Abstract
Published data were gathered for a meta-analysis to determine the difference in sperm parameters before and after administration of different types of coronavirus disease 2019 (COVID-19) vaccines, because the reproductive toxicity of COVID-19 vaccines has not yet been evaluated in clinical trials and COVID-19 has been associated with decreases in sperm quality. The preferred procedures for systematic reviews and meta-analyses were followed in the conduct and reporting of this study. The average sperm parameters of all sperm donors’ multiple sperm donations were compared before and after receiving various COVID-19 vaccinations. Semen volume, total sperm motility, total sperm count, morphological change, and sperm concentration were the primary outcome measures. We compiled and analyzed the results of six studies on total sperm motility, six studies on semen volume, six studies on sperm concentration, two studies on morphological change, and two studies on total sperm count. Parameter comparisons with patients who had and had not been vaccinated were only reported in one of the included studies. When different types of COVID-19 vaccine injections were compared, no discernible differences in parameters were observed. According to the available data, the parameters of semen are unaffected by inactivated or messenger RNA (mRNA) COVID-19 vaccinations. To support these findings, additional prospectively designed research is required.
Keywords: COVID-19, mRNA vaccine, sperm parameters, sperm quality, vaccine safety
INTRODUCTION
The coronavirus disease 2019 (COVID-19) outbreak was deemed a pandemic by the general director of the World Health Organization (WHO) in 2020, adversely affecting the lives of millions of people worldwide.1 The spread of this virus has resulted in severe diseases, countless fatalities, and serious medical, economic, and societal problems.2 In randomized controlled trials and real-world effectiveness study, vaccines have been shown to lower COVID-19 infections, transmission, hospitalizations, and fatalities.3 However, individuals are concerned about the potential harm of the two main types of COVID-19 vaccines used globally: the inactivated virus vaccine, and the viral mRNA vaccine. Scientists are still gathering safety data related to these types of vaccines.4 Aside from expected side effects, such as the usual low-grade fever or injection site soreness, serious adverse events have also been observed during the postmarketing surveillance phase.5,6 Despite clinical trials showing high efficacy and few adverse events, there is still much resistance to COVID-19 vaccinations.7
Among the nonrespiratory effects of COVID-19 infections, effects on male fertility are of concern,8 with poor spermatogenesis possibly reflecting the progression of COVID-19.9 Angiotensin-converting enzyme 2 (ACE2) receptors are expressed abundantly in spermatogonia, Leydig cells, and Sertoli cells, which may be direct viral targets, and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is known to react with them. Steroidogenesis and local vascular control are also regulated by ACE2.10,11 COVID-19 may affect testosterone levels in patients,12 which may lead to changes in spermatozoa.
In the USA, only 56% of people are willing to be vaccinated.13 Those unwilling to receive a COVID-19 vaccine have probably been influenced by social media, which have questioned the safety of the recently authorized vaccines and declared their impacts on fertility, despite the lack of scientific data.12,14
Although COVID-19 has been associated with deteriorating sperm quality, its toxicity to the reproductive system has not been clinically assessed.15 Previous studies have related sperm quality and fertility to the mortality and health of the population,16,17 thus vaccination may have significant effects on sperm parameters. To investigate this, we gathered relevant data for pooled analysis of sperm parameters before and after COVID-19 vaccination.
PATIENTS AND METHODS
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).18
Original study search
The databases of PubMed and Embase were combined to identify candidate studies, with COVID-19, vaccination, and sperm parameters used as search terms. Supplementary Table 1 includes a full description of the search technique. The reference of each candidate paper was located, and August 1, 2022, was the most recent search date. We chose papers that matched the following inclusion criteria for the meta-analysis following the PICOS (population, intervention, comparison, and outcomes principles). (1) P (population): individuals free of any conditions that could harm the quality of their spermatozoa; (2) I (intervention): COVID-19 vaccinations were the sole intervention (all vaccine types were considered in this review); (3) C (comparison): comparison between the population with and without vaccinations and the included patients themselves (before and after the vaccination); (4) O (outcomes): outcomes included relevant metrics, such as semen volume, sperm concentration, total sperm motility, and total motile sperm count. Some studies were excluded, such as reviews, conference abstracts, letters, and comments. For the retrospectively designed studies in the meta-analysis, we performed quality evaluations based on the Newcastle–Ottawa Scale (NOS).
Supplementary Table 1.
Sample searching strategy for different database
| Database | Searching strategy |
|---|---|
| PubMed | (((((((((((((((((((((((((((((((((((((COVID 19 [Title/Abstract]) OR (SARS-CoV-2 Infection[Title/Abstract])) OR (Infection, SARS-CoV-2[Title/Abstract])) OR (SARS CoV 2 Infection[Title/Abstract])) OR (SARS-CoV-2 Infections[Title/Abstract])) OR (2019 Novel Coronavirus Disease[Title/Abstract])) OR (2019 Novel Coronavirus Infection[Title/Abstract])) OR (2019-nCoV Disease[Title/Abstract])) OR (2019 nCoV Disease[Title/Abstract])) OR (2019-nCoV Diseases[Title/Abstract])) OR (Disease, 2019-nCoV[Title/Abstract])) OR (COVID-19 Virus Infection[Title/Abstract])) OR (COVID 19 Virus Infection[Title/Abstract])) OR (COVID-19 Virus Infections[Title/Abstract])) OR (Infection, COVID-19 Virus[Title/Abstract])) OR (Virus Infection, COVID-19[Title/Abstract])) OR (Coronavirus Disease 2019[Title/Abstract])) OR (Disease 2019, Coronavirus[Title/Abstract])) OR (Coronavirus Disease-19[Title/Abstract])) OR (Coronavirus Disease 19[Title/Abstract])) OR (Severe Acute Respiratory Syndrome Coronavirus 2 Infection[Title/Abstract])) OR (SARS Coronavirus 2 Infection[Title/Abstract])) OR (COVID-19 Virus Disease[Title/Abstract])) OR (COVID 19 Virus Disease[Title/Abstract])) OR (COVID-19 Virus Diseases[Title/Abstract])) OR (Disease, COVID-19 Virus[Title/Abstract])) OR (Virus Disease, COVID-19[Title/Abstract])) OR (2019-nCoV Infection[Title/Abstract])) OR (2019 nCoV Infection[Title/Abstract])) OR (2019-nCoV Infections[Title/Abstract])) OR (Infection, 2019-nCoV[Title/Abstract])) OR (COVID 19[Title/Abstract])) OR (COVID-19 Pandemic[Title/Abstract])) OR (COVID 19 Pandemic[Title/Abstract])) OR (Pandemic, COVID-19[Title/Abstract])) OR (COVID-19 Pandemics[Title/Abstract])) AND ((Vaccines[Title/Abstract]) OR (mRNA vaccines[Title/Abstract]))) AND ((Semen[Title/Abstract]) OR (sperm[Title/Abstract])) |
The search strategies used for the other databases were almost identical or slightly modified depending on the circumstances of each database
Data extraction
The effect of mRNA COVID-19 vaccinations on semen volume, sperm concentration, total sperm motility, and total sperm count before and after COVID-19 vaccinations was assessed. The data gathering procedures were double-checked by two coauthors (YCM and CC).
Statistical analyses
The data were processed with the program RevMan 5.4.1 (Cochrane Collaboration, London, UK). Means and standard deviations were calculated using an online calculator provided by Professor Tie-Jun Tong (https://www.math.hkbu.edu.hk/tongt/papers/median2mean.html).19,20 The inverse-variance (IV) combined mean difference (MD) method was used as the primary estimate for continuous variables. A random effects model was used to synthesize the data. All confidence intervals (CIs), unless otherwise stated, were set at 95%. We present the synthesized results in forest plots. Heterogeneity was evaluated using I2 and the P-value of the Q-test. Funnel plots were generated to evaluate possible publication bias. For the meta-analysis results, the trim-and-fill method was used to adjust any detected publication bias. Since data from different types of vaccines were pooled, subgroup analysis was necessary to provide more information. Statistical significance in this study was defined as two-tailed P < 0.05.
RESULTS
Study selection
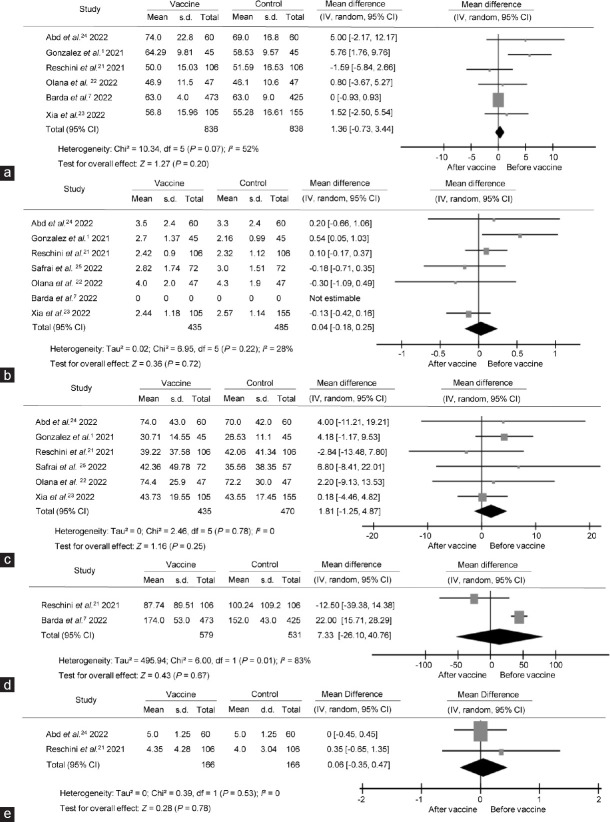
Seven screened studies in the database were assessed for quality and included in the quantitative analysis. The details on screening the articles are in the flowchart (Figure 1). Data from different studies were used for comparing semen variables before and after COVID-19 vaccination: six studies (comprising 1674 patients)1,7,21–24 for total sperm motility, six studies (920 patients)1,21–25 for semen volume and sperm concentration, two studies (1110 patients)7,21 for total sperm count, and two studies (332 patients)21,24 for morphological change. Out of seven included studies, five followed the WHO 5th edition, one study followed the WHO 6th edition, and one study did not report its methodology. We carefully read the manuals from the WHO and found that there were no obvious changes in parameters we were concerned with in this study (Table 1).
Figure 1.
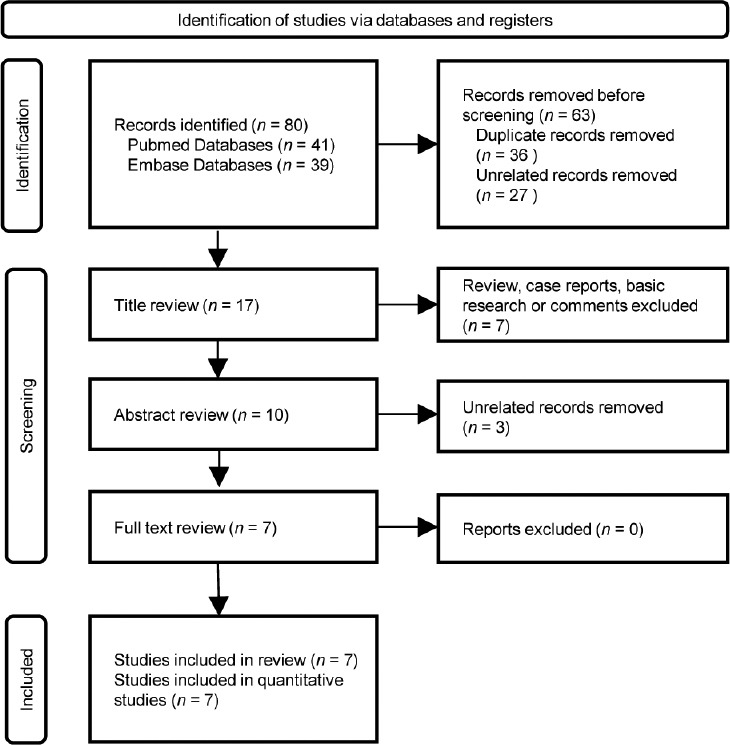
The flowchart of literature screening.
Table 1.
Basic information of included studies
| Study | Year | Country | Study design | Mean age (year) | Sample size (n) | Vaccine applied | Treatment involved | Intervention | Control | Semen analysis time point after second vaccine | Sperm parameter testing standard | Efficiency outcome assessment | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reschini et al.21 | 2022 | Italy | RCS | 39 | 212 | BNT162b2 and mRNA-1273 | Semen evaluation | COVID-19 vaccination | Before vaccination | 75 days | WHO 5th edition | Volume, total motility, sperm concentration, total spermatozoa | 7 |
| Gonzalez et al.1 | 2021 | Miami | RCS | 28 | 90 | BNT162b2 and mRNA-1273 | Semen analyses | mRNA COVID-19 vaccination | Before vaccination | 70 days | NR | Volume, total motility, sperm concentration | 7 |
| Barda et al.7 | 2022 | Israel | PCS | 27 | 898 | BNT162b2 | Semen analyses | mRNA COVID-19 vaccination | Before vaccination | 72 days | WHO 5th edition | Total motility, total spermatozoa | 6 |
| Safrai et al.25 | 2022 | Israel | RCS | 35.7 | 144 | BNT162b2 | Semen analysis | mRNA COVID-19 vaccination | Before vaccination | About 70 days | WHO 5th edition | Volume, sperm concentration | 7 |
| Xia et al.23 | 2022 | China | PCS | 31.8 | 260 | Inactivated vaccine | Semen analyses | mRNA COVID-19 vaccination | Patients didn’t receive vaccination | NR | WHO 5th edition | Volume, sperm concentration, total motility | 7 |
| Olana et al.22 | 2022 | Italy | PCS | 29.3 | 94 | BNT162b2 | Semen analyses | mRNA COVID-19 vaccination | Before vaccination | 3 months | WHO 5th edition | Volume, sperm concentration, total motility | 7 |
| Abd et al.24 | 2022 | UK | PCS | 37.2 | 120 | BNT162b2 | Semen analyses | mRNA COVID-19 vaccination | Before vaccination | 90 days | WHO 6th edition | Total motility, volume, sperm concentration | 7 |
RCS: retrospective cohort study; PCS: prospective cohort study; NOS: Newcastle–Ottawa Scale; WHO: World Health Organization; COVID-19: coronavirus disease 2019; NR: not report; mRNA: messenger RNA
Total motility comparison
There were no significant differences in total sperm motility before and after the COVID-19 vaccine (IV, MD: 1.36, 95% CI: −0.73–3.44, P = 0.20), and heterogeneity was observed (I2 = 52%, P = 0.07), as shown in Figure 2a. The funnel plot revealed that there was no significant publication bias in this comparison (Figure 3a). Main heterogeneity was introduced by Gonzalez et al.,1 and this may be caused by potential abstinence.
Figure 2.
Forest plots of sperm parameter comparison before and after the COVID-19 vaccine treatment. Forest plots of (a) total sperm motility, (b) semen volume, (c) sperm concentration, (d) total sperm count, and (e) sperm morphological change were compared accordingly. MD: mean difference; s.d.: standard deviation; CI: confidential interval; IV: inverse variance; df: degree of freedom.
Figure 3.
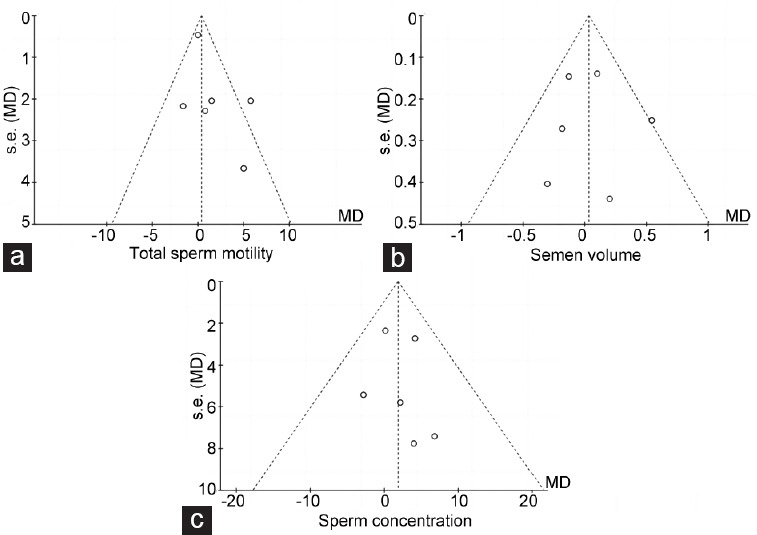
Funnel plots of sperm parameter comparisons. There was no linear trend in all three funnel plots of data points, and all points are relatively symmetrically distributed at both sides of the combined effect line. No significant publication bias was detected in (a) total sperm motility, (b) semen volume, and (c) sperm concentration. MD: mean difference; s.e.: standard error.
The volume of semen comparison
There were no differences in volume before and after COVID-19 vaccine (IV, MD: 0.04, 95% CI: −0.18–0.25, P = 0.72). No significant heterogeneity was detected (I2 = 28%, P = 0.22), as shown in Figure 2b. There was no significant publication bias detected in this comparison (Figure 3b). This combined result is robust, but considering that semen volume is largely affected by fluid, this parameter may mainly reflect the effect of vaccination on fluid-secreting glands.
Sperm concentration comparison
The primary results of each included study were stable, and no heterogeneity was detected (I2 = 0, P = 0.78). Overall synthetic results showed no differences in sperm concentration before and after the COVID-19 vaccine (IV, MD: 1.81, 95% CI: −1.25–4.87, P = 0.25; Figure 2c). There was no significant publication bias detected (Figure 3c). This result suggested that vaccine injection had no effect on the ability of seminiferous tubules to produce sperm cells.
Total sperm count comparison
There was no significant decrease in total sperm count after the COVID-19 vaccine (IV, MD: 7.33, 95% CI: −26.10–40.76, P = 0.67), but significant heterogeneity was found (I2 = 83%, P = 0.01), as shown in Figure 2d. This may weaken the robustness of pooling result. Since only two studies were included in this comparison, publication bias detection was omitted.
Morphological comparison
Three studies reported a sperm morphology comparison, among which one reported the abnormal rate, and two reported the normal rate. Therefore, we could only pool the comparison of the normal rate. There was no significant morphological change after the vaccination (IV, MD: 0.06, 95% CI: −0.35–0.47, P = 0.78) without significant heterogeneity (I2 = 0, P = 0.53; Figure 2e).
Subgroup analysis based on the different types of vaccines
In this meta-analysis, vaccine BNT162b, produced by Pfizer/BioNTech (Pfizer Inc., New York, NY, USA), was used in six of seven studies. Two studies used two different types of vaccines. There was only one study that applied inactivated vaccine. Considering the potential heterogeneity introduced by different vaccine types, we performed a subgroup analysis to provide more information (Table 2). The different types of vaccines did not alter the pooled results.
Table 2.
Subgroup analyses of comparison
| Subgroup on vaccine type | Pooled MD | Heterogeneity | ||
|---|---|---|---|---|
|
|
|
|||
| MD (95% CI) | P | I2 | P | |
| Total motility | ||||
| BNT162b2 | 0.11 (−0.79–1.01) | 0.81 | 0 | 0.38 |
| BNT162b2/mRNA-1273 | 2.12 (−5.08–9.32) | 0.56 | 84 | 0.01 |
| Inactivated | 1.52 (−2.50–5.54) | 0.46 | - | - |
| Sperm concentration | ||||
| BNT162b2 | 3.96 (−3.75–11.66) | 0.31 | 0 | 0.89 |
| BNT162b2/mRNA-1273 | 2.24 (−3.92–8.39) | 0.48 | 25 | 0.25 |
| Inactivated | 0.18 (−4.46–4.82) | 0.94 | - | - |
| Semen volume | ||||
| BNT162b2 | −0.13 (−0.52–0.26) | 0.51 | 0 | 0.68 |
| BNT162b2/mRNA-1273 | 0.27 (−0.15–0.69) | 0.21 | 57 | 0.13 |
| Inactivated | −0.13 (−0.42–0.16) | 0.38 | - | - |
MD: mean difference; CI: confidential interval; mRNA: messenger RNA; -: no value
DISCUSSION
With the identification of human coronaviruses 229E and OC43 in the late 1960s, coronaviruses began to be recognized as dangerous.26 Bats are very likely the original source of COVID-19 infection, and the virus can be transmitted to dogs and cats.27 Some coronaviruses cause serious and fatal infection symptoms in young children, the elderly, and the immunocompromised; however, human coronaviruses often cause mild symptoms similar to those of the common cold.28–30 Research on the possibility of mother-to-child transmission of COVID-19 through aerosols is in progress.31
The COVID-19 enters cells via its spike protein, which has an affinity for the sugar receptors in human cells.32 Routes for viral entry into host cells include the endocytic33 and nonendosomal.34 The former is the most common pathway, but variables such as the type of virus and the host cell dictate the entry mechanism.35 The SARS-CoV-2 can enter semen, and as such, it could affect semen cryopreservation and the quality of spermatozoa.36
There is currently no specific medication available to counter SARS-CoV-2’s rapid spread; vaccination is a crucial tool to limit infection.
BNT162b vaccines received emergency USA Federal Drug Authority (FDA) approval on December 11, 2020. These vaccines encode the SARS-CoV-2 spike protein through modified RNA, which can be modified to alter the spike protein’s three-dimensional form. SARS-CoV-2 maintains this form until it attaches to human cellular ACE2 receptors. Both vaccines are typically administered through deltoid injection. The Moderna vaccine is approved for those over 16 years of age, and the BNT162b vaccine for those over 18 years. Conventional inactivated virus vaccines are frequently used, which offer effective COVID-19 population protection.37 However, questions over their reproductive toxicity, such as in assisted reproduction technology,38 have led to vaccination hesitancy.39
There have been many interesting studies published on whether COVID-19 vaccines affect sperm quality. First, given that the inactivated vaccine does not contain any live viruses and only contains antigenic components, it can only trigger an immune response without transcription and replication of the viral genome. This process causes a minimal amount of systemic inflammation, which has no impact on the quality of spermatozoa.40 A recent study evaluated sperm parameters before and after COVID-19 mRNA vaccination and found no significant decrease in any parameters after vaccination.1 Surprisingly, Gonzalez et al.1 also observed that sperm concentration and semen volume improved after vaccination.1 Although a molecular mechanism underlying the vaccine cannot be ruled out, this phenomenon may be due to abstinence before the second sperm test.1 Another study examined the effects of the BNT162b2 vaccine on 43 males and found no differences in sperm parameters following vaccination.25 A more persuasive prospective comparative investigation demonstrated that vaccination did not directly influence detectable indicators of sperm in 75 fertile males 1–2 months following a second dose of the BNT162b vaccine.14 By contrast, other studies have suggested that the COVID-19 vaccine may have an impact on sperm quality. After receiving a second dose of the vaccine, a study using human sperm banks reported a large rise in total sperm count and a significant drop in total motile count, but no significant change in the motility percentage.7 Therefore, we carried out this meta-analysis to perform an integrated study of the reported data to obtain more reliable results.
After pooling all available data from databases, our meta-analysis revealed no discernible change in semen quality between the pre- and post-COVID-19 vaccination periods. As a result, our findings should help to allay worries about the potential of vaccination to reduce male fertility and to encourage more people to be immunized against COVID-19.
This study has the following limitations. First, it only demonstrates that different COVID-19 vaccine types do not change sperm quality, not that using vaccines will not affect fertility, as sperm metrics do not fully reflect fertility. Second, only seven studies were included. Therefore, there is a need for more primary research, particularly prospectively designed studies with large samples.
CONCLUSIONS
According to currently available data, neither inactivated nor mRNA COVID-19 vaccines have deleterious effects on semen parameters. More prospectively designed studies are needed to validate this conclusion.
AUTHOR CONTRIBUTION
YCM, CC, and XJ concepted and designed this study. YCM, CC, LYX, JW, and CY performed the literature screening. YCM, CC, and CY performed the data synthesis and further analysis. YCM and CC drafted the manuscript. XJ revised the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
ACKNOWLEDGMENTS
This article is supported by grants from the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD2018011), and Sichuan Provincial Department of Science and Technology (2022YFS0304).
REFERENCES
- 1.Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326:273–4. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harder T, Koch J, Vygen-Bonnet S, Külper-Schiek W, Pilic A, et al. Efficacy and effectiveness of COVID-19 vaccines against SARS-CoV-2 infection: interim results of a living systematic review, 1 January to 14 May 2021. Euro Surveill. 2021;26:2100563. doi: 10.2807/1560-7917.ES.2021.26.28.2100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klugar M, Riad A, Mekhemar M, Conrad J, Buchbender M, et al. Side effects of mRNA-based and viral vector-based COVID-19 vaccines among German healthcare workers. Biology (Basel) 2021;10:752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riad A, Pokorná A, Attia S, Klugarová J, Koščík M, et al. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10:1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riad A, Hocková B, Kantorová L, Slávik R, Spurná L, et al. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals (Basel) 2021;14:873. doi: 10.3390/ph14090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barda S, Laskov I, Grisaru D, Lehavi O, Kleiman S, et al. The impact of COVID-19 vaccine on sperm quality. Int J Gynaecol Obstet. 2022;158:116–20. doi: 10.1002/ijgo.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymen CM. The other side of COVID-19 pandemic: effects on male fertility. J Med Virol. 2021;93:1396–402. doi: 10.1002/jmv.26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TT, Hulme J, Tran HD, Vo TK, Vo GV. The potential impact of COVID-19 on male reproductive health. J Endocrinol Invest. 2022;45:1483–95. doi: 10.1007/s40618-022-01764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarra A, Albani E, Castellano S, Arruzzolo L, Levi-Setti PE. Coronavirus disease-19 infection: implications on male fertility and reproduction. Front Physiol. 2020;11:574761. doi: 10.3389/fphys.2020.574761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Xiao X, Zhang J, Zafar MI, Wu C, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salciccia S, Del Giudice F, Gentile V, Mastroianni CM, Pasculli P, et al. Interplay between male testosterone levels and the risk for subsequent invasive respiratory assistance among COVID-19 patients at hospital admission. Endocrine. 2020;70:206–10. doi: 10.1007/s12020-020-02515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szilagyi PG, Thomas K, Shah MD, Vizueta N, Cui Y, et al. National trends in the US public's likelihood of getting a COVID-19 vaccine-April 1 to December 8, 2020. JAMA. 2020;325:396–8. doi: 10.1001/jama.2020.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, et al. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod Biomed Online. 2022;44:145–9. doi: 10.1016/j.rbmo.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Best JC, Kuchakulla M, Khodamoradi K, Lima TF, Frech FS, et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health. 2021;39:489–95. doi: 10.5534/wjmh.200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Giudice F, Kasman AM, Ferro M, Sciarra A, De Berardinis E, et al. Clinical correlation among male infertility and overall male health: a systematic review of the literature. Investig Clin Urol. 2020;61:355–71. doi: 10.4111/icu.2020.61.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Giudice F, Kasman AM, Li S, Belladelli F, Ferro M, et al. Increased mortality among men diagnosed with impaired fertility: analysis of US claims data. Urology. 2021;147:143–9. doi: 10.1016/j.urology.2020.07.087. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J, Luo D, Weng H, Zeng XT, Lin L, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641–54. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 20.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 21.Reschini M, Pagliardini L, Boeri L, Piazzini F, Bandini V, et al. COVID-19 vaccination does not affect reproductive health parameters in men. Front Public Health. 2022;10:839967. doi: 10.3389/fpubh.2022.839967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olana S, Mazzilli R, Salerno G, Zamponi V, Tarsitano MG, et al. 4BNT162b2 mRNA COVID-19 vaccine and semen: what do we know? Andrology. 2022;10:1023–9. doi: 10.1111/andr.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia W, Zhao J, Hu Y, Fang L, Wu S. Investigate the effect of COVID-19 inactivated vaccine on sperm parameters and embryo quality in in vitro fertilization. Andrologia. 2022;54:e14483. doi: 10.1111/and.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abd ZH, Muter SA, Saeed RA, Ammar O. Effects of Covid-19 vaccination on different semen parameters. Basic Clin Androl. 2022;32:13. doi: 10.1186/s12610-022-00163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safrai M, Herzberg S, Imbar T, Reubinoff B, Dior U, et al. The BNT162b2 mRNA Covid-19 vaccine does not impair sperm parameters. Reprod Biomed Online. 2022;44:685–8. doi: 10.1016/j.rbmo.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leroy EM, Ar Gouilh M, Brugère-Picoux J. The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health. 2020;10:100133. doi: 10.1016/j.onehlt.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham RL, Baric RS. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J Virol. 2010;84:3134–46. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu SS, Chan KH, Chu KW, Kwan SW, Guan Y, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–9. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jevšnik M, Uršič T, Zigon N, Lusa L, Krivec U, et al. Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMC Infect Dis. 2012;12:365. doi: 10.1186/1471-2334-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S, Meyler P, Mozel M, Tauh T, Merchant R. Asymptomatic carriage and transmission of SARS-CoV-2: what do we know? Can J Anaesth. 2020;67:1424–30. doi: 10.1007/s12630-020-01729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li F. Structure, function, and evolution of coronavirus Spike proteins. Annu Rev Virol. 2016;3:237–61. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020;383:1920–31. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahbar Saadat Y, Hosseiniyan Khatibi SM, Zununi Vahed S, Ardalan M. Host serine proteases: a potential targeted therapy for COVID-19 and influenza. Front Mol Biosci. 2021;8:725528. doi: 10.3389/fmolb.2021.725528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yesudhas D, Srivastava A, Gromiha MM. COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection. 2021;49:199–213. doi: 10.1007/s15010-020-01516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paoli D, Pallotti F, Nigro G, Mazzuti L, Hirsch MN, et al. Molecular diagnosis of SARS-CoV-2 in seminal fluid. J Endocrinol Invest. 2021;44:2675–84. doi: 10.1007/s40618-021-01580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–22. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Giudice F, Belladelli F, Chen T, Glover F, Mulloy EA, et al. The association of impaired semen quality and pregnancy rates in assisted reproduction technology cycles: systematic review and meta-analysis. Andrologia. 2022;54:e14409. doi: 10.1111/and.14409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663–9. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 40.Soleimanpour S, Yaghoubi A. COVID-19 vaccine: where are we now and where should we go? Expert Rev Vaccines. 2021;20:23–44. doi: 10.1080/14760584.2021.1875824. [DOI] [PMC free article] [PubMed] [Google Scholar]