Abstract
This Review focuses on the mechanistic evidence for a link between obesity, dysregulated cellular metabolism and breast cancer. Strong evidence now links obesity with the development of 13 different types of cancer, including oestrogen receptor-positive breast cancer in postmenopausal women. A number of local and systemic changes are hypothesized to support this relationship, including increased circulating levels of insulin and glucose as well as adipose tissue-derived oestrogens, adipokines and inflammatory mediators. Metabolic pathways of energy production and utilization are dysregulated in tumour cells and this dysregulation is a newly accepted hallmark of cancer. Dysregulated metabolism is also hypothesized to be a feature of non-neoplastic cells in the tumour microenvironment. Obesity-associated factors regulate metabolic pathways in both breast cancer cells and cells in the breast microenvironment, which provides a molecular link between obesity and breast cancer. Consequently, interventions that target these pathways might provide a benefit in postmenopausal women and individuals with obesity, a population at high risk of breast cancer.
The number of new breast cancer cases has risen steadily over the past four decades and this trajectory is predicted to continue1–3. The increase has been attributed to a number of factors, including increased life expectancy, improved screening, older maternal age at first birth and obesity — defined in this Review as a BMI of ≥30 (BOX 1).
Box 1 | BMI as a measure of adiposity and metabolic dysfunction.
BMI, calculated as weight (kg)/height2 (m), correlates with the degree of adiposity in the majority of the population. Of course, this method is not perfect, and many studies describe exceptions: individuals with a nominally healthy BMI (BMI <25) who are hyper-adipose and metabolically unhealthy, and others with a BMI ≥30 who show no signs of metabolic dysfunction147–149. The shortcomings of BMI-based assessments of adiposity are especially apparent in athletes with low body fat and high muscle mass150, in some ethnic groups151 and in postmenopausal women152 (who experience decreases in lean mass and changes in body fat distribution). Nevertheless, BMI has provided important epidemiological insights into the influence of obesity on the risk of developing a number of diseases, including cancer.
Obesity rates in adults have tripled since 1975 and continue to rise worldwide4. In 2017–2018, the age-adjusted prevalence of obesity in adults in the US was 42.2%, with no difference between men and women5. According to a 2019 report by the Organization for Economic Cooperation and Development (OECD), obesity and its complications (which include chronic conditions such as diabetes mellitus) is expected to cost the US health system US$644 per person per year during 2020–2050 (REF.6), which represents 14% of total health expenditure in the USA (>US$210 billion each year). Moreover, the International Agency for Cancer Research has established evidence that links excess body fat to 13 types of cancer in humans, including postmenopausal breast cancer7. In postmenopausal women, the risk of breast cancer increases by 10% for every 5 BMI units above 25 (REF.7). The evidence for a link with obesity is strongest for oestrogen receptor-positive (ER+) subtypes of breast cancer8–10.
Obesity-related factors within the tumour and the breast microenvironment are now known to regulate several important metabolic pathways: phosphoinositide 3-kinase (PI3K)–RAC serine/threonine-protein kinase (AKT), hypoxia-inducible factor 1α (HIF1α), liver kinase B1 (LKB1)–AMP-activated protein kinase (AMPK) and p53. The identification of these metabolic pathways as key drivers of cancer growth has reignited the field of cellular metabolism. Dysregulated metabolism is not only a feature of tumour cells — metabolic pathways that are integral to the function of non-neoplastic cells in the breast microenvironment also support tumour growth.
This Review describes the emerging mechanistic evidence linking obesity to dysregulated cellular energetics in neoplastic and non-neoplastic cells in the tumour microenvironment. The evidence linking obesity to breast cancer is discussed along with the obesity-related factors, both systemic and produced locally within the breast adipose tissue, that are hypothesized to drive the growth of cancer. Mechanistic studies performed in human cells and mouse models are highlighted to illustrate the role of metabolic pathways in driving oestrogen production and changes in immune surveillance within the breast. Finally, the therapeutic approaches that directly or indirectly target metabolic pathways are discussed in terms of how they might be particularly beneficial for the treatment of obesity-related breast cancer.
Obesity-associated changes
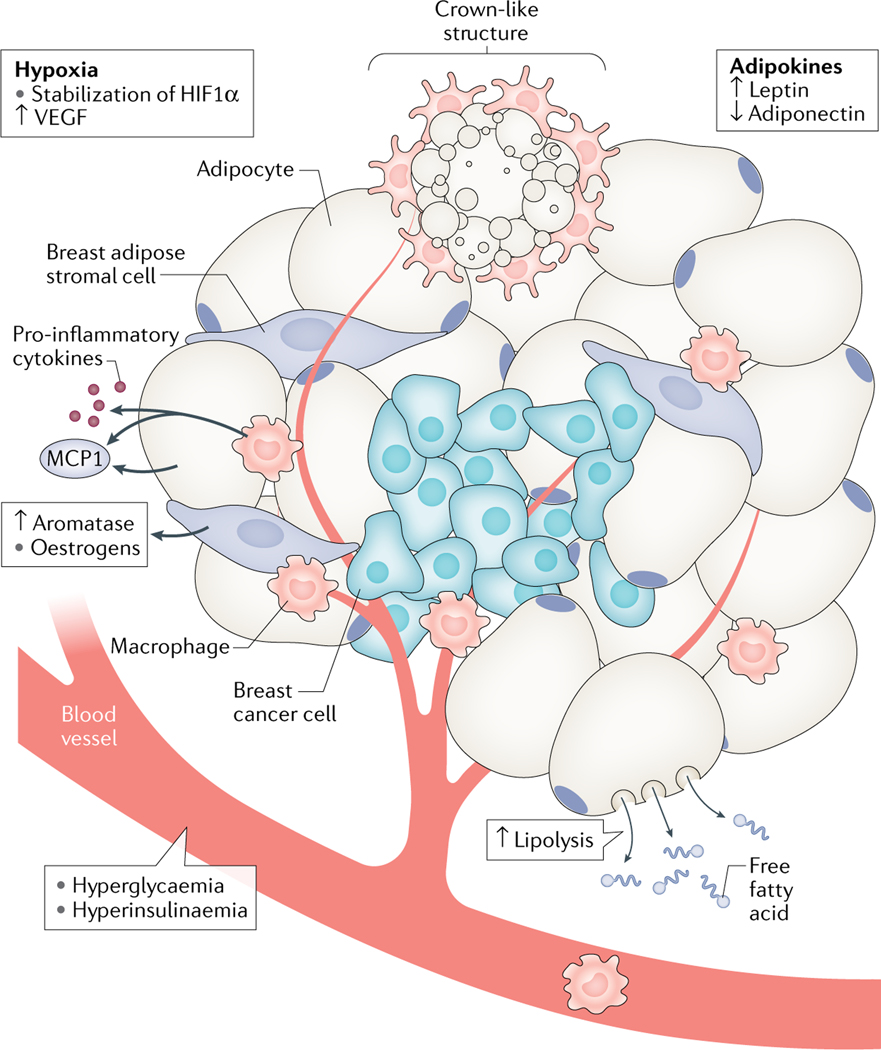
Obesity is associated with a number of systemic and local (adipose-specific) changes that are hypothesized to promote cancer growth (FIG. 1). The excess energy intake associated with the development of obesity can lead to hyperglycaemia and hyperinsulinaemia as well as to increases in fat mass. The expansion of adipose tissue requires the formation of new blood vessels that supply nutrients and oxygen to proliferating adipocyte precursors and enlarging adipocytes (reviewed elsehwere11). Angiogenesis is a normal function of tissue development and occurs as a result of the release of pro-angiogenic factors, including vascular endothelial growth factor (VEGF). However, adipocytes have a finite capacity to store lipid and, at large lipid droplet sizes, they become hypoxic11. Intracellular hypoxia leads to the stabilization of HIF1α, which is a key regulator of VEGF expression in most tissues. Interestingly, the expansion of adipose tissue in obesity and the stabilization of HIF1α in adipocytes are associated with fibrotic and inflammatory responses, rather than with a pro-angiogenic response, suggesting that the control of VEGF in adipose tissue is independent of HIF1α11.
Fig. 1 |. The breast microenvironment and key drivers of breast cancer in obesity.
Breast tumours are surrounded, and sometimes infiltrated, by breast adipose cells, including adipocytes, adipose stromal cells and immune cells. Obesity is associated with the expansion of adipose tissue and increased release of adipokines as well as with adipocyte dysfunction and cell death, which lead to the recruitment of immune cells and the release of inflammatory mediators. Oestrogen-producing adipose stromal cells respond to changes within the tumour microenvironment by increasing their expression of aromatase, leading to increased oestrogen production. Increased tissue biomass is also associated with both hypoxia and angiogenesis. Obesity-related hyperglycaemia and hyperinsulinaemia provide additional stimuli for breast cancer cell growth. HIF1α, hypoxia-inducible factor 1α; MCP1, monocyte chemoattractant protein 1; VEGF, vascular endothelial growth factor.
Adipocyte hypoxia and cell death lead to the release of chemokines, such as monocyte chemoattractant protein 1 (MCP1), which induce the recruitment of immune cells (including macrophages) that secrete inflammatory mediators12. Increased fat mass is associated with the increased production of adipokines by fat cells. The best-characterized of these adipokines is leptin, a peptide hormone that induces feelings of satiety in healthy individuals. In individuals with obesity, leptin is unable to perform this function owing to the development of leptin resistance in the central nervous system13. As such, levels of leptin are positively correlated with BMI.
The majority of breast cancers are ER+ and occur after menopause3,14. This fact might seem counterintuitive, considering that levels of circulating oestrogens plummet during the menopausal transition owing to the absence of developing and ovulatory ovarian follicles, which are the main source of endogenous oestrogens in women15. Circulating oestrogen levels in postmenopausal women of a healthy weight are thought to be below the levels required to activate ERs given that endometrial thickening does not occur in most of these women. Until 10–15 years ago, the limited sensitivity and specificity of standard assays meant that systemic oestrogen levels in postmenopausal women could not be reliably measured, as levels were often below the limit of detection16. However, techniques such as ultra-sensitive radioimmunoassay and liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS) can now reliably measure circulating hormone levels after menopause. These methods have shown that postmenopausal women with obesity have higher levels of circulating oestrogens than their peers without obesity17. These elevated circulating oestrogen levels are hypothesized to drive oestrogen-dependent malignancies of the uterus and breast and are a direct reflection of steroid production in the adipose tissue, the largest endocrine organ capable of producing oestrogens after menopause. The expression of aromatase — the rate-limiting enzyme in oestrogen biosynthesis — is consistently elevated in fat cells, including those of the breast, as a function of both menopause and BMI18–21. Intriguingly, levels of oestrogens in breast tissue are similar in premenopausal and postmenopausal women, despite the latter having circulating oestrogen levels 10–15 times lower than their premenopausal counterparts22.
Breast cancer growth is stimulated not only by the increased levels of obesity-associated factors but also by the loss of factors proposed to mitigate the obesity–breast cancer link, including the adipocyte-derived hormone adiponectin and the gut-derived peptide hormones ghrelin and non-acylated ghrelin23–27. Crosstalk between adipokines, sex hormones and inflammatory mediators and the interdependence of their production lead to a milieu conducive to breast cancer cell growth28–31. For example, many obesity-associated factors both regulate oestrogen production and directly interact with breast cancer cells, thereby promoting their growth.
Metabolic pathways
Obesity involves a sustained abundance of energy availability, whereas an increased energy demand is associated with uncontrolled cell proliferation (a hallmark of cancer). Metabolic pathways with important roles in maintaining cellular energy homeostasis are therefore often dysregulated in these two conditions. Much attention has been given to characterizing the metabolic changes that occur in cancer, including understanding how and why some cells shift their mode of energy production away from mitochondrial respiration and towards glycolysis, which is oxygen independent — a phenomenon termed the Warburg effect. Although glycolysis is much less efficient than respiration at producing ATP, the increased uptake of glucose, often in excess of what is required, enables cancer cells to generate not only sufficient levels of ATP but also metabolic intermediates that can be used for biosynthesis of the nucleotides, amino acids and fatty acids required for cell division. Any excess glycolytic byproducts are secreted into the extracellular space as lactate, which can also be used as a fuel source by adjacent proliferating (cancer and non-neoplastic) cells. Aerobic glycolysis also enables cells to generate energy under hypoxic conditions. The signalling pathways that regulate cellular metabolism to sustain cell growth and proliferation have been extensively reviewed elsewhere32–34.
Multiple proteins orchestrate the processes required for the efficient production of energy and biomass. For example, growth factors, via activation of the PI3K–AKT signalling pathway, stimulate the uptake of glucose and its metabolism to glucose-6-phosphate, which is required not only for cellular energy production but also for the biosynthesis of macromolecules such as amino acids, nucleotides and fatty acids. In a complementary fashion, high ATP levels suppress the energy sensor AMPK, leading to decreased β-oxidation of fatty acids and increased synthesis of cholesterol, proteins and fatty acids via effects on converging signalling pathways.
Extensive and elegant work in this field has shown that how these pathways interact and how they are regulated depend largely on the cellular context and can change with different cellular environments or during disease progression. The following sections provide an overview of the current literature on four important and interconnected pathways that are involved in the regulation of cellular metabolism (FIG. 2).
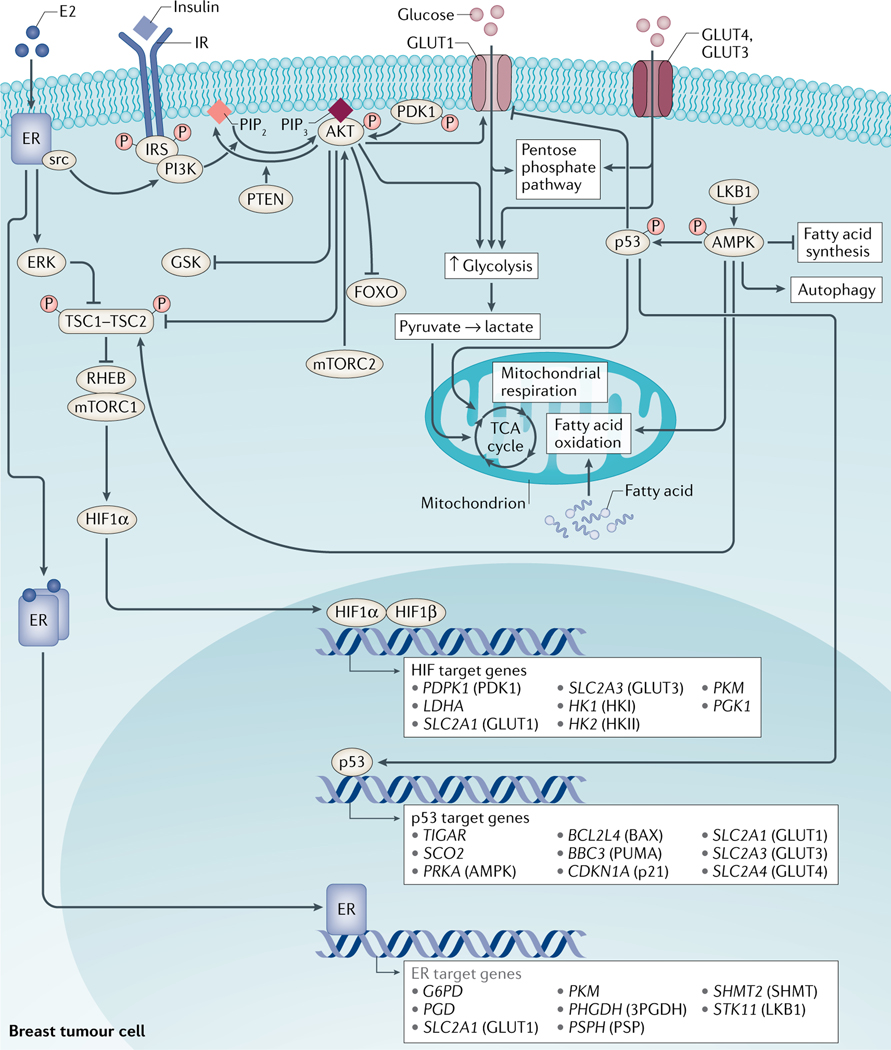
Fig. 2 |. Key metabolic pathways in breast cancer.
Several proteins orchestrate the regulation of energy metabolism in proliferating breast cancer cells and cells of the tumour microenvironment. Shifts in the mode of energy production from oxidative phosphorylation to aerobic glycolysis are tightly regulated by a number of proteins, including phosphoinositide 3-kinase (PI3K), RAC serine/threonine-protein kinase (AKT), mammalian target of rapamycin (mTOR) complex 1 (mTORC1), hypoxia-inducible factor 1α (HIF1α), liver kinase B1 (LKB1), AMP-activated protein kinase (AMPK) and p53. Activation of PI3K leads to the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which binds to and activates AKT. In turn, AKT, via effects on tuberous sclerosis complex 2 (TSC2), activates mTORC1, which leads to the increased translation of several proteins, including HIF1α. AKT and HIF1α stimulate aerobic glycolysis by regulating glucose uptake and glycolytic enzymes. Conversely, binding of AMP to AMPK results in the phosphorylation of AMPK by LKB1 and in the regulation of downstream targets, including the inhibition of fatty acid synthesis, stimulation of fatty acid oxidation and autophagy. AMPK can also phosphorylate (and thereby stabilize) p53. In turn, p53 has been implicated in the downregulation of GLUT1 expression, inhibition of glycolysis and stimulation of oxidative phosphorylation.
The PI3K–AKT pathway.
PI3K has a central role in transmitting external cues to regulate the metabolic processes required for cell division, including glucose metabolism and macromolecule biosynthesis34,35. Thus, mutations in PIK3CA and/or activation of PI3K signalling are among the most common changes observed in human cancers36. Part of the complexity of PI3K signalling resides in its multiple subunits, binding partners and downstream effectors. Insulin signals via class Ia PI3Ks, which are heterodimers consisting of catalytic p110 and regulatory p85 subunits. Upon binding to the insulin receptor, insulin triggers a phosphorylation cascade involving the autophosphorylation of insulin receptors, the phosphorylation of insulin receptor substrate (IRS) proteins and the recruitment of p85 to the IRS complex. Once localized to the plasma membrane, the p85–p110 PI3K heterodimer catalyses the formation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), an important second messenger that can bind to a wide range of proteins, including AKT. The binding of PIP3 to AKT facilitates the interaction of AKT with 3-phosphoinositide-dependent protein kinase 1, leading to the phosphorylation of AKT at Thr308 and its activation. At the plasma membrane, AKT can also be phosphorylated by mammalian target of rapamycin 2 (mTORC2) at Ser473, leading to further stimulation of AKT activity. Conversely, AKT signalling can be dampened by phosphatase and tensin homologue (PTEN), which dephosphorylates PIP3, yielding PIP2. The effects of the PI3K–AKT pathway on cellular metabolism are intricate (reviewed elsewhere)34 and include important coordinated direct and indirect effects of the PI3K–AKT pathway on regulation of the activity and expression of metabolic enzymes. Important downstream targets of this pathway include forkhead box proteins O1 and O3 (FOXO1 and FOXO3), tuberin (also known as tuberous sclerosis 2 protein (TSC2)), which is upstream of mammalian target of rapamycin (mTOR) complex 1 (mTORC1), and glycogen synthase kinase 3 (GSK3). The capacity of AKT to regulate glucose uptake and glycolytic enzymes is the basis of its ability to drive the switch to aerobic glycolysis37. Its role in regulating insulin-mediated glucose uptake via translocation of glucose transporter 4 (GLUT4) to the plasma membrane has been extensively characterized in insulin-responsive tissues, especially muscle and adipose tissue38.
In tumour cells, AKT promotes the expression and membrane localization of GLUT1, which increases glucose uptake to support their bioenergetic needs. AKT also directly phosphorylates several proteins involved in glucose metabolism, including hexokinase 2 (HK2), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB2) and pyruvate dehydrogenase kinase (PDK1), to name a few34. AKT also has important roles in protein and lipid synthesis as well as in the regulation of responses to increased oxidative stress. These contributions are often mediated by AKT-mediated regulation of its downstream effector mTORC1.
HIF1α.
Hypoxia, or low oxygen concentrations, can occur systemically as a result of vascular deficiencies or at the cellular level owing to the use of oxygen for essential biochemical reactions, including mitochondrial ATP biosynthesis via the electron transport chain.
As such, mechanisms have evolved to ensure adequate and rapid responses to changes in oxygen tension. HIF1α is an important mediator of responses to hypoxia. Under normoxic conditions, prolyl hydroxylases add hydroxyl groups to proline residues in the oxygen-dependent degradation domain of HIF1α. This change enables HIF1α to interact with von Hippel–Lindau protein, which ubiquitylates HIF1α, targeting it for proteasomal degradation. HIF1α is constitutively expressed and rapidly degraded under normoxic conditions but is stabilized when oxygen levels are low.
Under hypoxic conditions, HIF1α dimerizes with HIF1β, also known as the aryl hydrocarbon receptor nuclear translocator, and this complex binds to HIF target genes and regulates their expression. The shift in gene expression induced by HIF1α leads to many changes that promote the restoration of systemic and cellular oxygen levels, including the stimulation of angiogenesis. With regard to cellular metabolism, HIF1α maximizes the efficiency of the electron transport chain by causing a shift in the composition of cytochrome c oxidase subunits. HIF1α also acts directly on glycolysis and the tricarboxylic acid cycle by inducing the expression of lactate dehydrogenase A and PDK1. The net effect of these changes is that glucose is preferentially converted to lactate and flux through the tricarboxylic acid cycle is reduced. In the initial stages of cancer formation, vascular insufficiency in the context of increased tumour cell growth leads to oxygen and nutrient starvation. As a result, HIF1α stimulates both glucose uptake and glycolytic flux via increasing the expression of GLUT1, GLUT3, HK1, HK2, enolase 1, phosphoglycerate kinase 1, PKM2 and LDHA. In essence, HIF1α rewires cellular metabolism.
p53.
TP53 is one of the most frequently mutated genes in cancer and encodes the tumour suppressor p53, known as the ‘guardian of the genome’. Germline mutations in TP53 are associated with Li–Fraumeni syndrome; individuals with this syndrome have an increased lifetime risk of developing multiple types of cancers, including breast cancer. The best-characterized functions of p53 relate to its capacity to initiate cell-cycle arrest and apoptosis39. These effects are achieved through the transcriptional regulation of key effectors of the mitochondrial and death receptor-induced apoptotic pathways, which include the BCL2 family members apoptosis regulator BAX and PUMA as well as the cell cycle regulator cyclin-dependent kinase inhibitor 1 (CDKN1A, also known as p21). Under basal conditions, levels of p53 protein remain low owing to its ubiquitylation by E3 ubiquitin-protein ligase MDM2 and proteasomal degradation. Stress signals, including DNA damage and hypoxia, lead to post-translational modifications (phosphorylation and acetylation) that stabilize p53, thereby enabling this protein to undergo nuclear translocation and regulate p53 target genes.
In addition to the regulation of cell cycle arrest and apoptosis, p53 has important roles in the maintenance of cellular energy homeostasis, specifically in the regulation of glycolysis, mitochondrial respiration and fatty acid oxidation. This protein inhibits the expression of glucose transporters, including GLUT1, GLUT3 and GLUT4, and stimulates the expression of genes involved in oxidative phosphorylation, including TIGAR (encoding TP53-induced glycolysis regulatory phosphatase) and SCO2 (encoding protein SCO2 homologue, mitochondrial). Furthermore, p53 upregulates the expression of the β1 and β2 subunits of AMPK and some evidence also points to a direct interaction of p53 with the β1 promoter40. The net effect of p53 on metabolic activity favours mitochondrial respiration over aerobic glycolysis. Hence, the activation of p53 is predicted to inhibit the Warburg effect in cancer cells.
The LKB1–AMPK pathway.
Tumour suppressor LKB1, encoded by the STK11 gene, functions as a kinase and as a transcriptional regulator and has been implicated in the regulation of cell metabolism and cell cycle arrest. Notably, LKB1 interacts with p53 in the nucleus and regulates the transcription of p21 (REFS41,42) (a transcriptional target of p53). LKB1 also acts as an upstream kinase for AMPK and AMPK family members. AMPK is widely considered to be a master regulator of energy homeostasis. This serine/threonine kinase is a heterotrimer composed of α, β and γ subunits and regulates several physiological processes, including lipogenesis, fatty acid β-oxidation, cholesterol and protein synthesis, cell cycle arrest, and apoptosis43. AMPK functions as an energy sensor by interacting directly with AMP, ADP and ATP via its γ subunit. When bound to AMP or ADP, AMPK undergoes conformational changes that enable the phosphorylation of its α subunit at Thr172 by upstream kinases, including LKB1 (REFS44–46). Once activated, AMPK can phosphorylate several downstream targets: the phosphorylation of acetyl coenzyme A (CoA) carboxylase inhibits fatty acid synthesis; the phosphorylation of hamartin (also known as TSC1) and TSC2 inhibits protein synthesis and anabolic metabolism (via effects on mTOR); and the phosphorylation of p53 promotes mitochondrial respiration and causes cell cycle arrest and apoptosis. This capacity of AMPK to inhibit HIF1α and stimulate p53 is thought to directly oppose the Warburg effect39.
In human tissue samples, the phosphorylation of AMPK is higher in normal breast epithelial cells than in breast cancer cells, and breast tumours also exhibit reduced phosphorylation of acetyl-CoA carboxylase47. As such, AMPK was initially thought to act predominantly as a tumour suppressor. We now know that the role of AMPK in cancer is very complex48,49. For example, AMPK can induce autophagy in cells experiencing nutrient deprivation or hypoxia. This metabolic rescue is also accompanied by an increase in ATP production via fatty acid β-oxidation, which provides adequate nutrients for cancer cells that would otherwise undergo starvation-induced cell death.
The effects of mutations in oncogenes (such as PIK3CA, KRAS or BRAF) or tumour suppressors (such as TP53, PTEN, TSC1 or TSC2) that directly affect cancer cell metabolism have been extensively studied over the past 15 years. However, an important knowledge gap remains regarding how exogenous stimuli (those associated with obesity, for example) affect the already dysregulated metabolism of cancer cells and other cells in the tumour microenvironment. The following sections provide an overview of what is currently known in this underdeveloped area (FIG. 3).
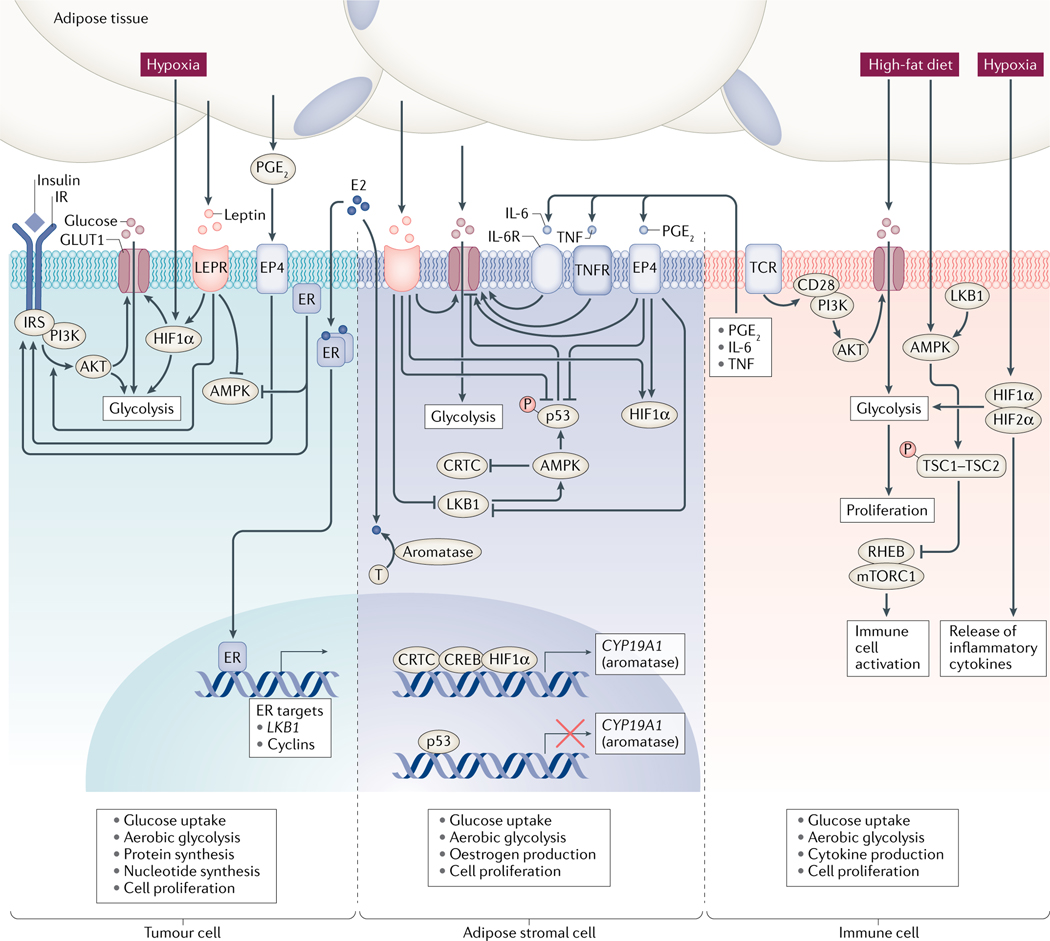
Fig. 3 |. Dysregulated metabolic pathways in breast cancer and adipose stromal cells in the context of obesity.
Dysregulation of several metabolic pathways contributes to breast cancer. However, our understanding of the role and regulation of these metabolic pathways in individuals with obesity is still in relative infancy. Several factors, including insulin, hypoxia, leptin and the inflammatory mediators prostaglandin E2 (PGE2), tumour necrosis factor (TNF) and IL-6, affect metabolic pathways either in breast cancer cells or in cells of the tumour microenvironment. In breast cancer cells, insulin, leptin and oestradiol (E2) stimulate PI3K–AKT signalling, whereas leptin and E2 suppress AMP-activated protein kinase (AMPK) signalling. Hypoxia induces the stabilization of hypoxia-inducible factor 1α (HIF1α), which (along with obesity-associated mediators) favours a shift towards aerobic glycolysis, increased glucose uptake, stimulation of protein and nucleotide synthesis, and cell proliferation. In adipose stromal cells, leptin, PGE2, TNF and IL-6 stimulate glucose transporter expression and glucose uptake. PGE2 and leptin also stimulate HIF1α, suppress LKB1–AMPK signalling and suppress p53 as well as stimulating the expression of aromatase, a crucial enzyme in oestrogen biosynthesis. The net effect of these changes favours the proliferation of cell lineages derived from adipose stromal cells, which could account for the desmoplasia observed in people with obesity. These proliferating adipose stromal cell-derived cells supply lactate and oestrogens that support further tumour cell growth. The effects of obesity on metabolic pathways in immune cells are still poorly characterized. However, hypoxia drives a shift to aerobic glycolysis via effects on HIF2α, and high-fat feeding in preclinical mouse models is associated with suppression of AMPK signalling. These findings suggest that obesity causes a metabolic shift that favours the rapid proliferation of immune cells and supports the increased production of cytokines that promote tumour growth.
Effects of obesity in breast cancer
In most cells, the stimulation of glucose uptake in response to extracellular cues, including growth factors, occurs either to satisfy increased energy demands or to contend with excess systemic energy availability. With increased energy intake, raised blood glucose levels trigger the secretion of insulin from glucose-sensing pancreatic β-cells. Insulin target tissues include muscle and adipose tissue, which store glucose as glycogen and lipids, respectively. As mentioned above, cancer cells are often intrinsically rewired to increase their uptake of glucose as a result of specific genetic mutations. However, increased glucose uptake also occurs as a result of (or even dependent on) the extracellular growth factors and hypoxia that develop as a result of cancer cell proliferation and the consequent restricted access to oxygen in the initial stages of tumour development. In obesity, a number of factors, including hypoxia and increased levels of insulin, oestrogens, inflammatory mediators, adipokines, and free fatty acids, affect metabolic pathways in cancer cells by increasing the glucose uptake and energy metabolism. These changes promote the growth of cancer cells.
Growth factors, including insulin, stimulate signalling cascades by activating receptor tyrosine kinases, many of which converge on the activation of PI3K and AKT. In turn, the activation of PI3K–AKT signalling leads to the stimulation of glucose uptake, aerobic glycolysis, protein synthesis, nucleotide biosynthesis and responses to oxidative stress50. In cancer cells, GLUT1 is the main glucose transporter that imports glucose51 and the localization of GLUT1 at the plasma membrane is maintained, at least in part, by the AKT-dependent downregulation of thioredoxin-interacting protein (TXNIP), which otherwise causes the endocytosis of GLUT1 (REFS52–54). PI3K and mTORC1 also stimulate the expression and translation of GLUT1 in addition to suppressing its turnover via endocytosis.
The hyperinsulinaemia and hyperglycaemia present in patients with type 2 diabetes mellitus (and by extension also in many individuals with obesity) have dramatic growth-promoting effects on cancer cells. These effects are particularly heightened in cells carrying PIK3CA mutations that further sensitize these cells to the effects of insulin. Insulin can also stimulate lipid accumulation; in MCF7 breast cancer cells, this effect was abrogated by rapamycin (an mTORC1 inhibitor)55. In HepG2 hepatocyte carcinoma cells, insulin stimulates cell metabolism via HIF1α-dependent effects on pyruvate kinase M2 (PKM2)56. Of note, PKM2 activity is actually reduced in these cells owing to increased levels of reactive oxygen species that induce the dissociation of its subunits. Therefore, the net effect of insulin is increased aerobic glycolysis and the accumulation of glycolytic intermediates, which are important supporters of cancer growth (although, as yet, this mechanism has not been confirmed in breast cancer cells). In individuals with obesity, levels of the gut-derived peptide hormones ghrelin (which stimulates appetite) and non-acylated ghrelin (which suppresses the proliferation of breast cancer cells25) are decreased. This effect was associated with the downregulation of MAPK and AKT signalling and was observed in serum-stimulated cancer cells as well as on those grown in 3D cultures25. These findings support the hypothesis that factors found in individuals of a healthy weight contribute to lowering cancer risk by inhibiting metabolic pathways that promote cancer cell growth.
Obesity also increases hypoxia in breast tissue, which is associated with the metabolic rewiring of cancer cells in ways that promote progression to more aggressive behaviour. In a syngeneic C57BL/6 mouse model of breast cancer, tumours in animals fed a high-fat diet had fewer blood vessels and a higher degree of hypoxia than those in animals fed a standard diet57. In these studies, hypoxia was measured using pimonidazole (which binds to thiol-containing proteins only in hypoxic cells) and by assessing HIF1α immunoreactivity and the expression of HIF1α target genes57. These observations are analogous to the results of human studies demonstrating that breast tumours from women with obesity seem to be more hypoxic than tumours from their lean counterparts58. However, in a study of 112 breast cancer tissue samples, BMI was not significantly associated with tumour HIF1α immunoreactivity (P = 0.063)58.
Both hypoxia and oestradiol stimulate the expression and activity of proteins linked to glycolysis, including GLUT1 and several enzymes involved in the conversion of glucose to lactate59–61. In cancer cells, oestrogens exert important metabolic effects. Although these effects are not exclusive to the obesity setting, the increased oestrogen levels in women with obesity are highly likely to contribute to the alterations in tumour cell metabolism and to the promotion of tumour growth. In MCF7 cells, the expression of LKB1 and phosphorylation of its downstream target AMPK are both repressed by oestrogens62. Intriguingly, treatment with oestradiol is associated with decreased binding of nuclear ER to the LKB1 promoter, decreased transcription and decreased mRNA expression62. Chronic exposure to insulin also primes breast cancer cells to the stimulatory effects of oestrogens on the expression of genes in the pentose phosphate and serine biosynthesis pathways, an effect that is reversed in the presence of the AMPK-activating compound 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR)28. In a complementary mechanism, oestradiol also increases both glucose uptake and aerobic glycolysis in breast cancer cell lines via the non-genomic effects of membrane-localized ERs on PI3K and AKT signalling60,63. Interestingly, these studies showed that the effect of oestradiol on glucose metabolism was dependent on glucose concentrations in the culture media. Specifically, oestradiol stimulated glycolysis under high glucose conditions whereas, in low glucose conditions, oestradiol caused a metabolic switch to mitochondrial respiration attributable to the activation of AMPK60.
Inflammatory mediators, in particular those produced by macrophages, are produced at increased levels in both obesity and cancer64,65. As the short half-life of prostaglandin E2 (PGE2) hinders its quantification, studies of the potential associations between PGE2 and breast cancer risk have relied on measurements of the PGE2 metabolite PGE-M64,66. One case–control study found that urinary levels of PGE-M were positively associated with breast cancer risk in postmenopausal women who were not taking NSAIDs, with hazard ratios (HR) of 2.1, 2.0 and 2.2 for the three highest quartiles of PGE-M levels64. A similar relationship has also been identified between breast cancer risk and IL-6 in two prospective cohorts and a meta-analysis (HR 1.10 for each natural log increase in IL-6 levels)65.
Few publications describe the effects of inflammatory mediators on the metabolic pathways described herein. However, prostaglandins are important drivers of cancer growth and, therefore, the rate-limiting enzyme in prostaglandin biosynthesis, cyclooxygenase 2 (COX2), has been proposed as a therapeutic target for many cancers66. Overexpression of COX2 in MCF7 cells is associated with the activation of both the PGE2 receptor EP4 subtype and PI3K signalling, which leads to the stimulation of proliferation, migration and invasion, and with an epithelial–mesenchymal transition phenotype67. IL-6 has been extensively studied for its role in driving insulin resistance. This cytokine suppresses cell proliferation via the stimulation of JAK–STAT signalling but also acts synergistically with epidermal growth factor receptor (EGFR) to increase MAPK and PI3K–AKT signalling, which leads to increased breast cancer cell migration68.
The close proximity of adipocytes to breast cancer cells is thought to be integral to breast cancer growth and progression and several adipokines and adipocyte-derived factors potentially contribute to this mechanism. For example, leptin levels are positively correlated with breast cancer risk69 and leptin acts via the leptin receptor to regulate both canonical (MAPK, PI3K) and non-canonical (PKC, JNK and p38 MAPK) signalling pathways. The best characterized of these pathways involve the activation of JAK–STAT signalling, although the effects of leptin on PI3K–AKT and AMPK signalling have also been studied extensively in metabolic tissues. In MCF-7 cells in vitro, leptin stimulates AMPK phosphorylation and fatty acid oxidation70. Conversely, leptin also decreases AMPK phosphorylation and prevents AMPK-mediated growth inhibition in multiple breast cancer cell lines71. Leptin can also stimulate cell proliferation by activating PI3K–AKT signalling71,72 and, in the context of disease progression, promotes epithelial–mesenchymal transition, a process that is dependent on the stimulation of PKM2 expression73. Leptin has other effects beyond metabolism, such as the stimulation of VEGF expression in breast cancer cells74. In human breast cancer and mouse mammary tumour cells, reporter assays showed that leptin caused the activation of HIF1α and NFκB and that these proteins were required for the leptin-dependent induction of VEGF. Interestingly, leptin expression in breast cancer cells in vitro can be stimulated by insulin, via its effects on HIF1α75,76, further adding to the complexity of the crosstalk between these obesity-associated factors.
Adiponectin levels are inversely associated with breast cancer risk77 and in vitro studies have provided some insights into the mechanisms that might explain these epidemiological findings. At the cellular level, adiponectin stimulates apoptosis by increasing the levels of p53 and BAX and suppresses cell cycle progression by inhibiting the expression of cyclin D1 (REFS78–80). Interestingly, adiponectin can also block the phosphorylation of AKT and GSK3β79. In other cell types, adiponectin stimulates fatty acid oxidation by causing the AMPK-dependent phosphorylation and inhibition of acetyl-CoA carboxylase, a process that requires the adapter protein deleted in colorectal cancer-interacting protein 13α (DIP13α, also known as APPL1)81. Both the antiproliferative effects of adiponectin and its capacity to suppress breast cancer cell migration and invasion are thought to be related, at least in part, to the stimulation of AMPK80,82.
Cancer-associated adipocytes release substrates for energy production that influence tumour cell metabolism and support their growth83. In response to cancer cell signals, adipocytes increase their rates of lipolysis and release free fatty acids that can be taken up by cancer cells84–86. Once inside these cells, fatty acid oxidation is uncoupled from ATP production and instead leads to the stimulation of AMPK84. Interestingly, the co-culture of MCF-7 or MDA-MB-231 breast cancer cells with lipid-replete 3T3-L1 adipocytes leads to increased glucose incorporation into lipid pools, an effect that is not seen when these cancer cell lines are co-cultured with lipid-depleted adipocytes86. This increase in fatty acid release from adipocytes is accompanied by an increased mitochondrial oxidative capacity of the breast cancer cells. Circulating levels of free fatty acids correlate with the increased proliferation and aggressiveness of ER+ breast cancer cells owing to the activation of ER and mTOR signalling87.
The breast cancer microenvironment
Adipose stromal cells.
The metabolic crosstalk that occurs between adipose stromal cells and breast cancer cells is thought to be critical for the support of tumour growth88. Considerable heterogeneity exists in tumours, whereby neoplastic cells and other cells within the tumour microenvironment exhibit various metabolic phenotypes. For example, some cells might predominantly use glycolysis whereas others rely on oxidative phosphorylation and this heterogeneity creates energetic synergy. Cancer-associated fibroblasts (also known as tumour-associated adipose stromal cells) have increased rates of glycolysis that drive the production of lactate, which is used as fuel by cancer cells. This energetic shift occurs, at least in part, as a consequence of stromal cell responses to cancer cell signals that induce oxidative stress, autophagy and increased expression of HIF1α. An altered cell metabolism can also occur as a result of proliferation and desmoplasia, which demand increased energy use to support the increase in biomass.
Inflammatory mediators associated with both obesity and breast cancer, including IL-6, TNF and PGE2, contribute to these phenotypes by increasing the expression of GLUT1 and GLUT3 in breast adipose stromal cells, which results in increased glucose uptake and cell proliferation89. Increased stromal cell proliferation is a common feature of breast cancer and desmoplasia is noticeable in the majority of tumours90. In one study, a high (≥50%) tumour stromal content was observed in 68% of the non-metastatic breast cancers examined (n = 574)91. The desmoplastic reaction, a term referring to features of increased stromal cell growth and/or increased extracellular matrix deposition, is seen not only around established tumours but also in normal tissues and is believed to contribute to tumour development. This hypothesis is supported by studies showing that increased breast density is associated with an increased risk of developing breast cancer and by mechanistic studies demonstrating that the loss of CD36 (the repression of which is associated with decreased fat accumulation and increased matrix deposition by adipose stromal cells) occurs even in the absence of tumour signals. Dense areas of the breast also have elevated expression of aromatase and are hypothesized to provide a hormonal milieu conducive to breast cancer cell growth92,93. Interestingly, the proliferation of adipose stromal cells and/or preadipocytes is also a feature of obesity94. In human tissues, cancer-associated adipose stromal cells and obesity-associated adipose stromal cells both exhibit a dysregulated expression of many proteins associated with metabolic dysfunction, including LKB1, AMPK, p53, HIF1α and PKM2 (REFS19,95–97).
Metabolic pathways and oestrogen.
Adipose tissue-derived oestrogens, including those synthesized in the breast, are hypothesized to drive the growth of ER+ breast cancers after menopause31. Aromatase, a crucial enzyme in oestrogen biosynthesis, is encoded by the CYP19A1 gene and its expression in breast adipose stromal cells is tightly regulated by both tumour-derived and adipose tissue-derived factors. The expression of CYP19A1 in humans is controlled by tissue-specific alternative promoters upstream of 11 untranslated first exons, not all of which have counterparts in mice98. As such, the regulation of aromatase expression in the adipose tissue of mice and humans is not wholly analogous and studies in the context of human obesity and breast cancer have relied largely on studies of isolated human cells99 and transgenic mice with mammary-specific expression of human aromatase100.
Interestingly, breast adipose stromal cells undergo a promoter-switching event in both obesity and breast cancer, whereby aromatase expression increases owing to a shift from promoter I.4 to the coordinated activation of promoters I.3 and PII. This switch has been ascribed to changes in external stimuli, including the increased production of inflammatory mediators and adipokines, and has offered numerous insights into the molecular regulation of aromatase with important consequences for the identification of potential therapeutic targets in obesity and breast cancer. For example, metabolic pathways have been implicated in the regulation of oestrogen production in breast adipose stromal cells in the context of both obesity and breast cancer. As the transcription of CYP19A1 via promoters I.3 and PII is dependent on CREB, an early link between obesity and breast cancer was established by the discovery that the activation of AMPK by LKB1 inhibits aromatase expression by causing cytoplasmic sequestration of CREB-regulated transcription co-activator 2 (CRTC2, also known as transducer of CREB protein 2 (TORC2))95. Subsequent studies found that CRTC1 and CRTC3 also bind to the PII promoter and stimulate the expression of aromatase in adipose stromal cells101.
Interestingly, the obesity-associated factors leptin and PGE2 are potent suppressors of LKB1–AMPK activity. They suppress both the expression of LKB1 and the phosphorylation of AMPK at Thr172 and instead stimulate the phosphorylation of AMPK at Ser485, an inhibitory site. This change leads to increased nuclear translocation and binding of CRTC1–CRTC3 proteins to aromatase promoter PII and increased expression of aromatase95. These observations contributed to the understanding not only of the mechanism of aromatase regulation in obesity and breast cancer in women but also of the increased risk of gynaecomastia in boys with Peutz–Jeghers syndrome (caused by germline mutations in STK11, which encodes LKB1). Boys with Peutz–Jeghers syndrome have an increased expression of aromatase in breast tissue as well as in the testes102. Importantly, adiponectin, a hormone associated with leanness, suppresses aromatase expression in breast adipose tissue via the activation of AMPK, suggesting that AMPK-activating drugs might inhibit tumour growth via this effect on aromatase. Consistent with this observation, the anti-hyperglycaemic agent metformin (which inhibits hepatic gluconeogenesis via the LKB1-dependent activation of AMPK103) also inhibits aromatase at pharmacologically relevant micromolar doses and does so in a promoter-selective manner104,105. Thus, the inhibition of aromatase promoters 1.3 and II but not I.4 by metformin would be expected to suppress the adipose-specific biosynthesis of oestrogens while leaving aromatase expression in bones and joints unaffected.
The aromatase promoters also include response elements for p53. A site located −441 to −435 bp upstream of the transcription start site binds to p53 with greatest affinity97. Mutations at this site increase aromatase gene transcription, suggesting that p53 acts as a transcriptional repressor of aromatase. In support of this observation, treatment with the small molecule RITA (reactivator of p53 and inducer of tumour cell apoptosis) prevents the HDM2-mediated degradation of p53 and thereby decreases levels of aromatase in breast adipose stromal cells. RITA also increases levels of SCO2, which is consistent with the induction of p53-driven metabolic activity in these cells. Additionally, a study of 14 women with Li–Fraumeni syndrome showed that these individuals had increased immunoreactivity to aromatase in their breast adipose stromal cells compared with women who had wild-type TP53. This finding might account, at least in part, for the increased risk of developing ER+ breast cancers in women with Li–Fraumeni syndrome97. Further studies of the links between dysregulated metabolism and aromatase regulation found that, in the presence of PGE2, the phosphorylation of p53 at Ser15 (a target site of both ATM and AMPK) was suppressed97. Phosphorylation at this site resulted in the dissociation of p53 from HDM2, leading to the stabilization of p53 (REF.97). Hence, the suppressed phosphorylation of p53 could account for the decreased nuclear abundance of p53 in adipose stromal cells from individuals with obesity19 and after treatment with PGE2 and leptin in vitro19,97.
HIF1α is another potent regulator of aromatase in adipose stromal cells96. The binding of HIF1α to a hypoxia response element that overlaps with the proximal CREB response element on the antisense strand of the aromatase promoter is associated with an increase in promoter PII activity. In addition to the accepted hypoxia-driven regulation of HIF1α, this finding also highlights an important alternative mechanism of HIF1α regulation: namely, that HIF1α is stabilized by PGE2 independently of oxygen tension and is required for the PGE2-mediated induction of aromatase in adipose stromal cells. Further studies demonstrated that leptin also stabilizes HIF1α, which interacts with PKM2 to regulate aromatase expression19. In support of these mechanistic observations, nuclear HIF1α immunoreactivity is positively correlated with aromatase immunoreactivity in breast adipose stromal cells in women and in the mammary fat pad of mice when assessed on a per-cell basis and as a function of obesity19,96.
Immune cells.
The regulation of metabolism in immune cells has an important role in providing the energy required by activated macrophages and proliferating T cells and is now also emerging as a key facet of the intricate regulation of immune responses in health and disease106. In immune cells, metabolic pathways contribute to cell survival and proliferation and are also critical for the production of inflammatory mediators, including cytokines. An evolutionary perspective on the relationship between immune and metabolic responses (published in 2006) describes common key regulatory molecules and signalling pathways that regulate both functions107.
Obesity is associated with chronic, low-grade inflammation, increased numbers of metabolically activated macrophages108,109, increased numbers of CD4+ and CD8+ lymphocytes110, and reduced numbers of regulatory T cells111,112. Several metabolic pathways, including those listed above, have been implicated in immune cell metabolism in the context of obesity. AMPK limits immune cell activation via effects on mTOR113 and, in mice, genetic deletion of the β1 subunit of AMPK in macrophages leads to increased intracellular levels of markers of inflammation as well as to increased adipose tissue inflammation and insulin resistance in animals fed a high-fat diet114. With the expansion of adipose tissue in obesity, local hypoxia drives increases in mRNA and protein levels of HIF1α and HIF2α in immune cells, promoting glycolysis and, more importantly, the release of inflammatory cytokines115,116. The T cell receptor can also activate CD28, which (similarly to receptor tyrosine kinases) then stimulates PI3K–AKT signalling, leading to a dramatic increase in glucose uptake to support the expansion of activated T cells117.
Metabolism-targeted therapies
Pharmacological approaches.
Targeting the mitochondrial electron transport chain is an anticancer approach that is gaining traction118. Metformin is the best-characterized mitochondrial electron transport chain complex I inhibitor in clinical use. In breast cancer cells, the cellular uptake of metformin requires organic cation transporter 3 (OCT3)119,120. Once inside the cell, inhibition of the mitochondrial electron transport chain decreases ATP production and leads to the activation of AMPK, which mediates many of the biological effects of metformin103.
Initial interest in this drug in the cancer setting came from the observation that patients with diabetes who took metformin had a reduced propensity to develop cancer121. In breast cancer cell lines in vitro, metformin suppresses the growth of cells that express LKB1 (REF.122). However, the doses required to achieve this effect exceed those attainable in humans. By contrast, the levels of metformin needed to inhibit aromatase expression in breast adipose stromal cells are lower than those in the blood of metformin-treated patients with diabetes104. Interestingly, metformin treatment also suppresses the growth of cancer cells in hyperinsulinaemic xenograft models, independent of the LKB1 status of the tumour cells123, which suggests that effects on host cells contribute to the antitumour effects of metformin in these models. Several clinical studies have noted promising effects of metformin on breast cancer incidence, the expression of proliferation marker Ki67 and complete pathological response rates124,125, which seem to depend, to a certain extent, on the tumour cells’ metabolic status. Interim results from the phase III MA.32 study, which is examining the effects of metformin in addition to current best practice, demonstrate that adjuvant metformin is associated with a considerable reduction in body weight, improvements in metabolic measures126 and decreased oestradiol levels in postmenopausal women with triple-negative breast cancer who are not receiving endocrine therapy127. The reductions in oestrogen levels were independent of baseline BMI and decreases in BMI, suggesting that the reductions in oestrogen levels associated with metformin treatment are at least partially independent of decreased fat mass. These findings highlight the potential of AMPK-activating drugs to suppress local and systemic drivers of breast cancer, including insulin, glucose, oestrogens and adipokines, and suggest that the potential anticancer effects of metformin should be considered in the clinical setting.
Mutations in the PIK3CA gene (which encodes the p110a subunit of PI3K) are common in breast cancer and are associated with the activation of PI3K–AKT signalling and hypersensitivity to insulin128. PI3K inhibitors suppress the catalytic activity of this enzyme and, as such, have shown benefit in some patients with breast cancer. Unfortunately, the use of PI3K inhibitors is associated with hyperglycaemia, which not only provides fuel for cancer cells but also causes a transient increase in pancreatic insulin secretion that can be prolonged in individuals with insulin resistance and overcomes the effects of PI3K inhibition in tumour cells129. Combining either dietary approaches or pharmacological interventions to manage hyperglycaemia with PI3K inhibitors might show promise in the management of breast cancers that carry PI3K mutations. In a preclinical study, treatment with the PI3K inhibitor buparlisib was accompanied by an increase in fluorodeoxyglucose uptake by tumour cells129. This observation could partly explain why patients with metastatic triple-negative breast cancer receiving buparlisib showed no confirmed objective response to this monotherapy in a phase II study130. In xenograft models, a ketogenic diet prevented the PI3K inhibitor-induced rebound activation of insulin signalling and led to tumour growth inhibition and improved survival129.
Lifestyle interventions.
Weight loss, achieved by either reduced caloric intake or exercise or a combination of both, is associated with a decrease in breast cancer risk and has profound effects on several obesity-related risk factors for breast cancer, including levels of sex hormones, inflammation and insulin131–134. A meta-analysis of six randomized controlled trials found beneficial effects of reduced caloric intake, alone and in combination with exercise, on levels of oestrogens, androgens and sex hormone-binding globulin (SHBG)132. Weight loss due to reduced caloric intake alone was associated with substantial decreases in the levels of total oestradiol (0.86; 95% CI 0.77–0.95), free oestradiol (0.73; 95% CI 0.66–0.81), free testosterone (0.91; 95% CI 0.84–0.98) and SHBG (1.20; 95% CI 1.06–1.36). Slightly better effects were observed when weight loss was achieved by reduced caloric intake combined with exercise. By contrast, weight loss achieved by exercise alone did not have any significant effects on sex hormone levels in this meta-analysis. In a separate meta-analysis, lifestyle modifications resulting in weight loss were associated with a 39% reduction in the relative risk of diabetes in an at-risk population. Another randomized controlled trial included 439 postmenopausal women with overweight or obesity who were randomly assigned to 1 year of caloric restriction (with the goal of 10% weight loss from baseline), aerobic exercise (225 min each week of moderate-to-vigorous exercise), combined diet and exercise, or a no-intervention control134. The results showed that caloric restriction leading to ≥5% weight loss, with or without exercise, was associated with reduced serum levels of several inflammatory markers, including C-reactive protein (measured by high-sensitivity assay), IL-6 and amyloid A.
Multiple observational studies have examined the relationship between physical activity and cancer risk135. Strong evidence links physical activity with a reduction in the risk of developing breast cancer. Physical activity is integrated into many lifestyle interventions that are standard of care for improving metabolic dysfunction and obesity and might also improve outcomes in patients with diabetes mellitus and individuals with obesity. Potential benefits of physical activity have also been proposed in the context of cancer prevention. Prospective randomized studies of diverse exercise regimens have been undertaken with, thus far, mixed results (reviewed elsewhere135). Improved definition of the dose and duration of exercise is thought to be critical for the implementation of guidelines on the use of exercise in the cancer prevention setting135,136. Additional clinical studies that incorporate lifestyle interventions are currently being undertaken, including the phase III randomized controlled Breast Cancer Weight Loss study137. This study will compare the effects of a telephone-based weight loss intervention with an educational control intervention in the adjuvant treatment of women with overweight or obesity who have early breast cancer. The researchers aim to assess the effects of these interventions on the levels of several serum biomarkers, including fasting insulin, adipokines and inflammatory markers. Little is known of the effect of lifestyle interventions on metabolic pathways in women.
In preclinical models, exercise reduces cancer initiation, growth and metastasis via effects on immune responses, energy metabolism, metabolic signalling pathways, apoptosis and the expression of metastasis-promoting genes138. For example, exercise abolishes the growth-stimulating effects of a high-fat diet on mammary tumours in mice by stimulating AMPK activity and inhibiting AKT, leading to the increased activity of p27Kip1, an important cyclin-dependent kinase inhibitor139. Weight loss is also associated with an increase in AMPK activity in the mammary glands of wild-type mice, suggesting that leanness has a protective effect against tumour formation140. In a similar breast cancer xenograft mouse model with diet-induced obesity, supplementation with vitamin D and calcitriol delayed mammary tumour penetrance141. These effects of vitamin D and calcitriol were hypothesized to be related to the observed reductions in oestrogen production and ER activity, decreased expression of PTGS2 and leptin receptor, increased adiponectin signalling, and stimulation of the LKB1–AMPK pathway. We must note, however, that preclinical studies in mice are often performed in young animals and involve excessive feeding or genetic manipulation. The observed changes in metabolic pathways remain to be confirmed in human studies.
Conclusions and future perspectives
Breast cancer treatments that focus only on cancer cell characteristics, such as hormone receptor expression or PIK3CA mutation status, are associated with an increased risk of incomplete response and disease progression in individuals with obesity who have elevated levels of factors that stimulate breast cancer growth (such as oestrogens, glucose and insulin)142,143. Obesity at the time of breast cancer diagnosis is a potential determinant of treatment efficacy and strong evidence now also suggests that several front-line therapies for breast cancer, including endocrine therapy and chemotherapy, cause weight gain and/or increase the risk of a number of metabolic diseases, including diabetes mellitus144,145. In women with obesity, prediabetes or metabolic syndrome, breast cancer treatment can exacerbate these underlying conditions, which are associated with reduced survival as well as with treatment resistance.
The identification of obesity-associated factors and metabolic pathways as important drivers of breast cancer has opened the door to a new class of therapeutics that target not only breast cancer cell biology but also the dysregulated metabolism of neoplastic and non-neoplastic cells in the tumour microenvironment. Several clinical studies have tested (or are currently testing) the effects of combining lifestyle interventions or pharmacological approaches aimed at treating or preventing obesity and/or metabolic dysfunction with breast cancer treatment125,146. Future studies should aim to characterize the dose and duration of interventions aimed at combating obesity and to refine the study populations most likely to benefit. Precision prevention studies, which focus on selecting high-risk study participants using breast cancer risk prediction models that integrate information beyond family history and reproductive factors (BMI, for example), have been proposed as an approach to provide invaluable insights into effective interventions while reducing the need for large sample sizes and long-term follow-up146. Contending with the obesity epidemic will similarly require a combination of lifestyle and pharmaceutical interventions to decrease the burden of obesity-associated cancer. We expect the results of ongoing clinical studies to shed some light on the effectiveness of such approaches.
Key points.
Strong evidence links obesity to the development of 13 types of cancer, including oestrogen receptor-positive breast cancer in postmenopausal women.
Metabolic pathways involving PI3K–AKT, HIF1α, LKB1–AMPK and p53 are key regulators of breast cancer cell metabolism and growth.
Obesity-associated factors drive metabolic alterations in both breast cancer cells and cells of the breast microenvironment that support tumour growth.
Therapies that target metabolic pathways might prove effective at treating and preventing breast cancer via effects on cancer cells, the tumour microenvironment and whole-body metabolism.
Acknowledgements
Research work by K.A.B. is supported by NIH grant R01 CA215797 and the Anne Moore Breast Cancer Research Fund.
Footnotes
Competing interests
The author declares no competing interests.
Peer review information
Nature Reviews Endocrinology thanks the (anonymous) reviewers for their contribution to the peer review of this work.
References
- 1.Smittenaar CR., Petersen KA., Stewart K. & Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 115, 1147–1155 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg PS, Barker KA & Anderson WF Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J. Natl Cancer Inst 107, djv159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heer E. et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob. Health 8, e1027–e1037 (2020). [DOI] [PubMed] [Google Scholar]
- 4.WHO. Obesity and Overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2020).
- 5.Hales CM, Carroll MD, Fryar CD & Ogden CL Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief no. 360 (National Center for Health Statistics, 2020). [PubMed] [Google Scholar]
- 6.OECD. The heavy burden of obesity: the economics of prevention. (OECD, 2019). [Google Scholar]
- 7.Lauby-Secretan B. et al. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med 375, 794–798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuhouser ML et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncol. 1, 611–621 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DSM et al. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 30, 1183–1200 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Munsell MF, Sprague BL, Berry DA, Chisholm G. & Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev 36, 114–136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corvera S. & Gealekman O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim. Biophys. Acta 1842, 463–472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engin A. The Pathogenesis of obesity-associated adipose tissue inflammation. Adv. Exp. Med. Biol 960, 221–245 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Friedman JM Leptin and the endocrine control of energy balance. Nat. Metab 1, 754–764 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Howlader N. et al. SEER Cancer Statistics Review, 1975–2017 https://seer.cancer.gov/csr/1975_2017/ (2020).
- 15.Burger HG, Hale GE, Robertson DM & Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum. Reprod. Update 13, 559–565 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Stanczyk FZ, Jurow J. & Hsing AW Limitations of direct immunoassays for measuring circulating estradiol levels in postmenopausal women and men in epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 19, 903–906 (2010). [DOI] [PubMed] [Google Scholar]
- 17.McTiernan A. et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity 14, 1662–1677 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Brown KA et al. Menopause Is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J. Clin. Endocrinol. Metab 102, 1692–1701 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahid H. et al. Leptin regulation of the p53-HIF1α/PKM2-aromatase axis in breast adipose stromal cells: a novel mechanism for the obesity-breast cancer link. Int. J. Obes 42, 711–720 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris PG et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res 4, 1021–1029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misso ML et al. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause 12, 210–215 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Miller WR & O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids 50, 537–548 (1987). [DOI] [PubMed] [Google Scholar]
- 23.Docanto MM et al. Ghrelin and des-acyl ghrelin inhibit aromatase expression and activity in human adipose stromal cells: suppression of cAMP as a possible mechanism. Breast Cancer Res. Treat 147, 193–201 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Au CC et al. Des-acyl ghrelin inhibits the capacity of macrophages to stimulate the expression of aromatase in breast adipose stromal cells. J. Steroid Biochem. Mol. Biol 170, 49–53 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Au CC et al. Three-dimensional growth of breast cancer cells potentiates the anti-tumor effects of unacylated ghrelin and AZP-531. eLife 9, e56913 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au CC, Furness JB & Brown KA Ghrelin and breast cancer: emerging roles in obesity, estrogen regulation, and cancer. Front. Oncol 6, 265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macis D, Guerrieri-Gonzaga A. & Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int. J. Epidemiol 43, 1226–1236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wairagu PM et al. Insulin priming effect on estradiol-induced breast cancer metabolism and growth. Cancer Biol. Ther 16, 484–492 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naimo GD, Gelsomino L, Catalano S, Mauro L. & Ando S. Interfering role of ERα on adiponectin action in breast cancer. Front. Endocrinol 11, 66 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ando S, Naimo GD, Gelsomino L, Catalano S. & Mauro L. Novel insights into adiponectin action in breast cancer: evidence of its mechanistic effects mediated by ERα expression. Obes. Rev 21, e13004 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Bhardwaj P. et al. Estrogens and breast cancer: mechanisms involved in obesity-related development, growth and progression. J. Steroid. Biochem. Mol. Biol 189, 161–170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J. & Thompson CB Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell. Biol 20, 436–450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzig S. & Shaw RJ AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol 19, 121–135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoxhaj G. & Manning BD The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves MD, Hopkins BD & Cantley LC Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med 379, 2052–2062 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Lawrence MS et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elstrom RL et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Jaldin-Fincati JR, Pavarotti M, Frendo-Cumbo S, Bilan PJ & Klip A. Update on GLUT4 vesicle traffic: a cornerstone of insulin action. Trends Endocrinol. Metab 28, 597–611 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Simpson ER & Brown KA p53: protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 75, 5001–5007 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Feng Z. et al. The regulation of AMPK β1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 67, 3043–3053 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Zeng PY & Berger SL LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 66, 10701–10708 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y. et al. LKB1 deficiency-induced metabolic reprogramming in tumorigenesis and non-neoplastic diseases. Mol. Metab 44, 101131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie DG & Alessi DR LKB1 and AMPK and the cancer-metabolism link — ten years after. BMC Biol. 11, 36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawley SA et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol 2, 28 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods A. et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol 13, 2004–2008 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Shaw RJ et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadad SM et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 9, 307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zadra G, Batista JL & Loda M. Dissecting the dual role of AMPK in cancer: from experimental to human studies. Mol. Cancer Res 13, 1059–1072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardie DG The LKB1-AMPK pathway — friend or foe in cancer? Cancer Cell 23, 131–132 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Barthel A. et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem 274, 20281–20286 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Adekola K, Rosen ST & Shanmugam M. Glucose transporters in cancer metabolism. Curr. Opin. Oncol 24, 650–654 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waldhart AN et al. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep. 19, 2005–2013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu N. et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell 49, 1167–1175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parikh H. et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 4, e158 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volinsky N. et al. Signalling mechanisms regulating phenotypic changes in breast cancer cells. Biosci. Rep 35, e00178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iqbal MA et al. Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2. Mol. Cancer 12, 72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bousquenaud M, Fico F, Solinas G, Ruegg C. & Santamaria-Martinez A. Obesity promotes the expansion of metastasis-initiating cells in breast cancer. Breast Cancer Res. 20, 104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai Q. et al. Positive correlation between the expression of hEag1 and HIF-1α in breast cancers: an observational study. BMJ Open 4, e005049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drabovich AP, Pavlou MP, Dimitromanolakis A. & Diamandis EP Quantitative analysis of energy metabolic pathways in MCF-7 breast cancer cells by selected reaction monitoring assay. Mol. Cell Proteom. 11, 422–434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Mahony F, Razandi M, Pedram A, Harvey BJ & Levin ER Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol. Endocrinol 26, 2058–2070 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imbert-Fernandez Y. et al. Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3). J. Biol. Chem 289, 9440–9448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown KA et al. LKB1 expression is inhibited by estradiol-17β in MCF-7 cells. J. Steroid. Biochem. Mol. Biol 127, 439–443 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Ko BH, Paik JY, Jung KH & Lee KH 17β-estradiol augments 18F-FDG uptake and glycolysis of T47D breast cancer cells via membrane-initiated rapid PI3K-Akt activation. J. Nucl. Med 51, 1740–174 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Kim S, Taylor JA, Milne GL & Sandler DP Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev. Res 6, 511–518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heikkila K. et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 20, 15–26 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Wang D. & DuBois RN. Urinary PGE-M: a promising cancer biomarker. Cancer Prev. Res 6, 507–510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.George RJ, Sturmoski MA, Anant S. & Houchen CW EP4 mediates PGE2 dependent cell survival through the PI3 kinase/AKT pathway. Prostaglandins Other Lipid Mediat. 83, 112–120 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Badache A. & Hynes NE Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 61, 383–391 (2001). [PubMed] [Google Scholar]
- 69.Gui Y. et al. The association between obesity related adipokines and risk of breast cancer: a meta-analysis. Oncotarget 8, 75389–75399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanquer-Rossello MDM, Oliver J, Sastre-Serra J, Valle A. & Roca P. Leptin regulates energy metabolism in MCF-7 breast cancer cells. Int. J. Biochem. Cell Biol. 72, 18–26 (2016). [DOI] [PubMed] [Google Scholar]
- 71.El-Masry OS, Al-Sakkaf K, Brown BL & Dobson PR Differential crosstalk between the AMPK and PI3K/Akt pathways in breast cancer cells of differing genotypes: leptin inhibits the effectiveness of AMPK activation. Oncol. Rep 34, 1675–1680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frankenberry KA, Skinner H, Somasundar P, McFadden DW & Vona-Davis LC Leptin receptor expression and cell signaling in breast cancer. Int. J. Oncol 28, 985–993 (2006). [PubMed] [Google Scholar]
- 73.Wei L. et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J. Exp. Clin. Cancer Res 35, 166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez-Perez RR et al. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell Signal 22, 1350–1362 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartella V. et al. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 68, 4919–4927 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Cascio S. et al. Mechanism of leptin expression in breast cancer cells: role of hypoxia-inducible factor-1α. Oncogene 27, 540–547 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Miyoshi Y. et al. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 9, 5699–5704 (2003). [PubMed] [Google Scholar]
- 78.Dos Santos E. et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol. Rep 20, 971–977 (2008). [PubMed] [Google Scholar]
- 79.Wang Y. et al. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 66, 11462–11470 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Dieudonne MN et al. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun 345, 271–279 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Mao X. et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 8, 516–523 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Taliaferro-Smith L. et al. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 28, 2621–2633 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu Q. et al. Cancer-associated adipocytes: key players in breast cancer progression. J. Hematol. Oncol 12, 95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang YY et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoy AJ, Balaban S. & Saunders DN Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol. Med 23, 381–392 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Balaban S. et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5, 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madak-Erdogan Z. et al. Free fatty acids rewire cancer metabolism in obesity-associated breast cancer via estrogen receptor and mTOR signaling. Cancer Res. 79, 2494–2510 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Martinez-Outschoorn U, Sotgia F. & Lisanti MP Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin. Oncol 41, 195–216 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Docanto MM, Ham S, Corbould A. & Brown KA Obesity-associated inflammatory cytokines and prostaglandin E2 stimulate glucose transporter mRNA expression and glucose uptake in primary human adipose stromal cells. J. Interferon Cytokine Res. 35, 600–605 (2015). [DOI] [PubMed] [Google Scholar]
- 90.DeClerck YA Desmoplasia: a response or a niche? Cancer Discov. 2, 772–774 (2012). [DOI] [PubMed] [Google Scholar]
- 91.de Kruijf EM et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res. Treat 125, 687–696 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Vachon CM et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res. Treat 125, 243–252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huo CW et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 17, 79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Harmelen V. et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord 27, 889–895 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Brown KA et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 69, 5392–5399 (2009). [DOI] [PubMed] [Google Scholar]
- 96.Samarajeewa NU et al. HIF-1α stimulates aromatase expression driven by prostaglandin E2 in breast adipose stroma. Breast Cancer Res. 15, R30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X. et al. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: a novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer Res. 75, 645–655 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Chow JD, Simpson ER & Boon WC Alternative 5´-untranslated first exons of the mouse Cyp19A1 (aromatase) gene. J. Steroid Biochem. Mol. Biol 115, 115–125 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Ackerman GE, Smith ME, Mendelson CR, MacDonald PC & Simpson ER Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J. Clin. Endocrinol. Metab 53, 412–417 (1981). [DOI] [PubMed] [Google Scholar]
- 100.Zhao H. et al. A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology 153, 2701–2713 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samarajeewa NU, Docanto MM, Simpson ER & Brown KA CREB-regulated transcription co-activator family stimulates promoter II-driven aromatase expression in preadipocytes. Horm. Cancer 4, 233–241 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ham S. et al. Overexpression of aromatase associated with loss of heterozygosity of the STK11 gene accounts for prepubertal gynecomastia in boys with Peutz-Jeghers syndrome. J. Clin. Endocrinol. Metab 98, E1979–E1987 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Shaw RJ et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown KA, Hunger NI, Docanto M. & Simpson ER Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res. Treat 123, 591–596 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Samarajeewa NU, Ham S, Yang F, Simpson ER & Brown KA Promoter-specific effects of metformin on aromatase transcript expression. Steroids 76, 768–771 (2011). [DOI] [PubMed] [Google Scholar]
- 106.O’Neill LA, Kishton RJ & Rathmell J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol 16, 553–565 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hotamisligil GS Inflammation and metabolic disorders. Nature 444, 860–867 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Kratz M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang H. et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol 185, 1836–1845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu J, Zhao J, Meng H. & Zhang X. Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front. Immunol 10, 1173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med 25, 141–151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Neill LA & Hardie DG Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493, 346–355 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Galic S. et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest 121, 4903–4915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boutens L. et al. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 61, 942–953 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choe SS et al. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63, 3359–3371 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Frauwirth KA et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002). [DOI] [PubMed] [Google Scholar]
- 118.Vasan K, Werner M. & Chandel NS Mitochondrial metabolism as a target for cancer therapy. Cell. Metab 32, 341–352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cai H, Everett RS & Thakker DR Efficacious dose of metformin for breast cancer therapy is determined by cation transporter expression in tumours. Br. J. Pharmacol 176, 2724–2735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wheaton WW et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR & Morris AD Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown KA, Samarajeewa NU & Simpson ER Endocrine-related cancers and the role of AMPK. Mol. Cell Endocrinol. 366, 170–179 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Algire C. et al. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 30, 1174–1182 (2011). [DOI] [PubMed] [Google Scholar]
- 124.Grossmann ME, Yang DQ, Guo Z, Potter DA & Cleary MP Metformin treatment for the prevention and/or treatment of breast/mammary tumorigenesis. Curr. Pharmacol. Rep 1, 312–323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samuel SM, Varghese E, Kubatka P, Triggle CR & Busselberg D. Metformin: the answer to cancer in “a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast cancer. Biomolecules 9, 846 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goodwin PJ et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J. Natl Cancer Inst. 107, djv006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pimentel I. et al. The effect of metformin vs placebo on sex hormones in CCTG MA.32. J. Natl Cancer Inst. 10.1093/jnci/djaa082 (2020). [DOI] [Google Scholar]
- 128.Millis SZ, Ikeda S, Reddy S, Gatalica Z. & Kurzrock R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2, 1565–1573 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Hopkins BD et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garrido-Castro AC et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 22, 120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eliassen AH, Colditz GA, Rosner B, Willett WC & Hankinson SE Adult weight change and risk of postmenopausal breast cancer. JAMA 296, 193–201 (2006). [DOI] [PubMed] [Google Scholar]
- 132.de Roon M. et al. Effect of exercise and/or reduced calorie dietary interventions on breast cancer-related endogenous sex hormones in healthy postmenopausal women. Breast Cancer Res. 20, 81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haw JS et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med 177, 1808–1817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Imayama I. et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 72, 2314–2326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iyengar NM & Jones LW Development of exercise as interception therapy for cancer: a review. JAMA Oncol. 10.1001/jamaoncol.2019.2585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McTiernan A. et al. Physical activity in cancer prevention and survival: a systematic review. Med. Sci. Sports Exerc. 51, 1252–1261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ligibel JA et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer 3, 37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW & Jones LW Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 76, 4032–4050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Theriau CF, Shpilberg Y, Riddell MC & Connor MK Voluntary physical activity abolishes the proliferative tumor growth microenvironment created by adipose tissue in animals fed a high fat diet. J. Appl. Physiol 121, 139–153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qin Y. et al. Weight loss reduces basal-like breast cancer through kinome reprogramming. Cancer Cell Int. 16, 26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Swami S. et al. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr. Relat. Cancer 23, 251–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanker AB, Kaklamani V. & Arteaga CL Challenges for the clinical development of PI3K inhibitors: strategies to improve their impact in solid tumors. Cancer Discov. 9, 482–491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ligibel JA & Winer EP Aromatase inhibition in obese women: how much is enough? J. Clin. Oncol 30, 2940–2942 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Brown KA, Andreopoulou E. & Andreopoulou P. Endocrine therapy-related endocrinopathies - biology, prevalence and implications for the management of breast cancer. Oncol. Hematol. Rev 16, 17–22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van den Berg MM et al. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer 17, 259 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brown JC & Ligibel JA Lifestyle interventions for breast cancer prevention. Curr. Breast Cancer Rep. 10, 202–208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsatsoulis A. & Paschou SA Metabolically healthy obesity: criteria, epidemiology, controversies, and consequences. Curr. Obes. Rep 9, 109–120 (2020). [DOI] [PubMed] [Google Scholar]
- 148.Batsis JA et al. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int. J. Obes 40, 761–767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sahakyan KR et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann. Intern. Med 163, 827–835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grier T, Canham-Chervak M, Sharp M. & Jones BH Does body mass index misclassify physically active young men? Prev. Med. Rep 2, 483–487 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heymsfield SB, Peterson CM, Thomas DM, Heo M. & Schuna JM Jr Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev 17, 262–275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Banack HR, Wactawski-Wende J, Hovey KM & Stokes A. Is BMI a valid measure of obesity in postmenopausal women? Menopause 25, 307–313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]