Abstract
Background
An estimated 2.4 million babies died within the first 28 days of life in 2020. The third leading cause of neonatal death continues to be neonatal sepsis. Sepsis-causing bacterial pathogens vary temporally and geographically and, with a rise in multidrug-resistant organisms (MDROs), pose a threat to the neonatal population.
Methods
This was a single-center, retrospective study of very low birth weight (VLBW) infants with late-onset sepsis (LOS) admitted to a neonatal unit in South Africa. We aimed to calculate the prevalence of multidrug-resistant (MDR) infections in this population. The data collected included demographic and clinical characteristics, length of hospital stay, risk factors for MDRO and mortality, and microbiology results. Logistic regression was used to assess the association between prespecified risk factors with MDR infections and mortality.
Results
Of 2570 VLBW infants admitted, 34% had LOS, of which 33% was caused by MDROs. Infection with Acinetobacter spp., Pseudomonas spp., extended-spectrum beta-lactamase Klebsiella spp., or Escherichia coli was associated with the highest mortality in the LOS cohort. Infants with congenital infections (adjusted odds ratio [aOR], 5.13; 95% CI, 1.19–22.02; P = .028) or a history of necrotizing enterocolitis (aOR, 2.17; 95% CI, 1.05–4.49; P = .037) were at significantly higher risk for MDR infections.
Conclusions
More than one-third of LOS cases in VLBW infants were caused by MDROs in this study. MDR infections cause substantial neonatal mortality. Antimicrobial stewardship programs, infection control protocols, and ongoing surveillance are needed to prevent further emergence and spread of MDR infections worldwide.
Keywords: neonatal sepsis, sub-Saharan Africa, very low birthweigth infants
Despite significant progress in decreasing global childhood mortality rates in the last 3 decades, the progress in decreasing neonatal mortality rates (NMRs) has plateaued. In 2020 alone, 2.4 million babies died within the first month of life [1, 2]. Disparities in NMRs exist across regions and countries, with Sub-Saharan Africa and Southeast Asia accounting for 79% of the total burden of neonatal deaths [2, 3]. In South Africa, for example, the neonatal mortality rate in 2018 was 11 deaths for every 1000 live births, compared with 3.5 deaths for every 1000 live births in the United States [4]. Furthermore, reported NMRs in low- to middle-income countries (LMICs) are likely inaccurate. South African databases and global agencies report neonatal death rates; however, these estimates are variable, which highlights the need for more accurate methods, such as additional estimation techniques [5, 6].
Among neonates, sepsis continues to be the third leading cause of global mortality, preceded by prematurity and intrapartum-related events [7]. These top 3 global causes of neonatal death have remained the same for decades. Rhoda et al. reported causes of neonatal death in South Africa from the years 2012 to 2016 and estimated that ∼13% of all neonatal deaths among babies weighing >1 kg were due to infection [5]. The bacterial pathogens responsible for such infections change, both geographically and temporally [8]. Although epidemiological data from low- to middle-income countries (LMICs) are insufficient at this time, we have strong evidence to suggest that different causative organisms exist among developed and developing countries [9, 10]. As the microbiological patterns change with time, this ongoing phenomenon gives rise to pathogens resistant to first-line antibiotic therapy, impacting morbidity and mortality of neonates today and in the future.
The emergence of multidrug-resistant (MDR) infections that began in the mid-1990s has now become an urgent matter. The World Health Organization (WHO) has claimed antimicrobial resistance (AMR) as one of the top 10 global public health threats facing humanity [11]. A major challenge to addressing AMR is understanding the true burden, especially in regions where data and surveillance are limited [12]. In a South African neonatal unit, Ballot et al. reviewed multidrug-resistant Enterobacterales (MDRE) and found an increasing number of MDRE isolates between 2013 and 2015 and the emergence of carbapenem-resistant Enterobacterales [13]. Many neonatal intensive care units (NICUs) across other LMICs experienced a similar emergence of resistant pathogens; therefore, interventions to help delay the emergence and outbreaks of resistant pathogens are a pressing matter [14–16]. Infection prevention and surveillance of neonatal sepsis are crucial to conserve the efficacy of currently used antibiotics and delay the progression of AMR [17–19]. Furthermore, identifying risk factors for the development of antimicrobial-resistant infections among neonates, specifically the very low birthweight (VLBW) infant population, will help us in our effort to prevent significant morbidity and mortality. Additionally, epidemiological studies in LMICs are crucial in estimating the impact AMR has on NMRs and reducing existing disparities [20].
This study aims to estimate the prevalence of MDR infections in VLBW infants with late-onset sepsis (LOS) in the NICU at the Charlotte Maxeke Academic Hospital in Johannesburg, South Africa. Additionally, we aim to identify risk factors associated with MDR infections and increased mortality and to identify the distribution of bacterial pathogens and their associated mortality rate in infants with LOS.
METHODS
Study Design and Patients
In this single-center, retrospective cohort study, we identified VLBW infants admitted to the NICU at Charlotte Maxeke Academic Hospital in Johannesburg, South Africa, between January 2015 and December 2020. Infants with a birth weight between 401 and 1500 g and a gestational age >22 weeks who were admitted within 48 hours of birth were included. We identified infants with LOS (n = 869) and those with multidrug-resistant organisms (MDROs), as defined below. All infants with positive blood and cerebrospinal fluid (CSF) cultures meeting MDRO definitions were included. Infants with an isolated positive urine and/or respiratory culture were excluded unless a concomitant blood and/or CSF infection was detected. Patients with >1 episode of LOS were classified as infants with MDRO if that patient had at least 1 bacterial isolate that met criteria as MDRO in any 1 episode of LOS. If a patient cultured the same pathogen repeatedly, the bacterial pathogen was only included in the data analysis a single time.
Data Collection
We analyzed data collected from an existing neonatal database created in Research Electronic Data Capture (REDCap) and hosted by the University of Witwatersrand [21]. The data collected included maternal/neonatal demographic and clinical characteristics, length of stay, risk factors for MDRO and mortality, microbiology results, and all-cause mortality.
Definitions
Infants classified as “outborn” were born at home or at a different facility and referred to the Charlotte Maxeke Hospital NICU. Early-onset sepsis (EOS) was defined as culture-proven sepsis within 72 hours of life. Late-onset sepsis (LOS) was defined as a single positive blood or CSF culture after 72 hours of life, including infants with coagulase-negative Staphylococcus (CoNS) spp. Bacterial isolates classified as MDRO included methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacterales, extended-spectrum beta-lactamase (ESBL)–producing Klebsiella species, or any gram-negative bacterium with nonsusceptibility to at least 1 antimicrobial drug in 3 or more classes. Further, patients were subclassified into 2 groups: (1) MDRO infants with at least 1 bacterial isolate that met criteria for MDRO and (2) non-MDRO infants with bacterial isolates that did not meet criteria for MDRO. Necrotizing enterocolitis (NEC) was defined as modified Bell's stage 2 or 3 [22]. Congenital infection was defined as a child infected at birth with 1 of the following: Toxoplasma gondii, Rubella, Treponema pallidum (syphilis), cytomegalovirus, herpes simplex, Zika, parvovirus b19, or varicella zoster. Mortality was defined as all-cause mortality during hospitalization.
Statistical Analysis
Categorical data, presented by frequency tables and percentages, were compared using Pearson's chi-square test. Continuous variables, presented in terms of medians and interquartile ranges (IQRs), were compared between groups using the Mann-Whitney U test.
We used univariate logistic regression analysis to assess the risk factors associated with mortality in the LOS cohort and to compare baseline variables between MDRO and non-MDRO patients within the LOS cohort. We then used multivariate logistic regression to investigate the risk factors associated with LOS caused by MDRO, adjusted for birthweight, gestational age, sex, and length of stay in the NICU. Variables included in the final model were prespecified and selected based on clinical knowledge and literature review. Odds ratios (ORs) and 95% CIs were calculated. A P value <.05 was interpreted as statistically significant. All statistical analyses were performed using Stata (version 16.1; StataCorp LLC, College Station, TX, USA).
Patient Consent
This study did not require patient consent and was reviewed and approved by the Human Research Ethics Committee of the University of the Witwatersrand and the Institutional Review Board of Vanderbilt University Medical Center.
RESULTS
A total of 2780 VLBW infants were admitted to the Charlotte Maxeke NICU during the study period (Table 1). Two hundred ten infants (7.5%) did not meet inclusion criteria and were excluded from the study (gestational age <22 weeks n = 7 and transferred to outside facility n = 203). Of the 2570 VLBW infants who were included, the median maternal age was 29 years. Of the mothers, 1927 (75%) attended at least 1 antenatal care visit before delivery. Approximately 30% (n = 780) of mothers had known HIV infection, of whom ∼93% were receiving antiretroviral therapy. Maternal hypertension was also common (26%).
Table 1.
Maternal and Neonatal Characteristics
| Total (n = 2570) | No. (%) |
|---|---|
| Maternal characteristics | |
| Maternal age, median [IQR], y | 29 [24–33] |
| Antenatal care | |
| Yes | 1927 (75) |
| No | 512 (19.9) |
| Unknown | 131 (5.1) |
| Chorioamnionitis | 68 (2.6) |
| Maternal hypertension (chronic or PIH) | 673 (26.2) |
| Maternal HIV infection | 780 (30.4) |
| Maternal antiretroviral therapy | 728 (93.3) |
| Maternal syphilis (RPR positive) | 51 (2) |
| Maternal tuberculosis | 21 (<1) |
| Maternal diabetes | 32 (1.2) |
| Prenatal steroids | 695 (27) |
| Neonatal characteristics | |
| Sex | |
| Male | 1225 (47.7) |
| Female | 1345 (52.3) |
| Gestational age, median [IQR], wk | 29 [27–30] |
| Birth weight, median [IQR], g | 1100 [900–1300] |
| Total days in NICU, median [IQR] | 28 [11–47] |
| Place of birth | |
| Inborn | 2122 (82.6) |
| Outborna | 440 (17.2) |
| Major birth defect | 57 (2.2) |
| Congenital HIV | 19 (<1) |
| Congenital Infection | 45 (1.8) |
| LOS | 869 (33.8) |
| LOS caused by MDRO | 287 (33) |
| Death | 872 (34) |
Abbreviations: CMJAH, Charlotte Maxeke Johannesburg Academic Hospital; IQR, interquartile range; LOS, late-onset sepsis; MDRO, multidrug-resistant organism; NICU, neonatal intensive care unit; PIH, pregnancy induced hypertension; RPR, rapid plasma reagent.
Children classified as “outborn” were born at home or a different facility and referred and admitted to CMJAH.
Among the infants enrolled, 52% were female. The median birth weight was 1100 g, and the median length of time in the NICU was 28 days. Approximately 34% (n = 869) were characterized as having LOS, with a prevalence of 287 (33%) LOS cases caused by MDROs. The prevalence of LOS caused by MDROs in the entire cohort was ∼11%. A total of 872 (34%) VLBW infants died during their hospitalization, compared with 119 (41%) among the infants with LOS caused by MDROs.
Types of Pathogens
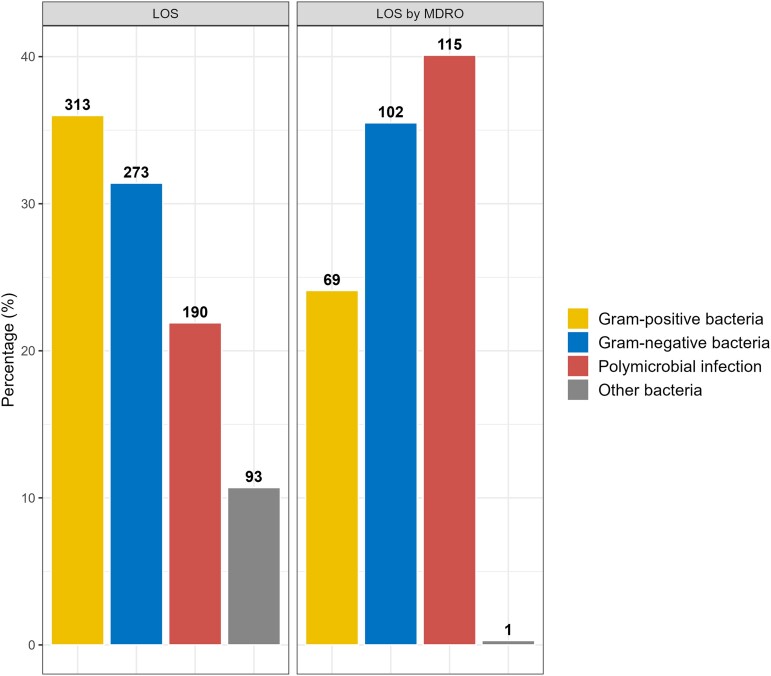
A total of 1305 bacterial pathogens were isolated from 869 patients classified as LOS; 313 (36%) were gram-positive bacteria, 273 (31.4%) gram-negative bacteria, 190 (21.9%) polymicrobial, and 93 (10.7%) other bacteria (Figure 1). The most common bacterial isolate was CoNS (333/1305; 25.5%), followed by Klebsiella spp. (274/1305; 21%), Acinetobacter spp. (158/1305; 12%), and MRSA (130/1305; 10%). The most common bacterial isolates causing polymicrobial infections were CoNS (121/190; 64%), Klebsiella spp. (118/190; 62%), and MRSA (61/190; 32%). The 2 most common bacterial isolates categorized as “other bacteria” were Serratia spp. (29/133; 22%) and extremely drug-resistant (XDR) Acinetobacter baumannii (25/133; 19%).
Figure 1.
Pathogen distribution in LOS and MDRO groups. Abbreviations: LOS, late-onset sepsis; MDRO, multidrug-resistant organism.
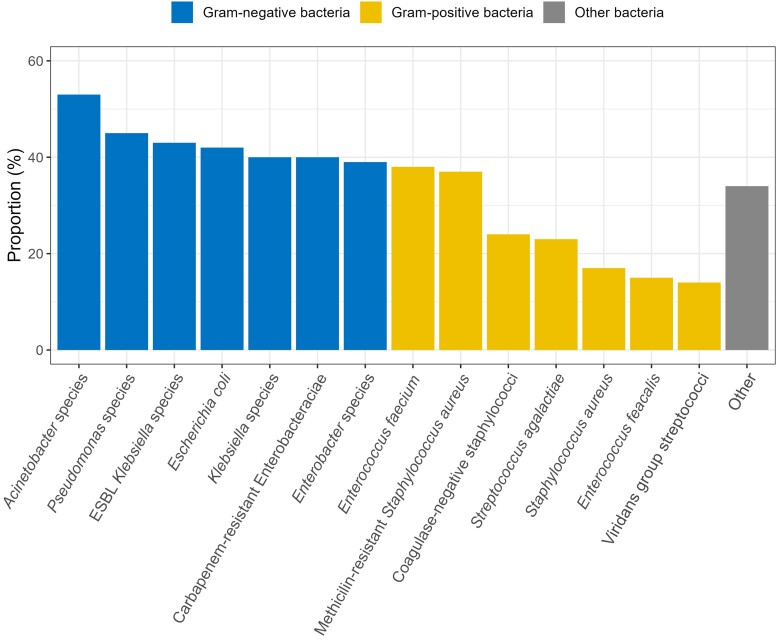
Among the LOS cohort, the highest mortality occurred among patients infected with gram-negative bacteria. Acinetobacter spp., Pseudomonas spp., ESBL Klebsiella spp., and Escherichia coli were the pathogens with the highest mortality, at 53%, 45%, 43%, and 42%, respectively (Figure 2).
Figure 2.
Proportion of deaths in LOS by isolated bacterial pathogens. Abbreviations: ESBL, extended-spectrum beta-lactamase; LOS, late-onset sepsis.
Two hundred eighty-seven (33%) of the 869 patients with LOS had MDRO infections. The pathogen distribution in the MDRO subgroup consisted of 69 (24.1%) gram-positive bacteria, 102 (35.5%) gram-negative bacteria, 115 (40.1%) polymicrobial infections, and 1 (0.3%) other bacterium (Figure 1).
Mortality Risk Factors
We assessed the risk factors for all-cause mortality in this cohort of VLBW infants with LOS (Table 2). Univariate logistic regression revealed a 12% lower likelihood of death for each 1-week increase in gestational age (OR, 0.88; 95% CI, 0.82–0.94; P = .001) and a roughly 20% lower likelihood of death for each 100-g increase in birthweight (OR, 0.81; 95% CI, 0.75–0.87; P = .001). VLBW infants who received respiratory support at 36 weeks had a 74% lower likelihood of mortality (OR, 0.26; 95% CI, 0.18–0.37; P = .001). Infants with LOS who required mechanical ventilation during their hospitalization had a nearly 3.5-fold higher likelihood of death (OR, 3.56; 95% CI, 2.67–4.75; P ≤ .001), while those diagnosed with a congenital infection had a roughly 4-fold higher likelihood of death (OR, 4.29; 95% CI, 1.10–16.7; P = .036). Infants with LOS who had a prior episode of EOS were 1.58 times more likely to die, though this was not statistically significant (OR, 1.58; 95% CI, 0.92–2.70; P = .094).
Table 2.
Neonatal Risk Factors and Mortality in LOS Cohort
| OR | 95% CI | P Value | |
|---|---|---|---|
| Gestational age (per 1-wk increase) | 0.88 | [0.82–0.94] | <.001 |
| Birth weight (per 100-g increase) | 0.81 | [0.75–0.87] | <.001 |
| Sex | |||
| Female | 1.0 | [0.76–1.32] | .979 |
| Male | 1.0 | [0.76–1.32] | .979 |
| Resuscitation in delivery room | 0.89 | [0.62–1.28] | .545 |
| Respiratory support after initial resuscitation | 2.86 | [0.33–24.6] | .338 |
| Respiratory support at 36 wk | 0.26 | [0.18–0.37] | <.001 |
| Early-onset sepsis | 1.58 | [0.92–2.70] | .094 |
| Mechanical ventilation | 3.56 | [2.67–4.75] | <.001 |
| Maternal chorioamnionitis | 0.96 | [0.42–2.20] | .933 |
| Maternal HIV | 1.12 | [0.83–1.50] | .468 |
| Congenital HIV | 1.35 | [0.30–6.1] | .693 |
| Congenital infectiona | 4.29 | [1.10–16.7] | .036 |
| Fungal sepsis | 1.05 | [0.73–1.50] | .791 |
Abbreviations: LOS, late-onset sepsis; OR, odds ratio.
Toxoplasmosis (Toxoplasma gondii), rubella virus, syphilis (Treponema pallidum), cytomegalovirus, herpes simplex, parvovirus B19, Zika virus, varicella zoster virus.
MDRO Risk Factors
Among the cohort of infants with LOS, we compared infants with MDRO infections with those with non-MDRO infections (Table 3). Infants in the MDRO group had a longer median length of stay (48 days) compared with the non-MDRO group (43 days; P = .004). The proportion of infants who died before discharge was higher in the MDRO group when compared with the non-MDRO group, 41% and 34%, respectively (P = .036). The MDRO group had more infants with the following diagnoses: patent ductus arteriosus (25%; P = .01), respiratory support at 36 weeks (37%; P < .001), and NEC (27%; P < .001). There was a higher proportion of infants with EOS and fungal sepsis in the MDRO group, though neither was statistically significant (7% vs 6% and 22% vs 16%, respectively).
Table 3.
Neonatal Characteristics and Risk Factors by MDRO Status
| Variables | MDRO (n = 287), No. (%) |
Non-MDRO (n = 582), No. (%) |
P Value |
|---|---|---|---|
| Clinical characteristics | |||
| Sex | |||
| Male | 138 (32) | 296 (68) | .592 |
| Female | 149 (34) | 286 (66) | … |
| Gestational age, median [IQR], wk | 28.5 [27–30] | 28 [27–30] | .267 |
| Birth weight, median [IQR], g | 1020 [890–1230] | 1070 [905–1230] | .227 |
| Length of stay, median [IQR], d | 48 [25–70] | 43 [20–62] | .004 |
| Death before discharge | 119 (41) | 199 (34) | .036 |
| Risk factors | |||
| Respiratory distress syndrome | 262 (91) | 536 (92) | .652 |
| Patent ductus arteriosus | 71 (25) | 101 (18) | .010 |
| Ibuprofen treatment | 28/71 (39) | 31/101 (31) | .015 |
| PDA ligation | – | 2/101 (2) | – |
| Respiratory support at 36 wk | 107 (37) | 139 (24) | <.001 |
| Steroids for chronic lung disease | 89 (33) | 161 (30) | .315 |
| Necrotizing enterocolitis (stage 2 or 3) | 78 (27) | 98 (17) | <.001 |
| NEC surgery | 33/78 (42) | 32/98 (33) | .002 |
| Congenital HIV (positive birth PCR) | 4 (1.6) | 3 (0.6) | .177 |
| Early-onset sepsis | 21 (7) | 37 (6) | .574 |
| Fungal sepsis | 62 (22) | 94 (16) | .056 |
| Any surgery | 47 (16) | 65 (11) | .031 |
Abbreviations: IQR, interquartile range; MDRO, multidrug-resistant organism; NEC, necrotizing enterocolitis; PCR, polymerase chain reaction; PDA, patent ductus arteriosus.
In multivariate logistic regression, we assessed prespecified maternal and neonatal risk factors that are associated with LOS caused by MDRO infections (Table 4). When adjusted for birth weight, gestational age, sex, and length of NICU stay, only congenital infection (aOR, 5.13; 95% CI, 1.19–22.02; P = .028) and prior history of NEC (aOR, 2.17; 95% CI, 1.05–4.49; P = .037) were significantly associated with being diagnosed with an MDRO infection.
Table 4.
Maternal/Neonatal Risk Factors Associated With LOS Caused by MDRO
| aOR | 95% CI | P Value | |
|---|---|---|---|
| Maternal chorioamnionitis | 0.76 | [0.07–7.84] | .817 |
| Maternal HIV | 1.02 | [0.56–1.85] | .952 |
| Respiratory distress syndrome | 1.59 | [0.43–5.90] | .491 |
| Mechanical ventilation | 0.89 | [0.50–1.59] | .705 |
| Respiratory support at 36 wk | 1.27 | [0.691–2.35] | .438 |
| Early-onset sepsis | 0.54 | [0.16–1.91] | .343 |
| Fungal sepsis | 0.76 | [0.35–1.66] | .497 |
| Congenital infection | 5.13 | [1.19–22.02] | .028 |
| Necrotizing enterocolitis | 2.17 | [1.05–4.49] | .037 |
| Patent ductus arteriosus | 0.90 | [0.44–1.85] | .782 |
Abbreviations: aOR, adjusted odds ratio (adjusted for birth weight, gestational age, sex, length of hospital stay); LOS, late-onset sepsis; MDRO, multidrug-resistant organism.
DISCUSSION
In this study, we found that VLBW infants admitted to a representative NICU in South Africa had a significantly high mortality rate of 34%. One-third of the VLBW infants with LOS were infected with an MDRO. South African studies have shown increasing AMR rates and AMR’s impact on morbidity and mortality across NICUs [13, 23, 24].
Gram-positive bacteria were the most common LOS isolates, and this was largely due to isolation of CoNS affecting 25% of all infants with LOS. Despite the possibility of blood culture contamination with skin commensal flora, VLBW infants contribute disproportionately to CoNS-related mortality, in contrast to full-term infants who usually suffer milder symptoms [25, 26]. If CoNS isolates were excluded from the analysis, Acinetobacter spp. were the leading cause of LOS in this cohort. Extensively resistant Acinetobacter arising in NICUs worldwide is now one of the WHO's top priorities for research and development of new antibiotics. Several studies have identified risk factors associated with extensively drug-resistant Acinetobacter baumannii infections (XDRABIs) in neonatal units. A. baumannii is a difficult pathogen to eradicate once present in a hospital setting given its ability to form strong biofilms [27]. Perovic et al. reported >4800 cases of A. baumannii from 2017 to 2019, with the majority (60%) of cases from Gauteng province and 41% occurring in infants [28]. Length of stay, use of antimicrobials (specifically glycopeptides and aminoglycosides), mechanical ventilation, use of total parenteral nutrition (TPN), and the presence of central venous access have been identified as independent risk factors for XDRABIs [29–31]. Although gram-positive bacteria were most commonly isolated in this cohort of VLBWs with LOS, the highest mortality occurred with gram-negative infections, which is consistent with other studies [32]. When we studied the subpopulation of infants with MDR infections, polymicrobial infections were the most common, followed by gram-negative bacteria and, third, gram-positive bacteria. The distribution of pathogens was different among the subgroup with MDR infections when compared with the LOS cohort. The main pathogens responsible for LOS at any given institution can change with time, allowing for the emergence of dangerous MDROs. Establishing infection control protocols, antimicrobial stewardship programs, and ongoing surveillance are key components to combatting the threat these MDROs pose on VLBW infants.
When we studied prespecified risk factors associated with mortality among the LOS cohort, the younger, smaller infants and those requiring mechanical ventilation had a higher risk of mortality, as expected. Interestingly, infants requiring respiratory support at 36 weeks postmenstrual age (PMA) were significantly less likely to die. Further analysis revealed that most infants who died within this cohort were <36 weeks PMA, meaning that the infants who reached 36 weeks were more likely to survive to discharge. Among the subgroup of infants with MDRO infections, infants with congenital infections and those with a history of NEC were associated with development of an MDRO infection. Infants with congenital infections and NEC are usually exposed to prolonged antimicrobial therapy that can last several weeks. Prolonged antimicrobial therapy can lead to increasing rates of NEC [33], antimicrobial resistance, altered intestinal microbiome, and mortality in premature infants.
This study had several limitations as it was conducted at a single center. The available data were limited and did not include patient information on important risk factors associated with LOS such as central vascular access and antimicrobial exposure. Additionally, we did not describe the susceptibility patterns for all isolates. All CoNS isolates were included in the study given that the data-collecting method was not robust enough to differentiate a contaminant from CoNS-related sepsis. Lastly, paper charts were used for data collection at the time of discharge; therefore, temporal information was not available to conduct a time-to-event analysis for bacterial pathogens and the outcome of mortality. For example, if a patient had >1 episode of LOS with >1 bacterial pathogen isolated, we could not determine which bacterial pathogen was associated with the outcome of interest, mortality.
In conclusion, our study showed that LOS caused by MDROs is a serious challenge in NICUs of developing countries. More than one-third of VLBW infants with LOS had at least 1 MDRO isolated from a blood or CSF culture. From our data, we conclude that gram-negative isolates have the highest burden of mortality in this patient population, which coincides with previous studies. Antimicrobial stewardship programs, infection control protocols, ongoing surveillance, rapid diagnostic tests, and novel treating agents for MDROs are crucial. Research efforts should prioritize the development of new antibiotics and should include the neonatal population in the dosing evaluations needed for these efforts to impact the MDRO burden among these infants. Additionally, the implementation of electronic medical records, electronic prescribing, and adaptation of standardized and comprehensive reporting in LMICs would help monitor the burden of LOS and MDR infections.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Contributor Information
Genesis Licona, Division of Neonatology, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Daynia Ballot, Division of Neonatology, Department of Pediatrics, Charlotte Maxeke Academic Hospital, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Troy D Moon, Division of Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Ritu Banerjee, Division of Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Gustavo Amorim, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Alexander G Agthe, Division of Neonatology, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jörn-Hendrik Weitkamp, Division of Neonatology, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1. World Health Organization. Newborn mortality . Available at: https://www.who.int/news-room/fact-sheets/detail/levels-and-trends-in-child-mortality-report-2021. Accessed May 20, 2022.
- 2. You D, Hug L, Ejdemyr S, et al. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet 2015; 386:2275–86. [DOI] [PubMed] [Google Scholar]
- 3. Sharrow D, Hug L, You D, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob Health 2022; 10:e195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Bank . World Bank open data. Available at: https://data.worldbank.org. Accessed June 13, 2023.
- 5. Rhoda N, Velaphi S, Gebhardt GS, Kauchali S, Barron P. Reducing neonatal deaths in South Africa: progress and challenges. S Afr Med J 2018; 108(3a):s9. [Google Scholar]
- 6. Damian DJ, Njau B, Lisasi E, Msuya SE, Boulle A. Trends in maternal and neonatal mortality in South Africa: a systematic review. Syst Rev 2019; 8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wattal C, Kler N, Oberoi JK, Fursule A, Kumar A, Thakur A. Neonatal sepsis: mortality and morbidity in neonatal sepsis due to multidrug-resistant (MDR) organisms: part 1. Indian J Pediatr 2020; 87:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaidi AKM, Thaver D, Ali SA, Khan TA. Pathogens associated with sepsis in newborns and young infants in developing countries. Pediatr Infect Dis J 2009; 28:S10–8. [DOI] [PubMed] [Google Scholar]
- 10. Zelellw DA, Dessie G, Worku Mengesha E, Balew Shiferaw M, Mela Merhaba M, Emishaw S. A systemic review and meta-analysis of the leading pathogens causing neonatal sepsis in developing countries. BioMed Res Int 2021; 2021:6626983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antimicrobial resistance . Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed May 8, 2022.
- 12. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399:629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ballot DE, Bandini R, Nana T, et al. A review of multidrug-resistant Enterobacteriaceae in a neonatal unit in Johannesburg, South Africa. BMC Pediatr 2019; 19:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herindrainy P, Rabenandrasana MAN, Andrianirina ZZ, et al. Acquisition of extended spectrum beta-lactamase-producing Enterobacteriaceae in neonates: a community based cohort in Madagascar. PLoS One 2018; 13:e0193325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berglund B, Hoang NTB, Lundberg L, et al. Clonal spread of carbapenem-resistant Klebsiella pneumoniae among patients at admission and discharge at a Vietnamese neonatal intensive care unit. Antimicrob Resist Infect Control 2021; 10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saleem AF, Ahmed I, Mir F, Ali SR, Zaidi AK. Pan-resistant Acinetobacter infection in neonates in Karachi, Pakistan. J Infect Dev Ctries 2010; 4:30–7. [PubMed] [Google Scholar]
- 17. Dramowski A, Velaphi S, Reubenson G, et al. National Neonatal Sepsis Task Force launch: supporting infection prevention and surveillance, outbreak investigation and antimicrobial stewardship in neonatal units in South Africa. S Afr Med J 2020; 110:360–3. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. WHO strategic priorities on antimicrobial resistance . Available at: https://www.who.int/publications-detail-redirect/9789240041387. Accessed May 25, 2022.
- 19. Centers for Disease Control and Prevention . Addressing health equity across AR threats. Published April 4,2022. Available at: https://www.cdc.gov/drugresistance/solutions-initiative/stories/ar-health-equity.html. Accessed May 25, 2022.
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg 1978; 187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mbelle NM, Feldman C, Sekyere JO, et al. Pathogenomics and evolutionary epidemiology of multi-drug resistant clinical Klebsiella pneumoniae isolated from Pretoria, South Africa. Sci Rep 2020; 10:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reddy K, Bekker A, Whitelaw AC, Esterhuizen TM, Dramowski A, Duse AG. A retrospective analysis of pathogen profile, antimicrobial resistance and mortality in neonatal hospital-acquired bloodstream infections from 2009–2018 at Tygerberg Hospital, South Africa. PLoS One 2021; 16:e0245089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchant EA, Boyce GK, Sadarangani M, Lavoie PM. Neonatal sepsis due to coagulase-negative staphylococci. Clin Dev Immunol 2013; 2013:586076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong Y, Speer CP, Glaser K. Beyond sepsis: Staphylococcus epidermidis is an underestimated but significant contributor to neonatal morbidity. Virulence 2018; 9:621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weinberg SE, Villedieu A, Bagdasarian N, Karah N, Teare L, Elamin WF. Control and management of multidrug resistant Acinetobacter baumannii: a review of the evidence and proposal of novel approaches. Infect Prev Pract 2020; 2:100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perovic O, Duse A, Chibabhai V, et al. Acinetobacter baumannii complex, national laboratory-based surveillance in South Africa, 2017 to 2019. PLoS One 2022; 17:e0271355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar A, Randhawa VS, Nirupam N, Rai Y, Saili A. Risk factors for carbapenem-resistant Acinetobacter baumanii blood stream infections in a neonatal intensive care unit, Delhi, India. J Infect Dev Ctries 2014; 8:1049–54. [DOI] [PubMed] [Google Scholar]
- 29. Tekin R, Yolbaş İ, Bozkurt F, et al. Risk factors for extensively drug-resistant Acinetobacter baumannii in neonatal patients. J Pediatr Infect Dis 2021; 16:031–5. [Google Scholar]
- 30. Al Jarousha AMK, El Jadba AHN, Al Afifi AS, El Qouqa IA. Nosocomial multidrug-resistant Acinetobacter baumannii in the neonatal intensive care unit in Gaza City. Palestine. Int J Infect Dis 2009; 13:623–8. [DOI] [PubMed] [Google Scholar]
- 31. Shah J, Jefferies AL, Yoon EW, Lee SK, Shah PS, Network CN. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks’ gestation. Am J Perinatol 2015; 32:675–82. [DOI] [PubMed] [Google Scholar]
- 32. Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States . 2019. Available at: https://stacks.cdc.gov/view/cdc/82532. Accessed March 15, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.