Abstract
Aims
To study aetiologies of in-hospital cardiac arrests (IHCAs) and their association with 30-day survival.
Methods and results
Observational study with data from national registries. Specific aetiologies (n = 22) of IHCA patients between April 2018 and December 2020 were categorized into cardiac vs. non-cardiac and six main aetiology categories: myocardial ischemia, other cardiac causes, pulmonary causes, infection, haemorrhage, and other non-cardiac causes. Main endpoints were proportions in each aetiology, 30-day survival, and favourable neurological outcome (Cerebral Performance Category scale 1–2) at discharge. Among, 4320 included IHCA patients (median age 74 years, 63.1% were men), approximate 50% had cardiac causes with a 30-day survival of 48.4% compared to 18.7% among non-cardiac causes (P < 0.001). The proportion in each category were: myocardial ischemia 29.9%, pulmonary 21.4%, other cardiac causes 19.6%, other non-cardiac causes 11.6%, infection 9%, and haemorrhage 8.5%. The odds ratio (OR) for 30-day survival compared to myocardial ischemia for each category were: other cardiac causes OR 1.48 (CI 1.24–1.76); pulmonary causes OR 0.36 (CI 0.3–0.44); infection OR 0.25 (CI 0.18–0.33); haemorrhage OR 0.22 (CI 0.16–0.3); and other non-cardiac causes OR 0.56 (CI 0.45–0.69). IHCA caused by myocardial ischemia had the best favourable neurological outcome while those caused by infection had the lowest OR 0.06 (CI 0.03–0.13).
Conclusion
In this nationwide observational study, aetiologies with cardiac and non-cardiac causes of IHCA were evenly distributed. IHCA caused by myocardial ischemia and other cardiac causes had the strongest associations with 30-day survival and neurological outcome.
Keywords: IHCA, CPR, AED, Aetiology
Graphical Abstract
Graphical Abstract.
Introduction
In-hospital cardiac arrest (IHCA) is a major health concern associated with high mortality and neurologic disabilities among survivors. The incidence of IHCA varies between 1.5 and 7 per 1000 hospital admissions depending on country, with a somewhat higher reported incidence in the US compared to Europe.1–4 The 30-day survival among reported IHCA patients varies between 15% and 34%.3–8
To reduce the incidence and improve outcome for patients suffering from IHCA, early recognition of clinical deterioration as well as recognition and treatment of the aetiology of the cardiac arrest is important.9,10 IHCA is often preceded by hours or days of clinical deterioration.10 Identification of the aetiology by the hospital's rapid response team (RRT) is often challenging. In the majority of cases, recognition of the aetiology is based on knowledge of the most common aetiologies of cardiac arrest and symptoms presented before the arrest.11 However, due to the emergency of the situation, information of the specific patient in medical charts and reported by staff is often difficult to fully grasp.
Improved identification of aetiology of IHCA is important as it affects both the potential to use specific treatments and outcome.9,10 Previous studies have shown acute myocardial infarction to be the most common aetiology of IHCA.9,10,12 Approximately 50% of the aetiology in patients with IHCA have in previous studies been of cardiac cause and 50% of non-cardiac cause.11–13 Most cardiac arrest studies are in the setting of out-of-hospital cardiac arrest, focusing on patients with a presumed cardiac cause with an initial shockable rhythm.14–16 A presumed cardiac cause is shown beneficial compared to non-cardiac causes as it is more prone to have an initial shockable rhythm for which defibrillation is an effective initial treatment.2,4,12,17 Patients presenting with an initial non-shockable rhythm are less well studied both in the in-hospital and out-of-hospital setting. These patients are associated with a higher mortality, and it is important to early assess potentially reversible causes to improve outcome in this group.9
Since 2005, the Swedish Registry of Cardiopulmonary Resuscitation (SRCR) for IHCA have collected data in a structured manner regarding aetiologies of the arrest, resuscitation factors, and outcomes in this patient group. In 2018, the aetiology categories for IHCA were expanded from eight to 22 categories to better understand the underlying factors, allowing a more detailed analysis of the causes behind IHCA and the outcomes in these patients. The aim of the present study is to study the proportion of aetiologies (main categories and detailed) of IHCA and to study their association to return of spontaneous circulation (ROSC), 30-day survival, and favourable neurologic outcome at discharge.
Method
Study design and ethics
This is an observational study based on data from the SRCR as well as the Cause of Death Registry and the Swedish Inpatient Registry from the National Board of Health and Welfare. The study was approved by the Swedish Ethical Review Authority, (Dnr 2021-00260).
The SRCR is a national quality registry with the primary aim to improve knowledge and quality of care among patients who suffer from cardiac arrest in Sweden. The registration of IHCA started in 2005 and since then the number of participating hospitals has increased. From 2018, the registry includes all 73 emergency hospitals in Sweden.18 In 2018, the SRCR expanded the number of categories for recording the aetiology of IHCA, from eight to 22 categories. This change enabled a more detailed description of the aetiology of IHCA. Data in the registry are collected according to the Utstein template however the Utstein template does not specify how aetiology of IHCA should be reported.19,20 Patients with a do not resuscitate order are not included in the SRCR.
National Board of Health and Welfare
The Swedish Inpatient Registry includes the primary and secondary discharge diagnoses based on ICD10 for inpatient care throughout Sweden.21 The Inpatient Registry includes hospitalizations since 1987 and has been validated.22 Since 1991, all deaths in Sweden are reported to the Cause of Death Registry and approximately 92 000 deaths are reported yearly.23
Patient inclusion and exclusion criteria
The study included patients ≥18 years of age who suffered from IHCA, received cardiopulmonary resuscitation (CPR), and were registered in the SRCR between 18th of April 2018 and 31st December 2020. Patients with ongoing CPR upon admission to the hospital were excluded. In cases where the patient experienced cardiac arrest more than once, only the first cardiac arrest was included.
Registration of IHCA aetiology
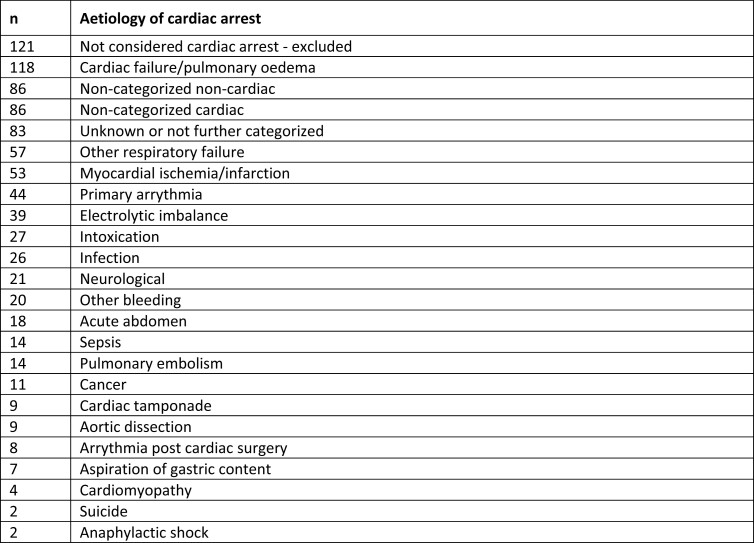
Before April 2018, the aetiology of cardiac arrest was recorded in eight categories in the SRCR. In April 2018, the number of categories expanded to 22 categories. The instructions to the reporting nurse or physician regarding aetiologies to the cardiac arrest is to register the ‘primary triggering cause of the cardiac arrest’. For each patient with IHCA, only one aetiology category can be chosen. Figure 1 presents the 22 aetiologies reported to the SRCR. In this study, the 22 categories were grouped into six main categories: myocardial ischemia, other cardiac causes, pulmonary causes, infection, haemorrhage, and other non-cardiac causes. These six main categories were then further categorized in cardiac and non-cardiac group (Figure 1). Supplementary material online, Figure S1 presents the SRCR's explanation of each of the 22 categories.
Figure 1.
Categorisation and frequencies of aetiologies among patients who experienced IHCA during the study period. *Aetiology categories added by the authors.
Of the patients who had the IHCA aetiology registered as ‘other’ (n = 879), 856 (97.4%) could be categorized into one of the 22 standard categories by reading free text added by the reporting nurse or physician. Four additional aetiology categories were added: cardiac failure/pulmonary oedema, non-categorized cardiac cause (for example aortic stenosis or procedure related), electrolyte imbalance, and non-categorized non-cardiac cause (for example liver failure, ketoacidosis, hypovolemia). Patients who could not be categorized were excluded from further analyses. Figure 1 and Figure 2 show a summary of the categorization of cardiac arrest registered as ‘other’.
Figure 2.
Aetiologies primarily categorized as “other” but with complimentary information from the SRCR categorized in the 26 aetiology categories.
Data collection and linkage
As described in previous studies, the registration in the SRCR occurs in two steps.24 The first part of the registration is performed by a nurse or physician who were present at the scene of the cardiac arrest. This part contains information regarding treatments given during the resuscitation, place of the cardiac arrest, critical delay times, initial rhythm, if the patient was monitored at the time of the arrest, and if the patient received ROSC. As these variables most often are collected at the time of the acute event, situations may occur where there are limitations in the possibility to report some of the variables.
After death or 30-days, a registered nurse or physician affiliated with the SRCR fills in the second part of the registration based on information in patient's medical chart. This part contains information of probable aetiology of the cardiac arrest, clinical condition hours prior to the cardiac arrest, treatment after resuscitation, 30-day survival, Cerebral Performance Category (CPC) score at hospital discharge, and previous medical history.
Patients who suffered from IHCA during the study period were identified in the SRCR. By using the 12-digit personal identification number, unique for all Swedish citizens, data from the Cause of Death Registry and the Swedish Inpatient Registry from the National Board of Health and Welfare were merged seamless.
Outcomes
We present frequency of IHCA aetiologies, ROSC, 30-day survival, and favourable neurological outcome at hospital discharge. ROSC was defined as sustained ROSC for a minimum of 20 min. Favourable neurological outcome was considered as having a CPC score of 1–2 at hospital discharge. CPC-score is a five-point scale ranging from 1–5, CPC 1 representing good cerebral performance and CPC 5 representing brain death.
Data analysis and statistical methods
Baseline characteristics were reported using median with appropriate dispersions measures. The two aetiology groups, cardiac and non-cardiac group, were compared using Chi square test for dichotomous variables and Wilcoxon two-sample test for continuous variables. When the aetiologies were categorized in the six main categories, analysis were performed using multiple logistic regression analysis. A P < 0.05 was considered significant.
The association between aetiology and outcome was analysed with multivariable logistic regression model, described using odds ratio (OR) with 95% confidence interval and presented as forest plots. In the adjusted models, missing data were imputed using multiple imputation by chain equations (mice). Regression analyses were performed on 10 imputed datasets and the results were pooled using Rubin's rule. Data was assumed to be missing at random. The same models with complete cases analysis are presented in supplement. Cardiac arrest due to myocardial ischemia was set as reference. Three different models are presented. Model 1 presents the unadjusted 30-day survival. Model 2 presents adjusted 30-day survival, confounders adjusted for include sex, age, and comorbidity. Model 3 presents adjusted 30-day survival and include model 2 and intermediate variables known to affect outcome including if CPR was started within 1 min, if the patient was monitored or witnessed at the time of the arrest, and the location of the cardiac arrest. Initial rhythm was not adjusted for, as it is closely related to aetiology and will increase net bias by overadjustment of the model. The same models were used for ROSC and favourable neurological outcome CPC 1–2 at hospital discharge. The statistical analysis was performed using R 4.1.3.
Results
Baseline characteristics
A total of 5906 patients suffered from IHCA and were included in the study of whose aetiologies were registered for 4324 patients (73.2%). The median age of all patients with a registered aetiology were 74 years, and 2729/4324 (63.1%) were men. The proportion of male patients in the cardiac group was higher compared to the non-cardiac group (68% vs. 58.2%, P < 0.001). Patients with cardiac arrest due to a non-cardiac cause had significantly more comorbidities compared to cardiac arrest of a cardiac cause (Tables 1 and 2). In the six main aetiology categories cardiac arrest due to other non-cardiac causes were the youngest group with a median age of 72 years (Table 3). Apart from hypertension, cancer was the second most common comorbidity in all four aetiology categories in the non-cardiac group. Cardiac arrest due to pulmonary cause had the highest proportion of asthma and Chronic Obstructive Pulmonary Disease. Cardiac arrest due to infection had the highest proportion of diabetes (Table 3). Information regarding initial cardiac rhythm, time, and treatment of IHCA of the six main aetiology categories is presented in (Table 4). Basic characteristics among patients in all 26 aetiologies are presented in Supplementary material online, Tables S1:1–S1:4 and S2:1–S2:4.
Table 1.
Basic characteristics presented in the cardiac and non-cardiac groups
| Cardiac cause (%) | Non cardiac cause (%) | P-value | SMD | Missing (%) | |
|---|---|---|---|---|---|
| n | 2138 | 2182 | |||
| Sex (Women) | 689 (32.2) | 906 (41.5) | <0.001 | 0.194 | 0.0 |
| Age [median (IQR)] | 74 [66, 81] | 73 [65, 80] | 0.020 | 0.113 | 0.0 |
| Comorbidity | |||||
| Cancer | 700 (32.7) | 886 (40.6) | <0.001 | 0.164 | 0.0 |
| Diabetes | 641 (30.0) | 642 (29.4) | 0.712 | 0.012 | 0.0 |
| Renal failure | 374 (17.5) | 489 (22.4) | <0.001 | 0.123 | 0.0 |
| Heart failure | 733 (34.3) | 636 (29.1) | <0.001 | 0.111 | 0.0 |
| Myocardial infarction | 1185 (55.4) | 528 (24.2) | <0.001 | 0.673 | 0.0 |
| Asthma/COPD | 300 (14.0) | 479 (22.0) | <0.001 | 0.207 | 0.0 |
| Addiction | 253 (11.8) | 411 (18.8) | <0.001 | 0.195 | 0.0 |
| Psychiatric comorbidity | 154 (7.2) | 280 (12.8) | <0.001 | 0.188 | 0.0 |
| Hypertension | 1357 (63.5) | 1381 (63.3) | 0.927 | 0.004 | 0.0 |
| Location | <0.001 | 1.048 | 0.0 | ||
| Emergency department | 274 (12.8) | 328 (15.0) | |||
| Catheterization lab | 478 (22.4) | 17 (0.8) | |||
| Other | 44 (2.1) | 38 (1.7) | |||
| Cardiac care unit (CCU) | 482 (22.5) | 116 (5.3) | |||
| Intermediate care unit (IMCU) | 23 (1.1) | 85 (3.9) | |||
| Intensive care unit (ICU) | 144 (6.7) | 228 (10.4) | |||
| Clinic, laboratory, radiology department | 67 (3.1) | 135 (6.2) | |||
| Operation room (OR) | 26 (1.2) | 57 (2.6) | |||
| General ward | 600 (28.1) | 1178 (54.0) | |||
Table 2.
Information of initial cardiac rhythm, circumstances, time, and treatment of IHCA presented in the cardiac and non-cardiac groups
| Cardiac cause (%) | Non cardiac cause (%) | P-value | SMD | Missing (%) | |
|---|---|---|---|---|---|
| n | 2138 | 2182 | |||
| Initial cardiac rhythm | <0.001 | 0.879 | 18.7 | ||
| Asystole | 596 (31.0) | 794 (46.5) | |||
| Pulseless electrical activity (PEA) | 472 (24.6) | 761 (44.5) | |||
| Ventricular fibrillation (VF) | 586 (30.5) | 97 (5.7) | |||
| Pulseless ventricular tachycardia (VT) | 268 (13.9) | 57 (3.3) | |||
| Witnessed | 1905 (89.2) | 1701 (78.5) | <0.001 | 0.295 | 0.6 |
| ECG monitored | 1641 (77.6) | 953 (44.6) | <0.001 | 0.719 | 1.5 |
| Event times | |||||
| Recognition—call ≤1min | 1408 (84.7) | 1408 (78.9) | <0.001 | 0.149 | 19.2 |
| Recognition—CPR ≤1min | 1780 (93.8) | 1835 (89.8) | <0.001 | 0.145 | 8.8 |
| Recognition—def ≤3min | 754 (79.4) | 127 (38.6) | <0.001 | 0.911 | 72.4 |
| Treatment at the scene of the arrest | |||||
| CPR started before arrival of RRT | 1625 (91.2) | 1761 (92.1) | 0.404 | 0.029 | 14.0 |
| Chest compression | 1539 (98.3) | 1703 (99.4) | 0.005 | 0.102 | 23.6 |
| Defibrillation performed | 1025 (49.0) | 358 (16.8) | <0.001 | 0.728 | 3.0 |
| Intubation | 831 (39.8) | 1291 (60.8) | <0.001 | 0.468 | 3.4 |
| Epinephrine | 1152 (55.0) | 1629 (76.3) | <0.001 | 0.491 | 2.7 |
| Post cardiac arrest treatment | |||||
| PCI | 509 (41.5) | 10 (1.0) | <0.001 | 1.062 | 48.6 |
| ICD | 113 (9.2) | 2 (0.2) | <0.001 | 0.418 | 48.6 |
| Pacemaker during hospital stay | 268 (21.8) | 19 (2.0) | <0.001 | 0.629 | 48.6 |
| Coronary angiography | 571 (46.5) | 29 (3.0) | <0.001 | 1.066 | 48.6 |
| CABG during hospital stay | 25 (2.0) | 1 (0.1) | <0.001 | 0.183 | 48.6 |
Table 3.
Basic characteristics presented in the six main aetiology categories
| Cardiac or non-cardiac group | Cardiac group | Non-cardiac group | Missing (%) | ||||
|---|---|---|---|---|---|---|---|
| 6 main aetiology categories | Myocardial ischemia (%) | Other cardiac cause (%) | Pulmonary causes (%) | Infection (%) | Haemorrhage (%) | Other non-cardiac (%) | |
| N | 1291 (29.9) | 847 (19.8) | 923 (21.4) | 389 (9) | 367 (8.5) | 503 (11.6) | |
| Sex (Women) | 406 (31.4) | 283 (33.4) | 394 (42.7) | 139 (35.7) | 146 (39.8) | 227 (45.1) | 0.0 |
| Age [median (IQR)] | 74 [65, 81] | 74 [66, 81] | 74 [65, 80] | 74 [66, 82] | 75 [66, 80] | 72 [59, 79] | 0.0 |
| Comorbidity | |||||||
| Cancer | 416 (32.2) | 284 (33.5) | 386 (41.8) | 150 (38.6) | 142 (38.7) | 208 (41.4) | 0.0 |
| Diabetes | 376 (29.1) | 265 (31.3) | 265 (28.7) | 146 (37.5) | 85 (23.2) | 146 (29.0) | 0.0 |
| Renal failure | 180 (13.9) | 194 (22.9) | 183 (19.8) | 116 (29.8) | 76 (20.7) | 114 (22.7) | 0.0 |
| Heart failure | 345 (26.7) | 388 (45.8) | 305 (33.0) | 151 (38.8) | 78 (21.3) | 102 (20.3) | 0.0 |
| Myocardial infarction | 854 (66.2) | 331 (39.1) | 225 (24.4) | 115 (29.6) | 90 (24.5) | 98 (19.5) | 0.0 |
| Asthma/COPD | 158 (12.2) | 142 (16.8) | 256 (27.7) | 84 (21.6) | 51 (13.9) | 88 (17.5) | 0.0 |
| Addiction | 139 (10.8) | 114 (13.5) | 164 (17.8) | 66 (17.0) | 58 (15.8) | 123 (24.5) | 0.0 |
| Psychiatric comorbidity | 80 (6.2) | 74 (8.7) | 112 (12.1) | 51 (13.1) | 31 (8.4) | 86 (17.1) | 0.0 |
| Hypertension | 784 (60.7) | 573 (67.7) | 589 (63.8) | 267 (68.6) | 235 (64.0) | 290 (57.7) | 0.0 |
| Location | 0.0 | ||||||
| Emergency department | 181 (14.0) | 93 (11.0) | 133 (14.4) | 34 (8.7) | 89 (24.3) | 72 (14.3) | |
| Catherization lab | 410 (31.8) | 68 (8.0) | 6 (0.7) | 0 (0.0) | 7 (1.9) | 4 (0.8) | |
| Other | 24 (1.9) | 20 (2.4) | 16 (1.7) | 2 (0.5) | 4 (1.1) | 16 (3.2) | |
| Cardiac care unit (CCU) | 287 (22.2) | 195 (23.0) | 63 (6.8) | 13 (3.3) | 17 (4.6) | 23 (4.6) | |
| Intermediate care unit (IMCU) | 10 (0.8) | 13 (1.5) | 39 (4.2) | 25 (6.4) | 10 (2.7) | 11 (2.2) | |
| Intensive care unit (ICU) | 64 (5.0) | 80 (9.4) | 87 (9.4) | 65 (16.7) | 27 (7.4) | 49 (9.7) | |
| Clinic, laboratory, radiology department | 30 (2.3) | 37 (4.4) | 36 (3.9) | 16 (4.1) | 37 (10.1) | 37 (10.1) | |
| Operation room (OR) | 6 (0.5) | 20 (2.4) | 16 (1.7) | 8 (2.1) | 13 (3.5) | 20 (4.0) | |
| General ward | 279 (21.6) | 321 (37.9) | 527 (57.1) | 226 (58.1) | 163 (44.4) | 262 (52.1) | |
Table 4.
Information of initial cardiac rhythm, circumstances, time, and treatment of IHCA presented in the six main aetiology categories
| Cardiac or non-cardiac group | Cardiac group | Non-cardiac group | |||||
|---|---|---|---|---|---|---|---|
| 6 main aetiology categories | Myocardial ischemia (%) | Other cardiac cause (%) | Pulmonary causes (%) | Infection (%) | Haemorrhage (%) | Other non-cardiac (%) | Missing (%) |
| n | 1291 (29.9) | 847 (19.8) | 923 (21.4) | 389 (9) | 367 (8.5) | 503 (11.6) | |
| Initial cardiac rhythm | 18.7 | ||||||
| Asystole | 287 (24.6) | 309 (40.9) | 323 (45.5) | 159 (50.8) | 120 (41.4) | 192 (48.5) | |
| Pulseless electrical activity (PEA) | 300 (25.7) | 172 (22.8) | 343 (48.3) | 122 (39.0) | 152 (52.4) | 144 (36.4) | |
| Ventricular fibrillation (VF) | 443 (38.0) | 143 (18.9) | 23 (3.2) | 24 (7.7) | 14 (4.8) | 36 (9.1) | |
| Pulseless ventricular tachycardia (VT) | 137 (11.7) | 131 (17.4) | 21 (3.0) | 8 (2.6) | 4 (1.4) | 24 (6.1) | |
| Witnessed | 1170 (90.8) | 735 (86.9) | 734 (80.0) | 290 (75.5) | 294 (80.8) | 383 (76.4) | 0.6 |
| ECG monitored | 1041 (81.6) | 600 (71.4) | 387 (43.0) | 178 (46.4) | 174 (48.5) | 214 (43.2) | 1.5 |
| Event times | |||||||
| Recognition—call ≤1min | 835 (85.6) | 573 (83.4) | 582 (77.1) | 249 (80.6) | 262 (84.2) | 315 (77.0) | 19.2 |
| Recognition—CPR ≤1min | 1068 (94.0) | 712 (93.4) | 776 (90.1) | 335 (90.8) | 316 (91.3) | 408 (87.4) | 8.8 |
| Recognition—def ≤3min | 526 (80.6) | 228 (76.8) | 43 (37.1) | 25 (37.3) | 17 (32.1) | 42 (45.2) | 72.4 |
| Treatment at the scene of the arrest | |||||||
| CPR started before arrival of RRT | 951 (90.2) | 674 (92.7) | 753 (92.4) | 310 (94.5) | 299 (90.6) | 399 (90.7) | 14.0 |
| Chest compression | 894 (97.8) | 645 (99.1) | 728 (99.5) | 300 (99.7) | 288 (99.0) | 387 (99.5) | 23.6 |
| Defibrillation performed | 694 (54.8) | 331 (40.0) | 117 (13.0) | 76 (20.2) | 59 (16.3) | 106 (21.6) | 3.0 |
| Intubation | 547 (43.3) | 284 (34.5) | 556 (62.1) | 230 (60.4) | 236 (66.3) | 269 (55.0) | 3.4 |
| Epinephrine | 734 (57.9) | 418 (50.4) | 707 (78.3) | 311 (81.0) | 284 (78.7) | 327 (67.3) | 2.7 |
| Post cardiac arrest treatment | |||||||
| PCI | 492 (59.9) | 54 (9.0) | 4 (0.7) | 1 (0.5) | 2 (1.4) | 6 (1.9) | 48.6 |
| ICD | 44 (5.4) | 86 (14.3) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 3 (1.0) | 48.6 |
| Pacemaker during hospital stay | 78 (9.5) | 236 (39.3) | 7 (1.3) | 6 (3.1) | 1 (0.7) | 15 (4.8) | 48.6 |
| Coronary angiography | 493 (60.0) | 137 (22.8) | 18 (3.4) | 2 (1.0) | 3 (2.1) | 25 (8.0) | 48.6 |
| CABG during hospital stay | 24 (2.9) | 4 (0.7) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 48.6 |
Frequency of aetiologies
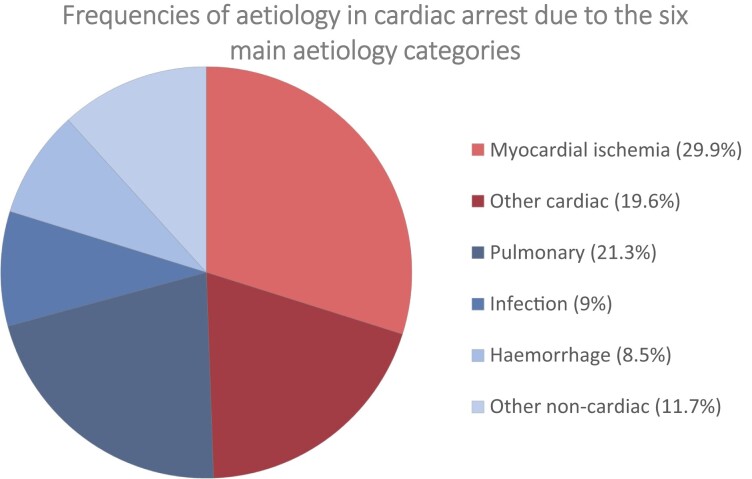
The distribution between the cardiac group and the non-cardiac group were 49.4% vs. 50.6% (Table 1). The most common aetiology was myocardial ischemia (29.9%) followed by pulmonary causes (21.3%), other cardiac causes (19.6%), other non-cardiac causes (11.7%), infection (9%), and haemorrhage (8.5%) (Figure 3). Supplementary material online, Figures S2 and S3 present the frequencies of all 26 aetiologies divided into cardiac and non-cardiac causes.
Figure 3.
Frequency distribution of the six main aetiology categories.
Thirty-day survival
The cardiac group had significantly higher 30-day survival (48.4% vs. 18.7%, P < 0.001) compared to the non-cardiac group (Table 5). In the six main aetiology categories, cardiac arrest with an aetiology of other cardiac causes had the highest 30-day survival (53.5%) followed by cardiac arrest due to myocardial ischemia (43.8%) (Table 6). Lowest crude 30-day survival was seen in cardiac arrest due to haemorrhage (14.7%). Overall, 30-day survival in all 26 aetiology categories varied between (2.6% and 75%) and are presented in Supplementary material online, Tables S3:1–S3:4.
Table 5.
Outcomes presented in the cardiac and non-cardiac group
| Cardiac cause (%) | Non cardiac cause (%) | P-value | Missing (%) | |
|---|---|---|---|---|
| n | 2138 | 2182 | ||
| ROSC (%) | 1361 (64.4) | 1108 (51.6) | <0.001 | 1.8 |
| 30-day survival (%) | 1018 (47.6) | 472 (21.6) | <0.001 | 0.0 |
| CPC (%) | <0.001 | 72.7 | ||
| CPC 1–2 | 900 (89.5) | 321 (73.0) | ||
| CPC 3–5 | 42 (4.2) | 88 (20.0) | ||
| Unknown CPC | 64 (6.4) | 31 (7.0) |
Table 6.
Outcomes presented in the six main aetiology categories
| Cardiac or non-cardiac group | Cardiac group | Non-cardiac group | P | ||||
|---|---|---|---|---|---|---|---|
| 6 main aetiology categories | Myocardial ischemia (%) | Other cardiac cause (%) | Pulmonary causes (%) | Infection (%) | Haemorrhage (%) | Other non-cardiac (%) | |
| n | 1291 (29.9) | 847 (19.6) | 923 (21.3) | 389 (9) | 367 (8.5) | 503 (11.7) | |
| ROSC (%) | 784 (61.5) | 577 (68.9) | 503 (55.5) | 184 (47.5) | 130 (35.8) | 291 (59.0) | <0.001 |
| 30-day survival (%) | 565 (43.8) | 453 (53.5) | 203 (22.0) | 63 (16.2) | 54 (14.7) | 152 (30.2) | <0.001 |
| CPC (%) | <0.001 | ||||||
| CPC 1–2 | 515 (91.2) | 385 (87.3) | 138 (72.3) | 32 (54.2) | 44 (88.0) | 107 (76.4) | |
| CPC 3–5 | 18 (3.2) | 24 (5.4) | 43 (22.5) | 18 (30.5) | 4 (8.0) | 23 (16.4) | |
| Unknown CPC | 32 (5.7) | 32 (7.3) | 10 (5.2) | 9 (15.3) | 2 (4.0) | 10 (7.1) | |
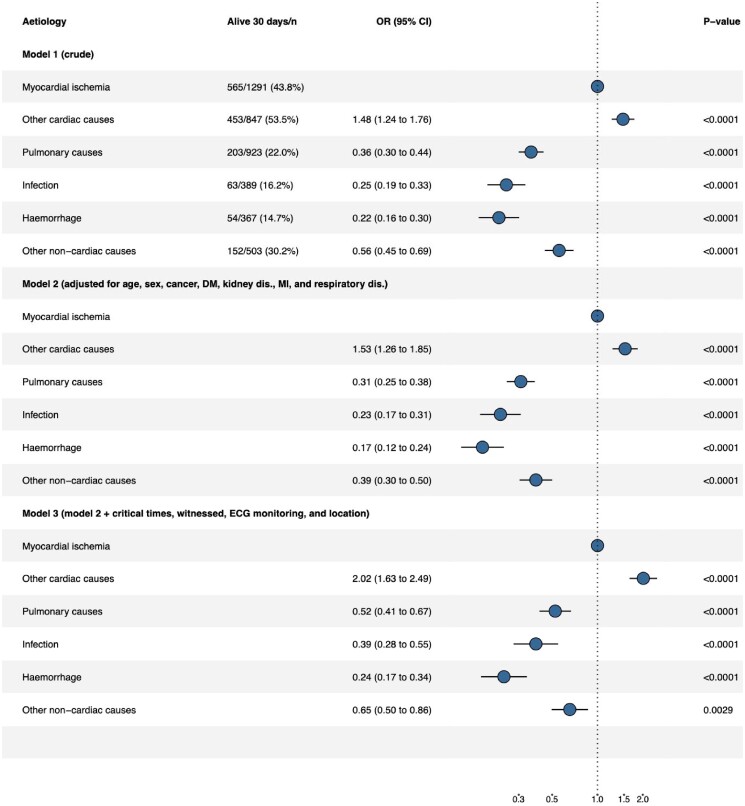
Figure 4 , model 1 presents the OR of unadjusted 30-day survival among patients who suffer from IHCA. All four aetiologies in the non-cardiac group had a lower probability of 30-day survival compared to the two aetiologies in the cardiac group. The OR for 30-day survival compared to myocardial ischemia were, for other cardiac causes OR 1.48 (CI 1.24–1.76), pulmonary causes OR 0.36 (CI 0.3–0.44), infection OR 0.25 (CI 0.18–0.33), haemorrhage OR 0.22 (CI 0.16–0.3), and other non-cardiac causes OR 0.56 (CI 0.45–0.69). When adjusting for confounders as well as when adjusting for confounders and intermediates, the lower 30-day survival among aetiologies in the non-cardiac group (pulmonary, infection, haemorrhage, and other non-cardiac causes) remained (Figure 4, model 2 and 3). Complete case analysis is presented in Supplementary material online, Figure S4.
Figure 4.
Forrest plot of 30-day survival. Model 1, unadjusted 30-day survival. Model 2, adjusted for confounders. Model 3, adjusted for confounders and intermediates.
Return of spontaneous circulation
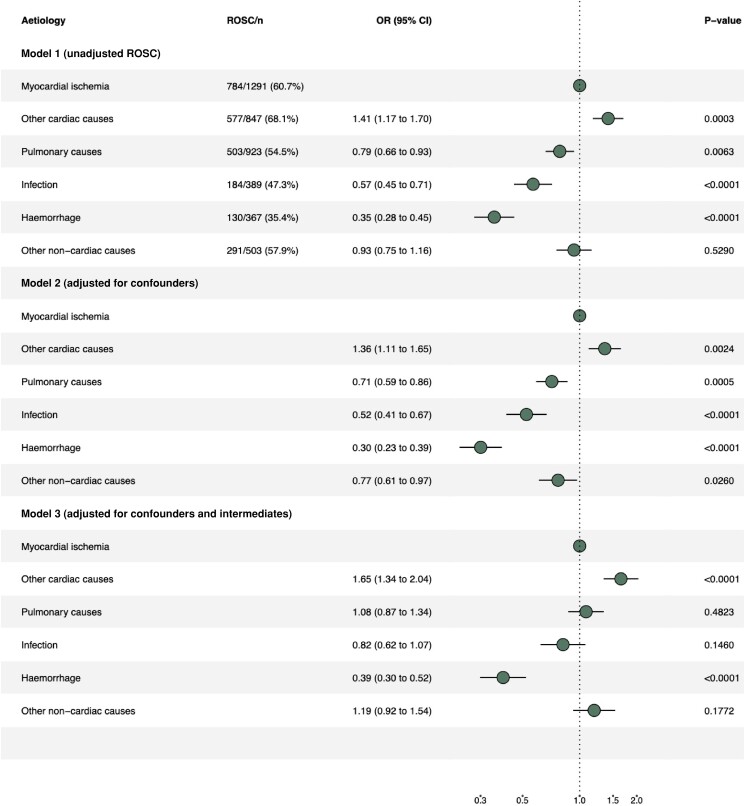
Cardiac arrest due to cardiac cause had significantly higher ROSC (65.3% vs. 49.9%, P < 0.001), compared to cardiac arrest due to non-cardiac cause (Table 5). Among the six main aetiology categories, ROSC were highest among patients who suffered from cardiac arrest due to other cardiac causes (68.9%). Cardiac arrest due to haemorrhage had the lowest ROSC (35.8%) (Table 6). Overall, in the detailed aetiology categories, ROSC were ranging between 20.4% and 95.5% (cardiac arrest due to aortic dissection having the lowest and cardiac arrest due to anaphylactic shock having the highest proportion of ROSC) (see Supplementary material online, Tables S3:1–S3:4).
When adjusting for confounders, the proportion of ROSC in all four aetiologies in the non-cardiac group had a lower OR of ROSC compared to myocardial ischemia (Figure 5, model 2). The lower OR of ROSC seen among cardiac arrests due to infection, pulmonary cause, and other non-cardiac causes were no longer significant when adjusting for both confounders and intermediates (Figure 5, model 3). However, cardiac arrest due to haemorrhage still had a lower OR for ROSC compared to cardiac arrest due to myocardial ischemia. Complete case analysis is presented in Supplementary material online, Figure S5.
Figure 5.
Forrest plot of ROSC. Model 1, unadjusted ROSC. Model 2, adjusted for confounders. Model 3, adjusted for confounders and intermediates.
Favourable neurological outcome, Cerebral Performance Category score
The cardiac group had significantly higher favourable neurological outcome (89.8% vs. 72.8%, P < 0.001) compared to the non-cardiac group (Table 5). The highest proportion of favourable neurological outcome at hospital discharge among survivors were seen among patients with an aetiology of myocardial ischemia, with 91.2% of survivors having a CPC-score of 1–2 (Table 6). Among patients with pulmonary cause, 72.3% had favourable neurological outcome at discharge. The lowest proportion of favourable neurological outcome was seen among patients who suffered from cardiac arrest due to infection (54.2%). In the detailed aetiology categories, CPC-score of 1–2 at hospital discharge, were ranging between 42.9 and 100% (cardiac arrest due to stroke having the lowest and cardiac arrest due to aortic dissection having the highest of CPC-score 1–2) (Supplementary material online, Tables S3:1–S3:4).
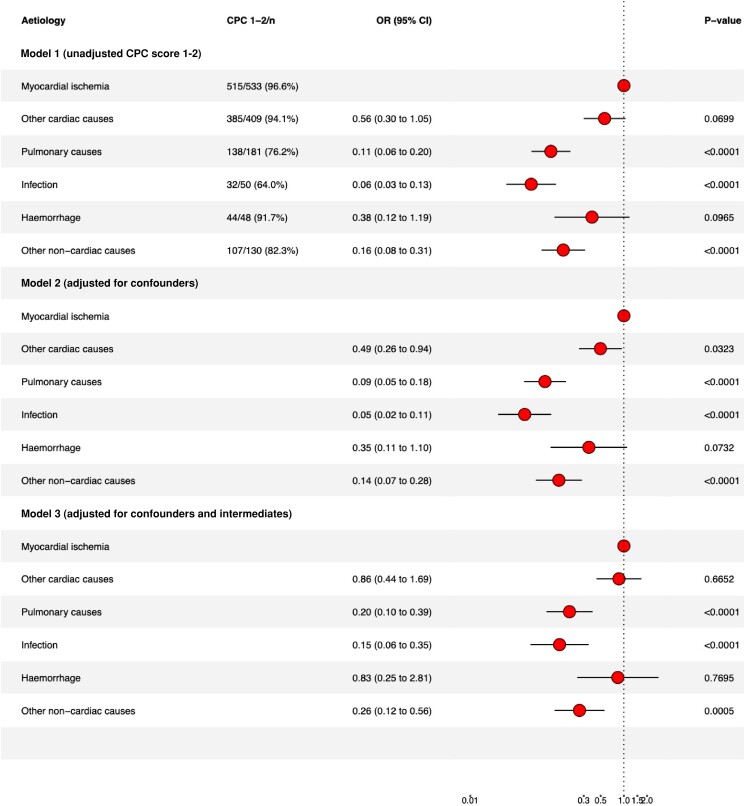
In the adjusted analysis, the lower OR of favourable neurological outcome among cardiac arrest due to infection, pulmonary, and other non-cardiac causes remained when adjusting for confounders as well as when adjusting for confounders and intermediates (Figure 6). Complete case analysis is presented in Supplementary material online, Figure S6.
Figure 6.
Forrest plot of favourable neurological outcome among survivors (CPC-score 1–2). Model 1, unadjusted CPC-score 1–2. Model 2, adjusted for confounders. Model 3, adjusted for confounders and intermediates.
Discussion
Identifying aetiology may enable prevention of the cardiac arrest and has implication on treatment and outcome. Apart from correctly performed CPR, urgent work must be done to treat the underlying pathophysiology leading up to the cardiac arrest. Many cardiac arrests are considered preventable in retrospective reviews. To prevent cardiac arrest and improve survival, it is important to have a system both for identifying patients at risk of deteriorating to cardiac arrest and to have a RRT with knowledge of appropriate interventional responses. Studies have shown that the benefit of recognizing the aetiology of cardiac arrest is more pronounced in non-cardiac causes and non-shockable rhythms which may correspond to possible reversible aetiologies.9,17 Cardiac arrest due to non-cardiac causes is a more heterogenic group and treatment of the underlying cause is more diverse and not as obvious as for myocardial ischemia where revascularization is gold standard. For non-cardiac causes, treatment such as thrombolysis may be vital for a patient with cardiac arrest due to pulmonary embolism but devastating for a patient with dissecting aortic aneurysm or intracerebral bleeding. Also, some aetiologies have especially low 30-day survival and among other aetiologies patients have an increased risk of surviving with neurological impairment. This is important knowledge for clinicians both in the treatment of the patient peri and post cardiac arrest, but also in the communication with relatives. These findings are important and support that there is a need for a universal categorization of the aetiology of IHCA.
The main findings of this nationwide study of IHCA with more granular data on aetiologies compared to previous studies were that cardiac arrest due to myocardial ischemia and pulmonary cause were the two most common causes with considerable differences in ROSC, 30-day survival, and neurologic outcomes. Patients whose cardiac arrest was caused by myocardial ischemia and other cardiac causes had the highest proportion of ROSC and 30-day survival. When comparing outcomes among all registered aetiologies, there was a wide range in the proportion of achieving ROSC (20.4–95.5%), 30-day survival (2.6–75%), and CPC 1–2 at discharge (42.9–100%). In the comparison between the cardiac and non-cardiac group, the main finding was that the differences in outcome between the two groups are most pronounced in the 30-day survival and functional neurologic outcome, not in ROSC.
The distribution of the six main aetiology categories in our study may be difficult to compare with other studies as there is not a uniform way to report aetiologies of IHCA and thus the frequency and aetiology categories differs between studies.19,25 In addition, the Utstein template for IHCA from 2019 does not specify the reporting of aetiology.19 Other studies have reported a prevalence of cardiac cause of 50–63% and pulmonary cause of 11–42%,4,12,26–28 thus, similar to our data. The prevalence of haemorrhage and infection as causes of IHCA is also similar.4,9,12,13,25,29 The study by Hessulf et al. is one of few studies from the SRCR that report frequencies of aetiologies of IHCA prior to the change from eight to 22 categories in 2018.30 In Hessulf's study 25% of the IHCA were due to primary arrhythmia, 34% due to myocardial ischemia, 11% due to respiratory failure, 7% due to hypotension and 4% due to acute pulmonary oedema. The remaining 19% did not have an aetiology registered and classified as missing. Compared to the study by Hessulf et al., the non-cardiac group in our study was significantly larger and we were also able to classify the non-cardiac group more carefully.
Wallmuller et al. present a detailed description of aetiologies among IHCA and their relation to CPC-score at six-months.12 When comparing their data with our results (CPC 1–2 at hospital discharge), the proportion of favourable neurological outcome in our study were lower among patients with an aetiology of myocardial ischemia and most non-cardiac causes, but higher among other cardiac causes. However, these results are difficult to compare as the definition of aetiologies, the way data were retrieved, and the time from cardiac arrest to neurological evaluation were different.12
An important finding in our study was that patients with non-cardiac causes had worse outcomes, in particular 30-day survival and functional neurologic outcome, compared to patients with cardiac cause of IHCA. This is in accordance with previous studies.4,12,26 Cardiac arrests in the non-cardiac group more often occurred in general wards, in unmonitored patients, were less often witnessed, and more often had an initial non-shockable rhythm. The four aetiologies in the non-cardiac group also had a lower proportion of patients receiving CPR within one minute from recognition of the cardiac arrest compared to the cardiac group. A longer time of no circulation increases the hypoxic injury in all organs and especially the brain by each minute of prolonged delay. In addition, a delayed start of CPR increases the risk of conversion from a shockable to a non-shockable rhythm. All the above factors have in previous studies been associated with worse outcome.30–33 Patients with cardiac arrest due to infection, pulmonary, and other non-cardiac causes more often had symptoms of hypoxia one hour prior to cardiac arrest further increasing the risk of hypoxic organ damage and global ischemic injury post ROSC leading to multiorgan failure and a more severe post resuscitation syndrome. This may partly explain the more pronounced difference between ROSC and 30-day survival seen in the four aetiologies in the non-cardiac group as compared with the cardiac group.
We found some aetiologies with especially low 30-day survival including cardiac arrest due to aspiration, cancer, infection, sepsis, aortic dissection, cerebrovascular cause, and acute abdomen. An aetiology of aspiration and cancer has previously been shown to have particularly low survival.24,30 The treatment of the underlying cause in patients who suffer from cardiac arrest due to acute abdomen or aortic dissection, is immediate surgery. To perform surgery in a patient who recently was resuscitated after a cardiac arrest is high-risk performance and the low survival among these patients is thus not surprising. Sepsis is, regardless of cardiac arrest, associated with a high mortality. Cardiac arrest due to sepsis has multiple potential causes with multiple organ failure including circulatory failure, respiratory failure, vasoplegia, and metabolic derangement as potential causes.34 With early recognition of sepsis, acute abdomen, gastric impairment leading to aspiration, cardiac arrest due to these aetiologies may be preventable. Cardiac arrest due to cerebrovascular cause and cancer does not have a distinct treatment for the underlying cause and the survival for patients with these aetiologies will probably remain poor.
To be able to compare results and treatment of IHCA it is important to acknowledge the heterogenicity among cardiac arrests due to cardiac and non-cardiac causes. Thus, there is a need for harmonization when it comes to reporting aetiologies of IHCA.
Strengths and limitation
The SRCR is a mandatory national register with high coverage, ensuring high quality data.
However, the study has several limitations. First, the aetiology of cardiac arrest is not always obvious, and the aetiology in a substantial proportion of the cardiac arrests will remain unknown unless autopsy is performed. In this study, 27% of the cardiac arrests had an unknown aetiology and this effects the internal validity. Second, the registration of data at the scene of the arrest such as critical delay times, treatment, and initial rhythm is challenging in acute situations and it is important to acknowledge that registry data have limitations in regard to recognition, collection, and encoding. However, this is true for the majority of registry studies especially when involving acute situations. This is considered as a random misclassification bias and would generally not contribute to systematic errors. Third, the cause of cardiac arrest may be multifactorial, in the SRCR only one aetiology can be chosen. Fourth, several of the aetiology categories pre-specified in the SRCR, such as infection and cancer are difficult to pathophysiologically explain as the triggering cause of the cardiac arrest. For the aetiologies infection and cancer, this rather indicates symptoms prior to the arrest rather than the final trigger of the cardiac arrest. The categorization of aetiologies of IHCA in SRCR is not standardised in the same way as in out-of-hospital cardiac arrest and further emphasises the need of a universal aetiology categorization.
Conclusion
In this nationwide observational study of IHCA, the distribution between cardiac and non-cardiac causes were similar, but with a substantial difference in 30-day survival. Cardiac arrest caused by myocardial ischemia and other cardiac causes had the strongest associations with ROSC, 30-day survival and neurologic outcome compared to other categories. There is a need for a universal categorization of the aetiology of IHCA to be able to compare outcome, treatment, and intervention for IHCA in future studies.
Supplementary Material
Acknowledgements
Study concept and design: M.A., J.H., A.R., P.N. Study selection and data extraction: A.R., J.H., P.N. Statistical analysis and interpretation of the data: M.A., M.J., A.R., P.N. Drafting of the manuscript: M.A., P.N. Ethical approval: M.A., P.N. Critical revision of the manuscript for important clinical advice and intellectual content: P.N., J.H., J.H., S.F., A.C., M.T., P.L. All authors approved the final manuscript.
Contributor Information
Malin Albert, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden.
Johan Herlitz, Centre for Prehospital Research, Faculty of Caring Science, Work Life and Social Welfare, University of Borås, SE-501 90 Borås, Sweden.
Araz Rawshani, Department of Molecular and Clinical Medicine, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden.
Sune Forsberg, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden.
Mattias Ringh, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden.
Jacob Hollenberg, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden.
Andreas Claesson, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden.
Meena Thuccani, Department of Anaesthesiology and Intensive Care Medicine, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Peter Lundgren, Centre for Prehospital Research, Faculty of Caring Science, Work Life and Social Welfare, University of Borås, SE-501 90 Borås, Sweden; Department of Molecular and Clinical Medicine, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden; Department of Cardiology, Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Martin Jonsson, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden.
Per Nordberg, Department of Clinical Science and Education, Södersjukhuset, Centre for Resuscitation Science, Karolinska Institutet, Sjukhusbacken 10, 118 83 Stockholm, Sweden; Functional Perioperative Medicine and Intensive Care, Karolinska University Hospital, Stockholm, Sweden.
Lead author biography
 Malin Albert is a doctoral student at the research group ‘Centre for Resuscitation Science’ at the Department of Clinical Science and Education, Södersjukhuset, Karolinska Institutet, Sweden. The title of the doctoral project is ‘aetiology, treatment, and prognostication of cardiac arrest’ and this article will be a part of the doctoral project. Malin Albert is also a last year resident physician at the department of anaesthesiology and intensive care at Södersjukhuset, Stockholm.
Malin Albert is a doctoral student at the research group ‘Centre for Resuscitation Science’ at the Department of Clinical Science and Education, Södersjukhuset, Karolinska Institutet, Sweden. The title of the doctoral project is ‘aetiology, treatment, and prognostication of cardiac arrest’ and this article will be a part of the doctoral project. Malin Albert is also a last year resident physician at the department of anaesthesiology and intensive care at Södersjukhuset, Stockholm.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
Swedish Heart Lung foundation with grant numbers 20200463 (PN) and 20190068 (JH).
Data availability
Data are available upon request from the author M.A.
References
- 1. Morrison LJ, Neumar RW, Zimmerman JL, Link MS, Newby LK, McMullan PW Jr, Hoek TV, Halverson CC, Doering L, Peberdy MA, Edelson DP. Strategies for improving survival after in-hospital cardiac arrest in the United States: 2013 consensus recommendations: a consensus statement from the American Heart Association. Circulation 2013;127:1538–1563. [DOI] [PubMed] [Google Scholar]
- 2. Merchant RM, Berg RA, Yang L, Becker LB, Groeneveld PW, Chan PS; American Heart Association’s Get With the Guidelines-Resuscitation Investigators . Hospital variation in survival after in-hospital cardiac arrest. J Am Heart Assoc 2014;3:e000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nolan JP, Soar J, Smith GB, Gwinnutt C, Parrott F, Power S, Harrison DA, Nixon E, Rowan K. Incidence and outcome of in-hospital cardiac arrest in the United Kingdom National Cardiac Arrest Audit. Resuscitation 2014;85:987–992. [DOI] [PubMed] [Google Scholar]
- 4. Radeschi G, Mina A, Berta G, Fassiola A, Roasio A, Urso F, Penso R, Zummo U, Berchialla P, Ristagno G, Sandroni C. Incidence and outcome of in-hospital cardiac arrest in Italy: a multicentre observational study in the piedmont region. Resuscitation 2017;119:48–55. [DOI] [PubMed] [Google Scholar]
- 5. Grasner JT, Herlitz J, Tjelmeland IBM, Wnent J, Masterson S, Lilja G, Bein B, Böttiger BW, Rosell-Ortiz F, Nolan JP, Bossaert L, Perkins GD. European Resuscitation Council Guidelines 2021: epidemiology of cardiac arrest in Europe. Resuscitation 2021;161:61–79. [DOI] [PubMed] [Google Scholar]
- 6. Adamski J, Nowakowski P, Gorynski P, Onichimowski D, Weigl W. Incidence of in-hospital cardiac arrest in Poland. Anaesthesiol Intensive Ther 2016;48:288–293. [DOI] [PubMed] [Google Scholar]
- 7. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 8. Cummins RO, Ornato JP, Thies WH, Pepe PE. Improving survival from sudden cardiac arrest: the “chain of survival” concept. A statement for health professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation 1991;83:1832–1847. [DOI] [PubMed] [Google Scholar]
- 9. Bergum D, Haugen BO, Nordseth T, Mjølstad OC, Skogvoll E. Recognizing the causes of in-hospital cardiac arrest—A survival benefit. Resuscitation 2015;97:91–96. [DOI] [PubMed] [Google Scholar]
- 10. Saarinen S, Nurmi J, Toivio T, Fredman D, Virkkunen I, Castrén M. Does appropriate treatment of the primary underlying cause of PEA during resuscitation improve patients’ survival? Resuscitation 2012;83:819–822. [DOI] [PubMed] [Google Scholar]
- 11. Bergum D, Nordseth T, Mjølstad OC, Skogvoll E, Haugen BO. Causes of in-hospital cardiac arrest—incidences and rate of recognition. Resuscitation 2015;87:63–68. [DOI] [PubMed] [Google Scholar]
- 12. Wallmuller C, Meron G, Kurkciyan I, Schober A, Stratil P, Sterz F. Causes of in-hospital cardiac arrest and influence on outcome. Resuscitation 2012;83:1206–1211. [DOI] [PubMed] [Google Scholar]
- 13. Tirkkonen J, Hellevuo H, Olkkola KT, Hoppu S. Aetiology of in-hospital cardiac arrest on general wards. Resuscitation 2016;107:19–24. [DOI] [PubMed] [Google Scholar]
- 14. Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Bělohlavek J, Callaway C, Cariou A, Eastwood G, Erlinge D, Hovdenes J, Joannidis M, Kirkegaard H, Kuiper M, Levin H, Morgan MPG, Nichol AD, Nordberg P, Oddo M, Pelosi P, Rylander C, Saxena M, Storm C, Taccone F, Ullén S, Wise MP, Young Paul, Friberg H, Nielsen N. Targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest (TTM2): a randomized clinical trial-rationale and design. Am Heart J 2019;217:23–31. [DOI] [PubMed] [Google Scholar]
- 15. Elfwen L, Lagedal R, Nordberg P, James S, Oldgren J, Böhm F, Lundgren P, Rylander C, van der Linden J, Hollenberg J, Erlinge D, Cronberg T, Jensen U, Friberg H, Lilja G, Larsson I-M, Wallin E, Rubertsson S, Svensson L. Direct or subacute coronary angiography in out-of-hospital cardiac arrest (DISCO)—an initial pilot-study of a randomized clinical trial. Resuscitation 2019;139:253–261. [DOI] [PubMed] [Google Scholar]
- 16. Desch S, Freund A, Akin I, Behnes M, Preusch MR, Zelniker TA, Skurk Carsten, Landmesser Ulf, Graf Tobias, Eitel Ingo, Fuernau Georg, Haake Hendrik, Nordbeck Peter, Hammer Fabian, Felix Stephan B., Hassager Christian, Engstrøm Thomas, Fichtlscherer Stephan, Ledwoch Jakob, Lenk Karsten, Joner Michael, Steiner Stephan, Liebetrau Christoph, Voigt Ingo, Zeymer Uwe, Brand Michael, Schmitz Roland, Horstkotte Jan, Jacobshagen Claudius, Pöss Janine, Abdel-Wahab Mohamed, Lurz Philipp, Jobs Alexander, de Waha-Thiele Suzanne, Olbrich Denise, Sandig F, König IR, Brett S, Vens M, Klinge K, Thiele H. Angiography after out-of-hospital cardiac arrest without ST-segment elevation. N Engl J Med 2021;385:2544–2553. [DOI] [PubMed] [Google Scholar]
- 17. Soar J, Bottiger BW, Carli P, Couper K, Deakin CD, Djarv T, Lott C, Olasveengen T, Paal P, Pellis T, Perkins GD, Sandroni C, Nolan JP. European Resuscitation Council Guidelines 2021: adult advanced life support. Resuscitation 2021;161:115–151. [DOI] [PubMed] [Google Scholar]
- 18. Araz Rawshani JH, Lindqvist J, Aune S, Strömsöe A. Årsrapport 2021. Svenska hjärtlungräddningsregistret; 2021.
- 19. Nolan JP, Berg RA, Andersen LW, Bhanji F, Chan PS, Donnino MW, Lim SH, Ma MH, Nadkarni VM, Starks MA, Perkins GD, Morley PT, Soar J. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Template for In-Hospital Cardiac Arrest: a consensus report from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia). Resuscitation 2019;144:166–177. [DOI] [PubMed] [Google Scholar]
- 20. Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, Deakin CD, Finn JC, Gräsner J-T, Hazinski MF, Iwami T, Koster RW, Lim SH, Ma MH-M, McNally BF, Morley PT, Morrison LJ, Monsieurs KG, Montgomery W, Nichol G, Okada K, Hock Ong ME, Travers AH, Nolan JP; Utstein Collaborators . Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Resuscitation 2015;96:328–340. [DOI] [PubMed] [Google Scholar]
- 21. Det statistiska registrets framställning och kvalitet, Patientregistret. In: Socialstyrelsen MoHaW, editor. Website of the Ministry of Health and Welfare2022. p. 1–24.
- 22. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson P. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Munter J. Det statistiska registrets framställning och kvalitet, dödsorsaksregistret. In: Socialstyrelsen MoHaW, editor. Website of the Ministry of Health and Welfare2022. p. 1–15.
- 24. Albert M, Herlitz J, Rawshani A, Ringh M, Claesson A, Djarv T, Nordberg P. Cardiac arrest after pulmonary aspiration in hospitalised patients: a national observational study. BMJ Open 2020;10:e032264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allencherril J, Lee PYK, Khan K, Loya A, Pally A. Etiologies of in-hospital cardiac arrest: a systematic review and meta-analysis. Resuscitation 2022;175:88–95. [DOI] [PubMed] [Google Scholar]
- 26. Hirlekar G, Karlsson T, Aune S, Ravn-Fischer A, Albertsson P, Herlitz J, Libungan B. Survival and neurological outcome in the elderly after in-hospital cardiac arrest. Resuscitation 2017;118:101–106. [DOI] [PubMed] [Google Scholar]
- 27. Perman SM, Stanton E, Soar J, Berg RA, Donnino MW, Mikkelsen ME, Edelson DP, Churpek MM, Yang L, Merchant RM; American Heart Association's Get With the Guidelines®—Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators . Location of in-hospital cardiac arrest in the United States—variability in event rate and outcomes. LIDJ Am Heart Assoc 2016;5:e003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riley LE, Mehta HJ, Lascano J. Single-center in-hospital cardiac arrest outcomes. Indian J Crit Care Med 2020;24:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tran S, Deacon N, Minokadeh A, Malhotra A, Davis DP, Villanueva S, Sell RE. Frequency and survival pattern of in-hospital cardiac arrests: the impacts of etiology and timing. Resuscitation 2016;107:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hessulf F, Karlsson T, Lundgren P, Aune S, Stromsoe A, Södersved Källestedt ML, Djärv T, Herlitz J, Engdahl J. Factors of importance to 30-day survival after in-hospital cardiac arrest in Sweden—a population-based register study of more than 18,000 cases. Int J Cardiol 2018;255:237–242. [DOI] [PubMed] [Google Scholar]
- 31. Thoren A, Rawshani A, Herlitz J, Engdahl J, Kahan T, Gustafsson L, Djärv T. ECG-monitoring of in-hospital cardiac arrest and factors associated with survival. Resuscitation 2020;150:130–138. [DOI] [PubMed] [Google Scholar]
- 32. Fernando SM, Tran A, Cheng W, Rochwerg B, Taljaard M, Vaillancourt C, Rowan KM, Harrison DA, Nolan JP, Kyeremanteng K, McIsaac DI, Guyatt GH, Perry JJ. Pre-arrest and intra-arrest prognostic factors associated with survival after in-hospital cardiac arrest: systematic review and meta-analysis. BMJ 2019;367:l6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med 2010;38:101–108. [DOI] [PubMed] [Google Scholar]
- 34. Morgan RW, Fitzgerald JC, Weiss SL, Nadkarni VM, Sutton RM, Berg RA. Sepsis-associated in-hospital cardiac arrest: epidemiology, pathophysiology, and potential therapies. J Crit Care 2017;40:128–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the author M.A.