Abstract
The penis is a vital organ of perception that transmits perceived signals to ejaculation-related centers. The penis consists of the glans penis and penile shaft, which differ considerably in both histology and innervation. This paper aims to investigate whether the glans penis or the penile shaft is the main source of sensory signals from the penis and whether penile hypersensitivity affects the whole organ or only part of it. The thresholds, latencies, and amplitudes of somatosensory evoked potentials (SSEPs) were recorded in 290 individuals with primary premature ejaculation using the glans penis and penile shaft as the sensory areas. The thresholds, latencies, and amplitudes of SSEPs from the glans penis and penile shaft in patients were significantly different (all P < 0.0001). The latency of the glans penis or penile shaft was shorter than average (indicating hypersensitivity) in 141 (48.6%) cases, of which 50 (35.5%) cases were sensitive in both the glans penis and penile shaft, 14 (9.9%) cases were sensitive in the glans penis only, and 77 (54.6%) cases were sensitive in the penile shaft only (P < 0.0001). There are statistical differences in the signals perceived through the glans penis and the penile shaft. Penile hypersensitivity does not necessarily mean that the whole penis is hypersensitive. We classify penile hypersensitivity into three categories, namely, glans penis, penile shaft, and whole-penis hypersensitivity, and we propose the new concept of penile hypersensitive zone.
Keywords: neuroelectrophysiology, perceptive zone, premature ejaculation, sensitivity, sensory perception
INTRODUCTION
The penis, as the most critical organ of perception for men during sexual intercourse, converts stimuli such as touch, temperature, and pressure sensations into electrical signals sent to ejaculation-related centers. The etiology of premature ejaculation (PE) is multifactorial, and high sensitivity of the glans penis and serotonin (5-hydroxytryptamine [5-HT]) disorders within the central nervous system (CNS) are thought to be the two leading pathophysiological causes of PE.1,2 Penile hypersensitivity affects sensory perception through the penis, which determines the final sensory signal to the ejaculation-related centers. Ejaculation latency time (ELT) is usually the longest during intercourse, shorter in the laboratory, and the shortest during masturbation.3 This phenomenon suggests that ejaculation is a complex process and that PE should be studied as a systemic problem. In addition to environmental factors and central cortical influences, the intensity of penile stimulation (i.e., female intravaginal pressure) and penile sensitivity should be considered.
The penis is composed of the glans penis and the penile shaft. There are significant differences in histology and innervation between the glans penis and the penile shaft. To date, there are few studies on whether this difference affects their sensory function, and whether penile hypersensitivity affects the glans penis, penile shaft, or the whole organ.
Suppose there is a difference in perceptual sensitivity between the glans penis and the penile shaft, whether the glans penis or the penile shaft perceives a given stimulus will inevitably affect the sensory signals transmitted to the ejaculation-related centers during vaginal intercourse. Thus, it is important to understand the relative sensitivity of the glans penis and the penile shaft. Penile sensitivity is an issue that cannot be avoided in studying ejaculation disorders. Through quantitative sensory testing (QST), Salonia et al.4 found that the cold sensation threshold of the penile shaft was not significantly different between primary premature ejaculation (PPE) patients and healthy controls, whereas PPE patients had a markedly lower cold threshold than controls at the glans penis. This suggests a sensory difference between the glans penis and the penile shaft.4 However, QST is occasionally questioned for its potential subjectivity; thus, instead of a difference in the activation of sensory neurons, patients with PPE may have major alterations in how these afferent impulses from the periphery are processed centrally, leading to changes in the perception and response elicited by sensory inputs.5,6 As a result, it is necessary to apply accurate detection to avoid the above psychological factors as much as possible. Somatosensory evoked potentials (SSEPs) have been recognized in recent years as an objective and effective method to measure penile sensitivity in PE.7
This study recorded the threshold, latency, and amplitude of SSEPs in 290 PPE patients in response to localized stimulation of the glans penis or the penile shaft. We analyzed the difference between the glans penis and the penile shaft sensitivity and whether both sites were consistently hypersensitive in PPE patients. We further assessed the degree of hypersensitivity in the entire penis, in the glans penis and penile shaft specifically). This study is a large study to analyze the sensory function and hypersensitivity of the glans penis and penile shaft by neuroelectrophysiological methods.
PARTICIPANTS AND METHODS
Inclusion criteria
All 290 patients who attended the First Hospital of Dalian Medical University (Dalian, China) between July 2017 and March 2021 were collected. The inclusion criteria were as follows: (1) patients were over 24 years old; (2) patients’ sexual partners were female and had been engaging in regular intercourse for more than 6 months; (3) ejaculation often or always occurred before or within approximately 1 min of vaginal penetration from the first sexual experience; (4) premature ejaculation diagnostic tool (PEDT) score ≥11; and (5) no surgery had been performed on the penis.
Exclusion criteria
The following candidates were excluded: (1) patients with neurological disease or genital malformations; (2) patients with diseases of the endocrine system (diabetes, hyperthyroidism, etc.); (3) patients with erectile dysfunction (ED); (4) patients with secondary premature ejaculation (SPE); (5) patients with chronic prostatitis; (6) patients with mental health conditions; or (7) PE patients who had been taking selective serotonin reuptake inhibitors (SSRIs) for a long period (>2 months).
Examination method
This study was approved by the Ethics Review Board of the First Hospital of Dalian Medical University (Approval No. PJ-KS-KY-2022-361), and oral informed consent was obtained from all patients. The examination was performed using a method previously described in the literature. The patients were relaxed and free of any disturbances. The lighting and temperature were maintained at comfortable levels.
The patients were informed of the primary examination procedure and precautions for the examination so that they could cooperate fully. Each patient was positioned supine, exposing the external genitalia and right forearm. Saline was used to clean the glans penis, and penile surface. An electromyography/evoked potential instrument (Synergy 10-conductor, Oxford, England) was used to record electrophysiological data. Positive and negative ring electrodes were placed at the coronal sulcus and the root of the penis, respectively. The ground electrode was placed at the right wrist. Electrodes were placed at the central cephalic (Cz) and median forehead (FPz) sites after being coated with conductive paste to reduce impedance. The recording electrode and reference electrodes were placed so that the electrode impedance was less than 5 kΩ. Patients were allowed a 5-min adaptation period before recording.8
The patient was instructed to relax, and the current was slowly increased, starting from 0 mA. The biological sensory threshold of dorsal nerve somatosensory evoked potentials (DNSEPs) was established as the intensity of the stimulation current that evoked a slight pins-and-needles sensation in the patient’s penile shaft. The current setting was increased to 3 times this threshold, and the superposition was performed 200 times. The latency of the DNSEP was recorded as the time required for the first evoked potential (EP) wave to appear. The amplitude was recorded as the DNSEP wave amplitude at this point.
The placement and settings of the ground electrode, scalp recording electrodes, and reference electrode for glans penis somatosensory evoked potentials (GPSEPs) were the same as those for DNSEPs.
Statistical analyses
The data were statistically analyzed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). First, the Kolmogorov‒Smirnov test was used to test the normality of continuous variables. Variables conforming to a normal distribution were expressed as the mean ± standard deviation (s.d.). When variables had a skewed distribution, the median (interquartile range [IQR]) was used. The latency and biosensory threshold of the glans penis and penile shaft conformed to a normal distribution. The glans penis and penile shaft amplitude conform to a skewed distribution. The two groups were compared by Student’s t-test if the normality assumption was met and the Mann‒Whitney U test was used if met skewed distribution. Correlations between the two groups were shown by Pearson correlation or Spearman correlation, as dictated by the variable distribution. The consistency of hypersensitivity between the glans penis and the penile shaft was assessed using McNemar’s test. P < 0.05 indicates a statistically significant difference unless otherwise specified.
RESULTS
General data
Neurophysiological data were collected from 290 patients with PPE who attended the First Hospital of Dalian Medical University between July 2017 and March 2021. The biosensory threshold, latency, and amplitude of DNSEPs and GPSEPs were recorded. The characteristics of the overall patients are shown in Table 1.
Table 1.
Characteristics of 290 patients
| Characteristic | Value |
|---|---|
| Age (year), mean±s.d. | 29.04±4.42 |
| Height (cm), mean±s.d. | 174.92±4.52 |
| Body mass (kg), mean±s.d. | 71.21±5.43 |
| PEDT score, mean±s.d. | 14.20±2.14 |
| Not married, n (%) | 202 (69.7) |
| Smoking, n (%) | 137 (47.2) |
| Alcohol consumption, n (%) | 228 (78.6) |
PEDT: premature ejaculation diagnostic tool; s.d.: standard deviation
Analysis of the differences between the glans penis and the penile shaft
Biosensory thresholds, latencies, and amplitudes were recorded for the glans penis and the penile shaft. The biosensory thresholds (mean ± s.d.) of the glans penis and penile shaft were 1.97 ± 0.69 mA and 2.38 ± 0.85 mA, respectively; the latencies (mean ± s.d.) of the glans penis and penile shaft were 43.46 ± 3.15 ms and 39.21 ± 2.49 ms, respectively; and the amplitudes (median [IQR]) of the glans penis and penile shaft were 1.40 (1.00, 2.10) μV and 1.10 (0.75, 1.60) μV, respectively. The biosensory threshold in the penile shaft was significantly higher than that in the glans penis (P < 0.0001), the latency in the glans penis was significantly longer than that in the penile shaft (P < 0.0001), and the amplitude in the glans penis was lower than that in the penile shaft (P < 0.0001), as shown in Figure 1.
Figure 1.

Comparison of biosensory thresholds, latency periods, and amplitudes between the glans penis and penile shaft. (a) The biosensory threshold of the glans penis was lower than that of the penile shaft. (b) The latency of the glans penis was longer than that of the penile shaft. (c) The evoked potential wave amplitude of the penile shaft was lower than that of the glans penis.
Correlation analysis between the glans penis and the penile shaft
Correlation analysis was performed on the SSEP latency, biosensory threshold, and amplitude of the glans penis and the penile shaft. The Pearson correlation coefficient of the biosensory threshold between the glans penis and the penile shaft was weak, at 0.3795 (P < 0.001); the Spearman correlation coefficients of the latency and amplitude between the glans penis and the penile shaft were both moderate, at 0.6198 and 0.5439, respectively (P < 0.001; Figure 2).
Figure 2.

Correlations of the glans penis and penile shaft. (a) There was a weak positive correlation between the biosensory thresholds of the glans penis and penile shaft. (b) A significant positive correlation was found between the latency of the glans penis and that of the penile shaft. (c) The correlation coefficient of amplitude between the glans penis and penile shaft was significant.
Relationship between the length of the penile shaft and latency
To investigate the relationship between the length of the penile shaft and latency, we randomly selected 20 patients. The negative electrode was placed at the root of the penile shaft, the positive electrode was at the coronal sulcus of the penis, and the rest of the method was the same as above. The measured penile shaft latency value (mean ± s.d.) was recorded as 39.93 ± 2.39 ms. The negative electrode was then moved to the middle of the penile shaft, the latency (mean ± s.d.) was rerecorded (39.88 ± 2.41 ms), and the difference between the two was compared. The results showed that the difference between the two was not statistically significant (P = 0.979; Figure 3), suggesting that the penile shaft length did not affect DNSEP latency.
Figure 3.
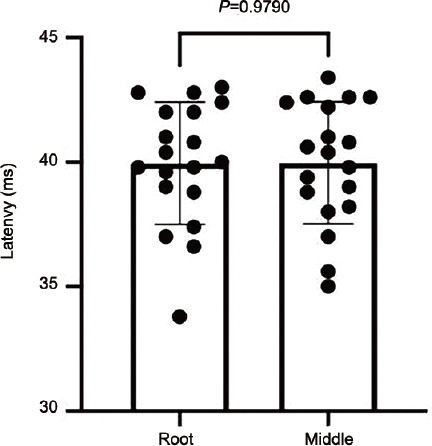
The latency difference between the root of the penile shaft and the middle of the penile shaft center was no statistically significant.
Comparison of the hypersensitivity consistency of the glans penis and penile shaft
Patients with low GPSEP latency (≤40.83 ms) and DNSEP latency (≤39.03 ms) are defined as having hypersensitivity of the penis.9 Glans penis or penile shaft hypersensitivity was observed in 141 (48.6%) cases, of which 50 (35.5%) cases were hypersensitive in both the glans penis and penile shaft of the penis, 14 (9.9%) cases were hypersensitive in the glans penis only, and 77 (54.6%) cases were hypersensitive in the penile shaft only. There was a difference in the concordance of hypersensitivity between the glans penis and the penile shaft (P<0.0001; Table 2).
Table 2.
Comparison of the hypersensitivity consistency of the glans and shaft
| Variable | GPSEP, n (%) | McNemar’s test | |
|---|---|---|---|
|
| |||
| Normal | Hypersensitive | ||
| DNSEP | |||
| Normal | 149 (51.4) | 14 (4.8) | P<0.0001 |
| Hypersensitive | 77 (26.6) | 50 (17.2) | |
DNSEP: dorsal nerve somatosensory evoked potential; GPSEP: glans penis somatosensory evoked potential
DISCUSSION
The measurement of SSEPs is one method of examining penile neurophysiology. SSEPs are the result of stimulation of a peripheral part of the body, processing of the information by the brain, and production of bioelectrical changes in the corresponding part of the brain that can be detected at a relatively fixed time interval and in a specific phase with a given stimulus.10 Penile SSEPs are divided into GPSEPs and DNSEPs. This study’s test indexes included penile SSEP threshold, latency, and amplitude.
This study analyzed the penile neurophysiological findings of 290 PPE patients, and significant differences were found in the biosensory thresholds, latencies, and wave amplitudes between the glans penis and the penile shaft. The biological sensory threshold of the glans penis was lower than that of the penile shaft. In contrast, the latency of the glans penis was longer, and the amplitude of the glans penis was lower, suggesting different sensitivity.
The dorsal penile nerve arises from the pudendal nerve in the pelvis and gives rise to two branches of nerve fibers. The first branch runs along the middorsal line of the penis and terminates at the glans penis. The other spreads ventrally and dorsally from the main trunk of the dorsal penile nerve to the penis and terminates in and on the corpus spongiosum.11 The nerve fibers of the dorsal penile nerve travel dorsal to the penile shaft, parallel with each other to the distal penis, showing a two-dimensional linear relationship, occasionally penetrating the white membrane at the penile shaft to enter the corpus cavernosum, i.e., the penetrating branches. The distal dorsal penile nerve enters the glans penis and travels to the glans penis in a three-dimensional structure. The nerve distribution is denser than that at the penile shaft, and the latter is distributed in a two-dimensional manner. The nerve fibers emerging from the dorsal penile nerve at the glans penis have been demonstrated to run directly through or parallel to the subdermis, forming a sensory plexus and distributing free nerve endings uniformly to the skin surface.12 Immunohistochemical studies of dorsal penile nerves have revealed that most nerve fibers in the distal third of the penis are somatosensory nerve fibers (indicated by positive immunostaining with a specific antibody against peripheral myelin protein-22 [PMP22]). In contrast, the fibers in the proximal two-thirds of the penis are primarily visceral motor nerve fibers (with positive immunostaining of nitric oxide synthase [NOS], tyrosine hydroxylase [TH], and vesicular acetylcholine transporter [VAChT]).13 Therefore, the study suggests that the dorsal penile nerve in the glans penis is mainly responsible for relaying afferent sensory information. In contrast, the dorsal penile nerve in the penile shaft is accountable for afferent sensory information. This segment of the nerve may be associated with erection and detumescence, owing to its recognized anatomical differences from the distal segment.14 Furthermore, the diameter of nerve fibers in the glans penis is significantly smaller than that of nerve fibers in the penile shaft, resulting in lower resistance and faster conduction speed. These studies suggest differences in the innervation between the glans penis and the penile shaft.
The surface of the glans penis is composed of stratified squamous epithelium and a dense connective tissue layer, equivalent to the skin’s dermis. In contrast, the penile shaft is composed of keratinized squamous epithelium.15 The receptors of the glans penis are almost entirely composed of free nerve endings (FNEs), which also contain Meissner’s corpuscles.16 The glans penis does not possess sensory receptors unique to the skin, so it is not sensitive to light touch.17 Although both branches of nerves are derived from the dorsal penile nerve, there are differences in the receptors, which may be why the biological sensory thresholds of the two are correlated, but the correlation is weak.
Some studies have suggested that the length of the penile shaft affects dorsal penile nerve latency.18–20 Herbaut et al.19 and Kiwamoto et al.20 used pharmacological erection and penile traction to demonstrate that penile length affects dorsal penile nerve latency. In this study, 20 patients were randomly selected. When the stimulation position of the positive electrodes was changed, we found no difference in the latency measure where the positive electrode was connected to the middle of the penile shaft and the coronal sulcus compared with the root of the penis, the coronal sulcus. We concluded that the length of the penile shaft might not be a factor affecting latency. It was not meaningful to compare the difference in nerve latency by changing the distance between the dorsal penile nerve stimulation electrodes.
Guo et al.21 classified patients with PE into three groups based on the intravaginal ejaculation latency time (IELT) and recorded vibratory thresholds using a biothesiometer. They demonstrated that penile hypersensitivity through a biothesiometer was associated with a short IELT. The viewpoint from Guo et al.21 was consistent with the finding from Xia et al.22 The latter team performed neuroelectrophysiological tests on patients and observed penile hypersensitivity in patients with PE and found that lidocaine could alleviate it.22 Rowland et al.23 classified patients into four groups: primary PE, secondary PE with erectile dysfunction, ED alone, and normal sexual function. Their results suggested no penile hypersensitivity in premature ejaculation; however, they observed that ejaculation latency is associated with the threshold. Perhaps, the inconsistent view of penile sensitivity from Rowland et al.23 was related to their insufficient sample size, or perhaps penile sensitivity was not subdivided before the study. Thus, this study may be much more significant. It was found that the glans penis and the penile shaft have different sensitivities and that penile hypersensitivity is not the whole penis but only a part of the penis. Xia et al.22 also found that patients with primary delayed ejaculation (DE) appear to have a penile shaft rather than glans penis hyposensitivity and hyperexcitability, and adaptation to a specific masturbatory technique (higher and idiosyncratic) may be related to the causes of primary DE. Therefore, it may be more reasonable to subdivide the penile hypersensitive zone into the glans penis only, the penile shaft, or the whole penis. Therefore, a new concept of the penile hypersensitive zone is proposed to clarify the hypersensitive penile zone for better research.
When the vagina is inserted and filled with the penis, contraction of the vaginal muscle produces a significant “grip” effect on the penis. The distal vagina, clitoris, and urethra share blood supply and innervation, called the clitourethrovaginal (CUV) complex.24 These components function as a unit during intercourse. Vaginal orgasm is mainly a rhythmic contraction produced in the lower 1/3 of the vagina.25 In 2005, Guaderrama et al.26 used infusion manometry to obtain vaginal pressure profiles in 14 asymptomatic women. They concluded that the intravaginal pressure was highest in the mid-zone over 3–4 cm. Peak pressure occurred in the vaginal canal, approximately 2 cm cranial to the hymen.26 This article referred to the area producing intense pressure on the inserted penis as the vital vaginal pressure area (VVPA).
When the penis is inserted into the vagina, the vaginal muscles produce a significant clenching effect on the penis. The penis is the most crucial perceptor during sexual intercourse that converts mechanical stimuli such as pressure into electrical signals and transmits them to ejaculation-related centers. The length of the VVPA theoretically cannot completely cover the erectile penis at least 10 cm after erection, so only part of the erectile penis can receive stimulation from the VVPA. Moreover, the stimulation felt in this zone may be the most effective stimulation, which is more critical to the IELT. However, penile hypersensitivity may not affect the whole penis but only a part of the penis, indicating that the glans penis and the penile shaft have different perceptibility. Thus, we propose a new mechanism through a penile effective perceptive zone. This article referred to the area enwrapped by VVPA as a penile effective perceptive zone (PEPZ). During vaginal intercourse, PEPZ being or not being the penile hypersensitive site will affect final perception signals and IELT. According to the above theory, the study of IELT must take into account the match between the penile hypersensitive zone and the PEPZ. This may explain the phenomenon that we also found in the clinic: early ejaculation occurred recurrently or consistently in specific conditions (sexual intercourse with a particular mate or in a particular position) and seldom occurred in intercourse without these conditions. The hyposensitivity and hyperexcitability of the penile shaft and using the penile shaft as PEPZ may cause the pathology of primary DE. Simultaneously, some patients with unusual masturbation patterns, such as prone masturbation, exhibit symptoms of anejaculation,27 which may be related to the match between the penile insensitive zone and the PEPZ. We may modulate the final perceived stimulus by matching the penile hypersensitive site and the PEPZ and thus affect the IELT, which would be a new approach to treating ejaculatory disorders. Of course, psychology is also an important factor, such as in the treatment of ED,28 which should also be considered in treatment. This study found that nearly half of the patients with PPE had a penile hypersensitive zone, avoiding this hypersensitive zone as the PEPZ may prolong the IELT. Meanwhile, using this hypersensitive zone as the PEPZ may promote ejaculation.
This study only explored the penile nerve electrophysiology of the PPE glans penis and penile shaft in the flaccid state through electrical stimulation. It did not examine the electrophysiological differences under pressure or temperature stimulation. We also did not explore the effect of vaginal and penile secretion on the degree of lubrication caused by sexual intercourse, which cannot imitate entirely actual intravaginal intercourse. In addition, normal control data in this study were referred to Xia et al.,22 which may lead to errors due to different operators. Further research will be conducted on the above aspects in the future.
CONCLUSION
In this paper, through a large sample of SSEPs, we discovered that penile hypersensitivity could sometimes be localized to part of the penis instead of spanning the entire organ. Classifying penile hypersensitivity according to its location (the glans penis, the penile shaft, or the whole penis) may be more clinically reasonable than ignoring this distinction. Therefore, we propose the new concept of the penis hypersensitivity zone. Adjusting the match between the penile hypersensitive zone and PEPZ may be a new method to treat ejaculation disorders.
AUTHOR CONTRIBUTIONS
LZ and LTW designed this study and analyzed the data. QZT, CLS, KNW, and WRL provided samples. LZ, LTW, and TJ participated in the statistical analysis and drafting of the manuscript. TJ and HJ reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTEREST
All authors declare no competing interests.
REFERENCES
- 1.Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, et al. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE) Sex Med. 2014;2:60–90. doi: 10.1002/sm2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donatucci CF. Etiology of ejaculation and pathophysiology of premature ejaculation. J Sex Med. 2006;3:303–8. doi: 10.1111/j.1743-6109.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 3.Vanden Broucke H, Everaert K, Peersman W, Claes H, Vanderschueren D, et al. Ejaculation latency times and their relationship to penile sensitivity in men with normal sexual function. J Urol. 2007;177:237–40. doi: 10.1016/j.juro.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 4.Salonia A, Saccà A, Briganti A, Del Carro U, Dehò F, et al. Quantitative sensory testing of peripheral thresholds in patients with lifelong premature ejaculation:a case-controlled study. J Sex Med. 2009;6:1755–62. doi: 10.1111/j.1743-6109.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- 5.Breda G, Xausa D, Giunta A, Tamai A, Silvestre P, et al. Nomogram for penile biothesiometry. Eur Urol. 1991;20:67–9. doi: 10.1159/000471664. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett G, Stewart JD, Tamblyn R, Abrahamowicz M. Normal distributions of thermal and vibration sensory thresholds. Muscle Nerve. 1998;21:367–74. doi: 10.1002/(sici)1097-4598(199803)21:3<367::aid-mus11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Sun Z, Liao Z, Zheng Q, Chen J, Lv B, et al. A study of differences in penile dorsal nerve somatosensory evoked potential testing among healthy controls and patients with primary and secondary premature ejaculation. J Sex Med. 2021;18:732–6. doi: 10.1016/j.jsxm.2021.01.186. [DOI] [PubMed] [Google Scholar]
- 8.Xin ZC, Choi YD, Rha KH, Choi HK. Somatosensory evoked potentials in patients with primary premature ejaculation. J Urol. 1997;158:451–5. [PubMed] [Google Scholar]
- 9.Yang B, Hong Z, Luse DC, Han Y, Sun G, et al. The diagnostic role of neurophysiological tests for premature ejaculation:a prospective multicenter study. J Urol. 2022;207:172–82. doi: 10.1097/JU.0000000000002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia JD, Zhou LH, Han YF, Chen Y, Wang R, et al. A reassessment of penile sensory pathways and effects of prilocaine-lidocaine cream in primary premature ejaculation. Int J Impot Res. 2014;26:186–90. doi: 10.1038/ijir.2014.5. [DOI] [PubMed] [Google Scholar]
- 11.Yang CC, Bradley WE. Peripheral distribution of the human dorsal nerve of the penis. J Urol. 1998;159:1912–6. doi: 10.1016/S0022-5347(01)63194-X. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Koh KS, Song WC. Macro/microscopic distribution of the dorsal nerve of penis in human glans penis. J Anat. 2020;237:849–53. doi: 10.1111/joa.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diallo D, Zaitouna M, Alsaid B, Quillard J, Ba N, et al. The visceromotor and somatic afferent nerves of the penis. J Sex Med. 2015;12:1120–7. doi: 10.1111/jsm.12851. [DOI] [PubMed] [Google Scholar]
- 14.Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- 15.Halata Z, Munger BL. The neuroanatomical basis for the protopathic sensibility of the human glans penis. Brain Res. 1986;371:205–30. doi: 10.1016/0006-8993(86)90357-4. [DOI] [PubMed] [Google Scholar]
- 16.García-Mesa Y, García-Piqueras J, Cobo R, Martín-Cruces J, Suazo I, et al. Sensory innervation of the human male prepuce:Meissner's corpuscles predominate. J Anat. 2021;239:892–902. doi: 10.1111/joa.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munger BL, Ide C. The structure and function of cutaneous sensory receptors. Arch Histol Cytol. 1988;51:1–34. doi: 10.1679/aohc.51.1. [DOI] [PubMed] [Google Scholar]
- 18.Yang CC, Bradley WE, Berger RE. The effect of pharmacologic erection on the dorsal nerve of the penis. Muscle Nerve. 1997;20:1439–44. doi: 10.1002/(sici)1097-4598(199711)20:11<1439::aid-mus12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Herbaut AG, Sattar AA, Salpigides G, Nogueira MC, Wespes E. Sensory conduction velocity of dorsal nerve of the penis during pharmacoerection:a more physiological technique? Eur Urol. 1996;30:60–4. doi: 10.1159/000474146. [DOI] [PubMed] [Google Scholar]
- 20.Kiwamoto H, Kanda H, Onishi N, Esa A, Sugiyama T, et al. [Measurement of nerve conduction velocity of the dorsal nerve of the penis] Hinyokika Kiyo. 1988;34:1007–10. [Article in Japanese] [PubMed] [Google Scholar]
- 21.Guo L, Liu Y, Wang X, Yuan M, Yu Y, et al. Significance of penile hypersensitivity in premature ejaculation. Sci Rep. 2017;7:10441. doi: 10.1038/s41598-017-09155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia JD, Han YF, Pan F, Zhou LH, Chen Y, et al. Clinical characteristics and penile afferent neuronal function in patients with primary delayed ejaculation. Andrology. 2013;1:787–92. doi: 10.1111/j.2047-2927.2013.00119.x. [DOI] [PubMed] [Google Scholar]
- 23.Rowland DL, Haensel SM, Blom JH, Slob AK. Penile sensitivity in men with premature ejaculation and erectile dysfunction. J Sex Marital Ther. 1993;19:189–97. doi: 10.1080/00926239308404903. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell HE, Eizenberg N, Rahman M, Cleeve J. The anatomy of the distal vagina:towards unity. J Sex Med. 2008;5:1883–91. doi: 10.1111/j.1743-6109.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- 25.Jannini EA, Buisson O, Rubio-Casillas A. Beyond the G-spot:clitourethrovaginal complex anatomy in female orgasm. Nat Rev Urol. 2014;11:531–8. doi: 10.1038/nrurol.2014.193. [DOI] [PubMed] [Google Scholar]
- 26.Guaderrama NM, Nager CW, Liu J, Pretorius DH, Mittal RK. The vaginal pressure profile. Neurourol Urodyn. 2005;24:243–7. doi: 10.1002/nau.20112. [DOI] [PubMed] [Google Scholar]
- 27.Ma GC, Zou ZJ, Lai YF, Zhang X, Zhang Y. Regular penis-root masturbation, a novel behavioral therapy in the treatment of primary premature ejaculation. Asian J Androl. 2019;21:631–4. doi: 10.4103/aja.aja_34_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Zhang XB, Liao ZC, Tang ZY, Li DJ. Is mild erectile dysfunction associated with severe psychological symptoms in Chinese patients with moderate-to-severe chronic prostatitis/chronic pelvic pain syndrome? Asian J Androl. 2021;23:319–24. doi: 10.4103/aja.aja_71_20. [DOI] [PMC free article] [PubMed] [Google Scholar]


