Abstract
Particulate-filtering respirators (PFRs) have been recommended as a practical personal-level intervention to protect individuals from the health effects of particulate matter exposure. However, the cardiovascular benefits of PFRs including improvements in key surrogate endpoints remain unclear. We performed a systematic review and meta-analysis of randomized studies (wearing versus not wearing PFRs) reporting the effects on blood pressure (BP) and heart rate variability (HRV). The search was performed on January 3, 2022 to identify published papers until this date. We queried three English databases, including PubMed, Web of Science Core Collection and Scopus. Of 527 articles identified, eight trials enrolling 312 participants (mean age ± standard deviation: 36 ± 19.8; 132 female) met our inclusion criteria for analyses. Study participants wore PFRs from 2 to 48 h during intervention periods. Wearing PFRs was associated with a non-significant pooled mean difference of −0.78 mmHg (95% confidence interval [CI]: −2.06, 0.50) and −0.49 mmHg (95%CI: −1.37, 0.38) in systolic and diastolic BP (SBP and DBP). There was a marginally significant reduction of mean arterial pressure (MAP) by nearly 1.1 mmHg (95%CI: −2.13, 0.01). The use of PFRs was associated with a significant increase of 38.92 ms2 (95%CI: 1.07, 76.77) in pooled mean high frequency (power in the high frequency band (0.15–0.4 Hz)) and a reduction in the low (power in the low frequency band (0.04–0.15Hz))-to-high frequency ratio [−0.14 (95%CI: −0.27, 0.00)]. Other HRV indices were not significantly changed. Our meta-analysis demonstrates modest or non-significant improvements in BP and many HRV parameters from wearing PFRs over brief periods. However, these findings are limited by the small number of trials as well as variations in experimental designs and durations. Given the mounting global public health threat posed by air pollution, larger-scale trials are warranted to elucidate more conclusively the potential health benefits of PFRs.
Keywords: Air pollution, Blood pressure, Heart rate variability, Particulate-filtering respirators
1. Introduction
Cardiovascular disease (CVD) is the leading cause of global mortality, contributing to approximately 31% (18.6 million) of premature deaths worldwide in 2019 (Basu et al., 2017; Collaborators and Ärnlöv, 2020; Frumkin and Haines, 2019; Hadley et al., 2018a; Hadley et al., 2018b; Lelieveld et al., 2019; Mensah et al., 2019; Rajagopalan and Landrigan, 2021; Roth et al., 2020). It has been estimated that approximately 19% of all cardiovascular deaths (over 3 million) and 21% of all stroke deaths (more than 1.1 million) are attributable to long- and short-term exposures to ambient fine particulate matter ≤2.5 μm (PM2.5) (Faridi et al., 2021; Hadley et al., 2018a; Schraufnagel et al., 2019). Short-term elevations in ambient PM2.5 exposures (over hours to days or weeks) increase the likelihood of myocardial infarctions, stroke, arrhythmias and heart failure by 1%–3% within a few days (Al-Kindi et al., 2020; Bevan et al., 2020; Newman et al., 2020; Rajagopalan et al., 2018; Rajagopalan and Landrigan, 2021; Walzer et al., 2020). While regulations that lower air pollution at a population-level improve public health (Bard et al., 2019), 99% of the global population remain exposed to annual PM2.5 levels above the updated World Health Organization Air Quality Guidelines (WHO AQGs) (Rajagopalan et al., 2020; Rajagopalan and Landrigan, 2021). One proposed measure with the potential to help protect this vast number of individuals is the use of high-efficiency PFRs (e.g., N95 respirators) (Allen and Barn, 2020; Bard et al., 2019; Faridi et al., 2020; Giles et al., 2011; Rajagopalan et al., 2020). Although PFRs could provide some protection in regions with comparatively good air quality (e.g., Canada and United States), larger health benefits from their usage are more likely to occur in more heavily-polluted locations (e.g., China and Iran) (Bard et al., 2019; Faridi et al., 2019; Langrish et al., 2009a; Laumbach et al., 2015; Morishita et al., 2019; Shi et al., 2016; van Dorn, 2017; Zhang et al., 2016). This stands to reason because the degree of exposure reduction is potentially much greater in regions with higher ambient PM concentrations (Kaufman et al., 2020).
To date, a few randomized crossover trials (RCTs) have been published demonstrating improvements in key surrogate markers of cardiovascular health, including BP and HRV, in response to wearing PFRs (Bard et al., 2019). However, results have been mixed and thus the overall evidence to support their usage remains inconclusive. Additionally, a recently published meta-analysis only reported the pooled trial results regarding the effect of wearing PFRs on BP(Han et al., 2021b). We believe that the global public has a right to make informed decisions based upon sound scientific evidence regarding the merits of undertaking measures to protect themselves from the harmful effects of ambient air pollution, particularly its most notable marker (PM2.5). Therefore, we conducted a systematic review and meta-analysis of published RCTs focusing on two established surrogate cardiovascular endpoints, BP and HRV, given that they are well-known to be negatively impacted by PM2.5 (Rajagopalan et al., 2018; Rajagopalan et al., 2020) and are both linked to adverse CVD outcomes.
2. Methods
2.1. Search strategy
We conducted a systematic search of the articles based on the Preferred Reporting and Items for Systematic Review and Meta-Analysis (PRISMA) criteria. The search was performed on May 29, 2021 and updated on January 3, 2022 to identify the published articles until this date. The PICOS including: Participants, Intervention, Comparisons, Outcomes, and Study design are provided in Table S1 (Supplemental file). To access the relevant studies, we queried three English databases, including PubMed, Web of Science Core Collection and Scopus using the following search keywords: respirator (“respirator”, “particulate- filtering respirators”, “mask”, “N95”, “N99” “facemask”, “N95 Respirator”, “respiratory protective device”, “filtering face piece respirator”, “respirator air-purifying”, “disposable particulate respirator”), blood pressure (“cardiovascular”, “blood pressure”, “SBP”, “DBP”, “systolic blood pressure”, “diastolic blood pressure”, “arterial pressure”, “aortic blood pressure”), heart rate variability (“heart rate variability”, “HRV”, “cardiopulmonary”), particulate matter (“air pollution”, “particulate matter”, “Ultrafine Particle”, “air pollutant”, “PM”, “PM2.5”, “PM10”, “UFP”), and RCT (“Randomized crossover”, “Randomized crossover trials”, “RCT”, “Randomized Double-Blind Crossover Trial”, “Randomized Single-Blind Crossover Trial”, “crossover”). To combine the above- mentioned search key terms Boolean operators such as “OR” and “AND” were used. Full search strategy for PubMed, Scopus, and Web of Science is presented in Table S2.
2.2. Study inclusion and exclusion criteria
Our inclusion criteria were as follows: 1) study design: any types of RCTs; 2) intervention: any types of PFRs (N95 and N99) or facemasks; 3) subjects: humans, with no limitation on age, sex or medical history; 4) outcomes: BP outcome including SBP, DBP, MAP and/or HRV outcome including (SDNN: standard deviation of all the normal-to-normal intervals; rMSSD: root mean square of successive differences between adjacent NN intervals; pNN50: percentage of number of NN interval with difference ≥50 ms; LF: power in the low frequency band (0.04–0.15Hz); HF: power in the high frequency band (0.15–0.4 Hz); the ratio of LF to HF, HR: heart rate); 5) full-length peer-reviewed studies; and 6) language: English.
2.3. Article selection
Firstly, two authors of the paper (S.F and F.Y) screened all articles, independently and in duplicate. We selected eligible articles based on the title and abstract, if they fulfilled all aforementioned inclusion and exclusion criteria. Then, if the title and abstract of studies did not provide sufficient detail for a decision, two authors reviewed the full text of articles independently. To justify the exclusion of any article via a rationale form, a more rigorous second round of screening of all selected articles has been made by S.F. and F.Y, and any conflict and discrepancies between the preceding reviewers on the studies was resolved by M.S.H and M.SH through verbal discussion and consensus.
2.4. Data extraction
S.F and F.Y independently extracted the detailed information on the characteristics of studies, including study ID, country and city of the studies, study design, number of participants and their characteristics (age, sex and body mass index (BMI)), intervention duration and washout period, study duration, health outcomes measured. We extracted detailed information on the PFRs wore by the participants. Additionally, the picture of the PFRs worn by the participants in the reviewed studies is presented in Figure S1 (Supplemental file). Detailed information regarding PM2.5 levels, BP and HRV measurement protocols during the intervention periods in the included studies are presented in Table S3–S5. To estimate the effect of PFR intervention on cardiovascular outcomes, we extracted the means and standard deviations (SDs) of the reported cardiovascular outcomes between intervention and control periods. If the preceding data were not stated, we calculated SDs from standard errors, 95% CIs or ranges based on Cochrane Handbook for Systematic Reviews of Interventions (Cumpston et al., 2019). For the studies that only reported mean, median and interquartile range of BP and HRV indices between intervention and control periods, the SDs were estimated according to an approach developed by the study of Wan et al., (2014) (Wan et al., 2014).
2.5. Outcomes
The health outcomes assessed were changes in SBP, DBP, MAP, HF, LF, the ratio of LF to HF, SDNN, pNN50, rMSSD and HR in association with PFRs use.
2.6. Statistical analysis and risk of bias assessment
To assess between-study heterogeneity and variation, we used Cochran’s Q test and tau2, respectively (Pedersen et al., 2014). I2 is the proportion of total variation in the point estimates that is attributable to between-study heterogeneity rather than within-study error (Coory, 2010). Given the limited statistical power of Cochran’s Q test when the number of included studies is small like our study, we decided to investigate the effects of wearing PFR versus not wearing PFR on the changes of BP and HRV indices and their 95% confidence intervals (95% CIs) using random-effect meta-analysis model, as a conservative approach (Pedersen et al., 2014). We also expected that there were considerable variations among the included studies based on participants characteristics (e.g., age, sex and ethnicity), PM2.5 levels, measures of BP and HRV monitoring, and the characteristics of PFRs (e.g., efficiency and respiration resistance). As a result, we used the random-effect meta-analysis model as a conservative approach. To evaluate publication bias, we used funnel plots and egger tests (Pedersen et al., 2014). To identify the potential influential study and explore the robustness of the findings of our meta-analysis to the exclusion of the study, we conducted sensitivity analyses by repeating meta-analyses after removing one study at a time and comparing the combined estimates with and without that study (Pedersen et al., 2014). It should be highlighted that we analyzed the pooled data of all studies in which the participants wore their PFRs for 2, 4, 24 and 48 h. Also, we conducted the subgroup meta-analyses stratified by mean age of participants (the trials with the participants above versus below 60 years), duration of wearing PFRs (the studies in which the participants wore their PFRs for 24 and 48 h versus the trials in which the subjects used the PFRs for 2 and 4 h) and ambient PM2.5 levels (24-h PM2.5 concentrations above versus below 25 μg m−3). Though the number of studies (2 trials versus 3 or 4) were incomparable for several outcomes in the sub-group meta-analyses, nonetheless, we believe conducting these analyses may helpful for designs of future studies and/or to provide suggestive scientific evidence. All meta-analyses were performed using the Review Manager Software (version 5.4).
2.7. Risk of bias assessment
To conduct the risk of bias assessment (RoB), we explored five domains (Figure S6) as follow: 1. bias arising from the randomization process; 2. bias due to deviations from intended intervention; 3. bias due to missing outcome data; 4. Bias in measurement of the outcome; and 5. bias in selection of the reported result (Higgins et al., 2019). The RoB was conducted independently by two authors (S.F and F.Y), and any conflict and discrepancies between the preceding reviewers was resolved by M.S.H and M.SH through verbal discussion and consensus. The RoB for the included studies in our meta-analysis was assessed by using the Cochrane Collaboration’s online tool; (RoB2 tool, https://mcguinlu.shinyapps.io/robvis/).
3. Results
3.1. Overview of the included studies
Our systematic search retrieved 527 studies, of which 205 duplicates were removed. Then, we excluded 322 studies based on the title and abstract or a brief screening of the full text of article. Based on our inclusion and exclusion criteria mentioned above, the remaining 12 articles underwent a full-text review in detail, after which four articles were excluded (Fig. 1). Eight RCTs were eligible for our meta-analysis, enrolling a total of 312 participants (mean age: 36; 132 female) (Table 1). The average (±SD) SBP/DBP of participants across trials was 115.7/72.6 (±7.3/4.5) mmHg during the PFR intervention and 117.3/ 73.0 ± (8.6/5.3) mmHg without the PFR. Six RCTs were conducted in the highly polluted countries with the 24-h mean concentrations more than 25 μg m−3 (five ones in China and another in Iran) and two studies conducted in the USA and South Korea. The mean PM2.5 concentrations in the trials (Table S3) were 57.1 ± 37.3 μg m−3 (range 9.2–140 μg m−3). All trials recruited healthy individuals, except for one which included patients with coronary heart disease. Eight RCTs investigated changes in SBP and DBP, whereas 5 evaluated changes of MAP. Six RCTs analyzed changes in HF, LF, SDNN and HR and 5 trials the ratio of LF to HF, rMSSD and pNN50.
Fig. 1.
Flowchart illustrating the stages of the literature search.
Table 1.
Summary of characteristics of studies included in this systematic review and meta-analysis.
| Study | Country/City | Study design | # of study participants (include to analyses) | Study participants | Sex(F/M) | Age (Ave ± SD) | BMI (Ave ± SD) | Intervention duration | Study duration | Health outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Han et al. (2021a) | China/Tianjin | Randomized, Double-Blinded, Crossover Intervention Study (participants were blinded and the research assistants equipping BP and HRV measurement devices). | 39 (39) | Healthy university students | F: 20 M: 19 | 22.7 (range: 20–29) | 21.0 (range: 15.6–29.4) | 2-h near- roadway during the early morning rush hour | November 2014 to January 2015 | SBP, DBP, HR, HRV (SDNN, rMSSD, pNN50, HF, LF and VLF) |
| Faridi et al. (2021) | Iran/Tehran | Randomized Crossover Study | 30 (26) | Healthy college students | M: 26 | 25.0 ± 2.6 | 23.2 ± 3.04 | 48-h in indoor dormitory; a week washout period | February 14th to 23rd, 2019 | SBP, DBP, PPa, MAP, SDNN, rMSSD, pNN50, VLFb, TPc, TBd, LF, HF, LF/HF ratio, HR), blood biomarkers (hs- TnTe, WBCf, HGBg, HCTh and platelet) |
| Lim et al. (2020) | Korea/Seoul | Quasi-Experimental Study (Randomization) | 21 (21) | Elderly without any clinically- diagnosed Chronic diseases | F: 21 | 72.7 ± 3.8 | Height: 152.5 ± 5.3 and Weight: 56.6 ± 6.5 | 3 weeks (6 days) wear mask until the health examination and for as long as possible except while eating, sleeping, and washing | May 2018 (three weeks) | SBP, DBP, MAP, PP, lung function (FEV1i, FVC j, FEV1/FVCk, and FEF25–75l). |
| Morishita et al. (2019) | USA/Michigan | Randomized Single-Blind Crossover Study | 50 (50) | Nonsmoking healthy adults aged 18–65 years | F: 36 M: 14 | 36 ± 14 | 26.1 ± 4.7 | 2-h near- roadway exposures; a weekend washout period | During warm months (May to October) 2017–2018 to avoid exposures to excessively cold temperatures | SBP, DBP, aortic hemodynamics, SDNN, LF, HF, LF/HF ratio |
| Yang et al. (2018b) | China/ Beijing | Randomized Crossover Study | 40 (39) | Healthy college students | F: 18 M: 21 | 21.2 ± 1.7 |
21.6 ± 1.7 | 4-h in subway system; a 2- week washout period | From 11 March to May 28, 2017 | SBP, DBP, MAP, PP, SDNN, rMSSD, pNN50, TP, LF, HF, LF/HF ratio, HR |
| Shi et al. (2017) | China/ Shanghai | Randomized Crossover Trial | 30 (24) | Healthy college students | F: 11 M: 13 | 23 | 22 ± 4 | 48-h in campus dormitory rooms; a 3- week washout period | From 21 March to April 13, 2014 | SBP, DBP, SDNN, SDANN, rMSSD, pNN50, VLF, LF, HF, LF/HF ratio, HR, Circulating biomarkers (Fibrinogen, P- selectin, VCAM-1 m, ET-1 n and vWF) |
| Langrish et al. (2012) | China/ Beijing | Prospective Randomized Open Blinded End Point (PROBE) Crossover | 102 (98) | Patients with a history of coronary heart disease | F: 13 M: 85 | 62 ± 7 | 26 ± 3 | wear the PFR for 24 h prior to the study day and 24 h of the study day; a week washout period | Between March and May 2009 | SBP, DBP, MAP, SDNN, rMSSD, pNN50, LF, HF, HF/LF ratio, LFn, HFn, HR, Ischemic burden (Inferior (II) territory, Anterior (V2) territory, Lateral (V5) territory, Sum (II + V2 + V5) |
| Langrish et al. (2009b) | China/Beijing | Open-Label Cross-Over Randomized Controlled Trial | 15 (15) | Healthy volunteers | F: 13 M: 2 | Median: 28 (min-max: 20–45) | 20.5 (95% CI, 19.3–21.7) | wear the PFR for 24 h prior to the study day and 24 h of the study day; a week washout period | August 2008 | SBP, DBP, MAP, Triangular Index, SDNN, rMSSD, pNN50, LF, HF, HF/LF ratio, LFn, HFn, HR |
Standard deviation of the averages of NN intervals in all 5-min segments of the entire recording.
Percentage of number of NN interval with difference ≥50 ms
Lower in the very low frequency band (0.01–0.04 Hz).
Total power.
C-reactive protein.
Platelet factor.
VonWillebrand Factor.
α2-macroglobulin.
α-acid glycoprotein.
Serum amyloid protein.
Tumor necrosis factor.
Granulocyte macrophage colony-stimulating factor.
Interferon Gamma.
Pulse pressure.
Power in the very low frequency band (0.01–0.04 Hz).
The area under the spectral curve between 0.01 and 0.4 Hz.
Total beats.
, high-sensitive cardiac troponin.
White Blood Cells.
Hemoglobin.
Hematocrit.
Forced expiratory volume in 1 Second [L].
Forced vital capacity [L].
The ratio of FEV1 to FVC [%].
Forced expiratory flow at 25%–75% of the FVC [L/s]).
Vascular cell adhesion molecule-1.
Endothelin-1.
Table 2 provides detailed information on the PFRs used by the participants in the included studies. Except for one study (Lim et al., 2020), other publications characterized the types of PFRs used by participants and whether it had an exhalation valve. As shown in Table 2 and Figure S1, six studies have used PFRs with a one-way exhalation valve to lessen the buildup of heat, moisture, and CO2 inside the respirator in the breathing zone, whereas participants in two studies (Faridi et al., 2021; Han et al., 2021a) wore PFRs without an exhalation valve. Among seven studies mentioned above, in one study (Morishita et al., 2019), PFRs had a micro-fan in addition to one-way exhalation valve (Figure S1). In the included studies, participants were asked to wear their PFRs for 2, 4, 24 and 48 h in different situations (in the near-roadway site, traveling by the underground subway, in campus dormitory rooms and outdoor, walking in city center and near-roadway). Except for 3 studies (Faridi et al., 2021), (Lim et al., 2020) and (Shi et al., 2017), the studies did not report detailed metrics of compliance, such as the actual duration (or percentage of trial time) participants wore the PFRs during each intervention period. In addition, two studies (Faridi et al., 2021; Langrish et al., 2009a) assessed the efficiency of PFRs, while other studies reported the efficiency per their manufacturers. With two exceptions (Faridi et al., 2021; Han et al., 2021a), participants in the trials were educated on how to wear PFRs to ensure a proper facial fit to minimize the penetration of PM through gaps. Four studies (Lim et al., 2020; Shi et al., 2017; Yang et al., 2018b; Zhang et al., 2019) formally assessed the facial fit of PFRs. With the exception of one study (Lim et al., 2020), facial fit was assessed by quantitative fit testing. In general, most studies reported some degree of patient intolerance with wearing the PFRs. A low to high respiratory resistance was reported on average by patients in five studies. Only one study reported that the PFRs were completely comfortable and tolerable.
Table 2.
Detailed information of the PFRs used by the participants in the included studies.
| Study | Types of PFRs used by participants (did the used PFR had an exhalation valve?) | Time use of the PFRs by the participants in each intervention period | Have the efficiency/ penetration of PFRs been measured by the included studies? | Have the participants been instructed to wear PFRs accurately for ensuring a perfect fit with their face to minimize the penetration of PM through the gaps between the face and the PFRs? | Have the fit testing of PFRs been measured in the studies? | Were the participants ask to complete symptom questionnaire or data collection form on the respiration resistance, headache, difficulty in breathing when using the PFRs, comfortability/ intolerability of PFRs or other symptoms? If yes, what were their responses? |
|---|---|---|---|---|---|---|
| Han et al. (2021a) | N95 (8210 N95 Respirator, 3M Science, MN, United States) without exhalation valve | 2 h | No (They reported that “ The N95 mask we used in this study was bought online, with the filtration efficiency on particles no less than 95%”). | No | No | No |
| Faridi et al. (2021) | Biomask (without exhalation valve) | The PFR intervention group was asked to wear their PFRs at all times while awake as much as possible when in the dormitory and when they were outdoors. The participants have reported that they wore the PFR between 10.2 and 11.1 h while awake during the interventions. | Yes (The efficiency of PFR used by the participants has been measured based on experimental set-up developed by the study against ambient PM1, PM2.5 and PM10 in a traffic-affected urban site in Tehran). The average filtering effectiveness of the used PFR was 83.5%, 68.1%, 46.1% and 32.2% in terms of ambient particle number concentration, PM10, PM2.5 and PM1 mass concentrations, respectively. This PFR reduced exposure to ambient PM10 in the range of 51.7–100.3 μg m−3, with a mean value of 94.6 μg m−3. The PFR reduced ambient PM2.5 and PM1 by 25.7–43.5 μg m−3 and 14.7–21.8 μg m−3, with mean values of 29.0 and 18.2 μg m−3, respectively. | Yes | No | Yes (More than 80% of participants reported increased respiratory resistance while wearing the PFR due to a lack of an exhalation valve. Of 26 participants, 22 and 23 subjects reported very high to moderate respiratory resistance and difficulty in breathing when using the PFR. In addition, 23 of participants stated that the PFR was very high to moderate intolerable due to its rigid ear loop clamps. Nine college students also reported very high to moderate headache while wearing the PFR. |
| Lim et al. (2020) | Disposable particulate respirators (PNTD, Mungyeong, Korea) (There is no information on whether PFR had the exhalation valve in the article.) | The participants wore PFRs for six consecutive days (excluding time spent eating, sleeping, and bathing). | No (They reported that “These particulate respirators are capable of filtering more than 80% of 0.6 μm nonoil particulates, meeting the Korea Food and Drug Administration KF80 standards”)). | Yes | Yes (Mask Fitting Tester MT-03 (SIBATA Science Technology, Saitama, Japan) was used. Out of 21 participants, only 14.3% (n = 3), 19% (n = 4) and 29% (n = 6) of participants showed less than 50% of leaking rate or passed each fitting test in the first, second and third leak tests. | No (One participant reported that she did not wear the PFR one morning while heading to the health examination for the experimental period. Another subject reported that she could not wear the respirator all the time because of intolerance to wearing the particulate respirator at home.) |
| Morishita et al. (2019) | N95 respirator (a new Dettol SiTi shield Protect Plus Smart Mask) with a micro- fan to lessen the buildup of heat, moister, and CO2 inside the respirator in the breathing zone | 2 h | No (It has been reported that the PFR used by the participants validated and approved by the National Institutes of Occupational Safety and health (NIOSH)). | Yes | No | No |
| Yang et al. (2018b) | 3M respirator (9002V), the used PFR had an exhalation valve. | 4 h | No | Yes | Yes (Before the study, PFRs fit tests were conducted by using the TSI PortaCount Pro+8038 (TSI Inc., USA)), in accordance with the Occupational Safety and Health Administration (OSHA). The participants with the integrated fit factor >100 were included to participate in the study. | No |
| Shi et al. (2017) | Disposable particulate respirators (8210V; 3M™), the used PFR had an exhalation valve. | The participants were equally randomized into two groups and wore particulate- filtering respirators for 48 h The intervention group was required to wear their PFRs for all the time they were outdoors and as much as possible when they were indoors. It has been reported that the participants wore their respirators for more than 90% of their time outdoors and 82% of their time indoors, on average. | No (It has been reported that the PFR used by the participants validated and approved by the National Institutes of Occupational Safety and health (NIOSH)). | Yes | Yes (Before the intervention study, qualitative respirator fit testing on the face-to- respirator seal was performed using the 3M™ Qualitative Fit Test Apparatus FT-30 (3M™, USA). Additionally, the subjects positioned the respirators and then placed the professional testing hood on their heads. A bitter-tasting agent was sprayed into the hood. If the subject did not taste the bitter agent at all, the respirators were worn correctly. No detection of bitter taste in any test was considered as formally accepted. | Yes (The scores reported by the participants for the comfortability of respirators during the study period was 5 on average (on a scale from 0 to 10 referring to the worst comfort to the best)). |
| Langrish et al. (2012) | Dust Respirator 8812; 3M, St. Paul, MN, USA The used PFR had an exhalation valve. | Subjects were asked to wear the PFRs for 24 h before the PFRs study day, in addition to wearing it during the 24 h study day, and were given instructions to wear the PFRs at all times while outdoors and as much as possible when indoors. | No (It has been reported that the PFR used by the participants validated and approved by the EN149:2001 FFP1 European Standard (British Standards Institute, 2001)). | Yes | No | Yes (All subjects have stated that they tolerated the PFR intervention well, scoring the comfort of the PFR as 0.64 ± 1.06 on a 0–10 scale (0 represents completely comfortable, and 10, intolerable)). |
| Langrish et al. (2009b) | Dust Respirator 8812; 3M, St. Paul, MN, USA The used PFR had an exhalation valve. | When randomized to wear the PFRs, subjects were asked to wear the PFRs for 24 h prior to the study day and 24 h of the study day. Subjects were asked to wear the PFRs at all times when outside, and as much as possible whilst indoors. | Yes (It has been reported that the penetration of PFR has been measured using an experimental set-up against fresh diesel exhaust particulate (a mass concentration of 75 ± 12 μg m−3 (as measured by gravimetric analysis) and a particle number concentration equal to 500,000 particles/cm3)). The penetration for the used PFR was approximately 3.5%. | Yes | No | Yes (The participants have reported that the PFR was generally well tolerated with an average score of 24.8% (95% CI, 16.2–33.3%); 0% being completely tolerable and 100% being intolerable). Additionally, the subjects did report slightly greater difficulty breathing (increased resistance to respiration) whilst walking although this did not reduce the level of exercise undertaken by the subjects. |
3.2. Outcomes of PFRs on BP and HRV indices
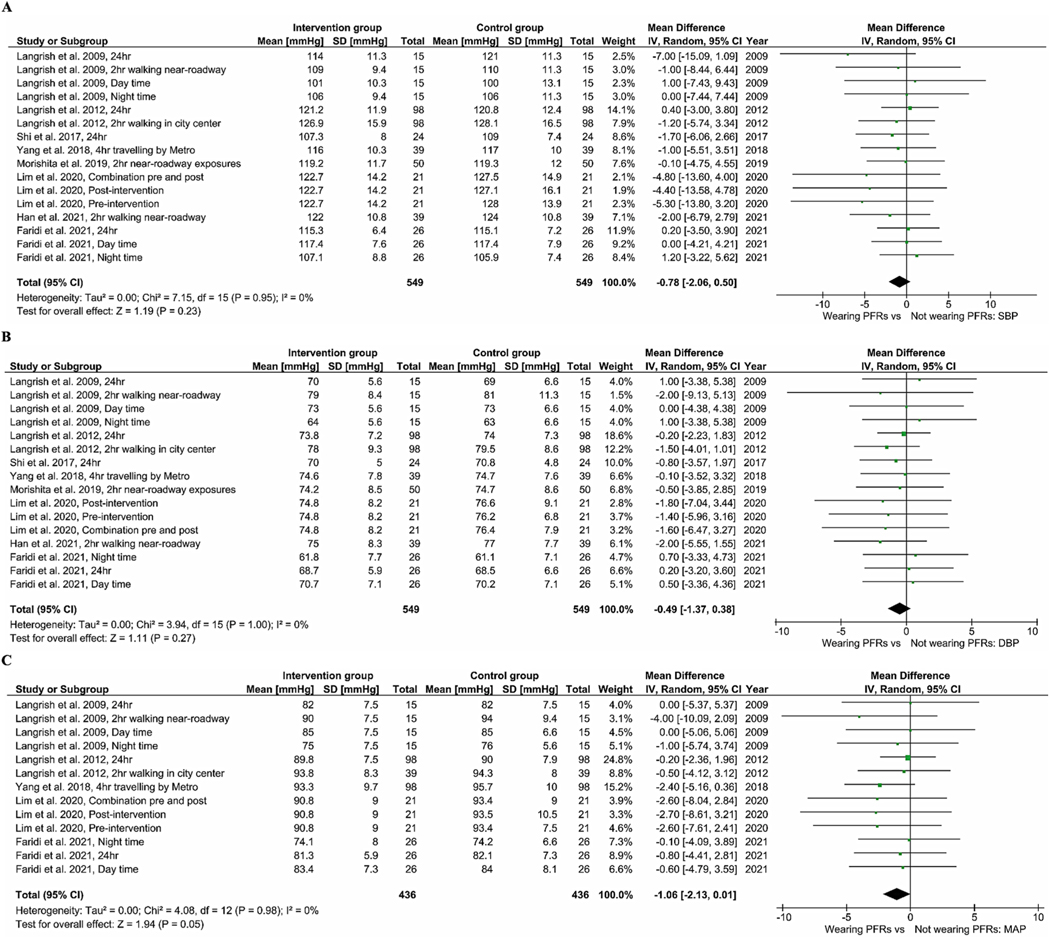
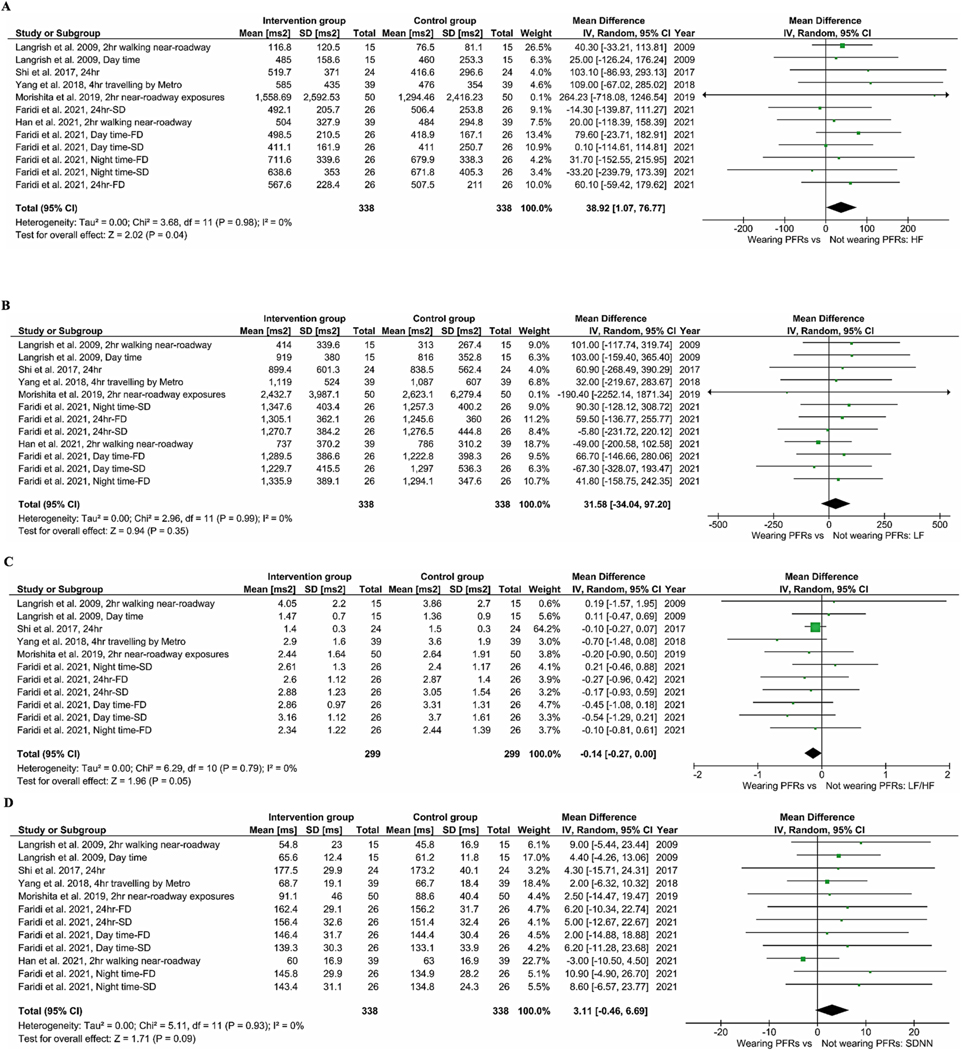
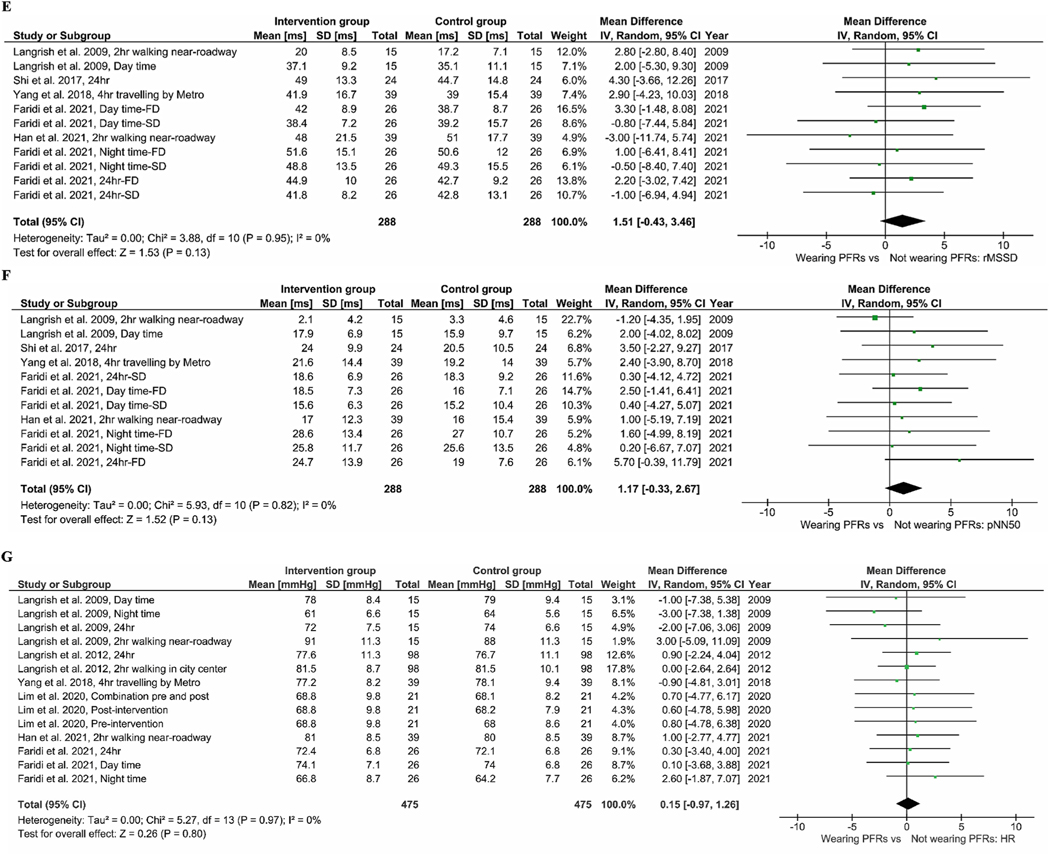
Fig. 2A–C gives the BP results from our meta-analysis. Wearing PFRs was associated with a pooled mean difference of −0.78 mmHg (95% CI: −2.06, 0.50), −0.49 mmHg (95% CI: −1.37, 0.38), and −1.06 mmHg (95% CI: −2.13, 0.01) in SBP (Fig. 2A), DBP (Fig. 2B) and MAP (Fig. 2C), respectively. The results of meta-analysis of pooled data of all trials for HRV indices are presented in Fig. 3A–G. Across all seven RCTs for the indices of HF and LF, the use of PFRs was associated with an increase of 38.92 ms2 [(95% CI: 1.07, 76.77); p-value = 0.04] and 31.58 ms2 [(95% CI: −34.04, 97.2); p-value = 0.35] in pooled mean HF (Fig. 3A) and LF (Fig. 3B), respectively. In terms of the ratio of LF to HF (Fig. 3C), a reduction of −0.14 [(95% CI: −0.27, 0.00); p-value = 0.05] was found. There was no statistically significant difference in pooled mean SDNN [3.11 ms (95% CI: −0.46, 6.69), p-value = 0.09] (Fig. 3D). We did find a non-significant improvement in rMSSD (Fig. 3E) resulting from the use of PFRs 1.51 ms [(95% CI: −0.43, 3.46), p-value = 0.13]. The use of PFRs was associated with a non-significant increase of 1.17 (95% CI: −0.33, 2.67) in pNN50 (Fig. 3F) and HR of 0.15 bpm (95% CI: −0.97, 1.26) (Fig. 3G). For elderly participants (>60 years old), wearing PFRs was associated with a pooled mean reduction of −1.22 mmHg (95% CI: −3.62, 1.18), −0.92 mmHg (95% CI: −2.30, 0.45) and −0.89 mmHg (95% CI: −2.48, 0.71) in SBP (Figure S2–A), DBP (Figure S2–B) and MAP (Figure S2–C), respectively. Although not statistically significant, there was a trend towards greater BP reductions in the elderly participants (Figure S2 A–C) in comparison to the younger participants (Figure S3 A–C). Similar trends were observed for HR [0.47 bpm (95%CI: −1.23, 2.17) in the elderly −0.09 bpm (95%CI: −1.57, 1.38) in the younger participants], as shown in Figure S2D and Figure S3D. In the meta-analysis of trials with mean ambient PM2.5 levels below 25 μg m−3 (Figure S4), the use of PFRs led to a non-significant decrease of −2.26 mmHg (95%CI: −5.69, 1.17) and −1.14 mmHg (95%CI: −3.29, 1.02) in SBP and DBP. Compared to trials with mean PM2.5 levels above 25 μg m−3 (Figure S4 and 5), there was a tendency for larger BP reductions. In the meta- analysis of trials in which the participants wore their PFRs for 24 and 48 h (Table S6), the use of PFRs led to a non-significant decrease of −0.71 (95% CI: −2.17, 0.74) mmHg and −0.41 mmHg (95% CI: −1.39, 0.56) in SBP and DBP and increase of 36.46 ms2 (95% CI: −3.95, 76.86) in HF. By contrast, trials in which the participants wore their PFRs for 2 and 4 h (Table S6), there were the tendencies for larger BP reductions and HF increase.
Fig. 2.
Meta-analysis of the effects of wearing PFR on BP. The mean difference estimates (95%CIs) are shown for SBP (A), DBP (B) and MAP (C).
Fig. 3.
Meta-analysis of the effects of wearing PFR on HRV indices. The mean difference estimates (95%CIs) are shown for HF (A), LF (B), LF:HF ratio (C), SDNN (D), rMSSD (E), pNN50 (F) and HR (G).
3.3. Results of bias assessment and publication bias
Based on the Cochrane Collaboration’s online tool (https://mcguinlu.shinyapps.io/robvis/) for assessing the risk of bias (RoB2 tool), seven studies were assessed as having “low” or “some concerns” risk of bias and only one study had “high” risk of bias (Figure S6). Figure S7 and S8 reveal the Begg funnel plots and Eggers’ tests for the BP and HRV indices, respectively. The Egger’s test and Begg funnel plots suggested no sign of publication bias for BP outcomes, except for SBP (p-value = 0.007). The Egger’s test did not provide evidence of publication bias among RCTs for all of HRV indices. Begg funnel plots were not markedly asymmetrical for either BP parameters or HRV indices. Based on Cochran’s Q test (Chi2), heterogeneity was not found for all of BP and HRV outcomes between studies (P-value > 0.05) and also tau2 and I2 index were 0.0 and 0%, respectively.
3.4. Sensitivity analyses
Given SBP, DBP, LF, rMSSD and HR, we did not observe any notable changes in our pooled estimates after removing studies one-by-one (Table S7). However, for LF/HF ratio (Langrish et al., 2009b), pNN50 (Langrish et al., 2009b) and SDNN (Han et al., 2021a) when the influential study was removed the pooled estimates were statistically significant and wearing the PFRs versus not wearing them reduced LF/HF by −0.15 (95% CI: −0.29, −0.01), increased pNN50 and SDNN by 1.85% (95% CI: 0.07, 3.64) and 4.91 ms (95% CI: 0.84, 8.97), respectively. Moreover, for HF when two influential studies of (Morishita et al., 2019) and (Han et al., 2021a) were removed the pooled estimates showed that wearing the PFRs versus not wearing them increased HF by 38.58 ms2 (95% CI: 0.70, 76.46) and 40.45 ms2 (95% CI: 1.10, 79.80), respectively.
4. Discussion
Nearly the entire global population (99%) is exposed to annual PM2.5 levels above the updated WHO AQGs (Rajagopalan et al., 2020; Rajagopalan and Landrigan, 2021). As such, there is a growing need to consider personal-level interventions to prevent the adverse health effects, especially in regions with poor air quality. PFRs have been proposed as one potentially viable measure; however, their real-world effectiveness could be variable and of uncertain value despite growing usage in some regions (e.g., China and Iran) (Huang and Morawska, 2019). It is therefore of critical importance to validate their health benefits, especially in relation to reducing cardiovascular risk (Bard et al., 2019; Hadley et al., 2018a; Rajagopalan et al., 2018; van Dorn, 2017). Public-health bodies such as the WHO, as well as the American Heart Association and the European Society of Cardiology have acknowledged the potential usefulness of PFRs, yet have made no formal promulgations supporting their usage due to a paucity of evidence (Bard et al., 2019; Brook et al., 2017; Hadley et al., 2018a; Hadley et al., 2018b; Huang and Morawska, 2019). To provide sufficient scientific evidence on the effectiveness of wearing PFRs to reducing cardiovascular risk, several trials from across the world have investigated the effect of use of them on BP and HRV outcomes and one recently published meta-analysis only reported the pooled trial results regarding the effect of wearing PFRs on BP (Han et al., 2021b). Though this published meta-analysis paper is well designed and written, another major limitation for this study is that the authors have not conducted sensitivity analyses as one of the most important sections for each systematic review and meta-analysis study (Han et al., 2021b). Consequently, we performed this systematic review and meta-analysis to reveal simultaneously the effect of wearing PFRs on BP and HRV, both well-established surrogate markers predictive of adverse health outcomes that are negatively influenced by PM2.5 (Rajagopalan et al., 2018). Additionally, we have conducted three sub-group meta-analyses of usage PFRs on both BP and HRV with new interpretations to provide suggestive scientific evidence for designs of future studies. Also, we have discussed on the probable biological mechanisms of improvement of BP and HRV due to wearing PFRs. Finally, we have explored and reported the findings of several studies assessed the efficiency of PFRs against ambient PM air pollution to introduce several suggestions to manufacturers and wearers for improving the efficiency of PFRs as a practical personal-level intervention against ambient PM air pollution and its health consequences.
Our meta-analysis provides suggestive evidence that PFRs have the potential to be protective for cardiovascular health. Wearing PFRs for a few hours to days modestly lowered SBP and DBP; however, the results were not statistically significant except for MAP. PFRs were further associated with some improvements in HRV indices, a few of which were significantly improved (HF and LF to HF ratio) for all included trials. Additionally, when the influential study was removed the pooled estimates were statistically significant and wearing the PFRs versus not wearing them increased pNN50 and SDNN. There were also trends for enhanced benefits (e.g., greater BP reductions) in older participants and in trials with PM2.5 levels below 25 μg m−3. While the former finding is not surprising as elderly people are more sensitive to air pollution (Rajagopalan et al., 2018), the latter result may appear unexpected at first consideration. However, it could be explained by the well-established exposure-response curve that is steeper at lower levels of ambient PM2.5 (Rajagopalan et al., 2018). As a consequence, greater health benefits should accrue from interventions that reduce exposures at lower ambient levels. For example, a PFR that reduces PM2.5 exposure by 110 μg/m3 (from 150 to 40 μg/m3) may yield a comparable or even smaller benefit than one that reduces exposure by only 30 μg/m3 (from 40 to less than 10 μg/m3) (Faridi et al., 2021; Hadley et al., 2018a; Rajagopalan et al., 2018).
Interestingly, based on our subgroup meta-analysis for duration of wearing PFRs, the pooled-effect size of wearing PFRs for the studies in which the participants wore them for 2 and 4 h were higher than that of the studies in which the subjects wore them for 24 and 48 h. Several potential factors might explain this finding (Faridi et al., 2021; Guan et al., 2018). In the longer studies, participants may not wear respirators at all the time during the study, particularly while asleep and eating (Faridi et al., 2021; Lim et al., 2020; Shi et al., 2016). Consequently, this may have obviated any health benefits not only during the night but also the following day (Faridi et al., 2021). Additionally, it is plausible that longer wearing the respirators and the increased respiratory resistance mitigated potential health benefits (Faridi et al., 2021). While the observed decreases in BP in our meta-analysis were small, the potential public health benefits should not be discounted. It has been estimated that a decline of 5 mmHg SBP in a population will reduce mortality from stroke, coronary heart disease and all-cause mortality by 14%, 9%, and 7%, respectively (Adler et al., 2021; Walzer et al., 2020; Whelton, 2002). Thus, a short-term decrease of nearly 1 mmHg in SBP may yield important public health benefits if sustainable in thousands, or even hundreds of thousands of patients (Walzer et al., 2020; Whelton, 2002). Patients with hypertension generally enjoy significantly larger reductions in BP following lifestyle interventions (e.g., decreased sodium intake, exercise) than normotensives. We posit that wearing a PFR may produce a greater BP-lowering benefit among patients with overt hypertension. HRV is a well-documented measure of cardiac autonomic modulation in healthy individuals and patients with cardiovascular disorders (Huang et al., 2021; Magari et al., 2001), and a reduced HRV is a predictor of increased risk for CVD mortality and morbidity (Breitner et al., 2019; Gold et al., 2000; Pieters et al., 2012). It is plausible that the significant reduction in LF/HF we observed reflects a favorable change in autonomic balance that could be responsible for mediating the decreases in BP and/or have other direct benefits (e.g., prevent arrhythmias) that reduce cardiovascular risk (Faridi et al., 2021; Hadley et al., 2018a; Newman et al., 2020).
4.1. Probable biological mechanisms of improvement of BP and HRV due to wearing PFRs
Note that the biological mechanisms eliciting the advancement of CVD as well as the subsequent adverse CV events observed after exposure to PM2.5 are not yet understood in detail (Brook and Rajagopalan, 2021; Rajagopalan et al., 2018; Rankin et al., 2021). The observed reactions in response to inhaled PM2.5 air pollution have been discussed in several informative reviews, and include five pathways as follows: 1) pulmonary and systemic oxidative stress and inflammation leading to systemic inflammation, 2) vascular changes and endothelial dysfunction, 3) an increase in thrombogenicity and decrease in fibrinolysis, 4) changes in cardiac electrophysiological properties, and 5) autonomic imbalance with a shift to a relative increase in sympathetic outflow (Brook and Rajagopalan, 2021; Rajagopalan et al., 2018; Rankin et al., 2021). Among the pathways, the most relevant mechanism through which short-term PM2.5 exposures may contribute to acute cardiovascular events, consistent with the time frame of the herein reviewed studies, is autonomic imbalance which can directly alter systemic hemodynamics (e.g., increase BP) and/or promote arrhythmogenesis (Bevan et al., 2020; Brook and Rajagopalan, 2020; Rajagopalan et al., 2020). The assessment of autonomic tone in humans is difficult. Alterations in HRV parameters represent complex integrated responses in time and frequency domains that can provide insights into cardiac autonomic functioning (Brook and Rajagopalan, 2021; Rajagopalan et al., 2018; Rankin et al., 2021). Full details are reviewed elsewhere (Rajagopalan et al., 2018; Rankin et al., 2021) and are beyond the scope of this article; however, our findings (in particular reduced LF/HF ratio by wearing PFRs) are generally consistent with particle exposures promoting autonomic balance favoring sympathetic activity (Brook and Rajagopalan, 2021; Rankin et al., 2021). We recognize that characterizing autonomic activity/balance in humans is a complex issue. There are numerous complicating factors at the physiological level (e.g., discordant responses between organs, time-dependent changes, and direct versus baroreflex-mediated compensations) as well as limitations inherent to all available methodologies whether evaluating direct (e.g., muscle sympathetic activity [MSA]), indirect or organ-specific (e.g., HRV) or “whole-body” responses (e.g., circulating/urinary catecholamines, metabolomic profiling) (Grassi and Esler, 1999). HRV has its own weakness and interpretation of the findings from a physiological standpoint are not without controversies (Hayano and Yuda, 2019). The biological basis for generating HRV, in particular at the frequency domain, are not simple nor completely understood. We acknowledge that while our findings (i.e., increased LF/HF) might be consistent with heightened sympathetic tone, a full understanding of the underlying etiology cannot be provided by our observational analysis of prior reports. However, when taken together with the totality of prior evidence supporting heightened sympathetic tone in response to PM exposure, such as from direct MSA recording (Rankin et al., 2021) and metabolomic profiling responses (Li et al., 2017), we believe our findings are at least consistent with this speculation. Wearing PFRs (including N95 or N99 PFRs) have been proposed as an affordable and feasible personal-level interventions to control/manage the changes in the abovementioned pathways posed by ambient PM2.5 air pollution for atherosclerotic CVD and reducing its consequences (Allen and Barn, 2020; Bard et al., 2019; Faridi et al., 2020; Giles et al., 2011; Rajagopalan et al., 2020).
5. Limitations and recommendations for future research
There are some notable limitations of our meta-analysis including the relatively small number and variable quality of original studies with small sample sizes. The correct time frame of wearing the PFRs required to derive a benefit is not known and we homogenized different time frames in this analysis. Perhaps longer-term exposure reductions are required to derive benefits such as has been seen in the trials of portable air cleaners with an average intervention period of nearly 2 weeks (Walzer et al., 2020). Many studies also show lag periods of responses with BP changing one or a few days following exposure. These responses would have been missed by the current trials. Other factors that raise BP and are co-exposures often with PM such as noise were not accounted for and may prohibit a reduction in BP by PFRs (especially for urban and roadway trials). Gaseous air pollutants (specifically NO2, SO2 and O3) linked to the increase of SBP and DBP over short-term exposures (Yang et al., 2018a) are not reduced. The trials were all open label single blinded. No control or sham mask was used and therefore some bias could be introduced. A better understanding of effect modifiers of responses is also needed. We could not account for the negative influence of discomfort on mitigating the health benefits. However in the study by Morishita et al. (2019) we did not find that wearing an N95 per se for 2 h caused enough discomfort to raise BP in a quiet setting. This may not apply to all types of masks however as a special mask with an exhalation valve and micro-fan were used in this PFR. Finally, for studies longer than a few hours, especially those lasting 1–2 days, patients could not be expected to wear PFRs indoors or at all times (e.g., eating and sleeping). This would lead to an incomplete exposure reduction throughout the course of a day. Marrying PFR usage while outdoors with the use of indoor protective measures such as portable air cleaners, especially while sleeping, may provide superior and complete 24-h protection.
6. Conclusions
Our meta-analysis shows that wearing PFRs has the potential to lower BP and improve HRV. However, the paucity of brief and relatively limited trials prohibits the capacity to establish firm conclusions. Given the mounting global public health threat posed by air pollution, future large-scale trials testing the real-world health benefits of PFRs are warranted.
Supplementary Material
Acknowledgments
This study was financially supported by Institute for Environmental Research (IER), Tehran University of Medical Sciences (grant number: 1400-2-110-54266).
Footnotes
Author statement
Sasan Faridi: Conceptualization, Data curation, Resources, Formal analysis, Software, Investigation, Writing – original draft, Writing – review & editing; Robert D Brook: Conceptualization, Data curation, Writing – original draft, Writing – review & editing; Fatemeh Yousefian: Conceptualization, Data curation, Investigation, Mohammad Sadegh Hassanvand: Writing – review & editing; Ramin Nabizadeh Nodehi: Data curation, Formal analysis; Mansour Shamsipour: Data curation, Formal analysis; Sanjay Rajagopalan: Writing – review & editing; Kazem Naddafi: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2022.119109.
This paper has been recommended for acceptance by Dr. Payam Dadvand.
References
- Adler A, Agodoa L, Algra A, Asselbergs FW, Beckett NS, Berge E, Black H, Brouwers FP, Brown M, Bulpitt CJ, 2021. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet 397, 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S, 2020. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat. Rev. Cardiol. 17, 656–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RW, Barn P, 2020. Individual-and household-level interventions to reduce air pollution exposures and health risks: a review of the recent literature. Curr. Environ. Health Rep. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard RL, Ijaz MK, Zhang JJ, Li Y, Bai C, Yang Y, Garcia WD, Creek J, Brook RD, 2019. Interventions to reduce personal exposures to air pollution: a primer for health care providers. Global Heart 14, 47–60. [DOI] [PubMed] [Google Scholar]
- Basu R, Malig B, Broadwin R, Ebisu K, Gold EB, Qi L, Derby C, Green RS, 2017. Association between gaseous air pollutants and inflammatory, hemostatic and lipid markers in a cohort of midlife women. Environ. Int. 107, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan GH, Al-Kindi S, Brook RD, Münzel T, Rajagopalan S, 2020. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler. Thromb. Vasc. Biol. 120, 315219. ATVBAHA. [DOI] [PubMed] [Google Scholar]
- Breitner S, Peters A, Zareba W, Hampel R, Oakes D, Wiltshire J, Frampton MW, Hopke PK, Cyrys J, Utell MJ, 2019. Ambient and controlled exposures to particulate air pollution and acute changes in heart rate variability and repolarization. Sci. Rep. 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Newby DE, Rajagopalan S, 2017. The global threat of outdoor ambient air pollution to cardiovascular health: time for intervention. JAMA Cardiol. 2, 353–354. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, 2020. Inhaling hypertension: clearing the air to prevent cardiometabolic diseases. Am. Heart Assoc. 76 (1), 32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, 2021. Getting Sympathetic about Air Pollution Exposure. Am Heart Assoc, e021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G, Ärnlöv J, 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coory MD, 2010. Comment on: heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 39, 932–932. [DOI] [PubMed] [Google Scholar]
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J, 2019. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10, ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridi S, Brook RD, Hassanvand MS, Nodehi RN, Shamsipour M, Tajdini M, Naddafi K, Sadeghian S, 2021. Cardiovascular health effects of wearing a particulate-filtering respirator to reduce particulate matter exposure: a randomized crossover trial. J. Hum. Hypertens. 1–11. [DOI] [PubMed] [Google Scholar]
- Faridi S, Niazi S, Yousefian F, Azimi F, Pasalari H, Momeniha F, Mokammel A, Gholampour A, Hassanvand MS, Naddafi K, 2019. Spatial homogeneity and heterogeneity of ambient air pollutants in Tehran. Sci. Total Environ. 697, 134123. [DOI] [PubMed] [Google Scholar]
- Faridi S, Nodehi RN, Sadeghian S, Tajdini M, Hoseini M, Yunesian M, Nazmara S, Hassanvand MS, Naddafi K, 2020. Can respirator face masks in a developing country reduce exposure to ambient particulate matter? J. Expo. Sci. Environ. Epidemiol. 30, 606–617. [DOI] [PubMed] [Google Scholar]
- Frumkin H, Haines A, 2019. Global environmental change and noncommunicable disease risks. Annu. Rev. Publ. Health 40. [DOI] [PubMed] [Google Scholar]
- Giles LV, Barn P, Künzli N, Romieu I, Mittleman MA, van Eeden S, Allen R, Carlsten C, Stieb D, Noonan C, 2011. From good intentions to proven interventions: effectiveness of actions to reduce the health impacts of air pollution. Environ. Health Perspect. 119, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R, 2000. Ambient pollution and heart rate variability. Circulation 101, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M, 1999. How to assess sympathetic activity in humans. J. Hypertens. 17, 719–734. [DOI] [PubMed] [Google Scholar]
- Guan T, Hu S, Han Y, Wang R, Zhu Q, Hu Y, Fan H, Zhu T, 2018. The effects of facemasks on airway inflammation and endothelial dysfunction in healthy young adults: a double-blind, randomized, controlled crossover study. Part. Fibre Toxicol. 15, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley MB, Baumgartner J, Vedanthan R, 2018a. Developing a clinical approach to air pollution and cardiovascular health. Circulation 137, 725–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley MB, Vedanthan R, Fuster V, 2018b. Air pollution and cardiovascular disease: a window of opportunity. Nat. Rev. Cardiol. 15, 193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Zhao R, Zhang N, Xu J, Zhang L, Yang W, Geng C, Wang X, Bai Z, Vedal S, 2021a. Acute Cardiovascular Effects of Traffic-Related Air Pollution (TRAP) Exposure in Healthy Adults: A Randomized, Blinded, Crossover Intervention Study. Environmental Pollution, 117583. [DOI] [PubMed] [Google Scholar]
- Han C, Lim Y-H, Hong Y-C, 2021b. Particulate Respirator Use and Blood Pressure: A Systematic Review and Meta-Analysis. Environmental Pollution, 117574. [DOI] [PubMed] [Google Scholar]
- Hayano J, Yuda E, 2019. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthropol. 38, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, 2019. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tang M, Li H, Wen J, Wang C, Gao Y, Hu J, Lin J, Chen R, 2021. Particulate matter air pollution and reduced heart rate variability: how the associations vary by particle size in Shanghai, China. Ecotoxicol. Environ. Saf. 208, 111726. [DOI] [PubMed] [Google Scholar]
- Huang W, Morawska L, 2019. Face Masks Could Raise Pollution Risks. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Kaufman JD, Elkind MS, Bhatnagar A, Koehler K, Balmes JR, Sidney S, Burroughs Peña MS, Dockery DW, Hou L, Brook RD, 2020. Guidance to reduce the cardiovascular burden of ambient air pollutants: a policy statement from the American Heart Association. Circulation 142, e432–e447. [DOI] [PubMed] [Google Scholar]
- Langrish JP, Li X, Wang S, Lee MM, Barnes GD, Miller MR, Cassee FR, Boon NA, Donaldson K, Li J, 2012. Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environ. Health Perspect. 120, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Mills NL, Chan JK, Leseman DL, Aitken RJ, Fokkens PH, Cassee FR, Li J, Donaldson K, Newby DE, 2009a. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part. Fibre Toxicol. 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish JP, Mills NL, Chan JK, Leseman DL, Aitken RJ, Fokkens PH, Cassee FR, Li J, Donaldson K, Newby DE, 2009b. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part. Fibre Toxicol. 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumbach R, Meng Q, Kipen H, 2015. What can individuals do to reduce personal health risks from air pollution? J. Thorac. Dis. 7, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, Klingmüller K, Pözzer A, Poschl U, Fnais M, Daiber A, Münzel T, 2019. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H, 2017. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation 136, 618–627. [DOI] [PubMed] [Google Scholar]
- Lim YH, Kim W, Choi Y, Kim HC, Na G, Kim HR, Hong YC, 2020. Effects of particulate respirator use on cardiopulmonary function in elderly women: a Quasi-experimental study. J. Kor. Med. Sci. 35, e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC, 2001. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 104, 986–991. [DOI] [PubMed] [Google Scholar]
- Mensah GA, Roth GA, Fuster V, 2019. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. [DOI] [PubMed] [Google Scholar]
- Morishita M, Wang L, Speth K, Zhou N, Bard RL, Li F, Brook JR, Rajagopalan S, Brook RD, 2019. Acute blood pressure and cardiovascular effects of near-roadway exposures with and without N95 respirators. Am. J. Hypertens. 32, 1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JD, Rajagopalan S, Levy P, Brook RD, 2020. Clearing the air to treat hypertension. J. Hum. Hypertens. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P, 2014. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension 64, 494–500. [DOI] [PubMed] [Google Scholar]
- Pieters N, Plusquin M, Cox B, Kicinski M, Vangronsveld J, Nawrot TS, 2012. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: a meta-analysis. Heart 98, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Al-Kindi SG, Brook RD, 2018. Air pollution and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 72, 2054–2070. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brauer M, Bhatnagar A, Bhatt DL, Brook JR, Huang W, Münzel T, Newby D, Siegel J, Brook RD, 2020. Personal-level protective actions against particulate matter air pollution exposure: a scientific statement from the American heart association. Circulation 142, e411–e431. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Landrigan PJ, 2021. Pollution and the heart. N. Engl. J. Med. 385, 1881–1892. [DOI] [PubMed] [Google Scholar]
- Rankin GD, Kabéle M, Brown R, Macefield VG, Sandström T, Bosson JA, 2021. Acute exposure to diesel exhaust increases muscle sympathetic nerve activity in humans. J. Am. Heart Assoc. 10, e018448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, 2020. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung S-H, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, 2019. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies’ environmental committee, Part 2: air pollution and organ systems. Chest 155, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Lin Z, Chen R, Wang C, Yang C, Cai J, Lin J, Xu X, Ross JA, Zhao Z, 2016. Cardiovascular benefits of wearing particulate-filtering respirators: a randomized crossover trial. Environ. Health Perspect. 125, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Lin Z, Chen R, Wang C, Yang C, Cai J, Lin J, Xu X, Ross JA, Zhao Z, 2017. Cardiovascular benefits of wearing particulate-filtering respirators: a randomized crossover trial. Environ. Health Perspect. 125, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorn A, 2017. Clearing the air: do facemasks protect health? Lancet Respir. Med. 5, 555–556. [DOI] [PubMed] [Google Scholar]
- Walzer D, Gordon T, Thorpe L, Thurston G, Xia Y, Zhong H, Roberts TR, Hochman JS, Newman JD, 2020. Effects of home particulate air filtration on blood pressure: a systematic review. Hypertension 76, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T, 2014. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton P, 2002. National high blood pressure education program coordinating committee: primary prevention of hypertension: clinical and public health advisory from the national high blood pressure education program. JAMA 288, 1882–1888. [DOI] [PubMed] [Google Scholar]
- Yang B-Y, Qian Z, Howard SW, Vaughn MG, Fan S-J, Liu K-K, Dong G-H, 2018a. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ. Pollut. 235, 576–588. [DOI] [PubMed] [Google Scholar]
- Yang X, Jia X, Dong W, Wu S, Miller M, Hu D, Li H, Pan L, Deng F, Guo X, 2018b. Cardiovascular benefits of reducing personal exposure to traffic-related noise and particulate air pollution: a randomized crossover study in the Beijing subway system. Indoor Air 28, 777–786. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li L, Gao W, Wang Y, Yao X, 2016. Interventions to reduce individual exposure of elderly individuals and children to haze: a review. J. Thorac. Dis. 8, E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chu M, Zhang J, Duan J, Hu D, Zhang W, Yang X, Jia X, Deng F, Sun Z, 2019. Urine metabolites associated with cardiovascular effects from exposure of size-fractioned particulate matter in a subway environment: a randomized crossover study. Environ. Int. 130, 104920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.