Abstract
Human immunodeficiency virus type 1 (HIV-1) variants resistant to protease inhibitors often display a reduced replicative capacity as a result of an impairment of protease function. Such fitness-impaired viruses display Gag precursor maturation defects. Here, we report that some protease inhibitor-resistant viruses also display abnormalities in the processing of reverse transcriptase (RT) by the protease. In three recombinant viruses carrying resistant protease sequences from patient plasma, we observed a marked decrease in the amount of mature RT subunits and of particle-associated RT activity compared to their parental pretherapy counterparts. We investigated the possibility that a decrease in the amount of particle-associated mature RT could affect the sensitivity of the corresponding virus to RT inhibitors. We observed a twofold increase of sensitivity to zidovudine (AZT) when a virus which carried AZT mutations was processed by a resistant protease. Interestingly, the presence of AZT-resistance mutations partially rescued the replication defect associated with the mutated protease. The interplay between resistance to protease inhibitors and to RT inhibitors described here may be relevant to the therapeutic control of HIV-1 infection.
The efficacy of protease inhibitors (PI) in suppressing human immunodeficiency virus type 1 (HIV-1) replication in treated patients, particularly when used in combination with other antiretroviral agents, has been extensively documented (3, 11, 14, 21). In the course of treatment, however, resistant viral variants can arise in a fraction of the patient population as a consequence of the accumulation of mutations in the protease (8, 9, 13, 22–24, 27, 28). Most resistance-associated mutations are not observed in PI-naive viral isolates, suggesting that they confer a selective disadvantage to the virus in the absence of drug (4, 18, 30). Accordingly, we and others have described a significant reduction in viral replicative capacity consecutive to the development of PI resistance (5, 10, 15, 16, 20, 26, 29, 31, 32). The observed reduction in infectivity is due to a reduced cleavage efficiency by the resistant proteases at several cleavage sites in Gag, which results in the accumulation of partially cleaved precursor molecules in the viral particles (20, 29, 31). Interestingly, resistant proteases exhibit distinct processing impairment profiles, indicating that specific resistance mutations differentially affect cleavage at distinct sites (20, 31). Mutations in Gag cleavage sites arise under the selective pressure of PI, both in tissue culture and in infected patients. In particular, mutations that target the two cleavage sites that surround the p1 spacer peptide were shown to partially compensate for resistance-associated loss of viral fitness by providing better substrates for the mutated protease (12, 20, 32). Although Gag is the most abundant substrate of the viral protease, we previously described a marked reduction of particle-associated mature reverse transcriptase (RT) in viral particles processed by a ritonavir-resistant protease, demonstrating that cleavage at sites in Pol can also be affected (31). Here, we better characterize the reduction of particle-associated mature RT in three PI-resistant viruses, and we analyze the effects of this reduction on RT activity and sensitivity to reverse transcriptase inhibitors (RTI). Additionally, we show that zidovudine (AZT) resistance mutations in the RT can partially rescue the replicative defect of a PI-resistant virus.
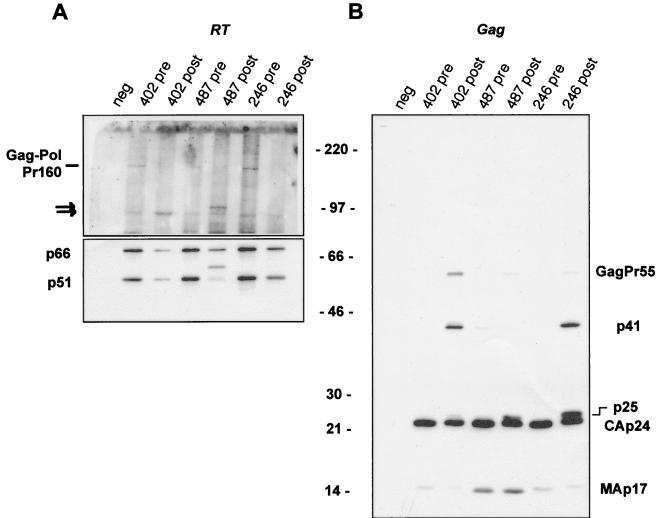
pNL4-3-derived molecular clones of HIV carrying inhibitor-resistant proteases from plasma virus were compared to isogenic clones harboring the corresponding pretherapy protease sequences. Procedures for PCR amplification and cloning of protease sequences were as previously described (20, 31). Three patients were selected based on incidental evidence of possible reduction of RT quantity in viral particles. We first evaluated the amount of the mature RT subunits (p66 and p51) in particles produced after transfection of HeLa cells with the protease-reconstructed viral clones. Particle-associated material was normalized for HIV-1 p24 antigen content in each virus pair and analyzed by Western blotting with monoclonal antibodies that recognize RT (Fig. 1A) or the Gag products MA and CA (Fig. 1B). A significant reduction in the amount of both RT subunits was observed for the three viruses that carry resistant proteases (Fig. 1A, lower panel). Such a decrease in the amount of mature RT subunits is likely to contribute to the marked reduction in viral fitness observed for these three viruses (20, 31). Interestingly, two distinct RT maturation patterns could be observed, suggesting that different mechanisms can lead to impaired RT processing. For viruses 402-post and 246-post, we observed a parallel reduction of both RT subunits, indicating that cleavage at the RT internal site (which separates the polymerase domain from the RNase H domain) was not affected (Fig. 1A, lower panel). For virus 487-post, the p51 subunit was almost completely replaced by a higher molecular mass product (of approximately 56 kDa), suggesting that the virus 487-resistant protease cleaved an alternative sequence in the RNase H domain more efficiently than the normal polymerase-RNase H cleavage site (Fig. 1A, lower panel).
FIG. 1.
Virus particle-associated material in the supernatant of transfected HeLa cells was analyzed by Western blotting with monoclonal antibodies that recognize the RT subunits p66 and p51 (A) or the gag-encoded MA and CA proteins (B). The top half of the Western blot shown in panel A was deliberately overexposed to allow the detection of the Gag-Pol precursor molecules. Arrows point to Gag-Pol cleavage intermediates. The molecular mass marker (in kilodaltons) is indicated for both panels A and B. Pre and post indicate that the viral protease was cloned from a pretherapy or postresistance patient isolate, respectively. neg, mock-transfected cells.
We expected that the reduction in the amount of mature RT in viral particles consecutive to reduced protease activity would be associated with the accumulation of uncleaved Gag-Pol precursor molecules. Instead, for the three virus pairs analyzed here, we found decreased amounts of particle-associated Gag-Pol precursor in viruses with resistant proteases (Fig. 1A, upper panel). Furthermore, in these viruses we observed either an increase (virus 402-post) in the intensity of a partially cleaved precursor band (Fig. 1A, upper panel) or a shift in the size of this cleavage intermediate (virus 487-post). Altogether, these observations suggest that the Gag-Pol precursor of resistant viruses may be less stable and can be processed with different specificity in the presence of a functionally impaired protease.
Consistent with our previous description, Gag cleavage was also impaired in the -post viruses, which were characterized by the accumulation of distinct Gag cleavage intermediates (Fig. 1B). In particular, cleavage intermediates corresponding to p41 (MA-CA) and p25 (CA-p2), as well as the full-length Gag precursor (GagPr55), were readily detected (Fig. 1B).
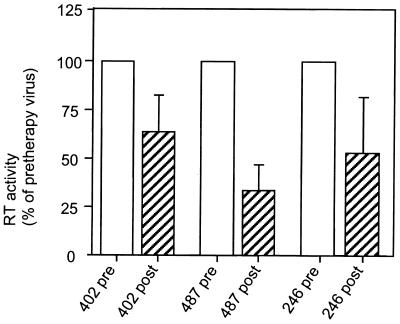
To evaluate the effect of improper cleavage of the RT subunits on RT function, we compared RT activity in viral particles carrying pretherapy or the corresponding resistant protease. To this end, HIV-1 p24 antigen and exogenous RT activity [on poly(rA)-oligo(dT)] were measured in the pelletable fraction of supernatant from transfected HeLa cells. A marked reduction in RT activity was observed for the 402-, 487-, and 246-post viruses (Fig. 2). No significant difference was observed for other resistant PR-reconstructed viruses analyzed (data not shown) for which the resistance-associated loss of infectivity was less intense and was associated only with Gag precursor processing defects. In all viruses studied, the reduction of RT activity correlated with the profile of particle-associated mature RT subunits.
FIG. 2.
Virus particle-associated RT activity measured in a poly(rA)-oligo(dT) assay. The RT activity of each pair of pretherapy and resistant (-post) viruses was expressed as a percentage of the pretherapy virus. Open columns represent viruses carrying pretherapy protease alleles, and hatched columns represent viruses carrying resistant proteases. The measurements were repeated at least three times, and the averages and standard deviations are indicated.
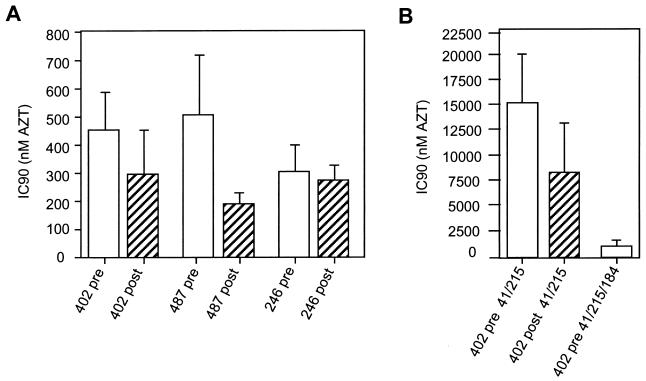
Considering that nucleoside analogue RTI are thought to act as competitive inhibitors of RT in addition to their effect on DNA chain termination (2), we reasoned that changes in the amount of particle-associated RT could affect sensitivity of the virus to RTI. Since the reduction of particle-associated RT is the consequence of resistance to PI, such possibility would have interesting implications given that current anti-HIV therapy regimens combine PI and RTI treatment. We first compared the sensitivity to AZT of RTI-naive viruses carrying pretherapy or resistant RT-maturation-impaired proteases. We used transfected HeLa cell supernatant to infect P4 (HeLa-CD4, LTR-LacZ) cells (7) to measure the 90% inhibitory concentration (IC90) in a single-cycle assay. Target cells were pretreated with increasing concentrations of AZT (0, 1.6, 8, 40, 200, 1,000, 5,000, and 25,000 nM) for 3 h and infected in the presence of AZT for 24 h, after which cells were lysed and β-galactosidase activity was measured by a colorimetric assay as previously described (31). The infectious titer for each AZT concentration was compared to that in the absence of AZT, and IC90 values for the six viral clones were calculated. As shown in Fig. 3A, the presence of resistant protease produced a reduction of 35 and 62% of the IC90 for virus 402 and 487, respectively. A discordant phenotype was instead observed for virus 246, for which we did not find significant differences in the IC90 between the pretherapy and the PI-resistant clone. However, this discordance could be explained in part by the observed particularly high sensitivity to AZT of the virus 246-pre (Fig. 3A). We next analyzed the effect of impaired RT processing on AZT resistance level of a virus carrying AZT resistance mutations. To this end, we inserted by site-directed mutagenesis the typical AZT resistance mutations M41L and T215Y in the RT coding sequence of the two molecular clones reconstructed with the protease alleles from patient 402, producing the clones 402-pre-41/215 and 402-post-41/215. In the presence of AZT resistance mutations (Fig. 3B) the increase in AZT sensitivity due to impaired RT cleavage was more pronounced (45% reduction in the IC90). Although significant, such a reduction of the IC90 is not quite as dramatic as the previously described resensitization to AZT consequent to the development of the 3TC resistance mutation M184V in the presence of AZT resistance mutations (17, 19, 25). In our assay, addition of the M184V mutation to the 402-pre-41/215 viral clone resulted in a 93% reduction of AZT resistance (Fig. 3B). Addition of the M184V mutation affects all RT molecules and results in an almost complete resensitization, while in the case of resensitization due to reduced RT maturation, mature RT molecules should still be fully resistant, leading to a milder resensitization phenomenon.
FIG. 3.
Effect of impaired processing by resistant protease on AZT resistance on viruses carrying wild-type (A) or mutated (B) RT sequences. IC90 values for AZT were calculated in a single-cycle assay, measurements were repeated at least three times, and the averages and standard deviations are indicated. Open columns represent viruses carrying pretherapy protease alleles, and hatched columns represent viruses carrying resistant proteases. The mutated residues in the RT coding region are indicated.
Interestingly, the sensitivities to 3TC for virus 402-pre and 402-post were similar (data not shown), suggesting that the reduction in the quantity of mature RT molecules observed for virus 402-post more specifically affects HIV sensitivity to AZT. Similarly, resistance to AZT appears to be remarkably sensitive to associated mutations in the RT (17, 19, 25). Therefore, it could be that the function or the amounts of mature RT are particularly rate limiting in the AZT resistance phenotype, compared to resistance to other nucleoside analogues.
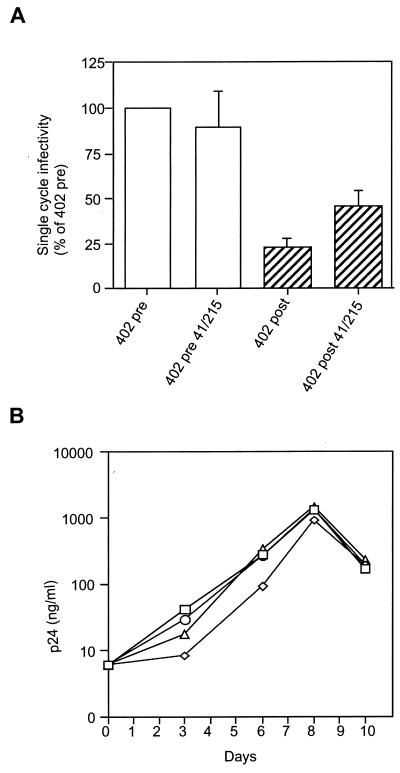
Comparing RT sensitivity of viruses 402-post and 402-post-41/215 revealed that the single-cycle infectivity of the viral clone 402-post was reproducibly improved in the presence of AZT resistance mutations. To quantify this phenotype, we analyzed in more detail the infectivity of the 402-derived viral clones in the absence of AZT (Fig. 4A). Consistent with our previous finding (31), virus 402-post displayed a marked reduction of infectivity due to the resistant protease (Fig. 4A). Interestingly, a twofold increase in infectivity was measured for viral clone 402-post in the presence of the M41L and T215Y mutations (Fig. 4A). These RT mutations had no effect on drug-free infectivity in virus carrying pretherapy protease (Fig. 4A). The rescue of infectivity was confirmed in a virus propagation assay. MT4 cells (106 cells in 5 ml) were infected with supernatant from transfected HeLa cells (corresponding to 30 ng of HIV-1 p24 antigen). Virus replication was followed by measuring the accumulation of HIV-1 p24 in the supernatant of infected cells for 10 days (Fig. 4B). The replication kinetics of virus 402-post was consistently slower than that of 402-pre. Such a delay was reproducibly but only partially compensated for by the AZT resistance mutations in virus 402-post-41/215, in agreement with the single-cycle data. Again, no significant difference was observed for virus 402-pre in the presence and in the absence of AZT resistance mutations. A possible explanation may be found in the previously described increase of RT processivity associated with some AZT resistance mutations, T215Y among them (1, 6). We reasoned that reduced amounts of RT per particle would be partially compensated for by a more processive enzyme. On the other hand, the increase in infectivity consequent to AZT resistance mutations was not perceptible when the viral precursor molecules were cleaved by a PI-naive protease, maybe because complete processing of RT by a wild-type protease provides optimal conditions for replication. The possibility that mutations in RT could improve cleavage by the resistant protease, reducing the infectivity defect, was ruled out since no increase in the virus-associated mature RT was detected by Western blotting analysis (data not shown).
FIG. 4.
Partial rescue of infectivity by AZT resistance mutations. Infectivity of 402-derived viral clones with and without mutations in the RT was analyzed in a single-cycle assay and expressed as percent of infectivity of the 402-pre virus (pretherapy protease and wild-type RT) (A). Open columns represent viruses carrying pretherapy protease alleles, and hatched columns represent viruses carrying resistant proteases. Three independent experiments were performed, and the averages and standard deviations are shown. (B) Replication kinetics of the 402-derived viral clones in MT4 cells. Squares represent 402-pre, circles correspond to 402-pre-41/215, triangles correspond to 402-post-41/215, and diamonds represent 402-post.
Although Gag precursor is quantitatively the prevalent substrate for the HIV-1 protease, the effect of impaired cleavage of viral enzymes could have considerable effects on viral replication. The three virus pairs studied here were characterized by a strong difference in infectivity between the pretherapy and post resistance protease-reconstructed molecular clones (20, 31). The relative impact of aberrant cleavage in Gag or Gag-Pol may be difficult to dissect. Only for virus 487-post did we know that the replicative defect was largely due to incomplete Gag cleavage, since we previously showed that mutations in Gag cleavage sites that improved processing also substantially rescued infectivity (20). It would be of interest to determine whether substrate adaptation could restore maturation of the RT subunits, as is the case for Gag domains. The alteration of cleavage specificity due to the development of PI resistance is particularly evident for virus 487-post. In this case, resistance mutations in the protease did not only affect the efficiency of cleavage at normal RT sites but also determined the preferred recognition of an alternative site in the RNase H domain and the almost complete absence of cleavage at the regular site between the polymerase and RNase H domains. The 487-post protease (obtained from a patient treated with saquinavir in combination with dideoxycytosine) contains five resistance mutations (L10I, M36I, G48V, I84V, and L90M), two of which target residues around the active site and could directly influence the affinity for different substrates. It is interesting to note that virus 246-post was also selected during saquinavir (monotherapy) treatment, but it displays a processing profile clearly distinct from that of virus 487-post. Virus 246-post harbors the resistance mutations M46I, G48V, and L90M. These observations and the previously described deleterious effect of the mutation G48V on precursor cleavage (20) suggest a role for the combination of the G48V and the I84V mutation in determining the modified cleavage specificity of virus 487-post. Analysis of protein profiles generated by protease carrying single resistance mutations could help in determining the residues involved in the recognition of the alternative cleavage site. Finally, the RT maturation profile of virus 402-post (obtained from a patient treated with ritonavir, AZT, and dideoxycytosine) is quite similar to that of the 246-post virus, although the accumulation of Gag cleavage intermediates clearly differentiates these two viruses. The protease of virus 402-post contains the L10I, K20R, M36I, M46I, A71V, and V82A resistance mutations, two of which (L10I and M36I) were present in the pretherapy virus as well, and the unusual F53L amino acid change.
Further elucidation of the interplay between resistance to PI and to RTI consequent to the altered functionality of the two enzymes could prove relevant to the therapeutic control of HIV-1 infection.
Acknowledgments
We thank Olivier Schwartz (Institut Pasteur) for helpful discussion and for his help with protein analysis and Luc Montagnier for his support.
This work was supported in part by the Agence Française de Recherches sur le Sida (ANRS). F.M. is the recipient of a fellowship from ANRS.
REFERENCES
- 1.Arion D, Borkow G, Kaushik N, Parniak M. Fifth Conference on Retroviruses and Opportunistic Infections, Chicago, Ill. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-2′-deoxythymidine (AZT), abstr. 32. [Google Scholar]
- 2.Arts E J, Wainberg M A. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1996;40:527–540. doi: 10.1128/aac.40.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 4.Barrie K A, Perez E E, Lamers S L, Farmerie W G, Dunn B M, Sleasman J W, Goodenow M M. Natural variation in HIV-1 protease, Gag p7 and p6, and protease cleavage sites within Gag/Pol polyproteins: amino acid substitutions in the absence of protease inhibitors in mothers and children infected by human immunodeficiency virus type 1. Virology. 1996;219:407–416. doi: 10.1006/viro.1996.0266. [DOI] [PubMed] [Google Scholar]
- 5.Borman A, Paulous S, Clavel F. Resistance of HIV-1 to protease inhibitors: selection of resistance mutations in the presence and in the absence of the drug. J Gen Virol. 1996;77:419–426. doi: 10.1099/0022-1317-77-3-419. [DOI] [PubMed] [Google Scholar]
- 6.Caliendo A M, Savara A, An D, DeVore K, Kaplan J C, D’Aquila R T. Effects of zidovudine-selected human immunodeficiency virus type 1 reverse transcriptase amino acid substitutions on processive DNA synthesis and viral replication. J Virol. 1996;70:2146–2153. doi: 10.1128/jvi.70.4.2146-2153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F. HIV-1 reverse transcription: a termination step at the center of the genome. J Mol Biol. 1994;241:651–662. doi: 10.1006/jmbi.1994.1542. [DOI] [PubMed] [Google Scholar]
- 8.Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryelski L J, Graham D J, Laird D, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodakewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;70:8270–8276. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Hobbins H L, Roth E, Shivaprakash M, Titus D L, Yang T, Teppler H, Squires K E, Deutsch P J, Emini E A. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995;374:569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 10.Croteau G, Doyon L, Thibeault D, McKercher G, Pilote L, Lamarre D. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J Virol. 1997;71:1089–1096. doi: 10.1128/jvi.71.2.1089-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks S G, Smith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 12.Doyon L, Poulin F, Pilote L, Clouette C, Thibeault D, Croteau G, Lamarre D. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J Virol. 1996;70:3763–3769. doi: 10.1128/jvi.70.6.3763-3769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson J W. The not-so-great escape. Nat Struct Biol. 1995;2:523–529. doi: 10.1038/nsb0795-523. [DOI] [PubMed] [Google Scholar]
- 14.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 15.Ho D D, Toyoshima T, Mo H, Kempf D J, Norbeck D, Chen C M, Wideburg N E, Burt S K, Erickson J W, Singh M K. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J Virol. 1994;68:2016–2020. doi: 10.1128/jvi.68.3.2016-2020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan A, Michael S, Wehbie R, Knigge M, Paul D, Everitt L, Kempf D, Norbeck D, Erickson J, Swanstrom R. Selection of multiple human immunodeficiency virus type 1 variants that encode viral proteases with decreased sensitivity to an inhibitor of the viral protease. Proc Natl Acad Sci USA. 1994;91:5597–5601. doi: 10.1073/pnas.91.12.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavlick M F, Shirasaka T, Kojima E, Pluda J M, Hui F, Jr, Yarchoan R, Mitsuya H. Genotypic and phenotypic characterization of HIV-1 isolated from patients receiving (–)-2′,3′-dideoxy-3′-thiacytidine. Antiviral Res. 1995;28:133–146. doi: 10.1016/0166-3542(95)00044-m. [DOI] [PubMed] [Google Scholar]
- 18.Kozal M, Shah N, Shen N, Yang R, Fucini R, Merigan T, Richman D, Morris D, Hubbell E, Chee M, Gingeras T. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 19.Larder B A, Kemp S D, Harrigan P R. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995;269:696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- 20.Mammano F, Petit C, Clavel F. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J Virol. 1998;72:7632–7637. doi: 10.1128/jvi.72.9.7632-7637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowitz M, Cao Y, Hurley A, et al. Abstracts of the XI International Conference on AIDS. XI International Conference on AIDS Society, Vancouver, British Columbia, Canada. 1996. Triple therapy with AZT, 3TC and ritonavir in 12 subjects newly infected with HIV-1, abstr. Th. B. 933. [Google Scholar]
- 22.Markowitz M, Mo H, Kempf D J, Norbeck D W, Bhat T N, Erickson J W, Ho D D. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J Virol. 1995;69:701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellors J W, Larder B A, Schinazi R F. Mutations in HIV-1 reverse transcriptase and protease associated with drug resistance. Int Antiviral News. 1995;3:8–13. [Google Scholar]
- 24.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 25.Nijhuis M, Schuurman R, de Jong D, van Leeuwen R, Lange J, Danner S, Keulen W, de Groot T, Boucher C A. Lamivudine-resistant human immunodeficiency virus type 1 variants (184V) require multiple amino acid changes to become co-resistant to zidovudine in vivo. J Infect Dis. 1997;176:398–405. doi: 10.1086/514056. [DOI] [PubMed] [Google Scholar]
- 26.Nijhuis M, Schuurman R, Schipper P, de Jong D, van Bommel T, de Groot T, Molla A, Borleffs J, Danner S, Boucher C. Abstracts of the International Discussion Meeting on HIV Population Dynamics, Variation and Drug Resistance. Edinburgh, United Kingdom. 1997. Reduced replication potential of HIV-1 variants initially selected under ritonavir therapy is restored upon selection of additional substitutions, abstr. 23. [Google Scholar]
- 27.Patick A, Mo H, Markowitz M, Appelt K, Wu B, Musick L, Kalish V, Kaldor S, Reich S, Ho D, Webber S. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob Agents Chemother. 1996;40:292–297. doi: 10.1128/aac.40.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts N A. Drug-resistance patterns of saquinavir and other HIV proteinase inhibitors. AIDS. 1995;9:S27–S32. [PubMed] [Google Scholar]
- 29.Rose R, Gong Y, Greytok J, Bechtold C, Terry B, Robinson B, Alam M, Colonno R, Lin P. Human immunodeficiency virus type 1 viral background plays a major role in development of resistance to protease inhibitors. Proc Natl Acad Sci USA. 1996;93:1648–1653. doi: 10.1073/pnas.93.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winslow D L, Stack S, King R, Scarnati H, Bincsik A, Otto M J. Limited sequence diversity in the HIV type 1 protease gene from clinical isolates and in vivo susceptibility to HIV protease inhibitors. AIDS Res Hum Retroviruses. 1995;11:107–113. doi: 10.1089/aid.1995.11.107. [DOI] [PubMed] [Google Scholar]
- 31.Zennou V, Mammano F, Paulous S, Mathez D, Clavel F. Loss of viral fitness associated with multiple Gag and Gag-Pol processing defects in human immunodeficiency virus type 1 variants selected for resistance to protease inhibitors in vivo. J Virol. 1998;72:3300–3306. doi: 10.1128/jvi.72.4.3300-3306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Imamichi H, Imamichi T, Lane H, Falloon J, Vasudevachari M, Salzman N. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J Virol. 1997;71:6662–6670. doi: 10.1128/jvi.71.9.6662-6670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]