Abstract
Despite the growing recognition that gastrointestinal (GI) dysfunction is prevalent in Parkinson’s disease (PD) and occurs as a major prodromal symptom of PD, its cellular and molecular mechanisms remain largely unknown. Among the various types of GI cells, enteric glial cells (EGCs), which resemble astrocytes in structure and function, play a critical role in the pathophysiology of many GI diseases including PD. Thus, we investigated how EGCs respond to the environmental pesticides rotenone (Rot) and tebufenpyrad (Tebu) in cell and animal models to better understand the mechanism underlying GI abnormalities. Both Rot and Tebu induce dopaminergic neuronal cell death through complex 1 inhibition of the mitochondrial respiratory chain. We report that exposing a rat enteric glial cell model (CRL-2690 cells) to these pesticides increased mitochondrial fission and reduced mitochondrial fusion by impairing MFN2 function. Furthermore, they also increased mitochondrial superoxide generation and impaired mitochondrial ATP levels and basal respiratory rate. Measurement of LC3, p62 and lysosomal assays revealed impaired autolysosomal function in ECGs during mitochondrial stress. Consistent with our recent findings that mitochondrial dysfunction augments inflammation in astrocytes and microglia, we found that neurotoxic pesticide exposure also enhanced the production of pro-inflammatory factors in EGCs in direct correlation with the loss in mitochondrial mass. Finally, we show that pesticide-induced mitochondrial defects functionally impaired smooth muscle velocity, acceleration, and total kinetic energy in a mixed primary culture of the enteric nervous system (ENS). Collectively, our studies demonstrate for the first time that exposure to environmental neurotoxic pesticides impairs mitochondrial bioenergetics and activates inflammatory pathways in EGCs, further augmenting mitochondrial dysfunction and pro-inflammatory events to induce gut dysfunction. Our findings have major implications in understanding the GI-related pathogenesis and progression of environmentally linked PD.
Keywords: rotenone, tebufenpyrad, enteric glial cells, enteric nervous system, reactive oxygen species, autophagy
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease, affecting around 6.1 million people globally. According to a systematic analysis for the Global Burden of Disease Study 2016, PD is one of the fastest-growing neurological disorders in prevalence, disability, and deaths, imposing a heavy global burden on public health as well as socio-economic development (Dorsey et al., 2018). Loss of dopaminergic neurons in the substantia nigra (SN) of the mid-brain and the widespread accumulation of intracellular α-synuclein protein aggregates are usually considered proof for the definitive diagnosis of idiopathic PD (Armstrong & Okun, 2020; Poewe et al., 2017). Although the etiopathogenesis of PD is still ambiguous, increasing evidence over the past decade suggests environmental factors as a trigger for idiopathic PD (Fleming, 2017; Song et al., 2019). Diagnosing PD currently relies on clinically evident motor symptoms, which arise only after the loss of >50% of dopaminergic neurons during the progression of PD, suggesting its onset occurs much earlier than the key PD features (Dijkstra et al., 2014; Poewe et al., 2017; Poirier et al., 2016). This would explain the development of non-motor symptoms several years preceding the motor symptoms in PD. The non-motor symptoms of PD are varied, ranging from neuropsychiatric symptoms like depression and cognitive dysfunction, sleep disorders, hyposmia, autonomic symptoms including bladder and erectile dysfunction, gastrointestinal (GI) symptoms like constipation, and pain-related sensory symptoms (Chaudhuri & Schapira, 2009; Klingelhoefer & Reichmann, 2015).
Identifying early biomarkers of PD has become a crucially important challenge. Although the mechanisms are largely unknown, GI dysfunction could potentially serve as a disease indicator (Poirier et al., 2016). The common symptoms of GI impairments include dysphagia, gastroparesis, prolonged gastrointestinal transit time, and constipation (Cersosimo et al., 2013). Recently, GI pathogenesis in PD has been linked to aggregates of α-synuclein fibrils found in the enteric nervous system (ENS) (Chaudhuri & Schapira, 2009).
The growing number of evidence-based studies in the last couple of decades linking the gut and brain through bidirectional communication between the ENS and CNS has fueled its current prominence in the field of PD (Arotcarena et al., 2020; Ghaisas et al., 2016). The ENS, along with enteric glial cells (EGCs), functions as a semi-autonomous nervous system that maintains epithelial barrier function and transmucosal fluid movement, interacts with the immune and endocrine systems, and regulates GI motility through its motor innervation of the smooth muscles of the small and large intestines (Furness, 2012; Pellegrini et al., 2015). Within the ENS, enteric neurons do not function alone in manifesting the GI dysfunction in PD patients, as gliosis mediated by EGCs, commonly referred to as astrocytes of the gut, also contributes to the gut pathogenesis (Clairembault et al., 2015; Gershon & Rothman, 1991). Braak’s hypothesis states that a neurotoxic agent initiating α-synuclein aggregation in the submucosal plexus of the gut promotes enteric gliosis and a local proinflammatory response, leading to the cascades of PD’s pathological process (Braak et al., 2006). Indeed, EGCs are known to be involved in the pathophysiology of numerous diseases. EGCs have a fundamental role in the inflammatory process, immune responses, and bowel function in inflammatory bowel disease and jejunoileitis (Klingelhoefer & Reichmann, 2015). Recent data suggest reactive enteric gliosis and its proinflammatory phenotype to be a causative factor in PD-related ENS dysfunction (Benvenuti et al., 2020). Despite this, the pertinent role of EGCs in PD pathology with respect to mitochondrial function is still largely unknown and the exact mechanisms of enteric glial activation and enteric neuronal loss in PD, including enteric dopaminergic neurons, are yet to be determined.
Mitochondrial dysfunction, due to the inhibition of mitochondrial complex I in the nigrostriatal system, is a major aspect of PD pathophysiology, and agents inhibiting mitochondrial complex I are specifically used in this study to detail GI-associated pathogenesis in models of PD. The mitochondrial complex I inhibitor rotenone (Rot) is a naturally occurring isoflavone insecticide extracted from the roots of Derris elliptica and Lonchocarpus utilis (Soloway, 1976) and has been used in fisheries and agriculture. Rot readily crosses the bloodbrain barrier because its highly lipophilic, and even low-dose exposure can produce PD pathogenic hallmarks (Betarbet et al., 2000; Hernández-Romero et al., 2012). While epidemiological evidence links PD to Rot exposure (Tanner et al., 2011), studies have also implicated that exposure to Rot decreases the number of nigral TH neurons, reduces striatal dopamine levels, and increases α-synuclein-positive cytoplasmic inclusions in the SN of various rodent models (Johnson & Bobrovskaya, 2015). Tebufenpyrad (Tebu) is another class of toxicant used as a pyrazole greenhouse acaricide, and similar to Rot, is a mitochondrial complex I inhibitor (Sherer et al., 2007) that is known to morphologically and functionally damage mitochondria in SH-SY5Y and HeLa cells (Chen et al., 2017). Our previous work emphasizes that treatment with Rot and Tebu induces oxidative stress and structural and functional mitochondrial defects in dopaminergic (Charli et al., 2016) and microglial cells (Sarkar et al., 2017). This present study delineates the effects of these environmental pesticides on EGCs and the ENS to demonstrate that exposure to certain neurotoxicants causes mitochondrial dysfunction, proinflammatory signaling, autophagic dysfunction in EGCs, and ultimately ENS dysfunction.
Materials and Method
Cell culture and treatment paradigm
CRL-2690 cell culture: The rat immortalized EGC line (CRL-2690) purchased from ATCC (ATCCR CRL-2690™) was originally isolated from the rat myenteric plexus and exhibits characteristic GFAP, S-100 and vimentin immunoreactivity (Rühl et al., 2001). CRL-2690 EGCs were cultured in DMEM (Gibco) medium containing 10% FBS (Gibco), 1% glutamine (Invitrogen), 1% penicillin and streptomycin (Gibco) (10% DMEM media) and maintained at 37°C and 5% CO2. For treatments, cell cultures were exposed to serum-free DMEM with 1% glutamine, 1% penicillin and streptomycin containing 1 μM of Rot (Sigma) or 1 μM Tebu (Sigma) reconstituted in dimethyl sulfoxide (DMSO) (Fisher Scientific).
Primary enteric mixed culture: Entire GI tracts isolated from 8–10 embryonic (E14-E15) C57BL/6 mice were collected in DMEM medium with 1% glutamine, 1% penicillin and streptomycin and then cut into fine pieces as per our previous publication (Ghaisas et al., 2021). The resultant suspension was then enzymatically digested in serum-free DMEM containing collagenase (0.2 mg/mL) (Sigma) and neutral protease (0.2 mg/mL) (Worthington, NJ) at 37°C for 30 min. The tissue sediment was then subjected to trypsin-EDTA (Gibco) digestion for 20 min followed by 10% DMEM media to halt trypsinization. The suspension was centrifuged at 250 × g for 5 min and reconstituted in 10% DMEM medium till a single cell suspension was obtained. Cells were then filtered through a 40-μm cell strainer and plated on poly-D-lysine (Sigma) and laminin (Sigma) pre-coated plates. After 24 h, media was changed to DMEM/F-12 medium containing 50 ng/mL GDNF (Sigma), 1% N2 (Gibco), 2% B27 (Gibco), 1% glutamine, and 1% penicillin and streptomycin supplements and cultured up to 3 wk at 37°C and 5% CO2. Half of the media was changed every 2 d. Pesticide treatments of primary cultures were carried out at a concentration of 200 nM for both Rot or Tebu in 2% FBS in DMEM with 1% glutamine, 1% penicillin and streptomycin for 6 h at 37°C and 5% CO2.
Animal studies
Male Lewis rats (6 to 7 months old) were purchased from Hilltop Lab Animals, Inc. Rats were housed on a 12-hour light cycle with ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at Iowa State University. Rot (2.8 mg/kg/d) was injected intraperitoneally once daily for 4 d and the animals under ketamine and xylazine anesthesia were transcardially perfused with PBS followed by 4% PFA (paraformaldehyde) solution (0.1 M phosphate-buffered saline, pH 7.4) for histological procedures. Rot dose and time point were selected based on previous publications (Cannon et al., 2009; Sarkar et al., 2017).
MTS cell viability assay
Cell viability was measured using the Cell Titer 96® AQueous Non-Radioactive Cell Proliferation (MTS assay) kit from Promega. Following treatment with Rot or Tebu in 96-well tissue plates, each well of CRL-2690 cells (plated at 20,000 cells/well) were incubated with 10 μL MTS reagent as per manufacturers protocol at 37°C and 5% CO2 for 45 min. The resultant formation of formazan crystals was dissolved with 25 μL of DMSO and absorbance was measured spectrophotometrically at 490 nm.
Live-cell staining
For experiments using MitoTracker Green (200 nM), MitoTracker Red (200 nM), MitoSox Red (5 μM) and LysoTracker Green (50 nM) dyes (all from Molecular Probes), cells were stained with the respective dye for 45–60 min as described previously (Charli et al., 2016; Sarkar et al., 2017). Cells were treated either on PDL-coated coverslips (plated at 150,000 cells/well) or in 96-well plates (plated at 150,000 cells/well) and following treatment with the toxicants in their respective concentrations, cells were washed twice with HBSS (Gibco) and then the respective dye was added according to the dilution recommended by the manufacturer. After staining, cells were washed and fixed for immunocytochemistry (ICC). A Cytation 3 real-time imaging system (BioTek) was used for MitoSox Red imaging. Changes in mitochondrial structure were quantified using the ImageJ plugin “mitochondrial morphology”.
Measurement of mitochondrial oxygen consumption
Mitochondrial oxygen consumption was measured using a Seahorse XF96 Extracellular Flux Analyzer (Seahorse Bioscience) as described previously (Charli et al., 2016; Heinz et al., 2017; Sarkar et al., 2017). CRL-2690 cells were plated at 90,000 cells/well on a 24-well Seahorse plate followed by treatment with the respective pesticide for 6 h. A Mito Stress report generator was used for the analysis for which 0.75 μM oligomycin, 1 μM FCCP, and 0.5 μM Rot/antimycin were used.
Quantitative reverse transcription PCR (RT-qPCR)
Following treatment, RNA was extracted using the TRIZOL reagent (Invitrogen) as per the manufacturer’s protocol. Conversion of cDNA was performed using a High-Capacity cDNA Synthesis kit (Applied Biosystems). RT-qPCR was carried out on an Applied Biosystems QuantStudio 3 system with the SYBR Master Mix (Applied Biosystems). QuantiTect Primer Assays (Qiagen) for genes coding for the proteins IL-6, NOS2, TNFSF-12, PGP9.5, TH and D2R were used for RT-qPCR with 18S rRNA as the housekeeping gene. Dissociation curves were run for all completed SYBR Green reactions and fold change was calculated using the ΔΔCt method.
Western blotting
Following treatments with Rot or Tebu for 6 h, cells were lysed by sonicating in a modified RIPA buffer. The proteins were normalized and 60 μg of protein was loaded in each lane and separated using a 12–15% SDS-PAGE gel. After transfer, nitrocellulose membranes were blocked with LI-COR blocking buffer. The membranes were then incubated overnight at 4oC with the primary antibody, followed by incubation with IRDye-tagged secondary antibodies. The membranes were scanned using the LI-COR Odyssey Classic imaging system. The primary antibodies used for immunoblotting include anti-p62 (1:1000, ab-56416). The β-actin antibody was used as a loading control.
Immunocytochemistry (ICC) and Immunohistochemistry (IHC)
ICC: After being treated for 6 h with the respective pesticide, cells plated at 150,000 cells/well were fixed in 4% paraformaldehyde (Fisher Scientific) for 15–30 min followed by blocking with 1.5% BSA (Sigma), 0.05% Tween (BioRad), and 0.5% Triton (Sigma) for 1 h. Primary antibodies were prepared in 1% BSA and incubated overnight at 4°C. The following primary antibodies were used: anti-MFN2 (1:500, cs-D2D10), anti-iNOS (1:500, ab-3523) and anti-LC3 (1:500, ab-48394). After incubating in the appropriate secondary antibody for 1 h at RT, cell nuclei were stained with Hoechst (Molecular Probes) (1:5000) and mounted on slides using Fluoromount mounting medium (Sigma). Slides were dried overnight and then imaged using a Nikon Eclipse C1 microscope or a Keyence BZ-X800 microscope. For co-localization, a 3D color plotter (Image J analysis software) was used to plot all the different colors seen in the RGB images.
IHC: After treatment, rats were perfused as mentioned in the animal study. The entire fixed colon was removed and was additionally post-fixed in 4% PFA for 24 h. Tissues were then embedded in paraffin and sectioned at 5 μm thickness. Paraffin-embedded colon sections were deparaffinized and subjected to antigen retrieval in citrate buffer. Sections were then incubated in blocking reagent (10% normal goat serum, 1% BSA and 0.5% Triton X-100 in PBS) for 1 h before incubating in the appropriate primary antibodies anti-iNos (1:500) and anti-PGP9.5 (1:500, ab-10404). Sections were then incubated in the appropriate secondary antibody for 1 h at RT, followed by cell nuclei staining with Hoechst (1:5000) and slide-mounting using Fluoromount mounting medium and then imaged using a Keyence BZ-X800 microscope.
Statistical analysis
All statistical analyses were performed using Prism 7.05 (GraphPad Software). Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test for comparing all treatment groups with that of the control. For the correlation assessment in Fig. 3, we used linear regression analysis. Differences with p ≤ 0.05 were considered statistically significant.
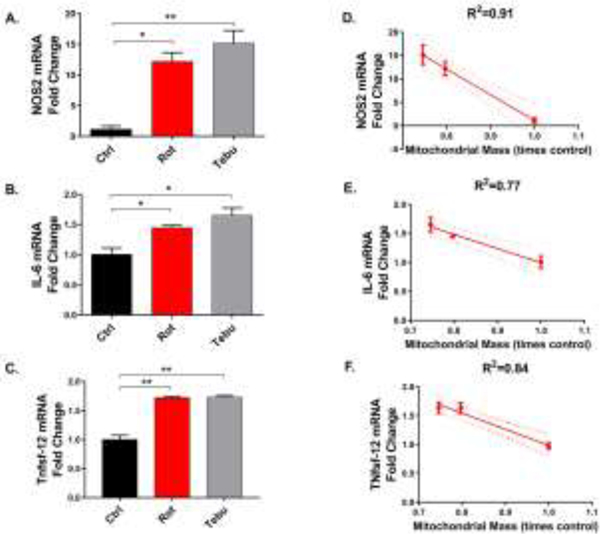
Figure 3: Rotenone (Rot) and tebufenpyrad (Tebu) treatments upregulate proinflammatory factors in enteric glial cells (EGCs).
EGCs treated with Rot and Tebu at 1 μM for 6 hrs probed for proinflammatory markers showed increased mRNA expression of (A) Nos2, (B) Il-6, and (C) Tnfsf-12. Correlation plots between inflammatory markers and mitochondrial mass of pesticide-treated EGCs showing inverse correlations between mitochondrial mass and (D) NOS2, (E) IL-6, and (F) TNFSF-12. Data analyzed via one-way ANOVA and represented as mean±SEM and correlation coefficient *p≤0.05, **p <0.01.
Results
Rot and Tebu treatments induce mitochondrial dysregulation in EGCs
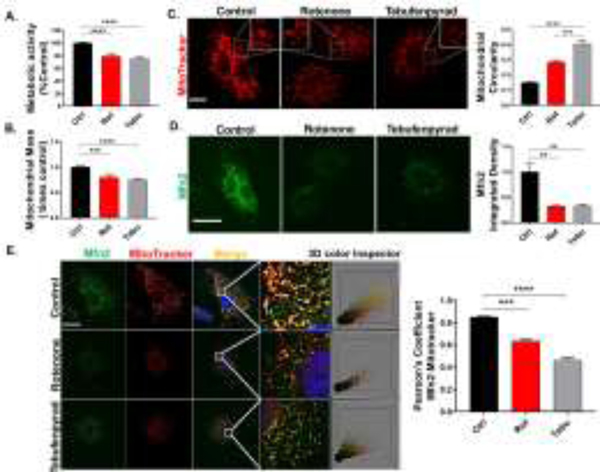
Our initial test to determine the metabolic activity of the rat CRL-2690 EGCs following Rot and Tebu treatments through MTS assay shows significantly decreased metabolic activity in the treatment groups as compared to the control (Fig. 1A). Since both Rot and Tebu are considered to be mitochondrial inhibitors (by IRAC), we next determined the extent of their effects on mitochondrial morphology in CRL-2690 EGCs. Using MitoTracker Green FM as an indicator of mitochondrial mass and health (Krohn et al., 1999; Métivier et al., 1998), we show that mitochondrial mass was considerably reduced in CRL-2690 cells treated with Rot and Tebu at the dose of 1 μM for 6 h when compared to control cells (Fig. 1B). Then, using MitoTacker Red to visualize mitochondrial morphology, we found that the pesticide-treated groups lost their tubular mitochondrial structure, appearing more circular indicating increased mitochondrial fragmentation as seen in the ICC image and the quantification of mitochondrial circularity (Fig. 1C). Depending upon respiratory conditions, mitochondria undergo cycles of fission and fusion to regulate their function and maintain a dynamic population of cellular mitochondria (Chan, 2012; Westermann, 2012). Since dysregulated mitochondrial fusion can cause the mitochondrial fragmentation and abnormal mitochondrial physiology seen in PD pathogenesis (Arduíno et al., 2011; Pham et al., 2012), we investigated the state of mitochondrial fusion under pesticide treatment by probing CRL-2690 EGCs with the mitofusin protein MFN2, which is a mitochondrial outer membrane GTPase crucial for mitochondrial fusion (Filadi et al., 2018). MFN2 protein levels were greatly reduced in the treatment groups (Fig. 1D), which indicates that neurotoxic pesticide treatments can inhibit mitochondrial fusion in EGCs. A similar trend is also seen in our Western blot results (Fig. S1). A co-localization ICC study further reveals a correlated decrease in both MFN2 and MitoTracker Red in the treatment groups, which was evaluated by Pearson’s correlation analysis (Fig. 1E). The 3D color plot further illustrates the degree of colocalization and its marked reduction in the treatment groups seen by the merging of red and green channels. Collectively, these results show a dysregulation in mitochondria of EGCs treated with Rot and Tebu.
Figure 1: Rotenone (Rot) and tebufenpyrad (Tebu) treatments induce mitochondrial dysregulation in enteric glial cells (EGCs).
(A) Metabolic activity was measured through an MTS assay on EGCs after being treated with Rot and Tebu at 1 μM for 6 hrs. (B) MitoTracker Green assay showing decreased mitochondrial mass in pesticide-treated groups. (C) Mitotracker Red ICC showing decreased fluorescence intensity and increased circularity as a sign of altered mitochondrial mass and in the Rot and Tebu groups. Scale bar, 25 μm. Zoomed images are shown as inserts in their respective figures. Right: Quantification of mitochondrial circularity measured through ImageJ. (D) Decreased Mfn2 immunoreactivity in EGCs treated with the pesticides. Scale bar, 25 μm. Right: Quantification of the integrated density of Mfn2 (E) Colocalization of MFN2 and MitoTracker Red and associated Pearson’s correlation coefficient and the respective 3D color plot of the focused area. Scale bar, 25 μm. Data analyzed via one-way ANOVA and represented as mean±SEM, **p <0.01, ***p<0.001, ****p<0.0001.
Rot and Tebu alter mitochondrial dynamics and increase oxidative stress in EGCs
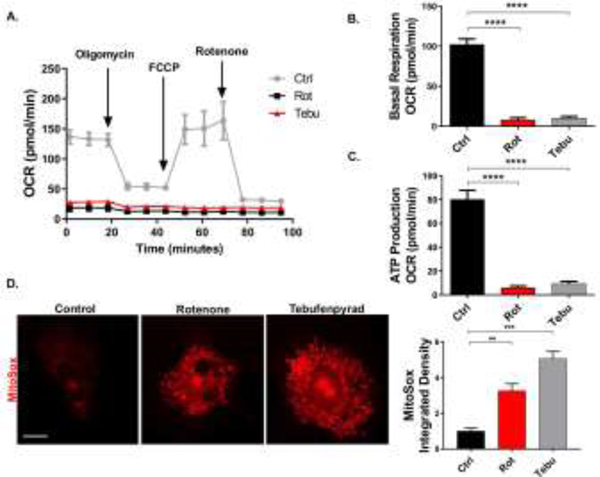
After demonstrating their effects on mitochondrial fission and fusion in EGCs, we investigated the effects of Rot and Tebu on other mitochondrial functional parameters. CRL-2690 EGCs were similarly treated with 1 μM Rot and Tebu for 6 h, and mitochondrial bioenergetics was assessed through oxygen consumption rate (OCR), basal mitochondrial respiration and ATP-linked respiration as measured using an XFe24 extracellular flux analyzer (Seahorse Bioscience). Both pesticides decreased OCR capacity, basal mitochondrial respiration and ATP production in CRL-2690 cells, indicating the state of functionally damaged mitochondria (Fig. 2A-C). Impaired mitochondrial function is known to be associated with free radical production such as reactive oxygen species (ROS) (Murphy, 2009). To determine whether EGC mitochondrial dysfunction results in higher cellular ROS production, we probed CRL-2690 cells with the MitoSox dye. Our ICC results and their quantification show that mitochondrial superoxide increased significantly in pesticide-treated EGCs (Fig. 2D). These findings suggest that the neurotoxic pesticides Rot and Tebu impair mitochondrial dynamics and induce mitochondrial oxidative stress in EGCs.
Figure 2: Rotenone (Rot) and tebufenpyrad (Tebu) alter mitochondrial dynamics and increase oxidative stress in enteric glial cells (EGCs).
EGCs treated with Rot and Tebu at 1 μM for 6 hrs in Seahorse Mito Stress assay show reduced (A) oxygen consumption rate, (B) basal respiration, and (C) ATP production. (D) MitoSox assay shows increased mitochondrial superoxide produced in the EGCs exposed to pesticides. Scale bar, 25 μm. Right: Quantification of the integrated density of MitoSox. Data analyzed via one-way ANOVA and represented as mean±SEM, **p <0.01, ***p<0.001, ****p<0.0001.
Rot and Tebu treatments upregulate proinflammatory factors in EGCs
After confirming that Rot and Tebu induce mitochondrial functional and morphological deficits in EGCs, we next investigated their contributions to the inflammatory response in EGCs. EGCs help maintain bowel function, and their ablation in the gut results in fatal jejunoileitis (Bush et al., 1998). Furthermore, intestinal inflammation is strongly associated with the pathophysiology of PD (Houser & Tansey, 2017). We found that CRL-2690 EGCs exposed to 1 μM Rot or Tebu for 6 h showed increased mRNA expression of the proinflammatory proteins NOS-2, IL-6 and TNFSF12 (TNF Superfamily Member 12) (Fig. 3A-C), which also were inversely correlated with mitochondrial mass, further supporting that Rot- and Tebu-induced mitochondrial dysregulation is strongly associated with the activation of inflammation in EGCs (Fig. 3D-F).
Rot and Tebu treatments impair autophagy in EGCs
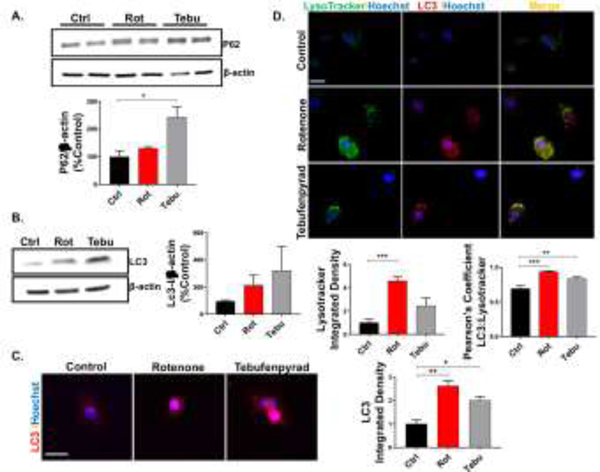
Next, we tested if the mitochondrial impairment and inflammation induce the progression of the cell cycle towards autophagy, as it is the primary route of elimination of dysfunctional organelles like mitochondria (Pan et al., 2008). We determined the levels of two autophagy markers, p62 and LC-3, in Rot- and Tebu-treated CRL-2690 EGCs. Klionsky et al. (2016) reported that an increase in LC3 expression without a decrease in p62 indicates a disrupted autophagic flux. Our Western blot analysis shows that pesticide treatments increased the expression of p62 protein, however, the increase was statistically significant only in Tebu-treated EGCs (Fig. 4A). Next, we found the autophagy marker LC3 increased in both pesticide-treated groups when compared with the control (Fig. 4B-C). Our co-localization study reveals a correlated increase in the fluorescence intensity of LysoTracker Green and punctated LC3 in Rot and Tebu-treated CRL-2690 EGCs (Fig. 4D). Here we found enlarged and clustered lysosomes, seen through LysoTracker Green indicating a dysregulated state (Hockey et al., 2015) in Rot- and Tebu-treated groups along with a significantly increased Pearson’s correlation coefficient. Together, these results indicate that Rot and Tebu treatments inhibit an autophagic response in EGCs.
Figure 4: Rotenone (Rot) and tebufenpyrad (Tebu) treatments impair autophagy in enteric glial cells (EGCs).
EGCs treated with Rot and Tebu at 1 μM for 6 hrs probed for autophagic markers. (A-B) Representative Western blot and densitometric analysis showing an increase in (A) p62 and (B) LC3 marker in pesticide-treated group. (C) ICC showing increased LC3. Scale bar, 25 μm. Right: Quantification of the integrated density of LC3. (D) ICC showing colocalization of LysoTracker dye and LC3 and increased expression of both in the pesticide group and the associated Pearson’s correlation coefficient. Scale bar, 50 μm. Below: Quantification of the integrated density of LysoTracker green and Pearson’s correlation coefficient between LC3 and LysoTracker green. Data analyzed via one-way ANOVA and represented as mean±SEM, *p≤0.05, **p <0.01, ***p<0.001.
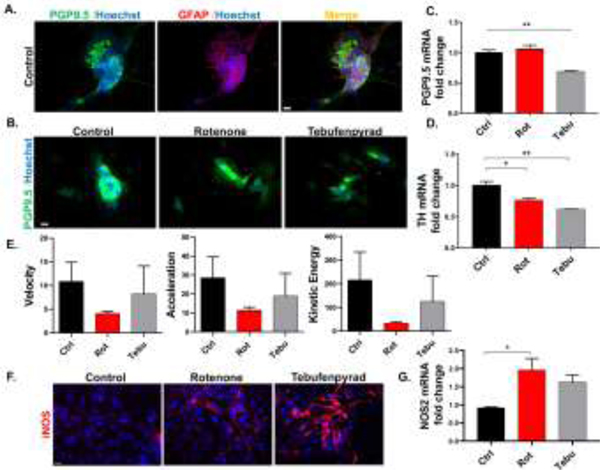
Rot and Tebu causes inflammation with neuronal and functional loss in primary ENS cultures
We followed our experiments in CRL-2690 EGCs by studying the effects of Rot and Tebu in the ENS using primary enteric mixed cultures. The primary enteric mixed cultures from E14–15 C57BL mice were grown in 6-well plates with specific neurotrophic factors as specified in Methods. Once the primary cultures had established a functioning interconnected network between smooth muscle cells that exhibited “gut-like” peristaltic movement in the culture dishes, governed by GFAP+ enteric glia and PGP9.5+ enteric neurons (Fig. 5A), we treated the wells with Rot and Tebu (200 μM each) for 6 h. Our ICC results show reduced expression of pan-neuronal marker PGP9.5 in primary enteric mixed cultures in Rot- and Tebu-treated groups (Fig. 5B), while our qPCR data from the primary enteric mixed cultures showed significantly decreased expression PGP9.5 only in Tebu-treated cells (Fig. 5C). PD patients were reported to have decreased TH immunoreactivity and dopamine concentration in the colon, when compared to control patients, as far back as Singaram et al. (1995). Supporting this, mRNA expression of Th was significantly reduced by both Rot and Tebu in the dopaminergic neuronal cells of our primary enteric mixed cultures (Fig. 5D). These effects translated into a functional deficit in the primary enteric mixed cultures that was discernible at the scale of peristaltic contractions exhibited by the primary enteric mixed cultures as viewed in Video-1 (Ctrl), −2 (Rot) and −3 (Tebu). We measured the peristaltic movements in video-recordings of primary enteric mixed culture using the TRACKER software and found that Rot- and Tebu-treated cultures had decreased motility as measured by velocity, acceleration and kinetic energy (Fig. 5E). Like EGCs, pesticide-treated primary enteric mixed cultures had increased expression of iNOS at both the protein and mRNA levels as seen by ICC and qPCR, respectively (Fig. 5F-G). Collectively, these results indicate that Rot and Tebu exert neuronal and functional loss with inflammatory changes in mouse primary enteric mixed cultures.
Figure 5: Rotenone (Rot) and tebufenpyrad (Tebu) cause inflammation with neuronal and functional loss in the enteric nervous system (ENS).
(A) ICC analysis showing primary mouse mixed enteric neuronal culture expressing neuronal marker PGP 9.5 and glial marker GFAP. (B) ICC analysis showing decreased expression of PGP 69.5 in ENS-treated with Rot and Tebu at 200 nM for 6 h. (C-D) RT-qPCR analyses showing reduced mRNA expression in Tebu-treated ENS group for (C) Pgp9.5 and (D) Th. (E) Tracker analysis of video-recordings of primary ENS treated with the pesticides shows reduced velocity, acceleration and kinetic energy, which correlated with the decreased peristaltic function in the pesticide group when compared with the control as seen in Video 1 (Control), 2 (Rot) and 3 (Tebu). (F-G) ENS treated with Rot and Tebu at 200 nM for 6 h shows inflammatory changes as seen through increased expression of NOS2 in (F) ICC analysis and (G) RT-qPCR analysis of mRNA. Scale bar, 20 μm. Data represented as mean±SEM and analyzed via Student’s t test and correlation coefficient *p<0.05, **p <0.01.
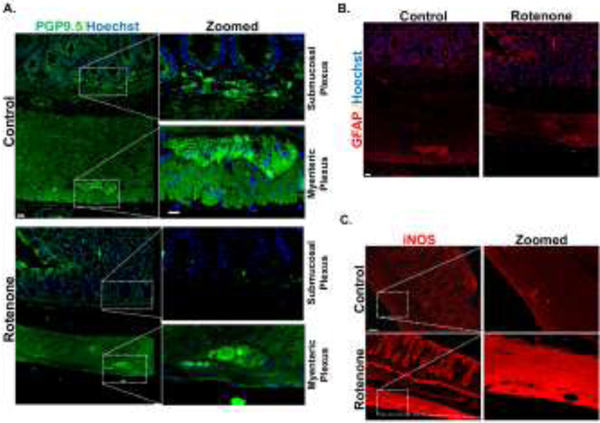
Enteric neuronal degeneration in the submucosal plexus in a rat Rot model of PD
Finally, we investigated the effects of neurotoxic pesticide exposure on the ENS in vivo using a Rot rat model of PD. Here, the rats were injected with 2.8 mg/kg/d of Rot for 4 days and sacrificed after 3 months (Sarkar et al., 2017). In Rot-treated rats, the enteric neuronal population of the myenteric plexus did not show marked changes in the expression of PGP9.5, whereas the submucosal plexus showed reduced expression (Fig. 6A). GFAP expression in the rat colon did not differ significantly between the Rot and control groups (Fig. 6B). Additionally, the Rot-treated rat colon also showed increased expression of the inflammatory marker iNOS in the myenteric plexus (Fig. 6C). Overall, our in vivo data corresponds well with our primary culture results in showing how a mitochondria-impairing pesticide treatment alters gut physiology.
Figure 6: Enteric neuronal degeneration in the submucosal plexus in a rat rotenone (Rot) model of PD.
(A) IHC of rat colon treated with Rot (2.8mg/kg/i.p) showing decreased expression of PGP 9.5 in the submucosal plexus. (B) IHC of rat colon treated with Rot showing no significant change in expression of GFAP in the myenteric plexus. (C) IHC of rat colon treated with Rot showing increased expression of iNOS in the myenteric plexus (n=2). Scale bar, 20 μm.
Discussion
Environmental toxicant-induced mitochondrial dysfunction as an initiating factor of gut pathology has not been studied in depth. Although solid evidence exists supporting CNS mitochondrial dysfunction in neurodegenerative diseases like PD, the cause-and-effect role of dysfunctional processes of the second brain in the gut, the ENS, remains less certain. Our present study ascertained that inducing mitochondrial dysregulation by exposing EGCs to the mitochondria complex-1-targeting pesticides Rot and Tebu leads to a series of mitochondrial quality control signaling events affecting the ENS. We employed these pesticides to study the main mediator of gut pathology, EGCs, to explore their functional response in the face of environmentally induced PD. Our work clearly shows that neurotoxic pesticides can alter mitochondrial functioning and dynamics and induce proinflammatory cytokines accompanied by an impaired autophagic flux in EGCs. We then demonstrated a possible EGC-induced loss of function in primary enteric mixed cultures.
Complex-1 inhibition triggers events leading to dopaminergic degeneration in PD (Schapira et al., 1990). When we exposed rat CRL-2690 EGCs to potent complex-1 inhibitors Rot and Tebu, it resulted in decreased metabolic activity in these cells. We and others (Betarbet et al., 2000; Charli et al., 2016; Gao et al., 2002; Sarkar et al., 2017) have previously shown these pesticides to induce increased mitochondrial fission and reduced mitochondrial fusion in dopaminergic and microglial cells. In our present study, the pesticides dramatically impaired EGC mitochondrial function as measured by reduced mitochondrial mass, increased mitochondrial fragmentation-associated circularity, and reduced expression of the mitochondrial fusion protein MFN2. The resulting imbalance in mitochondrial fission-fusion dynamics has been reported in models of genetic and environmentally induced PD (Deng et al., 2008; Sarkar et al., 2018). Not only has reduced MFN2 been linked to the degeneration of dopaminergic neurons and the associated motor deficits, but also its overexpression protects against dopaminergic neuronal loss (Pham et al., 2012; Zhu et al., 2017). Furthermore, mitochondria-produced ROS, particularly superoxide, is linked with oxidative damage to mitochondrial proteins and the resultant impairment of ATP generation (Murphy, 2009). Corroborating this, we found that the pesticides greatly increased mitochondrial superoxide generation, and compromised mitochondrial bioenergetics as seen through decreased OCR and ATP production.
Our treatment of CRL-2690 cells with pesticides also increased the expression of the autophagic substrate p62 and the autophagy protein LC3. Increasing LC3 levels imply an increased autophagosome formation, but the lack of decreased p62 infers an impaired autophagy-mediated protein degradation and thus a probable blockage of the autophagic flux (Klionsky et al., 2016; Lambelet et al., 2018; Lynch-Day et al., 2012; Yoshii & Mizushima, 2017). Disrupting the autophagic pathway, whether due to environmental, age-related or genetic factors, can lead to the accumulation of p62 and fragmented mitochondria and ultimately neuronal cell death (Alvarez-Erviti et al., 2010; Zhang et al., 2014). Interestingly, in an in vitro model of paraquat-induced PD, (González-Polo et al., 2007), reported finding normal autophagy early during paraquat exposure but then impaired autophagy later after more prolonged exposure.
Within the ENS, EGCs structurally and functionally play a vital role in maintaining the integrity of the ENS, similar to astrocytes in the CNS, and are capable of responding to inflammatory insults in the ENS (Bush et al., 1998; Coelho-Aguiar et al., 2015; Ferri et al., 1982; Gershon & Rothman, 1991; K. R. Jessen & Mirsky, 1983; Kristjan R. Jessen & Mirsky, 1980; Rühl et al., 2001). Inflammation of the gut is a major pathology of many neurodegenerative diseases. Liu et al. (2021) report a 44% increased risk of developing PD in IBD-diagnosed patients. Inflammation has been implicated as the primary initiating factor of PD supporting Braak’s hypothesis of the gut-brain-axis (Braak et al., 2003; Houser & Tansey, 2017), and the EGC’s role during the inflammatory response in the neuromuscular system of the gut has been previously emphasized (Geboes et al., 1992). Supporting this view, we found an upregulation of the proinflammatory proteins NOS-2, IL-6 and TNFSF-12 at the gene level in pesticide-treated EGCs.
Damage to the intestinal tissue through pathogens or environmental substances can evoke an enteric inflammatory response, which in turn leads to altered CNS function (Ghaisas et al., 2016; Houser & Tansey, 2017). In our study, pesticide-induced glial inflammation could be associated with ENS inflammation, which would later lead to CNS inflammatory manifestations in vivo. Transmission of enteric neuronal pathology through the vagal nerve to the CNS in PD is implicated across many models (Holmqvist et al., 2014; Kim et al., 2019; Uemura et al., 2018). Chronic ingestion of low-dose Rot can cause α-synuclein pathology in the mouse ENS ganglia followed by its accumulation in both the dorsal motor nucleus of the vagal nerve and the SN, and surgical intervention with hemivagotomy decreased the loss of dopaminergic neurons and α-synuclein accumulation (Svensson et al., 2015; Tysnes et al., 2015). Supporting this work, an epidemiological study also reported an association between truncal vagotomy and decreased risk of PD (Fleming et al., 2004). Rot has also been linked with decreased TH immunoreactivity and microglial activation in addition to behavioral impairments (Pan-Montojo et al., 2010). Debates over the origin of PD pathology in the gut either point to the submucosal plexus as the region of origin (Shannon et al., 2012; Singaram et al., 1995)or have been met with contradicting reports of no changes in the calcium responses and mitochondrial membrane potential and lack of loss in the submucosal neurons in the ENS in PD (Desmet et al., 2017). Our present findings support the hypothesis of the probable initiation of the enteric neuronal dysregulation through the mitochondrial dysfunction of EGCs as observed from our primary culture studies. Another study has validated that the effects of the mitochondrial complex I inhibitor Rot follow Braak’s hypothesis, resulting in α-synuclein accumulation and phosphorylation in ENS, which then progressed to the intermediolateral nucleus in the spinal cord (IML) and dorsal motor nucleus of the vagus (DMV) and then to the SN in C57BL/6J mice (Pan-Montojo et al., 2010). Murakami et al. (2015) show that systemic Rot exposure without direct local exposure to the ENS, through subcutaneous administration, induced EGC activation and neuronal degeneration in the ascending colon of C57BL/6J mice, while previous work (Schaffernicht et al., 2021) has shown intestines from mice treated orally with Rot resulted in altered receptor-based enteric neuronal physiology. Differential regional and subtype-specific vulnerability of the ENS to mitochondrial dysfunction has been shown in transgenic mice with mitochondrial defects in enteric neuronal and glial cells induced through targeted deletion of the mitochondrial transcription factor A (Tfam) gene in midbrain dopaminergic neurons, demonstrating that healthy mitochondrial function in the ENS is necessary for normal GI motility (Viader et al., 2011).
Since EGCs are major functional and structural supporters of the ENS, and actively participate in neuron-glia crosstalk in regulating gut physiology (Grubišić & Gulbransen, 2017; Sharkey, 2015), we further explored how our findings of mitochondrial dysfunction in EGCs affect the ENS by conducting similar pesticide exposure experiments in primary mixed enteric cultures. Here, we discovered that mitochondria-impairing toxicants greatly reduced multiple metrics related to peristaltic movements. Previous work (Yang et al., 2018) has shown that, in addition to decreased colon motility and fecal water content, oral administration of Rot in mice altered fecal microbiota, specifically in the phyla Bacteroidetes and Firmicutes. Indeed, one of the most early non-motor symptoms of PD is GI motility dysfunction, and our results were in line with this concept and Greene et al.’s (2009) report that chronic Rot-treated rats exhibit impaired GI longitudinal muscle contraction. Reduced GI motility in PD has been associated with an underlying inflammation and neuronal loss. Our probing of pesticide-treated mixed culture for proinflammatory and neuronal markers revealed increased expression of Nos-2, and diminished expression of the enteric neuronal marker PGP9.5, and the dopaminergic marker TH. Our cell culture findings were further supported by our in vivo study, where we found that the colon of Rot-treated rats showed neuronal loss in the submucosal plexus and increased levels of the inflammatory marker iNOS. Our previous publication (Charli et al., 2016) shows that, similar to Rot, Tebu also induces ROS in dopaminergic neurons. Hence, we expect Tebu to elicit similar enteric neuronal degeneration in rats. Since we only used male rats, our results are subject to potential sex-specific biases and must be interpreted accordingly. Since human and mouse enteric nervous systems share a core transcriptional program for neurotransmitters and transcriptional factors (Drokhlyansky et al., 2020), we hypothesis similar glial and neuronal pathologies in humans exposed to these environmental pesticides.
Although, further studies to strengthen our findings can be conducted with either ENS treated with conditioned media from pesticide-exposed EGCs or through co-culture of EGCs and enteric neurons as a means to identify the factors present in the conditioned media that would affect the ENS. This would shed light especially on the neuron-glia crosstalk in the event of EGC mitochondrial damage in the gut. Overall, our study demonstrates that mitochondrial dysfunction in ECGs can induce autophagic dysregulation and proinflammatory response thereby affecting gut motility.
Supplementary Material
Acknowledgment
This study was supported by the National Institute of Health grants ES026892, ES027245, NS100090, and NS088206. The W. Eugene and Linda Lloyd Endowed Chair and Eminent Scholar and the Armbrust endowment to AGK and the Salisbury Endowed Professorship to A.K are also acknowledged.
Abbreviations:
- EGCs
Enteric glial cells
- MFN2
Mitofusion 2
- LC3
Microtubule-associated protein 1A/1B-light chain 3
- p62
ubiquitin-binding protein
- ENS
Enteric nervous system
- TH
Tyrosine hydroxylase
- GFAP
Glial fibrillary acidic protein
- DMEM
Dulbecco’s modified eagle medium
- EDTA
Ethylenediaminetetraacetic acid
- GDNF
Glial cell line-derived neurotrophic factor
- PDL
Poly-D-lysine
- HBSS
Hank’s balanced salt solution
- FCCP
Trifluoromethoxy carbonylcyanide phenylhydrazone
- D2R
Dopamine 2 receptor
- PGP9.5
Protein gene product 9.5
- IRAC
Insecticide resistance action committee
Footnotes
CRediT authorship contribution statement
Bharathi N Palanisamy: Conceptualization, Methodology, Investigation, Validation, Writing - Original Draft. Souvarish Sarkar, Emir Malovic, Manikandan Samidurai, and Adhithiya Charli: Investigation, Validation. Gary Zenitsky, Huajun Jin, and Vellareddy Anantharam: Writing - Review & Editing. Arthi Kanthasamy and Anumantha Kanthasamy: Conceptualization, Supervision.
Conflict of Interest: AGK and VA have an equity interest in PK Biosciences Corporation located in Ames, IA. AGK also has an equity interest in Probiome Therapeutics located in Ames, IA. The terms of this arrangement have been reviewed and approved by ISU in accordance with its conflict of interest policies. The other authors declare no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, & Schapira AHV (2010). Chaperone-mediated autophagy markers in Parkinson disease brains. Archives of Neurology, 67(12), 1464–1472. 10.1001/archneurol.2010.198 [DOI] [PubMed] [Google Scholar]
- Arduíno DM, Esteves AR, & Cardoso SM (2011). Mitochondrial fusion/fission, transport and autophagy in Parkinson’s disease: When mitochondria get nasty. In Parkinson’s Disease (Vol. 2011). Hindawi Limited. 10.4061/2011/767230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, & Okun MS (2020). Diagnosis and Treatment of Parkinson Disease: A Review. JAMA, 323(6), 548–560. 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- Arotcarena ML, Dovero S, Prigent A, Bourdenx M, Camus S, Porras G, Thiolat ML, Tasselli M, Aubert P, Kruse N, Mollenhauer B, Damas IT, Estrada C, Garcia-Carrillo N, Vaikath NN, El-Agnaf OMA, Herrero MT, Vila M, Obeso JA, … Bezard E (2020). Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain, 143(5), 1462–1475. 10.1093/brain/awaa096 [DOI] [PubMed] [Google Scholar]
- Benvenuti L, D’antongiovanni V, Pellegrini C, Antonioli L, Bernardini N, Blandizzi C, & Fornai M (2020). Enteric glia at the crossroads between intestinal immune system and epithelial barrier: Implications for parkinson disease. In International Journal of Molecular Sciences (Vol. 21, Issue 23, pp. 1–16). MDPI AG. 10.3390/ijms21239199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov A. v., & Greenamyre JT (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nature Neuroscience, 3(12), 1301–1306. 10.1038/81834 [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RAI, Bohl J, & del Tredici K (2006). Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neuroscience Letters, 396(1), 67–72. 10.1016/j.neulet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, & Braak E (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24(2), 197–211. 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, & Sofroniew M. v. (1998). Fulminant jejuno-ileitis following ablation of enteric gila in adult transgenic mice. Cell, 93(2), 189–201. 10.1016/S0092-8674(00)81571-8 [DOI] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, & Greenamyre JT (2009). A highly reproducible rotenone model of Parkinson’s disease. Neurobiology of Disease, 34(2), 279–290. 10.1016/J.NBD.2009.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutiérrez C, Micheli FE, & Benarroch EE (2013). Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. Journal of Neurology, 260(5), 1332–1338. 10.1007/s00415-012-6801-2 [DOI] [PubMed] [Google Scholar]
- Chan DC (2012). Fusion and fission: Interlinked processes critical for mitochondrial health. Annual Review of Genetics, 46, 265–287. 10.1146/annurev-genet-110410-132529 [DOI] [PubMed] [Google Scholar]
- Charli A, Jin H, Anantharam V, Kanthasamy A, & Kanthasamy AG (2016). Alterations in mitochondrial dynamics induced by tebufenpyrad and pyridaben in a dopaminergic neuronal cell culture model. NeuroToxicology, 53, 302–313. 10.1016/j.neuro.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, & Schapira AH (2009). Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. In The Lancet Neurology (Vol. 8, Issue 5, pp. 464–474). Elsevier. 10.1016/S1474-4422(09)70068-7 [DOI] [PubMed] [Google Scholar]
- Chen T, Tan J, Wan Z, Zou Y, Afewerky HK, Zhang Z, & Zhang T (2017). Effects of commonly used pesticides in China on the mitochondria and ubiquitin-proteasome system in Parkinson’s disease. International Journal of Molecular Sciences, 18(12). 10.3390/ijms18122507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairembault T, Leclair-Visonneau L, Neunlist M, & Derkinderen P (2015). Enteric glial cells: New players in Parkinson’s disease? Movement Disorders, 30(4), 494–498. 10.1002/mds.25979 [DOI] [PubMed] [Google Scholar]
- Coelho-Aguiar J de M, Bon-Frauches AC, Gomes ALT, Veríssimo CP, Aguiar DP, Matias D, Thomasi BB de M, Gomes AS, Brito GA de C, & Moura-Neto V (2015). The Enteric Glia: Identity and Functions. Glia, 63(6), 921–935. 10.1002/glia.22795 [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, & Guo M (2008). The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 105(38), 14503–14508. 10.1073/pnas.0803998105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet AS, Cirillo C, Tack J, Vandenberghe W, & Berghe vanden P. (2017). Live calcium and mitochondrial imaging in the enteric nervous system of parkinson patients and controls. ELife, 6. 10.7554/eLife.26850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra AA, Voorn P, Berendse HW, Groenewegen HJ, Rozemuller AJM, & van de Berg WDJ (2014). Stage-dependent nigral neuronal loss in incidental Lewy body and parkinson’s disease. Movement Disorders, 29(10), 1244–1251. 10.1002/mds.25952 [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC, Ansha MG, Brayne C, Choi J-YJ, Collado-Mateo D, Dahodwala N, Do HP, Edessa D, Endres M, Fereshtehnejad S-M, Foreman KJ, Gankpe FG, Gupta R, Hankey GJ, … Murray CJL (2018). Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology, 17(11), 939–953. 10.1016/S1474-4422(18)30295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drokhlyansky E, Smillie CS, van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, Dionne D, Cuoco MS, Goder-Reiser MN, Sharova T, Kuksenko O, Aguirre AJ, Boland GM, Graham D, Rozenblatt-Rosen O, Xavier RJ, & Regev A (2020). The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell, 182(6), 1606–1622.e23. 10.1016/J.CELL.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, & Polak JM (1982). Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature, 297(5865), 409–410. 10.1038/297409a0 [DOI] [PubMed] [Google Scholar]
- Filadi R, Pendin Di, & Pizzo P (2018). Mitofusin 2: From functions to disease. In Cell Death and Disease (Vol. 9, Issue 3, pp. 1–13). Nature Publishing Group. 10.1038/s41419-017-0023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM (2017). Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. In Current environmental health reports (Vol. 4, Issue 2, pp. 192–199). Springer. 10.1007/s40572-017-0143-2 [DOI] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, & Chesselet MF (2004). Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Experimental Neurology, 187(2), 418–429. 10.1016/j.expneurol.2004.01.023 [DOI] [PubMed] [Google Scholar]
- Furness JB (2012). The enteric nervous system and neurogastroenterology. In Nature Reviews Gastroenterology and Hepatology (Vol. 9, Issue 5, pp. 286–294). Nat Rev Gastroenterol Hepatol. 10.1038/nrgastro.2012.32 [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, & Liu B (2002). Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. Journal of Neuroscience, 22(3), 782–790. 10.1523/jneurosci.22-03-00782.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes K, Rutgeerts P, Ectors N, Mebis J, Penninckx F, Vantrappen G, & Desmet VJ (1992). Major histocompatibility class II expression on the small intestinal nervous system in Crohn’s disease. Gastroenterology, 103(2), 439–447. 10.1016/0016-5085(92)90832-J [DOI] [PubMed] [Google Scholar]
- Gershon MD, & Rothman TP (1991). Enteric glia. Glia, 4(2), 195–204. 10.1002/glia.440040211 [DOI] [PubMed] [Google Scholar]
- Ghaisas S, Harischandra DS, Palanisamy B, Proctor A, Jin H, Dutta S, Sarkar S, Langley M, Zenitsky G, Anantharam V, Kanthasamy A, Phillips GJ, & Kanthasamy A (2021). Chronic Manganese Exposure and the Enteric Nervous System: An in Vitro and Mouse in Vivo Study. Environmental Health Perspectives, 129(8). 10.1289/EHP7877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaisas S, Maher J, & Kanthasamy A (2016). Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. In Pharmacology and Therapeutics (Vol. 158, pp. 52–62). Elsevier Inc. 10.1016/j.pharmthera.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Polo RA, Niso-Santano M, Ortíz-Ortíz MA, Gómez-Martín A, Morán JM, García-Rubio L, Francisco-Morcillo J, Zaragoza C, Soler G, & Fuentes JM (2007). Relationship between autophagy and apoptotic cell death in human neuroblastoma cells treated with paraquat: Could autophagy be a “brake” in paraquat-induced apoptotic death? Autophagy, 3(4), 366–367. 10.4161/auto.4194 [DOI] [PubMed] [Google Scholar]
- Greene JG, Noorian AR, & Srinivasan S (2009). Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Experimental Neurology, 218(1), 154–161. 10.1016/j.expneurol.2009.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubišić V, & Gulbransen BD (2017). Enteric glia: the most alimentary of all glia. Journal of Physiology, 595(2), 557–570. 10.1113/JP271021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, & Ellinger-Ziegelbauer H (2017). Mechanistic Investigations of the Mitochondrial Complex i Inhibitor Rotenone in the Context of Pharmacological and Safety Evaluation. Scientific Reports, 7(1), 1–13. 10.1038/srep45465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Romero MC, Delgado-Cortés MJ, Sarmiento M, de Pablos RM, Espinosa-Oliva AM, Argüelles S, Bández MJ, Villarán RF, Mauriño R, Santiago M, Venero JL, Herrera AJ, Cano J, & Machado A (2012). Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. NeuroToxicology, 33(3), 347–360. 10.1016/j.neuro.2012.01.018 [DOI] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Cristina Brailoiu G, Brailoiu E, Futter CE, Schapira AH, Marchant JS, & Patel S (2015). Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. Journal of Cell Science, 128(2), 232–238. 10.1242/JCS.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang ZY, Roybon L, Melki R, & Li JY (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathologica, 128(6), 805–820. 10.1007/s00401-014-1343-6 [DOI] [PubMed] [Google Scholar]
- Houser MC, & Tansey MG (2017). The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? In npj Parkinson’s Disease (Vol. 3, Issue 1, pp. 1–9). Nature Publishing Group. 10.1038/s41531-016-0002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, & Mirsky R (1980). Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature, 286(5774), 736–737. 10.1038/286736a0 [DOI] [PubMed] [Google Scholar]
- Jessen KR, & Mirsky R (1983). Astrocyte-like glia in the peripheral nervous system: An immunohistochemical study of enteric glia. Journal of Neuroscience, 3(11), 2206–2218. 10.1523/jneurosci.03-11-02206.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, & Bobrovskaya L (2015). An update on the rotenone models of Parkinson’s disease: Their ability to reproduce the features of clinical disease and model gene-environment interactions. In NeuroToxicology (Vol. 46, pp. 101–116). Elsevier. 10.1016/j.neuro.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, & Ko HS (2019). Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron, 103(4), 627–641.e7. 10.1016/j.neuron.2019.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhoefer L, & Reichmann H (2015). Pathogenesis of Parkinson disease—the gut–brain axis and environmental factors. Nature Reviews Neurology, 11(11), 625–636. 10.1038/nrneurol.2015.197 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Arozena AA, Adachi H, Adams CM, Adams PD, Adeli K, Adhihetty PJ, Adler SG, Agam G, Agarwal R, Aghi MK, Agnello M, Agostinis P, Aguilar P. v., Aguirre-Ghiso J, … Zughaier SM (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). In Autophagy (Vol. 12, Issue 1, pp. 1–222). Taylor and Francis Inc. 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn AJ, Wahlbrink T, & Prehn JHM (1999). Mitochondrial depolarization is not required for neuronal apoptosis. Journal of Neuroscience, 19(17), 7394–7404. 10.1523/jneurosci.19-17-07394.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambelet M, Terra LF, Fukaya M, Meyerovich K, Labriola L, Cardozo AK, & Allagnat F (2018). Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis article. Cell Death and Disease, 9(2), 1–15. 10.1038/s41419-017-0121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Sjölander A, Pedersen NL, Ludvigsson JF, Chen H, Fang F, & Wirdefeldt K (2021). Irritable bowel syndrome and Parkinson’s disease risk: register-based studies. Npj Parkinson’s Disease, 7(1). 10.1038/s41531-020-00145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch-Day MA, Mao K, Wang K, Zhao M, & Klionsky DJ (2012). The role of autophagy in Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine, 2(4). 10.1101/cshperspect.a009357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I, & Kroemer G (1998). Cytofluorometric detection of mitochondrial alterations in early CD95/Fas/APO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunology Letters, 61(2–3), 157–163. 10.1016/S0165-2478(98)00013-3 [DOI] [PubMed] [Google Scholar]
- Murakami S, Miyazaki I, Miyoshi K, & Asanuma M (2015). Long-Term Systemic Exposure to Rotenone Induces Central and Peripheral Pathology of Parkinson’s Disease in Mice. Neurochemical Research, 40(6), 1165–1178. 10.1007/s11064-015-1577-2 [DOI] [PubMed] [Google Scholar]
- Murphy MP (2009). How mitochondria produce reactive oxygen species. In Biochemical Journal (Vol. 417, Issue 1, pp. 1–13). Biochem J. 10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Kondo S, Le W, & Jankovic J (2008). The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain, 131(8), 1969–1978. 10.1093/brain/awm318 [DOI] [PubMed] [Google Scholar]
- Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, & Funk RHW (2010). Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE, 5(1). 10.1371/journal.pone.0008762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini C, Antonioli L, Colucci R, Ballabeni V, Barocelli E, Bernardini N, Blandizzi C, & Fornai M (2015). Gastric motor dysfunctions in Parkinson’s disease: Current preclinical evidence. In Parkinsonism and Related Disorders (Vol. 21, Issue 12, pp. 1407–1414). Elsevier Ltd. 10.1016/j.parkreldis.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Pham AH, Meng S, Chu QN, & Chan DC (2012). Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Human Molecular Genetics, 21(22), 4817–4826. 10.1093/hmg/dds311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, & Lang AE (2017). Parkinson disease. Nature Reviews Disease Primers, 3(1), 1–21. 10.1038/nrdp.2017.13 [DOI] [PubMed] [Google Scholar]
- Poirier AA, Aubé B, Côté M, Morin N, di Paolo T, & Soulet D (2016). Gastrointestinal dysfunctions in Parkinson’s disease: Symptoms and treatments. In Parkinson’s Disease (Vol. 2016). Hindawi Publishing Corporation. 10.1155/2016/6762528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl A, Trotter J, & Stremmel W (2001). Isolation of enteric glia and establishment of transformed enteroglial cell lines from the myenteric plexus of adult rat. Neurogastroenterology and Motility, 13(1), 95–106. 10.1046/j.1365-2982.2001.00246.x [DOI] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, Rokad D, Zenitsky G, Jin H, Anantharam V, Kanthasamy AG, & Kanthasamy A (2018). Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. NeuroToxicology, 64, 204–218. 10.1016/j.neuro.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A, Palanisamy BN, Rokad D, Jin H, Anantharam V, Kanthasamy A, & Kanthasamy AG (2017). Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. Npj Parkinson’s Disease, 3(1). 10.1038/s41531-017-0032-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffernicht G, Shang Q, Stievenard A, Bötzel K, Dening Y, Kempe R, Toussaint M, Gündel D, Kranz M, Reichmann H, Vanbesien-Mailliot C, Brust P, Dieterich M, Funk RHW, Ravens U, & Pan-Montojo F (2021). Pathophysiological Changes in the Enteric Nervous System of Rotenone-Exposed Mice as Early Radiological Markers for Parkinson’s Disease. Frontiers in Neurology, 12. 10.3389/FNEUR.2021.642604/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, & Marsden CD (1990). Mitochondrial Complex I Deficiency in Parkinson’s Disease. Journal of Neurochemistry, 54(3), 823–827. 10.1111/j.1471-4159.1990.tb02325.x [DOI] [PubMed] [Google Scholar]
- Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, & Kordower JH (2012). Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Movement Disorders, 27(6), 709–715. 10.1002/mds.23838 [DOI] [PubMed] [Google Scholar]
- Sharkey KA (2015). Emerging roles for enteric glia in gastrointestinal disorders. In Journal of Clinical Investigation (Vol. 125, Issue 3, pp. 918–925). American Society for Clinical Investigation. 10.1172/JCI76303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov A. v., Yagi T, Matsuno-Yagi A, Miller GW, & Greenamyre JT (2007). Mechanism of toxicity of pesticides acting at complex I: Relevance to environmental etiologies of Parkinson’s disease. Journal of Neurochemistry, 100(6), 1469–1479. 10.1111/j.1471-4159.2006.04333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaram C, Gaumnitz EA, Torbey C, Ashraf W, Quigley EMM, Sengupta A, & Pfeiffer R (1995). Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. The Lancet, 346(8979), 861–864. 10.1016/S0140-6736(95)92707-7 [DOI] [PubMed] [Google Scholar]
- Soloway SB (1976). Naturally occurring insecticides. Environmental Health Perspectives, vol.14, 109–117. 10.1289/ehp.7614109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Charli A, Luo J, Riaz Z, Jin H, Anantharam V, Kanthasamy A, & Kanthasamy AG (2019). Mechanistic interplay between autophagy and apoptotic signaling in endosulfan-induced dopaminergic neurotoxicity: Relevance to the adverse outcome pathway in pesticide neurotoxicity. Toxicological Sciences, 169(2), 333–352. 10.1093/toxsci/kfz049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, & Sørensen HT (2015). Vagotomy and subsequent risk of Parkinson’s disease. Annals of Neurology, 78(4), 522–529. 10.1002/ana.24448 [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, & Langston JW (2011). Rotenone, Paraquat, and Parkinson’s Disease. Environmental Health Perspectives, 119(6), 866–872. 10.1289/ehp.1002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes O-B, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH, & Reichmann H (2015). Does vagotomy reduce the risk of Parkinson’s disease? Annals of Neurology, 78(6), 1011–1012. 10.1002/ana.24531 [DOI] [PubMed] [Google Scholar]
- Uemura N, Yagi H, Uemura MT, Hatanaka Y, Yamakado H, & Takahashi R (2018). Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Molecular Neurodegeneration, 13(1). 10.1186/s13024-018-0257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, Wright-Jin EC, Vohra BPS, Heuckeroth RO, & Milbrandt J (2011). Differential regional and subtype-specific vulnerability of enteric neurons to mitochondrial dysfunction. PLoS ONE, 6(11). 10.1371/journal.pone.0027727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2012). Bioenergetic role of mitochondrial fusion and fission. Biochimica et Biophysica Acta - Bioenergetics, 1817(10), 1833–1838. 10.1016/j.bbabio.2012.02.033 [DOI] [PubMed] [Google Scholar]
- Yang X, Qian Y, Xu S, Song Y, & Xiao Q (2018). Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Frontiers in Aging Neuroscience, 9(JAN). 10.3389/FNAGI.2017.00441/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii SR, & Mizushima N (2017). Monitoring and measuring autophagy. In International Journal of Molecular Sciences (Vol. 18, Issue 9). MDPI AG. 10.3390/ijms18091865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Duan C, & Yang H (2014). Defective Autophagy in Parkinson’s Disease: Lessons from Genetics. In Molecular Neurobiology (Vol. 51, Issue 1, pp. 89–104). Humana Press Inc. 10.1007/s12035-014-8787-5 [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang W, Wang C, Siedlak SL, Fujioka H, & Tang B (2017). Mfn2 protects dopaminergic neurons exposed to paraquat both in vitro and in vivo: Implications for idiopathic Parkinson’s disease. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1863(6), 1359–1370. 10.1016/j.bbadis.2017.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.