Abstract
Noninvasive ventilation (NIV) is a common modality employed to treat acute respiratory failure. Most data guiding its use is extrapolated from adult studies. We sought to identify clinical predictors associated with failure of NIV, defined as requiring intubation. This single-center retrospective observational study included children admitted to pediatric intensive care unit (PICU) between July 2014 and June 2016 treated with NIV, excluding postextubation. A total of 148 patients was included. Twenty-seven (18%) failed NIV. There was no difference between the two groups with regard to age, gender, comorbidities, or etiology of acute respiratory failure. Those that failed had higher admission pediatric risk of mortality ( p = 0.01) and pediatric logistic organ dysfunction ( p = 0.002) scores and higher fraction of inspired oxygen (FiO 2 ; p = 0.009) at NIV initiation. Failure was associated with lack of improvement in tachypnea. At 6 hours of NIV, the failure group had worsening tachypnea with a median increase in respiratory rate of 8%, while the success group had a median reduction of 18% ( p = 0.06). Multivariable Cox's proportional hazard models revealed FiO 2 at initiation and worsening respiratory rate at 1- and 6-hour significant risks for failure of NIV. Failure was associated with a significantly longer PICU length of stay (success [2.8 days interquartile range (IQR): 1.7, 5.5] vs. failure [10.6 days IQR: 5.6, 13.2], p < 0.001). NIV can be successfully employed to treat acute respiratory failure in pediatric patients. There should be heightened concern for NIV failure in hypoxemic patients whose tachypnea is unresponsive to NIV. A trend toward improvement should be closely monitored.
Keywords: critical care, acute respiratory failure, pediatric acute respiratory distress syndrome, noninvasive ventilation, BiPAP, CPAP
Introduction
Etiologies of respiratory failure in children are diverse and include diagnoses such as asthma, pneumonia, postoperative respiratory failure, bronchiolitis, neuromuscular weakness, and indirect lung injury. There may be components of hypoxemia, hypercarbia, or both. NIV is a modality to support children with respiratory failure 1 but its use is highly variable. The heterogeneity of respiratory failure and lack of evidence-guiding NIV use in children can make it challenging to optimize practice. Much of the data regarding the use of NIV in children is extrapolated from adult studies which have shown a benefit in patients with neuromuscular diseases, 2 community-acquired pneumonia, 3 severe hypoxemic respiratory failure, 4 and obstructive processes. 3 4 Other adult studies have shown that patients with acute respiratory distress syndrome (ARDS) or community-acquired pneumonia are less likely to benefit from NIV. 5 6 There is likely a role for NIV in the pediatric population; however, it is challenging to determine which patients are most likely to be successfully treated with NIV. 7 Several studies, supporting NIV use, have found that early NIV may prevent clinical worsening 8 or decreased need for intubation. 9 10 Nevertheless, there has also been a suggestion there is no reduction in intubations or improvement in outcomes. 11
Further research is needed to understand which patients yield the most benefit from NIV for optimal application of this therapy. Identifying the predictors associated with failure of treatment modalities like NIV is critical to avoid delaying life-saving therapies. Previous studies have examined factors associated with failure of NIV in adults and children. 5 12 13 14 15 16 Risk factors for NIV failure in children, such as multiorgan dysfunction, illness acuity scores, hemodynamic instability, hypoxemia, fraction of inspired oxygen (FiO 2 ), and underlying oncology diagnosis have been reported. 12 13 14 15 16 17 18 However, there is even less pediatric information available than in adults where the reported risk factors are variable and many of the sample sizes are small (i.e., <100 patients). This study sought to examine clinical predictors with specific consideration for hypoxemia as a risk for NIV failure in the pediatric population in a larger cohort of patients. Since patients with ARDS have been recognized as potentially poor NIV candidates 5 18 19 and as children with hypoxemia are at risk of developing pediatric acute respiratory distress syndrome (PARDS), with a mortality rate of up to 35%, 20 21 22 we hypothesized that markers of hypoxemia are associated with NIV failure.
Methods
This is a single-center retrospective cohort study of children, age ≤18 years, admitted to a quaternary care pediatric intensive care unit (PICU), who were treated with positive pressure delivered noninvasively. We defined this a priori as either bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP) between July 2014 and June 2016 and will refer to these modes through the remainder of the manuscript generally as NIV. NIV was delivered using a nasal mask, nasal prongs, or full-face mask. The interface was chosen by respiratory therapist according to the child's size and to achieve minimal air leak. There is not an NIV initiation protocol in use at this center; thus the NIV settings were selected for each patient by the physician in charge of that patient's care. There is not an institutional sedation protocol for patients on NIV. In general, it is the practice at this institution to attempt NIV without sedation. Children were excluded from the study if they were on NIV postextubation, had a limited code status, had a tracheostomy, were on home NIV (on baseline settings and baseline duration), and/or were admitted to the cardiac intensive care unit. If a patient was admitted to the PICU requiring NIV on multiple occasions, only the first admission was included. Institutional review board approval (protocol number: 1702133310) was obtained and consent was waived.
Demographic data including age, gender, weight, comorbidities, admission pediatric logistic organ dysfunction (PELOD) 23 24 and pediatric risk of mortality (PRISM) 25 scores, and primary admission diagnosis was collected. Vital signs and blood gases obtained at initiation of NIV, 1 hour (if 1-hour data were not available, we used data up to 2-hour post-NIV), and 6 hours after initiation of NIV were also collected. When a patient had both 1-hour and 2-hour vital signs, the 1-hour vitals were included in the analysis. Failure of NIV was defined as need for intubation and invasive mechanical ventilation (IMV). Decisions for intubation were at the discretion of the medical care team.
Markers of oxygenation were calculated to quantify hypoxemia and as part of the diagnosis of PARDS for those that failed NIV and required IMV. Saturation from pulse oximetry (SpO 2 ):FiO 2 ratios were calculated when SpO 2 ≤ 97%. 26 Oxygen saturation index (OSI) was calculated for patients who were receiving IMV and had a SpO 2 ≤ 97%. Oxygenation index (OI) was calculated on patients receiving IMV and who had an arterial blood gas.
OI = (P aw × FiO 2 )/P a O 2
OSI = (P aw × FiO 2 )/SpO 2 (where P aw is mean airway pressure)
PARDS was defined using diagnostic criteria from the pediatric acute lung injury consensus conference (PALICC). 27 Additional therapies, such as need for extracorporeal life support, continuous renal replacement therapy, inhaled nitric oxide, and high-frequency oscillatory ventilation were also collected.
Statistical Analysis
Continuous variables are reported as medians (interquartile ranges [IQRs]) and were compared with Mann–Whitney U -test. Categorical variables are reported as frequencies (percent) and were compared with Chi-square or Fisher's exact test. Variables were further evaluated for an association with NIV failure using logistic regression, and odds ratios (OR) and 95% confidence intervals (CIs) were calculated. Due to nonlinearity in the logit for some continuous variables, Receiver operating characteristics (ROC) curves were calculated, and Youden's index was used to identify optimal cut points for categorizing variables or variables were categorized by accepted clinical age-related norms (i.e., normal heart rate vs. tachycardia). Vital signs were analyzed as percent change from initiation and then categorized as improving or worsening. Unadjusted and adjusted Cox's proportional hazards models were used for the time to event analysis. Cumulative incidence functions were also constructed for significant variables. A p -value of less than 0.05 was considered statistically significant.
Results
In the cohort of 148 pediatric patients admitted to the PICU and placed on NIV for acute respiratory failure or acute on chronic respiratory failure, 27 (18.2%) failed NIV and required IMV. The median age was 7.2 years (1.4–13.6 years). Age, gender, admission diagnoses, and underlying comorbidities were not associated with failure of NIV. Patients who failed NIV had higher PRISM and PELOD scores at admission ( Table 1 ). The majority (83.8%) of patients were started on BiPAP and with the remainder started on CPAP. The starting mode of NIV was not associated with failure ( p = 0.99). Starting median inspiratory and expiratory pressures were not associated with failure. Starting median inspiratory pressure for those that failed was 12 cm H 2 O (IQR: 10.0, 15.0) compared with those that were successful treated with a median of 12 cm H 2 O (IQR: 12.0, 14.8), p = 0.897. Expiratory pressures were also similar with those that failed having a median of 6 cm H 2 O (IQR: 6.0, 8.0) versus those who were successful having a median of 6 cm H 2 O (IQR: 6.0, 6.0), p = 0.129.
Table 1. Demographics.
| Demographics | Entire cohort ( n = 148) | NIV success ( n = 121) | NIV failure ( n = 27) | p -Value |
|---|---|---|---|---|
| Gender (female) n (%) | 68 (44.6) | 55 (45.4) | 13 (48.1) | 0.8 |
| Age (y) Median (IQR) |
7.1 (1.4, 13.6) | 7.2 (1.9, 14.0) | 6.3 (0.5, 11.5) | 0.14 |
| Admission diagnosis n (%) | 0.25 | |||
| Pneumonia | 31 (21.1) | 26 (21.7) | 5(18.5) | |
| Bronchiolitis/viral illness | 37 (25.2) | 29 (24.2) | 8 (29.6) | |
| Sepsis | 5 (3.4) | 3 (2.5) | 2 (7.4) | |
| Upper airway obstruction | 17 (11.6) | 14 (1.7) | 3 (11.1) | |
| Post-op spinal fusion | 7 (4.8) | 7 (5.8) | 0 (0) | |
| Asthma | 27 (18.4) | 25 (20.8) | 2 (7.4) | |
| Other | 23 (15.6) | 16 (13.3) | 7 (15.6) | |
| Comorbidities n (%) | ||||
| Prematurity | 27 (20.0) | 21 (18.2) | 6 (28.6) | 0.37 |
| pHTN | 6 (3.9) | 5 (3.9) | 1 (3.7) | 1.00 |
| Chronic pulmonary disease | 72 (48.6) | 61 (50.4) | 11 (40.7) | 0.34 |
| Heart disease | 6 (4.1) | 4 (3.3) | 2 (7.4) | 0.30 |
| Oncologic disease | 8 (5.4) | 7 (5.8) | 1 (3.7) | 1.00 |
| Immunosuppression | 12 (8.1) | 8 (6.6) | 4 (14.8) | 0.23 |
| Neurologic condition | 55 (37.2) | 46 (38) | 9 (33.3) | 0.83 |
| Genetic condition | 24 (16.2) | 20 (16.5) | 4 (14.8) | 1.00 |
| Airway anomaly | 10 (6.8) | 9 (7.4) | 1 (3.7) | 0.69 |
| PRISM Median (IQR) |
0.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 3.0 (0.0, 3.0) | 0.01 |
| PELOD Median (IQR) |
2.0 (0.0, 10.0) | 1.0 (0.0, 10.0) | 10.0 (1.0, 11.0) | 0.002 |
Abbreviations: IQR, interquartile range; NIV, noninvasive ventilation; pHTN, pulmonary hypertension; Post-op, postoperative; PRISM, pediatric risk of mortality; PELOD, pediatric logistic organ dysfunction.
Note: Categorical variables are presented as counts with (%) and continuous variables are presented as medians with (IQR).
In a univariable analysis, those who failed had a higher median FiO 2 at initiation of NIV than those who were successfully treated without IMV ( p = 0.01; Supplementary Table S1 ; available in the online version). Those who failed NIV had a lower SpO 2 :FiO 2 ratio at initiation but did not reach statistical significance: 158.3 (89, 194) versus 290.0 (143.8, 310; p = 0.06). As early as 1 hour of post-NIV initiation, those who were successfully treated with NIV demonstrated improvement in tachypnea. One hour following initiation, the failure group had a median respiratory rate of 36 (24, 52) versus the success group 29 (21, 40; p = 0.05). At 1 hour of NIV, the failure group had worsening tachypnea with a median increase of 2.4% while the success group had a median reduction of 15.8% ( p = 0.15). At 6 hours of NIV, the failure group had worsening tachypnea with a median increase in respiratory rate of 8%, while the success group had a median reduction in respiratory rate of 18% ( p = 0.06). The percent change in respiratory rate as an isolated variable had an area under curve (AUC) of 0.67. Changes in heart rate and oxygen saturations were not associated with NIV failure. ( Supplementary Table S1 ; available in the online version).
Unadjusted Cox's proportional hazard testing was conducted on variables at initiation, 1 hour of NIV, and 6 hours of NIV ( Table 2 ). On unadjusted analysis, those on FiO 2 over 45% at initiation of NIV had an increased risk of failing ( p = 0.007; Fig. 1A ). Additionally, while tachypnea at initiation was not associated with NIV failure, having a worsening respiratory rate at 1 and 6 hours of NIV use was associated with increased risk for NIV failure ( Fig. 1B and 1C ). Heart rate, SpO 2 , SpO 2 :FiO 2 , PELOD, and PRISM were not significant on unadjusted analysis ( Table 2 ).
Table 2. Unadjusted variables for risk of NIV failure.
| Vital sign | Unadjusted hazard ratio (95% confidence interval) | p -Value |
|---|---|---|
| Heart rate (HR) | ||
| Tachycardia at initiation a | 0.93 (0.41, 2.14) | 0.863 |
| Worsening HR at 1 hour | 1.00 (0.42, 2.37) | 0.991 |
| Worsening HR at 6 hours | 2.32 (0.78, 6.71) | 0.130 |
| Respiratory rate (RR) | ||
| At initiation | 0.83 (0.37, 1.84) | 0.644 |
| Worsening RR at 1 hour | 2.91 (1.21, 7.00) | 0.017 |
| Worsening RR at 6 hours | 4.55 (1.44, 14.34) | 0.010 |
| Saturation from pulse oximetry (SpO 2 ) | ||
| At initiation | 0.93 (0.84, 1.02) | 0.102 |
| Worsening SpO 2 at 1 hour | 1.43 (0.53, 3.91) | 0.484 |
| Worsening SpO 2 at 6 hours | 0.60 (0.21, 1.73) | 0.343 |
| Fraction of inspired oxygen (FiO 2 ) ≥ 45% | ||
| At initiation | 3.77 (1.43, 9.95) | 0.007 |
| At 1 hour | 1.74 (0.74, 4.11) | 0.205 |
| At 6 hours | 2.67 (0.90, 7.98) | 0.078 |
| SpO 2 :FiO 2 b | ||
| At initiation | 0.99 (0.98, 1.00) | 0.075 |
| Worsening SpO 2 :FiO 2 at 1 hour | 2.26 (0.46, 11.19) | 0.319 |
| Worsening SpO 2 :FiO 2 at 6 hours | 9.45 (0.85, 105.12) | 0.068 |
| Acuity scores | ||
| PELOD | 1.04 (0.98, 1.10) | 0.197 |
| PRISM | 1.06 (0.97, 1.61) | 0.175 |
Abbreviations: NIV, noninvasive ventilation; PRISM, pediatric risk of mortality; PELOD, pediatric logistic organ dysfunction.
Tachycardia defined by being above age-specific norms.
Only 46 patients with valid data available.
Fig. 1.
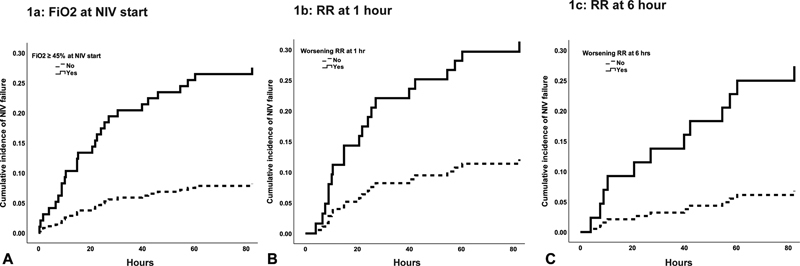
Cumulative incidence functions of NIV failure by FiO 2 > 45% at NIV initiation ( A ), respiratory rate at 1 hour of NIV ( B ), and respiratory rate at 6 hours of NIV ( C ). FiO 2 , fraction of inspired oxygen; NIV, noninvasive ventilation; RR, respiratory rate.
Multivariable Cox's proportional hazard models were constructed for worsening respiratory rate (RR) at 1 hour and 6 hours of post-NIV initiation. While not significant on unadjusted testing, PELOD was incorporated into the model to adjust for level of acuity. In these models, FiO 2 ≥ 45% at initiation and worsening respiratory rate at 1 and 6 hours remained significant risks for failure of NIV ( Table 3 ).
Table 3. Adjusted Cox's proportional hazard models for NIV failure.
| Model for risk of failing NIV | HR (95% CI) | p -Value |
|---|---|---|
| Hour 1 of NIV | ||
| Increasing respiratory rate from initiation to +1 hour | 3.5 (1.4, 8.5) | 0.006 |
| FiO 2 at initiation ≥0.45 | 3.0 (1.1, 8.5) | 0.033 |
| PELOD | 1.1 (0.99, 1.11) | 0.110 |
| Hour 6 of NIV | ||
| Increasing respiratory rate from initiation to +6 hour | 5.6 (1.7, 18.6) | 0.004 |
| FiO 2 at initiation ≥0.45 | 10.8 (1.4, 85.4) | 0.025 |
| PELOD | 1.1 (0.99, 1.2) | 0.087 |
Abbreviations: CI, confidence interval; FiO 2 , fraction of inspired oxygen; HR, hazard ratio; NIV, noninvasive ventilation; PELOD, pediatric logistic organ dysfunction.
Failure of NIV was associated with longer PICU stay ( p < 0.0001) and longer hospital stay ( p < 0.0001; Table 4 ). Seventeen patients were diagnosed with PARDS. Of the patients diagnosed with PARDS, 13 (76%) failed NIV and required IMV. Of those who failed NIV, nearly half were diagnosed with PARDS. There was a single mortality in the cohort. Of note, this patient failed NIV and was diagnosed with PARDS.
Table 4. Outcomes.
| Outcome | Entire cohort ( n = 148) | NIV success ( n = 121) | NIV failure ( n = 27) | p -Value |
|---|---|---|---|---|
| Diagnosis of PARDS n (%) |
17 (11.5) | 4 (3.3) | 13 (48.1) | <0.0001 |
| Mortality n (%) |
1 (0.7) | 0 (0.0) | 1 (3.7) | 0.03 |
| Hospital LOS (d) Median (IQR) |
8.9 (5.2, 15.7) | 8.0 (4.8, 12.7) | 16.3 (9.1, 22.8) | <0.0001 |
| PICU LOS (d) Median (IQR) |
3.7 (2.1, 6.8) | 2.8 (1.7, 5.5) | 10.6 (5.6, 13.2) | <0.0001 |
Abbreviations: IQR, interquartile range; LOS, length of stay; NIV, noninvasive ventilation; PARDS, pediatric acute respiratory distress syndrome; PICU, pediatric intensive care unit.
Note: Results for categorical variables are presented in counts (%).
None of the patients in this cohort were documented to have experienced adverse events such as skin breakdown, emesis, aspiration, or epistaxis.
Discussion
This study of pediatric patients with acute respiratory failure describes the practice regarding utilization of noninvasive positive pressure ventilation at a single center. We found that elevated FiO 2 at initiation, lack of improvement in tachypnea, and low SpO 2 :FiO 2 ratio were predictors of failure. When placed in a multivariable model to obtain a set of clinical variables that could aid in predicting NIV failure, the best multivariable model combined worsening tachypnea at 6 hours and FiO 2 at initiation of 45% or higher. There were more patients who developed PARDS in the NIV failure group. Also, those who failed NIV had longer PICU length of stay and had increased mortality.
The use of NIV is common in the pediatric population with a variable success rate of 55 to 96%. 28 Several studies have identified that patients with bronchiolitis, asthma, airway obstruction, and sickle cell disease with acute chest syndrome generally respond well to NIV. 1 16 28 However, there are also disease- and patient-specific factors that influence NIV failure and success. This study confirmed that patients with higher illness severity are more likely to fail NIV. 14 16 28
Similar to other studies that have shown hypoxemia 5 13 15 25 or presence of ARDS 14 is a predictor for failure of NIV, our study showed that FiO 2 at initiation was a marker for failure. It is interesting that even though FiO 2 at initiation is statistically different between the groups, both groups that are successfully treated and those that failed have improvement in FiO 2 by 6 hours and the FiO 2 at this time point is no longer significantly different. This could be due to a sample size or it could suggest that FiO 2 alone is not a strong predictor of success. It may be that it is not FiO 2 but severity of hypoxemia that is a key predictor of failure. While the SpO 2 :FiO 2 ratio was not statistically different, this particular analysis is challenged by the numerous missing data points secondary to the SpO 2 > 97%, making the SpO 2 :FiO 2 calculation invalid. 26 In these cases, as we do not have PaO 2 data, it is difficult to determine the degree of hypoxemia. With the numerous saturations over 97%, it is possible that patients were delivered an unnecessarily high FiO 2 creating the possibility of lung injury secondary to hyperoxia. However, we suspect that hypoxemia also likely plays a role in NIV failure and this is supported by the high rate of PARDS in our failure group.
It has been reported the severity of hypoxemia at PARDS diagnosis in those utilizing NIV was strongly indicative of likelihood of intubation. Using the PALICC definition, we found that NIV failure was associated with PARDS. Strikingly, this study found the incidence of PARDS in those that failed NIV was 48% which is much higher than an international study on the incidence and epidemiology of PARDS that found an overall PARDS incidence of 3% for all PICU patients. 29 Clearly, there is an association between PARDS and NIV failure. It is challenging to determine at what point PARDS develops (i.e., before or after NIV failure) as PALICC requires a full facemask for the diagnosis of NIV PARDS and many of our patients were only treated with a nasal mask. It is commonly accepted that PARDS involves a decreased functional residual capacity, increased dead space, reduced lung compliance, and impaired gas exchange, and that currently recommended management is focused on a high PEEP strategy. 30 While those that failed NIV were sicker at the start given higher admission PELOD and PRISM scores, it is possible that NIV may not be able to obtain the necessary positive end-expiratory pressure (PEEP) to recruit the lungs and prevent worsening lung compliance seen in PARDS. Additionally, in this cohort, the median PEEP used was only 6 but this was not associated with NIV failure. When combining degree of hypoxemia as assessed by FiO 2 at initiation and worsening respiratory rate at 6 hours, there was a stronger multivariable model to predict NIV failure. One potential explanation for this increased risk of PARDS is that there may be more derecruitment in patients that are tachypneic and hypoxemic. Another possible explanation is patient self-inflicted lung injury described in adults with ARDS who are managed with NIV. This injury is thought to be caused by the significant respiratory effort generated by the patient resulting in unregulated tidal volume and swings in transpulmonary pressures. 31 32 These variables may also just reflect severity of lung injury. Either way, these simple and readily available clinical predictors can be easily implemented at the bedside.
Given that over 80% of patients in this study were successfully treated with NIV, it represents an important modality in the treatment of acute respiratory failure in pediatrics. However, it is imperative to have a mechanism in place to identify those who are at the highest risk of failing. We constructed two multivariable models that each incorporate two easily identifiable criteria at two time points. Both models were predictive of failure. Importantly, the model gains strength as the lack of improvement continues illustrating the importance of trending an examination overtime in those who have an elevated FiO 2 at initiation. An initiation FiO 2 ≥ 45 should heighten the concern of the bedside nurse, respiratory therapist, or clinician that the patient is at risk for failure especially in conjunction with a worsening tachypnea at 1 hour or certainly 6 hours following initiation. This simple model can be easily applied in different PICUs with varying levels of resources and staffing.
Limitations
This study has some limitations. As a retrospective study and not a standardized protocol, the specific factors that led to intubation can be difficult to ascertain. The variables we identified of FiO 2 and respiratory rate are also likely to influence the decision to intubate. Further, important variables such as blood gas data and mask leak were not universally available retrospectively. The sample size may not have significant statistical power to detect a difference for some variables studied. The heterogeneity of disease processes and lack of an NIV protocol also limit the conclusions of the study. While some of the limitations are tempered by the large cohort and the center encompassing patients from a large and diverse catchment area, it would be important for future prospective studies to consider these limitations in the design.
Conclusion
Overall, it is important to carefully consider each patient when utilizing NIV. In this cohort, having an initial FiO 2 ≥ 45% and an increasing respiratory rate at 1 hour and 6 hours of NIV were associated with an increased risk of NIV failure. While these markers deserve further investigation in a prospective cohort, if validated, they have the benefit of being simple clinical predictors that can be easily used at the bedside.
Conflict of Interest None declared.
Authors' Contributions
C.M.R. conceptualized and designed the study, coordinated and supervised data collection, interpretation and analysis, and approved the final manuscript as submitted. A.K.B. conceptualized and designed the study, collected the data, drafted the initial manuscript, and approved the final manuscript as submitted.
A.L.B., B.D.L., R.L.L., and A.I.C. conceptualized and designed the study, supervised data collection, and approved the final manuscript as submitted. E.A.M. performed the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Supplementary Material
References
- 1.Morley S L. Non-invasive ventilation in paediatric critical care. Paediatr Respir Rev. 2016;20:24–31. doi: 10.1016/j.prrv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Bott J, Carroll M P, Conway J Het al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease Lancet 1993341(8860):1555–1557. [DOI] [PubMed] [Google Scholar]
- 3.Confalonieri M, Potena A, Carbone G, Porta R D, Tolley E A, Umberto Meduri G.Acute respiratory failure in patients with severe community-acquired pneumonia. A prospective randomized evaluation of noninvasive ventilation Am J Respir Crit Care Med 1999160(5 pt 1):1585–1591. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer M, Esquinas A, Leon M, Gonzalez G, Alarcon A, Torres A. Noninvasive ventilation in severe hypoxemic respiratory failure: a randomized clinical trial. Am J Respir Crit Care Med. 2003;168(12):1438–1444. doi: 10.1164/rccm.200301-072OC. [DOI] [PubMed] [Google Scholar]
- 5.Peter J V, Moran J L, Phillips-Hughes J, Warn D. Noninvasive ventilation in acute respiratory failure--a meta-analysis update. Crit Care Med. 2002;30(03):555–562. doi: 10.1097/00003246-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 6.FLORALI Study Group ; REVA Network . Frat J P, Thille A W, Mercat A et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 7.Cheifetz I M.Invasive and noninvasive pediatric mechanical ventilation Respir Care 20034804442–453., discussion 453–458 [PubMed] [Google Scholar]
- 8.García-Salido A, Mastro-Martínez I, Cabeza-Martín B et al. Respiratory failure in children with hemato-oncological diseases admitted to the PICU: a single-center experience. J Pediatr Hematol Oncol. 2015;37(06):449–454. doi: 10.1097/MPH.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 9.Fortenberry J D, Del Toro J, Jefferson L S, Evey L, Haase D. Management of pediatric acute hypoxemic respiratory insufficiency with bilevel positive pressure (BiPAP) nasal mask ventilation. Chest. 1995;108(04):1059–1064. doi: 10.1378/chest.108.4.1059. [DOI] [PubMed] [Google Scholar]
- 10.Padman R, Lawless S T, Kettrick R G. Noninvasive ventilation via bilevel positive airway pressure support in pediatric practice. Crit Care Med. 1998;26(01):169–173. doi: 10.1097/00003246-199801000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Delclaux C, L'Her E, Alberti C et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284(18):2352–2360. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 12.Dohna-Schwake C, Stehling F, Tschiedel E, Wallot M, Mellies U. Non-invasive ventilation on a pediatric intensive care unit: feasibility, efficacy, and predictors of success. Pediatr Pulmonol. 2011;46(11):1114–1120. doi: 10.1002/ppul.21482. [DOI] [PubMed] [Google Scholar]
- 13.Bernet V, Hug M I, Frey B. Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr Crit Care Med. 2005;6(06):660–664. doi: 10.1097/01.pcc.0000170612.16938.f6. [DOI] [PubMed] [Google Scholar]
- 14.Essouri S, Chevret L, Durand P, Haas V, Fauroux B, Devictor D. Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med. 2006;7(04):329–334. doi: 10.1097/01.PCC.0000225089.21176.0B. [DOI] [PubMed] [Google Scholar]
- 15.Joshi G, Tobias J D. A five-year experience with the use of BiPAP in a pediatric intensive care unit population. J Intensive Care Med. 2007;22(01):38–43. doi: 10.1177/0885066606295221. [DOI] [PubMed] [Google Scholar]
- 16.Mayordomo-Colunga J, Medina A, Rey C et al. Predictive factors of non invasive ventilation failure in critically ill children: a prospective epidemiological study. Intensive Care Med. 2009;35(03):527–536. doi: 10.1007/s00134-008-1346-7. [DOI] [PubMed] [Google Scholar]
- 17.James C S, Hallewell C P, James D P, Wade A, Mok Q Q. Predicting the success of non-invasive ventilation in preventing intubation and re-intubation in the paediatric intensive care unit. Intensive Care Med. 2011;37(12):1994–2001. doi: 10.1007/s00134-011-2386-y. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz-Bonet J I, Flor-Macián E M, Brines J et al. Predictive factors for the outcome of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med. 2010;11(06):675–680. doi: 10.1097/PCC.0b013e3181d8e303. [DOI] [PubMed] [Google Scholar]
- 19.ENIVA Study Group . He H, Sun B, Liang L et al. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care. 2019;23(01):300. doi: 10.1186/s13054-019-2575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yehya N, Thomas N J. Relevant outcomes in pediatric acute respiratory distress syndrome studies. Front Pediatr. 2016;4:51. doi: 10.3389/fped.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pediatric Acute & Critical Care Medicine Asian Network (PACCMAN) . Wong J J, Phan H P, Phumeetham S et al. Risk stratification in pediatric acute respiratory distress syndrome: a multicenter observational study. Crit Care Med. 2017;45(11):1820–1828. doi: 10.1097/CCM.0000000000002623. [DOI] [PubMed] [Google Scholar]
- 22.Pediatric Acute Respiratory Distress syndrome Incidence and Epidemiology (PARDIE) Investigators ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Khemani R G, Smith L, Lopez-Fernandez Y M et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7(02):115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leteurtre S, Duhamel A, Grandbastien B, Lacroix J, Leclerc F.Paediatric logistic organ dysfunction (PELOD) score, Lancet 2006367897(9514):, author reply 900–902 [DOI] [PubMed]
- 24.Leteurtre S, Martinot A, Duhamel Aet al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study Lancet 2003362(9379):192–197. [DOI] [PubMed] [Google Scholar]
- 25.Bilan N, Galehgolab B A, Emadaddin A, Shiva Sh. Risk of mortality in pediatric intensive care unit, assessed by PRISM-III. Pak J Biol Sci. 2009;12(06):480–485. doi: 10.3923/pjbs.2009.480.485. [DOI] [PubMed] [Google Scholar]
- 26.Khemani R G, Patel N R, Bart R D, III, Newth C JL. Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest. 2009;135(03):662–668. doi: 10.1378/chest.08-2239. [DOI] [PubMed] [Google Scholar]
- 27.Pediatric Acute Lung Injury Consensus Conference Group . Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(05):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaret P, Mulder A, Loeckx I, Trippaerts M, Lebrun F. Non-invasive ventilation is useful in paediatric intensive care units if children are appropriately selected and carefully monitored. Acta Paediatr. 2015;104(09):861–871. doi: 10.1111/apa.13057. [DOI] [PubMed] [Google Scholar]
- 29.Pediatric Acute Lung Injury Consensus Conference Group Khemani R G, Smith L S, Zimmerman J J, Erickson S.Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference Pediatr Crit Care Med 201516(5, suppl 1):S23–S40. [DOI] [PubMed] [Google Scholar]
- 30.Heidemann S M, Nair A, Bulut Y, Sapru A. Pathophysiology and management of acute respiratory distress syndrome in children. Pediatr Clin North Am. 2017;64(05):1017–1037. doi: 10.1016/j.pcl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LUNG SAFE Investigators ; ESICM Trials Group . Bellani G, Laffey J G, Pham T et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195(01):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 32.Grieco D L, Menga L S, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85(09):1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


