Abstract
Aortic aneurysm and dissection (AAD) are a group of insidious and lethal cardiovascular diseases that characterized by seriously threatening the life and health of people, but lack effective nonsurgical interventions. Alterations in metabolites are increasingly recognized as universal features of AAD because metabolic abnormalities have been identified not only in arterial tissue but also in blood and vascular cells from both patients and animal models with this disease. Over the past few decades, studies have further supported this notion by linking AAD to various types of metabolites such as those derived from gut microbiota or involved in TCA cycle or lipid metabolism. Many of these altered metabolites may contribute to the pathogenesis of AAD. This review aims to illustrate the close association between body metabolism and the occurrence and development of AAD, as well as summarize the significance of metabolites correlated with the pathological process of AAD. This provides valuable insight for developing new therapeutic agents for AAD. Therefore, we present a brief overview of metabolism in AAD biology, including signaling pathways involved in these processes and current clinical studies targeting AAD metabolisms. It is necessary to understand the metabolic mechanisms underlying AAD to provides significant knowledge for AAD diagnosis and new therapeutics for treatment.
Keywords: Metabolites, Aortic aneurysm and dissection, Gut microbes, TCA cycle, Clinical applications.
Introduction
Aortic disease is a large group of vascular diseases with high mortality rates, in addition to coronary heart disease (CHD) and peripheral artery disease1. The incidence of primary aortic diseases, including aortic aneurysm and dissection (AAD), increased from 2.49/100,000 to 2.78/100,000 between 1990 and 2010, with higher mortality rates in older individuals and males2. Excluding underlying genetic alterations, sporadic AAD is associated with smoking, hypertension, old age, and male gender3. Mechanically, the pathogenesis of AAD involves vascular smooth muscle cell (VSMC) phenotype switch, endothelial dysfunction, inflammation, cells apoptosis, and degradation of the extracellular matrix (ECM)4. D-dimer is of great significance in the diagnosis of AAD, which is widely used in clinical practice for AAD risk assessment with their increase suggesting the presence of AAD5. Unfortunately, classical biomarkers can only partially explain the distribution of AAD risk in the general population and numerous metabolic drivers of the AAD risk are underestimated. Additionally, while computed tomography angiography remains the clinical standard for AAD diagnosis and monitoring, they are less suitable for mass screening and large-scale trials6.
Although drug therapy, such as β-adrenergic receptor antagonists and angiotensin receptor type I antagonists, can alleviate the enlargement of aneurysms, no effective medication has been found to prevent the occurrence and progression development of AAD in clinical practice7. This indicates that the current lack of accurate biomarkers and effective targets for the diagnosis and therapy limits the prevention and treatment of AAD8. It is therefore necessary to further explore the potential molecular targets of AAD in order to develop more effective early prevention strategies, thereby reducing the associated morbidity and mortality.
To date, numerous studies have illustrated that metabolites that are involved in vascular injury and repair by interacting with AAD risk factors are the most prominent factors driving AAD. In this context, the potential for reducing AAD mortality and improving prognosis through the use of metabolic therapy is great. Although an increasing number of metabolomics studies, an emerging analysis technique, have linked AAD to different low-molecular-weight-metabolites (LMWMs) 9, 10, there is still a lack of a comprehensive understanding of the metabolic profile of AAD has hampered our understanding of the biology of the hallmark metabolites and their role in AAD pathogenesis. A deeper understanding of the essential function of metabolites in the pathophysiology of AAD may remain crucial for achieving significant breakthroughs in the diagnosis and treatment of AAD. This review summarizes recent advances in this field and aims to provide a review of the existing literature on the relationship between metabolites and AAD, thereby discussing their regulatory role in the development of AAD as well as the current status and future outlook on their potential as diagnostic markers and therapeutic targets.
Metabolites as candidate biomarkers of AAD
Metabolomics is a burgeoning field in the life sciences, the emergence of which is inevitable for life science research11. It utilizes a combination of advanced techniques in analytical chemistry and comprehensive statistical methods to fully characterize the metabolome, focusing on the qualitative and quantitative analysis of LMWMs with a relative molecular weight of less than 1500 in a certain organism or cell12. Metabolites are the ultimate products of gene expression, generated by the action of metabolic enzymes. They reflect variations in the genome, transcriptome, and proteome and indicates the endpoint of the 'omics' cascade. The metabolome interacts with and actively modulates all other 'omics' levels (Figure 1). Although metabolites are small molecules compared to genes or proteins, the importance of metabolites that should not be underestimated is due to the fact that they are commonly present in a given organ elle, cell, organ, biofluid or organism rather than dead cells13.
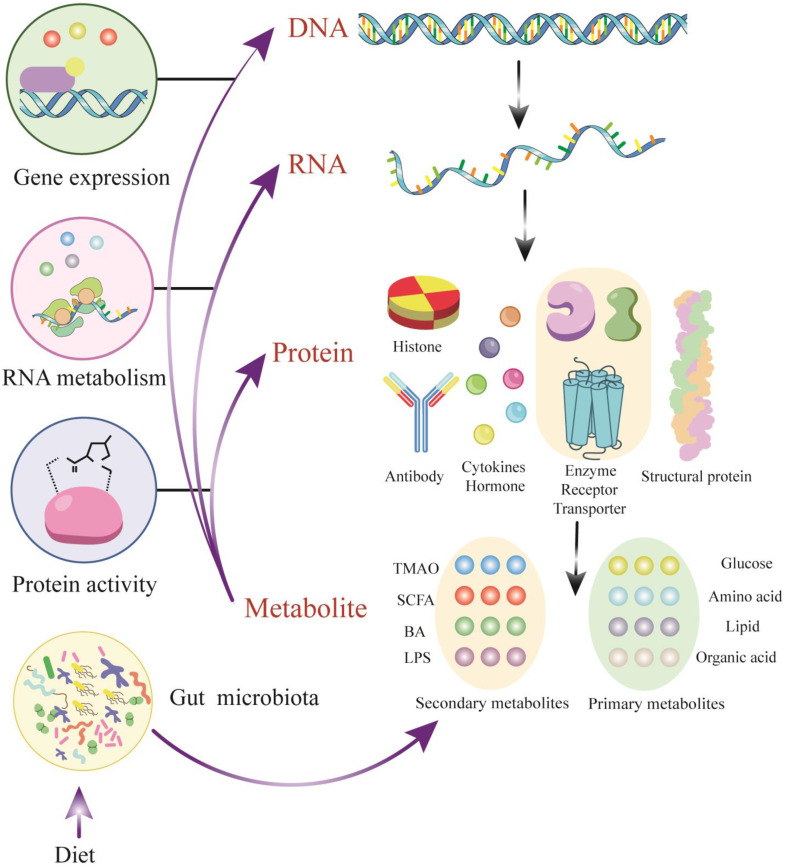
Figure 1.
Metabolites - active modulators of gene and protein activity. DNA transcription and RNA translation yield proteins with a broad range of structure and function. A subset of proteins including enzymes and transporters plays a key role in modulating metabolites, which are derived from endogenous metabolism and exogenous sources such as diet and the microbiome. Metabolites could actively regulate protein activity, serve as signaling molecules for transcription factors and be involved in epigenetic regulation. Abbreviations: TMAO, trimethylamine N-oxide; SCFA, short-chain fatty acid; BA, bile acid; LPS, lipopolysaccharide; DNA, Deoxyribonucleic Acid; RNA, Reibonucleic Acid.
LMWMs in the metabolome contain both endogenous sources from genetics and exogenous sources which mainly including nutrition, drugs, and environment. Endogenous metabolites are highly conserved and represent the collections of similar LMWMs, albeit at different concentrations, in different species14. However, exogenous metabolites are highly variable and mainly reflect dietary and environmental factors, as well as the composition of the microbiome. Both endogenous and exogenous LMWMs fulfill an essential function in normal and pathological processes15. Given that the metabolome is composed of thousands of individual chemicals attaching to diverse chemical classes16, more extensive and precise equipment is needed to ensure the accuracy of metabolome measurements compared with genomics or proteomics. Nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS), typically coupled to gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-mass spectrometry (LC-MS), as well as capillary electrophoresis-mass spectrometry (CE-MS), and ion mobility spectrometrymass spectrometry (IMS-MS), are the most commonly used detection and analysis techniques17.
Recent studies have shown that metabolites do not only maintain normal physiological functions of living organisms, they can serve as regulators of pathological processes in diseases such as cardiovascular disease (CVD)18. Endogenous metabolites, also known as primary metabolites, are encoded by the host genome and they are responsible for the growth, development, and crucial physiological functions of the body19. On the other hand, secondary metabolites are often referred to as exogenous metabolites from diet or environment that may not be essential for growth and development or even harmful20, 21. Endogenous metabolites and exogenous metabolites play important roles in the pathogenesis of AAD and have potential as new biomarkers for diagnosis, gaining popularity in various areas of medical research. Elevated plasma succinate levels were shown to reliably differentiate patients with AAD from those with acute myocardial infarction (AMI) or pulmonary embolism (PE) when presenting with chest pain, suggesting that succinate could be used as a biomarker for pre-diagnosis9. Elevated levels of trimethylamine N-oxide (TMAO) were found to be associated with the incidence and growth of abdominal aortic aneurysms (AAA) in two case-control cohorts. Furthermore, there was a significant correlation between higher TMAO concentrations and abdominal aortic diameter22. Additionally, impaired metabolism of aromatic microbial metabolites (AMMs), as detected by levels in the blood before and after surgery, was found to be associated with postoperative complications in AA patients23. In summary, metabolomics analysis appears to improve the risk stratification and early identification of AAD, while also holding promises for enhancing our understanding of the pathophysiological processes that lead to AAD.
Gut microbiota-derived metabolite implications in AAD
Characteristics of the gut microbiota
The term 'microbiota', a hidden endocrine organ, contributes to 150 times more genetic information than the entire human genome, which could date back to early 1900s24. Various parts of the human body coexist with a large number of microbes composed of bacteria, yeasts, and viruses in the gut, skin, mouth and lungs. Generally, the gastrointestinal tract is considered to host the largest and most variable number of microbes25. It is widely acknowledged that gut microbiota is the most essential microbiota for maintaining our health, which is comprised of six phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia) 26. Among them, Firmicutes and Bacteroidetes make up the vast majority of healthy intestinal environments in adults. The Firmicutes/ Bacteroidetes ratio is defined as a signature indicator that leads to exert healthy intestinal microbiota27. Meanwhile, gut bacteria play important roles in food fermentation, pathogen resistance, immune response stimulation, and vitamin production.
Gut microbiota dysbiosis and AAD
In a healthy state, the human intestine barrier serves as a defense to protect against the translocation of microorganisms or microbial products into the bloodstream. It has been maintained by the tight junctions of epithelial cells, mucus production, mucosal immunity, and the gut-vascular barrier28. In AAD patients, it has been observed that the serum levels of diamine oxidase (DAO) reflecting impaired gut barrier function at an early stage were significantly higher 29. The presence of microbiota dysbiosis can lead to leaky intestines. For example, LPS from most Gram-negative bacteria increase intestinal permeability30. Microbiota dysbiosis refers to an unbalanced interaction between host and microbe, which is mainly manifested in two aspects including altered composition of gut microbiota, and gut microbiota-derived signaling molecules. Ultimately, this leads to the pathophysiological diseases such as CVDs including atherosclerosis, myocardial infarction (MI), hypertension, and atrial fibrillation (AF)31-33. According to previous studies, only a few have investigated the changes in gut microbiota between patients with AAD and healthy participants. Gut microbiota diversity did not differ significantly between patients with AAD and atherosclerosis, however, AAD patients had higher relative abundance of Leuconostocaceae and Faecalibacterium while lower levels of Firmicuteria and Selenomonadales34. A recent study revealed significant changes in the composition of gut microbiota in Ang II-induced AAA mice35. Akkermansia was reduced in AAA mice, and Odoribacter, Helicobacter and Ruminococcus were significantly positively correlated with the abdominal aortic diamete, indicating their potential role in the progression of AAA 35. Tian et al. revealed gut microbiota dysbiosis in AAA patients, which was associated with the occurrence of AAA36. Surprisingly, R. intestinalis alleviated AAA by inhibiting neutrophil infiltration and phenotypic switching of VSMCs.
Consequently, the constituent species of the intestinal flora and their relative abundance are factors that may modify susceptibility to the development of AAD. Although there are few studies clarifying the specific underlying mechanism of intestinal flora changes affecting AAD, which cannot fully clarify the crucial cause-effect relationships, it still suggests the potential of gut microbiota as therapeutic applications target for AAD.
The crosstalk of dietary habits and metabolites in the development of AAD
Diet plays a crucial role in shaping the compositional and functional characteristics of gut microbiota in both humans and animals. Various studies have shown that the absorption of nutrients from food is influenced in part by the composition of an individual's gut microbiota, which in turn is shaped by food37. Caloric restriction (CR), which involves a long-term reduction in total caloric intake without malnutrition, is the only known strategy to reliably improve health and longevity in most living organisms38. Meanwhile, CR has been reported as a nonpharmacological intervention to prevent AAA formation39. Gao et al. suggested that CR reduces the occurrence of AA by maintaining p53-mediated mitochondrial function. However, in the absence of p53, CR inversely promoted AAA formation accompanied by smooth muscle cell mitochondrial dysfunction40. Moreover, the ketogenic diet significantly reduced the incidence of AAA expansion and rupture in mice, accompanied by a significant reduction in inflammatory cytokine content and infiltrated macrophages in AAA tissue41. In addition, a retrospective cross-sectional cohort study in the United States revealed a correlation between fruit and vegetable intake and AAA risk42. Similarly, a prospective cohort study carried out in Sweden found that only fruit intake was associated with AAA risk43. Furthermore, another prospective Swedish cohort study emphasized the importance of fibre and plant foods for reducing AAA risk. High consumption of fruits, berries, and vegetables, particularly leaf vegetables, were linked to decreased AAA incidence44. Conversely, a high-fat diet increases AAA diameter significantly and the risk of rupture by accelerating chronic inflammation45. In conclusion, recent studies are beginning to reveal the biological mechanisms that mediate the beneficial effects of healthy dietary patterns of AAD. However, studies on the role of dietary habits and metabolites in the development of AAD are still in the preliminary stages and more mechanistic studies are needed to understand the interactions between dietary habits, microbiome and metabolites in the key molecular pathways regulating AAD.
Microbiota-Derived Metabolites and AAD
The gut microbiota participate in the uptake of nutrition and energy, and producing multifarious metabolites to maintain physiological function. There are a number of microbiota-derived metabolites, including short-chain fatty acid (SCFA), bile acid (BA), TMAO, amino acid derivatives, vitamins, lipopolysaccharide (LPS), antioxidants, and hormones in the gut46. Microbiota-derived metabolites have been demonstrated to be able to penetrate through the gut mucosa layer into the bloodstream to migrate to distant organs, leading to exert synergistic effects in healthy individuals and have significant impacts on the regulation of CVD47. The first research revealing the possible causality between the microbiota-derived metabolite and CVD focused on TMAO, derived from L-carnitine, promotes atherosclerosis.48 Emerging evidence supports that microbiota-derived metabolites, discussed below, are correlated with the occurrence and recovery of AAD (Figure 2).
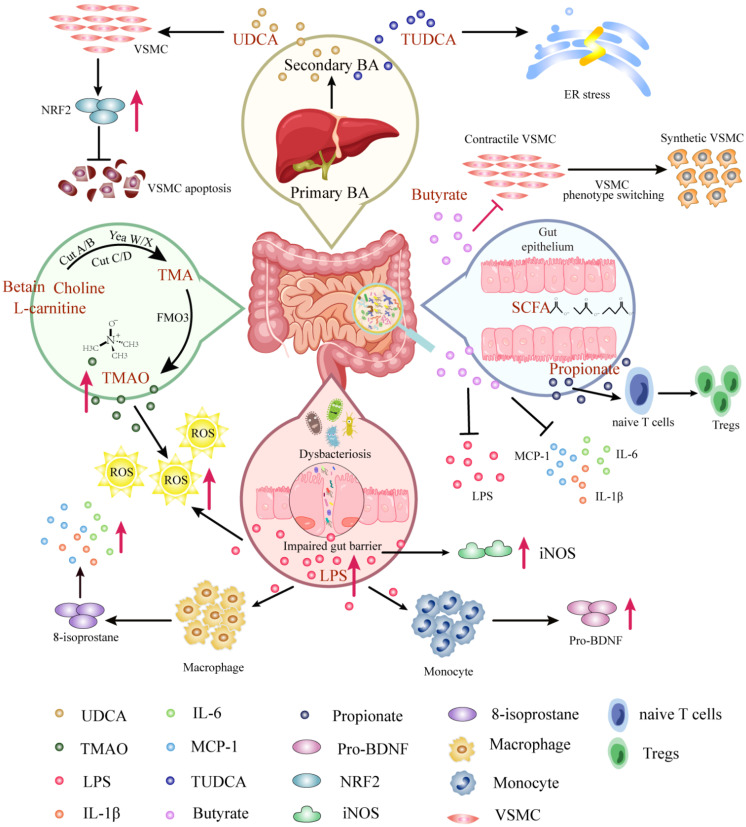
Figure 2.
Mechanisms of gut microbiota-derived metabolites in AAD. Molecular pathways and host receptors that link gut microbiota-derived metabolites with AAD and cellular phenotypes, mainly including TMAO, SCFA, BA, and LPS. Abbreviations: VSMC, vascular smooth muscle cell; UDCA, ursodeoxycholic acid; TUDCA, tauroursodeoxycholic acid; BA, bile acid; SCFA, short-chain fatty acid; ER, endoplasmic reticulum; NRF2, nuclear factor erythroid 2; TMA, trimethylamine; TMAO, trimethylamine N-oxide; FMO3, flavin lpscontaining monooxygenase 3; LPS, lipopolysaccharide; ROS, reactive oxygen species; IL-6, interleukin-6; IL-1β, interleukin-1β; iNOS, ; pro-BDNF, brain-derived neurotrophic factor precursor.
The intestinal flora produces trimethylamine (TMA) from metabolism of choline, lecithin and L-carnitine by the fermentation of dairy products such as eggs, fish and meat. Then TMA is converted to TMAO via the flavin-containing monooxygenase 3 (FMO3) enzyme in the liver49. Undoubtedly, TMAO is the most distinguished gut microbiota-derived metabolite which leads to AAD. It was found that TMAO levels were significantly higher in AAD patients compared to healthy people, while their precursor carnitine, choline and betaine levels were significantly lower10. Consistently, experimental studies proposed that TMAO promoted AAA formation by inducing ROS accumulation and aggravating aortic smooth muscle cell senescence in mice50. Overall, the existing researches showed a dose-positive correlation between TMAO concentration and the occurrence of AAD, which was directly involved in the pathogenesis of AAD. However, detailed and mechanism investigations are needed to understand the interaction between TMAO production and AAD risks.
Gut microbiota participates in bile acid (BA) metabolism, generating associated unconjugated and secondary BAs51. A key contribution of BAs has been proved to facilitate emulsifying and absorbing fat-soluble dietary nutrients. The primary BAs (e.g., chenodeoxycholic acid) are modified via intestinal flora and bile salt hydrolase in the ileum, then they will be converted by BA 7α dehydrogenation, dihydroxyacetone, or authorization to yield secondary BAs (e.g., ursodeoxycholic acid), numerous of which serve as hormone-like roles52. More recent studies have shown some structurally specific BAs play additional crucial part, including but not limited in regulating AAD. Ursodeoxycholic Acid (UDCA) has been shown to inhibit oxidative stress by up-regulating nuclear factor erythroid 2 (Nrf2) expression in VSMCs, which contribute to the decrease of VSMCs apoptosis, leading to suppress acute aortic dissection formation53. Alternatively, the tauroursodeoxycholic acid (TUDCA) formed by condensation between the carboxyl group of UDCA and the amino group of taurine, palys a role in attenuating Angiotensin II induced AAD formation by restraining endoplasmic reticulum stress54. It has been suggested that UDCA and TUDCA have clinical potential to be novel targets for AAD therapy.
LPS is an endotoxin originating from Gram-negative bacteria, especially Escherichia genera55. When the gut barrier is impaired, LPS can enter the host circulation where recognized by toll-like receptors (TLRs) on the cells surface, inducing the release of proinflammatory cytokines to result in a proinflammatory host state56. While the role of LPS directly on the pathogenesis of AAD has not been investigated, antibodies against Porphyromonas gingivalis (Pg) and Aggregatibacter actinomycetemcomitans (Aa), LPS, and elevated CRP levels are present in all AAA patients. Notably, the presence of Pg in saliva correlated significantly with LPS levels in blood57. Indirectly, a suppressed cytokine response was observed in monocyte-derived macrophages treated with LPS in the plasma of AAA patients via reducing 8-isoprostane. The evidence illustrated that macrophages exhibited a recalcitrant phenotype during AAD pathology featuring inhibition of inflammatory cytokine production following LPS challenge58. In addition, LPS induced the monocyte supernatanta significant release of brain-derived neurotrophic factor precursor (proBDNF), which was upregulated in M2-like monocytes of AD patients59. Overall, LPS may contribute to an essential trigger for the activation of the inflammatory response, increasing the risk of AAD by reducing the ability to produce pro-inflammatory mediators that augment the immune response. On the other hand, LPS has been demonstrated to induce iNOS expression in arterial walls in AAA, and LPS-induced vascular inflammation fulfills an essential function in aneurysm initiation60.
SCFAs are produced by intestinal flora and play a role in the fermentation of resistant dietary carbohydrates, which can then enter the circulation and regulate host physiological processes61. SCFAs primarily consist of acetate, propionate, and butyrate, and their main receptors of action are intracellular specific G protein-coupled receptors (GPR) 41 and histone deacetylases62. It has been proposed that the butyrate produced by Roseburia intestinalis can ameliorate intestinal barrier function to counteract endotoxemia and provide protection against atherosclerosis63, suggesting that SCFAs might prevent CVD progression. In addition, SCFAs can mitigate the inflammation response induced by LPS and regulate the elevated blood pressure that is involved in AAD risk factors, suggesting that SCFAs have a theoretical protective effect against AAD. For instance, Tian et al. discovered that butyrate suppressed neutrophil infiltration and neutrophil extracellular traps formation to reduce the expression of inflammatory factors and matrix metalloproteinases (MMPs), then hinder the phenotypic switching of VSMCs in AAA36. Moreover, propionate plays a protective role to alleviates AAA by expanding the pool of regulatory T cells (Tregs) in the colonic lamina propria (cLP), which promotes Tregs recirculation through colonic draining lymph nodes to the inflamed aorta64. Nevertheless, additional investigation is needed to assess the prophylactic potential for AAD.
Amino acid metabolism in AAD
The metabolism of amino acids has been established to be critical for AAD. Raman microspectroscopy analysis of aortic tissue from mice and humans revealed that alterations in amino acid metabolites, including phenylalanine, tyrosine, tryptophan, cysteine, aspartate, and glutamate, occur in aortic tissue from ascending thoracic aortic aneurysms (aTAA)65. Another study demonstrated a correlation between amino acid metabolites and AAA growth. Significant reductions in four amino acids, including histidine, aspartate, leucine, and isoleucine, were found in abdominal aortic aneurysms, but no abdominal aortic aneurysm growth rates were found66. Plasma amino acid profiles were markedly different in patients with AAD compared with patients with coronary artery disease. Amino acid metabolites such as histidine, glycine, serine, and citric acid differed in the AD group. Targeted metabolomic studies of angiotensin II-induced AAD in Ldlr-/- mice showed that polyamines and asymmetric dimethylarginine (ADMA) were elevated in mouse aortic tissue. Furthermore, decreases in glutamine, glycine, taurine, and carnitine concentrations and increases in branched-chain amino acid (BCAA) concentrations were observed in the blood of AA patients67. Overall, these studies suggest that amino acid metabolites have the potential to exploit a promising new and non-invasive diagnostics for AAD.
Homocysteine
Homocysteine (Hcy) is one of the most investigated amino acids in AAD. It is known that the only origin of Hcy in the human body is the dietary protein methionine (Met), one of the eight essential amino acids68. Hcy is generally not involved in the genetic code and is therefore considered a nonprotein-producing amino acid69, 70. Due to various factors such as genetic or nutritional deficiencies, Hcy cannot be metabolized to Met or Cys, which results in the accumulation of Hcy and its protein-related metabolites in the human organism, inducing a variety of pathological states71. Over the past years, research in the field of homocysteine has experienced incredible development, focusing on seizing the significance of homocysteine and its metabolites in CVDs.
Hyperhomocysteinemia (HHcy), elevated concentration of Hcy in the blood, has been confirmed the role in AAD. We identified 7 clinical studies aimed at shedding light on the association between HHcy and patients with AAD compared to normal participants (Table 1). We identified some common traits of these studies: (1) Serum total homocysteine (tHcy) concentrations and aortic tissue Hcy expression were abnormally elevated in the patients with AAD than in healthy controls. (2) The proportion of HHcy was significantly higher in the patients with AAD than in normal controls. (3) Hcy damages the wall of thinner abdominal aortic aneurysm segments. (4) HHcy tended to be associated with a greater risk of AAD patients. The cohort suggests that HHcy is an independent risk factor for AAD and larger prospective cohort studies are warranted to validate these findings.
Table 1.
The association between Hcy and patients with AAD.
| Reference | Cohort size | Type of AAD | Cohort background | Altering catabolites in AAD |
|---|---|---|---|---|
| Eftihia et al191 | 31 aortic dissection (AD) patients, 30 aortic aneurysm (AA) patients, and 20 controls | AAD | Greece | Hcy↑ Folate ↓ |
| Sakshi et al192 | 142 abdominal aortic aneurysm (AAA) patients and 279 controls | AAA | Sweden | Hcy↑ |
| Yuen et al193 | 12,203 men including 318 AAA patients | AAA | Australia | tHcy↑ |
| Deng et al194 Liu et al195 |
73 AAA patients and 219 controls, 155 AAA patients and 310 controls |
AAA | China | HHcy is an independent risk factor for AAA |
| Crystal et al196 | 30 AAA patients and 31 controls | AAA | China | Hcy in AAA tissues↑ |
| Aldona et al197 | 36 patients treated for AAA repair | AAA | Poland | Hcy in damaged arterial wall↑ |
Consistent with these studies, studies conducted in animal models suggested that Hcy act as a risk factor to worsen the prognosis of AAD in experimental models. Primarily, excessive Met which is the only source of Hcy exacerbates the development of AAA in Rats72. Hcy, serving as a partial agonist of the angiotensin II type 1 (AT1) receptor, directly induced the AT1 receptor activation via the Arg167 and Cys289 sites, which aggravated aneurysmal vascular injuries in AAA mice73. Similarly, Liu et al. detected that adventitial fibroblast from Apoe-/- mice supplemented with excess Hcy in drinking water exaggerated inflammation due to the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4, which increased the incidence of AAA74. Hcy can also contribute to vascular inflammation by inducing the production of pathogenic anti-β2GPI that derived by B cell, aggravating AAA formation, the β2GPI antigen expressed on ECs formed immune complexes with anti-β2GPI antibodies and might participate in HHcy-aggravated AAA formation75. In addition to playing a role in AAA by participating in systemic immune imbalance, Hcy can also modulate local autoimmune processes. Hcy was investigated to cause the imbalance between follicular helper T cells (Tfh) and T helper cell 17 (Th17) via up-regulating the expression of inflammasomes which participated in humoralnfactor-induced CD4 cell imbalance, resulting in inflammasome-induced local autoimmunity in AAA76. Additionally, moderately elevated homocysteine does not contribute to thoracic aortic aneurysm in mice77. Taken together, excessive Hcy can aggravate the occurrence and development of AAA, but physical high homocysteine levels, not within the scope of wild type mice induced abnormal aortic growth. Therefore, targeting homocysteine levels is an attractive intervention goal which can be achieved by manipulating the rise of Hcy levels in physiological range to inhibit the development of AAA (Figure 3A).
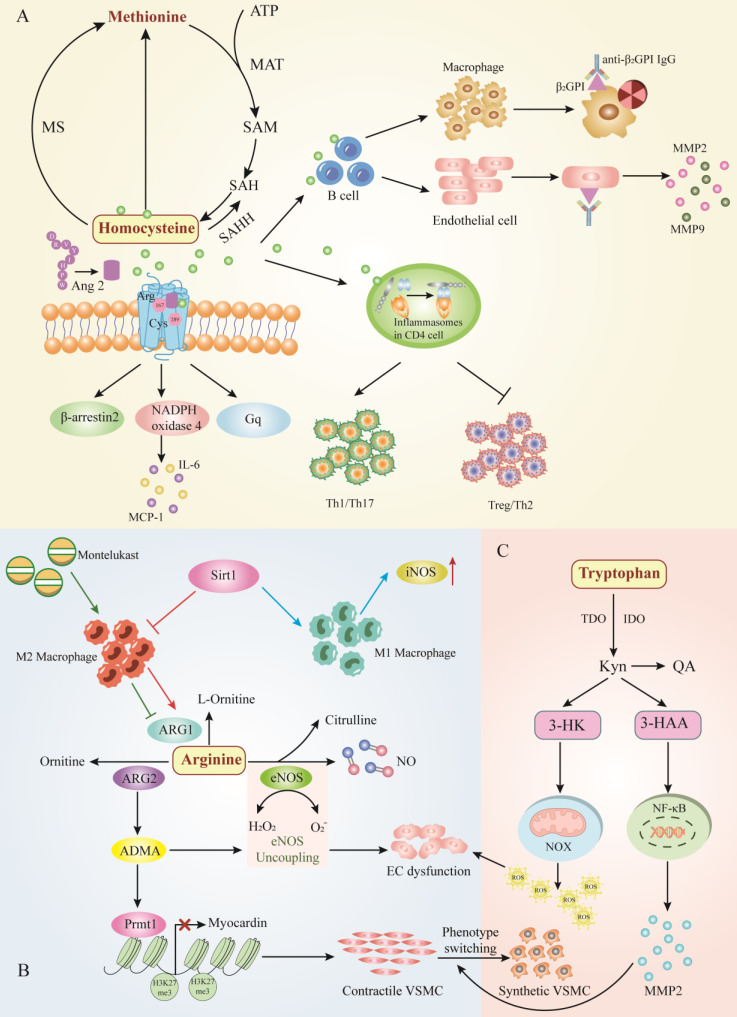
Figure 3.
The major amino acid metabolic pathway involves AAD. A.Homocysteine metabolism, B. Arginine metabolism, and C. Tryptophan metabolism. Hcy exacerbates vascular inflammation by inducing immune cells such as B cells and T cells, thereby increasing the incidence of AAD. Abbreviations: ATP, adenosine triphosphate; MAT, methionine adenosyltransferase; SAM, s-adenosylmethionine; SAH, S adenosyl L homocysteine; MS, methionine synthetase; SAHH, S adeno sylhom ocysteine hydrolase; Tfh, follicular helper T cells; Th17, T helper cell 17; NADPH, nicotinamide adenine dinucleotide phosphate; β2GP1, β2-Glycoprotein 1; MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9; MCP-1, monocyte chemoattractant protein 1; IL-6, interleukin-6; Arg, arginase; Cys, cysteine; Ang 2, angiopoietin 1; iNOS, inducible nitric oxide synthase; Sirt1, Sirtuin1; ARG1, arginase 1; ARG2, arginase 2; NO, nitric oxide; ADMA, asymmetric dimethylarginine; eNOS, endothelial nitric oxide synthase; EC, endothelial cell; NOX, NAD(P)H oxidase; 3-HK, 3-hydroxykynurenine; Kyn, kynurenine; QA, quinolinic acid; 3-HAA, 3-hydroxyaminobranoic acid; TDO, tryptophan-2,3-dioxygenase; IDO, indoleamine 2, 3-dioxygenase.
Arginine
Arginine a semi-essential amino acid that serves as a substrate for nitric oxide synthase (NOS) and arginase (ARG), the prominent NOS isoform is endothelial-type nitric oxide synthase (eNOS) in aortic vessels, which converts ARG to nitric oxide (NO)78. NO, acting as a vasodilator, plays an endothelial protective role in the cardiovascular system79. However, ARG catabolizes arginine to ornithine and urea, which affects the bioavailability of arginine and thus limits NO production in the AAD.
The main product of classically activated macrophages (M1) is NO, which requires the infusion of arginine and oxygen. The products of the inducible nitric oxide synthase (iNOS) reaction are citrulline and NO. Nevertheless, alternatively activated macrophages (M2) do not produce NO but instead utilize arginase 1 (ARG1) to hydrolyze large amounts of imported arginine80. Recent studies have shown that ARG1 could target the polarization of macrophages to play an essential role in the pathogenesis of AAA. Sirtuin (SIRT1)-deficiency increased iNOS induced by pro-inflammatory M1 molecules and decreased anti-inflammatory molecules produced by M2, accelerating Ang II-induced AAA formation81. Interestingly, montelukast significantly induced infiltration of M2 to enhance the expression of the ARG1, which suppresseed aneurysmal dilation. Consistently, increased the protein expression of ARG1 inhibited MMPs, resulting in a significant reduction in the diameter of AAA82. In conclusion, the balance between macrophage activation and polarization is vital for AAD pathogenesis. Targeting this balance, arginine might represent a new prognostic or therapeutic target in aneurysmal disease.
Arginine is synthetic from citrulline via the cytosolic enzymes argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL). Citrulline can be produced from a variety of sources: (1) from arginine via NOS catalysis, (2) Substitution from ornithine by decomposition of proline or glutamine/glutamic acid, (3) from ADMA, it is catalyzed by dimethylarginine dimethylamine hydrolase (DDAH)83. Glutamine in tiny intestinal epithelial cells produce citrulline, which is subsequently converted to arginine with the assistance of renal tubular cells. A randomized controlled clinical trial manifested that glutamine supplementation during the perioperative period attenuated the impaired renal arginine synthesis after open aortic surgery and compensated for the suppressive effects of ischemia-reperfusion injury79. However, to date there is no definitive evidence to assess whether glutamine supplementation affects AAD, and there are reports that excessive glutamine circulation to the TCA cycle leads to an abnormal pro-inflammatory response84. It is questionable whether glutamine induces an inflammatory response to the vessel wall in AAD. Moreover, protein arginine methyltransferase 1 (Prmt1) is the predominant enzyme that catalyzes the asymmetric arginine dimethylation of proteins, which is the source of ADMA. Prmt1 induces the switch from contractile to synthetic VSMC by regulating myocardin levels and asymmetric dimethyl-H4R3 histone modifications, leading to downregulation of VSMC contractile gene expression and upregulation of synthetic gene expression, resulting in the formation of AAD85.
The recoupling of eNOS alleviated expansion of aortic roots and abdominal aortas, then animal models of endothelial dysfunction and eNOS uncoupling increased the rate of AAA formation and increased eNOS augmented the risk of aortic dissection. The increase of eNOS-derived ROS and the decrease of NO production may be the main causes of endothelial dysfunction in AAD86, 87. Nw-nitro-L-arginine methylester (L-NAME), serving as NOS inhibitor, induced NO suppression to cause severe endothelial dysfunction, which could accelerate the onset of dissection but not aneurysm88. At the same time, a randomized, single-blind, multi-centre, and placebo-controlled trial indicated that patients receiving perindopril which contained 10 mg arginine showed similar blood pressure lowering effects as amlodipine, but perindopril did not affect the overall rate of AAA growth during 2 years of follow-up89. Improving NO bioavailability might suggest a potential treatment strategy in AAD, and arginine metabolic pathway may be the link between NO bioavailability and AAD (Figure 3B).
Tryptophan
Tryptophan (Trp) is another essential amino acid that can be obtained from dietary consumption, which has been proven to influence AAD. Trp is essential for protein synthesis and therefore for normal cellular homeostasis. There are two pathways that process Trp into other metabolites, including the 5-hydroxytryptamine (5-HT) pathway and the kynurenine (Kyn) pathway. Hepatic tryptophan-2,3-dioxygenase (TDO) maintains physiological concentrations of Trp and Kyn at modulated levels via the kynurenine pathway (KP)90. It has been found that tryptophan catabolic processes to acetyl-CoA, tryptophan catabolic processes to kynurenine, and tryptophan catabolic process were enriched in the human AAA group91.
During tryptophan catabolism, tryptophan is further metabolized to L-kynurenine (Kyn) via indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and TDO to produce N-formylkynurenine. Kyn is further catabolized to the active metabolites 3-hydroxykynurenine (3HK), 3-hydroxyaminobromic acid (3-HAA), kynurenine acid (KA), quinolinic acid (QA), and the essential pyridine nucleotide end product nicotinamide adenine dinucleotide+ (NAD+)92. The accumulating evidence demonstrates that Trp has an essential contribution to the execution of AAD development. It has been identified that kynurenine pathway activity is enhanced in aortic atherosclerotic aneurysm. Metabolite levels of tryptophan, kynurenine, and QA were enhanced in the walls of the aneurysm, and inhibition of kynurenine increased IDO1 levels in cultured macrophage93. Furthermore, the expression of 3-HAA, IDO, and kynureninase were obviously augmented in human AAA samples, IDO deletion in vivo restrained AngII-induced AAA in Apoe-/- mice and low density lipoprotein receptor-deficient mice fed with high fat diet94, 95. Mechanistically, 3-HAA contributes to increased NF-κB-Mediated MMP2 expression in HASMCs, leading to promote phenotypic switching of VSMCS which accelerated angiotensin II-induced AAA formation in mice94. On the other hand, ECs dysfunction and apoptosis are important pathophysiological processes in the development of AAD, and 3-HK has been shown to induce endothelial cell apoptosis and endothelial dysfunction by increasing NAD(P)H oxidase (NOX)-derived ROS. Therefore, inhibition of 3-HK formation may be a prospective therapeutic intervention to prevent AAD96 (Figure 3C). Overall, a causal relationship between Kyn pathway activation and AAD development has been established, suggesting that Trp-derived metabolites may be diagnostic biomarkers for AAD and may have the potential to be therapeutic for AAD.
Taurine
Taurine, mainly derived from seafood such as shellfish, is a non-protein amino acid with a distinctive sulfonic acid composition97. Evidence suggests that taurine affects a variety of cellular functions, including osmolarity, oxidation, and bile acid binding. At the same time, taurine has anti-inflammatory effects and has been proved to have the benefit of suppressing the renin-angiotensin system in CVDs. Populations with higher meat intake exhibited higher CVD related mortality compared to those with higher seafood intake98. It is possible that the amount of taurine in seafood is much higher than that in meat, suggesting that taurine fulfill an essential function in preventing CVD.
Research has identified a correlation between taurine and vascular dysfunction. Vascular dysfunction was attenuated in type 2 diabetes patients after taurine supplementation in a clinical study, indicating that taurine therapy reverses endothelial dysfunction, which is the central pathway for aortic aneurysms and aortic dissection. Supplementation with taurine is proved to alleviate myeloperoxidase-mediated oxidative stress, leading to the mitigation of AAA formation in mice99. In the aortic valve interstitial cells (AVICs) isolated from patients with type A dissection without leaflet disease, taurine attenuated PCM-induced osteogenic differentiation of AVICs by reducing alkaline phosphatase (ALP) activity in a dose-dependent manner100. Therefore, taurine supplementation may elicit a beneficial role in the progression of AAD and further investigations are required to confirm whether taurine supplementation is a feasible adjunct treatment of AAD.
Lipid metabolism in AADs
Polyunsaturated fatty acids (PUFA)
Fatty acids are the major components of dietary fat, which is composed mainly of saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA)101. An initial observational study demonstrated lower mortality from CVDs in people with a high intake of ω -3 PUFA102,103 and thus attracted attention to the cardiovascular protective effects of PUFA.102, 103.
PUFAs are further divided into the omega-6 PUFAs and the omega-3 PUFAs according to the location and function of the double bond. Linoleic acid and arachidonic acid (AA) belong to the ω-6 PUFAs, while linolenic acid, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) belong to the ω-3 PUFAs104 (Figure 4). The Mendelian randomization analysis of FA genetic data illustrated the role of circulating PUFAs on cardiovascular diseases risk105. Higher plasma alpha-linolenic acid, linoleic acid, and oleic acid levels were correlated with a lower probability of large artery stroke and venous thromboembolism, while higher levels of arachidonic acid and stearic acid were associated with a higher probability of occurrence106. Higher genetically predicted DHA levels were associated with higher risk of CVDs endpoints, and the levels of DHA were risk factors for AAD.
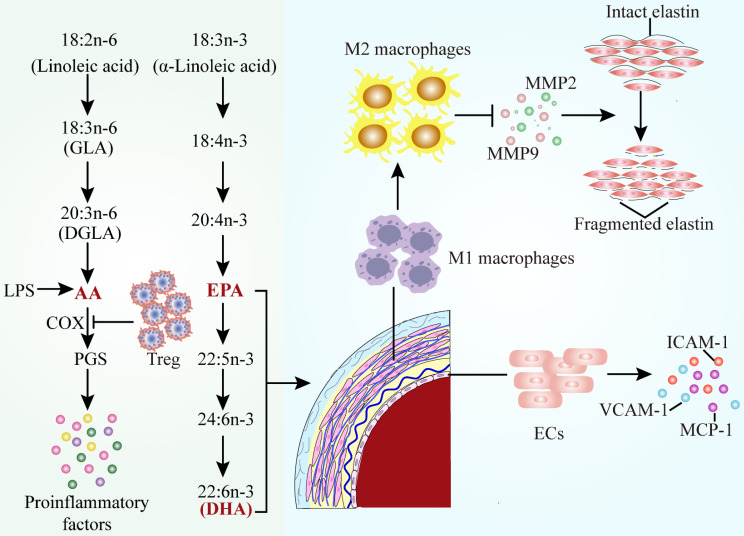
Figure 4.
Alterations of polyunsaturated fatty acids metabolic pathways in AAD. The classification, formation of polyunsaturated fatty acids and their function in AAD. Decreased concentrations of AA, EPA and DHA lead to the occurrence of proinflammatory events in AAD. Abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ECs, endothelial cells; Treg, regulatory T cells; GLA, gamma linolenic acid; DGLA, dihomo gamma linolenic acid; PGs, prostaglandins; COX, cyclooxygenase; LPS, lipopolysaccharide; MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9; VCAM-1, vascular cell adhesion molecule 1; ICAM-1, intercellular adhesion factor 1; MCP-1, monocyte chemoattractant protein 1.
AAD are chronic inflammatory diseases, which are initiated by the accumulated macrophages, release of inflammatory factors, and oxidative stress. In consequence, pro-inflammatory AA seems to play an important role in AAD. The pro-inflammatory response observed in AAD could be due to the lack of anti-inflammatory AA results107. Therefore, it is necessary to investigate whether the combined application of PUFA is more suitable for the prevention of AAD in humans.
Arachidonic Acid Metabolism
AA is one of the most abundant PUFAs in the human body and serves as a component of cell membrane phospholipid bilayers, an inflammatory mediator and a vasodilator or constrictor. Endogenous AA is mainly involved in the production of various bioactive derivatives of arachidonic acid, which can be metabolized by three enzyme systems, COX, cytochrome P450 (CYP) enzymes and lipoxygenase (LOX)108. The imbalance between thromboxane A2 (TXA2) and prostacyclin synthesis is attributed to differential expression of isoform proteins of COXs, which suggests the alteration in AA metabolism. It has been observed that the concentration of arachidonic acid is decreased, which is connected with an enhancement of COX2, CYP450 and 5-LOX, leading to the formation of the aortic aneurysm109. The RBC proportion of arachidonic acid increased prevalence of AAA and the risk of surgical repair, indicating the potential of AA served as a prognostic biomarker. It has been also reported that the level of AA and thrombin mediated platelet reactivity could represent a switch of vascular inflammation in patients after major vascular surgery110.
COX-2 can catalyse converting of AA to prostaglandins (PGs), modulating the expression of pro-inflammatory chemokines to regulate cell functions. COXs and PGs are considerable inflammatory mediators which are associated with AAD111. COX-2 is generally not detectable in most physiological states, whereas it can be rapidly triggered in response to various inflammatory stimuli (e.g. LPS or Ang II) and COX-2 expression is strongly characterized in AAD112. Compared to healthy individuals, COX-2 expression is visibly increased in AAD patients113. COX-2 knockdown significantly alleviated the incidence and severity of Ang II-induced AAA in mice. Moreover, Liu et al. detected that CD4+CD25+ regulatory T cells (Tregs) adoptive transferred to suppress the level of COX-2 in AAA aortic tissues, indicating that Tregs protected against AAA by suppression of the COX-2 expression114.
Omega -3 Polyunsaturated Fatty Acid
Interest in omega-3 (ω-3) PUFAs has escalated in recent years because of their various roles in health promotion and disease risk reduction. ω-3 PUFAs include EPA, DHA, α-linolenic acid (ALA), stearidonic acid (SDA), and docosapentaenoic acid (DPA). ω-3 PUFAs with health benefits ascribed mainly to EPA and DHA, which are primarily obtained from the diet such as the body lipids of fatty fish and the liver of white lean fish115. Multiple studies have been conducted on the effects of ω-3 PUFAs on dominating cardiovascular diseases, such as MI, stroke, AF, coronary heart disease, heart failure (HF), and AAD116.
A randomized controlled trial demonstrated that erythrocytes from patients with AAA exhibit an alternating FA profile characterized by an anomalous ratio of n-6 FAs with inflammatory state, reflecting the effects of systemic inflammation and oxidative stress. However, the inflammatory parameters and n-6 fatty acid status were significantly improved afterω-3 PUFA supplementation, indicating that dietary fatty acids conduct a potential therapeutic intervention for AAA117. Additionally, supplementation of ApoE-/- mice with a diet high in n-3 PUFA content protected the mice against pro-inflammatory and oxidative stress responses following short-term infusion with Ang II118. ω-3 PUFA also commonly occurr in tree nuts. Previous studies have reported that administrating ω-3 PUFAs can limit AAA development and severity within experimental animal models119. Based on this previous experimental evidence, it was investigated that a diet enriched with tree nuts could limit the development and severity of AAA120.
EPA plays a protective role in abdominal aortic aneurysm, animal models and macrophages by multiple pathways. In the patients with surgical repair of AAA, EPA/AA ratio was lower than those in healthy Japanese subject, which was negatively correlated with the maximum AAA diameter. Low serum EPA levels and EPA/AA ratio were associated with the size and growth rate of AAA121. The protective effects and underlying mechanisms of EPA in AAA have been identified (Figure 4): (1) increased anti-inflammatory property of macrophages due to lessened inflammatory factor such as MMP2 and MMP9 levels122-124 (2) attenuated of mesenchymal stem cell (MSC) dysfunctions125 (3) suppressed the weakening of the vascular wall by inhibiting the elastin fiber degradation caused by nicotine126 (4) inhibition of endothelial cell-mediated inflammatory factors107. AAA is associated with inflammation and oxidative stress, the latter of which contributes to activation of macrophages, a prominent cell type in AAA. Meanwhile, a recent study found that DHA decreased the concentration of TNF-α and interleukin-6 (IL-6) in macrophage supernatants and increased glutathione peroxidase activity and heme oxygenase-1 expression. The improvements in macrophage oxidative stress status serve as a stimulus for further investigation of DHA in patients with AAA127.
Nitro-fatty acids (NO2-FAs)
Nitro-fatty acids (NO2-FAs), electrophilic lipid molecules, are endogenously synthesized via the reaction of unsaturated fatty acids (USFAs) with NO or nitrite-derived nitrogen dioxide free radicals128. NO2-OA has been shown to confer substantial cardioprotection through supressing the activation of NF-κB. Given that NO2-FAs are preferred in terms of synthetic properties and stability, it emerged in two Phase II studies including patients with PAH (NCT03449524) and focal segmental glomerulosclerosis (NCT03422510). Importantly, NO2-OA has been investigated to attenuate ascending aortic dilation and wall stiffening in Marfan syndrome (MFS) mice by inhibiting the overactivation of ERK1/2, Smad2 and NF-κB in the aorta, thus reducing elastin fragmentation, cell apoptosis and collagen deposition induced by matrix metalloproteinase 2129. Correspondingly, NO2-OA exerts a protective effect on the observable decrease of extracellular matrix degradation, inflammatory cytokine release, and macrophage infiltration in AAA model129. With the current studies, the data might expedite the use of NO2-OA for AAA therapy.
Other Lipid Metabolites in AADs
It is well established that oxidative stress and inflammation are the two main mechanisms leading to AAD, and the modification of cell membrane phospholipids has been proved in AAD130. Lysophosphatidylcholines (LPCs) and sphingolipids were significantly altered in AAD patients compared with healthy controls. Furthermore, three sphingolipids, including sphingosine, phytosphingosine, and ceramide, were specifically significantly decreased in Stanford type A AAD patients. It can be utilized as potential biomarkers for the diagnosis of AAD and to distinguish Stanford type A from Stanford type B131. Under oxidative stress, PUFA-containing phospholipids and cholesterol esters in cellular membrane and lipoproteins can be readily oxidized through a free radical-induced lipid peroxidation (LPO) process to form a complex mixture of oxidation products. The association of serum lipid peroxidation products with disease severity in AAA patients has been determined132. Macrophages in the vascular wall could amplificate the local inflammatory response by secreting proinflammatory cytokines and degradation of ECM by producing proteases to promote the occurrence of AAA. GW4869, an inhibitor of sphingomyelinase, ameliorated extracellular vesicles (EVs) generated by T lymphocytes and reduced macrophage migration and infiltration, thereby inhibiting the development of AAA in elastase-deficient mice133. The presence of lipid-protein interactions in the membrane provides substrates lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA). Autotaxin (ATX) directly interacting with integrin on the target cell surface to regulate cell function as a secreted protein, lysophosphatidase D (lysoPLD) which catalyzes the conversion of LPA of LPC based on its catalytic phosphodiesterase (PDE) domain134. Plasma ATX concentration is up-regulated in elastase-induced AAA mouse model, ATX interacting with T cells by binding to integrin α4 activated the FAK/Src-RhoA signaling pathway to promote T cell migration, which in turn recruits T cells into the vessel wall and induces vascular inflammation, thereby accelerating the pathogenesis of AAA135. Additionally, bioactive lipids play a central role in AAA by regulating coagulation and its associated inflammation. Coagulation factors assemble on phospholipid (PL) membranes to induce thrombin generation to promote hemostasis occurrence, aminoPL (aPL) and enzymatically oxidized PL (eoxPL) work together to achieve coagulation136. Mice with AAA were shown to have increased intravascular coagulation and formation of large amounts of eoxPL. Instead of activating circulating clotting factor consumption and depletion to reduce AAA, coagulation is kept away from the vessel wall due to eoxPL deficiency137. Complex interactions between bleeding and thrombosis through the vessel wall or in the circulation can drive or prevent AAA development, providing new insights into AAA pathogenesis and treatment. Importantly, plasma phospholipid transfer protein (PLTP), another cardiovascular risk factor, has been shown to regulate the coagulation process. Mice with PLTP deficiency showed reduced VSMC depletion, elastin degradation and macrophage infiltration, and reduced expression and release of tissue matrix metalloproteinase. PLTP deficiency was associated with a significantly lower incidence and less severe degree of AAA expansion. PLTP may be of particular clinical relevance due to its dual proinflammatory and hyperlipidemic effects. However, it remains to be determined whether the PLTP blockade may constitute a relevant approach for AAA prevention138. Indeed, HDL is known to display beneficial antielastase activity139, and plasma HDL cholesterol levels are reported to be inversely associated with the incidence of AAA140.
TCA Cycle and Electron Transport Chain (ETC)
Cellular metabolism mainly involves the utilization of carbohydrates, proteins, and fats for energy synthesis, and when metabolic processes are used to produce energy, the final step is oxidative phosphorylation141. Molecules from these processes are used in the tricarboxylic acid (TCA) cycle to generate substrates that enter the ETC for oxidative phosphorylation. The ETC, located in the inner mitochondrial membrane, receives electrons donated by NADH and FADH2 produced by the TCA cycle at complex I (NADH: ubiquinone oxidoreductase) or complex II (succinate dehydrogenase), respectively142, generating cellular ATP. In brief, Complexes I and II, with additional the flavoprotein-ubiquinone oxidoreductase, transfer electrons to ubiquinone (coenzyme Q). Subsequently, electrons are transferred sequentially to Complex III (coenzyme Q: cytochrome c reductase), cytochrome c, Complex IV, and eventually to molecular oxygen, the terminal electron acceptor. Complex V consists of two distinct domains, including an extracellular domain (known as F1) and a transmembrane domain (known as FO). Proton movement from the membrane gap through FO is coupled to rotation, resulting in the synthesis of ATP by the addition of a phosphate and ADP at the F1 site, which ultimately leads to produce ATP143.
There has been evidence that ETC is defective in AAD. A microarray study identified 47 significantly differentially expressed genes in AAA patients compared with controls. GO functional analysis revealed that these genes play important roles in electron transport chain144. Moreover, the protein level of Complexes I through IV was significantly increased in Fibulin4R/R aortas compared with Fibulin-4+/+, whereas Complex V had no significant change. In Fibulin-4R/R mice, the extracellular matrix protein Fibulin-4 was significantly reduced, which results in decreased blood ketone and hepatic fatty acid levels, indicating dysregulated metabolism to promote thoracic aortic aneurysm formation145. It has been observed that CR exacerbated Ang II-induced AAA formation in p53-/- mice. Notably, knockout of p53 promoted Ang II-induced AAA via inhibiting the expression of cytochrome C oxidase assembly protein 2 (SCO2) which is a component of the complex IV.40 Accordingly, the enzymatic activities of citrate synthase and cytochrome C oxidase, serving as biomarkers of mitochondrial respiratory chain functionality, were significantly reduced in the AAA aorta146. Consistently, the transcriptomics and metabolic analysis of aortas from an MFS mouse model revealed that mitochondrial complexes were reduced. Supplementation with mitochondrial metabolism NAD precursor nicotinamide riboside improves mitochondrial function to reverse aortic aneurysm147. Taken together, the absence of ETC might aggravate the occurrence of AAA, suggesting mitochondrial boosting strategies as a potential treatment to managing aortic aneurysms and therapies targeting ETC could benefit patients with AAA.
The TCA cycle, also known as the citric acid cycle or the Krebs cycle, is a series of reactions in a closed loop that forms a metabolic engine within cells (Figure 5). While the TCA cycle is vital of the oxidation of substrates for energy production (ATP), TCA cycle metabolites have been demonstrated to serve as signaling molecules with functions regulating chromatin modifications, DNA methylation, the hypoxic response, and immunity, leading to change cell function and fate in diverse diseases148. Sodium pyruvate (SP), a substrate of the TCA cycle, has been demonstrated to be the potential target for hypoxic-ischemic encephalopathy. SP improved cerebral metabolism via maintaining ATP levels and inhibiting intracellular reactive oxygen species (ROS) levels to alleviate brain damage and enhance neurological performance149. Mechanistically, it has been found that visnagin bound to mitochondrial malate dehydrogenase (MDH2) which was a key enzyme in the TCA cycle, resulting in reducing doxorubicin-induced cardiomyocytes apoptosis and increasing cardiac contractility in mice150. Furthermore, a maladaptive increase in anaplerosis via malic enzyme 1 (ME1) in TCA is associated with reduced GSH content. In hypertrophic rat hearts, inhibition of ME1 expression can reduce pyruvate carboxylation, reduce lactate accumulation, and restore GSH content, thereby normalizing hypertrophy151.
Figure 5.
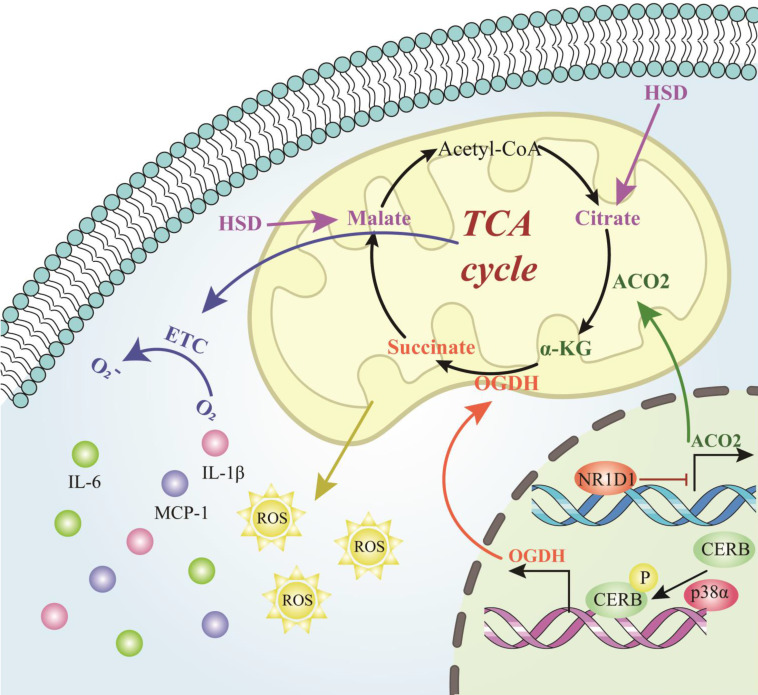
Role of tricarboxylic acid cycle-related cellular metabolites in AAD. The process of the intracellular tricarboxylic acid cycle and the role of the corresponding metabolites in AAD. The process figures those molecules and reactions found in the TCA cycle in mitochondria. Abbreviations: TCA cycle, tricarboxylic acid cycle; HSD, high salt diet; α-KG, alpha‑ketoglutarate; ACO2, aconitase-2; Acetyl-CoA, acetyl coenzyme A; OGDH, oxoglutarate dehydrogenase; ROS, reactive oxygen species; ETC, electron transport chain; IL-6, interleukin-6; IL-1β, interleukin-1β; MCP-1, monocyte chemoattractant protein 1; NR1D1, NR subfamily 1 group D member 1; CREB, cyclic adenosine monophosphate-responsive element-binding protein 1.
Hypertension is an important risk factor for aortic aneurysm and aortic dissection. When the body is under the level of hypertension for a long time, it will lead to the change of blood flow of the patient, which in turn can cause the change of the ratio of elastic fibers to collagen and morphology of the aortic wall, increase the shear stress of the blood vessel, increase the stiffness of the blood vessel wall, and eventually cause AAD152. High salt diet (HSD) significantly weakened the TCA cycle, and attenuated the antioxidant system in the renal medulla, which finally contributed to salt-sensitive hypertension153. Metabolomic profiling was performed that three serous TCA-cycle-associated compounds, including decreased malic acid and citric acid, as well increased fumaric acid were differentially detected in salt-sensitive rats treated with HSD153. Alpha‑ketoglutarate (α-KG) has been reported to exert significant impacts on AAA. α-KG significantly suppressed the aneurysmal dilation by attenuating the oxidative stress, macrophage infiltration, elastin degradation and collagen fibers remodeling via inhibiting PXDN/HOCL/ERK signaling pathways154. Moreover, NR1D1 contributes to AAA formation by targeting the mitochondrial TCA cycle enzyme aconitase-2 (ACO2). The activity of the key TCA cycle enzyme ACO2 in both human and mouse AAA samples were decreased, which mediated the negative effects of NR1D1 on mitochondrial function and AAA formation. Supplementation with α-KG, a downstream metabolite of ACO2, rescued mitochondrial dysfunction and protected against AAA formation155. Additionally, untargeted metabolomics identifies succinate as a biomarker and therapeutic target in AAD. Plasma succinate concentrations were increased in patients with AAD which can be used as a biomarker for diagnosis and allow reliable differentiation from AMI and PE when patients present with chest pain. Mechanistically, oxoglutarate dehydrogenase (OGDH) is a key upstream TCA enzyme for direct synthesis of succinate, inhibition of OGDH reduces the expression of inflammatory factors in macrophages to increase the mitochondrial ROS level in the vasculature, further aggravated AAD formation targeting p38a-CREB-OGDH axis9. Collectively, the results of the present study provided evidence that the TCA cycle metabolites played powerful parts in AAD formation and progression and were identified as potential novel targets for AAD treatment.
The Role of Other AAD-associated Metabolites
It is well established that in addition to the AAD-associated metabolites we have described above, there are numerous additional metabolites that play a crucial role in the pathogenesis of AAD.
Sex is a prominent risk factor for AAA, and Ang II induces AAA formation to a greater degree in male than in female mice. It has been found that a cytochrome P450 1B1generated metabolite of testosterone, 6βhydroxytestosterone (6βOHT), contributed to Ang II-induced AAA development in male mice, which provides a bridge between sex and metabolites in AAD pathogenesis156. Additionally, vitamin D deficiency could exacerbate AA development via regulating the genes involved in extracellular matrix remodeling157.
Uric acid (UA) is a purine derivative and a well-established CVD risk marker. Serum uric acid is associated with AD in Chinese men158, 159, and it can serve as an indicator for the differential diagnosis of AD and CAD or a marker of oxidative stress in dilatation of the ascending aorta160, 161. It has been speculated that a non-linear correlation was determined between serum UA and in-hospital mortality of patients with acute type A aortic dissection162. When serum uric acid > 260 µmol/L, it showed a positive correlation with in-hospital mortality. Further clinical studies are necessary to investigate whether UA is an independent risk factor for AAD.
Polyphenols are naturally occurring compounds found largely in fruits, vegetables, cereals, and beverages. Numerous polyphenols including resveratrol and curcumin have been shown to be associated with AAD. Resveratrol attenuated AD by increasing endothelial barrier function through the SIRT1 pathway163. Meanwhile, curcumin, radix astragali, resveratrol, Baicalein, and Salvianolic acid have been demonstrated to be potential treatments for AAA164.
Metabolic profile and potential therapies for AAD
Previous studies have shown that the metabolic profile of AAD patients is significantly different from that of healthy participants or patients with other diseases. Therefore, we summarized the metabolic profile of AAD patients to contribute to provide potential targets for AAD clinical treatment (Table 2). Overall, the main observations were that the AAs and phosphatidylcholine levels were decreased165, and the metabolism of sphingolipids, lysophospha-tidylcholine, cholesterol, and acylcarnitine was disturbed in AAA patients compared with healthy subjects166. A combination of salicylic acid, pregnenolone sulfate, 5-Lglutamyl-L-alanine, and 3-hexanone with mevalonic acid could serve as a biomarker to distinguish AAA and atherosclerosis167. On the other hand, the alterations of most lipid metabolism (phosphatidylcholine, lysophosphatidylcholines, triacylglycerols, fatty acids, acylcarnitines etc.)10, 131, 168 polyunsaturated fatty acids metabolism (EPA and DHA) and ceramide metabolism23 were further observed in AD patients but not in healthy individuals. Meanwhile, AD patients also show characteristic metabolic profiles compared to other diseases. It is well established that hypertension and CHD are important risk factors for AD, therefore, the level N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), ergothioneine, glycerophosphocholine, and Sphingofungin B in the peripheral blood of the AD patients were distinctly different compared to hypertension patients169. Plasma aminograms were significantly altered in patients with AD compared with CHD, especially in acute AD. Interestingly, among AD patients, different types of AD also present differential metabolic profiles. The contents of glutamate and phenylalanine were significantly changed in acute AD patients comparing with chronic AD patients170. Sphingolipids, including sphinganine, phytosphingosine, and ceramide, were reduced in the Stanford type A AAD patients, but not in the Stanford type B AAD patients131. As for the differences in metabolic profiles between AA and AD, Zhang et al. found 138 differential metabolites which were mainly involved in the galactose pathway, while sphingolipids, especially its core metabolite C18 ceramide, were significantly differentiated in thoracic aortic dissection (TAD) patients but not in thoracic aortic aneurysm (TAA) patients, which was an important step for better understanding the differences between AA and AD171. The above studies suggest that amino acid and lipid metabolism were expected to exploit a novel and non-invasive diagnosis for AAD.
Table 2.
Current metabolic profilings of AAD.
| Reference | Origin | Description | Study size | Substrate | Type of AAD | Metabolic profile and potential metabolic treatment targets |
|---|---|---|---|---|---|---|
| Lieberg et al165 | Estonia | Comparison of plasma metabolites between patients with AAA and aorta-healthy controls | AAA with fast yearly growth rate:39 AAA with slow yearly growth rate:39 Healthy subjects:79 |
Plasma | AAA | Amino acids and phosphatidylcholines metabolic pathway |
| Ji et al167 | China | Comparison of plasma metabolites between patients with AAA and AS patients | AAA patients:32 AS patients:32 |
Plasma | AAA | 2-Deoxy-D-ribose (2dDR) |
| Zeng et al10 | China | Comparison of serum metabolites between patients with Stanford Type A AAD and healthy individuals | Type A aortic dissection (TAAD) patients:19 Healthy individuals:20 |
Serum | AD | Phosphatidylcholine metabolic pathway |
| Zhou et al131 | China | Comparison of plasma metabolites between acute aortic dissection and healthy individuals | TAAD patients:20 Type B aortic dissection (TBAD) patients:15 Healthy subjects:20 |
Plasma | AD | Lysophosphatidylcholines and Sphingolipids metabolic pathway |
| Zhang et al171 | China | Comparison of metabolites secreted by AA aortic wall and AD aortic wall | AA patients:9 AD patients:9 |
Aortic tissue | AAD | Galactose metabolic pathway |
| Ren et al169 | China | Comparison of peripheral blood metabolites between AD patients and healthy individuals | AD patients:25 Hypertension patients:25 Healthy subjects:25 |
Peripheral blood | AD | Tryptophan, histidine, and glycerophospholipid metabolic pathway |
| Yang et al23 | China | Comparison of plasma metabolites between thoracic aortic aneurysm (TAA), thoracic aortic dissection (TAD) patients and healthy controls. | TAA patients:70 TAD patients:70 Healthy controls:70 |
Plasma | AAD | Sphingolipid metabolic pathway |
| Huang et al168 | China | Identification of plasma lipidomics for patients with aortic dissection | AD patients:35 Healthy subjects:32 |
Plasma | AD | Lipid metabolic pathway |
| Wang et al170 | China | Identification of plasma amino acid profile in patients with AD | CHD patients:11 Acute AD patients:11 Chronic AD patients:11 |
Plasma | AD | Amino acids metabolic pathway |
| Jiang et al198 | China | Comparison of serum oxylipin profiles between acute aortic dissection patients and healthy individuals | TAAD patients:10 TBAD patients:10 Healthy controls:10 |
Serum | AD | Arachidonic acid metabolism pathway |
| Cibrowski et al166 | Spain | Comparison of plasma metabolites between patients with aneurysms and healthy subjects | AA Patients: 30 Healthy subjects: 11 |
Plasma | AA | Sphingolipids, lysophospholipids, and cholesterol metabolic pathway |
In addition to the role of metabolites as biomarkers in the diagnosis of AAD, the metabolic changes of AAD suggest a novel metabolic pathway for the treatment of AAD. Targeting metabolism has been proposed in the treatment of AAD and shows some achievement. Chronic inflammation of the aorta has been reported to underlie AAA pathology, with infiltrating leukocytes producing cytokines and proteases that ultimately lead to deterioration of the vessel wall172. To date, anti-inflammatory drugs and immunosuppressive drugs have been demonstrated to exert on the inhibition of aneurysm formation. For example, celecoxib, sirolimus, and cyclosporine A have been shown to prevent aneurysm formation potently in the animal aneurysm models, whereas hydrocortisone induced aortic rupture and aneurysm formation in mice173-175. Notably, Azathioprine (Aza), an immunosuppressive drug, could promote metabolite 6-MP to modulate the activation of c-Jun-terminal-N-kinase (JNK) in ECs, leading to suppress aneurysm progression176. Targetting amino acid metabolism and blocking Hcy accumulation can also be viable treatments for AAD. OT-58, the most representative drug for HHcy treatment, has entered phase 1/2 clinical trials and might play a crucial role in the future treatment of AAA177-179. At the same time, a low methionine diet combined with vitamins B6, B12, and FA can reduce serum Hcy concentration in HHcy patients with AAA, which may have a protective effect on AAA180, 181. Several IDO inhibitors which fulfill a function in Tryptophan, such as Indoximod, Epacadostat, and Navoximod, have been approved for patients in anti-tumor therapy182, but there is a lack of research to assess the application of these drugs on AAA. Studies have found that Glycine (Gly) has protective effects on lowering blood pressure183, 184, and supplementing the diet with an appropriate dose of Gly could prevent cardiovascular diseases, suggesting that Gly may play a powerful part in the treatment of AAD by controlling blood pressure which is an important risk factor for AAD.
Based on previous studies on the relationship between lipid metabolism and AAA, it is possible to develop drugs for the prevention and treatment of AAA from the perspective of regulating lipid metabolism. High density lipoprotein (HDL) enhanced the level of annexin A1 (Anxa1) to inhibit endothelial cell inflammation, while Anxa1 has been shown to restrain the occurrence of acute aortic dissection by inhibiting the phenotypic transformation of vascular smooth muscle cells, suggesting that there may be drug targets for the treatment of AAD that target the inflammation response to HDL185, 186. Furthermore, previous clinical studies reported that proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors can reduce AAA risk, and PCSK9 inhibitors can reduce low-density lipoprotein cholesterol (LDL) by inhibiting degradation of the LDL receptor, whereas mutations in PCSK9 aggravated AAA development in mice187. Although their clinical effect on AAA has not been tested, it provides a new perspective for the clinical treatment of AAD.
Therefore, the risk of AAD formation can be assessed clinically by detecting the levels of corresponding metabolites in plasma or serum. For patients with a high risk of AAD, such as the elderly and hypertension, dietary advice can also be provided to achieve the purpose of early prevention. In addition, although the research on the recognized therapeutic drugs for AAD is still a wastland to be reclaimed, the metabolism-related experimental animal studies have provided numerous new clues for potential drug targets.
Conclusion and perspectives
Increasing evidence indicates a link between the metabolomics and AAD incidence. Multiple metabolites interact with the host through a variety of pathways and play an indispensable role in the occurrence and development of diseases. Altered metabolite composition or different metabolite concentrations might be responsible for AAD risk and its associated pathological changes. Thus, novel therapeutic targets for preventing and treating AAD have been developed harnessing the potential use of metabolites.
Metabolic abnormalities are found in human AAD and animal models of AAD. The metabolism of gut microbiota-derived secondary metabolites is characterized by increased circulating levels of TMAO and LPS, decreased levels of bile acids including UDCA and TUDCA, and decreased short-chain fatty acids such as butyrate. The above characteristics indicate that the differential expression of secondary metabolites caused by intestinal flora disorder are engaged in the pathological process of AAD, which makes it possible to target AAD therapy against intestinal flora. In addition, the differentially expressed primary metabolites were observed to be mainly involved in amino acids, phosphatidylcholines, galactose, ceramide, and polyunsaturated FAs metabolic pathways in AAD,131, 165, 171 which suggested potential disorders of lipolysis, FA synthesis, and amino acids transport in patients suffering from AAD. Arginine metabolism, which links NO production and TCA cycle, has a key role linking the vasoconstrictive phenotype of the disease with the metabolic abnormalities.188 Moreover, changes in TCA cycle intermediates can contribute to regulate ROS generation, driving alterations in the functions of key genes of AAD. Decreased levels of AA and ceramide metabolism disturbance in the AAA patients supply further evidence of the inflammatory pathway in the pathogenesis of AAD, such as NLRP3 pathway.189, 190 Therefore, therapeutic approaches targeting metabolic disorders might be relevant to address many cellular pathologic phenotypes in AAD.
The existing research suggests that efforts are being made to advance the potential application of metabolites in CVD. However, limitations exist in studying metabolites in AAD. First of all, gut microbiota plays a crucial role in regulating gut microbiota-derived secondary metabolites. Identifying specific bacteria present in AAD can help clarify the contribution of gut microbiota-derived metabolites to disease progression. Nevertheless, research on the gut microbiota profile of AAD remains limited. Secondly, while most current studies focus on exploring the metabolic profile of AAD, including differential metabolites and changes in metabolite concentrations, they lack elucidation of the mechanism by which metabolites cause AAD. Future studies should therefore prioritize investigating signaling pathways mediated by metabolites and their downstream functional consequences. Thirdly, there is an urgent need for personalized metabolic profiling. The analysis of spatial metabolic biomarkers in individual AAD patients, as opposed to other cardiovascular diseases, may significantly contribute to this endeavor. Fourthly, the clinical application of metabolites in the treatment of AAD is hindered by a lack of safe and effective conversion pathways. It seems that inhibitors targeting metabolite production and dietary supplementation are effective ways to implement, but large-scale population validation is lacking. Finally, most studies focus on the effects of various metabolites on AA, and there are few studies in this field of AD, and we speculated that it may be caused by the characteristics of acute onset and elevated mortality of AD. However, it is of great significance to strengthen the study of metabolomics of AD to improve the metabolic profile of aortic diseases.
The focus of future studies is to figure out how metabolites lead to AAD at the mechanistic level. This involves characterizing metabolites, microbes, and microbial-host interactions within complex microbiota ecosystems. Recognizing the role of gut microbiota and metabolites in both health maintenance and disease susceptibility offers a novel perspective that broadens our approach to AAD research and therapeutic interventions. Additionally, it is important to investigate potential genetic factors that may influence susceptibility to disease in combination with specific nutrient depletion/excess and the functional capacity of the microbiota. Finally, prospective cohort studies can bring metabolite research and dietary interventions into clinical practice. By measuring metabolic profiles in blood or urine to guide appropriate dietary recommendations and deliver targeted interventions, more translational findings will be achieved. These interventions may take various forms in the future, including dietary advice, inhibitors or agonists of metabolites, nonlethal microbial inhibitors or probiotics. Another alternative approach is to modulate gut microbiota to achieve changes in gut microbiota-derived metabolites, which is an active area of research.
Based on the above limitations and perspectives, it is beneficial to address the question of how metabolic abnormalities develop in AAD in the future, thus determining which pathways are ideal therapeutic targets. Fully understanding metabolic abnormalities of AAD over the course of the disease is the essential next step for developing therapies.
Acknowledgments
This work was supported by the Sponsored by Natural Science Foundation of Xinjiang Uygur Automous Region (No. 2022D01D66) and Postgraduate Research Innovation Project of Xinjiang Uygur Automous Region (No. XJ2023G158).
Author contributions
Qi Wang and Xiang Ma designed the review and wrote the initial draft of the manuscript. Gulinazi Yesitayi, Bingyan Liu and Dilixiati Siti contributed to drafting the manuscript. Mierxiati Ainiwan and Aliya Aizitiaili reviewed and approved the manuscript. All authors read and approved the final manuscript.
Abbreviations
- AAD
Aortic aneurysm and dissection
- CHD
coronary heart disease
- ECM
extracellular matrix
- LMWMs
low-molecular-weight-metabolites
- NMR
Nuclear magnetic resonance
- MS
mass spectrometry
- GC-MS
gas chromatography-mass spectrometry
- LC-MS
liquid chromatography-mass spectrometry
- CE-MS
capillary electrophoresis-mass spectrometry
- IMS-MS
ion mobility spectrometrymass spectrometry
- CVD
cardiovascular disease
- AMI
acute myocardial infarction
- PE
pulmonary embolism
- AAA
abdominal aortic aneurysms
- DAO
diamine oxidase
- AF
atrial fibrillation
- CR
Caloric restriction
- TMAO
trimethylamine N-oxide
- SCFA
short-chain fatty acid
- BA
bile acid
- LPS
lipopolysaccharide
- DNA
Deoxyribonucleic Acid
- RNA
Reibonucleic Acid
- VSMC
vascular smooth muscle cell
- UDCA
ursodeoxycholic acid
- TUDCA
tauroursodeoxycholic acid
- BA
bile acid
- SCFA
short-chain fatty acid
- ER
endoplasmic reticulum
- NRF2
nuclear factor erythroid 2
- TMA
trimethylamine
- FMO3
flavin lpscontaining monooxygenase 3
- LPS
lipopolysaccharide
- ROS
reactive oxygen species
- IL-6
interleukin-6
- IL-1β
interleukin-1β
- pro-BDNF
brain-derived neurotrophic factor precursor
- ATP
adenosine triphosphate
- MAT
methionine adenosyltransferase
- SAM
s-adenosylmethionine
- SAH
S adenosyl L homocysteine
- MS
methionine synthetase
- SAHH
S adeno sylhom ocysteine hydrolase
- Tfh
follicular helper T cells
- Th17
T helper cell 17
- NADPH
nicotinamide adenine dinucleotide phosphate
- β2GP1
β2-Glycoprotein 1
- MMP2
matrix metalloproteinase 2
- MMP9
matrix metalloproteinase 9
- MCP-1
monocyte chemoattractant protein 1
- Arg
arginase
- Cys
cysteine
- Ang 2
angiopoietin 2
- iNOS
inducible nitric oxide synthase
- Sirt1
Sirtuin1
- ARG1
arginase 1
- ARG2
arginase 2
- NO
nitric oxide
- ADMA
asymmetric dimethylarginine
- eNOS
endothelial nitric oxide synthase
- EC
endothelial cell
- NOX
NAD(P)H oxidase
- 3-HK
3-hydroxykynurenine
- Kyn
kynurenine
- QA
quinolinic acid
- 3-HAA
3-hydroxyaminobranoic acid
- TDO
tryptophan-2,3-dioxygenase
- IDO
indoleamine 2, 3-dioxygenase
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- Treg
regulatory T cells
- GLA
gamma linolenic acid
- DGLA
dihomo gamma linolenic acid
- PGs
prostaglandins
- COX
cyclooxygenase
- VCAM-1
vascular cell adhesion molecule 1
- ICAM-1
intercellular adhesion factor 1
- MCP-1
monocyte chemoattractant protein 1
- TCA cycle
tricarboxylic acid cycle
- HSD
high salt diet
- α-KG
alpha‑ketoglutarate
- ACO2
aconitase-2
- Acetyl-CoA
acetyl coenzyme A
- OGDH
oxoglutarate dehydrogenase
- ETC
electron transport chain
- NR1D1
NR subfamily 1 group D member 1
- CREB
cyclic adenosine monophosphate-responsive element-binding protein 1
- 6βOHT
6βhydroxytestosterone
- UA
Uric acid
- TAD
thoracic aortic dissection
- TAA
thoracic aortic aneurysm
- Aza
Azathioprine
- JNK
c-Jun-terminal-N-kinase
- HDL
High density lipoprotein
- PCSK9
proprotein convertase subtilisin-kexin type 9
- LDL
low-density lipoprotein cholesterol
References
- 1.Bossone E & Eagle K A. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nature Reviews Cardiology. 2020;18:331–48. doi: 10.1038/s41569-020-00472-6. [DOI] [PubMed] [Google Scholar]
- 2.Isselbacher E M, Preventza O, Black J H 3rd, 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022. [DOI] [PMC free article] [PubMed]
- 3.Safi H J & Estrera A L. Aortic dissection. Br J Surg. 2004;91:523–5. doi: 10.1002/bjs.4631. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty A, Li Y, Zhang C. et al. Programmed cell death in aortic aneurysm and dissection: A potential therapeutic target. J Mol Cell Cardiol. 2022;163:67–80. doi: 10.1016/j.yjmcc.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ainiwan M, Wang Q, Yesitayi G. et al. Identification of FERMT1 and SGCD as key marker in acute aortic dissection from the perspective of predictive, preventive, and personalized medicine. EPMA Journal. 2022;13:597–614. doi: 10.1007/s13167-022-00302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliga R R, Nienaber C A, Bossone E. et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging. 2014;7:406–24. doi: 10.1016/j.jcmg.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Nienaber C A & Clough R E. Management of acute aortic dissection. Lancet. 2015;385:800–11. doi: 10.1016/S0140-6736(14)61005-9. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y H, Lemaire S A, Webb N R. et al. Aortic Aneurysms and Dissections Series. Arterioscler Thromb Vasc Biol. 2020;40:e37–e46. doi: 10.1161/ATVBAHA.120.313991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui H, Chen Y, Li K, Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. 2021; 42: 4373-85. [DOI] [PMC free article] [PubMed]
- 10.Zeng Q, Rong Y, Li D. et al. Identification of Serum Biomarker in Acute Aortic Dissection by Global and Targeted Metabolomics. Ann Vasc Surg. 2020;68:497–504. doi: 10.1016/j.avsg.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Rinschen M M & Ivanisevic J. Identification of bioactive metabolites using activity metabolomics. 2019; 20: 353-67. [DOI] [PMC free article] [PubMed]
- 12.Wishart D S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol Rev. 2019;99:1819–75. doi: 10.1152/physrev.00035.2018. [DOI] [PubMed] [Google Scholar]
- 13.Laíns I, Gantner M, Murinello S. et al. Metabolomics in the study of retinal health and disease. Prog Retin Eye Res. 2019;69:57–79. doi: 10.1016/j.preteyeres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Lee N & Spears M E. Endogenous toxic metabolites and implications in cancer therapy. 2020; 39: 5709-20. [DOI] [PMC free article] [PubMed]
- 15.Zheng X, Cai X & Hao H. Emerging targetome and signalome landscape of gut microbial metabolites. Cell metabolism. 2022;34:35–58. doi: 10.1016/j.cmet.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Wu L, Chen J. et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. 2021;6:94. doi: 10.1038/s41392-020-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavelle A & Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. 2020; 17: 223-37. [DOI] [PubMed]
- 18.Jin L, Shi X, Yang J. et al. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein & cell. 2021;12:346–59. doi: 10.1007/s13238-020-00785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paapstel K & Kals J. Metabolomics of Arterial Stiffness. 2022; 12. [DOI] [PMC free article] [PubMed]
- 20.Witkowski M, Weeks T L & Hazen S L. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127:553–70. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talmor-Barkan Y, Bar N, Shaul A A, Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. 2022; 28: 295-302. [DOI] [PubMed]
- 22.Benson T W, Conrad K A, Li X S. et al. Gut Microbiota-Derived Trimethylamine N-Oxide Contributes to Abdominal Aortic Aneurysm Through Inflammatory and Apoptotic Mechanisms. Circulation. 2023;147:1079–96. doi: 10.1161/CIRCULATIONAHA.122.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Yang F, Luo M. et al. Metabolomic Profile Reveals That Ceramide Metabolic Disturbance Plays an Important Role in Thoracic Aortic Dissection. Frontiers in cardiovascular medicine. 2022;9:826861. doi: 10.3389/fcvm.2022.826861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ursell L K, Haiser H J, Van Treuren W. et al. The intestinal metabolome: an intersection between microbiota and host. Gastroenterology. 2014;146:1470–6. doi: 10.1053/j.gastro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zmora N, Suez J & Elinav E. You are what you eat: diet, health and the gut microbiota. Nature reviews Gastroenterology & hepatology. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 26.Tang W H, Kitai T & Hazen S L. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183–96. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng W Y, Wu C Y & Yu J. The role of gut microbiota in cancer treatment: friend or foe? 2020; 69: 1867-76. [DOI] [PMC free article] [PubMed]
- 28.Violi F, Cammisotto V, Bartimoccia S, Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat Rev Cardiol. 2022. pp. 1–14. [DOI] [PMC free article] [PubMed]
- 29.Gu J, Hu J, Qian H. et al. Intestinal Barrier Dysfunction: A Novel Therapeutic Target for Inflammatory Response in Acute Stanford Type A Aortic Dissection. J Cardiovasc Pharmacol Ther. 2016;21:64–9. doi: 10.1177/1074248415581176. [DOI] [PubMed] [Google Scholar]
- 30.Larabi A, Barnich N & Nguyen H T T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16:38–51. doi: 10.1080/15548627.2019.1635384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zhang S, Li B, Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. 2022; 118: 785-97. [DOI] [PubMed]
- 32.Marques F Z, Nelson E, Chu P Y. et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–77. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X, Li J, Guo J. et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6:66. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji L, Gu G C, Chen S L. et al. [Differences of Gut Microbiota Diversity between Patients with Abdominal Aortic Aneurysm and Atherosclerosis] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2021;43:677–84. doi: 10.3881/j.issn.1000-503X.13443. [DOI] [PubMed] [Google Scholar]
- 35.Xie J, Lu W, Zhong L. et al. Alterations in gut microbiota of abdominal aortic aneurysm mice. BMC Cardiovasc Disord. 2020;20:32. doi: 10.1186/s12872-020-01334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian Z, Zhang Y, Zheng Z. et al. Gut microbiome dysbiosis contributes to abdominal aortic aneurysm by promoting neutrophil extracellular trap formation. Cell host & microbe. 2022;30:1450–63. doi: 10.1016/j.chom.2022.09.004. e8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X & Gérard P. Diet-gut microbiota interactions on cardiovascular disease. Computational and structural biotechnology journal. 2022;20:1528–40. doi: 10.1016/j.csbj.2022.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madeo F, Carmona-Gutierrez D, Hofer S J. et al. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell metabolism. 2019;29:592–610. doi: 10.1016/j.cmet.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Wang T T, Zhang R. et al. Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J Exp Med. 2016;213:2473–88. doi: 10.1084/jem.20151794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao P, Zhang H, Zhang Q. et al. Caloric Restriction Exacerbates Angiotensin II-Induced Abdominal Aortic Aneurysm in the Absence of p53. Hypertension. 2019;73:547–60. doi: 10.1161/HYPERTENSIONAHA.118.12086. [DOI] [PubMed] [Google Scholar]
- 41.Sastriques-Dunlop S, Elizondo-Benedetto S, Arif B, Ketosis Prevents Abdominal Aortic Aneurysm Rupture Through CCR2 Downregulation and Enhanced MMP Balance. bioRxiv: the preprint server for biology. 2023. [DOI] [PMC free article] [PubMed]
- 42.Kent K C, Zwolak R M, Egorova N N. et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–48. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 43.Stackelberg O, Björck M, Larsson S C. et al. Fruit and vegetable consumption with risk of abdominal aortic aneurysm. Circulation. 2013;128:795–802. doi: 10.1161/CIRCULATIONAHA.112.000728. [DOI] [PubMed] [Google Scholar]
- 44.Bergwall S, Acosta S & Sonestedt E. Intake of fibre and plant foods and the risk of abdominal aortic aneurysm in a large prospective cohort study in Sweden. Eur J Nutr. 2020;59:2047–56. doi: 10.1007/s00394-019-02054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto K, Kugo H, Tanaka H. et al. The Effect of a High-Fat Diet on the Development of Abdominal Aortic Aneurysm in a Vascular Hypoperfusion-Induced Animal Model. J Vasc Res. 2018;55:63–74. doi: 10.1159/000481780. [DOI] [PubMed] [Google Scholar]
- 46.Lynch S V & Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–79. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 47.Kåhrström C T, Pariente N & Weiss U. Intestinal microbiota in health and disease. Nature. 2016;535:47. doi: 10.1038/535047a. [DOI] [PubMed] [Google Scholar]
- 48.Koeth R A, Wang Z, Levison B S. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shih D M, Zhu W, Schugar R C. et al. Genetic Deficiency of Flavin-Containing Monooxygenase 3 ( Fmo3) Protects Against Thrombosis but Has Only a Minor Effect on Plasma Lipid Levels-Brief Report. Arterioscler Thromb Vasc Biol. 2019;39:1045–54. doi: 10.1161/ATVBAHA.119.312592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J, Xu J, Shen S. et al. Trimethylamine N-Oxide Promotes Abdominal Aortic Aneurysm Formation by Aggravating Aortic Smooth Muscle Cell Senescence in Mice. J Cardiovasc Transl Res. 2022;15:1064–74. doi: 10.1007/s12265-022-10211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang F, Zheng X, Ma X, Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. 2019; 10: 4971. [DOI] [PMC free article] [PubMed]
- 52.Di Ciaula A, Garruti G, Lunardi Baccetto R. et al. Bile Acid Physiology. Ann Hepatol. 2017;16:s4–s14. doi: 10.5604/01.3001.0010.5493. [DOI] [PubMed] [Google Scholar]
- 53.Liu W, Wang B, Wang T. et al. Ursodeoxycholic Acid Attenuates Acute Aortic Dissection Formation in Angiotensin II-Infused Apolipoprotein E-Deficient Mice Associated with Reduced ROS and Increased Nrf2 Levels. Cell Physiol Biochem. 2016;38:1391–405. doi: 10.1159/000443082. [DOI] [PubMed] [Google Scholar]
- 54.Qin Y, Wang Y, Liu O. et al. Tauroursodeoxycholic Acid Attenuates Angiotensin II Induced Abdominal Aortic Aneurysm Formation in Apolipoprotein E-deficient Mice by Inhibiting Endoplasmic Reticulum Stress. Eur J Vasc Endovasc Surg. 2017;53:337–45. doi: 10.1016/j.ejvs.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Van Den Munckhof I C L, Kurilshikov A, Ter Horst R. et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. 2018;19:1719–34. doi: 10.1111/obr.12750. [DOI] [PubMed] [Google Scholar]
- 56.Kayagaki N, Wong M T, Stowe I B. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–9. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 57.Salhi L, Rijkschroeff P, Van Hede D. et al. Blood Biomarkers and Serologic Immunological Profiles Related to Periodontitis in Abdominal Aortic Aneurysm Patients. Frontiers in cellular and infection microbiology. 2021;11:766462. doi: 10.3389/fcimb.2021.766462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meital L T, Windsor M T, Maynard A E, Endotoxin Tolerance in Abdominal Aortic Aneurysm Macrophages, In Vitro: A Case-Control Study. Antioxidants. 2020. 9. [DOI] [PMC free article] [PubMed]
- 59.Shen W Y, Luo C, Reinaldo Hurtado P. et al. The regulatory role of ProBDNF in monocyte function: Implications in Stanford type-A aortic dissection disease. FASEB J. 2020;34:2541–53. doi: 10.1096/fj.201901905RR. [DOI] [PubMed] [Google Scholar]
- 60.Tan C K, Tan E H, Luo B. et al. SMAD3 deficiency promotes inflammatory aortic aneurysms in angiotensin II-infused mice via activation of iNOS. Journal of the American Heart Association. 2013;2:e000269. doi: 10.1161/JAHA.113.000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dalile B, Van Oudenhove L, Vervliet B, The role of short-chain fatty acids in microbiota-gut-brain communication. 2019; 16: 461-78. [DOI] [PubMed]
- 62.Zhao Y, Chen F, Wu W. et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–62. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brandsma E, Kloosterhuis N J, Koster M. et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ Res. 2019;124:94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F, Xia N, Guo S. et al. Propionate Alleviates Abdominal Aortic Aneurysm by Modulating Colonic Regulatory T-Cell Expansion and Recirculation. JACC Basic to translational science. 2022;7:934–47. doi: 10.1016/j.jacbts.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugiyama K, Marzi J, Alber J. et al. Raman microspectroscopy and Raman imaging reveal biomarkers specific for thoracic aortic aneurysms. Cell reports Medicine. 2021;2:100261. doi: 10.1016/j.xcrm.2021.100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lieberg J, Wanhainen A, Ottas A, Metabolomic Profile of Abdominal Aortic Aneurysm. Metabolites. 2021. 11. [DOI] [PMC free article] [PubMed]
- 67.Molina-Sánchez P, Del Campo L, Esteban V. et al. Defective p27 phosphorylation at serine 10 affects vascular reactivity and increases abdominal aortic aneurysm development via Cox-2 activation. J Mol Cell Cardiol. 2018;116:5–15. doi: 10.1016/j.yjmcc.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Hermann A & Sitdikova G. Homocysteine: Biochemistry, Molecular Biology and Role in Disease. 2021; 11. [DOI] [PMC free article] [PubMed]
- 69.Jakubowski H. Translational incorporation of S-nitrosohomocysteine into protein. J Biol Chem. 2000;275:21813–6. doi: 10.1074/jbc.C000280200. [DOI] [PubMed] [Google Scholar]
- 70.Jakubowski H. Homocysteine Editing, Thioester Chemistry, Coenzyme A, and the Origin of Coded Peptide Synthesis †. Life (Basel, Switzerland) 2017. 7. [DOI] [PMC free article] [PubMed]
- 71.Jakubowski H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol Rev. 2019;99:555–604. doi: 10.1152/physrev.00003.2018. [DOI] [PubMed] [Google Scholar]
- 72.Fan Y, Li N, Liu C. et al. Excessive Methionine Supplementation Exacerbates the Development of Abdominal Aortic Aneurysm in Rats. J Vasc Res. 2019;56:230–40. doi: 10.1159/000501313. [DOI] [PubMed] [Google Scholar]
- 73.Li T, Yu B, Liu Z. et al. Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nature communications. 2018;9:11. doi: 10.1038/s41467-017-02401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z, Luo H, Zhang L. et al. Hyperhomocysteinemia exaggerates adventitial inflammation and angiotensin II-induced abdominal aortic aneurysm in mice. Circ Res. 2012;111:1261–73. doi: 10.1161/CIRCRESAHA.112.270520. [DOI] [PubMed] [Google Scholar]
- 75.Shao F, Miao Y, Zhang Y. et al. B cell-derived anti-beta 2 glycoprotein I antibody contributes to hyperhomocysteinaemia-aggravated abdominal aortic aneurysm. Cardiovasc Res. 2020;116:1897–909. doi: 10.1093/cvr/cvz288. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Wei G, Cheng S. et al. Circulatory CD4-Positive T-Lymphocyte Imbalance Mediated by Homocysteine-Induced AIM2 and NLRP1 Inflammasome Upregulation and Activation Is Associated with Human Abdominal Aortic Aneurysm. J Vasc Res. 2020;57:276–90. doi: 10.1159/000508077. [DOI] [PubMed] [Google Scholar]
- 77.Roohi J, Kang B, Bernard D. et al. Moderately Elevated Homocysteine Does Not Contribute to Thoracic Aortic Aneurysm in Mice. The Journal of Nutrition. 2017;147:1290–5. doi: 10.3945/jn.117.251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visser M, Paulus W J, Vermeulen M A. et al. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur J Heart Fail. 2010;12:1274–81. doi: 10.1093/eurjhf/hfq158. [DOI] [PubMed] [Google Scholar]
- 79.Brinkmann S J, Buijs N & Vermeulen M A. Perioperative glutamine supplementation restores disturbed renal arginine synthesis after open aortic surgery: a randomized controlled clinical trial. 2016; 311: F567-75. [DOI] [PubMed]
- 80.Murray P J, Rathmell J & Pearce E. SnapShot: Immunometabolism. Cell metabolism. 2015;22:190–e1. doi: 10.1016/j.cmet.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Xu J, Liu Y. et al. Mouse macrophage specific knockout of SIRT1 influences macrophage polarization and promotes angiotensin II-induced abdominal aortic aneurysm formation. Journal of genetics and genomics = Yi chuan xue bao. 2018;45:25–32. doi: 10.1016/j.jgg.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 82.Lu Y, Ma Q, Tan H. et al. Specific inhibition of SHP2 suppressed abdominal aortic aneurysm formation in mice by augmenting the immunosuppressive function of MDSCs. Life Sci. 2021;265:118751. doi: 10.1016/j.lfs.2020.118751. [DOI] [PubMed] [Google Scholar]
- 83.Chen C L, Hsu S C, Ann D K, Arginine Signaling and Cancer Metabolism. Cancers (Basel) 2021. 13. [DOI] [PMC free article] [PubMed]
- 84.Kakimoto Y & Akazawa S. Isolation and identification of N-G,N-G- and N-G,N'-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970;245:5751–8. [PubMed] [Google Scholar]
- 85.Pyun J H, Ahn B Y, Vuong T A. et al. Inducible Prmt1 ablation in adult vascular smooth muscle leads to contractile dysfunction and aortic dissection. Exp Mol Med. 2021;53:1569–79. doi: 10.1038/s12276-021-00684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun J, Deng H, Zhou Z, Endothelium as a Potential Target for Treatment of Abdominal Aortic Aneurysm. 2018; 2018: 6306542. [DOI] [PMC free article] [PubMed]
- 87.Wang S, Wu J, Li X, CXCR6 Mediates Pressure Overload-Induced Aortic Stiffness by Increasing Macrophage Recruitment and Reducing Exosome-miRNA29b. J Cardiovasc Transl Res. 2022. [DOI] [PubMed]
- 88.Izawa-Ishizawa Y, Imanishi M, Zamami Y. et al. Development of a novel aortic dissection mouse model and evaluation of drug efficacy using in-vivo assays and database analyses. J Hypertens. 2019;37:73–83. doi: 10.1097/HJH.0000000000001898. [DOI] [PubMed] [Google Scholar]
- 89.Bicknell C D, Kiru G, Falaschetti E. et al. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK) Eur Heart J. 2016;37:3213–21. doi: 10.1093/eurheartj/ehw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gostner J M, Geisler S, Stonig M. et al. Tryptophan Metabolism and Related Pathways in Psychoneuroimmunology: The Impact of Nutrition and Lifestyle. Neuropsychobiology. 2020;79:89–99. doi: 10.1159/000496293. [DOI] [PubMed] [Google Scholar]
- 91.Guan X, Xin H, Xu M. et al. The Role and Mechanism of SIRT6 in Regulating Phenotype Transformation of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm. Comput Math Methods Med. 2022;2022:3200798. doi: 10.1155/2022/3200798. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Barik S. The Uniqueness of Tryptophan in Biology: Properties, Metabolism, Interactions and Localization in Proteins. International journal of molecular sciences. 2020. 21. [DOI] [PMC free article] [PubMed]
- 93.Nishimura M, Yamashita A, Matsuura Y. et al. Upregulated Kynurenine Pathway Enzymes in Aortic Atherosclerotic Aneurysm: Macrophage Kynureninase Downregulates Inflammation. Journal of atherosclerosis and thrombosis. 2021;28:1214–40. doi: 10.5551/jat.58248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q, Ding Y, Song P. et al. Tryptophan-Derived 3-Hydroxyanthranilic Acid Contributes to Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice In Vivo. Circulation. 2017;136:2271–83. doi: 10.1161/CIRCULATIONAHA.117.030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bader M, Metghalchi S, Vandestienne M. et al. Indoleamine 2 3-dioxygenase knockout limits angiotensin II-induced aneurysm in low density lipoprotein receptor-deficient mice fed with high fat diet. PLoS One. 2018;13:e0193737. doi: 10.1371/journal.pone.0193737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Q, Zhang M, Ding Y. et al. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res. 2014;114:480–92. doi: 10.1161/CIRCRESAHA.114.302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jakaria M, Azam S, Haque M E. et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox biology. 2019;24:101223. doi: 10.1016/j.redox.2019.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qaradakhi T, Gadanec L K, Mcsweeney K R, The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. 2020; 12. [DOI] [PMC free article] [PubMed]
- 99.Kim H W, Blomkalns A L, Ogbi M. et al. Role of myeloperoxidase in abdominal aortic aneurysm formation: mitigation by taurine. Am J Physiol Heart Circ Physiol. 2017;313:H1168–H79. doi: 10.1152/ajpheart.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng X, Li J M, Liao X B. et al. Taurine suppresses osteoblastic differentiation of aortic valve interstitial cells induced by beta-glycerophosphate disodium, dexamethasone and ascorbic acid via the ERK pathway. Amino Acids. 2012;43:1697–704. doi: 10.1007/s00726-012-1253-7. [DOI] [PubMed] [Google Scholar]
- 101.Das U N. Saturated Fatty Acids, MUFAs and PUFAs Regulate Ferroptosis. Cell chemical biology. 2019;26:309–11. doi: 10.1016/j.chembiol.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Bang H O, Dyerberg J & Sinclair H M. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr. 1980;33:2657–61. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 103.Kagawa Y, Nishizawa M, Suzuki M. et al. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. J Nutr Sci Vitaminol (Tokyo) 1982;28:441–53. doi: 10.3177/jnsv.28.441. [DOI] [PubMed] [Google Scholar]
- 104.D'helft J & Caccialanza R. Relevance of ω-6 GLA Added to ω-3 PUFAs Supplements for ADHD: A Narrative Review. 2022; 14. [DOI] [PMC free article] [PubMed]
- 105.Borges M C, Haycock P C, Zheng J. et al. Role of circulating polyunsaturated fatty acids on cardiovascular diseases risk: analysis using Mendelian randomization and fatty acid genetic association data from over 114,000 UK Biobank participants. BMC Med. 2022;20:210. doi: 10.1186/s12916-022-02399-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan S, Back M, Bruzelius M, Plasma Phospholipid Fatty Acids, FADS1 and Risk of 15 Cardiovascular Diseases: A Mendelian Randomisation Study. Nutrients. 2019. 11. [DOI] [PMC free article] [PubMed]
- 107.Das U N. Is Aortic Aneurysm Preventable? Journal of translational internal medicine. 2017;5:72–8. doi: 10.1515/jtim-2017-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou Y, Khan H, Xiao J, Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. International journal of molecular sciences. 2021. 22. [DOI] [PMC free article] [PubMed]
- 109.Soto M E, Guarner-Lans V, Herrera-Morales K Y. et al. Participation of Arachidonic Acid Metabolism in the Aortic Aneurysm Formation in Patients with Marfan Syndrome. Front Physiol. 2018;9:77. doi: 10.3389/fphys.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olechowski B, Khanna V, Mariathas M. et al. Changes in platelet function with inflammation in patients undergoing vascular surgery. Platelets. 2019;30:190–8. doi: 10.1080/09537104.2017.1392498. [DOI] [PubMed] [Google Scholar]
- 111.Walton L J, Franklin I J, Bayston T. et al. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.cir.100.1.48. [DOI] [PubMed] [Google Scholar]
- 112.Tani T, Ayuzawa R, Takagi T. et al. Angiotensin II bi-directionally regulates cyclooxygenase-2 expression in intestinal epithelial cells. Mol Cell Biochem. 2008;315:185–93. doi: 10.1007/s11010-008-9806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Camacho M, Dilmé J, Solà-Villà D. et al. Microvascular COX-2/mPGES-1/EP-4 axis in human abdominal aortic aneurysm. J Lipid Res. 2013;54:3506–15. doi: 10.1194/jlr.M042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu B, Kong J, An G. et al. Regulatory T cells protected against abdominal aortic aneurysm by suppression of the COX-2 expression. J Cell Mol Med. 2019;23:6766–74. doi: 10.1111/jcmm.14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shahidi F & Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annual review of food science and technology. 2018;9:345–81. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 116.Nogal A & Valdes A M. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. 2021; 13: 1-24. [DOI] [PMC free article] [PubMed]
- 117.Meital L T, Windsor M T, Ramirez Jewell R M L. et al. n-3 PUFAs improve erythrocyte fatty acid profile in patients with small AAA: a randomized controlled trial. J Lipid Res. 2019;60:1154–63. doi: 10.1194/jlr.P093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wales K M, Kavazos K, Nataatmadja M. et al. N-3 PUFAs protect against aortic inflammation and oxidative stress in angiotensin II-infused apolipoprotein E-/- mice. PLoS One. 2014;9:e112816. doi: 10.1371/journal.pone.0112816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Wang B, Li H. et al. Quercetin, a flavonoid with anti-inflammatory activity, suppresses the development of abdominal aortic aneurysms in mice. Eur J Pharmacol. 2012;690:133–41. doi: 10.1016/j.ejphar.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 120.Phie J, Moxon J V, Krishna S M. et al. A diet enriched with tree nuts reduces severity of atherosclerosis but not abdominal aneurysm in angiotensin II-infused apolipoprotein E deficient mice. Atherosclerosis. 2018;277:28–33. doi: 10.1016/j.atherosclerosis.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Aikawa T, Miyazaki T, Shimada K. et al. Low Serum Levels of EPA are Associated with the Size and Growth Rate of Abdominal Aortic Aneurysm. Journal of atherosclerosis and thrombosis. 2017;24:912–20. doi: 10.5551/jat.38315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fujishima R, Kugo H, Yanagimoto K. et al. Similar distribution of orally administered eicosapentaenoic acid and M2 macrophage marker in the hypoperfusion-induced abdominal aortic aneurysm wall. Food & function. 2021;12:3469–75. doi: 10.1039/d0fo03317k. [DOI] [PubMed] [Google Scholar]
- 123.Wang J H, Eguchi K, Matsumoto S. et al. The omega-3 polyunsaturated fatty acid, eicosapentaenoic acid, attenuates abdominal aortic aneurysm development via suppression of tissue remodeling. PLoS One. 2014;9:e96286. doi: 10.1371/journal.pone.0096286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshihara T, Shimada K, Fukao K. et al. Omega 3 Polyunsaturated Fatty Acids Suppress the Development of Aortic Aneurysms Through the Inhibition of Macrophage-Mediated Inflammation. Circ J. 2015;79:1470–8. doi: 10.1253/circj.CJ-14-0471. [DOI] [PubMed] [Google Scholar]
- 125.Kugo H, Enomoto H, Yanagimoto K. et al. Eicosapentaenoic acid is associated with the attenuation of dysfunctions of mesenchymal stem cells in the abdominal aortic aneurysm wall. Food & function. 2022;13:7540–7. doi: 10.1039/d2fo01102f. [DOI] [PubMed] [Google Scholar]
- 126.Kugo H, Zaima N, Onozato M. et al. Suppressive effects of dietary EPA-rich fish oil on the degradation of elastin fibers in the aortic wall in nicotine-administered mice. Food & function. 2017;8:2829–35. doi: 10.1039/c7fo00553a. [DOI] [PubMed] [Google Scholar]
- 127.Meital L T, Windsor M T, Perissiou M, Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci Rep. 2019. 9. [DOI] [PMC free article] [PubMed]
- 128.Schopfer F J & Khoo N K H. Nitro-Fatty Acid Logistics: Formation, Biodistribution, Signaling, and Pharmacology. Trends Endocrinol Metab. 2019;30:505–19. doi: 10.1016/j.tem.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nettersheim F S, Lemties J, Braumann S. et al. Nitro-oleic acid reduces thoracic aortic aneurysm progression in a mouse model of Marfan syndrome. Cardiovasc Res. 2022;118:2211–25. doi: 10.1093/cvr/cvab256. [DOI] [PubMed] [Google Scholar]
- 130.Zhong S, Li L, Shen X. et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med. 2019;144:266–78. doi: 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 131.Zhou X, Wang R, Zhang T. et al. Identification of Lysophosphatidylcholines and Sphingolipids as Potential Biomarkers for Acute Aortic Dissection via Serum Metabolomics. Eur J Vasc Endovasc Surg. 2019;57:434–41. doi: 10.1016/j.ejvs.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Shi F, Ma C, Ji C, Serum Lipid Oxidative Stress Products as Risk Factors Are the Candidate Predictive Biomarkers for Human Abdominal Aortic Aneurysms. 2020; 26: 1076029620932226. [DOI] [PMC free article] [PubMed]
- 133.Dang G, Li T, Yang D. et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox biology. 2022;50:102257. doi: 10.1016/j.redox.2022.102257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y, Chen Y C, Krummel M F. et al. Autotaxin through lysophosphatidic acid stimulates polarization, motility, and transendothelial migration of naive T cells. J Immunol. 2012;189:3914–24. doi: 10.4049/jimmunol.1201604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miao Y, Zhao Y, Han L. et al. NSun2 regulates aneurysm formation by promoting autotaxin expression and T cell recruitment. Cell Mol Life Sci. 2021;78:1709–27. doi: 10.1007/s00018-020-03607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mayr M.
- 137.Allen-Redpath K, Aldrovandi M, Lauder S N, Phospholipid membranes drive abdominal aortic aneurysm development through stimulating coagulation factor activity. 2019; 116: 8038-47. [DOI] [PMC free article] [PubMed]
- 138.Deckert V, Kretz B, Habbout A. et al. Development of abdominal aortic aneurysm is decreased in mice with plasma phospholipid transfer protein deficiency. Am J Pathol. 2013;183:975–86. doi: 10.1016/j.ajpath.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 139.Ortiz-Muñoz G, Houard X, Martín-Ventura J L. et al. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 2009;23:3129–39. doi: 10.1096/fj.08-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rizzo M, Krayenbühl P A, Pernice V. et al. LDL size and subclasses in patients with abdominal aortic aneurysm. Int J Cardiol. 2009;134:406–8. doi: 10.1016/j.ijcard.2007.12.082. [DOI] [PubMed] [Google Scholar]
- 141.Widlansky M E & Gutterman D D. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxidants & redox signaling. 2011;15:1517–30. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao R Z, Jiang S, Zhang L. et al. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int J Mol Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Robb E L, Hall A R, Prime T A, Control of mitochondrial superoxide production by reverse electron transport at complex I. 2018; 293: 9869-79. [DOI] [PMC free article] [PubMed]
- 144.Butt H Z, Sylvius N, Salem M K. et al. Microarray-based Gene Expression Profiling of Abdominal Aortic Aneurysm. Eur J Vasc Endovasc Surg. 2016;52:47–55. doi: 10.1016/j.ejvs.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 145.Van Der Pluijm I, Burger J, Van Heijningen P M. et al. Decreased mitochondrial respiration in aneurysmal aortas of Fibulin-4 mutant mice is linked to PGC1A regulation. Cardiovasc Res. 2018;114:1776–93. doi: 10.1093/cvr/cvy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zamorano-Leon J J, Serna-Soto M, Monux G. et al. Factor Xa Inhibition by Rivaroxaban Modified Mitochondrial-Associated Proteins in Human Abdominal Aortic Aneurysms. Ann Vasc Surg. 2020;67:482–9. doi: 10.1016/j.avsg.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Oller J, Gabandé-Rodríguez E, Ruiz-Rodríguez M J. et al. Extracellular Tuning of Mitochondrial Respiration Leads to Aortic Aneurysm. Circulation. 2021;143:2091–109. doi: 10.1161/CIRCULATIONAHA.120.051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bullo M, Papandreou C, Garcia-Gavilan J. et al. Tricarboxylic acid cycle related-metabolites and risk of atrial fibrillation and heart failure. Metabolism. 2021;125:154915. doi: 10.1016/j.metabol.2021.154915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan R, Rong Z, She Y. et al. Sodium pyruvate reduces hypoxic-ischemic injury to neonatal rat brain. Pediatr Res. 2012;72:479–89. doi: 10.1038/pr.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y, Asnani A, Zou L. et al. Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase. Sci Transl Med. 2014;6:266ra170. doi: 10.1126/scitranslmed.3010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lahey R, Carley A N, Wang X. et al. Enhanced Redox State and Efficiency of Glucose Oxidation With miR Based Suppression of Maladaptive NADPH-Dependent Malic Enzyme 1 Expression in Hypertrophied Hearts. Circ Res. 2018;122:836–45. doi: 10.1161/CIRCRESAHA.118.312660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu H, Du S, Fang B. et al. VSMC-specific EP4 deletion exacerbates angiotensin II-induced aortic dissection by increasing vascular inflammation and blood pressure. Proc Natl Acad Sci U S A. 2019;116:8457–62. doi: 10.1073/pnas.1902119116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zheng X, Zhao X, Jin Y. et al. High salt diet contributes to hypertension by weakening the medullary tricarboxylic acid cycle and antioxidant system in Dahl salt-sensitive rats. Biochimie. 2021;181:154–61. doi: 10.1016/j.biochi.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Liu J, Liu M, Feng J. et al. Alpha-ketoglutarate ameliorates abdominal aortic aneurysm via inhibiting PXDN/HOCL/ERK signaling pathways. J Transl Med. 2022;20:461. doi: 10.1186/s12967-022-03659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun L Y, Lyu Y Y, Zhang H Y, Nuclear Receptor NR1D1 Regulates Abdominal Aortic Aneurysm Development by Targeting the Mitochondrial Tricarboxylic Acid Cycle Enzyme Aconitase-2. 2022; 146: 1591-609. [DOI] [PMC free article] [PubMed]
- 156.Mukherjee K, Pingili A K, Singh P. et al. Testosterone Metabolite 6β-Hydroxytestosterone Contributes to Angiotensin II-Induced Abdominal Aortic Aneurysms in Apoe(-/-) Male Mice. Journal of the American Heart Association. 2021;10:e018536. doi: 10.1161/JAHA.120.018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nsengiyumva V, Krishna S M, Moran C S. et al. Vitamin D deficiency promotes large rupture-prone abdominal aortic aneurysms and cholecalciferol supplementation limits progression of aneurysms in a mouse model. Clin Sci (Lond) 2020;134:2521–34. doi: 10.1042/CS20200980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jiang W L, Qi X, Li X. et al. Serum uric acid is associated with aortic dissection in Chinese men. Int J Cardiol. 2016;202:196–7. doi: 10.1016/j.ijcard.2015.08.174. [DOI] [PubMed] [Google Scholar]
- 159.Zhang L, Zhou J & Jing Z. Serum uric acid might be associated with aortic dissection in Chinese men. Int J Cardiol. 2016;203:420–1. doi: 10.1016/j.ijcard.2015.10.185. [DOI] [PubMed] [Google Scholar]
- 160.Cai J, Zhang Y, Zou J, Serum uric acid could be served as an independent marker for increased risk and severity of ascending aortic dilatation in Behçet's disease patients. 2019; 33: e22637. [DOI] [PMC free article] [PubMed]
- 161.Esen A M, Akcakoyun M, Esen O. et al. Uric acid as a marker of oxidative stress in dilatation of the ascending aorta. Am J Hypertens. 2011;24:149–54. doi: 10.1038/ajh.2010.219. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Y, Xu X, Lu Y, Preoperative uric acid predicts in-hospital death in patients with acute type a aortic dissection. 2020; 15: 21. [DOI] [PMC free article] [PubMed]
- 163.Wang K, Zhao J, Zhang W. et al. Resveratrol Attenuates Aortic Dissection by Increasing Endothelial Barrier Function Through the SIRT1 Pathway. J Cardiovasc Pharmacol. 2020;76:86–93. doi: 10.1097/FJC.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiong J M, Liu H, Chen J. et al. Curcumin nicotinate suppresses abdominal aortic aneurysm pyroptosis via lncRNA PVT1/miR-26a/KLF4 axis through regulating the PI3K/AKT signaling pathway. Toxicology research. 2021;10:651–61. doi: 10.1093/toxres/tfab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lieberg J, Wanhainen A, Ottas A, Metabolomic Profile of Abdominal Aortic Aneurysm. 2021; 11. [DOI] [PMC free article] [PubMed]
- 166.Ciborowski M, Teul J, Martin-Ventura J L. et al. Metabolomics with LC-QTOF-MS permits the prediction of disease stage in aortic abdominal aneurysm based on plasma metabolic fingerprint. PLoS One. 2012;7:e31982. doi: 10.1371/journal.pone.0031982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ji L, Chen S, Gu G. et al. Discovery of potential biomarkers for human atherosclerotic abdominal aortic aneurysm through untargeted metabolomics and transcriptomics. Journal of Zhejiang University Science B. 2021;22:733–45. doi: 10.1631/jzus.B2000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang H, Ye G, Lai S Q. et al. Plasma Lipidomics Identifies Unique Lipid Signatures and Potential Biomarkers for Patients With Aortic Dissection. Frontiers in cardiovascular medicine. 2021;8:757022. doi: 10.3389/fcvm.2021.757022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ren Y, Tang Q, Liu W. et al. Serum Biomarker Identification by Mass Spectrometry in Acute Aortic Dissection. Cell Physiol Biochem. 2017;44:2147–57. doi: 10.1159/000485954. [DOI] [PubMed] [Google Scholar]
- 170.Wang L, Liu S, Yang W. et al. Plasma Amino Acid Profile in Patients with Aortic Dissection. Sci Rep. 2017;7:40146. doi: 10.1038/srep40146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang K, Pan X, Zheng J. et al. The metabolic analysis in human aortic tissues of aortic dissection. J Clin Lab Anal. 2022;36:e24623. doi: 10.1002/jcla.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shimizu K, Mitchell R N & Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–94. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 173.King V L, Trivedi D B, Gitlin J M. et al. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–43. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 174.Lawrence D M, Singh R S, Franklin D P. et al. Rapamycin suppresses experimental aortic aneurysm growth. J Vasc Surg. 2004;40:334–8. doi: 10.1016/j.jvs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 175.Esteban V, Méndez-Barbero N, Jiménez-Borreguero L J. et al. Regulator of calcineurin 1 mediates pathological vascular wall remodeling. J Exp Med. 2011;208:2125–39. doi: 10.1084/jem.20110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marinkovic G, Hibender S, Hoogenboezem M. et al. Immunosuppressive drug azathioprine reduces aneurysm progression through inhibition of Rac1 and c-Jun-terminal-N-kinase in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:2380–8. doi: 10.1161/ATVBAHA.113.301394. [DOI] [PubMed] [Google Scholar]
- 177.Xu W, Comhair S a A, Chen R. et al. Integrative proteomics and phosphoproteomics in pulmonary arterial hypertension. Sci Rep. 2019;9:18623. doi: 10.1038/s41598-019-55053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fijalkowska I, Xu W, Comhair S A. et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176:1130–8. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Archer S & Rich S. Primary pulmonary hypertension: a vascular biology and translational research "Work in progress". Circulation. 2000;102:2781–91. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- 180.Xu W, Koeck T, Lara A R. et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 2007;104:1342–7. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Asosingh K & Erzurum S. Mechanisms of right heart disease in pulmonary hypertension (2017 Grover Conference Series) Pulmonary circulation. 2018;8:2045893217753121. doi: 10.1177/2045893217753121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lanser L, Kink P, Egger E M. et al. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front Immunol. 2020;11:249. doi: 10.3389/fimmu.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stamler J, Brown I J, Daviglus M L. et al. Dietary glycine and blood pressure: the International Study on Macro/Micronutrients and Blood Pressure. Am J Clin Nutr. 2013;98:136–45. doi: 10.3945/ajcn.112.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.El Hafidi M, Pérez I & Baños G. Is glycine effective against elevated blood pressure? Curr Opin Clin Nutr Metab Care. 2006;9:26–31. doi: 10.1097/01.mco.0000196143.72985.9a. [DOI] [PubMed] [Google Scholar]
- 185.Zhou C & Lin Z. Anxa1 in smooth muscle cells protects against acute aortic dissection. 2022; 118: 1564-82. [DOI] [PubMed]
- 186.Pan B, Kong J, Jin J. et al. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating annexin A1 in vascular endothelial cells. Biochim Biophys Acta. 2016;1861:501–12. doi: 10.1016/j.bbalip.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 187.Guo Y, Tang Z, Yan B, PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Triggers Vascular Smooth Muscle Cell Senescence and Apoptosis: Implication of Its Direct Role in Degenerative Vascular Disease. 2022; 42: 67-86. [DOI] [PubMed]
- 188.Wu Q & Schapira M. Protein arginine methylation: from enigmatic functions to therapeutic targeting. 2021; 20: 509-30. [DOI] [PubMed]
- 189.Jia Y, Zhang L, Liu Z, Targeting macrophage TFEB-14-3-3 epsilon Interface by naringenin inhibits abdominal aortic aneurysm. 2022; 8: 21. [DOI] [PMC free article] [PubMed]
- 190.Ren P, Wu D, Appel R. et al. Targeting the NLRP3 Inflammasome With Inhibitor MCC950 Prevents Aortic Aneurysms and Dissections in Mice. Journal of the American Heart Association. 2020;9:e014044. doi: 10.1161/JAHA.119.014044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sbarouni E, Georgiadou P, Analitis A. et al. High homocysteine and low folate concentrations in acute aortic dissection. Int J Cardiol. 2013;168:463–6. doi: 10.1016/j.ijcard.2012.09.127. [DOI] [PubMed] [Google Scholar]
- 192.Vats S, Sundquist K, Wang X. et al. Associations of global DNA methylation and homocysteine levels with abdominal aortic aneurysm: A cohort study from a population-based screening program in Sweden. Int J Cardiol. 2020;321:137–42. doi: 10.1016/j.ijcard.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 193.Wong Y Y, Golledge J, Flicker L. et al. Plasma total homocysteine is associated with abdominal aortic aneurysm and aortic diameter in older men. J Vasc Surg. 2013;58:364–70. doi: 10.1016/j.jvs.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 194.Deng J, Liu J, Cao L, The Association between Hyperhomocysteinemia and Thoracoabdominal Aortic Aneurysms in Chinese Population. 2020; 2020: 4691026. [DOI] [PMC free article] [PubMed]
- 195.Liu J, Zuo S W, Li Y. et al. Hyperhomocysteinaemia is an independent risk factor of abdominal aortic aneurysm in a Chinese Han population. Sci Rep. 2016;6:17966. doi: 10.1038/srep17966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chan C Y T & Cheng S W K. Elevated homocysteine in human abdominal aortic aneurysmal tissues. Vasc Med. 2017;22:370–7. doi: 10.1177/1358863X17718260. [DOI] [PubMed] [Google Scholar]
- 197.Siennicka A, Zuchowski M, Chełstowski K. et al. Homocysteine-Enhanced Proteolytic and Fibrinolytic Processes in Thin Intraluminal Thrombus and Adjacent Wall of Abdominal Aortic Aneurysm: Study In Vitro. Biomed Res Int. 2018;2018:3205324. doi: 10.1155/2018/3205324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jiang Y, Tang X, Wang Y, Serum Oxylipin Profiles Identify Potential Biomarkers in Patients with Acute Aortic Dissection. Metabolites. 2022. 12. [DOI] [PMC free article] [PubMed]