Abstract
ERK1 and ERK2 mitogen-activated protein kinases (MAPK) play a critical role in regulation of cell proliferation and differentiation in response to mitogens and other extracellular stimuli. Mitogens and cytokines that activate MAPK in T cells have been shown to activate human immunodeficiency virus type 1 (HIV-1) replication. Little is known about the signal transduction pathways that activate HIV-1 replication in T cells upon activation by extracellular stimulation. Here, we report that activation of MAPK through the Ras/Raf/MEK signaling pathway enhances the infectivity of HIV-1 virions. Virus infectivity was enhanced by treatment of cells with MAPK stimulators, such as serum and phorbol myristate acetate, as well as by coexpression of constitutively activated Ras, Raf, or MEK (MAPK kinase) in the absence of extracellular stimulation. Treatment of cells with PD 098059, a specific inhibitor of MAPK activation, or with a MAPK antisense oligonucleotide reduced the infectivity of HIV-1 virions without significantly affecting virus production or the levels of virion-associated Gag and Env proteins. MAPK has been shown to regulate HIV-1 infectivity by phosphorylating Vif (X. Yang and D. Gabuzda, J. Biol. Chem. 273:29879–29887, 1998). However, MAPK activation enhanced virus infectivity in some cells lines that do not require Vif function. The HIV-1 Rev, Tat, p17Gag, and Nef proteins were directly phosphorylated by MAPK in vitro, suggesting that other HIV-1 proteins are potential substrates for MAPK phosphorylation. These results suggest that activation of the ERK MAPK pathway plays a role in HIV-1 replication by enhancing the infectivity of HIV-1 virions through Vif-dependent as well as Vif-independent mechanisms. MAPK activation in producer cells may contribute to the activation of HIV-1 replication when T cells are activated by mitogens and other extracellular stimuli.
The mitogen-activated protein (MAP) kinases ERK1 and ERK2 (also known as p44/42 MAPK and hereafter referred to as MAPK) are central components of signal transduction pathways activated by diverse extracellular stimuli. These serine and threonine kinases are present in all cell types and play a critical role in the regulation of cell proliferation and differentiation in response to mitogens and a wide variety of growth factors and cytokines (reviewed in references 3, 13, and 46). Upon activation, these closely related MAPK isoforms phosphorylate a large number of substrates, including transcription factors (e.g., c-Myc, c-Jun, NF-IL6, ATF-2, AP-1, and Elk-1), the epidermal growth factor (EGF) receptor, phospholipase A2, protein tyrosine phosphatase 2C, and cytoskeletal proteins (references 3, 13, and 46 and references therein). MAPK itself is activated by phosphorylation on threonine and tyrosine residues by the MAPK kinase (also known as MEK). The best understood mechanism for activation of MAPK is via activation of Ras by growth factor receptors or tyrosine kinases. Activation of Ras induces Raf-1 targeting to the membrane, leading to activation of Raf, which then phosphorylates and activates MEK (reviewed in references 9, 13, and 46). Ras-independent mechanisms have also been implicated in activation of MAPK (9). Activation of MAPK occurs during the G0/G1 transition and may be required for progression through the cell cycle (30, 34, 35, 46). Thus, MAPK serves to link stimuli from the cell surface to cellular events involved in proliferation and differentiation, including the cell cycle, generation of phospholipid messengers, transcription, and translation. Other MAP kinases in mammalian cells are JNK/SAPK and p38/HOG, which are activated by stress stimuli and inflammatory cytokines.
Several steps of the human immunodeficiency virus type 1 (HIV-1) virus life cycle depend on cellular activation by mitogenic stimuli (33). HIV-1 cannot replicate in quiescent T cells, which comprise the majority of circulating T cells in vivo (33, 56). Virus replication is blocked due to incomplete reverse transcription and lack of proviral DNA integration (56). Upon stimulation with mitogens, such as phytohemagglutinin (PHA) or interleukin 2, reverse transcription proceeds to completion and allows integration and virus production to occur (57). Mitogenic stimulation can also activate viral gene expression in cells that are latently infected and harbor integrated proviral DNA (20, 33). In contrast to the inability to replicate in quiescent T cells, HIV-1 can replicate in nondividing terminally differentiated macrophages (6, 19, 25, 50). Thus, mitogenic stimulation is not required to allow HIV-1 replication in all contexts.
Little is known about the cellular signal transduction pathways that activate HIV-1 replication in response to mitogenic stimulation. HIV-1 encodes Gag, Pol, and Env proteins, in addition to six regulatory proteins (Tat, Rev, Vif, Vpu, Vpr, and Nef). In mature virions, the p55Gag precursor is cleaved by the viral protease to form the p17Gag, p24Gag, p7Gag, and p6Gag proteins. Previous studies have shown that the HIV-1 p17Gag, p24Gag, Vif, Vpu, Rev, and Nef proteins are phosphorylated by cellular kinases in vitro and in vivo (2, 4, 7, 10, 24, 31, 45, 49, 54). Vif is phosphorylated and regulated by MAPK and other yet unknown kinases (54, 55). p17Gag, Nef, and Rev are phosphorylated by protein kinase C (PKC) (7, 24), and Vpu is phosphorylated by casein kinase II (44). Many kinases that phosphorylate and regulate the functions of HIV-1 proteins have not been identified. Understanding signal transduction pathways that regulate HIV-1 replication upon mitogenic stimulation is likely to provide important insights into mechanisms of virus replication and pathogenesis.
Mitogens and other extracellular stimuli that activate MAPK have been shown to activate HIV-1 replication (15, 20, 33, 36, 53). In this report, we show that activation of ERK1 and ERK2 MAPK by the Ras/Raf/MEK signaling pathway plays a role in HIV-1 replication by enhancing the infectivity of HIV-1 virions. The findings, together with the previous demonstration that MAPK can enhance HIV-1 infectivity by phosphorylating Vif (55), suggest the involvement of Vif-dependent as well as Vif-independent mechanisms. These findings suggest that activation of MAPK in the producer cell plays a role in regulation of HIV-1 replication by enhancing virion infectivity. This mechanism may contribute to the activation of HIV-1 replication when T cells are activated by mitogens and other extracellular stimuli.
Regulation of HIV-1 infectivity by the ERK MAPK pathway.
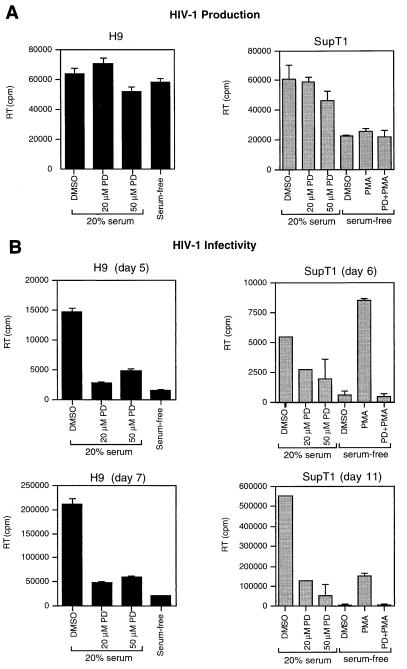
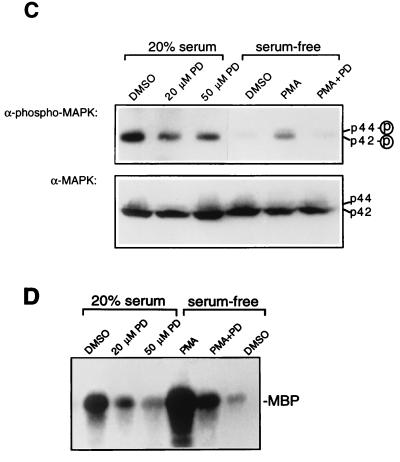
We initially examined the role of MAPK in HIV-1 replication by using PD 098059 [2-(2′-amino-3′-methoxyphenyl)oxanaphthalen-4-one], a specific inhibitor of the MAPK activating enzyme, MAPK kinase (MEK) (1, 14). Inhibition of MEK by PD 098059 prevents activation of MAPK and subsequent phosphorylation of MAPK substrates (14). The high degree of specificity of PD 098059 is indicated by its failure to inhibit more than 20 other kinases, including other MEK homologs (i.e., JNK/SAPK and p38/HOG kinase kinases) (1). Chronically infected H9 and SupT1 cells were serum starved for 24 h and then stimulated by addition of 20% fetal calf serum (FCS) in the presence or absence of PD 098059 (New England Biolabs) for 4 h (SupT1) or 6 h (H9). The virions produced in the culture supernatants were quantitated by reverse transcriptase (RT) assay (18), pelleted by centrifugation (1 h at 4°C at 14,000 × g), resuspended in fresh medium, and used to infect fresh H9 or SupT1 cells cultured in media with 10% FCS. Treatment of cells with PD 098059 did not significantly inhibit virus production in chronically infected cells (Fig. 1A). However, virus infectivity was inhibited by 75 to 90% as demonstrated by acute infection of fresh cells (Fig. 1B). Furthermore, the infectivity of virions was inhibited by more than 80 to 90% when virions were produced by serum-starved cells compared to serum-stimulated cells (Fig. 1B). The effect of serum starvation on virus infectivity was reversed by stimuli that activate MAPK, such as serum and phorbol myristate acetate (PMA) (Fig. 1B and data not shown). Conversely, serum or PMA enhancement of virus infectivity was abolished by treatment with PD 098059 (Fig. 1B). To confirm that PD 098059 blocked MAPK activation by serum and PMA in the virus-producing cells, MAPK activity was determined by immunoblotting with an anti-phosphorylated MAPK antibody which detects p44/42 MAPK only when activated by phosphorylation. As expected, serum or PMA stimulation induced MAPK activation in SupT1 cells, whereas PD 098059 inhibited MAPK activation (Fig. 1C). The total level of MAPK expression detected by immunoblotting with anti-ERK1 and anti-ERK2 was not significantly affected by treatment with serum, PMA, or PD 098059. We further confirmed activation of MAPK by serum and PMA by performing in vitro kinase assays as described previously (55), using myelin basic protein (MBP) as the MAPK substrate (Fig. 1D). The stronger activation by PMA in this experiment is due to the earlier time of occurrence of MAPK activation (1.5 h) compared to that (4 h) in the experiment for which results are presented in Fig. 1C. As expected, PD 098059 inhibited MAPK activation. Thus, inhibition of HIV-1 infectivity by PD 098059 is mediated by inhibition of MAPK activation rather than by reduction of MAPK expression. Together, these results suggest that MAPK or MAPK-activated pathways regulate the infectivity of HIV-1 virus particles.
FIG. 1.
Inhibition of HIV-1 infectivity by the MEK inhibitor PD 098059 (PD). H9 or SupT1 cells chronically infected with the HXB2 HIV-1 isolate were serum starved for 24 h, pretreated with the indicated concentrations of PD for 30 min, and then stimulated by addition of 20% FCS or 100 nM PMA. After incubation for 4 h (SupT1) or 6 h (H9) at 37°C, virus production was quantitated by measuring RT activity in the culture supernatants and virions were pelleted, resuspended in fresh medium, and used to infect fresh SupT1 cells cultured in media with 10% FCS. (A) Virus production in culture supernatants of chronically infected H9 and SupT1 cells. (B) Virus infectivity was determined by quantitation of RT activity in the supernatants of the acutely infected cells at the indicated time points. Values shown are expressed as 3H counts per minute per milliliter and are expressed as the means ± standard deviations of duplicates. As shown in panels C and D, PD inhibits MAPK activation by serum and PMA. (C) HIV-1-infected SupT1 cells were serum starved for 24 h and then stimulated for 4 h with 20% serum or 100 nM PMA in the presence or absence of PD at the indicated concentrations. For PMA-treated cells, PD was used at 50 μM. p44/42 MAPK activity and total MAPK expression levels were detected by immunoblotting equivalent amounts of protein in cell lysates with rabbit anti-phosphorylated MAPK (1:1,000 dilution; New England Biolabs) and rabbit anti-MAPK (anti-ERK1 and anti-ERK2, 1:1,500 dilution of each; Santa Cruz Biotechnology, Inc.), respectively, using the ECL system (Amersham) as described previously (55). (D) In vitro kinase assay for MAPK activity in cell lysates from SupT1 cells stimulated for 1.5 h with serum or PMA as described in panel C, using MBP as the MAPK substrate, was performed as described previously (55). DMSO, dimethyl sulfoxide.
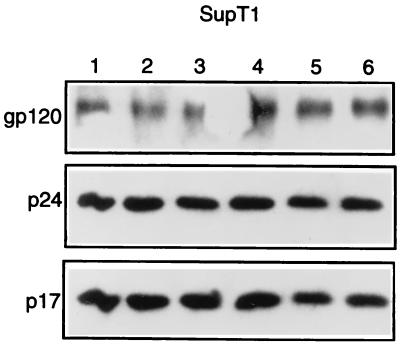
To determine whether PD 098059, serum, or PMA affect the levels of virion-associated Gag or Env proteins, equivalent amounts of HIV-1 virions were pelleted and analyzed for the levels of gp120Env, p24Gag, and p17Gag proteins by immunoblotting. Figure 2 shows that the levels of gp120Env, p24Gag, and p17Gag proteins incorporated into HIV-1 virions produced in SupT1 cells were similar under the experimental conditions shown in Fig. 1. Similar results were obtained for HIV-1 virions produced in H9 cells (data not shown). These results suggest that the regulation of virion infectivity by MAPK is not mediated by an effect on the levels of virion-associated Gag and Env proteins or processing of the p55Gag precursor.
FIG. 2.
Activation or inhibition of MAPK does not affect the levels of virion-associated Gag and Env proteins. Equivalent amounts of HIV-1 virions (15,000 cpm RT units) were pelleted from the supernatants of chronically infected SupT1 cells after serum starvation followed by stimulation with 20% FCS or 100 nM PMA in the presence or absence of PD 098059 as in the experiment for which results are presented in Fig. 1, lysed, and analyzed for the levels of virion-associated gp120Env, p24Gag, and p17Gag proteins by immunoblotting with rabbit anti-gp120Env (1:1,000 dilution; a gift of Richard Wyatt and Joseph Sodroski), rabbit anti-p24Gag (1:2,000 dilution; Intracell), or mouse monoclonal anti-p17Gag (1:2,000 dilution; Advanced Biotechnologies Inc.) as described in the legend for Fig. 1. Lanes 1 through 6 contain proteins from cells treated the same as indicated for the lanes (from left to right) in Fig. 1C.
Enhancement of HIV-1 infectivity by coexpression of constitutively activated Ras, Raf, and MEK.
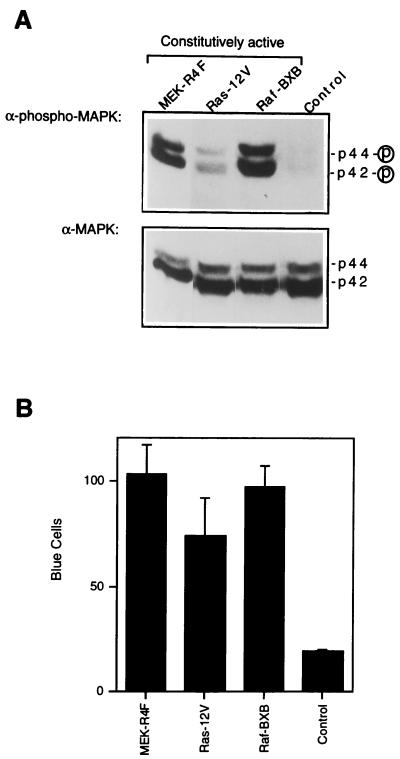
To further examine the role of the MAPK pathway in the regulation of HIV-1 infectivity, HIV-1 virions were produced in 293T cells cotransfected with 10 μg of pNL4-3 plasmid containing HIV-1 proviral DNA and 10 μg of a constitutively active kinase expressor plasmid for mutant MEK (pMEK-R4F, also called ΔN3/S218E/S222D; a gift of Natalie Ahn, University of Colorado, Boulder [32, 52]), H-Ras (pRSVRas12V; a gift of Alan Hall, University College London, London, United Kingdom [39]), or Raf-1 (pRaf-BXB; a gift of Ulf Rapp, University of Würzburg, Würzburg, Germany [16]). The MEK-R4F, Ras-12V, and Raf-BXB plasmids encode constitutively active forms of MEK, Ras, and Raf, respectively, and induce constitutive MAPK activation via the Ras/Raf/MEK pathway in the absence of extracellular stimulation (16, 32, 39, 52). Coexpression of these kinase mutants led to activation of MAPK in serum-starved 293T cells as determined by immunoblotting with anti-phosphorylated MAPK (Fig. 3A, upper panel) without affecting the total levels of MAPK expression as determined by immunoblotting with anti-ERK1 and anti-ERK2 (Fig. 3A, lower panel). The infectivity of virions produced in cells expressing constitutively activated MEK, H-Ras, or Raf-1 in the absence of extracellular stimulation was then examined by using the multinuclear activation of a galactosidase indicator (MAGI) assay, which allows quantitation of virion infectivity during a single round of infection on the basis of the ability of the viral Tat protein to transactivate the expression of an integrated β-galactosidase gene driven by the HIV-1 long terminal repeat (LTR) in HeLa CD4-LTR/β-Gal indicator cells (28, 40). The efficiency of a single round of virus infection is then determined by counting the number of blue cells or syncytia in situ. The infectivity of virions produced by 293T cells expressing constitutively active MEK, H-Ras, or Raf-1 was enhanced by approximately fivefold during a single round of infection compared to virions produced by control cells (Fig. 3B), providing further evidence that the activation of MAPK enhances HIV-1 infectivity.
FIG. 3.
Coexpression of constitutively activated Ras, Raf, or MEK enhances the infectivity of HIV-1 virions. (A) p44/42 MAPK activity (upper panel) and total MAPK expression levels (lower panel) in 293T cells transfected with constitutively active kinase mutants (MEK-R4F, Ras-12V, or Raf-BXB) or control vector pSG5 (Stratagene) DNA (control) and pNL4-3 containing HIV-1 proviral DNA by the calcium phosphate method. After transfection, cells were incubated with Dulbecco’s minimal essential medium containing 0.5% serum. At 48 h after transfection, cell lysates were prepared and equivalent amounts of proteins were subjected to electrophoresis and immunoblotting with anti-phosphorylated MAPK (α-phospho-MAPK) or anti-MAPK (anti-ERK1 and anti-ERK2) (α-MAPK). (B) MAGI assays were performed as described previously (28, 40) to quantitate the infectivity of HIV-1 virions produced in 293T cells under the same experimental conditions as used in the experiment for which activities are presented in panel A. Two days after transfection, culture supernatants containing virus were collected, passed through a 0.45-μm-pore-size filter, and assayed for RT activity. Equivalent amounts of virus stock (10,000 cpm RT units) were used to infect HeLa CD4-LTR/β-galactosidase indicator cells. After overnight incubation at 37°C, the medium was replaced with fresh medium. At 48 h after infection, cells were stained for β-galactosidase expression (28, 40). The total number of cells with blue nuclei in 16 random fields were counted under a microscope by using a 10× objective. A cluster of cells containing multiple blue nuclei was counted as one syncytium. Results are expressed as the means ± standard deviations of duplicates.
Inhibition of HIV-1 infectivity by MAPK antisense oligonucleotide.
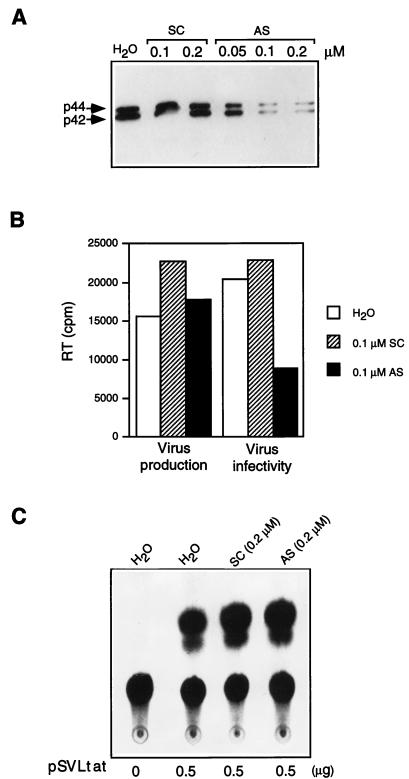
To further examine the role of MAPK in HIV-1 replication, an antisense phosphorothioate oligonucleotide (5′-GCCGCCGCCGCCGCCAT-3′) (42) (synthesized and purified by Oligos Etc.) that targets the MAPK gene at the initial translation codon was used to specifically deplete MAPK in cultured cells. The same oligonucleotide was previously shown to specifically inhibit MAPK expression in NIH 3T3 L1 cells (42). Treatment with the MAPK antisense oligonucleotide at 0.1 to 0.2 μM reduced MAPK levels in HeLa cells by 80 to 90% (Fig. 4A). The scrambled control oligonucleotide (5′-GGCCCGCTCGCGCACCC-3′) having the same nucleotide composition showed no effect, suggesting that inhibition of MAPK expression by the antisense oligonucleotide was specific. Similar results were observed with several other cell lines, including COS-1 and 293 cells (data not shown). Cell viability and the expression of other cellular proteins were not affected by the MAPK antisense under these conditions. At the time point when MAPK was maximally depleted (48 h), HeLa cells were transfected with HIV-1 proviral DNA. Virus production was not significantly affected by treatment with the MAPK antisense oligonucleotide (Fig. 4B). However, virions produced by the MAPK antisense oligonucleotide-treated cells showed a reduction in their infectivity as determined by infection of CEM cells (Fig. 4B). Similar results were obtained when SupT1 cells were used as targets for infection (not shown). A more significant reduction in virus infectivity (>10-fold) was observed when cells were treated at higher oligonucleotide concentrations (0.5 to 1.0 μM) (data not shown). However, nonspecific effects of the scrambled control oligonucleotide were observed at these higher concentrations. Treatment with the MAPK antisense oligonucleotide did not significantly inhibit expression of an HIV-1 LTR chloramphenicol acetyltransferase (CAT) reporter plasmid transactivated by coexpression of HIV-1 Tat (Fig. 4C), consistent with its lack of effect on virus production (Fig. 4B). These results provide further support for the idea that MAPK is involved in regulation of HIV-1 infectivity.
FIG. 4.
Inhibition of HIV-1 infectivity by a MAPK antisense oligonucleotide (AS) or scrambled control oligonucleotide (SC). (A) Depletion of p44/42 MAPK expression by AS. HeLa cells were treated with AS or SC at the indicated concentrations. HeLa cells were grown to 80% confluence in Dulbecco’s minimal essential medium (DMEM) containing 10% FCS in 35-mm-diameter six-well plates, and washed twice with serum-free DMEM. Appropriate concentrations of oligonucleotides in 125 μl of serum-free DMEM were preincubated at room temperature for 15 min with 125 μl of serum-free DMEM containing 40 μg of DOTMA (Lipofectin) (Gibco-BRL) per ml. This solution was then mixed with an additional 250 μl of serum-free DMEM. Cells were incubated with the mixture for 6 h at 37°C in the presence of 5% CO2. Subsequently, the medium containing DOTMA was removed and the incubation was continued for 48 h in fresh medium containing 10% FCS. After 48 h incubation, MAPK expression was determined by immunoblotting of equivalent amounts of total protein with anti-MAPK (anti-ERK1 [p44] and anti-ERK2 [p42]). (B) HeLa cells were transfected with 3 μg of pHXB2 containing HIV-1 proviral DNA following depletion of MAPK by treatment with MAPK antisense oligonucleotide as described for panel A. Virus production was determined by quantitation of RT activity in the supernatants of the transfected HeLa cell cultures at 48 h after transfection. Virus infectivity was determined by infection of CEM cells and quantitation of RT activity in the supernatants of the newly infected cells on day 7 after infection. (C) Treatment with AS does not inhibit expression of an HIV-1 LTR CAT reporter plasmid transactivated by coexpression of HIV-1 Tat. HeLa cells were treated with the oligonucleotides at 0.2 μM and cotransfected with 0.5 μg of pUIIIRCAT and 0 or 0.5 μg of the HIV-1 Tat expressor plasmid pSVLtat by the same method as described for panels A and B. Shown is CAT activity in the transfected cell lysate at 48 h posttransfection, as determined by the conversion of chloramphenicol to its acetylated forms.
Phosphorylation of HIV-1 proteins by MAPK.
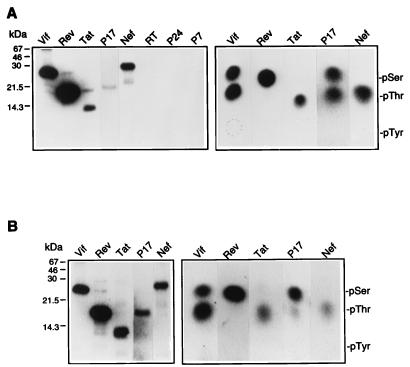
The demonstration of the involvement of MAPK in HIV-1 replication raised the possibility that HIV-1 proteins might be substrates for phosphorylation by MAPK. We previously demonstrated that HIV-1 Vif is phosphorylated and activated by MAPK (55). However, the experiments presented above show that MAPK activation in producer cells enhances virion infectivity in some cell lines that do not require Vif (i.e., SupT1, 293T, and HeLa cells), in addition to Vif-dependent cells (i.e., H9 cells) (12, 18, 26, 41, 51). To examine the possibility that other HIV-1 proteins are potential substrates for MAPK, we performed in vitro kinase assays as described previously (55) with recombinant p42 MAPK or MAPK immunoprecipitated from stimulated COS-1 cells using recombinant HIV-1 Vif, Rev, Tat, Nef, p17Gag, p24Gag, p7Gag, and RT proteins as substrates. We found that Vif, Rev, Tat, Nef, and p17Gag were phosphorylated in vitro by recombinant p42 MAPK (Fig. 5A) or by immunoprecipitated MAPK (Fig. 5B). Vif, Rev, and Nef were highly phosphorylated by recombinant MAPK, while Tat and p17Gag were phosphorylated at lower levels. In contrast, p24Gag, p7Gag, and RT were not phosphorylated by MAPK (Fig. 5A). Vif was phosphorylated on serine and threonine, Rev was phosphorylated on serine, and Nef and Tat were phosphorylated on threonine (Fig. 5A and B). p17Gag was phosphorylated on serine and threonine by recombinant p42 MAPK but was predominantly phosphorylated on serine by immunoprecipitated MAPK (Fig. 5A and B). These results demonstrate that HIV-1 Vif, Rev, Tat, Nef, and p17Gag proteins are directly phosphorylated by MAPK in vitro.
FIG. 5.
Phosphorylation of HIV-1 proteins by MAPK. In vitro kinase assays were performed using recombinant p42 MAPK (A) or MAPK immunoprecipitated from COS-1 cells stimulated with PMA (B). MAPK immunocomplexes were isolated from cell lysates by using anti-ERK1 and anti-ERK2. MAPK was incubated with 2 μg of each indicated recombinant HIV-1 protein for in vitro kinase assays performed in the presence of [γ-32P]ATP. Recombinant Rev and p7Gag were from the NIH AIDS Research and Reference Reagent Program, Tat and p24Gag were from Immunodiagnostics, Inc. (kindly provided by J. Raina), and p17Gag and Nef were from Intracell. Vif, Rev, Tat, p24Gag, and p7Gag were derived from the HXB2 HIV-1 isolate, p7Gag was derived from the MN HIV-1 isolate, and Nef was derived from the LAI HIV-1 isolate. The results of phosphorylated amino acid analysis performed as described previously (54) are shown on the right. The positions of phosphorylated Ser, Thr, and Tyr (pSer, pThr, and pTyr) markers are indicated. Phosphorylated amino acids detected in the recombinant HIV-1 proteins comigrated with pSer and pThr but not with pTyr.
Conclusions.
In this study, we present several lines of evidence indicating that the ERK MAPK signaling pathway plays a role in HIV-1 replication by enhancing the infectivity of HIV-1 virions. Treatment of cells with PD 098059, a specific inhibitor of MAPK activation (1, 14), markedly reduced the infectivity of HIV-1 virions without significantly affecting virus production. This resulted in subsequent inhibition of virus replication in the absence of the MAPK inhibitor. Similar results were observed when MAPK was depleted by treatment of cells with a MAPK antisense oligonucleotide. Further evidence linking MAPK to HIV-1 infectivity is the finding that the infectivity of HIV-1 virions was significantly enhanced by MAPK stimulators such as serum and PMA. Conversely, serum and PMA enhancement of virus infectivity was inhibited by PD 098059. Finally, the infectivity of HIV-1 virions was enhanced by coexpression of constitutively active H-Ras, Raf-1, and MEK mutants. These kinase mutants have much higher kinase activity than the parental wild-type kinases in the absence of extracellular stimulation (16, 32, 39, 52). As expected, expression of these active kinases induced activation of MAPK in unstimulated cells and the activation correlated with enhancement of HIV-1 infectivity. However, the level of MAPK activation produced by coexpression of Ras was lower than those induced by the other kinase mutants, suggesting that enhancement of virion infectivity by Ras may also involve MAPK-independent mechanisms. An alternative possibility is that enhancement of virion infectivity is not further increased once a certain threshold level of MAPK activation is reached. MEK activates and phosphorylates p44/42 MAPK but not the JNK/SAPK or p38/HOG MAP kinases (9). Interestingly, HIV-1 itself may activate the MAPK pathway (37, 43). Together, these findings indicate that the ERK MAPK signaling pathway plays an important role in regulation of HIV-1 replication by enhancing virus infectivity. This mechanism may contribute to the activation of HIV-1 replication when T cells are activated by mitogens and other extracellular stimuli.
Our studies suggest that activation of MAPK in the producer cell enhances HIV-1 infectivity through Vif-dependent mechanisms as well as through Vif-independent mechanisms. We previously demonstrated that MAPK phosphorylates Vif in vitro and in vivo on Thr 96 and Ser 165 (55). Furthermore, we showed that mutations of the highly conserved Thr 96 site result in loss of Vif function and inhibition of HIV replication and infectivity, suggesting that MAPK can regulate HIV-1 infectivity by phosphorylating Vif. In the present study, we show that MAPK enhances virion infectivity in several cell lines that do not require Vif function (i.e., SupT1, 293T, and HeLa cells) (12, 18, 26, 41, 51). Thus, the regulation of HIV-1 infectivity by MAPK also involves a Vif-independent mechanism that is likely to be important in cells which require Vif function as well as those that do not. Our data suggest that regulation of virion infectivity by the MAPK pathway is not mediated by an effect on virion incorporation of Gag and Env proteins or by processing of the p55Gag precursor. We found that the HIV-1 Rev, Tat, Nef, and p17Gag proteins were phosphorylated by MAPK in vitro. However, whether these HIV-1 proteins are phosphorylated by MAPK in vivo remains to be determined. p17Gag is phosphorylated on tyrosine and serine by as yet unknown cellular kinases prior to and during virus infection (6, 19). This facilitates its translocation to the nucleus and nuclear targeting of the viral preintegration complex in nondividing cells. Previous studies suggested that MAPK is associated with HIV-1 virions (8, 27). Furthermore, Jacqué et al. (27) demonstrated phosphorylation of p17Gag in vitro by virion-associated MAPK. Together, these findings raise the possibility that MAPK may regulate HIV-1 infectivity by phosphorylating one of the virion components, such as p17. However, we have not been able to confirm that virion incorporation of MAPK is specific, since we detected the same amount of MAPK in cellular membrane vesicles that copurify with mock virions (unpublished data). It is unlikely that the enhancement of virus infectivity by MAPK was due to an effect on Nef (21, 22), Rev, or Tat. The HXB2 Nef allele appears to be inactive (48, 58), and Rev does not significantly affect virus infectivity. Tat can stimulate reverse transcription and may thereby enhance virus infectivity (23). However, it seems unlikely that the enhancement of virus infectivity by MAPK is due to an effect on Tat, in view of our finding that inhibition of MAPK by an antisense oligonucleotide (Fig. 4C) or by PD 098059 (55a) does not appear to inhibit Tat function. It is also possible that MAPK may enhance virus infectivity by affecting cellular proteins or a virion-associated kinase(s) (6, 19). Further studies are required to elucidate the role of MAPK in specific steps of the virus life cycle.
Our studies show that MAPK regulates the infectivity of HIV-1 virions, but the results do not exclude the possibility that MAPK may also affect other steps of the virus life cycle. It has been reported that the Ras/Raf pathway is activated in HIV-infected monocytes and participates in the activation of transcription factor NF-κB (17), a key regulator of HIV-1 LTR expression. Since MAPK is a key component of the Ras/Raf pathway, MAPK may be directly involved in the activation of NF-κB or other transcription factors important for induced expression of HIV-1 in infected cells (5, 16). Other studies have demonstrated that the activation of Raf-1 induces expression of the HIV-1 LTR through NF-κB sites and stimulates HIV-1 replication in T cells (5, 38). However, our studies suggest that inhibition of MAPK by an antisense oligonucleotide (Fig. 4C) or by PD 098059 (55a) does not significantly affect the activity of the HIV LTR within the context of an HIV-1 LTR CAT reporter plasmid transactivated by coexpression of HIV-1 Tat. However, the effects of MAPK may be different in cells that are latently infected and harbor integrated proviral DNA. It is also possible that HIV-1 gene expression or other steps in the virus life cycle may be regulated by other MAPK family members. For example, p38/HOG MAPK has been shown to activate the HIV-1 LTR (29) and appears to be necessary for HIV-1 replication in T cells (11).
The involvement of MAPK in regulation of HIV-1 replication has implications for the pathogenesis of HIV-1 disease. HIV-1 replication is blocked in quiescent T cells due to incomplete reverse transcription (56). Our studies suggest that an additional mechanism which may help to explain why HIV-1 cannot replicate in quiescent T cells might be the unstimulated state of MAPK in producer cells. In vivo, the majority of circulating T cells are normally quiescent but can be activated in response to immune stimulation by cytokines or specific antigens. In HIV-1 infection, the immune system is stimulated during the early stages, when an immune response to HIV-1 is being mounted, and in the advanced stages, when opportunistic infections are frequent (33). The activation of MAPK as a consequence of immune stimulation may contribute to disease pathogenesis by enhancing virus infectivity and possibly processes occurring at other steps of the virus life cycle, thereby increasing the replication and dissemination of HIV-1.
Acknowledgments
We thank N. Ahn, U. Rapp, and A. Hall for plasmids, R. Wyatt and J. Sodroski for anti-gp120, J. Raina of Immunodiagnostics, Inc. (Bedford, Mass.), for Tat and p24Gag proteins, J. Sodroski and A. Engelman for critical reading of the manuscript, H. Park for assistance with plasmid purifications, and Y. Chen for assistance with transfections. The pNL4-3 plasmid (donated by Malcolm Martin) and HIV-1 Rev and p7 proteins were obtained from the NIH AIDS Research and Reference Reagent Program.
This work was supported by NIH grant AI36186. We also acknowledge support from the G. Harold and Leila Y. Mathers Charitable Foundation and, for supporting necessary core facilities, the Center for AIDS Research (AI28691) and Center for Cancer Research (AO6514). D.G. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel P. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Bandres J C, Luria S, Ratner L. Regulation of human immunodeficiency virus nef protein by phosphorylation. Virology. 1994;201:157–161. doi: 10.1006/viro.1994.1278. [DOI] [PubMed] [Google Scholar]
- 3.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodéus M, Marie-Cardine A, Bougeret C, Ramos-Morales F, Benarous R. In vitro binding and phosphorylation of human immunodeficiency virus type 1 Nef protein by serine/threonine protein kinase. J Gen Virol. 1995;76:1337–1344. doi: 10.1099/0022-1317-76-6-1337. [DOI] [PubMed] [Google Scholar]
- 5.Bruder J T, Heidecker G, Tan T-H, Weske J C, Derse D, Rapp U. Oncogene activation of HIV-LTR-driven expression via the NF-κB binding sites. Nucleic Acids Res. 1993;21:5229–5234. doi: 10.1093/nar/21.22.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnette B, Yu G, Felsted R L. Phosphorylation of HIV-1 gag proteins by protein kinase C. J Biol Chem. 1993;268:8698–8703. [PubMed] [Google Scholar]
- 8.Cartier C, Deckert M, Grangeasse C, Trauger R, Jensen F, Bernard A, Cozzone A, Desgranges C, Boyer V. Association of ERK2 mitogen-activated protein kinase with human immunodeficiency virus particles. J Virol. 1997;71:4832–4837. doi: 10.1128/jvi.71.6.4832-4837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1996;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 10.Cochrane A, Kramer R, Ruben S, Levine J, Rosen C A. The human immunodeficiency virus rev protein is a nuclear phosphoprotein. Virology. 1989;171:264–266. doi: 10.1016/0042-6822(89)90535-7. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P S, Schmidtmayerova Y, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey K J. The critical role of p38 MAP kinase in T cell HIV-1 replication. Mol Med. 1997;3:339–346. [PMC free article] [PubMed] [Google Scholar]
- 12.Courcoul M, Patience C, Rey F, Blanc D, Harmache A, Sire J, Vigne R, Spire B. Peripheral blood mononuclear cells produce normal amounts of defective Vif− human immunodeficiency virus type 1 particles which are restricted for the preretrotranscription steps. J Virol. 1995;69:2068–2074. doi: 10.1128/jvi.69.4.2068-2074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis R J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 14.Dudley D T, Pang L, Decker S J, Bridges A J, Salteil A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairhurst R M, Daeipour M, Amaral M C, Nel A E. Activation of mitogen-activated protein kinase/ERK-2 in phytohaemagglutinin in blasts by recombinant interleukin-2: contrasting features with CD3 activation. Immunology. 1993;79:112–118. [PMC free article] [PubMed] [Google Scholar]
- 16.Flory E, Hoffmeyer A, Smola U, Rapp U R, Bruder J T. Raf-1 kinase targets GA-binding protein in transcriptional regulation of the human immunodeficiency virus type 1 promoter. J Virol. 1996;70:2260–2268. doi: 10.1128/jvi.70.4.2260-2268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folgueira L, Algeciras A, MacMorran W S, Bren G D, Paya C V. The Ras-Raf pathway is activated in human immunodeficiency virus-infected monocytes and participates in the activation of NF-κB. J Virol. 1996;70:2332–2338. doi: 10.1128/jvi.70.4.2332-2338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Blanco M A, Cullen B R. Molecular basis of latency in pathogenic human viruses. Science. 1991;254:815–820. doi: 10.1126/science.1658933. [DOI] [PubMed] [Google Scholar]
- 21.Geleziunas R, Miller M D, Greene W C. Unraveling the function of HIV type 1 Nef. AIDS Res Hum Retroviruses. 1996;12:1579–1581. doi: 10.1089/aid.1996.12.1579. [DOI] [PubMed] [Google Scholar]
- 22.Greenway A, Azad A, Mills J, McPhee D. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J Virol. 1996;70:6701–6708. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrich D, Ulich C, Garcia-Martinez L F, Gaynor R. Tat is required for efficient HIV-1 reverse transcription. EMBO J. 1997;17:1224–1235. doi: 10.1093/emboj/16.6.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauber J, Bouvier M, Malim M H, Cullen B R. Phosphorylation of the rev gene product of human immunodeficiency virus type 1. J Virol. 1988;62:4801–4804. doi: 10.1128/jvi.62.12.4801-4804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinziger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Höglund S, Ohagen A, Lawrence K, Gabuzda D. Role of vif during packing of the core of HIV-1. Virology. 1994;201:349–355. doi: 10.1006/viro.1994.1300. [DOI] [PubMed] [Google Scholar]
- 27.Jacqué J-M, Mann A, Enslen H, Sharova N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimptom J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar S, Orsini M J, Lee J C, McDonnell P C, Debouck C, Young P R. Activation of the HIV-1 long terminal repeat by cytokines and environmental stress requires an active CSBP/p38 MAP kinase. J Biol Chem. 1996;271:30864–30869. doi: 10.1074/jbc.271.48.30864. [DOI] [PubMed] [Google Scholar]
- 30.Lavoie J N, L’Allemain G, Brunet A, Müller R, Pouysségur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 31.Luo T, Downing J R, Garcia J V. Induction of phosphorylation of human immunodeficiency virus type 1 Nef and enhancement of CD4 downregulation by phorbol myristate acetate. J Virol. 1997;71:2535–2539. doi: 10.1128/jvi.71.3.2535-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Van de Woude G F, Ahn N G. Transformation of mammalian cells by constitutively activated MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 33.McCune J M. Viral latency in HIV disease. Cell. 1995;82:183–188. doi: 10.1016/0092-8674(95)90305-4. [DOI] [PubMed] [Google Scholar]
- 34.Meloche S. Cell cycle reentry of mammalian fibroblasts is accompanied by the sustained activation of p44mapk and p42mapk isoforms in the G1 phase and their inactivation at the G1/S transition. J Cell Physiol. 1995;163:577–588. doi: 10.1002/jcp.1041630319. [DOI] [PubMed] [Google Scholar]
- 35.Pagès G, Lenormand P, L’Allemain G, Chambar J-C, Meloche S, Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perkins G R, Marvel J, Collins M K. Interleukin 2 activates extracellular signal-regulated protein kinase 2. J Exp Med. 1993;178:1429–1434. doi: 10.1084/jem.178.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popik W, Hesselgesser J E, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popik W, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rey I, Taylor-Harris P, van Erp H, Hall A. R-ras interacts with rasGAP, neurofibromin and c-raf but does not regulate cell growth or differentiation. Oncogene. 1994;9:685–692. [PubMed] [Google Scholar]
- 40.Rocancourt D, Bonnerot C, Jouin H, Emerman M, Nicolas J-F. Activation of a β-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990;64:2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai H, Shibata R, Sakuragi J, Sakuragi S, Kawamura M, Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1992;67:1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sale E M, Atkinson P G P, Sale G J. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmid-Antomarchi H, Benkirane M, Briettmayer V, Husson H, Ticchioni M, Devaux C, Rossi B. HIV induces activation of phosphatidylinositol 4-kinase and mitogen-activated protein kinase by interacting with T cell CD4 surface molecules. Eur J Immunol. 1996;26:717–720. doi: 10.1002/eji.1830260331. [DOI] [PubMed] [Google Scholar]
- 44.Schubert U, Schneider T, Henklein P, Hoffmann K, Berthold E, Hauser H, Pauli G, Porstmann T. Human-immunodeficiency-virus-type-1-encoded Vpu protein is phosphorylated by casein kinase II. Eur J Biochem. 1992;204:875–883. doi: 10.1111/j.1432-1033.1992.tb16707.x. [DOI] [PubMed] [Google Scholar]
- 45.Schubert U, Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J Virol. 1994;68:2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 47.Smith B L, Krushelnycky B W, Mochly-Rosen D, Berg P. The HIV Nef protein associates with protein kinase C theta. J Biol Chem. 1996;271:16753–16757. doi: 10.1074/jbc.271.28.16753. [DOI] [PubMed] [Google Scholar]
- 48.Terwilliger E F, Langhoff E, Gabuzda D, Zazopoulos E, Haseltine W. Allelic variation in the effects of the nef gene on replication of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1991;88:10971–10975. doi: 10.1073/pnas.88.23.10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veronese F D, Copeland T D, Oroszlan S, Gallo R C, Sarngadharan M G. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988;62:795–802. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Schwedler U, Song J, Aiken C, Trono D. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whalen A M, Galasinski S C, Shapiro P S, Nahreini T S, Ahn N G. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winston B W, Lange-Carter C A, Gardner A M, Johnson G L, Riches D W H. Tumor necrosis factor α rapidly activates the mitogen-activated protein kinase (MAPK) cascade in a MAPK kinase kinase-dependent, c-Raf-1-independent fashion in mouse macrophages. Proc Natl Acad Sci USA. 1995;92:1614–1618. doi: 10.1073/pnas.92.5.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X, Goncalves J, Gabuzda D. Phosphorylation of Vif and its role in HIV-1 replication. J Biol Chem. 1996;217:10121–10129. doi: 10.1074/jbc.271.17.10121. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Gabuzda D. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J Biol Chem. 1998;273:29879–29887. doi: 10.1074/jbc.273.45.29879. [DOI] [PubMed] [Google Scholar]
- 55a.Yang, X., and D. Gabuzda. Unpublished data.
- 56.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 57.Zack J A, Haislip A M, Krogstad P, Chen I S Y. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zazopoulos E, Haseltine W A. Effect of nef alleles on replication of human immunodeficiency virus type 1. Virology. 1993;194:20–27. doi: 10.1006/viro.1993.1230. [DOI] [PubMed] [Google Scholar]