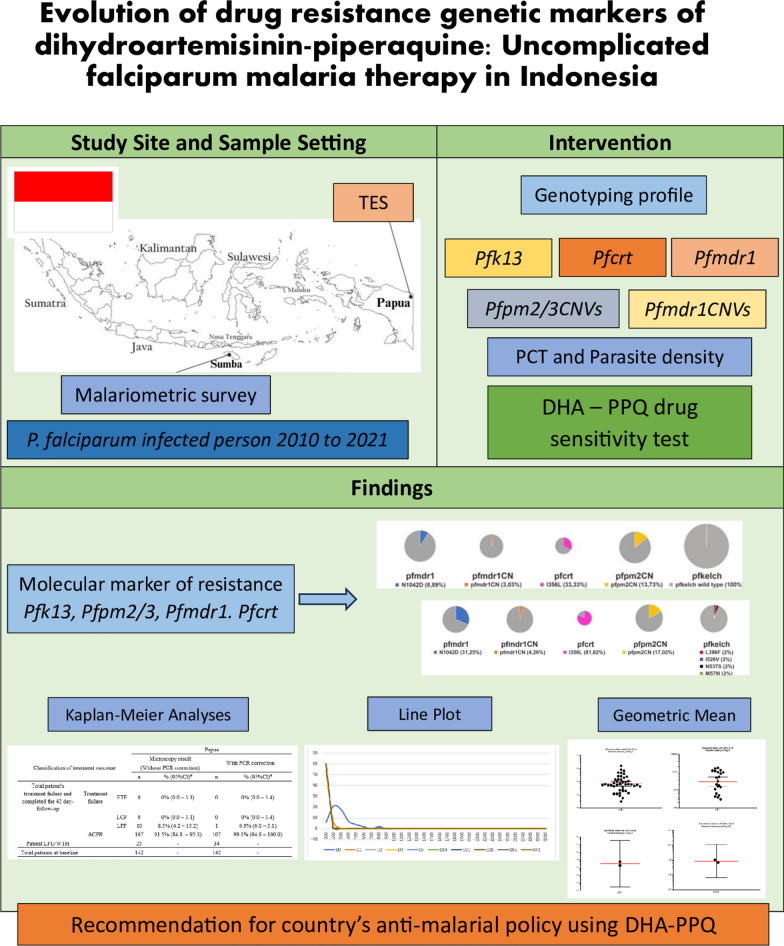
Abstract
Background
Dihydroartemisinin–piperaquine has been Indonesia’s first-line anti-malarial treatment since 2008. Annual therapeutic efficacy studies (TES) done in the last 12 years showed continued high treatment efficacy in uncomplicated Plasmodium falciparum malaria. Although these studies did not show evidence for artemisinin resistance, a slight increase in Late Treatment Failure was observed over time. It is highlight to explore the evolution of genetic markers for ACT partner drug resistance since adopting DHA–PPQ.
Methods
Dry blood spots were identified from a mass blood survey of uncomplicated falciparum malaria patients (N = 50) in Sumba from 2010 to 2018. Analysis of genotypic profile (N = 51) and a Therapeutic Efficacy Study (TES) from Papua (N = 142) from 2020 to 2021, 42-day follow-up. PCR correction using msp1, msp2, and glurp was used to distinguish recrudescence and reinfection. Parasite DNA from DBSs was used for genotyping molecular markers for antimalaria drug resistance, including in Pfk13, pfcrt, and pfmdr1, as well as gene copy number variation in pfpm2/3 and pfmdr1.
Results
The study revealed the absence of SNPs associated with ART resistance and several novel SNPs such as L396F, I526V, M579I and N537S (4.25%). In Sumba, the mutant haplotype SDD of pfmdr1 was found in one-third of the isolates, while only 8.9% in Papua. None of the pfcrt mutations linked to piperaquine resistance were observed, but 71% of isolates had pfcrt I356L. Amplification of the pfpm2/3 genes was in Sumba (17.02%) and Papua (13.7%), while pfmdr1 copy number prevalence was low (3.8%) in both areas. For the TES study, ten recurrences of infection were observed on days 28, 35, and 42. Late parasitological failure (LPF) was observed in 10/117 (8.5%) subjects by microscopy. PCR correction revealed that all nine cases were re-infections and one was confirmed as recrudescence.
Conclusion
This study revealed that DHA–PPQ is still highly effective against P. falciparum. The genetic architecture of the parasite P. falciparum isolates during 2010–2021 revealed single copy of Pfpm2 and pfmdr1 were highly prevalent. The slight increase in DHA–PPQ LTF alerts researchers to start testing other ACTs as alternatives to DHA–PPQ for baseline data in order to get a chance of achieving malaria elimination wants by 2030.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-023-04658-4.
Keywords: Plasmodium falciparum, Resistance, Pfk13, Pfmdr1, Pfcrt, Pfpm2/3
Background
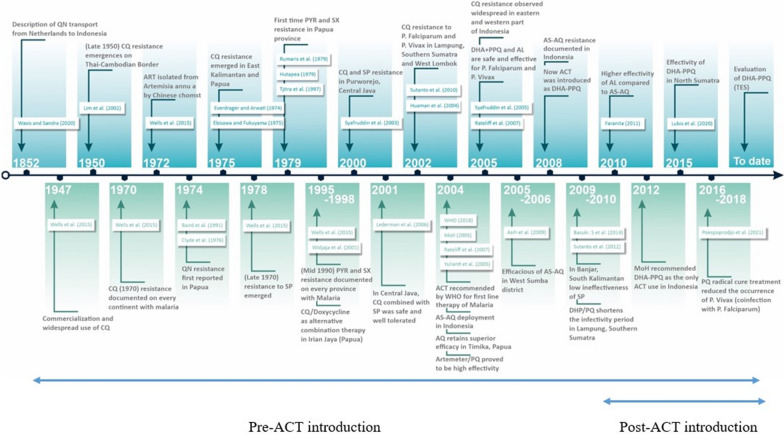
In Indonesia, increasing treatment failure rates in the treatment of uncomplicated falciparum malaria with chloroquine (CQ) and sulfadoxine–pyrimethamine (SP) has prompted a change to artemisinin-based combination therapy (ACT) as first-line anti-malarial treatment since 2004 [1]. ACT combines a potent but short-acting artemisinin (ART) component with a less potent but long-acting partner drug [2, 3]. Artesunate–amodiaquine (AS–AQ) was introduced first, but reports on poor tolerability and increasing treatment failure rates led to changing it to DHA–PPQ in 2008 [4–7].
Since 2010, this well-tolerated and effective regimen has also become the first-line treatment of other human malaria species, including Plasmodium vivax, due to increasing CQ resistance in this species [8]. Studies conducted between 1995 and 2002 in North Sumatra, West Kalimantan, North Sulawesi, West Nusa Tenggara, East Nusa Tenggara, and Papua have consistently demonstrated treatment failure for CQ in P. vivax infections. Since then, CQ has not been an effective treatment for acute vivax malaria [9–15]. Several studies have [4, 9, 16] documented excellent effectivity and tolerability for the DHA–PPQ treatment of uncomplicated malaria in Indonesia, including in Papua, Indonesia [17]. Artemisinin resistance in Plasmodium falciparum has not been established in Indonesia until now (Fig. 1).
Fig. 1.
Flowchart for samples set study
Therapeutic efficacy studies (TES) performed between 2011 and 2018 in South Sumatra, Central Kalimantan, West Kalimantan, North Sulawesi, Central Sulawesi, North Maluku, and East Nusa Tenggara have shown consistently high 42-day cure rates with DHA–PPQ for the treatment of uncomplicated falciparum malaria. No evidence was found for delayed parasite clearance, the hallmark of ART resistance [17]. From 2017 to 2018, molecular surveillance of K13 in Papua New Guinea samples revealed that all P. falciparum isolates carried the wild-type allele of K13 [18, 19]. Some other mutations, such as G453W (20%), V454C (20%), E455K (20%), and T474A (2.6%), were also observed at low frequencies [20]. An increased copy number of the pfpm2/3 gene has been detected in some Papua isolates surviving the DHA–PPQ [5].
Even though DHA–PPQ are still efficacious in treating malaria patients and there was no evidence of treatment failure, evaluation study for genotypic profile and additional measures, such as parasite clearance time (PCT) and parasite density should be regularly monitored. The interval between the patient’s first dose to the time of the first negative blood slide was called the PCT. According to a study in Papua, from April 2017 to April 2018, recurrent P. falciparum parasites were detected in 7 out of 102 cases that completed the 42-day follow-up and were classified as LTF at days 21, 35, and 42. Of the 7 LTF cases, one was re-infected with P. vivax, 2 were confirmed as recrudescent infections, and the remaining 4 were re-infections. No delay in parasite clearance or severe adverse reaction was observed in any study participant [5].
The efficacy of ACT depends on the sensitivity of the parasites for both components of the combination. Artemisinin resistance can be monitored by assessing the parasite clearance rate and the presence of SNPs in the Pfk13 gene, a well-established marker for ART resistance. Resistance to some of the ACT partner drugs can be monitored through molecular surveillance. These include: for PPQ SNPs in the pfcrt gene (position 343, 350, 353) and copy number variations (CNVs) of the P. falciparum Plasmepsin2/3 gene (pfpm2/3) and pfmdr1 gene. In addition for ART SNPs in the pfcrt gene (356).
The present study aims to assess the evolution of genetic markers for anti-malarial drug resistance following the adoption of DHA–PPQ as the first-line anti-malarial drug in 2010 for any uncomplicated malaria cases, the temporal dynamics trends of the evolution of pfk13, pfcrt, pfmdr1 genes also a copy number of pfpm2/3 and pfmdr1 from Sumba and Papua and observation of PCT including parasite density. This risk arises as more parasites may develop resistant to PPQ.
Methods
Study sites and sample collection
Plasmodium falciparum parasite DNA from filter paper blood spots was obtained from two different sample set studies as described in Fig. 2. The selected area for sample set studies was based on the fact that both sites had high annual parasite incidence (API). The total API in Jayapura and Keerom, Papua, from 2019 until 2021 was 95.43 and 383.01, 92.42 and 360.38, 73.08 and 254.93 cases per 1000 population, respectively. In West Sumba and Southwest Sumba, East Nusa Tenggara API in 2019–2021 showed 33.22 and 11.95, 33.09 and 24.21, 16.79 and 10.97 cases, respectively [21].
Fig. 2.
Time points history highlights of antimalaria drug resistance in Indonesia along with Southeast Asia. Cited are studies by Wasis and Sandra [65], MoH [66], Hutapea [67], Tjitra et al. [52], Wells [53], Lim et al. [54], Ebisawa and Fukuyama [68], Rumans et al. [69], WHO [51], Cylde et al. [70], Baird et al. [71], Poespoprodjo et al. [72], Yuliani et al. [73], Ratcliff et al. [4], Lederman et al. [74], Sutanto et al. [75], Syafruddin et al. [48], Syafruddin et al. [33], Basuki et al. [76]
Sample set 1 malariometric surveys
Fifty blood blots on filter paper (3 MM; Whatman, Hillsboro, OR, USA) containing approximately 25–50 μL blood equivalent were collected from Sumba during 2010–2018 through active case detection. Sample selection was based on the availability of the existing archived sample.
Sample set 2 therapeutics efficacy study (TES)
A therapeutic efficacy study was conducted in Papua Province from 2020 to 2021 by the Eijkman Institute for Molecular Biology, National Research and Innovation Agency, Cibinong, Indonesia, serves as part of DBS’s analysis for the current study. TES procedures followed World Health Organization (WHO) criteria. Briefly, smears and blots on filter paper (Whatman International Ltd., Maidstone, UK) were collected from finger pricks on days before enrolment, then on days 1, 2, 3, 7, 14, 21, 28, 35 and 42. The blood-spotted filter papers were allowed to dry completely, transferred into individual plastic bags, labelled, and stored at room temperature in a silica gel desiccator until further processing. 2749 subjects were screened through passive and active case detection, and 42% (1156/2749) were positive for malaria. One hundred forty-two who met the inclusion criteria samples were obtained from 768, a pool of P. falciparum malaria-infected individuals. The respondents were aged between 1 and 65 years, weighed more than 5 kg, and had a fever or history of fever in the preceding 24 h, with slide-confirmed malaria with a parasitemia of ≥ 500/µL asexual parasites for P. falciparum. Meanwhile, they were excluded with the following exclusion criteria: pregnant, had a history of allergy to the study drugs or studied drug’s derivative, had previously completed treatment with an anti-malarial drug in the preceding 2 weeks, or had a medical history of untreated hypertension or chronic heart, kidney, or liver disease [5]. All study participants were given a supervised treatment of DHA–PPQ from a primary health center containing 40 mg DHA and 320 mg PPQ per tablet and were administered once a day for three days, as a weight per dose regimen of 2.25 and 18 mg/kg of DHA–PPQ [26]. Treatment outcomes and a new infection were classified according to the WHO criteria [16, 17, 22].
In this study, fifty-one archived samples, consisting of 41 successful treatments and 10 treatment failure, were selected from one hundred forty-two subjects to analyze the genotypic profile of molecular markers associated with DHA–PPQ resistance treatment. As part of the Papua TES study, 142 subjects were evaluated for clinical and parasitological efficacy of DHA–PPQ during days 0, 1, 2, 3, 7, 14.21, 28, 35, 42 [22].
Genomic DNA preparation
DNA from all samples (including sample set 2, on the day of enrolment and day of recurrence) were extracted from the DBS samples using a Chelex-100 ion exchanger (Biorad Laboratories, Hercules, CA, USA) [22]. The genomic DNA obtained was purified following the Qiagen procedures.
Evaluation of mutations in Pfk13, Pfcrt and Pfmdr1
Polymorphisms in the Pfk13 gene were investigated using nested PCR amplification covering the gene’s propeller region [23], followed by sequencing with an ABI sequencer (Macrogen Inc, South Korea). The sequencing results were then aligned against the reference strain 3D7’s Pfk13 gene (PF13 0238) (NCBI reference sequence no. XM 001350122.1). The analysis was carried out using the BioEdit software (Abbott, CA, USA).
PCR: pfcrt was amplified from the DNA template to assess pfcrt mutations linked to PPQ resistance identified in a previous study [24, 25]. These were codons 343, 350, 353 and 356. On a 2% agarose gel, PCR product were visualized.
Pfmdr1 was amplified from the DNA template using nested PCR to assess pfmdr1 mutations, including the following SNPs: 1034, 1042 and 1246. The PCR amplicon was analysed on a 3% agarose gel under ultraviolet illumination. All PCR products were sent for DNA sequencing at 1st Base Inc. in Singapore for quality control [26, 27]. Details of amplification primers sequences and PCR product results are available in Additional file 1: Table S1, Fig. S1.
Assessment of PfPlasmepsin2/3 and pfmdr1 gene amplification
Relative quantitative real-time PCR (TaqMan real-time PCR) on an Applied Biosystems 7500 quantified pfpm 2/3 and pfmdr1 copy numbers (Roche Molecular Systems, Inc., USA). Previously disclosed primers and probes [28, 29], a BioRad CFX 96 thermocycler was used to amplify 20 μL in triplicate. Copy number estimates were 2−∆∆CT, where CT is the difference between the unknown sample’s threshold cycle (CT) and the reference sample’s CT. Runs are not interpretable of Ct values > 33 for pfpm2/3 or pftub or sample with a copy number estimate of < 0.5; reactions were repeated. As in previous studies, the main analysis used a cut-off copy number estimate of 1.5 to distinguish single-copy from multiple-copy pfpm2/3 and pfmdr1 gene carriage [28, 29].
Method for distinguishing between recrudescence and re-infection
The Plasmodium speciation and genotype of P. falciparum were determined using PCR. Genotypic analyses of the parasites at day 0 and the day of recurrence were conducted using the three markers recommended by the WHO: merozoite surface protein 1 (MSP1), MSP2, and glutamate-rich protein (GLURP) genes [26, 27]. Cases were categorized as re-infections as the genotypes of the parasites found on the day of recurrence differed from those found on day 0 (pre-treatment). The identical genotypes for the three markers could be either recrudescent [25, 28].
Analysis
Analysis was performed using Microsoft Office Excel basic functions and open-source software, RStudio version 2022.07.2+576, based on R version 4.2.2 [30, 31]. Significant differences in SNPs prevalence proportions each year during the study period were analyzed using the Fischer exact test for categorical variables or the Mann–Whitney U test for nonparametric comparisons. This study used the Excel Kaplan–Meier analysis template provided by the WHO. The results are expressed as success and failure cumulative incidence, with 95% CI.
Results
Demographic characteristics for TES samples as shown in Table 1. Dry blood spots from a mass blood survey of uncomplicated falciparum malaria patients (N = 50) in Sumba from 2010 to 2018 and fifty-one from TES from 2020 to 2021 in Papua to analyze the genotypic profile of molecular markers associated with DHA–PPQ resistance treatment. As part of the Papua TES study, one hundred forty-two subjects were evaluated for clinical and parasitological efficacy of DHA–PPQ during a 42-day follow-up (Table 2). No early treatment failure (ETF) was observed in Papua. However, ten patients out of 117 (8.5%) had a recurrent infection on days 28, 35 and 42 as late treatment failure (LTF) (Table 3).
Table 1.
Demographic characteristics from Papua TES samples
| Variable | Overall cases |
|---|---|
| Number of persons enrolled | 142 |
| Age group (years) | |
| Mean (SD) | 16.5 (12.4) |
| Range (y.o): | 2–53 |
| Adults | 7 |
| 5 to 15 | 34 |
| Under 5 | 6 |
| Gender | |
| Male [n (%)] | 72 (50.7%) |
| Female [n (%)] | 70 (49.3%) |
| Body temperature [°C, mean (SD)] | |
| Mean (SD) | 37.9 (1.1) |
| Range | 36–39.8 |
Table 2.
Treatment outcome from Papua TES during the 42 day of follow-up
| Classification of treatment outcome | Papua | |||
|---|---|---|---|---|
| Microscopy result (without PCR correction) | With PCR correction | |||
| n | % (95% CI)a | n | % (95% CI)a | |
| Total patient’s treatment failure and completed the 42 day-follow-up | ||||
| Treatment failure | ||||
| ETF | 0 | 0% (0.0–3.1) | 0 | 0% (0.0–3.4) |
| LCF | 0 | 0% (0.0–3.1) | 0 | 0% (0.0–3.4) |
| LPF | 10 | 8.5% (4.2–15.2) | 1 | 0.9% (0.0–5.1) |
| ACPR | 107 | 91.5% (84.8–95.8) | 107 | 99.1% (94.9–100.0) |
| Patient LFU/WTH | 25 | – | 34 | – |
| Total patients at baseline | 142 | – | 142 | – |
ETF early treatment failure, LCF late clinical failure, LPF late parasitological failure, LFU lost to follow up, WTH withdrawn
aKaplan–Meier analysis
Table 3.
Genotyping results of the parasites at day 0 and day of recurrence in P. falciparum Papua TES
| Isolate code | D0 strain MSP1a/MSP2b/GLURPc |
DR strain MSP1a/MSP2/bGLURPc |
Day of recurrence | Recurrent/reinfection |
|---|---|---|---|---|
| PAF 204 | K1/FC27/Code1 | K1/FC27/Code1 | D42 | Recrudescent |
| PAF 205 | K1/FC27/Code2 | K1-RO33/FC27/Code3 | D42 | Reinfection |
| PAF 213 | K1/FC27/Code1 | K1/3D7/Code1 | D42 | Reinfection |
| PAF 221 | K1/FC27/Code2 | K1-RO33/FC27/Code3 | D28 | Reinfection |
| PAF 222 | K1/FC27/Code3 | MAD20/3D7/Code3 | D42 | Reinfection |
| PAF 266 | K1/FC27/Code1 | K1/3D7/Code1 | D28 | Reinfection |
| PAF 274 | K1/MAD20/FC27Code2 | RO33/3D7/Code3 | D28 | Reinfection |
| PAF 307 | K1/FC27/Code3 | MAD20/3D7/Code3 | D35 | Reinfection |
| PAF 335 | K1/FC27/Code1 | K1/3D7/Code1 | D28 | Reinfection |
| PAF 358 | K1/MAD20/FC27Code2 | RO33/3D7/Code3 | D42 | Reinfection |
aMSP1 amplicon: K1 = 150–300 base pairs (bp); MAD20 = 150–400 bp; and RO33 = 120–230 bp
bMSP2 amplicon: FC27 = 250–700 bp; 3D7 = 280–780 bp
cGLURP amplicon: Code1 = 501–600 bp; Code2 = 601–700 bp; and Code3 = 701–800 bp
Single nucleotide polymorphisms in the Pfk13 gene
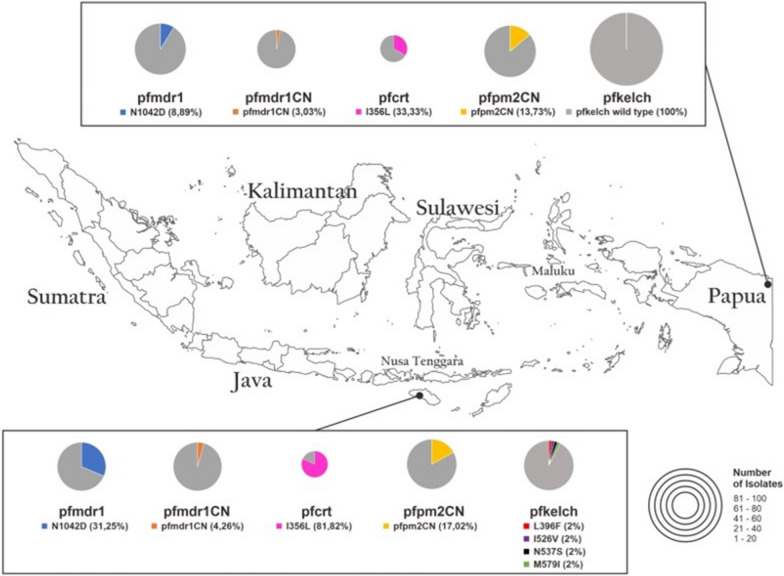
Of the 101 DNA specimens analysed, 94 gave full amplicons. None of the 20 SNPs previously reported to be associated with ART resistance were found. As shown in Fig. 3, the overall prevalence of the non-synonymous new mutant allele in BTB/POZ and the propeller domain was found in Sumba with a percentage of 4/94 (4.25%, 95% CI 0.94–1; see Additional file 1: Fig. S2, Table S2; Table 4) at positions L396F, I526V, N537S and M579I.
Fig. 3.
Map of Indonesia indicating molecular marker of resistance and sampling locations of the P. falciparum field isolates during observation study. Map source from Natural Earth (https://www.naturalearthdata.com) and modified according to data from the references
Table 4.
Summary of PfK13, pfcrt, pfmdr1 SNP frequencies, pfpm2/3 and pfmdr1 CNVs in Sumba and Papua
| Gene | Haplotype | (All) total | Total Sumba | Sumba | Total Papua | Papua | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year 2010 | 2011 | 2012 | 2015 | 2016 | 2017 | 2018 | Year 2020 | 2021 | |||||||||||||||||
| Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | Number | %a | ||
|
PfK13 Total n = 101 |
L396F | 1 | 1 | 1 | 20 | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| I526V | 1 | 1 | 1 | 16.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 16.7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| N537S | 1 | 1 | 1 | 20 | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| M579I | 1 | 1 | 1 | 20 | 0 | 0 | 0 | 0 | 1 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| WT | 97 | 96 | 46 | 92 | 20 | 100 | 5 | 100 | 2 | 40 | 5 | 100 | 4 | 100 | 5 | 100 | 6 | 100 | 51 | 100 | 27 | 100 | 24 | 100 | |
|
pfcrt Total n: 31 |
I356L | 22 | 71 | 18 | 95 | 3 | 15 | 3 | 60 | 4 | 80 | 3 | 60 | 2 | 50 | 3 | 60 | 0 | 0 | 4 | 33.3 | 2 | 50 | 2 | 25 |
| WT | 9 | 29 | 1 | 5.3 | 17 | 85 | 2 | 40 | 1 | 20 | 2 | 40 | 2 | 50 | 2 | 40 | 0 | 0 | 8 | 66.7 | 2 | 50 | 6 | 75 | |
|
pfmdr1 Total n = 93 Positions 1034, 1042, 1246 |
SDD | 19 | 20.4 | 15 | 31.3 | 3 | 15.8 | 4 | 80 | 2 | 40 | 3 | 75 | 1 | 33.3 | 2 | 40 | 0 | 0 | 4 | 8.9 | 3 | 10.3 | 1 | 6.3 |
| SND | 74 | 79.6 | 33 | 68.8 | 16 | 84.2 | 1 | 20 | 3 | 60 | 1 | 25 | 2 | 66.7 | 3 | 60 | 5 | 100 | 41 | 91.1 | 26 | 89.7 | 15 | 93.8 | |
|
pfpm2/3 CNV Total n = 98 |
Single copy | 83 | 84.7 | 39 | 83.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 75 | 2 | 40 | 2 | 33.3 | 44 | 86.3 | 24 | 77.4 | 20 | 100 |
| Multiple copies | 15 | 15.3 | 8 | 17.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 25 | 3 | 60 | 4 | 66.7 | 7 | 13.7 | 7 | 22.6 | 0 | 0 | |
|
pfmdr1 CNV Total n = 80 |
Single copy | 76 | 95.0 | 45 | 95.7 | 0 | 0 | 4 | 80 | 4 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 94 | 18 | 94.7 | 14 | 100 |
| Multiple copies | 3 | 3.8 | 2 | 4.3 | 0 | 0 | 1 | 20 | 1 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 5.3 | 0 | 0 | |
aFrequency data are presented as the percentage of sequences that carried a mutation out of the total number successfully sequence
Pfplasmepsin 2/3 and Pfmdr1 gene amplification
Pfplasmepsine 2/3 copy numbers were measured successfully in 98 samples. Since 2010, the National Malaria Control Programme, the Ministry of Health, Republic of Indonesia, has recommended DHA–PPQ as the first-line drug for uncomplicated malaria. After the deployment of DHA–PPQ, the prevalence of parasites with pfpm2/3 amplified slowly during 2016–2018 in Sumba (Table 4). Amplification of the pfpm2/3 genes in Sumba and Papua was found at 8/47 (17.02%) and 7/51 (13.7%), respectively (Table 4). Ten recurrent isolates from Papua showed no pfpm2/3 amplification (Table 5).
Table 5.
Summary of ten recurrent samples pfK13, pfmdr1 SNPs mutation, pfpm2/3 and pfmdr1 CNVs in Papua
| No. | ID sample | PfK13 | pfmdr1 SDD | pfpm2/3 CNVs | pfmdr1 CNVs |
|---|---|---|---|---|---|
| 1 | PAF 204 | WT | SND | Single copy | – |
| 2 | PAF 205 | WT | SND | Single copy | – |
| 3 | PAF 213 | WT | SND | Single copy | – |
| 4 | PAF 221 | WT | SND | Single copy | Single copy |
| 5 | PAF 222 | WT | SND | Single copy | Single copy |
| 6 | PAF 266 | WT | – | Single copy | Single copy |
| 7 | PAF 274 | WT | – | Single copy | Single copy |
| 8 | PAF 307 | WT | – | Single copy | – |
| 9 | PAF 335 | WT | – | Single copy | – |
| 10 | PAF 358 | WT | SDD | Single copy | – |
Eighty samples were successfully measured for pfmdr1 copy numbers, of which 3/80 (3.8%) were multiple copies. In Sumba and Papua, parasites with pfmdr1 amplification tend to disappeared after AS–AQ was replaced with DHA–PPQ in 2010 (Table 4). This study did not observe the concomitant amplification of both pfpm2/3 and pfmdr1 (Table 5).
Single nucleotide polymorphisms in the pfcrt gene
Only 31 of the 101 specimens were successfully PCR amplified for the pfcrt gene due to a lack of parasite DNA. None of the pfcrt mutations linked to piperaquine resistance were observed. Although the number of samples was small, approximately 71% (22/31) of all isolates from both study areas had pfcrt I356L, as shown in Table 4 and Additional file 1: Table S2.
Single nucleotide polymorphisms in the pfmdr1 gene
PCR amplicons of pfmdr1 were amplified from 90 samples (codons 1034, 1042, and 1246) obtained. Table 4 and Additional file 1: Table S2 show that approximately 79.6% (74/93) of isolates had the wild-type SND haplotype. In Sumba, the mutant haplotype SDD was found in one-third of the isolates (15/48; 31.3%); in Papua, the haplotype was found in 8.9% (4/45). In Papua, during 2 years of observation, prevalence decreased from 10.3% (3 of 29) to 6.3% (1 of 16) (Table 4). As both alleles (1034 and 1246) were observed at multiple locations in Indonesia across the region [32, 33], similar observations in pfmdr1 (1034C and 1246Y) were not found in any of the isolates examined.
Clinical and parasitological characteristics of Papuan TES
Analysis of one hundred forty-two P. falciparum-infected subjects (Table 1) 117 cases completed the 42 day-follow-up, and 25 cases were either lost to follow-up (LFU) or withdrawn (WTH). The classification of the treatment outcomes by PCR correction is presented in Table 3. At day 42, ACPR was noted in 91.5% (95% CI 84.8–95.8). Of the 10 LTF cases, nine were re-infections and one was confirmed as recrudescent (Table 3). Therefore, the PCR-corrected DHA–PPQ efficacy for falciparum was 99.1% (95% CI 94.9–100.0). No delay in parasite clearance at day 3 was observed in any isolates.
The PCT ranged from 1.5 to 35.7 days, with a median of 1 day (interquartile range [IQR], 1 to 2 days) and 38.5 days (IQR, 28 to 42 days) in the ACPR and recurrence groups, respectively. The dynamics of parasite density based on frequencies depict a decreasing trend during observations (Additional file 1: Fig. S3), although only one sample on the last day of observations had high parasitaemia. The imputed corrected geometric mean parasite densities (/μL) for detected infections were 707,326 parasites/mL (95% CI 469,080–1,066,577 parasites/mL) with standard deviation s = 2,891,848 (IQR: 299,600–1,525,600) in D0; 29,078 parasites/mL (95% CI 15,665–53,975 parasites/mL) with standard deviation s = 55,888 (IQR: 8800–106,800) in D1; 3326 parasites/mL (95% CI 3095–3,573,239 parasites/mL) with standard deviation s = 2715 (IQR, 1920–5760) in D2; 47,614 parasites/mL (95% CI 0.12–18 × 1010 parasites/mL) with standard deviation s = 80,554 (IQR, 17,280–131,200) in D42 (Additional file 1: Fig. S4).
Discussion
In Indonesia, AS–AQ was introduced in 2004 and poor tolerability was reported. Increasing treatment failure rates of AS–AQ led to drug policy change to DHA–PPQ in 2008. DHA–PPQ resistance has emerged in South-East Asia, posing a significant threat to malaria control and elimination efforts [34–36]. DHA–PPQ is well tolerated, with a faster ART derivative clearing parasites and the active compound PPQ removing the remaining parasites more slowly [37]. TES conducted in several parts of Indonesia [17, 19] revealed that DHA–PPQ is still highly effective, evidenced by the absence of delayed parasite clearance and low cases of parasite recurrence after 42-day observations.
Analysis of the pfk13 gene of P. falciparum isolates collected from 2010 to 2021 revealed the absence of mutations associated with ART resistance. However, several polymorphisms, such as L396F, I526V, N537S, and M579I, were found. Although not associated with ART resistance, these SNPs are newly found and have never been described in any endemic area [16, 18–20, 23, 24, 34–50]. It is still important to describe further the roles of the four SNPs. The average PCT result of 1.5 days and no delay in parasite clearance at day 3 was observed in any isolates. The TES results from Papua do not meet the WHO criteria for suspected ART resistance [51].
Resistance to PPQ has been associated with the increasing copy number of the pfpm2 gene [28, 52, 53] and more recently with mutant alleles of pfcrt [54–58]. This research result on 10 recurrent infection cases revealed no association of PPQ resistance with pfpm2/3 copy number (Table 5). Prior TES analysis in the southern region of Papua also did not identify any P. falciparum isolates carrying multiple copies of the pfpm2/3 gene [18]. Other studies proposed pfcrt mutations associated with PPQ resistance, namely 343, 350, or 353 [24, 25]. Those mutations were not observed in this study. After the deployment of DHA–PPQ, the prevalence of parasites with multiple copies of pfpm2/3 slowly increased from 2016 to 2018 and seemed to be relatively prevalent in no recurrent isolates. All this happened probably due to the selective exposure of DHA–PPQ over more than 10 years of adoption in Indonesia. The limited number of samples used in this study could also be a determining factor, so it will need further investigation to confirm. Although there was no evidence of an increased copy number of pfpm2/3 in recrudescence cases, the PPQ treatment still failed to eradicate the parasite from the blood and prevent reinfection during the follow-up period.
Surveillance in areas relying on DHA–PPQ as first-line anti-malarial treatment indicated that pfpm2/3 amplification was not the sole factor rendering PPQ resistance. The genetic background of circulating field isolates appeared to play a role in drug susceptibility [36, 37]. It is also supported by Fidock et al. [42] and Iwanaga et al. [59], specifically revealing that a drug-resistant strain’s successful production is directly generated in a drug-sensitive strain via in vitro study or genome-wide functional screening of drug resistance. These transformations were influenced by geographical origin (South East Asia, Africa and South America) and genetic background (haplotype allele or genotype of all other related genes), supporting the population survey results that the mutated pfcrt was possibly sufficient to confer resistance [60]. It was also suggested that the initial selection of pfpm2/3 and pfmdr1 CNVs, although a PPQ-resistant phenotype does not emerge, it developed a genetic background for novel pfcrt mutations [61].
Pfcrt is a 13-exon gene with several point mutations 74, 75, 76, 220, 271, 326, 356, 371 that connect with CQR [42] and 93, 97, 145, 218, 343, 350, 353 exclusively associated with PPQ resistance [36, 60, 62] located on chromosome 7 spanning from 36 kb segment. The overlapped region was correlated to the pfcrt gene and its regulatory elements such as the promoter and 3′ untranslated region (3′ UTR) responsible for regulating and activating the coding region [59]. The mutations in pfcrt might interfere with transporting the natural substrates out of the digestive vacuole, resulting in increased osmotic pressure. This phenotype was also observed in Dd2 parasites expressing the pfcrt mutations F145I, M343L, and G353V [60]. Not all novel pfcrt mutations exhibit a swollen DV phenotype, depending on the location of the mutated amino acids [63].
Intriguingly, pfcrt I356T/L mutation also increased ART IC50 values and resistance [38, 39], emphasizing the recent correlation of the I356T mutation in Southeast Asia with ART-resistant parasite populations in the pfk13 mutation.[40, 41]. Several pfcrt haplotype lines from many geographic regions serve as genetic backgrounds, with 356 alleles as one of them was associated with the development of ART resistance in P. falciparum parasites [38–40, 42]. The presence of moderate frequencies of pfcrt I356L 22/31 (71%) in the study site was possibly associated with long-time drug-selected pressure from DHA–PPQ treatment used and the availability of access to CQ in the private health sectors (non-malaria purposes) that could facilitate the evolution of ART resistance pfcrt alleles [16, 39].
Pfmdr1 was also proposed as a modulator for PPQ resistance. [28, 34] Resistance to PPQ was also associated with the amplification of pfmdr1. Another in-vitro study reported a correlation between a single copy number of pfmdr1 and P. falciparum isolates resistance to PPQ [35, 36, 43]. Plasmodium falciparum parasites might suffer a fitness disadvantage or reduced transmissibility if pfmdr1 gene is amplified more frequently [47]. By contrast, the multiple copy number of pfmdr1 was associated with MQ resistance [29, 37, 44, 64]. This study revealed that P. falciparum isolates in Indonesia predominantly posed single copy of pfmdr1 95%, suggesting reduced PPQ effectiveness. Previous studies observed a reduced prevalence of multicopy pfmdr1 since adopting DHA–PPQ [28, 37, 45]. However, the role of pfmdr1 remained controversial [43, 46].
Besides decreasing CNVs of pfmdr1, polymorphism in the pfmdr1 gene, such as N1042D increased by 76.9% before the introduction of PPQ treatment. The result in another eastern part of Indonesia SNP mutation S1034C was observed to reach 100% in 2010 [49]. After PPQ was introduced, the pfmdr1 S1034C, N1042D and D1246Y decreased significantly by 20.4%. There was a notable change in haplotype frequencies between the SND haplotype and mutant SDD haplotype (Table 4).
Conclusions
This study revealed that DHA–PPQ is still highly effective against P. falciparum. The genetic architecture of the parasite P. falciparum isolates during 2010–2021 revealed that pfmdr1 and pfpm2/3 single copy number were highly prevalent. The slight increase in DHA–PPQ LTF alerts researchers to the need to test alternative ACTs for baseline information in the event that DHA–PPQ will need to be replaced.
Supplementary Information
Additional file 1: Table S1. Primer sequences in this study. Figure S1. PCR product result for (A) K131 propeller domain (B) pfcrt exon 102 (C) pfmdr13 in electrophorese gel 2%. Figure S2. Chromatograms of sequence analysis on mutation position of the Kelch13 BTB/POZ and propeller domain in Sumba. The arrow shows the mutation position. Table S2. Summary of Confidence Interval (CI) 95% with lower and upper CI in each molecular marker associated with drug resistance. Figure S3. Line plot showing parasite density based on frequency per day observations in Papua. Figure S4. Geometric mean of parasite density during observation period 2020 to 2021 in Papua.
Acknowledgements
The authors are deeply grateful to Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Cibinong, Indonesia, for all the facilities provided, and Coordinator Director Office Global Malaria Programme WHO, Dr. Pascal Ringwald for his continued support in Therapeutic Efficacy Studies (TES) in Indonesia. Special grateful to Universitas Muhammadiyah Yogyakarta, Indonesia, for support of FVR in the graduate study program at Mahidol University.
Disclaimer
HHB and MDGB are staff members of the World Health Organization and are responsible for the views expressed in this publication, which do not necessarily reflect the decisions or policies of the World Health Organization.
Abbreviations
- P. falciparum
Plasmodium falciparum
- ACT
Artemisinin combination therapy
- DHA–PPQ
Dihydroartemisinin–piperaquine
- PPQ
Piperaquine
- CQ
Chloroquine
- SP
Sulfadoxine–pyrimethamine
- ART
Artemisinin
- LUM
Lumefantrine
- AS–AQ
Artesunate–amodiaquine
- PfK13
Plasmodium falciparum Kelch 13
- Pfcrt
Plasmodium falciparum chloroquine resistance transporter
- Pfmdr1
Plasmodium falciparum multidrug resistance 1
- Pfpm2/3
Plasmodium falciparum plasmepsine 2/3
- TES
Therapeutic Efficacy Study
- LTF
Late treatment failure
- ETF
Early treatment failure
- LCF
Late clinical failure
- LPF
Late parasitological failure
- LFU
Lost to follow up
- WTH
Withdrawal
- SNP
Single nucleotide polymorphism
- DBS
Dry blood spots
- MBS
Mass blood survey
- PCT
Parasite clearance time
- ACPR
Adequate clinical and parasitological responses
- API
Annual parasite incidence
- CT
Threshold cycle
- MSP1
Merozoite surface protein 1
- MSP2
Merozoite surface protein 2
- GLURP
Glutamate-rich protein
- IQR
Interquartile range
Author contributions
DS, PBSA, PC and MI concepted the study for genetic analysis. DS, HHB, and MDGB concepted the Therapeutic Efficacy Studies (TES) in Indonesia. FVR, PBSA, AD, MI, and DS drafted the original draft. FVR, PBSA, MI, IER, SW, RR, FKD, DHP, LP and HDP responsible to formal analysis, investigation, contributed in data curation, and design methodology. All authors read and approved the final manuscript.
Funding
In this study, sample collection and molecular assays were supported by Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Cibinong, Indonesia. TES samples collected in this study were supported by WHO. Several assays were funded by Universitas Muhammadiyah Yogyakarta, Indonesia. This study was supported by Mahidol University, MU’s Strategic Research Fund: fiscal year 2023 and part of the Mahidol-University Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of the United Kingdom (core grant 106698/B/14/Z) and Wellcome OA statement. This research was funded in whole, or in part, by the Wellcome Trust [220211]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Declarations
Ethics approval and consent to participate
Ethical review and approvals were granted by the Ethics Committee of the Faculty of Medicine, The University of Hasanuddin, Indonesia, Health Research Ethics Committee, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Yogyakarta, Indonesia and the Mahidol University Central Institutional Review Board, the Faculty of Tropical Medicine, Mahidol University (Approval No. MUTM 2012-045-05), Thailand. Therapeutic Efficacy Studies (TES) in Indonesia was registered with the clinical trial number ACTRN12622000248763.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ministry of Health . Buku Saku Tatalaksana Kasus malaria. Jakarta: Ministry of Health of the Republic of Indonesia; 2020. [Google Scholar]
- 2.WHO . Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 3.Karunajeewa H, Lim C, Hung TY, Ilett KF, Denis MB, Socheat D, et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin®) in Cambodian children and adults with malaria. Br J Clin Pharmacol. 2004;57:93–99. doi: 10.1046/j.1365-2125.2003.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung R, Laihad F, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–765. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asih PB, Dewi RM, Tuti S, Sadikin M, Sumarto W, Sinaga B, et al. Efficacy of artemisinin-based combination therapy for treatment of persons with uncomplicated Plasmodium falciparum malaria in West Sumba District, East Nusa Tenggara Province, Indonesia, and genotypic profiles of the parasite. Am J Trop Med Hyg. 2009;80:914–918. [PubMed] [Google Scholar]
- 6.Djatmiko W. Uji Efikasi Terapi Kombinasi artesunate+amodiaquine pada malaria Falciparum Tanpa Komplikasi di Kabupaten Banjarnegara Propinsi Jawa Tengah. Semarang: Universitas Diponegoro; 2005. http://eprints.undip.ac.id/12561/.
- 7.Hasugian A, Purba H, Kenangalem E, Wuwung R, Ebsworth E, Maristela R, et al. Dihydroartemisinin–piperaquine versus artesunate–amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin Infect Dis. 2007;44:1067–1074. doi: 10.1086/512677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Guideline for malaria. Geneva: World Health Organization; 2021. https://www.who.int/publications/i/item/guidelines-for-malaria
- 9.Sutanto I, Suprijanto S, Manoempil P, Baird JK. Resistance to chloroquine by Plasmodium vivax at Alor in the Lesser Sundas Archipelago in eastern Indonesia. Am J Trop Med Hyg. 2009;81:338–342. [PubMed] [Google Scholar]
- 10.Baird JK, Wiady I, Fryauff DJ, Sutanihardja MA, Leksana B, Widjaya H, et al. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am J Trop Med Hyg. 1997;56:627–631. doi: 10.4269/ajtmh.1997.56.627. [DOI] [PubMed] [Google Scholar]
- 11.Fryauff DJ, Soekartono, Tuti S, Leksana B, Suradi, Tandayu S, et al. Survey of resistance in vivo to chloroquine of Plasmodium falciparum and P. vivax in North Sulawesi, Indonesia. Trans R Soc Trop Med Hyg. 1998;92:82–83. doi: 10.1016/s0035-9203(98)90966-x. [DOI] [PubMed] [Google Scholar]
- 12.Baird J, Nalim MS, Basri H, Masbar S, Leksana B, Tjitra E, et al. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans R Soc Trop Med Hyg. 1996;90:409–411. doi: 10.1016/s0035-9203(96)90526-x. [DOI] [PubMed] [Google Scholar]
- 13.Fryauff DJ, Leksana B, Masbar S, Wiady I, Sismadi P, Susanti AI, et al. The drug sensitivity and transmission dynamics of human malaria on Nias Island, North Sumatra, Indonesia. Ann Trop Med Parasitol. 2002;96:447–462. doi: 10.1179/000349802125001249. [DOI] [PubMed] [Google Scholar]
- 14.Maguire J, Susanti A, Krisin, Sismadi P, Fryauff D, Baird J. The T76 mutation in the pfcrt gene of Plasmodium falciparum and clinical chloroquine resistance phenotypes in Papua, Indonesia. Ann Trop Med Parasitol. 2001;95:559–572. doi: 10.1080/00034980120092516. [DOI] [PubMed] [Google Scholar]
- 15.Fryauff DJ, Baird JK, Candradikusuma D, Masbar S, Sutamihardja MA, Leksana B, et al. Survey of in vivo sensitivity to chloroquine by Plasmodium falciparum and P. vivax in Lombok Indonesia. Am J Trop Med Hyg. 1997;56:241–244. doi: 10.4269/ajtmh.1997.56.241. [DOI] [PubMed] [Google Scholar]
- 16.Lubis IND, Wijaya H, Lubis M, Lubis CP, Beshir KB, Sutherland CJ. Plasmodium falciparum isolates carrying pfk13 polymorphisms harbor the SVMNT allele of pfcrt in Northwestern Indonesia. Antimicrob Agents Chemother. 2020;64:e02539–e2619. doi: 10.1128/AAC.02539-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lidia K, Deo DA, Pakan PD, Riwu M. Evaluation of therapeutic efficacy and safety of dihydroartemisinin–piperaquine in uncomplicated Plasmodium falciparum monoinfection in Timor Tengah Selatan District, Nusa Tenggara Timur, Indonesia. Southeast Asian J Trop Med Public Health. 2018;49:733–740. [Google Scholar]
- 18.Poespoprodjo JR, Kenangalem E, Wafom J, Chandrawati F, Puspitasari AM, Ley B, et al. Therapeutic response to dihydroartemisinin–piperaquine for P. falciparum and P. vivax nine years after its introduction in Southern Papua, Indonesia. Am J Trop Med Hyg. 2018;98:677–682. doi: 10.4269/ajtmh.17-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asih P, Rozi IE, Dewayanti FK, Wangsamuda S, Zulfah S, Robaha M, et al. Efficacy and safety of dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax malaria in Papua and Sumatra, Indonesia. Malar J. 2022;21:1–9. doi: 10.1186/s12936-022-04101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rachmad B. Isolasi Dan Identifikasi Mutasi Gen Pfk13 (Pf3d7_1343700) Sebagai Penanda Resistensi Artemisinin Pada Isolat Plasmodium falciparum Asal Lampung. Prosiding dalam rangka Rakernas XIV & Temu Ilmiah XXII. 2019. p. 25–40.
- 21.MoH. Informasi malaria Indonesia. Jakarta; 2021.
- 22.Syafruddin D. Institutional report: therapeutic efficacy study (TES) for first line anti-malarial drug (dihydroartemisinin piperaquine/DHP) in two sentinel sites in Indonesia: second phase. 2021.
- 23.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chenet SM, Okoth SA, Kelley J, Lucchi N, Huber CS, Vreden S, et al. Molecular profile of malaria drug resistance markers of Plasmodium falciparum in Suriname. Antimicrob Agents Chemother. 2017;61:e02655–e2716. doi: 10.1128/AAC.02655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelleau S, Moss EL, Dhingra SK, Volney B, Casteras J, Gabryszewski SJ, et al. Adaptive evolution of malaria parasites in French Guiana: reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc Natl Acad Sci USA. 2015;112:11672–11677. doi: 10.1073/pnas.1507142112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basco L, Ringwald P. Molecular epidemiology of malaria in Yaoundé, Cameroon. III. Analysis of chloroquine resistance and point mutations in the multidrug resistance 1 (pfmdr 1) gene of Plasmodium falciparum. Am J Trop Med Hyg. 1998;59:577–581. doi: 10.4269/ajtmh.1998.59.577. [DOI] [PubMed] [Google Scholar]
- 27.Price R, Cassar C, Brockman A, Duraisingh M, Van Vugt M, White N, Nosten F, Krishna S. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand (RFLP-RTPCR) Antimicrob Agents Chemother. 1999;43:2943–2949. doi: 10.1128/aac.43.12.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype–genotype association study [published correction appears in Lancet Infect Dis. 2018;18:829] Lancet Infect Dis. 2017;17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price RN, Uhlemann A-C, Brockman A, McGready R, Ashley E, Phaipun L, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R:A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2021. https://www.r-project.org/
- 31.Posit team. R studio: Integrated Development Environment for R [Internet]. Boston, MA:Posit Software, PBC; 2023. http://www.posit.co/
- 32.Syafruddin D, Asih PB, Wahid I, Dewi RM, Tuti S, Laowo I, et al. Malaria prevalence in Nias District, North Sumatra Province, Indonesia. Malar J. 2007;6:116. doi: 10.1186/1475-2875-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syafruddin D, Asih PB, Aggarwal SL, Shankar AH. Frequency distribution of antimalarial drug-resistant alleles among isolates of Plasmodium falciparum in Purworejo district, Central Java Province, Indonesia. Am J Trop Med Hyg. 2003;69:614–620. [PubMed] [Google Scholar]
- 34.Veiga M, Ferreira P, Malmberg M, Jörnhagen L, Björkman A, Nosten F, et al. pfmdr1 amplification is related to increased Plasmodium falciparum in vitro sensitivity to the bisquinoline piperaquine. Antimicrob Agents Chemother. 2012;56:3615–3619. doi: 10.1128/AAC.06350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, et al. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duru V, Khim N, Leang R, Kim S, Domergue A, Kloeung N, et al. Plasmodium falciparum dihydroartemisinin–piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabryszewski SJ, Modchang C, Musset L, Chookajorn T, Fidock DA. Combinatorial genetic modeling of pfcrt-mediated drug resistance evolution in Plasmodium falciparum. Mol Biol Evol. 2016;33:1554–1570. doi: 10.1093/molbev/msw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhingra SK, Gabryszewski SJ, Small-Saunders JL, Yeo T, Henrich PP, Nok S, et al. Global spread of mutant PfCRT and its pleiotropic impact on Plasmodium falciparum multidrug resistance and fitness. MBio. 2019;10:e02731-18. doi: 10.1128/mBio.02731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miotto O, Amato R, Ashley EA, MacInnis B, Almagro-Garcia J, Amaratunga C, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015;47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson TJ, Nair S, Nkhoma S, Williams JT, Imwong M, Yi P, et al. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J Infect Dis. 2010;201:1326–1330. doi: 10.1086/651562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muangnoicharoen S, Johnson DJ, Looareesuwan S, Krudsood S, Ward SA. Role of known molecular markers of resistance in the antimalarial potency of piperaquine and dihydroartemisinin in vitro. Antimicrob Agents Chemother. 2009;53:1362–1366. doi: 10.1128/AAC.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim P, Dek D, Try V, Eastman RT, Chy S, Sreng S, et al. Ex vivo susceptibility of Plasmodium falciparum to antimalarial drugs in western, northern, and eastern Cambodia, 2011–2012: association with molecular markers. Antimicrob Agents Chemother. 2013;57:5277–5283. doi: 10.1128/AAC.00687-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imwong M, Hien TT, Thuy-Nhien NT, Dondorp AM, White NJ. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect Dis. 2017;17:1022–1023. doi: 10.1016/S1473-3099(17)30524-8. [DOI] [PubMed] [Google Scholar]
- 46.Dhingra SK, Redhi D, Combrinck JM, Yeo T, Okombo J, Henrich PP, et al. A variant PfCRT isoform can contribute to Plasmodium falciparum resistance to the first-line partner drug piperaquine. MBio. 2017;8:e00303–e00317. doi: 10.1128/mBio.00303-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imwong M, Suwannasin K, Srisutham S, Vongpromek R, Promnarate C, Saejeng A, et al. Evolution of multidrug resistance in Plasmodium falciparum: a longitudinal study of genetic resistance markers in the greater Mekong subregion. Antimicrob Agents Chemother. 2021;65:e01121. doi: 10.1128/AAC.01121-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Syafruddin D, Asih PBS, Casey GJ, Maguire J, Baird JK, Nagesha HS, et al. Molecular epidemiology of Plasmodium falciparum resistance to antimalarial drugs in Indonesia. Am J Trop Med Hyg. 2005;72:174–181. [PubMed] [Google Scholar]
- 49.Ali M, Hidayatullah TA, Alimuddin Z, Sabrina Y. Sequence diversity of pfmdr1 and sequence conserve of pldh in Plasmodium falciparum from Indonesia: its implications on designing a novel antimalarial drug with less prone to resistance. Iran J Parasitol. 2013;8:522–529. [PMC free article] [PubMed] [Google Scholar]
- 50.Ibraheem ZO, Abd Majid R, Noor SM, Sedik HM, Basir R. Role of different Pfcrt and Pfmdr-1 mutations in conferring resistance to antimalaria drugs in Plasmodium falciparum. Malar Res Treat. 2014;2014:950424. doi: 10.1155/2014/950424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO. Artemisinin resistance and artemisinin-based combination therapy efficacy: a status report. Geneva: World Health Organization; 2018. https://apps.who.int/iris/handle/10665/274362.
- 52.Tjitra E, Gunawan S, Laihad F, Marwoto H, Sulaksono S, Arjoso S, et al. Evaluation of antimalarial drugs in Indonesia 1981–1995. Bull Penelitian Kesehatan. 1997;25:27–58. [Google Scholar]
- 53.Wells TN, Van Huijsduijnen RH, Van Voorhis WC. Malaria medicines: a glass half full? Nat Rev Drug Discov. 2015;14:424–442. doi: 10.1038/nrd4573. [DOI] [PubMed] [Google Scholar]
- 54.Lim P, Chy S, Ariey F, Incardona S, Chim P, Sem R, et al. pfcrt polymorphism and chloroquine resistance in Plasmodium falciparum strains isolated in Cambodia. Antimicrob Agents Chemother. 2003;47:87–94. doi: 10.1128/AAC.47.1.87-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohon AN, Alam MS, Bayih AG, Folefoc A, Shahinas D, Haque R, et al. Mutations in Plasmodium falciparum K13 propeller gene from Bangladesh (2009–2013) Malar J. 2014;13:431. doi: 10.1186/1475-2875-13-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siswantaro H, Ratcliff A, Kenangalem E, Wuwung M, Laihard F, Ebsworth P, et al. Efficacy of existing antimalarial drugs for uncomplicated malaria in Timika, Papua, Indonesia. Med J Indones. 2006;15:4. [Google Scholar]
- 57.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria [published correction appears in N Engl J Med. 2009 Oct 22;361:1714] N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, et al. The threat of artemisinin-resistant malaria. N Engl J Med. 2011;365:1073–1075. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwanaga S, Kubota R, Nishi T, Kamchonwongpaisan S, Srichairatanakool S, Shinzawa N, et al. Genome-wide functional screening of drug-resistance genes in Plasmodium falciparum. Nat Commun. 2022;13:6163. doi: 10.1038/s41467-022-33804-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross LS, Dhingra SK, Mok S, Yeo T, Wicht KJ, Kümpornsin K, et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun. 2018;9:3314. doi: 10.1038/s41467-018-05652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva M, Calçada C, Teixeira M, Veiga MI, Ferreira Multigenic architecture of piperaquine resistance trait in Plasmodium falciparum. Lancet Infect Dis. 2020;20:26–27. doi: 10.1016/S1473-3099(19)30689-9. [DOI] [PubMed] [Google Scholar]
- 62.Dhingra SK, Small-Saunders JL, Ménard D, Fidock Plasmodium falciparum resistance to piperaquine driven by PfCRT. Lancet Infect Dis. 2019;19:1168–1169. doi: 10.1016/S1473-3099(19)30543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boonyalai N, Vesely BA, Thamnurak C, Praditpol C, Fagnark W, Kirativanich K, et al. Piperaquine resistant Cambodian Plasmodium falciparum clinical isolates: in vitro genotypic and phenotypic characterization. Malar J. 2020;19:269. doi: 10.1186/s12936-020-03339-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim P, Alker AP, Khim N, Shah NK, Incardona S, Doung S, et al. Pfmdr1 copy number and arteminisin derivatives combination therapy failure in falciparum malaria in Cambodia. Malar J. 2009;8:1–9. doi: 10.1186/1475-2875-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WasisBaS E. Ecological study of Kina Tree (Cinchona spp.) and its benefits in overcoming the spread of malaria disease. Bogor: IPB University; 2020. [Google Scholar]
- 66.Faranita T. Pedoman Tatalaksana Kasus Malaria di Indonesia. 2005.
- 67.Hutapea AM. Treatment of falciparum malaria with combined sulfadoxine and pyrimethamine in Jayapura, Indonesia. Bull Penelit Kesehat. 1979;7:9–13. [Google Scholar]
- 68.Ebisawa I, Fukuyama T. Chloroquine resistance of Plasmodium falciparum in West Irian and East Kalimantan. Ann Trop Med Parasitol. 1975;69:275–282. doi: 10.1080/00034983.1975.11687011. [DOI] [PubMed] [Google Scholar]
- 69.Rumans LW, Dennis DT, Atmosoedjono S. Fansidar resistant falciparum malaria in Indonesia. Lancet. 1979;2:580–581. doi: 10.1016/s0140-6736(79)91633-7. [DOI] [PubMed] [Google Scholar]
- 70.Clyde DF, McCarthy VC, Miller RM, Hornick RB. Chloroquine-resistant falciparum malaria from Irian Jaya (Indonesian New Guinea) J Trop Med Hyg. 1976;79:38–41. [PubMed] [Google Scholar]
- 71.Baird JK, Basri H, Jones TR, Purnomo, Bangs MJ, Ritonga A. Resistance to antimalarials by Plasmodium falciparum in Arso Pir, Irian Jaya, Indonesia. Am J Trop Med Hyg. 2002;44:640–644. doi: 10.4269/ajtmh.1991.44.640. [DOI] [PubMed] [Google Scholar]
- 72.Poespoprodjo JR, Burdam FH, Candrawati F, Ley B, Meagher N, Kenangalem E, et al. Supervised versus unsupervised primaquine radical cure for the treatment of falciparum and vivax malaria in Papua, Indonesia: a cluster-randomised, controlled, open-label superiority trial. Lancet Infect Dis. 2021;22:367–376. doi: 10.1016/S1473-3099(21)00358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuliani M, Machfudz S, Sadjimin. Efikasi terapi artemeter dan primakuin versus klorokuin pada anak-anak penderita malaria tanpa komplikasi di wilayah Puskesmas Kokap, Samigaluh, Girimulyo Kabupaten Kulon Progo. Perpustakaan UGM. 2005;37.
- 74.Lederman ER, Maguire JD, Sumawinata IW, Chand K, Elyazar I, Estiana L, et al. Combined chloroquine, sulfadoxine/pyrimethamine and primaquine against Plasmodium falciparum in Central Java, Indonesia. Malar J. 2006;5:108. doi: 10.1186/1475-2875-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutanto I, Endawati D, Ling LH, Laihad F, Setiabudy R, Baird JK. Evaluation of chloroquine therapy for vivax and falciparum malaria in southern Sumatra, western Indonesia. Malar J. 2010;9:52. doi: 10.1186/1475-2875-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basuki S, Fitriah, Riyanto S, Budiono, Dachlan YP, Uemura H. Two novel mutations of pfdhps K540T and I588F, affecting sulphadoxine-pyrimethamine-resistant response in uncomplicated falciparum malaria at Banjar district, South Kalimantan Province, Indonesia. Malar J. 2014;13:135. doi: 10.1186/1475-2875-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primer sequences in this study. Figure S1. PCR product result for (A) K131 propeller domain (B) pfcrt exon 102 (C) pfmdr13 in electrophorese gel 2%. Figure S2. Chromatograms of sequence analysis on mutation position of the Kelch13 BTB/POZ and propeller domain in Sumba. The arrow shows the mutation position. Table S2. Summary of Confidence Interval (CI) 95% with lower and upper CI in each molecular marker associated with drug resistance. Figure S3. Line plot showing parasite density based on frequency per day observations in Papua. Figure S4. Geometric mean of parasite density during observation period 2020 to 2021 in Papua.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional files.