Abstract
Background
Recurrence of Clostridioides difficile infection (rCDI) is common, prolonging disease morbidity and leading to poor quality of life. We evaluated disease-specific health-related quality of life (HRQL) in patients with rCDI treated with fecal microbiota, live-jslm (REBYOTA [RBL]; Rebiotix) versus placebo.
Methods
This was a secondary analysis of a randomized, double-blind, placebo-controlled phase 3 study (PUNCH CD3). The disease-specific Clostridioides difficile Quality of Life Survey (Cdiff32) was administered at baseline and at weeks 1, 4, and 8. Changes in Cdiff32 total and domain (physical, mental, social) scores from baseline to week 8 were compared between RBL and placebo and for responders and nonresponders.
Results
Findings were analyzed in a total of 185 patients (RBL, n = 128 [69.2%]; placebo, n = 57 [30.8%]) with available Cdiff32 data. Patients from both arms showed significant improvements in Cdiff32 scores relative to baseline across all outcomes and at all time points (all P < .001); RBL-treated patients showed significantly greater improvements in mental domain than those receiving placebo. In adjusted analyses, RBL-treated patients showed greater improvements than placebo in total score and physical and mental domains (all P < .05). Similar improvement in mental domain was observed among responders, while nonresponders showed numerical improvements with RBL but not placebo.
Conclusions
In a phase 3 double-blinded clinical trial, RBL-treated patients reported more substantial and sustained disease-specific HRQL improvements than placebo-treated patients.
Clinical Trials Registration
ClinicalTrials.gov NCT03244644 (https://clinicaltrials.gov/ct2/show/NCT03244644).
Keywords: fecal microbiota, health-related quality of life, live-jslm, randomized clinical trial, Clostridioides difficile infection
We found significantly greater improvements in mental, physical, and overall health-related quality of life in patients with recurrent Clostridioides difficile infection treated with fecal microbiota, live-jslm (REBYOTA [RBL]) than in placebo-treated patients in a randomized, placebo-controlled trial (PUNCH CD3).
Clostridioides difficile infection (CDI) is a bacterial infection associated with >20 000 deaths per year and >$1 billion attributable healthcare cost annually in the United States [1, 2]. CDI causes symptoms ranging from diarrhea and abdominal cramping to life-threatening colitis and sepsis [3]. Despite treatment of primary CDI with standard-of-care antibiotics, recurrent CDI (rCDI) is common, and a history of recurrence is associated with up to 65% risk of future episodes [4–9]. rCDI increases morbidity and mortality rates [10, 11] and also substantially impairs patients’ health-related quality of life (HRQL) [12–14]. Patients with rCDI report greater disruptions to their daily life and work activities than those with a single episode and experience substantial psychological burdens due to anxiety and fear of future recurrences [14].
Fecal microbiota, live-jslm (REBYOTA) [RBL]; Rebiotix) is a live biotherapeutic product that restores the gut microbiome diversity and counters antibiotic-induced dysbiosis. Clinical trials have shown that RBL, following a standard of care antimicrobial, significantly reduced the rate of rCDI compared with placebo. RBL exhibited long-term efficacy and safety, with 92% of responders remaining CDI-free for up to 24 months [15–19]. The Clostridioides difficile Quality of Life Survey (Cdiff32) is a disease-specific instrument designed to identify quality of life impacts related to rCDI. Domains of the Cdiff32 include physical, mental, and social changes associated with rCDI. The objective of the current study was to analyze the Cdiff32 HRQL data collected in the PUNCH CD3 trial and to compare the HRQL in adults with rCDI between patients randomized to RBL and those randomized to placebo through 8 weeks after dosing [20].
METHODS
Data Source
PUNCH CD3 (NCT03244644) was a randomized, double-blinded, placebo-controlled phase 3 clinical trial evaluating the safety and efficacy of RBL versus placebo [21, 22]. The trial included adult patients (aged ≥18 years) with documented rCDI (ie, patients with ≥1 recurrence of CDI after a primary CDI episode, who had completed ≥1 round of standard-of-care oral antibiotic therapy or had ≥2 episodes of hospitalization due to severe CDI within the last year) who were taking or just prescribed antibiotics to control CDI-related diarrhea at the time of enrollment. Patients were randomized 2:1 to receive either 1 dose of RBL (active agent) or normal saline solution (placebo) and followed up for 8 weeks. A detailed description of the trial design is available from Khanna et al [22]. The trial was conducted in the United States and Canada according to the ethical principles of the Declaration of Helsinki, the Good Clinical Practice Guidelines, principles of informed consent, and requirements of publicly registered clinical trials.
We analyzed the as-observed data of the modified intention-to-treat (mITT) population of PUNCH CD3. The mITT population included all patients who successfully received blinded treatment but excluded those who withdrew before treatment, for whom treatment was attempted but not completed, or who discontinued before evaluation of treatment failure or success at week 8 if the reason for dropout was unrelated to CDI.
At week 8, patients who had no recurrence of CDI were categorized as responders, and those who had recurrence were categorized as nonresponders. At the investigator's discretion, patients who experienced a recurrence could have the option to receive a dose of open-label RBL, regardless of the initial treatment assignment. These patients who received another dose of RBL before week 8 were not included in the current analyses as the HRQL measure after receiving the second dose would not be reflective of the effect of the originally assigned treatment.
Patient Consent Statement
All aspects of this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol received institutional review board approval before its use and was conducted under a Food and Drug Administration Investigational New Drug application. All patients signed a written informed consent.
HRQL Instrument
HRQL was measured using the 32-item Cdiff32, a validated, disease-specific survey that quantifies changes in the HRQL using a total and 3 domain scores (physical, mental, and social) [20, 23]. Cdiff32 comprises 32 self-administered items about the impact of CDI in physical, mental, and social domains with 5-point Likert scale responses, typically ranging from strong disagreement to strong agreement about whether a CDI-specific concern affected the respondent in the past 7 days. The total and domain scores of Cdiff32 ranged from 0 (worst score) to 100 (best score) after aggregating and rescaling of items. The Cdiff32 was administered at baseline and at weeks 1, 4, and 8 during the double-blinded trial period.
Statistical Analyses
Analyses were restricted to patients in the mITT population who had Cdiff32 scores available at both baseline and week 8. Baseline patient demographics, disease characteristics, and Cdiff32 scores were summarized by treatment arm, using means and standard deviations (SDs) for continuous variables and counts and percentages for categorical variables. Total and domain Cdiff32 scores were summarized by treatment arm at weeks 1, 4, and 8 among patients with recorded Cdiff32 scores at baseline and the corresponding follow-up visit (week 1, 4 or 8, respectively) using mean and SD; mean scores were plotted by treatment arm over the 8-week treatment period.
Absolute scores at week 8 were compared with baseline scores using Wilcoxon rank sum tests by treatment arm and change from baseline to each week was calculated and compared between RBL and placebo at week 8 using Wilcoxon signed rank tests by treatment arm. Multivariable linear regressions were conducted for each Cdiff32 week 8 score with treatment group (placebo vs RBL) as the exposure variable of interest while controlling for the corresponding baseline Cdiff32 score and possible confounders of the treatment effect at baseline including sex (male vs female), age (in years), number of prior CDI episodes, prior treatment with fidaxomicin, prior proton pump inhibitor use, and indicators of common comorbid conditions (ie, metabolism and nutrition disorders, surgical and medical procedures, infections and infestations, gastrointestinal disorders, and psychiatric disorders).
Absolute scores at baseline and week 8 were summarized separately for responders and nonresponders by treatment arm and compared by means of Wilcoxon rank sum tests within each arm. Adjusted linear regressions of week 8 scores were conducted among responders, analogous to the adjusted regressions among all patients. All statistical analyses were performed using R 3.6.3 software (R Foundation for Statistical Computing).
RESULTS
Baseline Patient Characteristics
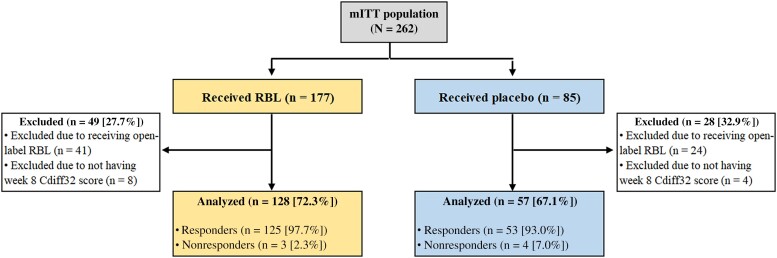
A total of 185 of 262 patients in the mITT population had both baseline and week 8 Cdiff32 scores and were included in this post hoc analysis, with 128 in the RBL arm (72.3% of the mITT RBL-treated patients) and 57 in the placebo arm (67.1% of the mITT placebo-treated patients). Seventy-seven patients were excluded, the majority (n = 65) owing to receipt of open-label RBL (41 in the RBL and 24 in the placebo arm) (Figure 1). Baseline demographics and disease characteristics across treatment arms are summarized in Table 1. Most patients were female (68.0% for RBL and 71.9% for placebo arm) and white (93.0% and 87.7%, respectively). The mean age (SD) was 60.8 (16.7) years for RBL and 57.0 (16.4) years for placebo. The mean numbers of prior CDI episodes were 3.2 and 3.0 for RBL and placebo, respectively, and 88.3% and 91.2% of patients received vancomycin as their standard of care antimicrobial before RBL and placebo, respectively. Given the trial design, the majority of patients who remained double-blind at week 8 were responders. At week 8, 178 of the 185 patients were responders (125 [97.7%] in the RBL and 53 [93.0%] in the placebo arm), and 7 patients were nonresponders (3 [2.3%] and 4 [7%], respectively). Responders were younger (average age, 59.3 vs 66.4 years for responders and nonresponders, respectively), and a smaller proportion were female (68.5% vs 85.7%, respectively).
Figure 1.
CONSORT diagram. Abbreviations: Cdiff32, Clostridioides difficile Health-related Quality-of-Life Survey; mITT modified intention-to-treat; RBL, fecal microbiota, live-jslm (REBYOTA; Rebiotix).
Table 1.
Patient Characteristics at Baseline
| Characteristic | Patients, No. (%)a | |
|---|---|---|
| Placebo Arm (n = 57) | RBL Arm (n = 128) |
|
| Demographic characteristics | ||
| Age, mean (SD), y | 57.0 (16.4) | 60.8 (16.7) |
| Age <65 y | 38 (66.7) | 67 (52.3) |
| Female sex | 41 (71.9) | 87 (68.0) |
| White race | 50 (87.7) | 119 (93.0) |
| Hispanic or Latino ethnicity | 2 (3.5) | 2 (1.6) |
| Disease characteristics | ||
| No. of CDI episodes before blinded treatment, mean (SD) | 3.0 (1.1) | 3.2 (1.1) |
| Prior hospitalization due to CDI | 9 (15.8) | 16 (12.5) |
| Antibiotics used at baseline | ||
| Vancomycin | 52 (91.2) | 113 (88.3) |
| Fidaxomicin | 4 (7.0) | 10 (7.8) |
| Other | 1 (1.8) | 5 (3.9) |
| Proton pump inhibitor use | 14 (24.6) | 23 (18.0) |
| Comorbid conditions | ||
| Surgical and medical procedures | 29 (50.9) | 83 (64.8) |
| Infections and infestations | 35 (61.4) | 76 (59.4) |
| Gastrointestinal disorders | 31 (54.4) | 69 (53.9) |
| Psychiatric disorders | 28 (49.1) | 69 (53.9) |
| Metabolic and nutrition disorders | 27 (47.4) | 67 (52.3) |
Abbreviations: CDI, Clostridioides difficile infection; RBL, fecal microbiota, live-jslm (REBYOTA; Rebiotix); SD, standard deviation.
Data represent no. (%) of patients unless otherwise specified. Patients include those from the modified intention-to-treat population with baseline and week 8 Clostridioides difficile health-related quality-of-life survey results. There were no significant differences between treatment arms.
CDI-Related HRQL Changes From Baseline by RBL Versus Placebo
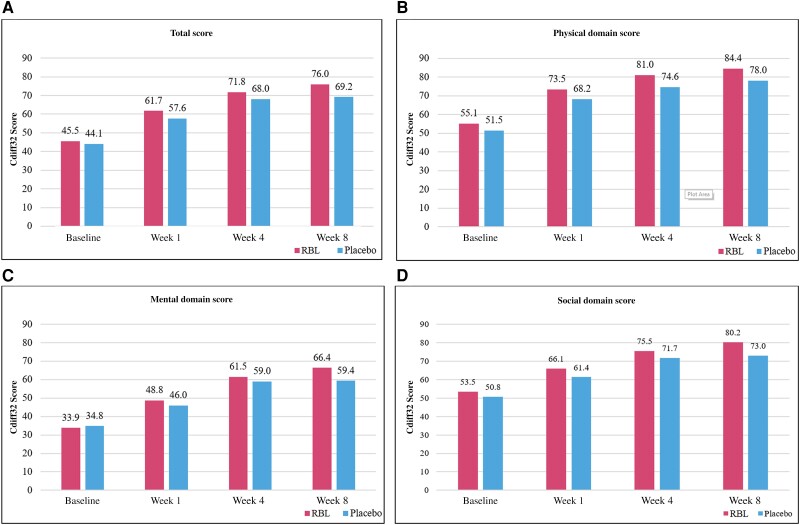
At baseline, Cdiff32 total scores (mean [SD]) were similar between RBL (44.2 [17.5]) and placebo (43.6 [21.3]). Cdiff32 total scores increased significantly from baseline to weeks 1, 4, and 8 in both arms (all P < .001) (Table 2 and Figure 2A). The mean (SD) change from baseline to week 8 was 6.0 (3.4) points higher for RBL (31.6 [21.4]) than for placebo (25.6 [21.4]) (P = .12). Adjusted multivariable analyses found a statistically significant difference of 7.2 (95% confidence interval, 1.2–13.2; P < .05) in favor of RBL compared with placebo at week 8 (Table 3).
Table 2.
Descriptive Summary of Clostridioides difficile Health-Related Quality-of-Life Survey Scores at Baseline and Weeks 1, 4, and 8
| Cdiff32 Domain | Cdiff32 Score, Mean (SD)a | Difference in Change From Baseline to wk 8 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | wk 1 | wk 4 | wk 8 | ||||||
| Placebo (n = 57) |
RBL (n = 128) |
Placebo (n = 76) |
RBL (n = 168) |
Placebo (n = 61) |
RBL (n = 135) |
Placebo (n = 57) |
RBL (n = 128) |
||
| Total | 43.6 (21.3) | 44.2 (17.5) | 57.6 (21.3) | 61.6 (18.3) | 68.0 (22.4) | 71.7 (18.7) | 69.2 (24.0) | 75.8 (18.1) | 6.0 (3.4) (P = .12) |
| Physical | 50.8 (23.5) | 53.2 (21.3) | 68.2 (22.5) | 73.3 (18.4) | 74.6 (23.7) | 81.0 (17.2) | 78.0 (23.0) | 84.4 (16.7) | 4.1 (3.8) (P = .30) |
| Mental | 34.5 (21.5) | 33.0 (16.9) | 46.0 (23.1) | 48.8 (21.6) | 59.0 (24.4) | 61.5 (22.7) | 59.4 (27.3) | 66.3 (21.8) | 8.5 (3.7) (P < .05) |
| Social | 50.1 (26.8) | 53.5 (23.4) | 61.4 (26.2) | 66.1 (23.2) | 71.7 (27.5) | 75.5 (22.3) | 73.0 (28.0) | 80.1 (21.1) | 3.6 (4.4) (P = .51) |
Abbreviations: Cdiff32, Clostridioides difficile Health-related Quality-of-Life Survey; RBL, fecal microbiota, live-jslm (REBYOTA; Rebiotix).
The sample size at baseline was restricted to patients who also had data at week 8. The sample size at week 1 was restricted to patients with both baseline and week 1 data, and the sample size at week 4 to patients with both baseline and week 4 data.
Figure 2.
Unadjusted Clostridioides difficile Health-related Quality-of-Life Survey (Cdiff32) scores (range, 0–100) during the blinded treatment period, stratified by treatment (modified intention-to-treat population, as-observed data) among all patients. A, Total score. B–D, Domain scores. The numbers of patients at baseline and at weeks 1, 4, and 8, were 176, 169, 136, and 129, respectively, for the RBL (fecal microbiota, live-jslm [REBYOTA; Rebiotix]) arm and 85, 76, 61, and 57 for the placebo arm.
Table 3.
Multivariable Adjusted Analyses for Week 8 Comparing RBL and Placebo Treatmenta
| Cdiff32 Domain | Difference in Cdiff32 Score, Point Estimateb (95% CI) (n = 185c) | P Value |
|---|---|---|
| Total | 7.2 (1.2–13.2) | <.05 |
| Physical | 6.6 (.8–12.3) | <.05 |
| Mental | 8.3 (1.4–15.3) | <.05 |
| Social | 6.5 (−.6 to 13.6) | .08 |
Abbreviations: Cdiff32, Clostridioides difficile Health-related Quality-of-Life Survey; CI, confidence interval; RBL, fecal microbiota, live-jslm (REBYOTA; Rebiotix).
The analyses included the following baseline covariates: respective baseline Cdiff32 score, treatment group, sex, age (in years), number of C difficile episodes before treatment, treatment with fidaxomicin, proton pump inhibitor use, metabolism and nutrition disorders, surgical and medical procedures, infections and infestations, gastrointestinal disorders, and psychiatric disorders.
The point estimates represent improvement in Cdiff32 total or domain score in patients given RBL versus placebo.
Sample sizes for the as-observed analysis differed across domain scores according to data availability.
Baseline mean values were similar for the physical, mental, and social domain scores between RBL and placebo groups (Table 2). Improvement from baseline was noted in week 1 and continued for weeks 4 and 8 for each domain (Table 2 and Figure 2B–2D). Adjusted multivariable analyses showed significantly improved domain scores for RBL for the physical (6.6 [95% confidence interval, .8–12.3]) and mental (8.3 [1.4–15.3]) domains compared with placebo at week 8 (both P < .05; Table 3).
CDI-Related HRQL in Responders and Nonresponders
Among responders, the Cdiff32 total score and the 3 domain scores showed statistically significant improvements at week 8 compared with their baseline scores in both treatment arms (Table 4). The multivariable analysis revealed significantly larger improvements from baseline to week 8 in the mental domain score for RBL-treated compared with placebo-treated patients (7.4 [.3–14.4] (mean [95% CI]); P < .05); the adjusted analyses for the total score and other domain scores also found numerically but not significantly larger improvements for RBL than for placebo.
Table 4.
Clostridioides difficile Health-Related Quality-of-Life Survey Component Scores at Baseline and Week 8 by Treatment Arm and Response Status
| Cdiff32 Domain |
RBL Arm | Placebo Arm | ||||||
|---|---|---|---|---|---|---|---|---|
| Cdiff32 Score, Mean (SD) | P Value | Cdiff32 Score, Mean (SD) | P Value | |||||
| Baseline | wk 8 | Change | Baseline | wk 8 | Change | |||
| Responders (n = 178)a | ||||||||
| Total | 43.7 (17.4) | 75.8 (18.3) | 32.1 (21.4) | <.001* | 42.4 (20.3) | 70.1 (23.2) | 27.7 (19.8) | <.001b |
| Physical | 52.6 (21.1) | 84.2 (16.9) | 31.7 (22.8) | <.001* | 49.9 (22.7) | 79.2 (21.6) | 29.3 (23.2) | <.001b |
| Mental | 32.7 (16.8) | 66.4 (22.0) | 33.7 (24.1) | <.001* | 33.0 (20.5) | 60.1 (27.0) | 27.0 (21.4) | <.001b |
| Social | 53.5 (23.6) | 80.2 (21.3) | 26.6 (27.9) | <.001* | 49.3 (26.5) | 73.6 (27.8) | 24.3 (27.3) | <.001b |
| Nonresponders (n = 7)a | ||||||||
| Total | 61.5 (16.7) | 76.3 (5.9) | 14.8 (16.4) | .42 | 59.0 (31.0) | 57.2 (35.0) | −1.8 (25.6) | .85 |
| Physical | 78.0 (14.9) | 91.1 (4.7) | 13.1 (19.3) | .42 | 62.5 (35.3) | 62.1 (37.8) | −0.5 (30.8) | .86 |
| Mental | 47.0 (19.6) | 60.7 (15.6) | 13.7 (16.2) | .18 | 54.9 (27.7) | 50.0 (33.2) | −4.9 (23.3) | .85 |
| Social | 54.2 (15.7) | 79.2 (3.6) | 25.0 (16.5) | .18 | 60.9 (33.2) | 65.6 (34.8) | 4.7 (22.5) | >.99 |
Abbreviations: Cdiff32, Clostridioides difficile Health-related Quality-of-Life Survey; RBL, fecal microbiota, live-jslm (REBYOTA; Rebiotix); SD, standard deviation.
At week 8, 178 patients were classified as responders (125 [97.7%] in the RBL and 53 [93.0%] in the placebo arm), and 7 were classified as nonresponders (3 [2.3%] in the RBL and 4 [7%] in the placebo arm).
Significant at P < .05.
Among the small number of nonresponders, numerical improvements from baseline to week 8 were observed for RBL-treated patients across all 4 Cdiff32 scores (Table 4). Although nonresponders comprised only a small number of patients (n = 7), all RBL-treated nonresponders had double-digit improvements in Cdiff32 scores (with changes [increases] in scores ranging from 13.1 to 25.0), while the placebo-treated nonresponders had lower Cdiff32 scores (with changes ranging from −4.9 to 4.7).
DISCUSSION
Patient-reported outcomes are important, independent clinical trial end points that provide a multidimensional view on the benefits of a particular treatment on patients’ overall health that can inform clinical and regulatory decisions [24]. HRQL is particularly relevant for CDI given its negative impact on patients’ physical and psychological well-being [6, 12, 25–29]. The efficacy of RBL for the prevention of rCDI has been proved in clinical trials [19, 23]. In this analysis of the phase 3, PUNCH CD3 clinical trial, patients with rCDI had a greater improvement in CDI-related quality of life when given RBL compared with placebo over the 8-week double-blind period. Improvements in HRQL were also observed in patients treated with another microbiome therapeutic during an 8-week trial period [30]. These data indicate that a microbiota-based live biotherapeutic treatment can not only improve the clinical benefit of reduced recurrence but can also improve HRQL, an important outcome for patients, clinicians, and regulatory agencies.
A positive and interesting finding from this study is that the improved HRQL with RBL versus placebo occurred despite the fact that most persons with further CDI episodes were excluded from this analysis owing to lack of data on Cdiff32. Inclusion criteria for this analysis required patients to remain blinded and complete baseline and week 8 Cdiff32 surveys. In the PUNCH CD3 trial, patients experiencing further CDI episodes were given the opportunity to receive an open-label dose of RBL at the investigator's discretion; this resulted in very few nonresponders in our analysis. Nevertheless, HRQL benefit with RBL was still observed, suggesting that greater HRQL benefits may be observed in real-world studies with RBL including both responders and nonresponders, although this hypothesis will need to be tested. These data suggest that the improved HRQL may be multicausal and that RBL may provide benefits beyond reduced CDI recurrence. CDI is precipitated by a disrupted microbiome associated with increased neurotransmitters, which play a role in anxiety and depression (eg, serotonin or gamma aminobutyric acid) [31, 32]. For example, mice without gut microbiota exhibit increased anxiety-related behavior on exposure to stressors [33]. Ingestion of live microbiota regulated emotional behavior and changed GABA receptor activity in a mouse [34]. How RBL modulates these neurotransmitters and provides improved HRQL will require further research.
The current study is among the first to analyze HRQL in patients with rCDI treated with RBL, a novel broad-spectrum microbiota restoration therapy. We used the Cdiff32, a CDI-specific survey originally developed using the SF-36 Health Survey as a comparator and validated in a separate study [23], which allowed us to capture patients’ HRQL with a comprehensive assessment of their quality of life. With other clinical trials noting positive benefits of using the Cdiff32 in clinical trials [30, 35], we propose that the Cdiff32 become a standard HRQL measurement survey in CDI clinical trials where appropriate.
Strengths and Limitations
This was a secondary analysis from a large, phase 3, double-blind, randomized clinical trial. We used the subset of patients from the mITT population who completed the Cdiff32 survey at baseline and at week 8. We did lose considerable sample size owing to the open-label treatment option for nonresponders; however, this provided us with a novel finding related to HRQL improvement among responders. Although the subanalysis of nonresponders provided interesting results, further research is warranted given the very small sample size in this group. We did not consider a missing data imputation approach (eg, last observation carried forward) so as to keep the data reported directly by patients intact, particularly given the proportion of patients without such data at week 8. Patients included in this study exhibited demographic characteristics (age, sex, and ethnicity) comparable to those in the overall mITT trial population. Although we studied patients up to 8 weeks after dosing, the durability of these changes beyond this time period will require further evaluation. Finally, because patients enrolled in clinical trials may differ from those in practice, the generalizability of the study results may be limited.
In conclusion, in this secondary analysis of a randomized clinical trial (PUNCH CD3), treatment with RBL, compared with placebo, was associated with more sustained and profound improvements in overall HRQL—specifically, in physical and mental health status. Our findings indicated that live biotherapeutic products such as RBL can positively affect patients’ HRQL, providing comprehensive benefit in the treatment of rCDI.
Acknowledgments
Medical writing assistance was provided by Yipeng Gao, PhD, and Janice Imai, employees of Analysis Group.
Author contributions. Conceptualization: A. G., M. Y., V. G. H., M. F., and H. W. Methodology: A. G., M. Y., V. G. H., M. F., and H. W. Data curation: A. G., A. H., and L. L. B. Validation: M. Y., V. G. H., M. F., and H. W. Formal analysis: M. Y., V. G. H., M. F., and H. W. Writing—original draft preparation: All authors. Writing—review and editing: All authors.
Data availability. The data sets generated and/or analyzed during the current study are not publicly available owing to the sensitive nature of the data.
Financial support. This work was supported by Ferring Pharmaceuticals.
Contributor Information
Kevin W Garey, University of Houston, Houston, Texas, USA.
Erik R Dubberke, Washington University, St Louis, Missouri, USA.
Amy Guo, Ferring Pharmaceuticals, Parsippany, New Jersey, USA.
Adam Harvey, Rebiotix, a Ferring Company, Roseville, Minnesota, USA.
Min Yang, Analysis Group Inc., Boston, Massachusetts, USA.
Viviana García-Horton, Analysis Group, Inc., New York, New York, USA.
Mirko Fillbrunn, Analysis Group Inc., Boston, Massachusetts, USA.
Hongjue Wang, Analysis Group Inc., Boston, Massachusetts, USA.
Glenn S Tillotson, GST Micro, North, Virginia, USA.
Lindy L Bancke, Rebiotix, a Ferring Company, Roseville, Minnesota, USA.
Paul Feuerstadt, Yale University School of Medicine, New Haven, Connecticut, USA; PACT-Gastroenterology Center, New Haven, Connecticut, USA.
References
- 1.Centers for Disease Control and Prevention. [. Accessed 23 January 2023.]. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf Antibiotic resistance threats in the United States, 2019. Available at:
- 2. Guh AY, Mu Y, Winston LG, et al. Trends in US burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho JM, Pardi DS, Khanna S, eds. Update on treatment of Clostridioides difficile infection. Mayo Clinic Proc 2020; 95:758–69. [DOI] [PubMed] [Google Scholar]
- 4. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hengel RL, Schroeder CP, Jo J, et al. Recurrent Clostridioides difficile infection worsens anxiety-related patient-reported quality of life. J Patient-Rep Outcomes 2022; 6:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alrahmany D, Ereshefsky BJ, El Nekidy WS, Harb G, Pontiggia L, Ghazi IM. Risk factors for recurrence of Clostridioides difficile in hospitalized patients. J Infect Public Health 2021; 14:1642–9. [DOI] [PubMed] [Google Scholar]
- 8. Cornely OA, Miller MA, Louie TJ, Crook DW, Gorbach SL. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(suppl 2):S154–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leong C, Zelenitsky S. Treatment strategies for recurrent Clostridium difficile infection. Can J Hosp Pharm 2013; 66:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med 2021; 9:2050312120986733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olsen M, Yan Y, Reske K, Zilberberg M, Dubberke E. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect 2015; 21:164–70. [DOI] [PubMed] [Google Scholar]
- 12. Heinrich K, Harnett J, Vietri J, Chambers R, Yu H, Zilberberg M. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Dig Dis Sci 2018; 63:2864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guillemin I, Marrel A, Lambert J, et al. Patients’ experience and perception of hospital-treated Clostridium difficile infections: a qualitative study. Patient-Patient-Centered Outcomes Res 2014; 7:97–105. [DOI] [PubMed] [Google Scholar]
- 14. Lurienne L, Bandinelli PA, Galvain T, Coursel CA, Oneto C, Feuerstadt P. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient-Rep Outcomes 2020; 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bancke L, Su X. Efficacy of investigational microbiota-based live biotherapeutic RBX2660 in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical studies. Open Forum Infect Dis 2021; 8(suppl 1):S100–1. [Google Scholar]
- 16. Braun T, Guthmueller B, Harvey AJ. Safety of investigational microbiota-based live biotherapeutic RBX2660 in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical studies. Open Forum Infect Dis 2021; 8(suppl 1):S611. [Google Scholar]
- 17. Orenstein R, Dubberke ER, Khanna S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis 2022; 22:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orenstein R, Dubberke E, Hardi R, et al. Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection: results of the PUNCH CD study. Clin Infect Dis 2016; 62:596–602. [DOI] [PubMed] [Google Scholar]
- 19. Dubberke ER, Lee C, Orenstein R, Khanna S, Hecht G, Fraiz J. Efficacy and safety of RBX2660 for the prevention of recurrent Clostridium difficile infection: results of the PUNCH CD 2 trial. Open Forum Infect Dis 2016. ; 3(suppl 1):1341. [Google Scholar]
- 20. Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol 2016; 50:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadeghi K, Downham G, Nhan E, Reilly J, Kardos A. Multiple recurrent Clostridiodes difficile infections: an evaluation of patient cases and economic impact at a community teaching hospital [poster]. Atlantic City, NJ: AtlantiCare Regional Medical Center, 2022. [Google Scholar]
- 22. Khanna S, Assi M, Lee C, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs 2022; 82; 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lapin B, Garey K, Wu H, et al. PCR150 validation of a health-related quality of life questionnaire in patients with recurrent Clostridioides difficile infection in ECOSPOR-III, a phase 3 randomized trial. Value Health 2022; 25:S569. [DOI] [PubMed] [Google Scholar]
- 24. Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 2018; 9:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes 2019; 17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han Z, Lapin B, Garey KW, Donskey CJ, Deshpande A. Impact of Clostridioides difficile infection on patient-reported quality of life. Infect Control Hosp Epidemiol 2022; 43:1339–1344. [DOI] [PubMed] [Google Scholar]
- 27. Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, Marcil-Héguy A, Nault V, Valiquette L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis 2016; 62:574–80. [DOI] [PubMed] [Google Scholar]
- 28. Vent-Schmidt J, Attara GP, Lisko D, Steiner TS. Patient experiences with Clostridioides difficile infection: results of a Canada-wide survey. Patient Prefer Adherence 2020; 14:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 30. Garey KW, Jo J, Gonzales-Luna AJ, et al. Assessment of quality of life among patients with recurrent Clostridioides difficile infection treated with investigational oral microbiome therapeutic SER-109: secondary analysis of a randomized clinical trial. JAMA Network Open 2023; 6:e2253570-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu L, Zhu G. Gut–brain axis and mood disorder. Front Psychiatry 2018; 9:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maiuolo J, Gliozzi M, Musolino V, et al. The contribution of gut microbiota–brain axis in the development of brain disorders. Front Neurosci 2021; 15:616883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crumeyrolle-Arias M, Jaglin M, Bruneau A, et al. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 2014; 42:207–17. [DOI] [PubMed] [Google Scholar]
- 34. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011; 108:16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kao D, Wong K, Franz R, et al. The effect of a microbial ecosystem therapeutic (MET-2) on recurrent Clostridioides difficile infection: a phase 1, open-label, single-group trial. Lancet Gastroenterol Hepatol 2021; 6:282–91. [DOI] [PubMed] [Google Scholar]