Abstract
The long-term value of immunopsychiatry will be based on its ability to translate basic science into effective clinical interventions. In this article, we discuss a key obstacle to achieving this important translational goal—namely, the preponderance of studies that are cross-sectional, or that have months-to-years long follow-up periods. Immunopsychiatric processes such as stress, inflammation, and depression symptoms are inherently dynamic and fluctuate over hours, days and weeks. This fact suggests that higher-density data collection with only days between measurements is necessary to capture—with adequate resolution—the actual dynamics of these systems, determine optimal time lags with which to observe associations between variables of interest, and maximize the translational potential of these data. To illustrate these points, we use pilot data from our own intensive longitudinal immunopsychiatric study. We then conclude by making several recommendations for future research. By learning how to better use existing data for dynamically informative studies as well as collecting intensive longitudinal data, we believe immunopsychiatry will be much better positioned to advance our causal understanding of the interplay between the immune system and health.
Keywords: inflammation, psychoneuroimmunology, immunopsychiatry, intensive longitudinal designs, within-person effects, dynamic modeling
The last 50 years of psychoneuroimmunology have provided compelling data connecting a plethora of psychological and immunological processes, including psychiatric reactions to cancer treatment1,2, regulation of emotions during acute immune responses3, interplay between social processes and immune biology4–6, inflammation and depression7, anti-inflammatory effects of psychotherapy8,9, and early life adversity and inflammatory stress responses10 among others. The next era of psychoneuroimmunology, in turn, will involve translating this basic research into clinical benefit whenever possible. This goal is particularly salient for immunopsychiatry, the subfield of psychoneuroimmunology that examines bidirectional associations between immune functioning and mental health.
In this article, we demonstrate how the typical temporal design of immunopsychiatry studies is a critical limitation to the clinical translatability of extant data. We then argue that more frequent assessments are necessary to advance our causal understanding of the interplay between immunology, psychopathology, and behavior. This is not to say that longer follow-up periods do not have merit for certain processes that take longer to unfold, such as cyclic relationships, but that rather that the dearth of shorter follow-up periods in psychoneuroimmunology is an important weakness to address. To address these points, we first briefly introduce the concepts of Simpson’s Paradox and ergodicity as motivating factors to consider research designs suited for within-person analysis. Second, we briefly summarize the current standards of longitudinal immunopsychiatric research. Third, we briefly review relevant psychometric and physiometric11 properties (i.e., the measurement properties of biological variables11,12) that underpin our call to improve study designs in immunopsychiatry. Fourth, we introduce the concept of intensive longitudinal designs and their potential methodological benefits in immunopsychiatry. Fifth, we present sample data from an ongoing intensive longitudinal immunopsychiatric study to concretely demonstrate several key motivations for this work. Finally, we conclude with recommendations on how to use existing longitudinal data to advance our temporal understanding of these processes and advocate for the increased use of intensive longitudinal data in immunopsychiatry. Throughout this article, we use the association between inflammation and depression as a running example, but the core methodological considerations we recommend apply to all research linking psychological, immunological, and behavioral processes.
Simpson’s Paradox and Ergodicity
Much social and behavioral research, psychoneuroimmunology included, attempts to identify population-level truths about associations of interest by testing between-person differences. Despite this fact, many key psychobiological processes of interest involve within-person change (e.g., Does this medication decrease symptoms? Does regular exercise improve immune health?). This is problematic, as much of what is observable at a population level in the social and medical sciences might not generalize to subgroups (i.e., Simpson’s Paradox13) or individuals14. Consider the following example from Ref 15. At a group-level, experienced typers are both faster and make fewer errors than non-experienced typers. However, if you look at data across time for any given person, the faster someone types, the more likely they are to make errors. This lack of group-to-individual generalizability is referred to as a nonergodic process. Conversely, ergodic processes are both equivalent between groups and individuals (i.e., homogeneous) and have constant means and variances overtime (i.e., stationary)16. These are widely considered to be unrealistic assumptions for psychological processes,14,16,17 which, by extension, renders them unrealistic for immunopsychiatric processes. Consequently, we advocate for the importance of conducting intensive longitudinal data collection as one key way to facilitate immunopsychiatric research that can adequately capture key within-person processes.
Current Longitudinal Immunopsychiatric Study Designs
A key limitation to the translational value of current immunopsychiatry studies rests on the frequency of their data collection and the resulting ability of those data to characterize the dynamics of the biopsychosocial systems they represent. Indeed, most immunopsychiatry studies use cross-sectional data, with the rest most commonly using longitudinal data collected in assessment lags of 6 months or longer. In a recent meta-analysis of longitudinal studies of the associations between inflammatory proteins and depression, for example, only 3 of the 38 studies included (8%) had follow-up lengths of less than 1 year, and no studies (0%) had a follow-up length of less than 6 months18. A second meta-analysis had a median follow-up length of 5 years19.
Indeed, many frequently cited longitudinal studies on the relation between two key constructs in immunopsychiatry—namely, inflammatory biology and depression—have assessment lags ranging from 5–12 years (e.g., Refs 20–23). Critically, meta-analyses of longitudinal studies have consistently found substantial heterogeneity between studies for the effect size between inflammatory proteins and depression18,19, underscoring the likelihood that one or more unaccounted factors are influencing observed effect sizes. Although follow-up duration was not a significant predictor of the interleukin (IL)-6 → depression association or C-reactive protein (CRP) → depression association in Ref. 18 (tumor necrosis factor (TNF)-α was not included in the meta-regression and Ref. 19 did not test follow-up duration as a meta-regression predictor)—we posit that one factor affecting effect sizes in longitudinal immunopsychiatric research is the variability in, and long duration of, the assessment lags.
It is widely accepted that the size of an effect depends on the time interval assessed24,25; however, very little research26 has investigated the temporal specificity of the inflammation → depression association in search of optimal time lags (i.e., when the effect size would be the largest). Ref. 21 found that temporal specificity of these associations differed by sex. Specifically, higher IL-6 and TNF-α predicted greater increases in depression symptoms at longer time lags for females, but for males, higher IL-8 predicted greater decreases in depression at longer time lags. Although not explicitly testing depression symptoms, results from a study measuring affect 5 times per day for 14 days leading up to a blood draw suggest that the association between negative affect and inflammation was stronger when affect and inflammation were measured closer in time27. Contrasting the two studies’ statistical methodology, it is plausible that the discrepant results are influenced by Ref. 21 predicting depression change scores—therefore, longer periods of time would be associated with greater likelihood of measurable variability in depression symptoms.
The lack of thorough evaluation of temporal specificity and optimal time lags is particularly problematic considering that time lags in psychological research are often biased toward being too long, leading to attenuated effect sizes28,29. Research also suggests that fields should initially risk over-sampling, instead of under-sampling, until optimal time lags are determined30. This is especially important when multiple parts of the system (e.g., different inflammatory proteins) might have different optimal time lags with a given outcome30.
This physiometric information is not only central to designing and analyzing future observational studies, but would also facilitate the development of interventions. Specifically, knowing the ideal time lag after which intervening on inflammation should improve depression symptoms (i.e., establishment of a temporal phenotype) will guide clinical expectations for time-to-improvement as well as selecting which immune mechanisms to target. For example, if two inflammatory processes have comparable effect sizes and side effect profiles, but one has a shorter optimal time lag to improvement in depression symptoms, the process with the shorter time-to-improvement would be a preferrable treatment target.
Immunopsychiatric Processes are Dynamic
Consideration of the temporal dynamics of immunopsychiatric processes is critical to optimizing study design. Dormann and Griffin28 demonstrate that temporal stability, the effect a variable has on itself over time, is a key factor in determining the optimal time lag between variables. Several immunopsychiatric theories hypothesize that inflammation is a mediator linking stress and depression31,32; therefore, efforts to quantify the causal relations between these variables must consider their stability. Before describing the following examples, we would like to reiterate that, formally, stability in this context refers to autoregressive effects (e.g., the effect of a variable on itself between time points); however, the following examples will use other approximations of temporal stability that are more common in the literature and/or might be more intuitively interpretable for most readers (e.g., re-test reliability, which is the correlation between two measurements at different points in time).
Meta-analytic evidence suggests that the most widely used self-report measure of stress, the Perceived Stress Scale, only has acceptable retest reliability*1 over about 6 weeks33. This is notably shorter than the standard assessment lag of immunopsychiatry studies; consequently, it is plausible that many longitudinal studies have underestimated the effect that stress has on immune functioning. Similarly, certain core features of depression, such as sad mood, feature fairly low stability, even in people with current Major Depressive Disorder34. Further, different depression symptoms feature different stabilities34. For example, in an ecological momentary assessment (EMA) study that assessed depression symptoms 5 times per day for 12 days, “low mood” was the least stable, whereas “wish to die” was the most temporally stable29. This result underscores the critical importance of temporally informed methodology to characterize inflammatory phenotypes of depression35–39. In this context, we believe intensive longitudinal data may provide even greater benefits for modeling immunological data than it does for self-report measures.
The temporal dynamics and stability of immune functioning vary at both the protein (e.g., C-reactive protein vs. IL-8) and process level (e.g., acute phase response) across a variety of scenarios (e.g., basal levels over months or years40, diurnal fluctuations across a day41, responses to acute stress42). Consider Ref.40, which tested the short-term (i.e., 120 minutes) and long-term (i.e., 18-month) reliability of seven inflammatory proteins measured using saliva samples. The reliability over just 120 minutes ranged from .51 ≤ r ≤ .81. In contrast, the reliability over 18 months, which is much more representative of standard psychoneuroimmunology research, was much lower, ranging from .04 ≤ r ≤ .32.
Considering these temporal dynamics might be critical for refining theory about how, and which facets of, immunology influence mental health (and vice-versa). This possibility is consistent with evidence that temporal features such as time of day of injury influence inflammatory responses to injury and length of hospital stay43. Digging deeper, it is well known that acute inflammatory reactions to stress, illness, and injury are themselves highly dynamic and involve a series of upregulations, migrations, and downregulations of various processes and proteins in what is commonly referred to as the inflammatory cascade44,45. The complexity of these dynamics is impossible to integrate into analyses, and consequently theory, using traditional approaches in immunopsychiatry. The inability to consider these nuances, already appreciated as critical to understanding recovery from physical injury and illness, might be a significant obstacle in the clinical translation of immunopsychiatry. Further, it is important to consider that the temporal dynamics of stress, the immune system, and depression symptoms might interact to determine optimal time lags.
Introduction to Intensive Longitudinal Data
As we have already alluded to, one strategy for addressing these methodological challenges involves collecting intensive longitudinal data. Intensive longitudinal data refer to frequent data collection at a small time scale, typically once every few days to multiple times per day. This methodology has facilitated advancements in the understanding of both how processes unfold in individuals (i.e., idiographic analyses46,47) as well as individual differences in within-person processes (i.e., change)48,49 in the psychological sciences.
Intensive longitudinal data can also reduce measurement error induced by recall/memory bias in traditional, retrospective self-report data (i.e., measuring affect daily for a week instead of asking participants to report how they felt the past week50) and can maximize ecological validity51. For an example that is particularly relevant to psychoneuroimmunology, one study found that only momentary (collected hourly) and daily assessments of stress predicted cortisol, which is widely considered the primary stress hormone and is a potent contributor to anti-inflammatory processes; in contrast, retrospectively reported stress levels—both across the past month and even just the previous 4 days—was unassociated with cortisol52. For constructs that are not highly stable over months or years, intensive longitudinal data methods enable the collection and analysis of data at timescales that more precisely complement how psychobiological systems naturally interact—maximizing the opportunity for causally, and thus clinically, relevant research.
Further, many outcomes of interest in immunopsychiatry—including psychosocial processes, immune biology, and mental health—are multiply determined and thus can have complicated trajectories that feature non-linear fluctuations. A scientist’s ability to characterize these trajectories is inherently restricted by the number of observations taken in a given study: one time point cannot reveal change; two time points can only reveal an increase or decrease; three time points can only detect increases, decreases, or a singular quadratic effect (increase then decrease or decrease then increase); and so forth. Truly, even if the actual temporal dynamics are known, they cannot be represented in the data if the frequency of assessment is insufficient (for an strong empirical example featuring simulated emotion dynamics, see Ref53). Consequently, we argue that the field of immunopsychiatry would benefit greatly from studies with both much shorter time lags and greater numbers of repeated observations per participant. Below, we present data from the first wave of an intensive longitudinal immunopsychiatric study to illustrate the benefits of this approach.
Mental Health & Immunodynamics of Social Stress (Project MHISS)
Preliminary data from an intensive longitudinal immunopsychiatric study, Project MHISS, provide an illustrative example of the notable benefits of using high frequency assessments. Project MHISS uses the transition to college for first-year college students to model biopsychosocial changes that occur in response to a naturally occurring social stressor. Participants in this study complete daily self-reports of stress (measured using the Perceived Stress Scale54) and depression symptoms (measured using the Inventory of Depression and Anxiety Symptoms-II55), and provide blood samples over 22 days. Participants’ first full day on campus is the 7th day of the protocol. Blood samples will not be immunoassayed until the end of data collection to limit batch effects; however, our preliminary analyses of the stress and symptom data from the first wave of data collection illustrate the benefits of collecting intensive longitudinal data for immunopsychiatry.
Determining the shape of the temporal unfolding of predictors and outcomes is a crucial step in longitudinal modeling of risk and resilience processes (for more information, see Case 4: “The Problem of Shape” in Ref56). Therefore, it is best to visualize trajectories of variables of interest before determining statistical modeling approaches (e.g., should there be a non-linear effect of time?). Consider Figure 1, which plots the trajectories of depression symptoms and perceived stress across the 22-day protocol. Note the red lines, which illustrate the trajectories if data were only collected on the three primary events of the study: study start, 7th day move-in, and study end. Both depression symptoms and stress appear to be on steady downward trajectories throughout the study. However, by overlaying the blue lines, which depict the daily data, a much more dynamic picture emerges.
Figure 1. Trajectories of Depression Symptoms and Stress During the Transition to College.
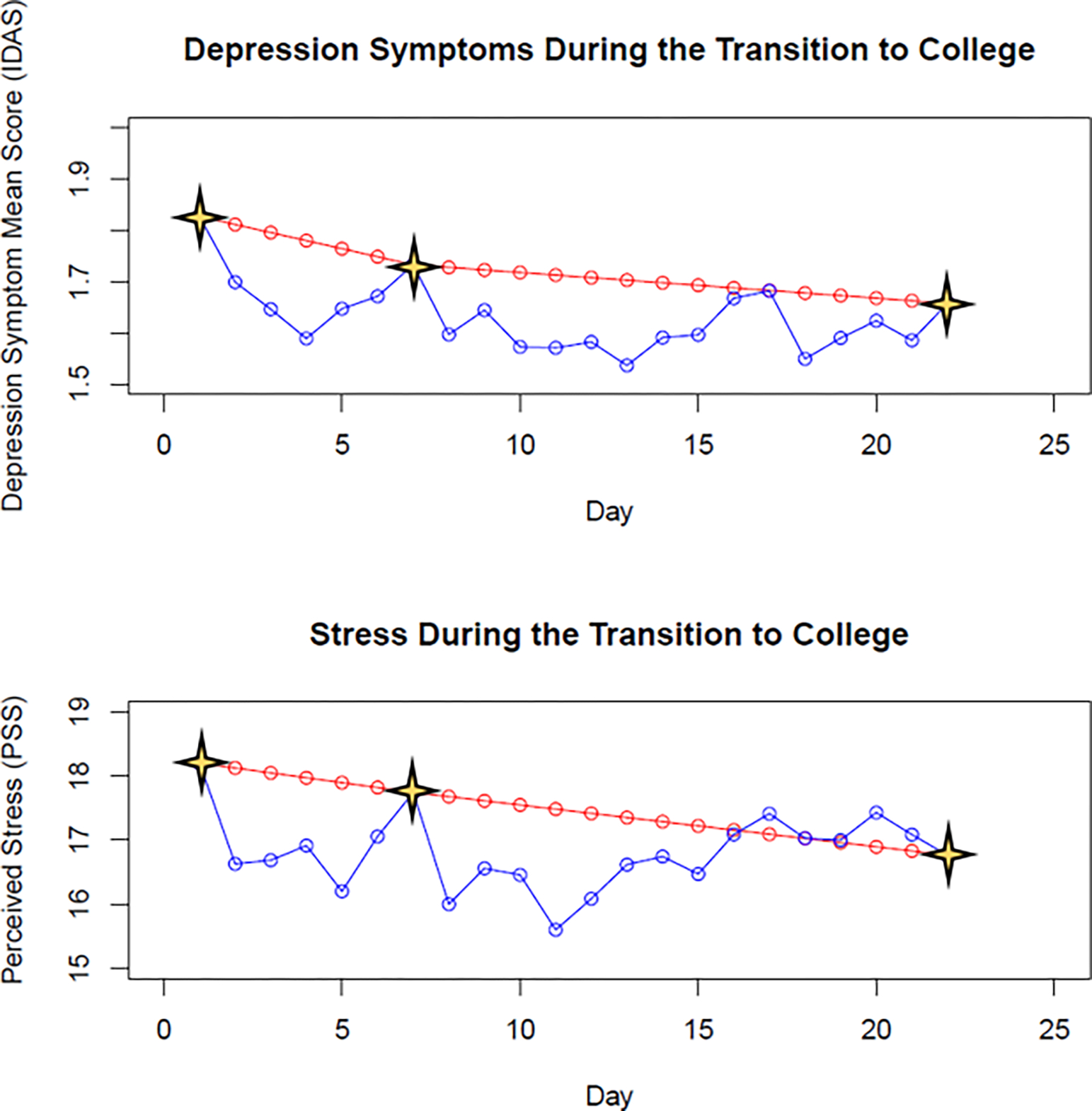
Stars indicate the three primary events of the study—namely, study start, moving onto campus, and study end. The red lines illustrate the trajectories of depression symptoms and stress if data were only collected and analyzed at these event time points. The blue lines illustrate the actual daily fluctuations in depression symptom and stress data throughout the study. Note: IDAS = Inventory of Depression and Anxiety Symptoms; PSS = Perceived Stress Scale.
As can be seen from Figure 1, both perceived stress and depression symptoms decrease throughout the first week but spike on students’ first days on campus. After moving onto campus, both stress and symptom levels decrease and stabilize for about a week before spiking again, potentially due to missing friends and family from home or adjustment to the start of classes. Interestingly, during these last few days of the study the trajectories for depression symptoms and stress diverge, with symptoms decreasing slightly and stabilizing while stress remained slightly elevated (relative to the first week on campus).
Further, it is important to highlight that change is a process that happens within-individuals over time. Visualizations and analyses at the group level (i.e., “nomothetic”) might obscure important nuances at individual level (i.e., “idiographic”). Consider Figure 2, which visualizes the depression symptom trajectories for the participant with the most variability (blue line) and the participant with the least variability (red line) in depression scores over the course of the study. Whereas the red line shows consistently negligible depression symptoms (regardless of timepoint), the blue line shows (a) much more change over time, (b) notably higher peak symptoms relative to Figure 1‘s group-based plots, and (c) a different trajectory than that suggested by Figure 1‘s group-based plots. Specifically, the group-based plots (Figure 1) reveal that depression symptoms were highest at the start of the study. However, this individual’s depression symptoms consistently fluctuated throughout data collection and peaked during the last week of the study. Critically, this lack of group-to-individual generalizability has inspired some researchers to call for more idiographic approaches in human subjects research14, giving rise to new methods that combine idiographic and nomothetic approaches such as group iterative multiple model estimation (GIMME)57. Proponents of these approaches highlight that many of the processes health scientists aspire to understand, such as disease detection, prevention, and treatment, all happen within specific individuals and argue that an overreliance on methods that aggregate across individuals obscures nuance that is critical for successfully translating basic research to clinical impact.
Figure 2. Individual Depression Symptom Trajectories.
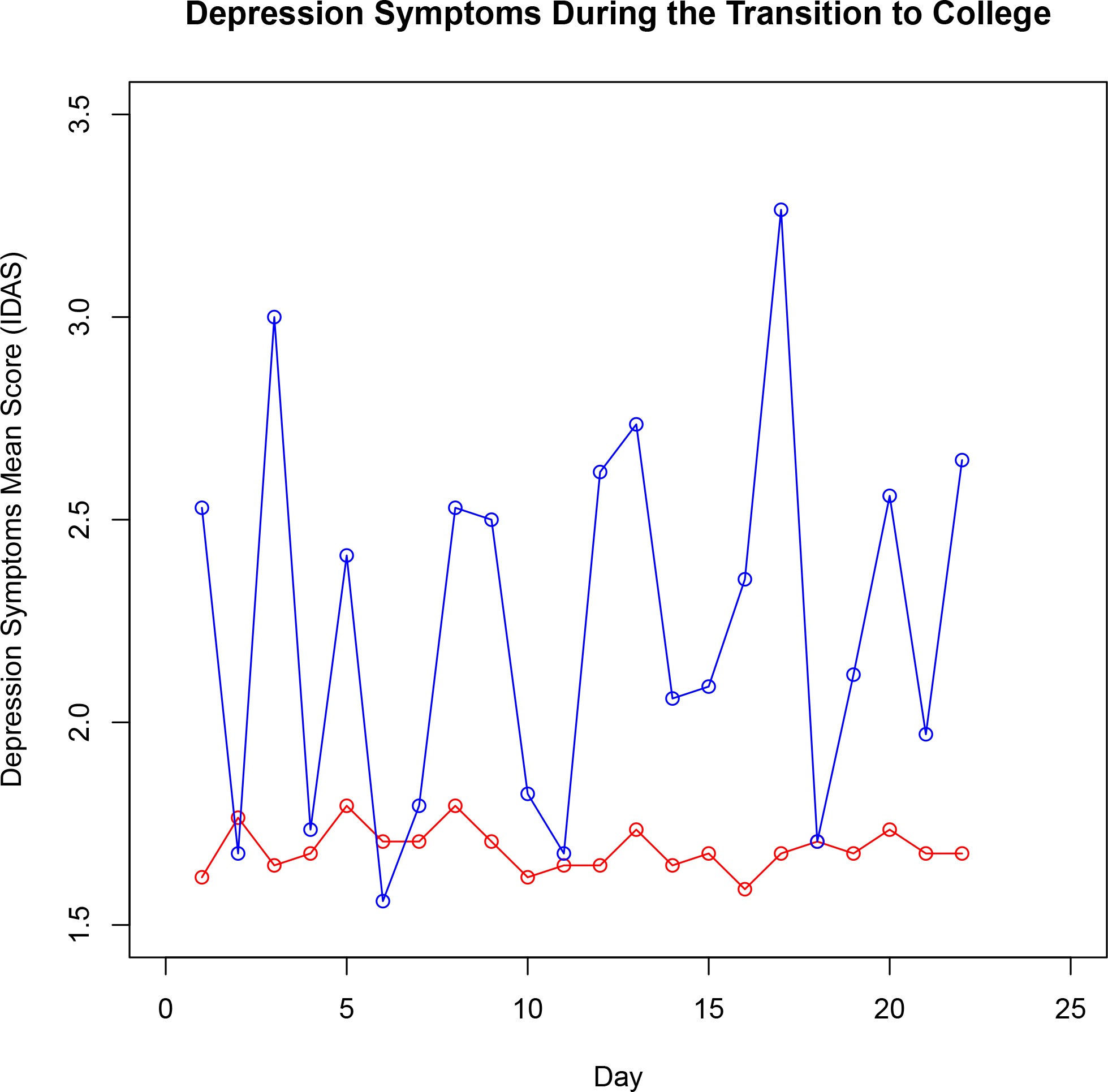
The trajectories for the participant with the greatest variability (blue line) and the participant with the least variability (red line) in depression symptoms. Note: IDAS = Inventory of Depression and Anxiety Symptoms.
Recommendations for Moving Forward
To help immunopsychiatry become a more temporally informed science, we conclude with several recommendations. First, existing longitudinal datasets—regardless of the duration between measurement occasions—should be leveraged to advance our temporal understanding of how immunopsychiatric variables change in isolation and in relation to one another over time. Examples include physiometrics11,12 such as temporal stability/reliability (e.g., retest reliability, intraclass correlation coefficients, stability estimates from a variable predicting itself over time28) and temporal specificity/optimal time lags to detect an association between two variables. It is important that this descriptive work specifies the timescale of the data tested to avoid general statements about stable/unstable or reliable/unreliable measures.
Second, existing data should be combined with analytic strategies that isolate within-person variance that can yield stronger inferences about change compared to between-person variance. One such methodology is the use of person-centered predictors, which separate each individual’s average levels of a variable (i.e., between-person variance) from their fluctuations overtime (i.e., within-person variance). For a more in-depth overview of this methodology, see Ref 58, and for an example of this methodology in psychoneuroimmunology research, see Ref7. Another relevant technique is the use of latent change score models, which estimate both latent change scores (i.e., the change in the “true score” between time points) and latent growth factors (i.e., the constant change across all measurement occasions). For guides and tutorials on latent change score models, see Refs 59,60, and for an example of this technique in psychoneuroimmunology research, see Ref. 61.
Finally, we recommend the collection of intensive longitudinal data for highly dynamic psychoneuroimmunological variables. Typically, intensive longitudinal designs for psychological data involve using web- or app-based surveys that are scheduled to prompt participants to complete self-report measures at pre-specified times, which is often referred to as EMA or experience sampling methods (ESM) (see Ref 62 for an introduction to a special section on EMA and ESM approaches). Frequent assessment of immune variables, in turn, can be done in several ways. First, standard venipuncture blood draws can be used. The benefits of this methodology are that it (a) is comparable to most psychoneuroimmunology studies, (b) might result in more consistent data collection and storage than alternatives due to the involvement of trained phlebotomists and blood draws being done in a laboratory setting, and (c) results in larger blood-volumes than microsampling procedures (described below), which allow for the assessment of more analytes with a single sample. However, high-intensity venipuncture blood draws would (typically) require frequent trips to a laboratory, thus significantly increasing burden for both participants and research staff. Additionally, it is not uncommon for some participants to have a strong aversion to needles, which can hurt and/or bias study recruitment efforts.
To address these limitations of standard venipuncture blood draws, some companies have developed micro-sampling devices that enable remote blood draws and which, in turn, permit less-invasive, high intensity immune assessment. Compared to standard venipuncture procedures, the micro-sampling devices have the benefits of typically being self-administered, removing the need to schedule in-person study visits and thus greatly reducing participant and research staff time and burden. This approach also has some limitations, such as logistical challenges including, but not limited to, shipping costs (for sending devices to and/or from participants), the lack of a controlled lab setting and associated confidence that proper blood draw procedures are followed, and potential variability in how samples are stored and/or handled before arriving to the lab for storage and analysis.
In weighing the pros and cons of these two approaches, for Project MHISS, we elected to use the latter method and achieved a 91% compliance rate for blood draw measurement for participants who completed the study, thus demonstrating its feasibility. Once data are collected, one particularly suitable and highly flexible statistical approach to analyzing intensive longitudinal data is dynamic structural equation modeling. The following references can serve as useful starting points for readers interested in learning more about this approach63–65. Additionally, differential time varying effect models can be useful in large intensive longitudinal datasets to identify optimal time lags between variables66.
In the end, data collected at longer timescales can be useful in some instances and, in fact, such data may be important for modeling certain associations that take longer to unfold or that have cyclic or bidirectional effects. In such circumstances, readers might want to consider the potential value of measurement-burst designs67, which combine both intensive longitudinal and longer scale assessments. Our final recommendation, therefore, is to make sure your study design and assessment frequency map on well to the hypothesized model you seek to test, taking into careful consideration knowledge about the rates at which each relevant variable can be expected to change over time.
Conclusion
In conclusion, what we have tried to highlight here is that there is presently a large disconnect between the months-to-years long follow-up periods typically seen in immunopsychiatry and what is known about the dynamics and stability of key constructs that are central to this field, including stress, immune functioning, and psychopathology, which change (sometimes quite substantially) in individuals over short amounts of time. Only by investing in high-density data will immunopsychiatry be able to determine what measurement frequency is ideal to maximize the translational value of this work. By learning how to better use existing data for research on temporal dynamics as well as collecting intensive longitudinal data, immunopsychiatric researchers will be much better positioned to advance our causal understanding of the interplay between the immune system, mental health, and the other psychological and behavioral outcomes, thus greatly enhancing the clinical and public benefit of the field as a whole.
Funding:
Daniel P. Moriarity and George M. Slavich were supported by grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. Additionally, Daniel P. Moriarity was supported by National Research Service Award F32 MH130149. These organizations had no role in planning, writing, editing, or reviewing this article, or in deciding to submit this article for publication.
Footnotes
It is important to note that stability differs from retest reliability in that stability refers to the effect of a variable on itself, but multiple factors (including stability) can influence the retest reliability of a variable. Therefore, citations incorporating retest reliability are useful, but imperfect examples of how temporal dynamics influence optimal time lags
Conflict of Interest: The authors declare no conflicts of interest with respect to this work.
References
- 1.Capuron L, Lawson DH, Nemeroff CB. Neurobehavioral Effects of Interferon-α in Cancer Patients: Phenomenology and Paroxetine Responsiveness of Symptom Dimensions. 26(5):10. [DOI] [PubMed] [Google Scholar]
- 2.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain, Behavior, and Immunity. 2004;18(3):205–213. doi: 10.1016/j.bbi.2003.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Hansson LS, Axelsson J, Petrovic P, et al. Regulation of emotions during experimental endotoxemia: A pilot study. Brain, behavior, and immunity. 2021;93:420–424. doi: 10.1016/j.bbi.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 4.Slavich GM. Social Safety Theory: Understanding social stress, disease risk, resilience, and behavior during the COVID-19 pandemic and beyond. Curr Opin Psychol. 2022;45:101299. doi: 10.1016/j.copsyc.2022.101299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavich GM. Social Safety Theory: A Biologically Based Evolutionary Perspective on Life Stress, Health, and Behavior. Annual Review of Clinical Psychology. 2020;16:265–295. doi: 10.1146/annurev-clinpsy-032816-045159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slavich GM, Roos LG, Mengelkoch S, et al. Social Safety Theory: Conceptual Foundation, Underlying Mechanisms, and Future Directions. Health Psychology Review. 2023;0(ja):1–65. doi: 10.1080/17437199.2023.2171900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriarity DP, Kautz MM, Mac Giollabhui N, et al. Bidirectional associations between inflammatory biomarkers and depressive symptoms in adolescents: Potential causal relationships. Clinical Psychological Science. 2020;8(4):690–703. doi: 10.1017/CBO9781107415324.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields GS, Spahr CM, Slavich GM. Psychosocial Interventions and Immune System Function: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry. 2020;77(10):1031–1043. doi: 10.1001/jamapsychiatry.2020.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Euteneuer F, Dannehl K, Del Rey A, Engler H, Schedlowski M, Rief W. Immunological effects of behavioral activation with exercise in major depression: An exploratory randomized controlled trial. Translational Psychiatry. 2017;7(5):1–10. doi: 10.1038/tp.2017.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between Plasma IL-6 Response to Acute Stress and Early-Life Adversity in Healthy Adults. Neuropsychopharmacology. 2010;35:2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segerstrom SC, Smith GT. Methods, variance, and error in psychoneuroimmunology research: The good, the bad, and the ugly. In: Segerstrom SC, ed. Oxford Handbook of Psychoneuroimmunology. Oxford U Press; 2012:421–432. [Google Scholar]
- 12.Moriarity DP, Alloy LB. Back to basics: The importance of measurement properties in biological psychiatry. Neuroscience and Biobehavioral Reviews. 2021;123:72–82. doi: 10.1016/j.neubiorev.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson EH. The Interpretation of Interaction in Contingency Tables. Journal of the Royal Statistical Society Series B (Methodological). 1951;13(2):238–241. [Google Scholar]
- 14.Fisher AJ, Medaglia JD, Jeronimus BF. Lack of group-to-individual generalizability is a threat to human subjects research. Proceedings of the National Academy of Sciences. 2018;115(27):E6106–E6115. doi: 10.1073/pnas.1711978115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamaker EL. Why researchers should think “within-person”: A paradigmatic rationale. In: Handbook of Research Methods for Studying Daily Life. The Guilford Press; 2012:43–61. [Google Scholar]
- 16.Molenaar PCM, Campbell CG. The New Person-Specific Paradigm in Psychology. Current Directions in Psychological Science. 2009;18(2):112–117. [Google Scholar]
- 17.Molenaar PCM. A Manifesto on Psychology as Idiographic Science: Bringing the Person Back Into Scientific Psychology, This Time Forever. Measurement: Interdisciplinary Research and Perspectives. 2004;2(4):201–218. doi: 10.1207/s15366359mea0204_1 [DOI] [Google Scholar]
- 18.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: Systematic review, meta-analysis, and meta-regression. Molecular Psychiatry. 2021;26(7):3302–3314. doi: 10.1038/s41380-020-00867-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Gimeno D, Kivimäki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological medicine. 2009;39(3):413–423. doi: 10.1017/S0033291708003723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zalli A, Jovanova O, Hoogendijk WJG, Tiemeier H, Carvalho LA. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology. 2016;233(9):1669–1678. doi: 10.1007/s00213-015-3919-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Biggelaar AHJ, Gussekloo J, de Craen AJM, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Experimental Gerontology. 2007;42(7):693–701. doi: 10.1016/j.exger.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 23.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain, Behavior, and Immunity. 2009;23(7):936–944. doi: 10.1016/j.bbi.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopwood CJ, Bleidorn W, Wright AGC. Connecting Theory to Methods in Longitudinal Research. Perspect Psychol Sci. 2022;17(3):884–894. doi: 10.1177/17456916211008407 [DOI] [PubMed] [Google Scholar]
- 25.Gollob HF, Reichardt CS. Taking Account of Time Lags in Causal Models. Child Development. 1987;58(1):80. doi: 10.2307/1130293 [DOI] [PubMed] [Google Scholar]
- 26.Moriarity DP, Mac Giollabhui N, Ellman LM, et al. Inflammatory proteins predict change in depressive symptoms in male and female adolescents. Clinical Psychological Science. 2019;7(4):754–767. doi: 10.1177/2167702619826586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham-Engeland JE, Sin NL, Smyth JM, et al. Negative and positive affect as predictors of inflammation: Timing matters. Brain, Behavior, and Immunity. 2018;74(August):222–230. doi: 10.1016/j.bbi.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dormann C, Griffin MA. Optimal time lags in panel studies. Psychological Methods. 2015;20(4):489–505. doi: 10.1037/met0000041 [DOI] [PubMed] [Google Scholar]
- 29.Dwyer J Statistical Models for the Social and Behavioral Sciences. 1st ed. Oxford University Press; 1983. Accessed February 19, 2023. https://www.amazon.com/Statistical-Models-Social-Behavioral-Sciences/dp/0195031458 [Google Scholar]
- 30.Boker SM, Nesselroade JR. A Method for Modeling the Intrinsic Dynamics of Intraindividual Variability: Recovering the Parameters of Simulated Oscillators in Multi-Wave Panel Data. Multivariate Behav Res. 2002;37(1):127–160. doi: 10.1207/S15327906MBR3701_06 [DOI] [PubMed] [Google Scholar]
- 31.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological bulletin. 2014;140(3):774–815. doi: 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EH. Review of the Psychometric Evidence of the Perceived Stress Scale. Asian Nursing Research. 2012;6(4):121–127. doi: 10.1016/j.anr.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 34.Baryshnikov I, Aledavood T, Rosenström T, et al. Relationship between daily rated depression symptom severity and the retrospective self-report on PHQ-9: A prospective ecological momentary assessment study on 80 psychiatric outpatients. Journal of Affective Disorders. 2023;324:170–174. doi: 10.1016/j.jad.2022.12.127 [DOI] [PubMed] [Google Scholar]
- 35.Moriarity DP, Slavich GM, Alloy LB, Olino TM. Hierarchical Inflammatory Phenotypes of Depression: A Novel Approach across Five Independent Samples and 27,730 Adults. Biological Psychiatry. Published online August 2022:S0006322322015268. doi: 10.1016/j.biopsych.2022.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriarity DP, Horn SR, Kautz MM, Haslbeck JM, Alloy LB. How handling extreme C-reactive protein (CRP) values and regularization influences CRP and depression criteria associations in network analyses. Brain, Behavior, and Immunity. 2021;91:393–403. doi: 10.1016/j.bbi.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried EI, von Stockert S, Haslbeck JMB, Lamers F, Schoevers RA, Penninx BWJH. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychological Medicine. Published online 2019. doi: 10.31234/osf.io/84ske [DOI] [PubMed] [Google Scholar]
- 38.Milaneschi Y, Lamers F, Berk M, Penninx BWJH. Depression Heterogeneity and Its Biological Underpinnings: Toward Immunometabolic Depression. Biological Psychiatry. 2020;88(5):369–380. doi: 10.1016/j.biopsych.2020.01.014 [DOI] [PubMed] [Google Scholar]
- 39.Moriarity DP, Alloy LB. Beyond diagnoses and total symptom scores: Diversifying the level of analysis in psychoneuroimmunology research. Brain, Behavior, and Immunity. 2020;89:1–2. doi: 10.1016/j.bbi.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shields GS, Slavich GM, Perlman G, Klein DN, Kotov R. The short-term reliability and long-term stability of salivary immune markers. Brain, Behavior, and Immunity. 2019;81(January 2020):650–654. doi: 10.1016/j.bbi.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349(1):82–90. doi: 10.1016/j.mce.2011.06.039 [DOI] [PubMed] [Google Scholar]
- 42.Szabo YZ, Slavish DC, Graham-Engeland JE. The effect of acute stress on salivary markers of inflammation: A systematic review and meta-analysis. Brain, Behavior, and Immunity. 2020;(October 2019):1–14. doi: 10.1016/j.bbi.2020.04.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaaqoq AM, Namas RA, Abdul-Malak O, et al. Diurnal Variation in Systemic Acute Inflammation and Clinical Outcomes Following Severe Blunt Trauma. Front Immunol. 2019;10:2699. doi: 10.3389/fimmu.2019.02699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavaillon JM, Adib-Conquy M. The Pro-Inflammatory Cytokine Cascade. Immune Response in the Critically Ill. Published online 2002:37–66. doi: 10.1007/978-3-642-57210-4_4 [DOI] [Google Scholar]
- 45.Megha KB, Joseph X, Akhil V, Mohanan PV. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine. 2021;91:153712. doi: 10.1016/j.phymed.2021.153712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabinowitz AR, Fisher AJ. Person-Specific Methods for Characterizing the Course and Temporal Dynamics of Concussion Symptomatology: A Pilot Study. Sci Rep. 2020;10(1):1248. doi: 10.1038/s41598-019-57220-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howe E, Bosley HG, Fisher AJ. Idiographic network analysis of discrete mood states prior to treatment. Counselling and Psychotherapy Research. 2020;20(3):470–478. doi: 10.1002/capr.12295 [DOI] [Google Scholar]
- 48.Hamaker EL, Wichers M. No Time Like the Present: Discovering the Hidden Dynamics in Intensive Longitudinal Data. Curr Dir Psychol Sci. 2017;26(1):10–15. doi: 10.1177/0963721416666518 [DOI] [Google Scholar]
- 49.Sperry SH, Walsh MA, Kwapil TR. Emotion dynamics concurrently and prospectively predict mood psychopathology. Journal of Affective Disorders. 2020;261:67–75. doi: 10.1016/j.jad.2019.09.076 [DOI] [PubMed] [Google Scholar]
- 50.Ben-Zeev D, Young MA, Madsen JW. Retrospective recall of affect in clinically depressed individuals and controls. Cognition and Emotion. 2009;23(5):1021–1040. doi: 10.1080/02699930802607937 [DOI] [Google Scholar]
- 51.Mehl M, Conner T. Handbook of Research Methods for Studying Daily Life. The Guilford Press; 2013. Accessed February 19, 2023. https://www.guilford.com/books/Handbook-of-Research-Methods-for-Studying-Daily-Life/Mehl-Conner/9781462513055 [Google Scholar]
- 52.Lazarides C, Ward EB, Buss C, et al. Psychological stress and cortisol during pregnancy: An ecological momentary assessment (EMA)-Based within- and between-person analysis. Psychoneuroendocrinology. 2020;121:104848. doi: 10.1016/j.psyneuen.2020.104848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haslbeck JMB, Ryan O. Recovering Within-Person Dynamics from Psychological Time Series. Multivariate Behavioral Research. 2022;57(5):735–766. doi: 10.1080/00273171.2021.1896353 [DOI] [PubMed] [Google Scholar]
- 54.Cohen S Perceived Stress Scale (PSS). Published online 1994. doi: 10.1007/978-3-030-39903-0_773 [DOI] [Google Scholar]
- 55.Watson D, O’Hara MW, Simms LJ, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess. 2007;19(3):253–268. doi: 10.1037/1040-3590.19.3.253 [DOI] [PubMed] [Google Scholar]
- 56.Cole DA, Maxwell SE. Statistical Methods for Risk-Outcome Research: Being Sensitive to Longitudinal Structure. Annual Review of Clinical Psychology. 2009;5(1):71–96. doi: 10.1146/annurev-clinpsy-060508-130357 [DOI] [PubMed] [Google Scholar]
- 57.Beltz AM, Wright AGC, Sprague BN, Molenaar PCM. Bridging the Nomothetic and Idiographic Approaches to the Analysis of Clinical Data. Assessment. 2016;23(4):447–458. doi: 10.1177/1073191116648209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falkenström F, Finkel S, Sandell R, Rubel JA, Holmqvist R. Dynamic models of individual change in psychotherapy process research. Journal of consulting and clinical psychology. 2017;85(6):537–549. [DOI] [PubMed] [Google Scholar]
- 59.Kievit RA, Brandmaier AM, Ziegler G, et al. Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Developmental Cognitive Neuroscience. 2018;33:99–117. doi: 10.1016/j.dcn.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klopack ET, Wickrama K(KAS). Modeling Latent Change Score Analysis and Extensions in Mplus: A Practical Guide for Researchers. Struct Equ Modeling. 2020;27(1):97–110. doi: 10.1080/10705511.2018.1562929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zainal NH, Newman MG. Increased Inflammation Predicts 9-Year Change in Major Depressive Disorder Diagnostic Status. J Abnorm Psychol. 2021;130(8):829–840. doi: 10.1037/abn0000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trull TJ, Ebner-Priemer UW. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: Introduction to the special section. Psychological Assessment. 2009;21(4):457–462. doi: 10.1037/a0017653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McNeish D, Hamaker EL. A primer on two-level dynamic structural equation models for intensive longitudinal data in Mplus. Psychological Methods. 2020;25:610–635. doi: 10.1037/met0000250 [DOI] [PubMed] [Google Scholar]
- 64.Sadikaj G, Wright AG, Dunkley DM, Zuroff DC, Moskowitz D. Multilevel Structural Equation Modeling for Intensive Longitudinal Data: A Practical Guide for Personality Researchers. In: The Handbook of Personality Dynamics and Processes.; 2021:855–885. [Google Scholar]
- 65.Hamaker E, Asparouhov T, Muthen B. Dynamic structural equation modeling as a combination of time series modeling, multilevel modeling, and structural equation modeling. In: The Handbook of Structural Equation Modeling. Vol 31. 2nd ed. Guilford Press; 2021. [Google Scholar]
- 66.Jacobson NC, Chow SM, Newman MG. The Differential Time-Varying Effect Model (DTVEM): A tool for diagnosing and modeling time lags in intensive longitudinal data. Behav Res Methods. 2019;51(1):295–315. doi: 10.3758/s13428-018-1101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ram N, Gerstorf D. Time-structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processes. Psychol Aging. 2009;24(4):778–791. doi: 10.1037/a0017915 [DOI] [PMC free article] [PubMed] [Google Scholar]


