Abstract
Background Lupus nephrtis in children is associated with high morbidity and mortality. The incidence of childhood systemic lupus erythematosus (SLE) ranges from 3.3 to 8.8/100000 children with a higher Asian preponderance. The predominance of SLE in female pediatric patients increases gradually with age to the values observed in adults.
Objectives To assess the clinical, immunological, and histopathological spectrum of childhood lupus nephritis in northeast India and explore the relationship between clinical, biochemical, serological, and histopathological findings.
Materials and Methods A retrospective descriptive study was performed over 8 years. Histopathology slides were reviewed by two pathologists, whereas other details were collected from patients' records.
Statistical Analysis Statistical analysis was based on the chi-square test and a p -value < 0.05 was considered statistically significant.
Results Fifty-three cases of lupus nephritis were included in the study. The patients' age ranged from 5 to 18 years with a mean age of 14.5 years and a female: male ratio of 6.5:1. Edema and hypertension were the commonest clinical presentations, whereas proteinuria was the commonest presenting laboratory parameter. Amongst all the immunological markers, dsDNA was the commonest. Histopathologically, predominantly study population belonged to class IV lupus nephritis. The patients with class IV showed a statistically significant correlation with proteinuria and hematuria at the time of diagnosis. Immunological markers, namely, ANA and anti-ds-DNA positivity were significantly associated with advanced renal histopathology.
Conclusion cSLE in northeast India presents mostly as Class IV LN presenting mostly with deranged laboratory parameters and preponderance of various immunological markers and clinical presentations.
Keywords: systemic lupus erythematosus, childhood lupus nephritis, class IV lupus nephritis
Introduction
Systemic lupus erythematosus (SLE) is a multisystem chronic inflammatory disorder of autoimmune origin predominately affecting the skin, joints, heart, lungs, and importantly kidneys. 1 Childhood SLE (cSLE) shows wide variation in its incidence and prevalence. Based on the demographic profile and age of the individuals, a comparatively higher frequency of cSLE cases is being reported amongst Asians, African Americans, Hispanics, and Native Americans. 2 It is a rare disease accounting for 15 to 20% of all the diagnosed cases of SLE, with an incidence of 0.3 to 0.9/100,000 children per year and a prevalence of 3.3 to 8.8/100,000. 3 4 5 The predominance of cSLE in female pediatric patients increases gradually with age to the values observed in adults. 6 7 8 9 Lupus nephritis in children is usually a severe manifestation of SLE and is associated with high morbidity and mortality. 10
The clinical presentation of lupus nephritis ranges from asymptomatic low-grade proteinuria to end-stage renal disease (ESRD). Unlike adults, the clinical features of SLE are often less conspicuous in the pediatric population with a significant proportion of children presenting with severe renal manifestation at onset, lacking adequate criteria to diagnose as SLE. 11 The International Society of Nephrology and Renal Pathology Society (ISN/RPS) graded lupus nephritis into six histological grades.
Our study is the first study conducted in northeast India emphasizing the epidemiological, clinical, immunological, and histopathological spectrum of cSLE. The relationship between the clinical, biochemical, serological, and histopathological findings is also studied in the present study.
Materials and Methods
This was a hospital-based retrospective descriptive study, spanning a period of 8 years (2012–2020) in the Department of Pathology. In all biopsy-proven cases with lupus nephritis in patients below 18 years of age, a minimum of 10 glomeruli was considered adequate. Only native kidney biopsies were included in the study. Clinical and laboratory data were retrieved from the medical record department of the hospital. Hematoxylin and eosin (H&E), Masson's trichrome (MT), periodic acid-Schiff (PAS), and Jones silver-stained slides were reviewed from the slide library of the department of histopathology. Data from immunofluorescence studies (IgA, IgG, IgM, C3, C1q, kappa, and lambda) were noted. Both histopathological and immunofluorescence slides were evaluated independently by two nephropathologists. A consensus was arrived after discussion in case of any disconcordance. All cases were classified according to the ISN/RPS classification system and statistical analysis was based on a chi-square test using the IBM SPSS Statistics Version 20 software. A p -value < 0.05 was considered statistically significant. All cases had informed written consent in their medical records about the use of individuals' data for academic purposes and all the patients' caretakers had consented to it in the past.
Results
A total of 53 biopsy-proven cases of lupus nephritis were included in the study. The patients' age ranged from 5 to 18 years. The mean age of presentation was 14.5 years. Out of the 53 cases, 46 were female and 7 were male with a female:male of 6.5:1. Amongst the clinical presentations, edema ( n = 20) and hypertension ( n = 20) were the most common presentation followed by skin lesions ( n = 17) and serous cavity effusion ( n = 16). Proteinuria ( n = 31), graded as 3+ ( n = 15), 2+ ( n = 10) and1+ ( n = 6) was the commonest laboratory parameters at presentation. Serum creatinine levels ranged from 0.4 mg/dL to 4.4 mg/dL with a mean of 1.4 mg/dL and serum creatinine levels were raised in 25 of the cases. Hematuria was seen in 23 of the cases, 21 cases showed microscopic hematuria, whereas only two cases showed macroscopic hematuria at presentation. Amongst all the immunological markers, dsDNA was the commonest presenting in 34 cases, followed by ANA with 33 positive cases.
Predominantly, the study population was classified as class IV lupus nephritis ( n = 34). No cases of class I and class V was noted. Histopathology showed mesangial hypercellularity, endocapillary proliferation, and wire loop lesions representing various classes of lupus nephritis ( Fig. 1 ). Immunofluorescence showed full house pattern with IgA, IgG, IgM, C3, C1q, Kappa, and lambda ( Fig. 2 ).
Fig. 1.
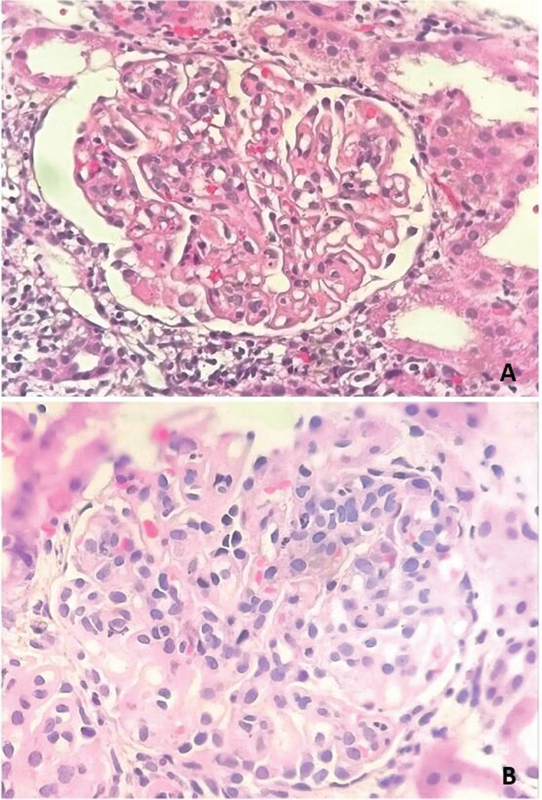
Histopathology of lupus nephritis showed ( A ) wire loop lesions and ( B ) mesangial hypercellularity (Hematoxylin and eosin, 200x and 400x).
Fig. 2.
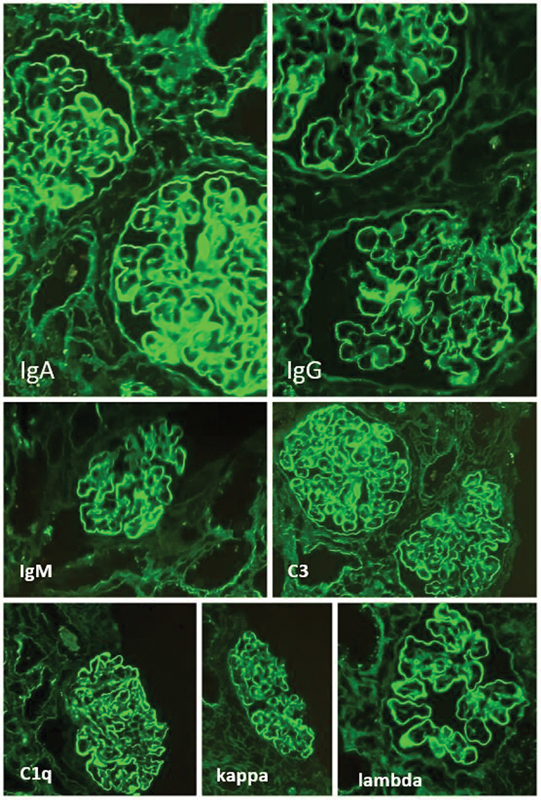
Immunofluorescence showing full house pattern for IgA, IgG, IgM, C3, C1q, kappa, and lambda (FITC, 100x and 200x).
Table 1 shows the relationship between the clinical, biochemical, serological, and histopathological findings of the study group. No statistically significant correlation between advanced histopathological class and age or gender in the study group was noted. However, significant correlation was noted for class IV LN patients presenting with proteinuria and hematuria at diagnosis. Moreover, ANA and anti-ds-DNA positivity were significantly associated with advanced renal histopathology.
Table 1. Relationship between clinical, biochemical, serological and histopathological findings.
| Histopathological class | II | III | IV | VI | p -Value | |
|---|---|---|---|---|---|---|
| Age (y) | <12 | 2 | 0 | 6 | 0 | 0.3 |
| >12 | 5 | 11 | 28 | 1 | ||
| Sex | Male | 1 | 2 | 4 | 0 | 0.9 |
| Female | 6 | 9 | 30 | 1 | ||
| S. creatinine | <1 mg/dL | 5 | 5 | 18 | 0 | 0.5 |
| >1 mg/dL | 2 | 6 | 16 | 1 | ||
| Proteinuria | 3+ | 0 | 2 | 12 | 1 | 0.01 |
| 2+ | 0 | 2 | 8 | 0 | ||
| 1+ | 0 | 4 | 2 | 0 | ||
| Nil | 7 | 3 | 12 | 0 | ||
| Hematuria | Present | 2 | 9 | 12 | 0 | 0.03 |
| Absent | 5 | 2 | 22 | 1 | ||
| Hypertension | Present | 2 | 6 | 12 | 0 | 0.5 |
| Absent | 5 | 5 | 22 | 1 | ||
| ANA | Positive | 1 | 7 | 24 | 1 | 0.003 |
| Negative | 6 | 4 | 10 | |||
| ds DNA | Positive | 2 | 10 | 21 | 1 | 0.04 |
| Negative | 5 | 1 | 13 | 0 | ||
Discussion
Our study illustrated clinical, biochemical, serological, and histopathological findings of LN in northeastern children of India. The etiopathogenesis of LN in children and adults is similar; however, the disease is more severe in pediatric populations. In our study, the prevalence of pediatric and adolescent lupus nephritis was found to be 11.3%, which was slightly higher than the prevalence seen in other studies 3 4 5 Patients' ages ranged from 5 to 18 years of age, which was similar to other studies that have found that LN is rare in children less than 5 years of age. 12 Female:male ratio was 6.5:1 in our study population, a finding almost similar to the study done by Samanta et al. 13 The prevalence of hypertension varies significantly across different studies, ranging from 30 to 50%. 14 15 16 In our study, hypertension was found in 37.7% of patients. In our study group, proteinuria was observed in 58.49% of cases ( n = 31) at presentation, microscopic hematuria and proteinuria are the most commonly identified abnormalities as seen in other Indian series. 17 18
Edema was the commonest presentation followed by skin lesions and serous cavity effusion in our study population. Most children clinically presented with serous cavity effusion, arthritis, and skin lesion. Despite a large number of clinical manifestations, the signs and symptoms of lupus nephritis do not always reflect the severity of the disease. Additionally, clinical findings do not predict the clinical development or the prognosis of patients with the disease. Therefore, kidney biopsy becomes an essential measure for assessing tissue involvement, categorizing lupus nephritis, and choosing the course of therapy
The current study showed class IV lupus nephritis was the most common histopathological finding (64.15%), similar to the results of a study done by Emre et al and Mittal et al. 19 20 We did not find any cases with class I or class V lupus nephritis in our study population a finding partially attributed to the lack of awareness among the caretakers of the patients and also to the unavailability of adequate health care facilities for prompt diagnosis. However medical literature notes a lower incidence of Class V LN in children. 21 In our study group, patients presenting with class IV LN were significantly associated with proteinuria, hematuria, and positivity for ANA and ds DNA, a finding consistent with a study done by Nandi et al and Jebali et al, where patients with Class IV LN were associated significantly with positive ds DNA. 14 17
Conclusion
SLE in the pediatric and adolescent population is a severe, chronic condition, and the overall mortality is high all over the world. Renal involvement is an important risk factor for mortality in patients with cSLE. A timely performed renal biopsy helps determine an accurate class of childhood lupus nephritis, which aids in better supportive care, selection of aggressive therapy, and use of newer drugs in selected cases. The current study helps in the documentation of various clinicopathologic spectra of childhood lupus nephritis in northeast India. The authors of the present study conclude that cSLE patients presenting as lupus nephritis in northeast India usually present with a higher histological grade with a statistically significant correlation concerning proteinuria and hematuria amongst the laboratory parameters, which points toward an aggressive clinical laboratory picture at presentation. There is an urgent need to improve aspects regarding clinical care and our understanding of various aspects associated with this disease, in particular regarding the evaluation of novel therapies as adjuncts to current treatment regimes.
Funding Statement
Funding None.
Conflict of Interest None declared.
Consent
The study was performed respecting the Declaration of Helsinki. Proper consent was taken for the study from the caretaker and identity of the patient was kept confidential.
References
- 1.Levy D M, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. 2012;59(02):345–364. doi: 10.1016/j.pcl.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malleson P N, Fung M Y, Rosenberg A M. The incidence of pediatric rheumatic diseases: results from the Canadian Pediatric Rheumatology Association Disease Registry. J Rheumatol. 1996;23(11):1981–1987. [PubMed] [Google Scholar]
- 3.Wenderfer S E, Ruth N M, Brunner H I. Advances in the care of children with lupus nephritis. Pediatr Res. 2017;81(03):406–414. doi: 10.1038/pr.2016.247. [DOI] [PubMed] [Google Scholar]
- 4.Kamphuis S, Silverman E D. Prevalence and burden of pediatric-onset systemic lupus erythematosus. Nat Rev Rheumatol. 2010;6(09):538–546. doi: 10.1038/nrrheum.2010.121. [DOI] [PubMed] [Google Scholar]
- 5.Oni L, Wright R D, Marks S, Beresford M W, Tullus K. Kidney outcomes for children with lupus nephritis. Pediatr Nephrol. 2021;36(06):1377–1385. doi: 10.1007/s00467-020-04686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira M V, Revelo M P, Bambirra E A. Lupic nephropathy in childhood: morphologic analysis of 18 cases [article in Portuguese] J Pediatr (Rio J) 1996;72(01):32–34. doi: 10.2223/jped.595. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal A, Srivastava P. Childhood onset systemic lupus erythematosus: how is it different from adult SLE? Int J Rheum Dis. 2015;18(02):182–191. doi: 10.1111/1756-185X.12419. [DOI] [PubMed] [Google Scholar]
- 8.Almaani S, Meara A, Rovin B H. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(05):825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraki L T, Benseler S M, Tyrrell P N, Harvey E, Hebert D, Silverman E D. Ethnic differences in pediatric systemic lupus erythematosus. J Rheumatol. 2009;36(11):2539–2546. doi: 10.3899/jrheum.081141. [DOI] [PubMed] [Google Scholar]
- 10.Sinha R, Raut S. Pediatric lupus nephritis: management update. World J Nephrol. 2014;3(02):16–23. doi: 10.5527/wjn.v3.i2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggiero B, Vivarelli M, Gianviti A et al. Lupus nephritis in children and adolescents: results of the Italian collaborative Study. Nephrol Dial Transplant. 2013;28(06):1487–1496. doi: 10.1093/ndt/gfs589. [DOI] [PubMed] [Google Scholar]
- 12.Al Salloum A A. Lupus nephritis in childhood. Saudi J Kidney Dis Transpl. 2003;14(01):43–56. [PubMed] [Google Scholar]
- 13.Samanta M, Nandi M, Mondal R et al. Childhood lupus nephritis: 12 years of experience from a developing country's perspective. Eur J Rheumatol. 2017;4(03):178–183. doi: 10.5152/eurjrheum.2017.16117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jebali H, Hajji M, Rais L, Hamida F B, Beji S, Zouaghi M K. Clinicopathological findings and outcome of lupus nephritis in Tunisian children: a review of 43 patients. Pan Afr Med J. 2017;27:153. doi: 10.11604/pamj.2017.27.153.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Abujam B, Gupta A et al. Childhood lupus nephritis in a developing country-24 years' single-center experience from North India. Lupus. 2015;24(06):641–647. doi: 10.1177/0961203315570166. [DOI] [PubMed] [Google Scholar]
- 16.Cameron J S. Lupus nephritis in childhood and adolescence. Pediatr Nephrol. 1994;8(02):230–249. doi: 10.1007/BF00865490. [DOI] [PubMed] [Google Scholar]
- 17.Nandi M, Mondal R. Renal involvement in childhood lupus: a study from Kolkata, India. Saudi J Kidney Dis Transpl. 2012;23(04):871–875. doi: 10.4103/1319-2442.98194. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal I, Kumar T S, Ranjini K, Kirubakaran C, Danda D. Clinical features and outcome of systemic lupus erythematosus. Indian Pediatr. 2009;46(08):711–715. [PubMed] [Google Scholar]
- 19.Emre S, Bilge I, Sirin A et al. Lupus nephritis in children: prognostic significance of clinicopathological findings. Nephron J. 2001;87(02):118–126. doi: 10.1159/000045899. [DOI] [PubMed] [Google Scholar]
- 20.Mittal A, Bamnawat H, Nalwa A et al. Pediatric onset lupus nephritis in western India-is it different from the rest of the country? Lupus. 2021;30(12):2008–2016. doi: 10.1177/09612033211045069. [DOI] [PubMed] [Google Scholar]
- 21.Wu J Y, Yeh K W, Huang J L. Early predictors of outcomes in pediatric lupus nephritis: focus on proliferative lesions. Semin Arthritis Rheum. 2014;43(04):513–520. doi: 10.1016/j.semarthrit.2013.07.005. [DOI] [PubMed] [Google Scholar]


