ABSTRACT
We report the screening and enrollment process for a phase I vaccine trial in Masaka, Uganda that investigated the safety and immunogenicity of a self-amplifying SARS-CoV-2 RNA vaccine amongst individuals with and without antibodies to SARS-CoV-2. Participant screening and enrollment were conducted between December 2021 and April 2022. Individuals were eligible if they were aged between 18 and 45 years, healthy, and never vaccinated against COVID-19. SARS-CoV-2 antibody status was determined using two point-of-care rapid tests, i.e. Multi G (MGFT3) and Standard Q (Standard Q COVID-19 IgM/IgG Plus). Data were entered and managed in OpenClinica. Analyses were performed and presented descriptively. A total of 212 individuals were screened and 43(20.3%) enrolled. The most common reasons for exclusion were ≥ grade 1 laboratory abnormalities (39, 18.4%), followed by discordant SARS-CoV-2 antibody results (23, 10.9%). While the first 38 participants were quickly enrolled over a period of 9 weeks, it took another 9 weeks to enroll the remaining five, as antibody negative participants became scarce during the surge of the Omicron variant. The SARS-CoV-2 antibody positivity rate was determined to be 60.8% and 84.4% in each half of the 18 months of screening respectively. The mean age (±Standard Deviation, SD) of screened and enrolled participants was 27.7 (±8.1) and 30.2 (±8.3) years respectively. We demonstrated that it is feasible to successfully screen and enroll participants for COVID-19 vaccine trials in Uganda in the time of a pandemic. Our experiences may be useful for investigators planning to undertake similar work in Africa.
KEYWORDS: COVID-19, SARS-CoV-2, vaccine, self-amplifying RNA, screening, enrolment
Introduction
Several vaccines based on mRNA, adenovirus vectors, and more recently, recombinant technologies are now available and are contributing to the control of the COVID-19 pandemic.1,2 The rate at which these vaccines moved from development to roll-out was unprecedented and has revolutionalised vaccine research. Apart from the phase 3 investigation of the ChAdOx-1 adenoviral vaccines in South Africa,3,4 most of these vaccines were investigated in other settings besides Africa. The opinion that Africa would be used as testing ground by western researchers to assess unsafe vaccines caused concern5; this having been exacerbated by the utterances of two researchers that shared the regrettable opinion that Africa was tailor made for COVID-19 vaccine research given its inadequate health infrastructure.6
As time has evolved, it has become clearer that it was necessary for Africa to participate in COVID-19 vaccine research. The reasons are many, including, that vaccine responses may vary amongst people based on genetic variations, as well as geographical differences.7 It is also possible that vaccine uptake in Africa would be higher if the vaccines utilized were investigated on the continent. A survey done among health workers in Uganda, Sierra Leone, and DRC found that vaccine uptake may be influenced by where the vaccine was developed and where clinical trials were conducted.8
We conducted the first COVID-19 vaccine trial in Uganda, to investigate the safety and immunogenicity of a self-amplifying ribonucleic acid (saRNA) vaccine encoding the S glycoprotein of SARS-CoV-2, the causative agent of COVID-19, in SARS-CoV-2 seronegative and seropositive Ugandan participants (COVAC Uganda trial). The saRNA vaccine platform is a new one, and if found to be safe and effective, may offer advantages compared to other traditional RNA vaccine platforms. These advantages include the opportunity to use smaller doses of a vaccine that amplifies itself intracellularly, thereby enabling vaccination of many more people compared to other RNA vaccines, as well as potentially lower costs of production. This vaccine platform developed at Imperial College London has demonstrated very good safety and immunogenicity profiles in non-human primates,9,10 and humans in a Phase 1 clinical trial “COVAC1,” conducted in the United Kingdom.11
We report the experiences of screening and enrollment for the COVAC Uganda trial. These experiences may inform future vaccine trials for COVID-19 and other infectious diseases, particularly those conducted in Africa.
Methods
Study design and setting
The study was a single center non-randomized phase 1 clinical trial to evaluate the safety and immunogenicity of LNP-nCOV saRNA-02 administered at 0 and 4 weeks in 42 individuals, seronegative (n = 21) and seropositive (n = 21) for SARS-CoV-2 antibodies. The study was conducted at MRC/UVRI & LSHTM Uganda Research Unit site in Masaka, approximately 120 km to the southwest of Kampala, Uganda’s capital.
Study population
The study population consisted of healthy adults (18–45 years), who were willing and able to provide informed consent, use a highly effective method of contraception (female participants)/avoid impregnating female partners (male participants) from screening until 18 weeks after last injection, avoid all other vaccines (including authorized COVID-19 vaccines) from within 4 weeks before the first injection through to 4 weeks after the second injection, comply with visit schedule, complete vaccine diaries and provide samples, and grant authorized persons access to their trial-related and other medical records. Individuals were excluded from the study if they were pregnant or lactating or had any of the following: a significant clinical history, abnormal physical finding on clinical examination, or presence of a disease that was active; a history of anaphylaxis or angioedema; active SARS-CoV-2 infection; indeterminate SARS-CoV-2 serostatus; history of severe or multiple allergies to drugs or pharmaceutical agents; history of severe local or general reaction to vaccination; previous receipt of an experimental or approved vaccine against COVID-19; receipt of any immunosuppressive agents within 18 weeks of screening; presence of antibodies to hepatitis C; presence of antibodies to HIV; ≥grade 1 abnormalities in routine laboratory parameters; participation in another clinical trial with an investigational drug/device; and immunization within 28 days of screening.
Community mobilization and recruitment strategies
The study Community Liaisons Team organized meetings with several communities “gatekeepers,” including the Community Advisory Board, village leaders, village health teams, and the Masaka district COVID-19 task force to provide information about the study and discuss possible approaches to recruitment. The members who attended these meetings came from different communities, including urban (around the city center) and semi-urban/rural settings (outside the city center). Subsequently, research assistants approached potential participants, initially focusing on those that came from settings near the research site, and invited them to the study site to attend general information sessions on clinical research and evaluation of drugs/vaccines, and to explore their interest in volunteering for research. These sessions were held in groups. Those that expressed interest to participate in clinical research were registered and invited to an individual session during which study staff provided specific information about the COVAC Uganda clinical trial. Subsequently, interested individuals were invited to undergo assessment for the trial.
To increase the likelihood of identifying SARS-CoV-2 seropositive individuals, we also approached persons who were known to have been diagnosed and treated for COVID-19. These individuals were identified from the Masaka district registry for COVID-19 patients managed under the home-based care model. SARS-CoV-2 seronegative individuals were recruited from the general population in Masaka city, with screening targeting individuals who had no known history of testing SARS-CoV-2 positive or COVID-19 diagnosis. With the onset of the SARS-CoV-2 Omicron variant wave in Uganda around January 2022, recruitment efforts were moved to more rural and sparsely populated communities in Masaka district to increase likely of identifying SARS-CoV-2 seronegative individuals. As noted previously, community “gatekeepers” in these settings had already been made aware of the trial.
Screening procedures
The screening procedures had a window of 6 weeks for each participant, during which consent was obtained, medical clinical assessment done, and protocol specified laboratory tests done.
Informed consent
Participants were provided with the following information using the study consent form which was available in English and Luganda:1) Purpose of the study, 2) why the participant was being asked to participate, 3) how the vaccine is made and previous research using the same candidate vaccine, 4) what was expected of study participants, 5) trial design, schedules, and study procedures, 6) potential side effects of the vaccine and risks/benefits of participating in the study, 7) data and specimen collection and storage, and 8) contact information for key study personnel and the chairperson of the Research and Ethics Committee in case one had questions or concerns. Participants were given the opportunity to ask questions, which were answered by study staff. A 10-question test of understanding was administered, and participants proceeded to sign the consent form if they answered eight or more questions correctly. Participants who failed to achieve this mark in the first instance were given an opportunity to go over the study information again and then repeat the test once. For illiterate participants, an impartial witness was required to be present throughout the consenting process and to sign the consent from. Contact information including home/work addresses and telephone contacts were collected at screening for purposes of locating participants if needed.
Eligibility assessment
A study clinician assessed the eligibility of each participant using their demographics, medical history including COVID-19 vaccination history, contraceptive use and other concomitant medications, physical examination, and laboratory test data. Laboratory tests included: a urine pregnancy test in female participants of childbearing potential, a full blood count, biochemistry (Liver function tests, creatinine, non-fasting blood sugar), hepatitis C serology, HIV serology, urinalysis (glucose, blood, white blood cells, nitrite, and protein) and SARS-CoV-2 serology. Participants with grade 1 hematology, biochemistry, or urinalysis abnormalities at the initial screening visit had the tests repeated once and could be enrolled into the study if the repeat result was normal. Grading was done using the FDA toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials.12
The kits used in this study for HIV testing (Abbott Determine HIV1/2, Manufactured by Abbott Diagnostics Medical Co. Ltd, 357 Matsuhidai, Matsudo-shi, Chiba, 270–2214, Japan; and HIV 1/2 STAT-PAK Manufactured by CHEMBIO DIAGNOSTIC SYSTEMS, INC. 3661 Horseblock Road Medford, New York 11,763 USA; and SD HIV 1/2 3.0 SD Standard Diagnostics, INC India) are able to detect HIV-1 and 2. The study intended to enroll healthy adults, and the trial clinicians had permission to deploy any tests that they may have considered necessary to exclude any suspected illnesses. We however do not rule out the possibility that some conditions, including inherited and acquired immune suppressing conditions, could have been missed. We consider this highly unlikely though given that these participants were adults in general good health.
SARS-CoV-2 serology
Blood obtained by venepuncture was tested using two SARS-CoV-2 serology rapid test kits: i) Multi G (MGFT3), Multi-G bvba 166 Lange Leemstraat 2018 Antwerpen – Belgium; ii) Standard Q (Standard Q COVID-19 IgM/IgG Plus), SD Biosensor, Inc., South Korea. The test kits detect the presence of IgM and IgG antibodies to SARS-CoV-2 in human serum, plasma, or whole blood and were shown to have ≥ 98% specificity and sensitivity in a validation performed in Uganda (unpublished data).
Participants were assigned SARS-CoV-2 seropositive status if both test kits showed the presence of SARS-CoV-2 antibodies. Similarly, participants were assigned SARS-CoV-2 seronegative status if both test kits showed absence of SARS-CoV-2 antibodies. The serostatus of a participant was considered indeterminate if they tested seropositive on one test kit and seronegative on another. Such participants were excluded from the trial (Figure 1). We used two tests in parallel to increase on performance, especially to ensure we also capture early infection since singly, these rapid diagnostic tests performed less well on IgM alone.13 Our sensitivity, specificity, and accuracy were determined for combined IgM/IgG (either or both IgM and igG).
Figure 1.
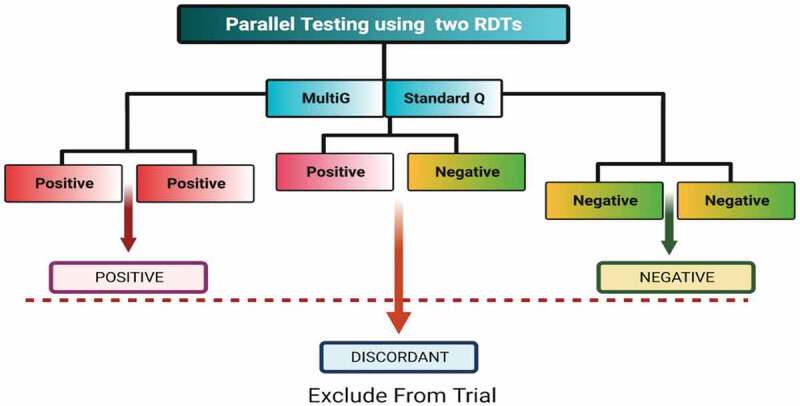
Algorithm for SARS-CoV-2 serology screening in the COVAC Uganda trial.
Enrolment procedures
At the enrollment visit, a study clinician asked about any new medical conditions or medications since the screening visit and, for female participants, any changes in contraceptive use, and conducted a physical examination. Enrollment was deferred for participants found to have a temperature over 37.5°C and those who had COVID-19-like symptoms. Such participants were investigated, managed, rescreened if the screening window had closed or if they were confirmed to have SARS-CoV-2 infection, and enrolled at a later date. Blood for immunogenicity assays was collected, and for female participants of childbearing potential, a urine pregnancy test was performed and a negative result confirmed prior to enrollment.
Ethical considerations and regulatory approval
The study was conducted in compliance with the protocol, Standard Operating Procedures, Good Participatory Practice, and International Conference on Harmonization-Good Clinical Practice (ICH-GCP) guidelines and applicable regulatory requirements. The study protocol and informed consent documents were reviewed and approved by the Uganda Virus Research Institute Research Ethics Committee (Ref: GC/127/829), the National Drugs Authority (Ref: CTA 0186), the Uganda National Council for Science and Technology (Ref: HS164/ES), the London School of Hygiene and Tropical Medicine Research Ethics Committee (Ref: 26510), and Imperial College London Research Ethics Committee (Ref: 21IC6703). All study procedures were conducted after obtaining written informed consent. Participants diagnosed with HIV infection and other chronic ailments were counseled and referred to local HIV care providers for comprehensive care and management. We required participants to avoid all other vaccines (including COVID-19 vaccines) from within 4 weeks before the first injection through to 4 weeks after the second injection. Those that wanted to receive ministry of health recommended vaccines thereafter were given adequate information and referral.
Data analysis
Data initially recorded on standard case report forms were entered and managed in REDCap and analyzed in STATA version 17.0 (StataCorp, College Station, TX). We used percentages and means (standard deviations) or medians (interquartile ranges) to summarize categorical and continuous data, respectively. Stratification by the study arms of some variables and cross tabulations was done. Proportions of participants screened, enrolled, and followed up were measured. Proportions of reasons for not enrolling a screened participant were also presented.
Results
The screening and enrollment profile is summarized in Figure 2.
Figure 2.
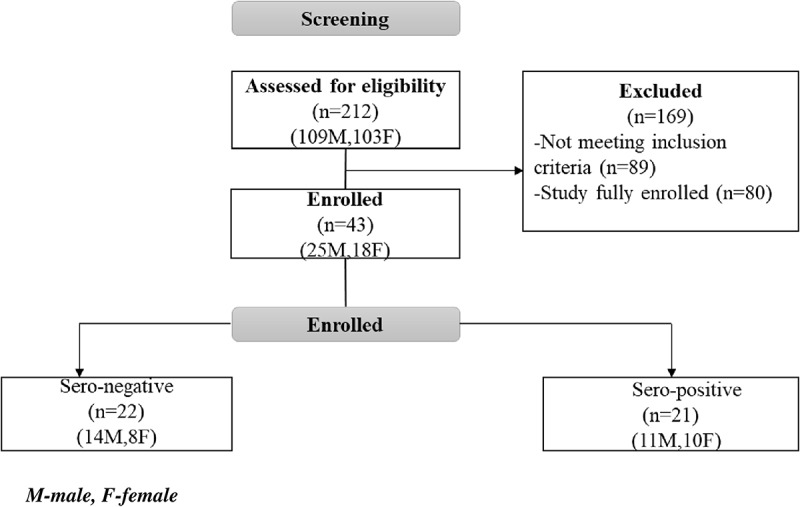
COVAC Uganda screening and enrollment profile.
While the study intended to enroll 42 participants, pre-vaccination samples for one SARS-CoV-2 seronegative participant were not obtained, due to a clinical error. As this participant would not contribute to the primary immunogenicity analyses, a decision was made to recruit an extra participant in the SARS-CoV-2 seronegative group. Therefore, a total of 43 participants were enrolled in the study.
Reasons for non-enrollment
The most common reasons for non-enrollment were grade 1 and above laboratory abnormalities (39, 18.4%), indeterminate SARS-CoV-2 serostatus (23, 10.9%). Eighty potentially eligible participants were not enrolled because they were screened later in the trial when the SARS-CoV-2 positive antibody arm had been fully enrolled, i.e., the study was only looking for antibody negative participants at this point to complete enrollment. The reasons for non-enrollment are summarized in Figure 3.
Figure 3.
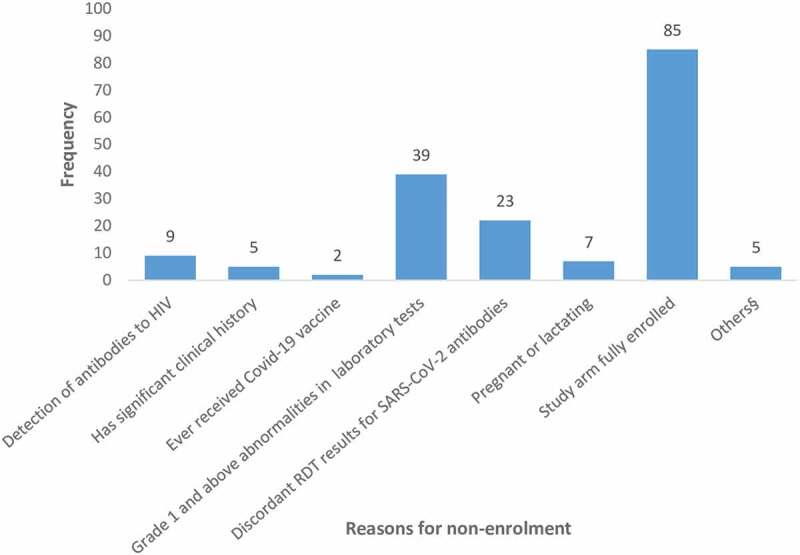
Graph showing reasons for screen failure (some participants had more than one reason for screen failure).
Characteristics of screened and enrolled participants
The mean age (±SD) of screened and enrolled participants was 27.7 (±8.1) and 30.2 (±8.3) years respectively. Majority of screened and enrolled participants belonged to the youngest age category (18–24 years), i.e., 97 (45.8%) and 15 (34.9%), respectively. Slightly more males than females were screened and enrolled in the trial [109(51.4%) males vs 103(48.6%) females (Screened); 25(58.1%) males vs 18(41.9%) females (enrolled)].
Use of injectable contraceptives and implant were the most popular methods of birth control among those screened (35, 16.5% for both), while implant was most popular among those enrolled 11 (25.6%). Thirty-eight (17.9%) of screened participants had preexisting chronic ailments, while these were present among 10 (23.3%) enrolled participants amongst whom these were not found important to preclude enrollment. Characteristics of the screened and enrolled participants are summarized in Table 1.
Table 1.
Characteristics of participants screened and enrolled in the COVAC Uganda trial.
| Variable | Screened N (%) | Enrolled |
||
|---|---|---|---|---|
| All N (%) |
SARS-CoV-2 seropositive N (%) |
SARS-CoV-2 seronegative N (%) |
||
| Number | 212 | 43 | 21 | 22 |
| Mean age in years (SD) | 27.7 (8.1) | 30.2 (8.3) | 33.0 (7.9) | 27.5 (7.8) |
| Age group | ||||
| 18–24 | 97(45.8) | 15(34.9) | 4(19.1) | 11(50.0) |
| 25–34 | 64(30.2) | 13(30.2) | 6(28.6) | 7(31.8) |
| 35–45 | 51(24.1) | 15(34.9) | 11(52.4) | 4(18.2) |
| Sex | ||||
| Male | 109(51.4) | 25(58.1) | 11(52.4) | 14(63.6) |
| Female | 103(48.6) | 18(41.9) | 10(47.6) | 8(36.4) |
| Contraceptive use for female or partner for males¥ | ||||
| Injectable | 35(32.7) | 9(34.6) | 5(35.7) | 4(33.3) |
| Implant | 35(32.7) | 11(42.3) | 7(50.0) | 4(33.3) |
| IUD | 8(7.5) | 1(13.9) | 0(0.0) | 1(8.3) |
| Oral pill | 3(2.8) | 1(13.9) | 1(7.1) | 0(0.0) |
| Other | 26(24.3) | 4(15.4) | 1(7.1) | 3(25.0) |
| None | 105(49.5) | 17(39.5 | 7(33.3) | 10(45.5) |
| Ever smoked? | ||||
| Never smoked | 203 (95.8) | 41(95.4) | 19(90.5) | 22(100.0) |
| Yes, current smoker | 3 (1.4) | 2(4.6) | 2(9.50) | 0(0.0) |
| Yes, but stopped | 6 (2.8) | 0(0.0) | 0(0.0) | 0(0.0) |
| Pre-existing chronic ailments | ||||
| Yes | 38 (17.9) | 10(23.3) | 8(38.1) | 2(9.1) |
| No | 174 (82.1) | 33(76.7) | 13(61.9) | 20(90.9)) |
| SARS-CoV-2 serostatus | ||||
| Negative | 45(21.2) | 22 (51.2) | - | 22 (100) |
| Positive | 144 (67.9) | 21 (48.8) | 21 (100) | - |
| Indeterminate | 23(10.9) | 0 (0.0) | - | - |
Rate of enrollment
While the first 38 participants were enrolled over a period of 9 weeks, a further 9 weeks were required to enroll the last 5 SARS-CoV-2 seronegative participants (Figure 4). The SARS-CoV-2 antibody positivity rate was 60.8% in the first 9 weeks of screening, and 84.4% in the last 9 weeks.
Figure 4.
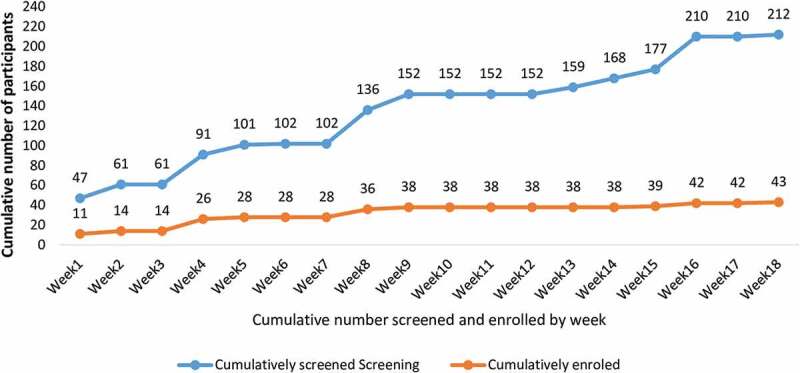
Comparison of cumulative screening and enrollment by weeks between (13th Dec 2021 and 13th April 2022).
Discussion
To our knowledge, COVAC Uganda is the first COVID-19 vaccine trial conducted in Uganda and also the first trial of a self-amplifying ribonucleic acid (saRNA) vaccine for COVID-19 in Africa. This study provides an opportunity to investigate responses to the new saRNA vaccine platform in an African population. Besides this, the need to investigate vaccines in diverse populations cannot be over emphasized, as vaccine responses may vary in different populations and environments.14–16 Africa has participated in identifying SARS-CoV-2 variants including variants of concern contributing to global surveillance and disease control responses.17 This capacity should preferably feed into vaccine development on the continent, but unfortunately, this opportunity has not yet been harnessed.18 Encouragingly, a mRNA vaccine hub was launched in 2022, which includes various countries in Africa.19 The hub was devised as an incubator for the development of Africa’s first COVID-19 vaccine, starting with crafting a unique version of Moderna’s COVID-19 vaccine. This technology would also be applied to the research of other vaccine candidates against infections such as malaria, tuberculosis, HIV, and other diseases that currently affect the region. Our study, a phase one trial in Africa with the investigational product being used in this population for the first time, required involvement of healthy adults. Our study design was based on the UK-based COVAC1 trial, which also had recruited participants aged 18–45 years.11 While this age group is quite frequently used in clinical trials in this phase, we are of the view that adults up to the age of 59 may be enrolled in similar trials if they are adequately assessed and found to be healthy.
In general, screening and recruitment of participants into this trial was successful with accrual achieved in a relatively short time. This was partly due to the low COVID-19 vaccination coverage in Uganda at the time of the study with approximately 50% having received at least 1 dose and 19% received fully dose,20 and the high SARS-CoV-2 seropositivity rate (68%) which ensured quick accrual of the SARS-CoV-2 seropositive group.
On the other hand, identification of SARS-CoV-2 seronegative participants was challenging particularly in the last half of the recruitment period where SARS-CoV-2 seropositivity rate was highest at 84.4%, compared to 60.8% in the first half (Table 2). Whereas the first 17 SARS-CoV-2 seronegative participants were recruited over a 9-week period, a similar duration was required to recruit the last five participants, with many potential participants screened out due to SARS-CoV-2 seropositivity. This can be attributed to the emergence of the highly transmissible Omicron variant in January 2022 and third COVID-19 wave in the country, that resulted in a substantial increase in the number SARS-CoV-2 infections.21,22 Additionally, the SARS-CoV-2 serostatus for 11% of screened individuals could not be determined as their Multi-G and Standard Q test results were discordant. As explained above, to increase the likelihood of identifying SARS-CoV-2 seronegative individuals, we focused our recruitment efforts away from the urban setting where most cases of COVID-19 were identified to rural communities with fewer cases. This experience strongly suggests that it may not be feasible to enroll individuals without prior exposure to SARS-CoV-2 in future COVID-19 vaccine studies.
Table 2.
Results of SARS-CoV-2 antibody testing across two time intervals.
| SARS-CoV-2 antibody results | First 9 weeks of screening (13th December 2021 to 14th February 2022) | Last 9 weeks (14th February 2022 to 13th April 2022) |
|---|---|---|
| Negative | 36 | 9 |
| Positive | 90 | 54 |
| Discordant | 22 | 1 |
| Total | 148 | 64 |
| Positivity Rate (%) | 60.8 | 84.4 |
Grade 1 and above laboratory abnormalities were a common reason for screen-failure in this trial. This study excluded all participants with grade 1 abnormalities irrespective of clinical significance. There is a suggestion that grading alone should not exclude participants from research participation without considering clinical significance.23 Another key issue to consider is that this study used the FDA toxicity grading scale,12 whose ranges may not be perfectly suited to this population. A study previously carried out in Uganda showed that 83% of participants that had been excluded from a HIV vaccine trial would have been enrolled if local ranges had been considered.24
We hereby summarize the challenges experienced during the set-up, screening, and enrollment for this study, and approaches that enabled us to overcome these in Table 3.
Table 3.
Challenges and mitigation strategies during screening and enrollment.
| Challenge | Solution/mitigation strategy |
|---|---|
| Multiple regulatory authorities’ approval was required prior to trial initiation. Regulatory approval was required from Uganda Virus Research Institute Research Ethics Committee (UVRI REC), the National Drugs Authority (NDA), the Uganda National Council for Science and Technology (UNCST), the London School of Hygiene and Tropical Medicine Research Ethics Committee (LSHTM REC), and Imperial College London Research Ethics Committee (ICL REC) | Prior engagements were made by the trial investigators with these authorities to make them aware that plans were under way to conduct this trial, and to expect study submissions. The site initially made application to the UVRI REC, followed by the NDA. On submission to NDA, request for joint review from the Ugandan authorities was made, Unfortunately, NDA notified the site that joint review was no longer possible since UVRI REC review had commenced already. Joint review would require that submissions are made to all Ugandan authorities at the same time, so that review could be done at the same time. This was a learning experience for the site. |
| Resentment of COVID-19 vaccines from the community. While this trial was able to enroll adequate numbers as had been planned, the experience from the community engagement activities showed that various potential participants who came for pre-screening sessions, did not return for subsequent visits including screening. The numbers may not have been captured for this publication, but research staff noticed that potential participants had more questions than usually asked for other trials, and a considerable number of attendees was not able to come for subsequent sessions. Many of these questions were derived from information received via social media. | The team endeavored to offer as much information as possible to inform participant decisions, addressed all questions asked as informed by research data including that from the UK version of this trial.11 The site has also established a very comprehensive community engagement strategy at the center of which is a competent Community Advisory Board (CAB), that supports conduct of clinical trials at the unit. The functions and operations of this community advisory board have been described elsewhere.24 |
| Evolution of the outbreak, with the emergency of variants of concern. As described in the text, the emergency of the Omicron variant toward the end of 2021 and beginning of 2022 made it extremely challenging to enroll SARS-CoV-2 antibody negative participants as the positivity rate increased rapidly (60.8% to 84.4%, based on data from this study). | The site had experience in successful community engagement prior to initiation of screening. When the challenge surfaced, it was facile to shift focus from the urban settings where initial screening had focused, to the rural settings where it was assumed that positivity rate would be lower given reduced mixing of people. |
| High screen outs due to grade 1 laboratory parameters. As already noted, the trial used the FDA toxicity grading scale, which had a number of parameters, especially hematological parameters graded as grade 1, that otherwise were in the normal range if assessed using local reference ranges. | Although this was not done for this trial, the solution to this would have been designing the protocol to allow for consideration of clinical significance in the assessment, especially for low grade laboratory parameters. The more useful and durable recommendation is for toxicity grading tables to be modified or created to grade laboratory parameters based on the populations in context. |
| Conflicting confirmation of serological results for participants screened. As indicated in the methods section, serological tests involved use of two kits, i.e., Multi G (MGFT3), and Standard Q (Standard Q COVID-19 IgM/IgG Plus). Unfortunately, 23(10.9%) of participants returned discordant results. By the time the trial was conducted, these were the only two kits that had been assessed and validated to be used in Uganda. The other challenge ensuing from this was communicating to participants with discordant results if they had been exposed to SARS-CoV-2 before or not. |
There was no practicable solution for this during the trial, apart from screening more participants to cover for the discordant results. The ideal solution would be encouraging more quick manufacture and testing of kits, including from local manufacturers where possible. Concerning communication of discordant results, the participants were informed that due to the challenge of only having two validated kits, we could not conclusively establish the exposure status of these participants. These participants were not enrolled in the trial, but were encouraged to go for routine vaccination at the government facilities where vaccines were available. |
This study has some limitations. First, we could not confirm the vaccination status of participants as the SARS-CoV-2 antibody testing kits do not distinguish between antibodies due to previous infection and antibodies elicited by a vaccine. Hence, it is possible that some vaccinated individuals may have been enrolled if they falsely reported that they had never been vaccinated. Second, the 42-day screening window was long such that it was possible for a person who was SARS-CoV-2 seronegative at screening to have seroconverted by the time of the enrollment visit. Moreover, we did not perform a repeat SARS-CoV-2 antibody test at the enrollment visit. However, samples stored from the enrollment visit are available and will be analyzed to determine SARS-CoV-2 serostatus at this visit, to aid interpretation of assays completed after enrollment. Thus, it is possible that the number of SARS-CoV-2 seronegative participants that will be reported from the main analysis of this work may be lower than that reported here.
In summary, we demonstrated that it is feasible to successfully screen and recruit participants for COVID-19 vaccine trials in Uganda. Our experiences, including handling of identified challenges and recommendations may be useful for investigators planning to undertake similar work in Africa.
Acknowledgments
The authors would like to thank all study participants, the MRC/UVRI & LSHTM Uganda Masaka Community Advisory Board, and all members of the study team at the MRC/UVRI & LSHTM Uganda Research Unit.
Funding Statement
This research is funded by the Department of Health and Social Care using UK Aid funding and is managed by the Engineering and Physical Sciences Research Council [EPSRC, grant number: EP/R013764/1]. The views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health and Social Care.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data from this trial will become available after final analyses have been done and reported, and details on how this data can be accessed will be shared in the final trial publication.
References
- 1.Korang SK, von Rohden E, Veroniki AA, Ong G, Ngalamika O, Siddiqui F, Juul S, Nielsen EE, Feinberg JB, Petersen JJ, et al. Vaccines to prevent COVID-19: a living systematic review with trial sequential analysis and network meta-analysis of randomized clinical trials. PLoS One. 2022;17:e0260733. doi: 10.1371/journal.pone.0260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farhud DD, Zokaei S.. A brief overview of COVID-19 vaccines. Iran J Public Health. 2021;50:i–8. doi: 10.18502/ijph.v50i7.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekker L-G, Garrett N, Goga A, Fairall L, Reddy T, Yende-Zuma N, Kassanjee R, Collie S, Sanne I, Boulle A, et al. Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study. Lancet. 2022;399(10330):1141–53. doi: 10.1016/S0140-6736(22)00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massinga Loembé M, Nkengasong JN. COVID-19 vaccine access in Africa: global distribution, vaccine platforms, and challenges ahead. Immunity. 2021;54(7):1353–62. doi: 10.1016/j.immuni.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samarasekera U. Feelings towards COVID-19 vaccination in Africa. Lancet Infect Dis. 2021;21:324–324. doi: 10.1016/S1473-3099(21)00082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Monde . Coronavirus: des spécialistes français s’excusent après leurs propos sur un test de vaccin en Afrique. 2020. https://www.lemonde.fr/afrique/article/2020/04/06/coronavirus-des-specialistes-francais-s-excusent-apres-leurs-propos-sur-un-test-de-vaccin-en-afrique_6035692_3212.html.
- 7.Garand M, Cai B, Kollmann TR. Environment impacts innate immune ontogeny. Innate Immun. 2016;23:3–10. doi: 10.1177/1753425916671018. [DOI] [PubMed] [Google Scholar]
- 8.Whitworth HS, Kitonsa J, Kasonia K, Tindanbil D, Kafeero P, Bangura J, Nije Y, Tetsa Teta D, Greenwood B, Kavunga-Membo H, et al. COVID-19 vaccine acceptability among healthcare facility workers in Sierra Leone, the democratic Republic of Congo and Uganda: a multi-centre cross-sectional survey. Int J Public Health. 2022;67:1605113. doi: 10.3389/ijph.2022.1605113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappaport AR, Hong S-J, Scallan CD, Gitlin L, Akoopie A, Boucher GR, Egorova M, Espinosa JA, Fidanza M, Kachura MA, et al. Low-dose self-amplifying mRNA COVID-19 vaccine drives strong protective immunity in non-human primates against SARS-CoV-2 infection. Nat Commun. 2022;13:3289. doi: 10.1038/s41467-022-31005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruggi G, Mallett CP, Westerbeck JW, Chen T, Lofano G, Friedrich K, Qu L, Sun JT, McAuliffe J, Kanitkar A, et al. A self-amplifying mRNA SARS-CoV-2 vaccine candidate induces safe and robust protective immunity in preclinical models. Mol Ther. 2022;30:1897–912. doi: 10.1016/j.ymthe.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock KM, Cheeseman HM, Szubert AJ, Libri V, Boffito M, Owen D, Bern H, O’Hara J, McFarlane LR, Lemm NM, et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. EClinicalMedicine. 2022;44:101262. doi: 10.1016/j.eclinm.2021.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA . Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007. Rockville, USA: U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. http://www.fda.gov/cber/guidelines.htm [Google Scholar]
- 13.Lutalo T, Nalumansi A, Olara D, Kayiwa J, Ogwang B, Odwilo E, Watera C, Balinandi S, Kiconco J, Nakaseegu J, et al. Evaluation of the performance of 25 SARS-CoV-2 serological rapid diagnostic tests using a reference panel of plasma specimens at the Uganda virus research institute. Int J Infect Dis. 2021;112:281–7. doi: 10.1016/j.ijid.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garand M, Cai B, Kollmann TR. Environment impacts innate immune ontogeny. Innate Immun. 2017;23(1):3–10. doi: 10.1177/1753425916671018. [DOI] [PubMed] [Google Scholar]
- 15.Nkurunungi G, Zirimenya L, Natukunda A, Nassuuna J, Oduru G, Ninsiima C, Zziwa C, Akello F, Kizindo R, Akello M, et al. Population differences in vaccine responses (POPVAC): scientific rationale and cross-cutting analyses for three linked, randomised controlled trials assessing the role, reversibility and mediators of immunomodulation by chronic infections in the tropics. BMJ Open. 2020;11(2):e040425. doi: 10.1136/bmjopen-2020-040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falahi S, Kenarkoohi A. Host factors and vaccine efficacy: implications for COVID-19 vaccines. J Med Virol. 2022;94(4):1330–5. doi: 10.1002/jmv.27485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen E, Ntoumi F, Hui DS, Abubakar A, Kramer LD, Obiero C, Tambyah PA, Blumberg L, Yapi R, Al-Abri S, et al. Emergence of new SARS-CoV-2 variant of concern omicron (B.1.1.529) - highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts. Int J Infect Dis. 2022;114:268–72. doi: 10.1016/j.ijid.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guleid FH, Oyando R, Kabia E, Mumbi A, Akech S, Barasa E. A bibliometric analysis of COVID-19 research in Africa. BMJ Glob Health. 2021;6(5):e005690. doi: 10.1136/bmjgh-2021-005690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryce E, Ong S. Covid-19 and mRNA technology are helping Africa fix its vaccine problems. Bmj. 2022;377:o1196. doi: 10.1136/bmj.o1196. [DOI] [PubMed] [Google Scholar]
- 20.Ndejjo R, Chen N, Kabwama SN, Namale A, Wafula ST, Wanyana I, Kizito S, Kiwanuka SN, Sambisa W, Tsai LL, et al. Uptake of COVID-19 vaccines and associated factors among adults in Uganda: a cross-sectional survey. BMJ Open. 2023;13(3):e067377. doi: 10.1136/bmjopen-2022-067377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 omicron variant: characteristics and prevention. MedComm. 2021;2(4):838–45. doi: 10.1002/mco2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bbosa N, Ssemwanga D, Namagembe H, Kiiza R, Kiconco J, Kayiwa J, Lutalo T, Lutwama J, Ssekagiri A, Ssewanyana I, et al. Rapid replacement of SARS-CoV-2 variants by delta and subsequent arrival of Omicron, Uganda, 2021. Emerg Infect Dis. 2022;28(5):1021–5. doi: 10.3201/eid2805.220121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Zhang Q, Liu Y, Zhang X, Cheng D, Li A, Chen Y, Zhu X, Su Y, Zhou H. Analysis of the reasons for screening failure in phase I clinical trials in China: a retrospective study of the clinical trials screening process. Ann Transl Med. 2021;9(20):1564. doi: 10.21037/atm-21-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eller LA, Eller MA, Ouma B, Kataaha P, Kyabaggu D, Tumusiime R, Wandege J, Sanya R, Sateren WB, Wabwire-Mangen F, et al. Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLoS One. 2008;3(12):e3919. doi: 10.1371/journal.pone.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this trial will become available after final analyses have been done and reported, and details on how this data can be accessed will be shared in the final trial publication.


