Summary
Background
On Aug 29, 2021, Operation Allies Welcome (OAW) was established to support the resettlement of more than 80 000 Afghan evacuees in the USA. After identification of measles among evacuees, incoming evacuee flights were temporarily paused, and mass measles vaccination of evacuees aged 6 months or older was introduced domestically and overseas, with a 21-day quarantine period after vaccination. We aimed to evaluate patterns of measles virus transmission during this outbreak and the impact of control measures.
Methods
We conducted a measles outbreak investigation among Afghan evacuees who were resettled in the USA as part of OAW. Patients with measles were defined as individuals with an acute febrile rash illness between Aug 29, 2021, and Nov 26, 2021, and either laboratory confirmation of infection or epidemiological link to a patient with measles with laboratory confirmation. We analysed the demographics and clinical characteristics of patients with measles and used epidemiological information and whole-genome sequencing to track transmission pathways. A transmission model was used to evaluate the effects of vaccination and other interventions.
Findings
47 people with measles (attack rate: 0·65 per 1000 evacuees) were reported in six US locations housing evacuees in four states. The median age of patients was 1 year (range 0–26); 33 (70%) were younger than 5 years. The age distribution shifted during the outbreak towards infants younger than 12 months. 20 (43%) patients with wild-type measles virus had rash onset after vaccination. No fatalities or community spread were identified, nor further importations after flight resumption. In a non-intervention scenario, transmission models estimated that a median of 5506 cases (IQR 10–5626) could have occurred. Infection clusters based on epidemiological criteria could be delineated into smaller clusters using phylogenetic analyses; however, sequences with few substitution count differences did not always indicate single lines of transmission.
Interpretation
Implementation of control measures limited measles transmission during OAW. Our findings highlight the importance of integration between epidemiological and genetic information in discerning between individual lines of transmission in an elimination setting.
Funding
US Centers for Disease Control and Prevention.
Introduction
After the fall of Kabul and the Taliban takeover of Afghanistan on August 15, 2021, the US Government established Operation Allies Welcome (OAW) to support Afghan evacuee resettlement in the USA. Because of persistently low measles vaccination coverage (66% for the first dose and 43% for the second dose in 2020)1 and an ongoing measles outbreak (25 988 clinical cases reported during January–November, 2021)2 in Afghanistan, the US Centers for Disease Control and Prevention (CDC) encouraged public health officials to maintain high vigilance for measles among Afghan evacuees.3
Although measles was declared to be eliminated in the USA in 2000,4 endemic measles persists in many countries,5 and importations continue to be a risk. In 2019, the USA reported the largest number of measles cases in more than 25 years, nearly losing its measles elimination status due to prolonged outbreaks in underimmunised communities after repeated importations.4,6,7 Furthermore, as in other countries,5,8 the COVID-19 pandemic led to decreases in administered vaccines in the USA.9 Although catch-up vaccination has occurred, prepandemic levels of vaccination have not been reached, and the impact of declines in immunisation on the US measles susceptibility landscape is unknown.
During OAW, more than 80 000 Afghan evacuees were temporarily housed at several overseas locations and transported to eight US military bases and a contracted isolation and quarantine hotel (Hotel A) before resettlement.10 After identification of measles among evacuees, three core strategies were implemented to mitigate the risk of measles during OAW: a pause on incoming international evacuee flights; mass measles, mumps, and rubella (MMR) vaccination of evacuees aged 6 months or older at US and overseas locations; and a quarantine to remain at these locations for 21 days post vaccination. We aimed to understand the patterns of measles virus transmission during OAW and the impact of the public health actions in limiting measles spread.
Methods
Study design and definitions
We conducted a measles outbreak investigation among Afghan evacuees who were resettled in the USA as part of OAW. Patients with measles were defined as individuals with an acute febrile rash illness occurring between Aug 29, 2021 (initiation of OAW), and Nov 26, 2021 (42 days, or two maximum incubation periods, after rash onset in the last patient), and either laboratory confirmation of infection or epidemiological link to a patient with measles with laboratory confirmation.11 Laboratory confirmation of measles infection is described in the appendix (p 5). Cases were categorised as an international importation if at least some of the patient’s exposure period (7–21 days before rash onset) occurred outside the USA and rash onset occurred within 21 days of entering the USA, with no known measles exposure in the USA during that time.11 This activity was reviewed by the CDC and was conducted in accordance with applicable federal law and CDC policy (45 CFR 46, 21 CFR 56; 42 USC 241d; 5 USC 552a; 44 USC 3501 et seq). The CDC determined that this investigation constituted an emergency public health activity; thus, it was exempt from institutional review board review and written informed consent was not required.
Procedures
Patients were identified upon intake physical examinations, when seeking care at on-site acute care clinics, or during wellness checks of barracks. Demographics, clinical presentation, and outcomes of patients were obtained through interviews with the patient or their parent or guardian, and by review of medical records, using case-investigation forms.
MMR vaccination was provided to all eligible infants (aged 6 months and older), children, and adults. Rates of MMR vaccine uptake among eligible evacuees were calculated using data documenting the daily number of MMR doses that were administered at each military base and Hotel A from Sept 9 to Oct 15, 2021 (the date of rash onset of the last patient). Evacuees were considered ineligible for vaccination if they were younger than 6 months or pregnant (appendix p 5).12
Outcomes
We evaluated patients with wild-type measles who developed rash after vaccine administration to assess the impact of the recommended 21-day quarantine after receipt of MMR vaccine. To distinguish between those who were likely to have been infected before vaccination from those who might have been exposed after vaccination, individual patient timelines were explored, including the date of MMR vaccination and inferred exposure period. To determine the most likely exposure dates, we used a lognormal probability distribution for the incubation period of measles derived from a historical dataset,13,14 with a median of 11·82 days (IQR 9·82–14·22) and a delay of 2 days between illness and rash onset.15
To assess the impact of control measures under different scenarios, we created a stochastic compartmental model to simulate potential outbreak trajectories at the five military bases that reported cases (Hotel A was modelled separately; appendix p 13). We compared a base-case scenario of vaccination as it occurred versus four specific intervention scenarios: no intervention; delay in vaccination by 7 days and 14 days; not lowering the age of MMR vaccine administration to include those aged 6–11 months; and not pausing flights from overseas locations (modelled as additional single importations to two domestic bases on Sept 10, 17, and 24). Simulated attack rates and the median (IQR) of the number of cases pooled across affected bases are presented. Sensitivity analyses varying several initial parameters of the model (increasing and decreasing baseline susceptibility, varying vaccine effectiveness of campaign doses, and exploring the full range of the basic reproduction number, R0) were performed.
Epidemiological clusters or groupings were determined based on shared locations (eg, flights, barracks, or base). We used contact tracing data to assess potential transmission events between cases. We plotted individual itineraries and explored overlap in the infectious and exposure periods and locations of cases. The exposure period was defined as 7–21 days before rash onset, and the infectious period as 4 days before to 4 days after rash onset.16 Potential infector-infectee pairs were identified based on known exposures, including shared flights, shared barracks, or being from the same family, along with appropriate delays from rash onsets of the potential infector to the potential infectee. Unrelated cases in different transmission chains within the USA were determined based on having differing arrival airports, domestic locations, and rash onset dates more than one maximum incubation period (21 days) apart.
Measles virus genotypes were determined by sequencing 450 C-terminal nucleotides of the measles virus nucleoprotein (N450), using standardised Sanger methods.17 Whole-genome sequencing was attempted on nasopharyngeal or oropharyngeal swab extracts from 43 individuals using probe hybridisation-enrichment RNA-Seq;18 41 sequencing attempts were successful and used in the final analyses alongside sequences from public data (n=114 for entire set). Bayesian phylogenies were computed using BEAST (version 2.6.3; appendix p 13),19 using a strict molecular clock with partitioning of coding and non-coding regions of the viral genome.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
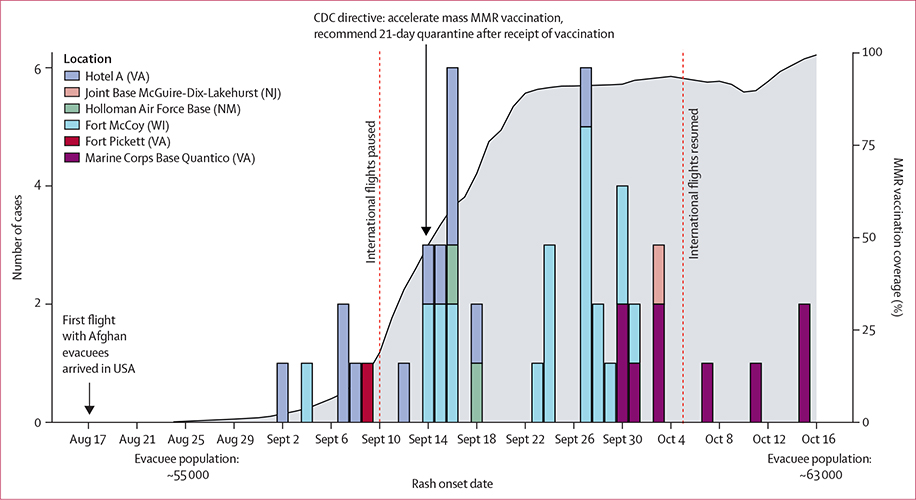
47 confirmed patients with measles were reported among 72 306 evacuees who had arrived in the USA by Nov 26, 2021 (crude attack rate: 0·65 per 1000 evacuees). Cases were reported in six domestic sites in four states; most were reported in Fort McCoy in Wisconsin, and Hotel A and Marine Corps Base (MCB) Quantico in Virginia (figure 1). Rash onsets ranged from Sept 2 to Oct 15, 2021.
Figure 1: Epidemiological curve of measles cases during Operation Allies Welcome, August–October, 2021.
Cases are shown according to location and date of rash onset. For two cases, the date of onset of rash could not be determined; therefore, the date that the case was identified is shown. Cases were reported in six locations: Hotel A (12 cases), Joint Base McGuire-Dix-Lakehurst (one case), Holloman Air Force Base (two cases), Fort McCoy (22 cases), Fort Pickett (one case), and Marine Corps Base Quantico (nine cases). The first flight with Afghan evacuees arrived on Aug 17, 2021. Flights from international locations were temporarily paused on Sept 10, 2021, and resumed on Oct 5, 2021 (indicated by vertical red dashed lines). The cumulative proportion of MMR vaccine doses administered to eligible Afghan evacuees in domestic sites is shown in grey; routine immunisation of arriving Afghan evacuees started on Aug 24, but vaccine efforts started to increase once cases were identified at domestic sites, and accelerated with the issuance of a directive for mass MMR vaccination across domestic and international sites on Sept 14. CDC=US Centers for Disease Control and Prevention. MMR=measles, mumps, and rubella.
The median age of patients was 1 year (range 0–26); 33 (70%) were younger than 5 years (appendix p 18). Before the midpoint of the outbreak (Sept 24), four (16%) of 25 patients were infants younger than 12 months; after Sept 24, 13 (59%) of 22 patients were infants younger than 12 months (p=0·0057 by χ2 test). All 47 patients were unvaccinated or had unknown vaccination status upon arrival to the USA (appendix p 25). 28 (60%) patients were admitted to hospital, and 26 (55%) experienced at least one complication. The high proportion of patients who were hospitalised might be indicative of the low threshold for measles-related hospitalisation in the USA. No deaths occurred.
After the identification of patients with measles at domestic sites, rapid scale-up of vaccination efforts, led by the Department of Defense, began across domestic military bases. All evacuation flights from overseas locations to the USA were temporarily halted on Sept 10, 2021. On Sept 14, the CDC issued a directive to the OAW Unified Coordination Group10 recommending: a formal pause on evacuation flights from overseas locations to the USA; the acceleration of mass MMR vaccination for all eligible evacuees aged 6 months or older who did not have contraindications, both domestically and internationally; and for evacuees to remain in quarantine (ie, on bases and overseas locations) for 21 days after receipt of the MMR vaccine. Efforts were also made to provide immunoglobulin to individuals who were ineligible for MMR vaccine (infants aged <6 months and seronegative pregnant women) at domestic sites.
At arriving airports, evacuees were screened for symptoms, and those presenting with measles-compatible symptoms were transported to a hospital for medical assistance and evaluation, then directly to Hotel A with their family unit for isolation and quarantine. Additionally, OAW staff and volunteers were recommended to be up to date on measles vaccination, and advance notification of hospitals receiving a suspected patient with measles was recommended so that the patient could be isolated on arrival.
Mass vaccination rapidly reached high coverage at domestic military bases and Hotel A: an estimated 91% of eligible evacuees were vaccinated by Sept 24, and an estimated 98% by Oct 15 (figure 1). Despite a large volunteer and interagency workforce present at domestic sites, and many of the patients with measles being evaluated at local hospitals, no measles cases were reported among military personnel, volunteers, or other staff; no measles cases resulting from transmission in health-care settings were reported; and no seeding of measles in US communities occurred. During the flight pause, four measles cases were reported among Afghan evacuees at Ramstein Air Base in Germany. OAW international flights resumed on Oct 5, 2021, with no additional measles cases identified among evacuees arriving after the pause.
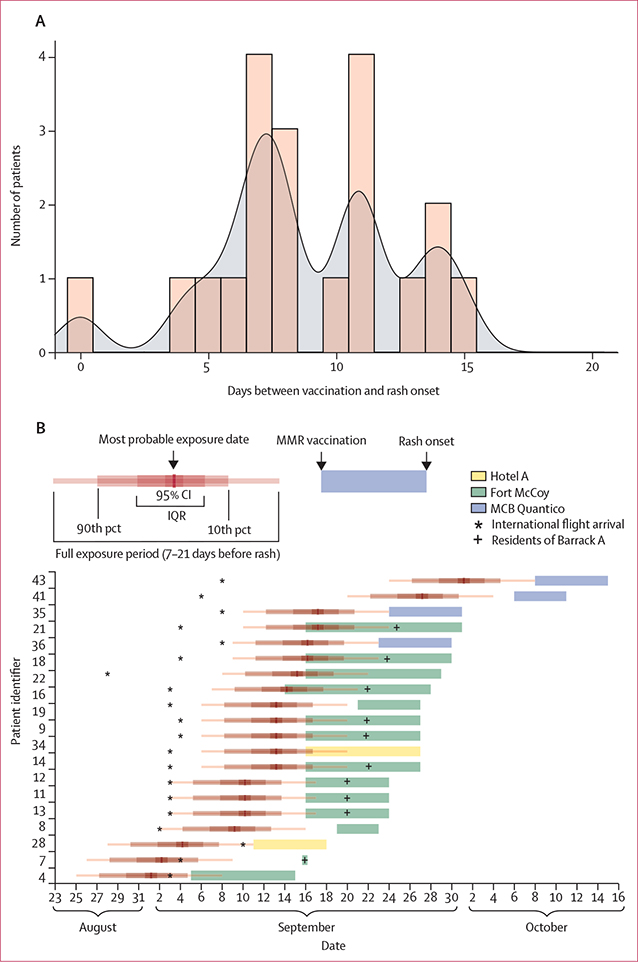
20 (43%) of 47 patients with wild-type measles virus detected received MMR vaccine before rash onset; rash onsets occurred a median of 8 days (range, 0–15) after vaccination, all within the 21-day quarantine period (figure 2A). For all 20 of these patients, at least part of their exposure period occurred before vaccination, including 17 (85%) for whom the exposure most likely occurred before vaccination (figure 2B). In three patients, the most likely exposure date occurred after vaccination; all three patients resided in Barrack A of Fort McCoy, where 12 other cases were reported. Among patients with longer times to rash after vaccination, exposure periods probably occurred just before or soon after vaccination, whereas in patients with shorter times to rash after vaccination, exposure periods probably occurred considerably before vaccination.
Figure 2: Length of time between MMR vaccine and onset of rash and timeline of probable exposure period for 20 patients who developed wild-type measles after receiving MMR vaccine during Operation Allies Welcome, August–October, 2021.
Genotype B3 was identified in all specimens. (A) Histogram and kernel density plot of the time in days between MMR vaccination and onset of rash. The kernel density has a probability of 0 after 17 days (bandwidth=0·8488, selected using unbiased cross validation). (B) Timeline for each of the patients, including date of arrival from overseas location, inferred exposure period, and the time between MMR vaccination and rash onset, colour-coded by domestic location. MCB=Marine Corps Base. MMR=measles, mumps, and rubella. pct=percentile.
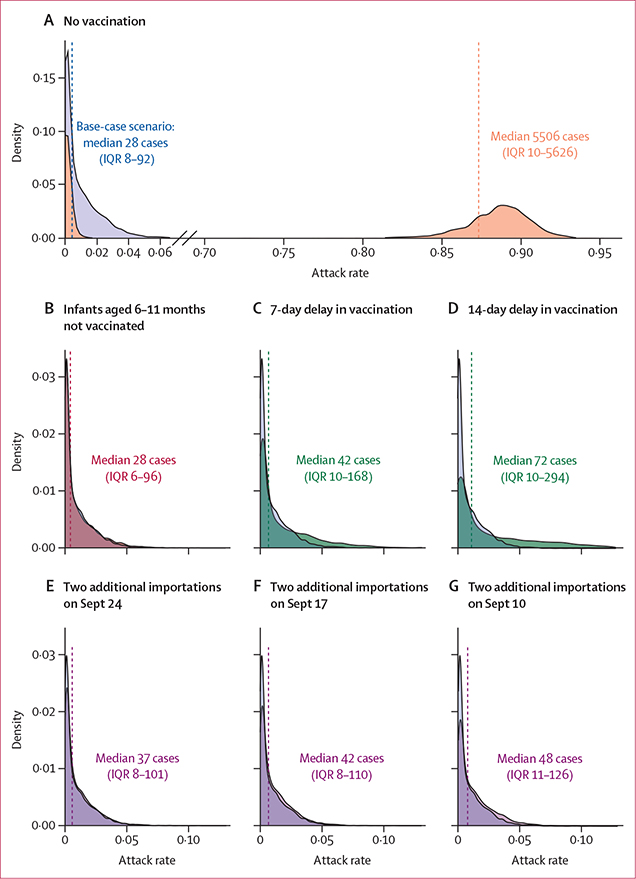
Modelling of a no-vaccination intervention scenario estimated a median attack rate of 87·33% (5506 cases [IQR 10–5626]); because of the stochastic nature of the model, there was a wide uncertainty range surrounding this estimate, including runs that led to no outbreak. By contrast, the base-case scenario (describing vaccination as it occurred) estimated a median attack rate of 0·44% (28 cases [6–96], compared with 35 patients with measles reported at these five bases in reality; figure 3, appendix pp 19–22). The largest increase in the absolute number of cases was seen in the scenario in which vaccination campaigns were delayed by 14 days (44 additional cases [median 72, IQR 10–294]; more cases were attributed by the model the longer the delay), followed by the scenario in which two additional importations occurred on Sept 10 (20 additional cases [48, 11–126]; more cases were attributed by the model the earlier the introductions occurred). Modelled scenarios showed no increase in the absolute number of cases when infants aged 6–11 months were excluded from vaccination. A combination of interventions had the greatest overall impact in limiting transmission (appendix p 22), and similarly showed that delays in vaccination would have led to the largest increase in attack rates, followed by additional importations (not pausing flights). The relative impact of modelled interventions was robust to varying the initial assumptions of the model, including varying the effectiveness of vaccine campaign doses, R0, and overall population susceptibility (appendix pp 26–28).
Figure 3: Model simulations of the probability distribution of measles attack rates among susceptible evacuees in five military bases with measles cases, under various scenarios.
(A) Mass vaccination not conducted, shown in orange (median attack rate 87·33%, IQR 0·15–89·23). (B) Age of measles, mumps, and rubella vaccine administration not lowered to 6 months (ie, those aged 6–11 months were not vaccinated, shown in red; median attack rate 0·44%, 0·10–1·53). (C) Vaccination delayed by 7 days (ie, vaccination uptake as it occurred but shifted by 7 days, shown in green; median attack rate 0·68%, 0·15–2·66). (D) Vaccination delayed by 14 days (ie, vaccination uptake as it occurred but shifted by 14 days, shown in green; median attack rate 1·14%, 0·15–4·67). (E–G) Two additional importations, one at Fort McCoy and one at MCB Quantico, on Sept 24 (median attack rate 0·58%, 0·12–1·61), Sept 17 (median attack rate 0·67%, 0·13–1·75), or Sept 10 (median attack rate 0·76%, 0·18–2·00); intervention scenarios are shown in purple. Each panel compares the base-case scenario (model results using vaccination uptake as it occurred), shown in blue (median attack rate 0·44%, IQR 0·12–1·46; in reality, 35 cases were observed in this outbreak across these five bases), with the modelled scenario. Vertical dashed lines represent median modelled cases. Five military bases with reported cases are included in these results (Fort McCoy, WI; MCB Quantico, VA; Holloman Air Force Base, NM; Joint Base McGuire-Dix-Lakehurst, NJ; and Fort Pickett, VA); total population, n=35 951, total estimated susceptible population, n=6305. Hotel A was modelled separately (appendix pp 19, 21). Medians and IQRs for the attack rates among susceptible evacuees and the total number of cases were generated from 1000 simulations per military base, which were pooled to create these combined estimates. MCB=Marine Corps Base.
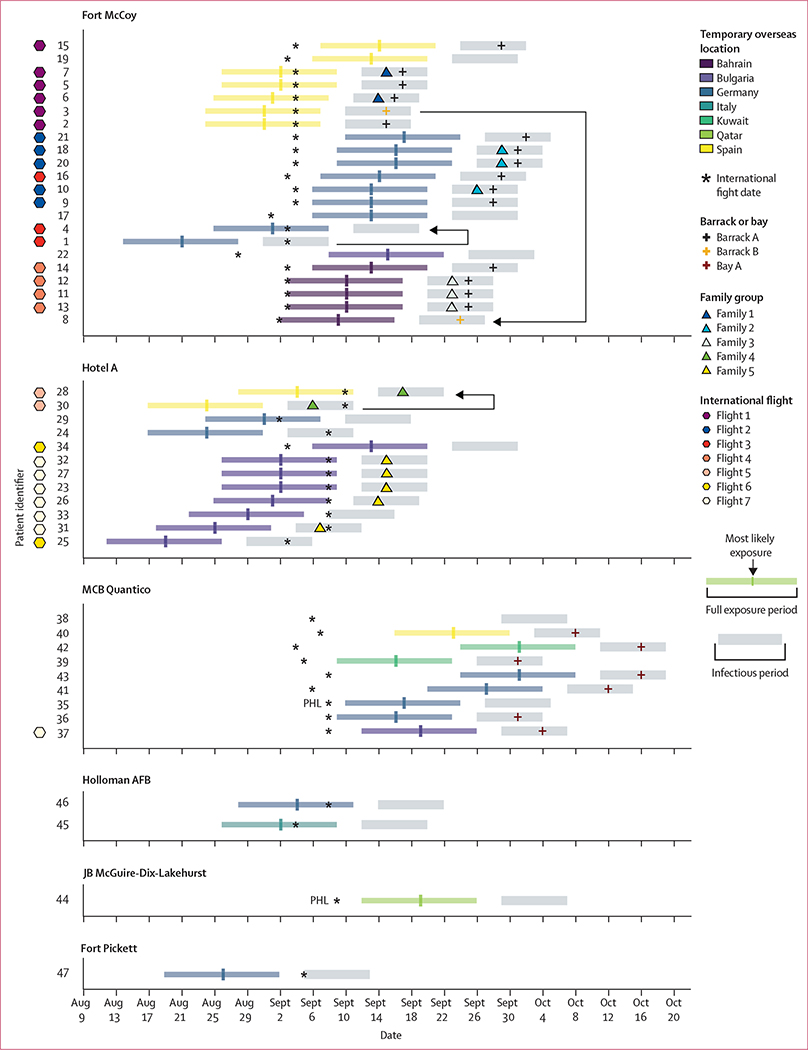
In an analysis of patterns of transmission, six separate infection clusters were identified based on arrival location (figure 4). Of the 47 patients with measles, 29 (62%) shared an incoming international evacuee flight with at least one other person with measles, and 45 (96%) transited through Dulles International Airport, of whom 18 (40%) had at least part of their exposure period occur on arrival. 20 (43%) cases were classified as importations; an additional five patients with measles had at least part of their exposure period occur outside the USA but were known to have been exposed to measles in the USA. The first patients identified at MCB Quantico and Joint Base McGuire-Dix Lakehurst had been at the bases for more than 21 days before rash onset, so the initial generation of cases was missed at these locations. Barrack A in Fort McCoy and Bay A in MCB Quantico were the most affected housing units. 15 (32%) of the patients were part of five family clusters. We identified three infector-infectee case-pairs (1→4, 3→8, and 30→28) and six case-pairs known to be unrelated (patient 35 unrelated to patients 1, 24, 25, 30, 31, and 47) based on epidemiological information (appendix p 15).
Figure 4: Trajectories and infectious and exposure periods of patients with measles during Operation Allies Welcome, August–October, 2021.
For each patient, we reviewed the overseas location before travel to the USA, flight information, US arrival date, receiving US airport, domestic location, barrack or bay housing, and information on relatives who were also patients. Each patient was assigned a number based on the order in which they were identified and plotted by their domestic and overseas location and date of onset of rash. For two patients, the date of onset of rash could not be determined, so the date that the patient was identified is shown. Exposure and infectious periods are shown for each patient to allow for a visual comparison of potential sources of infection among patients at various locations. Two patients arrived to the USA through PHL, the rest arrived through IAD (four times the number of evacuees arrived through IAD compared with PHL). Highly probable transmission pairs are noted by black lines, with arrows indicating the direction of transmission (1→4, 3→8, and 30→28). AFB=Air Force Base. IAD=Dulles International Airport. JB=Joint Base. MCB=Marine Corps Base. PHL=Philadelphia International Airport.
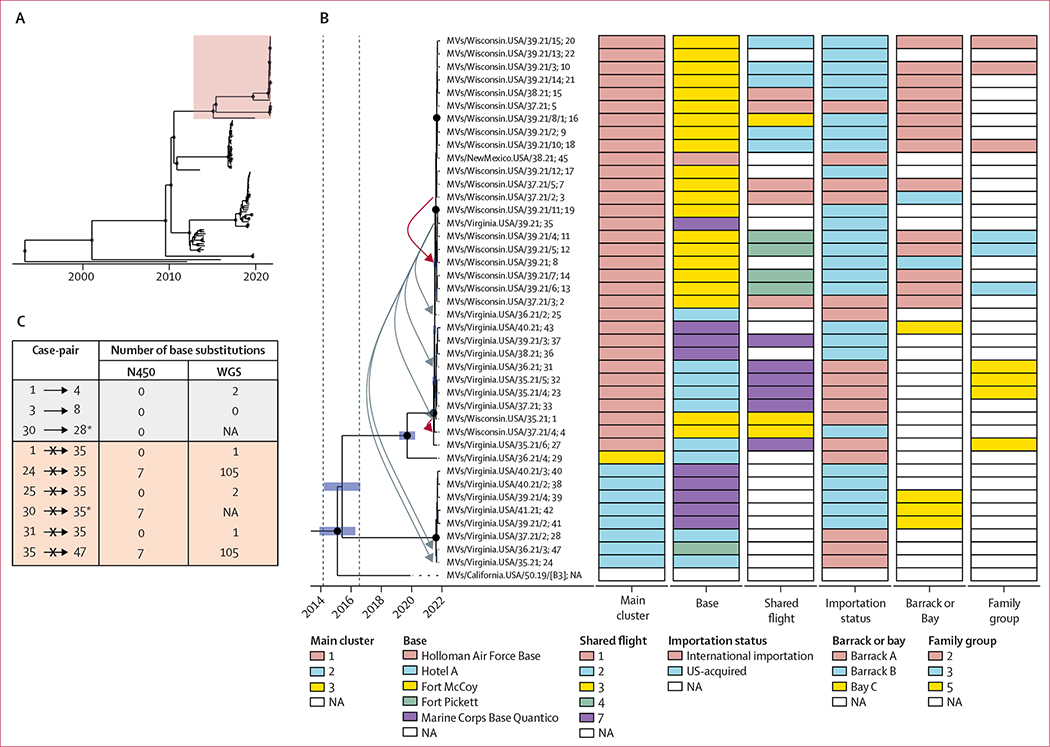
All viral sequences were found to be of genotype B3. The mean posterior clock rate was 4·98 × 10−4 substitutions per site per year (95% highest posterior density interval [HPD] 4·12 × 10−4 to 5·90 × 10−4), consistent with previously reported values for measles virus.20,21 The mean tree height (common ancestor age converted to decimal years) was 1993·288 (95% HPD 1988·963 to 1997·484). The mean common ancestor date of OAW specimens was 2015·408 (2014·157 to 2016·553). Whole-genome sequencing tree topologies showed importation of multiple genetically distinct measles virus lineages and distribution of these lineages across domestic arrival sites. Three well supported genetic groups were resolved, including one with cases in multiple domestic locations (ie, bases in Wisconsin, Virginia, and New Mexico; cluster 1, n=32 sequences; figure 5A, B). Conversely, some epidemiological groupings (eg, Hotel A, MCB Quantico, and Bay A) contained cases representing multiple viral lineages. In particular, Hotel A and MCB Quantico both housed cases from genetic clusters 1 and 3, indicating some diversity of imported viruses exceeding what would be expected by direct transmission during the outbreak period (figure 5B).
Figure 5: Phylogenetic data for viral whole genomes obtained from patients with measles during OAW, August–October, 2021.
(A) Complete topology of the phylogenetic tree obtained from all measles virus B3 sequences (n=114), of which the exploded lineage containing only OAW specimens is highlighted in pink. (B) Maximum clade credibility tree of measles virus sequences from OAW specimens for which WGS was successful, summarised using mean node heights in years pre-dating the most recent case. Very highly supported internal nodes (posterior probability >0·90) are shown as black dots. Inferred time in decimal calendar years of internal node (common ancestor) origins is shown as blue bars comprising the 95% HPD. 95% HPD for the common ancestor date of OAW specimens is denoted by vertical dashed lines. Cases are annotated with WHO measles sequence nomenclature; patient identifier. High-confidence transmission events informed by epidemiological information are depicted as red arrows. Transmission events of nil confidence are depicted as grey arrows. Base substitution counts for relevant exposure groupings, relative to the earliest ordinal case in that group, are shown in the appendix (pp 29–30). (C) Number of accumulated base substitutions from both the N450 region alone and the whole-genome sequence of proposed transmission pairs (1→4, 3→8, and 30→28) and unrelated control pairs. *Only N450 regions are shown because WGS for case 30 was not successful (appendix p 24). HPD=highest posterior density interval. NA=not available. OAW=Operation Allies Welcome. WGS=whole-genome sequencing.
Phylogenetic topology supported some high-confidence transmission linkages. Two such transmission pairs (1→4, 3→8) were substantiated by their close grouping in branch distance (figure 5B), and by the negligible accumulation of base substitutions (figure 5C). For a third pair (30→28), whole-genome sequencing was not available; tree distance and substitution counts were observed based on N450 only. Sequences obtained by whole-genome sequencing from three of the five family groups were narrowly clustered. Among control pairs (ie, cases not epidemiologically linked to case 35), sequencing analyses excluded relatedness to cases 24 and 47 by virtue of biologically implausible base substitution counts (figure 5C) and phylogenetic separation (figure 5B). However, sequencing analyses could not exclude relatedness between case 35 and cases 1, 25, and 31 because of negligible substitution counts and phylogenetic proximity.
Discussion
Identification of measles in a resettled population with low measles vaccination coverage, living in congregate settings, demanded immediate public health action. Our findings provide evidence that the rapid implementation of control measures curbed measles virus transmission after arrival to the USA. First, the decision to pause evacuee flights from overseas locations to allow for mass vaccination prevented additional importations while population immunity was building among evacuees, and was especially important given identification of international cases during the pause.22,23 Second, the high MMR vaccine coverage achieved across the age range and within 3 weeks of identification of the first case limited spread among evacuees and resulted in a very low case count relative to the population at risk (attack rates up to 25·5% have been observed in measles outbreaks in refugee settings with distinct levels of baseline immunity and rapidity of vaccine uptake).24 Furthermore, previous studies indicate that, in some emergency settings, in addition to infants and young children, targeting older children and adults during outbreak response immunisation is needed to achieve adequate levels of population immunity and stop measles virus transmission.25 Third, the 21-day quarantine post vaccination avoided seeding of neighbouring communities by patients incubating measles at the time they were vaccinated. Fourth, establishing an isolation and quarantine facility (Hotel A) permitted isolation of patients with measles who were symptomatic on arrival, as well as quarantining of their close contacts, preventing further introductions and transmission at bases. Finally, the change in age distribution over the course of the outbreak indicated the additional risk posed to infants, and the importance of lowering the age of MMR vaccination to 6 months in outbreaks with ongoing exposure to infants at highest risk for severe disease.16,26
Our model predictions corroborated these findings, showing increases in the predicted absolute number of cases if mass vaccination had been delayed or additional introductions had occurred before high levels of population immunity were reached. These results support previous observational and modelling studies highlighting the effectiveness of rapid and broad uptake of vaccination in limiting measles virus transmission in forcibly displaced populations.25,27 The lack of impact of lowering the age of vaccination to 6 months in the model was influenced by the small number of infants relative to the size of the population, the assumed baseline susceptibility among infants, and the rapid uptake of vaccination. We acknowledge that this resettlement effort was unique, and that some of the core mitigation strategies that were implemented (eg, halting flights and quarantine) might not be feasible or suitable in other forced displacement and resettlement contexts.
Quarantine periods are determined by the date of exposure. However, the high degree of mixing among evacuees made it difficult to ascertain exposures or their timing. Thus, to account for individuals who were already incubating measles at the time they were vaccinated, recommendations were based on the date of MMR vaccine receipt. A period of quarantine was important in preventing community spread because almost half of cases (infection with wild-type virus) were identified in recently vaccinated people. The length of quarantine used in elimination settings for the purposes of measles control emphasises disease prevention and is conservatively based on the maximum incubation period of measles (commonly 21 days is used).28 Although no patients developed rash after 15 days of being vaccinated during OAW, this finding is based on a small number of patients and does not capture the full range of incubation periods that have been previously described (7–21 days),14 or the rare instances when the incubation period has been suggested to exceed 21 days.14,29 A shorter duration of quarantine might be used in certain contexts depending on risk-benefit considerations. Because the start of quarantine was anchored on the date of vaccination, and MMR vaccine is more than 90% effective in preventing measles when given within 3 days after exposure,28 a quarantine of 18 days might have been sufficient during OAW. However, three evacuees in Barrack A, Fort McCoy, who might have been vaccinated near the time of exposure, developed measles, highlighting that in a congregate setting with high force of infection, the vaccine might fail, especially when given after exposure.
This investigation highlights the challenges with defining chains of measles virus transmission for the purpose of documenting maintenance of elimination. Re-establishment of endemic transmission is defined as occurring when a chain of transmission of a measles virus strain continues uninterrupted for at least 12 months.30 As such, national measles control programmes would optimally discern discrete transmission events to determine individual chains of transmission, which is difficult. During OAW, determining measles transmission chains with certainty was challenging because of overlapping infectious and exposure periods among multiple patients at various locations, as well as unidentified cases. In fact, even classification of cases as being imported or locally acquired was difficult due to our inability to distinguish between international, transit-associated, and domestic exposures. Similarly, the occurrence of multiple importations, implying the potential for multiple concurrent transmission chains, further complicates a clear delineation of chains of transmission, particularly when case burden is high.6,31 Thus, in most situations, the duration of transmission is based on the assumption that clusters of cases linked in time and space all form part of the same transmission chain. For surveillance purposes, cases associated with OAW were presumed to belong to six separate infection clusters, although there may have been more or fewer transmission chains.
Acquisition of complete sequences from measles cases is an area of active research to improve the identification of transmission pathways in post-elimination settings, where traditional short-window typing sequences (eg, N450) are increasingly homogeneous (appendix p 16).32 We observed considerable genetic diversity of sequences among imported cases and three separate genetic clusters. Because genetic clusters were not fully concordant with epidemiologic clusters, we show that cases assigned to an infection cluster by epidemiologic criteria could be further differentiated into smaller clusters by genetic analyses. For example, the two genetic clusters that were identified within the MCB Quantico infection cluster suggest these cases belonged to (at least) two separate chains of transmission, thus at least two importations were missed. Whole-genome sequencing was not as useful when evaluating individual transmission events, indicating some limit to the utility of whole-genome sequencing when assessing contemporaneous importations from the same source population. As expected, there were very few base-pair differences between infector-infectee case pairs. However, we also observed very few sequence differences between some unrelated case pairs, which implies the possibility of repeated introductions of the same lineage and that identical sequences would, in these cases, not necessarily signify a single line of transmission. Our study highlights the importance of integration between epidemiological findings and genetic data in documenting measles elimination.
Several limitations of our analyses are worth noting. Some under-reporting was evident, given generations were missed at two arrival bases. Determination of transmission links between infector-infectee pairs was not certain due to substantial mixing among evacuees, although these transmission links were supported by epidemiological and genetic data. There was considerable uncertainty both in the baseline level of measles susceptibility among evacuees and the estimate of R0 that were used in the models. Reassuringly, overall trends in modelling results were robust to modifications of initial parameters. Similarly, the assumption of homogeneous mixing in the model would not be reflective of more complex patterns of mixing among evacuees at domestic locations, and would overestimate final outbreak sizes, although our main comparisons of the interventions were against the vaccination scenario, which yielded a conservatively low attack rate, similar to what was observed.33 Given these limitations, the model trajectories should not be viewed as exact projections, but rather as a way to compare the impact of public health interventions under various scenarios. Phylogenetic results were well resolved and biologically plausible, but we note some instability of the model when non-coding genomic regions were considered independently. This was mitigated by inclusion of publicly available whole-genome sequencing data and use of less complex model settings.
Rapid implementation of control measures through a coordinated public health response prevented potentially thousands of cases in a vulnerable evacuee population, and avoided additional importations of measles and spread into US communities. Our investigation is unique in that an almost complete set of viral genomes was obtained from the individuals with measles, and we were able to show the benefits and limitations of whole-genome sequencing in discerning chains of measles virus transmission, which is important in the context of elimination. However, this in-depth molecular epidemiology work is often not possible in low-income and middle-income countries. Of note, the measles outbreaks associated with OAW occurred at a time when many US children had fallen behind on recommended immunisations because of the COVID-19 pandemic. We highlight the importance of preparedness and investment to control measles outbreaks in preserving elimination34 as we face the potential for an increase in measles cases due to the millions of infants and children worldwide who missed measles vaccines during the pandemic.5
Supplementary Material
Research in context.
Evidence before this study
Although endemic measles virus transmission was declared eliminated from the USA in 2000, measles remains endemic in many countries and continues to be a risk to the USA through international importations and subsequent spread in undervaccinated subpopulations. The duration of a measles virus transmission chain is the key factor determining maintenance of elimination status. Because of decreasing sequence diversity of circulating measles viruses globally, the current target window for typing sequences has become increasingly homogeneous, and has become less helpful in distinguishing between individual lines of transmission. Whole-genome sequencing has been proposed to aid in tracking of measles virus transmission pathways.
We searched PubMed for relevant articles published in English between Jan 1, 2011, and Aug 29, 2021, using the terms “([(measles[Title]) AND (refugee[Title/Abstract])] OR (resettlement[Title/Abstract])) OR (whole genome sequencing[Title/Abstract])”. The search yielded 34 studies. Six studies described measles outbreaks in refugee settlements that included some evaluation of the effectiveness of reactive vaccination campaigns. However, none occurred in an elimination context, in which evacuees had access to high levels of medical care in a controlled and secure environment; only one study used a mathematical model of measles transmission to measure the impact of vaccination; and none included assessments of other containment strategies. Four previous studies evaluated the utility of whole-genome sequencing in characterising measles outbreaks. None reported directly sequenced (unbiased) viral metagenomes, evaluated differences in sequences among cases with confirmed epidemiological links and cases with confirmed absence of epidemiological links, or situations in which several importations occurred from the same geographic location over a short period of time.
Added value of this study
We show that the rapid deployment of mass measles, mumps, and rubella vaccination is crucial in halting measles outbreaks among vulnerable migrant populations in congregate settings. We describe a change in the age distribution during the outbreak towards infants, highlighting the importance of lowering the age of vaccination to 6 months when transmission is sustained, as recommended in US Centers for Disease Control and Prevention, WHO, and Sphere guidelines. We show how the 21-day quarantine period provided a safety window to identify individuals who were already incubating measles at the time they were vaccinated, avoiding direct introductions of measles into US communities; almost half of cases (infection with wild-type virus) were identified in recently vaccinated people. Finally, our findings indicate that a short-term pause of incoming evacuee flights prevented additional importations while population immunity was building among evacuees. Our analysis allowed for prioritisation of these interventions in terms of their measurable impact on outbreak trajectory. Our dataset is unique, combining an almost complete set of whole viral genomes from measles cases with detailed contact tracing data. We show that infection clusters based on epidemiological criteria could be further delineated into smaller clusters using phylogenetic analyses; however, sequences with few nucleotide substitution count differences do not always indicate a single line of transmission.
Implications of all the available evidence
Well coordinated public health responses can successfully curb the morbidity and mortality associated with measles outbreaks in vulnerable resettled populations in both elimination and non-elimination settings. Our findings highlight the importance of investing in measles preparedness and of integration between genetic and epidemiological data, with future broad-reaching implications for characterising the epidemiology of measles in a post-elimination context.
Acknowledgments
Funding was provided by the US CDC. We acknowledge the teams of deployers, public health staff, Department of Defense personnel, Department of Homeland Security personnel, and volunteers who assisted with Operation Allies Welcome. We thank the epidemiology, clinical, and laboratory staff at local and state health departments throughout the USA, including Timothy Davis from the Wisconsin State Laboratory of Hygiene (Madison, WI, USA) and Jill Hacker from the California Department of Public Health (Richmond, CA, USA). We also acknowledge the staff at the Vaccine Preventable Disease Reference Centers of the Association of Public Health Laboratories, who conducted measles genotyping and sequencing. Certain bioinformatics tasks (next-generation sequence assembly and phylogenetic analyses) were performed on the CDC Aspen cluster, maintained by the CDC Offices of Scientific Computing and Advanced Molecular Detection (OAMD). We thank OAMD for access to these and other high-performance computational resources. Sequencing preparation tasks were performed by Cynthia Dixey (Goldbelt C6, Chesapeake, VA, USA), Elena Lopareva, Gimin Kim, and Anna-Rose Hutcheson (CDC, Atlanta, GA, USA). The methodology behind the measles susceptibility profile in Afghanistan was provided by James Goodson, Takudzwa Sayi, and Robert Perry (CDC, Atlanta, GA, USA). We also thank Christian Walker and Julie Hurst (US Department of Defense) for providing vaccination and pregnancy data, US Customs and Border Protection for providing flight details of affected patients, and Pritesh Gandhi (US Department of Homeland Security) for his thoughtful review of this paper. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC, the US Department of Health and Human Services, or the US Department of Defense.
Footnotes
Declaration of interests
We declare no competing interests.
For analysis scripts, metadata, and reproducibility information see https://data.cdc.gov/Models/Measles-Case-and-Genetic-Metadata-Operation-Allies/b8tp-jsmh
For the Centers for Disease Control and Prevention guidelines see https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm
For the WHO guidelines see https://www.who.int/publications/i/item/who-wer9217-205-227
For the Sphere guidelines see https://handbook.spherestandards.org/en/sphere/#ch001
See Online for appendix
Contributor Information
Nina B Masters, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA; Epidemic Intelligence Service, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Andrew S Beck, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Adria D Mathis, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Jessica Leung, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Kelley Raines, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Prabasaj Paul, Division of Healthcare Quality Promotion.
Scott E Stanley, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA; Office of the Joint Staff Surgeon, The Joint Staff, Department of Defense, Washington, DC, USA.
Alden L Weg, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA; Office of the Joint Staff Surgeon, The Joint Staff, Department of Defense, Washington, DC, USA.
Emily G Pieracci, Division of Global Migration and Quarantine.
Shannon Gearhart, Division of Global Migration and Quarantine.
Madina Jumabaeva, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA; Oak Ridge Institute for Science and Education (ORISE), Oak Ridge, TN, USA.
Bettina Bankamp, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Paul A Rota, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
David E Sugerman, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Paul A Gastañaduy, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Data sharing
Next-generation sequencing data are deposited to the National Center for Biotechnology Information under Bioproject PRJNA869081, which includes Sequence Read Archive, Genbank, and Biosample deposits. Accession numbers for these deposits are described in the appendix (p 31), alongside information for N450 genotyping windows, if available. We have made analysis scripts, metadata, and reproducibility information available on the CDC data repository.
References
- 1.WHO. Measles vaccination coverage, Afghanistan. 2022. https://immunizationdata.who.int/pages/coverage/mcv.html?CODE=AFG&ANTIGEN=MCV1&YEAR= (accessed April 19, 2022).
- 2.WHO. Afghanistan: infectious disease outbreaks situation report #15 (21 November 2021). Nov 22, 2021. https://www.humanitarianresponse.info/en/operations/afghanistan/document/afghanistan-infectious-disease-outbreaks-situation-report15-21 (accessed Dec 20, 2021). [Google Scholar]
- 3.Centers for Disease Control and Prevention. Guidance for clinicians caring for individuals recently evacuated from Afghanistan. Sept 20, 2021. https://emergency.cdc.gov/han/2021/pdf/CDC_HAN_452.pdf 2021 (accessed April 19, 2022). [Google Scholar]
- 4.Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16–17 March 2000. J Infect Dis 2004; 189 (suppl 1): S43–47. [DOI] [PubMed] [Google Scholar]
- 5.Dixon MG, Ferrari M, Antoni S, et al. Progress toward regional measles elimination—worldwide, 2000–2020. MMWR Morb Mortal Wkly Rep 2021; 70: 1563–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathis AD, Clemmons NS, Redd SB, et al. Maintenance of measles elimination status in the United States for 20 years despite increasing challenges. Clin Infect Dis 2022; 75: 416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel M, Lee AD, Clemmons NS, et al. National update on measles cases and outbreaks—United States, January 1–October 1, 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 893–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shet A, Carr K, Danovaro-Holliday MC, et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health 2022; 10: e186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 591–93. [DOI] [PubMed] [Google Scholar]
- 10.Masters NB, Mathis AD, Leung J, et al. Public health actions to control measles among Afghan evacuees during Operation Allies Welcome—United States, September–November 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 592–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Measles/rubeola 2013 case definition. 2013. https://ndc.services.cdc.gov/case-definitions/measles-2013 (accessed April 1, 2022).
- 12.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62: 1–34. [PubMed] [Google Scholar]
- 13.Klinkenberg D, Nishiura H. The correlation between infectivity and incubation period of measles, estimated from households with two cases. J Theor Biol 2011; 284: 52–60. [DOI] [PubMed] [Google Scholar]
- 14.Goodall EW. Incubation period of measles. BMJ 1931; 1: 73–74. [Google Scholar]
- 15.Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375: 1343–54. [DOI] [PubMed] [Google Scholar]
- 16.Gastanaduy P, Redd SB, Clemmons NS, et al. Measles. In: Roush SW, Baldy LM, Hall MAK, eds. Manual for the surveillance of vaccine-preventable diseases. Atlanta, GA: Centers for Disease Control and Prevention, 2013. [Google Scholar]
- 17.Bankamp B, Byrd-Leotis LA, Lopareva EN, et al. Improving molecular tools for global surveillance of measles virus. J Clin Virol 2013; 58: 176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metsky HC, Siddle KJ, Gladden-Young A, et al. Capturing sequence diversity in metagenomes with comprehensive and scalable probe design. Nat Biotechnol 2019; 37: 160–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouckaert R, Vaughan TG, Barido-Sottani J, et al. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 2019; 15: e1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penedos AR, Myers R, Hadef B, Aladin F, Brown KE. Assessment of the utility of whole genome sequencing of measles virus in the characterisation of outbreaks. PLoS One 2015; 10: e0143081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardy JL, Naus M, Amlani A, et al. Whole-genome sequencing of measles virus genotypes H1 and D8 during outbreaks of infection following the 2010 Olympic Winter Games reveals viral transmission routes. J Infect Dis 2015; 212: 1574–78. [DOI] [PubMed] [Google Scholar]
- 22.Buisson E Outbreak of measles amongst USA-bound Afghan evacuees. Dec 10, 2021. 10.31646/gbio.136 (accessed April 19, 2022). [DOI] [Google Scholar]
- 23.Svan JH. Thousands of Afghans at US bases in Europe receive measles and chickenpox vaccines. Sept 16, 2021. https://www.stripes.com/theaters/europe/2021-09-16/evacuees-at-ramstein-and-rhine-ordnance-barracks-to-get-measles-chickenpox-vaccines-2912710.html (accessed April 19, 2022). [Google Scholar]
- 24.Taylor WR. Measles in Vietnamese refugee children in Hong Kong. Epidemiol Infect 1999; 122: 441–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Colorado C, Mahamud A, Burton A, et al. Measles outbreak response among adolescent and adult Somali refugees displaced by famine in Kenya and Ethiopia, 2011. J Infect Dis 2014; 210: 1863–70. [DOI] [PubMed] [Google Scholar]
- 26.Strebel PM, Papania MJ, Gastañaduy PA, Goodson JL. Measles vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Plotkin’s vaccines, 7th edn. Amsterdam: Elsevier, 2018: 579–618.e21. [Google Scholar]
- 27.Chin T, Buckee CO, Mahmud AS. Quantifying the success of measles vaccination campaigns in the Rohingya refugee camps. Epidemics 2020; 30: 100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gastañaduy PA, Banerjee E, DeBolt C, et al. Public health responses during measles outbreaks in elimination settings: strategies and challenges. Hum Vaccin Immunother 2018; 14: 2222–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald TL, Durrheim DN, Merritt TD, Birch C, Tran T. Measles with a possible 23 day incubation period. Commun Dis Intell Q Rep 2012; 36: E277–80. [PubMed] [Google Scholar]
- 30.WHO. Framework for verifying elimination of measles and rubella. Jan 1, 2013. https://www.who.int/publications/i/item/framework-for-verifying-elimination-of-measles-and-rubella (accessed April 19, 2022). [PubMed] [Google Scholar]
- 31.Zucker JR, Rosen JB, Iwamoto M, et al. Consequences of undervaccination—measles outbreak, New York City, 2018–2019. N Engl J Med 2020; 382: 1009–17. [DOI] [PubMed] [Google Scholar]
- 32.WHO. The role of extended and whole genome sequencing for tracking transmission of measles and rubella viruses: report from the Global Measles and Rubella Laboratory Network meeting, 2017. Wkly Epidemiol Rec 2018; 93: 55–59. [PubMed] [Google Scholar]
- 33.Blumberg S, Lloyd-Smith JO. Inference of R(0) and transmission heterogeneity from the size distribution of stuttering chains. PLoS Comput Biol 2013; 9: e1002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winter AK, Lambert B, Klein D, et al. Feasibility of measles and rubella vaccination programmes for disease elimination: a modelling study. Lancet Glob Health 2022; 10: e1412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Next-generation sequencing data are deposited to the National Center for Biotechnology Information under Bioproject PRJNA869081, which includes Sequence Read Archive, Genbank, and Biosample deposits. Accession numbers for these deposits are described in the appendix (p 31), alongside information for N450 genotyping windows, if available. We have made analysis scripts, metadata, and reproducibility information available on the CDC data repository.