Abstract
In recent years, social research surrounding the consequences of infertility has increasingly focused on the male perspective; however, a gap exists in the understanding of men’s experiences of male infertility treatment. This review aims to synthesize the existing evidence concerning the psychological, social, and sexual burden of male infertility treatment on men, as well as patient needs during clinical care. A systematic search identified 12 studies that are diverse in design, setting, and methods. Psychological evaluations have found that urological surgery may have a lasting impact on infertility-specific stress, and treatment failure can lead to feelings of depression, grief, and inadequacy. Men tended to have an avoidant coping mechanism throughout fertility treatment, and their self-esteem, relationship quality, and sexual functions can be tied to outcomes of treatment. Partner bonds can be strengthened by mutual support and enhanced communication; couple separation, however, has been noted as a predominant reason for discontinuing male infertility treatment and may be associated with difficult circumstances surrounding severe male infertility. Surgical treatments can affect the sexual functioning of infertile men; however, the impact of testicular sperm extraction outcomes appears to be psychologically driven whereas the improvements after microsurgical varicocelectomy are only evident in hypogonadal men. Clinically, there is a need for better inclusion, communication, education, and resource provision, to address reported issues of marginalization and uncertainty in men. Routine psychosocial screening in cases of severe male infertility and follow-up in cases of surgical treatment failure are likely beneficial.
Keywords: male infertility, male infertility treatment, patient experience, patient-centered care, psychosocial, sexual
INTRODUCTION
The inability to conceive children causes significant personal suffering and social repercussions for both men and women. In recent years, social research concerning the consequences of infertility has increasingly focused on the male perspective, revealing that childless men are equally invested in experiencing parenthood as their female counterparts and suffer elevated anxiety due to the condition.1 Across North America, Europe, and Australia, 15% of couples are reported to be infertile and rates of male infertility range from 4.5% to 9%.2 Male factor infertility (MFI) is purely responsible for 30% of the total infertile couple population and is implicated, together with female factor, in another 20%.3 Men with MFI are a particularly vulnerable patient population, displaying a higher risk for sexual, emotional, and psychological strain compared to men in infertile couples without male factor involvement.4 Specific treatments for MFI exist and aim to improve semen quality and/or to extract viable sperm for subsequent fertilization through artificial reproductive technologies (ARTs). In general, medical treatments have a limited role except in specific endocrine disorders where hormonal manipulation can be effective in promoting sperm production.5 Invasive interventions include varicocele repair and surgical sperm retrieval techniques, such as testicular sperm extraction (TESE). For some men, especially those with severe forms of MFI, these treatment modalities are vital in their journey to achieving biological parenthood. Despite advances in research exploring the psychosocial perspectives of men diagnosed with MFI, previous systematic reviews have identified a gap in the understanding of men’s experiences during and after male infertility treatment.1,6 Given the increased vulnerability of this patient population, there is a need to bridge this knowledge gap to facilitate patient-centered care throughout the treatment process.
The objective of this review was to synthesize the existing evidence concerning the experiences of men receiving male infertility treatment, particularly, the psychological, social, and sexual burden of treatment, and male patient needs in clinical care. Additionally, this paper aimed to explore the implications of these studies on clinical practice as well as directions for future research.
MATERIALS AND METHODS
A systematic literature search was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and its protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration ID: CRD42022308538). Four electronic databases were used: Ovid MEDLINE, Web of Science, CINAHL, and PsycINFO. All English-language studies published before August 2022 were considered. The literature search and screening were performed by two reviewers independently (WW and JL). All titles and abstracts were screened for eligibility and discrepancies were resolved through discussion with a third reviewer (DJK). Studies which met the inclusion criteria were retrieved in full and references of all eligible studies were checked to identify others not retrieved by the electronic search. Terms used in the search included “male infertility”, “oligospermia”, “azoospermia”, “Klinefelter”, “treatment”, “surgery”, “sperm extraction”, “TESE”, “varicocele*”, “psych*”, “emotion”, “mental health”, “social”, “sexual*”, “quality of life”, “patient experience”, and “patient perspective”.
Primary studies with originally collected data, both quantitative and qualitative, were considered for inclusion. The focus population was men with an MFI diagnosis who were undergoing or have undertaken surgical or medical male infertility treatment. Studies with outcome measures concerning psychological, social, and sexual experiences related to treatment settings as well as patient needs were included.
Studies that did not specify an MFI diagnosis within the study population or disaggregate this data were excluded. Studies conducted in settings other than treatment for male infertility were excluded; this included those concerning men in the context of ART procedures without specification of male-specific treatment involvement.
RESULTS
From the electronic databases, 1171 records were obtained following the removal of duplicates. Thirty-six studies were retrieved for further analysis and 12 studies fulfilled the inclusion and exclusion criteria (Figure 1). The studied population, methodology, and scope of included studies are summarized in Table 1.
Figure 1.
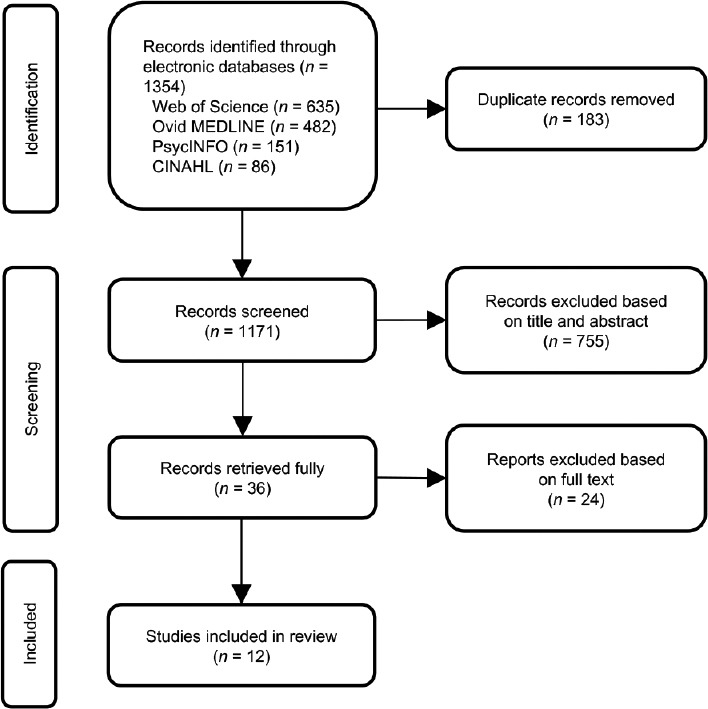
PRISMA flow diagram of study selection. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of included studies
| Study | Study population (number of participants/diagnosis/treatment received) | Methodology (study design/outcome measure[s]) |
|---|---|---|
| Quantitative studies on psychosocial implications of male infertility treatment | ||
| Bendayan et al.9 2022 (France) | 44 men/NOA/TESE | Prospective longitudinal/self-esteem, sexual health and functioning, dyadic adjustment, hormonal profile |
| Patel et al.7 2016 (India) | 300 men/male factor, female factor, mixed factor, idiopathic infertility/history of urological surgery | Cross-sectional/infertility-specific stress |
| Taniguchi et al.8 2018 (Japan) | 52 couples/NOA/micro-TESE 37 couples/OA/conventional TESE | Cross-sectional/HR-QOL |
| Quantitative studies on sexual implications of male infertility treatment | ||
| Akbal et al.13 2010 (Türkiye) | 66 men/NOA/TESE | Retrospective longitudinal/sexual functioning, anxiety and depression, hormonal profile |
| Najari et al.14 2017 (USA) | 34 men/MFI, sexual dysfunction/microsurgical varicocelectomy | Retrospective longitudinal/sexual health and functioning |
| Saylam et al.16 2020 (Türkiye) | 202 men/MFI with hypogonadism/microsurgical varicocelectomy | Retrospective longitudinal/sexual functioning, libido, hormonal profile |
| Taniguchi et al.12 2020 (Japan) | 152 men/NOA/micro-TESE 74 men/OA/conventional TESE | Cross-sectional/frequency of sexual intercourse |
| Zohdy et al.15 2011 (Egypt) | 103 men/MFI/microsurgical varicocelectomy 38 men/MFI/ART treatment | Prospective longitudinal/sexual functioning, hormonal profile |
| Qualitative studies on men’s experiences of male infertility treatment | ||
| Dancet et al.18 2010 (Belgium) | 17 men/azoospermia/TESE | Retrospective phenomenological/matters of importance in clinical care |
| Johansson et al.11 2011 (Sweden) | 8 men/OA/failed and terminated TESE-ICSI | Retrospective phenomenological/personal experiences regarding infertility and treatment |
| Stevenson et al.10 2019 (USA/UK) | 11 men/oligospermia, azoospermia/fertility urological management | Longitudinal phenomenological/adaptive challenges and adaptive work |
| Walschaerts et al.17 2013 (France) | 407 men/MFI/non-ART or ART treatment | Retrospective survey/reasons for treatment discontinuation |
ICSI: intracytoplasmic sperm injection; MFI: male factor infertility; OA: obstructive azoospermia; NOA: non-OA; TESE: testicular sperm extraction; micro-TESE: microdissection TESE; ART: artificial reproductive technology; HR-QOL: health-related quality of life
Psychological burden of male infertility treatment
The psychological states of men receiving male infertility treatment have been assessed in both quantitative and qualitative studies. Various dimensions have been evaluated, including stress, quality of life, and self-esteem, using self-report questionnaires in cross-sectional and longitudinal studies. Open accounts of challenges during treatment have also been analyzed in phenomenological studies of smaller sample size. The treatment settings were variable across these studies, and included TESE alone, TESE and intracytoplasmic sperm injection (ICSI), as well as broad or unspecified urological treatment. Despite the diversity in the study design, setting, and outcome variables, a consistent pattern of psychosocial strain and redress due to treatment processes is evident among infertile men.
Patel et al.7 studied 300 men, disaggregated by infertility factor, who have completed a nonvalidated psychological evaluation test for infertility. It was reported that men with a history of urological surgeries are seven times more likely than those without to experience significant infertility-specific stress requiring psychological support (odds ratio [OR] = 7.41, 95% confidence interval [CI]: 0.97–56.47, P = 0.05). These men described their experience of surgery, including repair of varicoceles and hydroceles, as highly distressing, which could have made a lasting impact on their attitude towards infertility. Other studies have focused on TESE specifically. Taniguchi et al.8 evaluated the health-related quality of life (HR-QOL) using the Short Form Health Survey (SF-8) in 89 infertile couples where the male partner had either nonobstructive azoospermia (NOA; n = 52) or obstructive azoospermia (OA; n = 37). Measured just before their TESE procedure, men with NOA scored significantly lower in the mental health domain compared to men with OA (mean ± standard deviation [s.d.]: 48.8 ± 6.8 vs 51.6 ± 6.6, P = 0.049). The study did not conduct follow-up assessment after TESE; thus, the impact of treatment outcomes was not assessed. This impact was elucidated by Bendayan et al.,9 who assessed the self-esteem of 44 NOA men 3 months before and 3 months after TESE. Patients who had successful TESE with viable sperm for ICSI (n = 24), had a significant improvement in overall self-esteem after the procedure (Coopersmith Self-Esteem Inventory [SEI] global score, mean ± standard error of the mean [s.e.m.]: 41.1 ± 2.0 to 44.0 ± 1.2, P = 0.01), largely due to a significant improvement in family-related self-esteem (mean ± s.e.m.: 6.7 ± 0.3 to 7.5 ± 0.2, P = 0.002). Conversely, patients with failed TESE (n = 20) had a significantly lower level of overall self-esteem after the procedure (mean ± s.e.m.: 39.5 ± 0.9 vs 43.8 ± 0.7, P < 0.001), with a significant decrease in all SEI domains of personal (mean ± s.e.m.: 22.2 ± 0.3 to 21.1 ± 0.5, P < 0.01), social (mean ± s.e.m.: 7.4 ± 0.2 to 6.1 ± 0.3, P < 0.01), professional (mean ± s.e.m.: 7.3 ± 0.2 to 6.2 ± 0.4, P < 0.01), and family-related (mean ± s.e.m.: 6.9 ± 0.4 to 6.1 ± 0.4, P < 0.01) self-esteem.
Another study approach was taken by Stevenson et al.10 and Johansson et al.,11 who conducted thematic analyses of challenges reported in semi-structured interviews of a total of 19 men who had received male infertility treatment. Recurring themes of avoidance and affective symptoms were identified. Throughout 6 months of treatment with a fertility-trained urologist, men with MFI avoided thinking about their infertility problem and disclosing their situation to friends and family, even distancing from friends with children altogether.10 This was echoed by men with OA who had failed and terminated ICSI following surgical sperm extraction, where the stress of contact with families that have children drove them to seek out families without children.11 Psychological symptoms of shock, depression, and grief progressed with diagnosis, treatment, and treatment failure.10 A feeling of inadequacy due to a threatened sense of masculinity was also reported, though this was followed by a feeling of redress and regain of self-esteem when sperm was detected through TESE.11
Impact of treatment on intimate relationships and sexual functioning
Several studies have exposed relationship dynamics throughout men’s infertility and treatment journey. Most of these studies9,12–16 have focused on the impact of male infertility treatment on male sexual functioning, measured by tools that also evaluated sexual health and well-being. Therefore, these studies have important implications on the sexual dynamics of intimate relationships. Other studies,9–11,17 though fewer, have highlighted emotional and communicative aspects of relationships through diverse study designs.
Phenomenological studies by Stevenson et al.10 and Johansson et al.11 have revealed that partners provide mutual support throughout their infertility journey, characterized by enhanced communication that has led to a strengthened bond. Men reported that they suppressed their emotional needs to support their partners, who they perceived to suffer more from the situation.11 The outcomes of treatment also had an impact on relationship quality. Bendayan et al.9 evaluated the relationship well-being of 44 men with NOA using the Dyadic Adjustment Scale (DAS) before and after TESE and as a function of TESE outcome. Men with sperm extracted successfully reported a significant improvement in the overall score after the procedure (mean ± s.e.m.: 120.3 ± 3.2 to 126.3 ± 2.5, P < 0.001), with significant improvements in their consensus, satisfaction, and affection subscores. In contrast, men with failed TESE had significantly lower overall scores (mean ± s.e.m.: 114.7 ± 3.0 vs 126.8 ± 1.6, P < 0.0001) as well as scores of all DAS domains after the procedure. The detrimental impact of infertility on intimate relationships can also be seen in a study examining reasons for discontinuation of fertility treatment in 407 men with MFI.17 Participants were split into those who had terminated the process following non-ART treatment (n = 218), including medical and surgical male infertility treatment, and those who discontinued ART treatment. It was revealed that the predominant reason for discontinuation of non-ART treatment was separation of the couple, whereas this was relatively uncommon in the ART treatment group (18% vs 7%, P ≤ 0.05). The authors reported that a semen sterility factor or azoospermia was mainly observed in the non-ART group, which could have played a role in the differences observed between the two groups.
Men’s sexual well-being and functioning have been evaluated in the treatment settings of surgical sperm extraction and varicocele repair. Taniguchi et al.12 examined the sexual activity of 226 men with NOA (n = 152) or OA before TESE and the factors which influenced this. The overall average sexual activity over a 1-month period was 3.6 times (mean ± s.d.: 3.6 ± 2.6 times), which was like that of the general male population aged 20–29 years in Japan. No significant differences in sexual activity were observed between the NOA and OA groups and the only factor which showed a correlation to sexual activity was marriage duration (r = −0.23; P < 0.01). The impact of the procedure on the sexual functioning of men with NOA was evaluated by other longitudinal studies that compared the International Index of Erectile Function (IIEF) scores and hormonal profiles before and after TESE. In 66 Turkish men with NOA, only those with failed TESEs (40.0%) reported a significant decrease in mean IIEF-5 scores 6 months after the procedure; those with successful TESEs reported an improvement that was insignificant.13 Furthermore, 12/13 (92.3%) of those who developed new-onset erectile dysfunction (ED) did not have sperm retrieved. Patients with new-onset ED also reported depression and anxiety according to the Hospital Anxiety and Depression Scale (HADS) and demonstrated a significant decrease in serum total testosterone (TT) levels (mean ± s.e.m.: 7.8 ± 2.0 ng ml−1 to 2.8 ± 2.0 ng ml−1, P < 0.001). The authors, therefore, concluded that unsuccessful TESE may have a negative impact on erectile function due to both hormonal and psychological factors. Bendayan et al.,9 however, found that the impact of TESE on sexual functions was in fact independent of serum TT in men with NOA (n = 44). Like the previous study, it was revealed that only TESE failure patients (45.0%) demonstrated significantly worse erectile function 3 months after the procedure compared to 3 months before (IIEF-15 erectile function domain, mean ± s.e.m.: 26.5 ± 0.9 vs 28.0 ± 0.7, P = 0.04). These men also had significantly lower scores in the IIEF-15 domains of sexual intercourse satisfaction and orgasmic function after the procedure. In contrast, TESE success patients reported significantly higher levels of intercourse satisfaction, orgasmic function, sexual desire, and overall satisfaction after the procedure. The current study differed from the previous in that it also compared pre- and postprocedural changes in serum TT between the two groups and found no significant differences, despite a significant decrease in serum TT in the overall study population after the procedure. It was, therefore, suggested that TESE outcomes have an impact on erectile function and other sexual functions, independent of changes in serum TT.
The relationship between infertile men’s sexual functioning and testosterone levels appears to be more nuanced with varicocele repair. In the USA, a retrospective review was performed examining the sexual health, measured by the clinically validated Male Sexual Health Questionnaire (MSHQ), and testosterone levels of 34 patients who had undergone microsurgical varicocelectomy.14 Half of these patients had presented due to infertility and the other half due to sexual dysfunction. Significant postoperative improvements were seen in various domains of MSHQ in the overall study population. However, this was mostly driven by the group of men with sexual dysfunction; infertile men did not have significant improvements in any domains of MSHQ, despite a significant increase in testosterone levels for both groups. Therefore, increased testosterone levels from microsurgical varicocelectomy did not influence the sexual health and function of infertile men in this study. The relationship between sexual functioning of infertile men and serum TT in the setting of microsurgical varicocelectomy was further investigated by Zohdy et al.15 and Saylam et al.16 using the IIEF. In the former study, 103 infertile men who underwent microsurgical varicocelectomy were compared with a control of 38 infertile men who refused surgical interventions and pursued ART instead.15 Both groups were also subdivided into hypogonadal (TT <300 ng dl−1) and eugonadal (TT ≥300 ng dl−1) men. Only hypogonadal men who underwent varicocelectomy (n = 49) showed significant improvements in both erectile function (IIEF-5, mean ± s.d.: 17.1 ± 2.6 to 19.7 ± 1.8, P < 0.001) and serum TT (mean ± s.d.: 219.3 ± 65.8 ng dl−1 to 358.1 ± 94.0 ng dl−1, P < 0.001) between initial visit and 6 months postoperation; no significant improvements in either parameters were observed in eugonadal infertile men who had varicocelectomy (n = 54) or in the control groups. There was also a significant decrease in the prevalence of hypogonadal men who suffered from ED (IIEF-5 <21) after varicocelectomy (85.7% to 55.1%, P = 0.001); no such changes were observed in any other groups. Similar patterns were observed by Saylam et al.16 in 202 infertile hypogonadal men (TT <3.5 ng ml−1), who also showed a significant improvement in erectile function (IIEF-EF, mean ± s.d.: 27.47 ± 2.96 to 28.61 ± 2.02, P < 0.001) and serum TT (mean ± s.d.: 2.55 ± 0.66 ng ml−1 to 3.72 ± 1.34 ng ml−1, P < 0.001) after microsurgical varicocelectomy. They also observed a significant increase in the prevalence of those who had normal erectile function (IIEF-EF ≥ 26) after the procedure (84.9% to 93.2%, P < 0.01), as well as a significant decrease in the prevalence of those who had decreased libido (20.3% to 4.5%, P < 0.001). It is consistent across these studies that microsurgical varicocelectomy may improve sexual functioning in infertile men with preoperative hypogonadism, but it is unknown whether improvement in sexual functioning is associated with successful treatment of subfertility with microsurgical varicocelectomy.
Male needs in fertility care
All three phenomenological studies10,11,18 included in the current study highlighted men’s subjective needs related to patient-centered care during treatment with remarkably consistent themes.
Provision of information regarding treatment is an important matter to men. Dancet et al.18 interviewed 17 men who had received TESE-related care and found that detailed information regarding the process of TESE and subsequent treatment, success rates, complications, and recovery were highly valued. Written information was appreciated, along with a clear, long-term treatment plan. The studies by Stevenson et al.10 and Johansson et al.11 also highlighted the need for information. Men struggled with uncertainty regarding the logistics and outcomes of treatment and wished for information on all available treatment options, including alternative approaches, such as donor sperm or adoption, to allow for constructive decision-making.
The need for emotional support and better communication from medical staff were also emphasized. There was a feeling of marginalization among men who have terminated ICSI following TESE.11 They felt that much of the clinical workup and treatment was not focused on male infertility and the cause of their azoospermia was not investigated, leaving unanswered questions that caused frustration. On the other hand, men reported positive associations with the treatment process when emotional support was provided by the immediate health-care team, especially in the form of direct and normalizing communication.10
DISCUSSION
Previous systematic reviews have identified a gap in the understanding of men’s experiences with infertility treatment, especially male-specific treatment.1,6 The findings of this systematic review have focused on the psychological, social, marital, and sexual dimensions of this critical stage of a man’s infertility journey. The included studies mostly concentrated on surgical treatments, and have elucidated the positive and negative psychosocial impacts of treatment outcomes, the burden and adaptive behaviors associated with the process, and patient needs in clinical care.
To ensure that the included studies accurately reflect the perspectives of infertile men who have personally experienced male-specific treatment, strict selection criteria were adopted for this systematic review. Notably, studies with an isolated focus on men in ART treatment were excluded due to the uncertainty of whether such participants have received male-specific infertility treatment. It is possible that the strict selection criteria have resulted in an underrepresentation of studies in this topic, especially in relation to medical treatments. Regardless, the findings were considerably consistent and have provided comprehensive insights that can guide clinical practice and future research.
There were a few limitations in the included studies which may have impacted the accuracy or relevance of findings. In the study by Patel et al.,7 the assertion that men with a history of urological surgery are more likely to experience infertility-specific stress was founded on an odds ratio with a wide confidence interval, which encompasses the possibility of no effect (95% CI: 0.97–56.47). The cross-sectional design of Taniguchi et al.8’s investigation into HR-QOL of men with NOA and OA receiving TESE sheds light on the impact of these conditions more so than the impact of the TESE procedure. Nonetheless, it allowed the identification of men with NOA as a particularly vulnerable patient population, likely due to limited treatment options and relatively poor treatment outcomes for this condition.19 Indeed, Bendayan et al.9 demonstrated that the outcome of TESE was of particular importance to these men, having a significant impact on their self-esteem, sexual functions, and relationship quality. Therefore, additional care should be taken throughout the clinical management of men with this condition, including routine screening of their psychological, relationship and sexual well-being, along with follow-up when treatment fails. Phenomenological studies have also provided important insights into the challenges experienced by infertile men receiving treatment. The avoidant coping mechanism highlighted by Stevenson et al.10 and Johansson et al.11 has been linked to more depressive symptoms compared to other coping strategies.20 Together with the affective symptoms described following failed treatment and the positive associations reported with emotional support from health-care teams, these phenomena further support the utility in providing or offering psychological services as part of clinical care.
The relationship dynamics of infertile men appear to be affected by circumstances surrounding infertility as well as from treatment. Available studies mostly focused on men’s sexual function and health, which have direct implications for the sexual dynamics of intimate relationships. TESE outcomes, both negative and positive, are associated with NOA men’s sexual functioning and relationship quality and appear to be more psychologically driven rather than hormonally. Patients should, therefore, be counseled regarding the possible impact TESE may have, not only on psychological well-being but also on sexual and relationship well-being. Varicocelectomy seems to improve the sexual functions of only infertile men who are hypogonadal preoperatively, which may be due to the normalization of serum TT for this population. Again, the association between successful surgical treatment of subfertility and sexual functioning is yet to be examined in the context of varicocelectomy. Evidence on the emotional and communicative aspects of relationships during treatment is less clear. Although phenomenological studies reported general improvements in partner bonds due to enhanced communication and support, the relatively high prevalence of couples who separated among men who discontinued the pursuit of parenthood after non-ART treatments highlights the burden of severe MFI. Again, this may be due to limited treatment options and outcomes for these conditions. Further studies are required to explore the emotional well-being of relationships during infertility treatment, especially for the more vulnerable patient groups, and verify the need to support not only the patient but also the couple confronting the issue together.
Men’s needs in the health-care setting were clear with complementary themes identified by phenomenological studies. Uncertainty regarding treatment and appreciation for information were important matters to infertile men. Clinicians should strive to equip patients with a comprehensive understanding of their condition, treatment processes, expected outcomes, and alternative options to allow for constructive planning and decision-making. “Planful problem-solving”, or the seeking of information and solutions, has been shown to be more common in men than women, and is associated with lower levels of infertility stress.21 Therefore, the facilitation of this adaptive coping behavior through adequate education and resource provision constitutes a crucial component of patient-centered care. Marginalization and appreciation for direct communication and support from clinicians were another set of complementary themes. The marginalization of men in the fertility setting has been well documented by other studies which focused on men’s experiences during ART treatment.22,23 In the study of Johansson et al.,11 this phenomenon was reported by men even when they were active participants of the treatment journey, requiring invasive TESE procedure themselves. This is an important insight that calls for attentive inclusion of the male counterpart during fertility treatment. It has been shown that infertile men feel involved as an equal partner when clinicians take the initiative to discuss the male partner’s concerns, provide information on the general experiences of infertile men, and provide the opportunity for men to talk about their wishes for fatherhood.22
Implications for clinical practice
Screening for psychological, social, marital and sexual well-being may provide valuable information to assist with clinical care throughout fertility treatment, and follow-up after failed treatment may assist in identifying those in need of specialized psychosocial support. This will likely benefit men with severe MFI and promote personal as well as relationship well-being in what can be a challenging period for a couple. Furthermore, health-care professionals should provide comprehensive education and written resources for patients who have treatment for MFI – not only from a medical standpoint but also from a psychosocial one. Continued communication with and inclusion of the male partner throughout the whole management process will also benefit the overall patient experience. These recommendations are pivotal in addressing the likely unmet psychosocial needs of infertile men.
CONCLUSION
This systematic review has synthesized the available evidence on men’s experiences of male infertility treatment. Psychological, social, marital, and sexual implications were highlighted, as well as male-specific needs in the clinical setting. These findings have clear implications for clinical practice as well as future research directions.
AUTHOR CONTRIBUTIONS
All authors contributed significantly to the conceptualization and design of this systematic review. WW conducted the first structured search of the literature, data extraction and analysis, and drafting of the manuscript, with editing completed by JL. JL conducted the second search and discrepancies were resolved through discussion among DJK, JL, and WW. All authors contributed to the synthesis and discussions of the results. DJK and KMS were instrumental in the coordination of the project. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests
REFERENCES
- 1.Fisher JR, Hammarberg K. Psychological and social aspects of infertility in men: an overview of the evidence and implications for psychologically informed clinical care and future research. Asian J Androl. 2012;14:121–9. doi: 10.1038/aja.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am. 2014;41:195–204. doi: 10.1016/j.ucl.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Smith JF, Walsh TJ, Shindel AW, Turek PJ, Wing H, et al. Sexual, marital, and social impact of a man's perceived infertility diagnosis. J Sex Med. 2009;6:2505–15. doi: 10.1111/j.1743-6109.2009.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karavolos S, Stewart J, Evbuomwan I, McEleny K, Aird I. Assessment of the infertile male. Obstet Gynaecol. 2013;15:1–9. [Google Scholar]
- 6.Dancet EA, Nelen WL, Sermeus W, De Leeuw L, Kremer JA, et al. The patients'perspective on fertility care: a systematic review. Hum Reprod Update. 2010;16:467–87. doi: 10.1093/humupd/dmq004. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Sharma PS, Narayan P, Nair BV, Narayanakurup D, et al. Distress in infertile males in Manipal-India: a clinic based study. J Reprod Infertil. 2016;17:213–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Taniguchi H, Matsuda T, Nakaoka Y, Morimoto Y. Health-related quality of life in infertile couples receiving testicular sperm extraction treatment. Int J Urol. 2018;25:164–5. doi: 10.1111/iju.13484. [DOI] [PubMed] [Google Scholar]
- 9.Bendayan M, Sais E, Alter L, Fathallah K, Jaoul M, et al. For patients with non-obstructive azoospermia, the outcome of testicular sperm extraction correlates with self-esteem, sexual health and the quality of the couple's relationship. Basic Clin Androl. 2022;32:3. doi: 10.1186/s12610-022-00153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson EL, McEleny KR, Moody E, Bailey DE. Applying the adaptive leadership framework for chronic illness to understand how American and British men navigate the infertility process. Health Psychol Open. 2019;6:1–11. doi: 10.1177/2055102919871647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson M, Hellström AL, Berg M. Severe male infertility after failed ICSI treatment: a phenomenological study of men's experiences. Reprod Health. 2011;8:4. doi: 10.1186/1742-4755-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniguchi H, Matsuda T, Nakaoka Y, Morimoto Y. Sexual activity of patients undergoing testicular sperm extraction. Sex Med. 2020;8:30–5. doi: 10.1016/j.esxm.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akbal C, Mangir N, Tavukçu HH, Ozgür O, Simşek F. Effect of testicular sperm extraction outcome on sexual function in patients with male factor infertility. Urology. 2010;75:598–601. doi: 10.1016/j.urology.2009.07.1330. [DOI] [PubMed] [Google Scholar]
- 14.Najari BB, Introna L, Paduch DA. Improvements in patient-reported sexual function after microsurgical varicocelectomy. Urology. 2017;110:104–9. doi: 10.1016/j.urology.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Zohdy W, Ghazi S, Arafa M. Impact of varicocelectomy on gonadal and erectile functions in men with hypogonadism and infertility. J Sex Med. 2011;8:885–93. doi: 10.1111/j.1743-6109.2010.01974.x. [DOI] [PubMed] [Google Scholar]
- 16.Saylam B, Çayan S, Akbay E. Effect of microsurgical varicocele repair on sexual functions and testosterone in hypogonadal infertile men with varicocele. Aging Male. 2020;23:1366–73. doi: 10.1080/13685538.2020.1769589. [DOI] [PubMed] [Google Scholar]
- 17.Walschaerts M, Bujan L, Parinaud J, Mieusset R, Thonneau P. Treatment discontinuation in couples consulting for male infertility after failing to conceive. Fertil Steril. 2013;99:1319–23. doi: 10.1016/j.fertnstert.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 18.Dancet EA, Spiessens C, Blocquiaux L, Sermeus W, Vanderschueren D, et al. Testicular biopsy before ART: the patients'perspective on the quality of care. Hum Reprod. 2010;25:3072–82. doi: 10.1093/humrep/deq262. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Xi Q, Pan Y, Jiang Y, Zhang H, et al. Pregnancy and neonatal outcomes in azoospermic men after intracytoplasmic sperm injection using testicular sperm and donor sperm. Med Sci Monit. 2018;24:6968–74. doi: 10.12659/MSM.912613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babore A, Stuppia L, Trumello C, Candelori C, Antonucci I. Male factor infertility and lack of openness about infertility as risk factors for depressive symptoms in males undergoing assisted reproductive technology treatment in Italy. Fertil Steril. 2017;107:1041–7. doi: 10.1016/j.fertnstert.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Peterson BD, Newton CR, Rosen KH, Skaggs GE. Gender differences in how men and women who are referred for IVF cope with infertility stress. Hum Reprod. 2006;21:2443–9. doi: 10.1093/humrep/del145. [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen AT, Madsen SA, Humaidan P. Psychological aspects of male fertility treatment. J Adv Nurs. 2013;69:1977–86. doi: 10.1111/jan.12058. [DOI] [PubMed] [Google Scholar]
- 23.Arya ST, Dibb B. The experience of infertility treatment: the male perspective. Hum Fertil. 2016;19:242–8. doi: 10.1080/14647273.2016.1222083. [DOI] [PMC free article] [PubMed] [Google Scholar]


