Abstract
The majority of health professionals now have genetic and genomic competencies and some are measured by certification standards. Nursing has a proud history of defining roles for nursing in informatics and genetics. In addition, the nursing professional organization, the American Nurses Association, has a Certification Center that has successfully achieved ISO 9001:2008 certification in the design, development, and delivery of global credentialing services which encompasses certification of advanced practice nurses in genetics. ISO 9001:2008 certification is the firmly established global standard for assuring stakeholders of an organization’s ability to satisfy quality-related requirements. However, despite the addition of genomics into the Informatics Scope and Standards of Practice, there is a need to define the integration of the genetic, genomics and other omics competencies into the informatics domain, especially the Electronic Health Record. Currently, there are also international and interprofessional activities and organizations that have established or are identifying competencies in genetics and genomics. There remains a need for more international collaborations to build upon the current resources and strategies implemented by several countries, to learn from each other, support each other, and to collaborate to answer questions and reduce duplication of efforts.
Keywords: Competencies in genetics, genomics and omics, Healthcare Professionals, Consumers, Integration into Electronic Health Records, Certifications, International efforts in genetics and genomics, Opportunities for international collaboration
1. Introduction
Nursing has a proud history spanning over a decade in developing competencies for nurses and certification of advanced practice level nurses in genetics. To begin with, two definitions are important: 1) Genetics is the study of individual genes and their impact on relatively rare single gene disorders, and 2) Genomics is the study of all the genes in the human genome together, including their interactions with each other, the environment, and other psychosocial and cultural factors [1]. The impact of genetics and genomics information and technology has the potential to improve the quality of care and result in safer practices at lower costs. These are so important to the future of the nursing profession, that the American Nurses Association included the concept of genetics and genomics in the new Nursing Informatics: Scope and Standards of Practice, 2nd edition. The statement specifies that informatics nurses must be able to: “Incorporate genetic and genomic technologies and informatics into practice.” and “Demonstrate in practice the importance of tailoring genetic and genomic information and services to clients based on their culture, religion, knowledge level, literature, and preferred language” [1].
2. Rapid Advances in Genetics, Genomics, and other Omics
There have been rapid advances over the past 25 years since the sequencing of the human genome [2]. The global community has engaged in the identification of human genetic variation through several projects such as: establishing the Encyclopedia of DNA Elements (ENCODE) to identify genomic functional elements; completed the International HapMap Project to identify genetic variation associated with human diseases, developed The Cancer Genome Atlas (TCGA) to identify genetic variation associated with cancer and cancer sub-types; and established the international 1000 Genomes Project to catalogue human genetic variation. Other United States (US) supported projects include finding Mendalian disease genes and supporting drug discovery and genomic underpinnings to drug metabolism. Because of these advances, we are now witnessing the application of these discoveries in clinical care.
The National Human Genome Research Institute (NHGRI) in the US is placing increased emphasis on validation and implementation of genomics into healthcare, in addition to the biology of genomes [3]. Programs focus on cancer genomics, pharmacogenomics, genomic medicine, newborn genomic analysis, clinical genomics information systems, and rare and/or undiagnosed genetic disease diagnostics. Additionally efforts surrounding genomic translation continue to grow, such as the IGNITE (Implementing GeNomics In PracTicE) initiative aimed at conducting projects designed to translate genomic information into clinical care [4]. Across the healthcare continuum the influences of genetics and genomics can be found in preconception/prenatal care, newborn screening, disease susceptibility, screening/diagnosis, prognosis and therapeutic decision, and monitoring disease burden and recurrence. Several publications in nursing journals and books have identified the major advances relevant to nursing and patient care, achieving outcomes, and improving safety at a reduced cost [5–7].
The prospect of Precision Medicine was greatly accelerated with the launch of the Precision Medicine Initiative [2]. Since then the biology of genes and genomes and the translation to health care and nursing practice applications has continued to expand. In the United States and elsewhere around the globe, Precision Medicine has been identified as a priority with funding secured to conduct vital research including the generation of large scale cohorts that include not just biospecimens but robust clinical and lifestyle data. The US has increased funding to accelerate the use of genomic variation information in healthcare with specific emphasis on cancer treatments and resistance. In addition, over 1 million Americans will be recruited to consent to study their biospecimens, diet, lifestyle, and other health information that is linked to their electronic health record (EHR). Effective implementation, however, has brought attention to the need for appropriate policy and regulation of information, the adequate preparation of the healthcare workforce to understand and use this new evidence, and the informatics infrastructure needed to manage these data.
3. New Advances in Pharmacogenomics are now Impacting the Safety and Quality of Healthcare Outcomes.
There are now several guidelines, workflows, and algorithms for determining the pharmacogenomics effects of several medications, their effectiveness, and the potential for adverse reactions even when the medications are administered at the correct dose [8, 9]. An international database to provide knowledge of the pharmacogenomics findings, called the Pharmacogenomics Knowledge Base (PharmGKB) it contains the evidence from literature, curated by experts, and disseminated on human genomics variation and potential differences in drug metabolism and responses [10]. Building on this evidence, PharmGKB and the Pharmacogenomics Research Network established a collaboration called the Clinical Pharmacogenetics Implementation Consortium (CPIC) to produce guidelines. The goal is to establish evidence based guidelines that are open access, peer reviewed, provide detailed drug and clinical information, and are updatable as the evidence base evolves. CPIC guidelines are amenable to integration into Electronic Health Records using Clinical Decision Support (CDS). To date, 33 CPIC guidelines with sufficient evidence for implementation into clinical practice are available on the website [9].
An important ongoing initiative focuses on workflow and algorithm pathways for the inclusion of the CPIC guidelines into the EHR. Hoffman et al. have developed a model workflow that supports CPIC guidelines, knowledge sources, and clinical decision support for pharmacogenomics integration into the EHR [8].
4. Formal competencies for nursing at all Academic Levels
Initial genetic and genomic competencies for US nursing defined a core of expected knowledge, skills, and attitudes required of all registered nurses regardless of academic preparation or specialties were published in January 2006 [11]. These competencies have since been revised to incorporate changes in technology and the addition of outcome indicators, and more recently competencies were developed for advanced practice [12, 13]. The most recent version of core competencies for all health professionals identified minimum levels of competencies, knowledge, skills, and attitudes from the National Coalition for Health Professional Education in Genetics (NCHPEG) [11]. These have been built upon similar to the nursing efforts by other disciplines including physician assistants, pharmacists, and most recently physicians [14–16]. Building on the work from NCHPEG, the Inter-Society Coordinating Committee for Practitioner Education in Genomics (ISCC) was established in 2013. Aims of the interprofessional ISCC is to help generate evidence and address provider competency gaps [17].
Not surprisingly, across all the disciplines with genomic competencies, there is considerable overlap. Competencies surrounding basic genetic concepts, assessments and indications for a genetic specialist referral, and ethical, legal, and social issues are underpinnings common across all disciplines. These commonalities support some interprofessional core educational activities.
In the international community, genomic based competencies have continued to develop. The United Kingdom (UK) recently updated their 2003 competency framework for nurses [18]. Building on the work in the US and UK, other countries have developed country specific competencies such as Japan and Brazil, or are moving in that direction [19].
5. Examples of Competencies in Nursing in Clinical Practice
Since genetic and genomic competencies have been established for all US registered nurses regardless of academic degree, clinical role, or specialty, implementation into clinical practice is important. A recent study of US Magnet® Recognition Program Hospital integration of genetics and genomics into practice demonstrated what strategies nurse leaders developed to diffuse this important information in order to improve nursing genomic competency thereby impacting quality and safety [20]. The project recruited twenty-one Magnet® hospitals and 2 control hospitals which participated in a one-year education intervention to help them understand the basic concepts sufficient enough to design and implement institutional integration efforts. Each hospital used several on-line and other resources to access information. Hospitals had to complete environmental scans to identify policies that needed to be changed, developed, or extended. Continuing education and staff development activities were found to be effective means to introduce this subject in practice. One of the most popular methods of incentivizing learning was a one-page monthly series called GeneSplash, a single concept learning tool that provided new discoveries and genetics and genomics and facts pertinent to diseases they managed. The Magnet® hospital study participants have partnered with the investigators to develop an online toolkit of all their strategies for use by other educators and administrators to facilitate implementation of these changes in the practice environment [21].
6. Certification of Advanced Practice Nurses in Genetics from the American Nurses Credentialing Center (ANCC) and ISO certified group
The American Nurses Credentialing Center (ANCC) has built on the work of the Genetic Nursing Credentialing Commission and developed a certification in Advanced Genetics Nursing [22]. In the US and worldwide, nurses who demonstrate genomic skills, knowledge and abilities, and who have a minimum of 1,500 practice hours in a genomic area within a 5 year period, and have 30 hours of continuing education credits within a 3 year period, are eligible to submit a portfolio for expert peer review and certification. The ANCC certifications focus on professional development; professional and ethical nursing practice; teamwork and collaboration; and quality and safety. These ANCC certifications are available worldwide since they have successfully achieved ISO 9001:2008 certification in the design, development, and delivery of global credentialing services and support products for nurses and healthcare organizations [22]. ISO 9001:2008 certification is the firmly established global standard for assuring stakeholders of an organization’s ability to satisfy quality-related requirements.
7. What are sources of information for Consumers?
One only has to listen to lay or social media through radio, television, internet, and advertising to find that consumers are being bombarded with information on where to test their genome, what they should look for at hospitals testing their genomics and pharmacogenomics, and determining their roots and ancestry through genetics. A reliable source for patients to obtain information on genetics and genomics is genome.gov/patients [23]. There they will find definitions of terms, policies, and resources to follow. Additionally, large private sector alliances continue to develop tools to improve consumers’ and patients’ knowledge about genetic services (http://geneticalliance.org/programs/geneslife) [24]. Additionally, the Center for Disease Control and Prevention (CDC) Public Health Genomics also offers a number or reliable resources (http://www.cdc.gov/genomics) [25].
8. Effective Integration of Genetics/Genomics, other Omics, and Pharmacogenomics into the Electronic Health Record (EHR)
Several genetic and genomic data and tools are recommended for integration into EHRs. These nursing informatics components for genomic implementation are included in Table 1. They are separated into entry level and advanced level components [4]. The entry level includes more users of the information resources, and the advanced level includes more developers, evaluators, and researchers of the information resources.
Table 1.
Nursing Informatics Components for Genomic Implementation Management at the Entry and Advanced levels.
| Entry level |
|---|
| 1. Facilitate Consumer Engagement in Consent – Signatures and Date |
| 2. Include Pedigree Maps for Family History and Family Values |
| 3. Use and access to Pharmacogenomics Knowledge Bases and other Data Repositories |
| 4. Use Diagnostic and Treatment and Path Report Protocols/Orders |
| 5. Use Evidence Guideline Databases |
| 6. Provide client with interpretive services for genomic tests |
| Advanced level |
| 1. Develop Data Standards to Ensure Privacy, Security, and Integrity for Big Data Analytics and Data Exchange |
| 2. Incorporate genetic/genomic/adverse reactions into Vocabulary and Terminology Standards to Monitor Quality of Outcomes and the Safety of Care |
| 3. Map to Pharmacogenomics Knowledge Bases and other Data Repositories |
| 4. Link to Advanced Biomarker Discovery Databases, Laboratory findings, Tissue databanks, and Imaging data |
| 5. Include Sequencing and Pathogen Discovery |
| 6. Link to Diagnostic and Treatment and Path Report Protocols/Orders |
| 7. Link to Evidence Guideline Databases |
| 8. De-identify Patients for Data Sharing |
| 9. Enhance EHRs and Mobile Devices to Promote Precision Medicine |
| 10. Develop New Algorithms and Workflows |
| 11. Identify Patients for Genetic/Genomic Services and Consults |
| 12. Develop Point of Care Computerized Clinical Decision Supports |
| 13. Participate on teams developing advanced security and cybersecurity solutions securing genomic data |
| 14. Conduct research data mining on genomic diagnosis, treatment, and symptom management |
| 15. Conduct research on patient access, diversity, and ethnic variation in patient care |
| 16. Identify health services research sites where patient access information on their genetics/genomics |
| 17. Develop new information access sources for clinicians and consumers |
A recent National Academy of Medicine Workshop Summary recommended links among genomics, clinical research and a learning health care system (LHCS). In a LCHS, the data from the patient, the genomics data, and other external repositories are integrated into the EHR [26]. They make a significant point that the regulations and policies of individual countries vary, challenging nurses to develop standards across continents to address informed consent and data sharing guidelines that address country specific society and cultural differences.
Several large studies are demonstrating the integration of genetic and genomic data into EHRs. The Electronic Medical Records and Genomics Network (eMERGE) is a project that supports the development of infrastructure for integration of genomic biorepository findings into the EHR to facilitate clinical implementation [26]. Several projects have resulted from eMERGE including: phenotyping algorithms available in an online public repository called Phenotype KnowledgeBase (PheKB) [27]; MyResults, a website where patients and families can learn more about their genetic results; eMERGE SPHINX, to facilitate drug and genomic variation discovery; PheWAS, designed to facilitate understanding of the variation in phenotypes associated with a single genotype [28]; as well as other efforts. eMERGE is also identifying actionable variants and including Clinical Decision Support (CDS) tools in the EHR in the program sites [29].
The Displaying and Integrating Genetic Information Through the EHR Action Collaborative (DIGITizE) working group at the Institute of Medicine (now called National Academy of Medicine- NAM) is developing implementation guides, Logical Observation Identifier Names and Codes (LOINC®) transfer codes, as well as other means to integrate the information into Electronic Health Records. This group includes companies such as Cerner, Epic, and Allscripts working together to implement genetics and genomic workflows into the EHR. SMART (Substitutable Medical Applications and Reusable Technology), the HL7 v3 message on clinical genomics information and family pedigree [30, 31], and FHIR (Fast Healthcare Interoperability Resources) are potentials to connect genomic information into the vendors’ EHRs [32, 33].
Another NHGRI supported project is called the Implementing GeNomics In pracTicE (IGNITE) Consortium [24]. The goal of this consortium is to create Genomic Medicine Pilot Demonstration projects that integrate genomics into the EHR and incorporate clinical decision support tools. There are currently multiple IGNITE sites and a coordinating center that focus on: 1) common health conditions including hypertension, kidney disease, and diabetes; 2) the family health history evaluation in diverse care settings applies an implementation science approach to the collection and evaluation of family history and development of implementation guidelines, and 3) the personalized medicine program with a pharmacogenomics focus that aims to include the development of best practices for genomic medicine implementation resulting from pharmacogenomics [4].
The Pharmacogenomics Research Network was established to conduct research on pharmacogenomics and evaluate the impact on clinical care of genetic variants predicting drug metabolism, toxicity and adverse drug effects. An international initiative is call the Pharmacogenomics for Every Nation Initiative (PGENI) which targets countries with good healthcare infrastructure but limited capacity to integrate pharmacogenomics into practice. PGENI is conducting research to ascertain what drugs and genetic variants would be most informative given ethnic variation. The intent is to establish country specific national formulary recommendations informed by genomic variation [34].
Figure 1 identifies the components of a simplified global strategy for moving genetically competent nursing informatics to the integration of genomics translated into precision clinical care. The process components include many subcomponents that detail multiple paths that can be taken by informatics nurses to assure integration into practice. The first building block is education/knowledge of genetics/genomics by all IT nurses because a lack of knowledge is an obstacle to translating genomics into information systems. The second building block is the data standards and vocabulary standards in order to utilize the data to measure quality, safety and for big data analytics. The third building block are the regulatory and policy changes needed nationally, globally, and locally to fulfill the inclusion of nursing with genomics into informatics nursing. The fourth component is nursing IT involvement in Health Information Technology at the stakeholder level, the vendor level, the repository level, and the development of tools integral to decision support. The fifth building block is developing a research agenda. A Genomic Nursing Science Blueprint has been developed by the US based on evidence gaps [35]. The sixth building block is the translation of genetic and genomic content into the EHR, mobile devices, and the Internet of Things for patients and practitioners.
Figure 1.
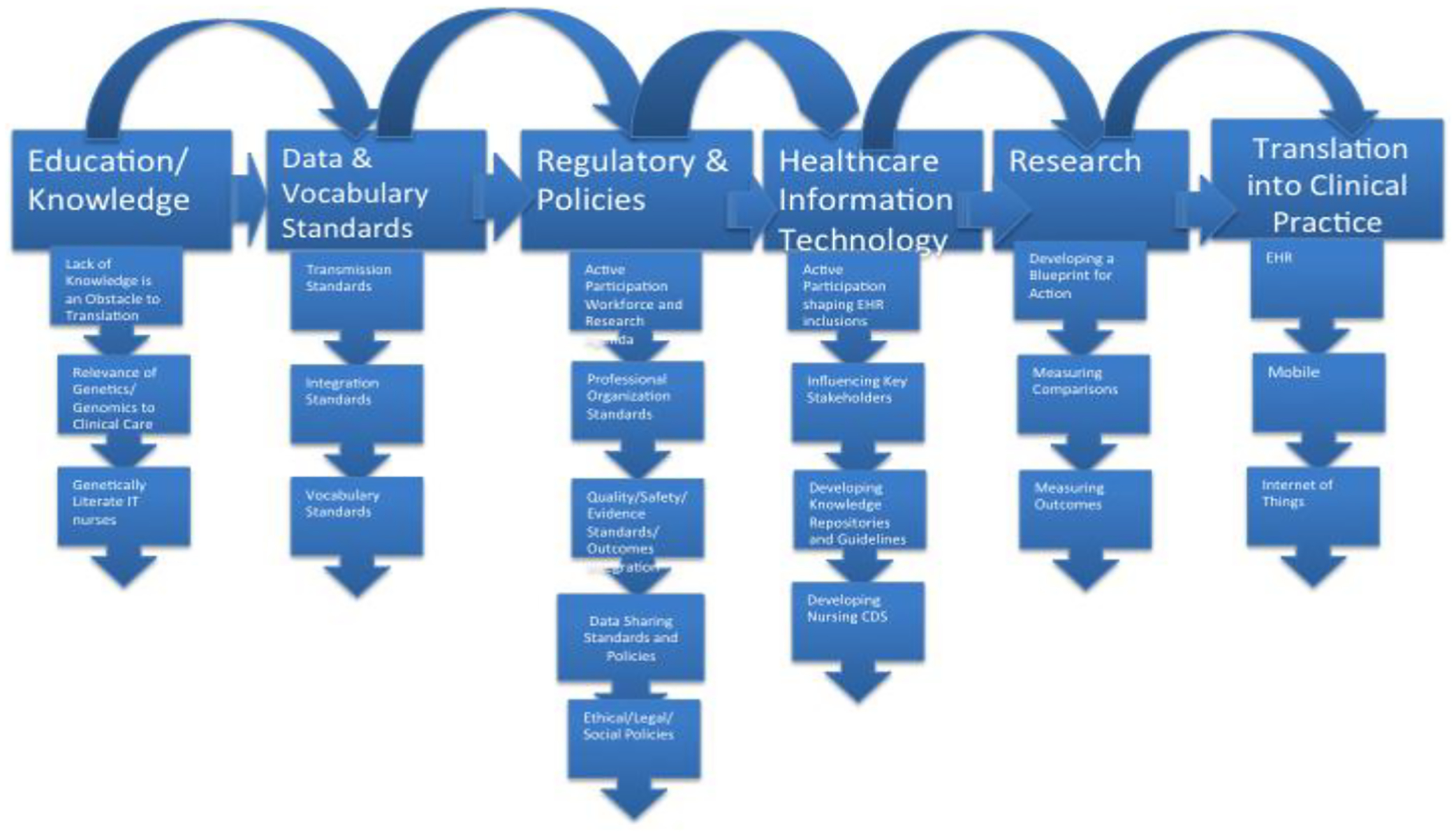
Components of a Global Process for Moving Genetically Competent IT Nurses toward Translation in Precision Clinical Care
9. Identification of Some Global Resources and Country Efforts in Genetics/Genomics
There are several areas where genomic medicine is entering a global environment. One effort, part of the NHGRI larger Genomic Medicine implementation initiative, was global in nature. Genomic Medicine VI (comprised of 50 leaders from 25 countries): Global Leaders in Genomic Medicine, met at the National Academy of Sciences in January 2014 [36]. They defined 4 major objectives:
Identify areas of active translation and implementation of genomic medicine,
Frame a policy agenda to advance the field,
Highlight nations with unique capabilities, and
Discuss opportunities for international collaborations.
Key issues that were identified included: the development of evidence for value of genomics in healthcare, ways to engage both institutional leaders and professionals in genomics, education of professionals and patients, effective integration of genomic results into EHR, and design of financial models that provide cost reductions rather that increases. Building on this work was the establishment of the Global Genomic Medicine Collaborative (G2MC) which is aimed at furthering global collaborations.
In nursing, similar international efforts are underway. The International Society of Nurses in Genetics (ISONG) is a truly international organization whose membership consists predominately of nurses with genetic/genomic expertise. The organizations mission surrounds development of their membership to increase research, clinical, and academic genomic integration as well as genomic information management [37].
A coalition of ISONG members collaborated to secure funding to begin the next steps for establishing a Global Genomics Nursing Alliance (G2NA) to explore strategies for knowledge mobilization and action. The first meeting of this initiative is now funded and nursing leaders globally will be meeting in January 2017 to learn from each other, share resources and expertise. The intent is to establish an Alliance to reduce duplication through sharing and building on work in other countries to accelerate the effective integration of genomics in to nursing practice and education. To facilitate that work the first meeting will include steps to establish a Maturity Matrix to guide and benchmark progress to accelerate integration of genomics into everyday healthcare practice.
There are several other global initiatives that are comprised of several countries and organizations throughout the world. Notable are the following:
The Global Alliance for Genomics and Health (GA4GH) is a network of over 375 institutions internationally that work together to create a common framework of harmonized approaches to enable secure data transfer and data sharing of genomic data linked to clinical data [38]. The International Rare Disease Research Consortium (IRDiRC) is a group of teams of researchers and organizations investing in rare diseases. It is the goal of the group to establish diagnostic tests for all rare diseases and create 200 new rare disease therapies by 2020 [39].
The European Union has identified several initiatives on the continent that seek to develop personalized medicine in the diagnosis and treatment of specific diseases. One initiative called the European Observatory on Personalized Medicine seeks to provide a closer link between research and innovation funding, and policy objectives by funding key initiatives in Personalized Medicine in Europe [40].
A listing of several genomics resources and URLs are provided in Table 2. These include programs, organizations and global initiatives mentioned in this chapter. They also include content about broad genomic programs, education specific programs for clinicians and patients, and detailed repositories and toolkits.
Table 2.
Genomics Programs and URLs Mentioned in this Chapter
| Global Genomics Programs | URL |
|---|---|
| Human genomics strategy group: Building on our inheritance—Genomic technology in healthcare | www.2ov.uk/2overnment/uploads/system/uploads/attachment_data/file/213705/dh_132382.pdf |
| European Science Foundation. Forward look: Personalised medicine for the European citizen | www.esf.org/uploads/media/Personalised_Medicine.pdf |
| Genome Canada: 2012 Large-scale applied research project competition in genomics and personalized health | www.genomecanada.ca/en/portfolio/research/2012-competition.aspx |
| European Association for Predictive, Preventive, and Personalised Medicine (EPMA) | www.epmanet.eu |
| European Commission: EuroBioForum observatory | www.eurobioforum.eu/2028/observatory |
| Genomic Medicine Alliance | www.senomicmedicinealliance.org |
| International rare diseases research consortium (IRDiRC). | www.irdirc.org |
| Eurogentest: Harmonizing genetic testing across Europe | www.eurogentest.org |
| National human genome research institute | www.genome.gov |
| Genomics England | www.genomicsengland.co.uk |
| Belgian medical genomics initiative (BeMGI) | www.bemgi.be |
| Database of genotypes and phenotypes (dbGaP) | www.ncbi.nlm.nih.gov/gap |
| PharmGKB: The pharmacogenomics knowledgebase | www.pharmgkb.org |
| Genatak: Pioneering personalized genomic medicine | www.genatak.com |
| Implementing genomics in practice (IGNITE) | www.ignite-genomics.org/IGNITE_ABOUT.html |
| Australian New Zealand cLinical trials registry | www.anzctr.org.au |
| E.U.Clinical TRials register | www.clinicaltrialsregister.eu |
| U.S. NIH Clinical Trials Registry | https://clinicaltrials.gov |
| National Health and Medical Research Council of Australia: Principles for the translation of “omics”-based tests from discovery to health care | http://consultations.nhmrc.gov.au/files/consultations/drafts/attaevidentialstandardsdocument.pdf |
| A guide to the Exome Aggregation Consortium data set, MacArthur lab, Massachusetts General Hospital | http://macarthurlab.org/2014/11/18/a-guide-to-the-exome-aggregation-consortium-exac-data-set |
| Clinical Genomics Resource (ClinGen) | www.clinicalgenome.org |
| European translational information and knowledge management services (ETRIKS) | www.etriks.org |
| National coalition for health professional education in genetics: Core competencies for all health professionals (2007) | http://www.nchpeg.org/index.php?option=com_content&view=article&id=237&Itemid=84 |
| Coursera and University of California, San Francisco. Genomic and precision medicine | www.coursera.org/course/genomicmedicine |
| The EuroGenTest clinical utility gene cards | www.eurogentest.org/index/php?id=668 |
| Pharmacogenomics knowledge base (PharmGKB): International consortium for antihypertensives pharmacogenomics studies (ICAPS) | www.pharmgkb.org/page/icap |
| Pharmacogenetics for every nation initiative (PGENI) | www.pgeni.org |
| Global alliance for genomics and health (GA4GH) | http://genomicsandhealth.org |
| U.S. National Academy of Medicine (formerly Institute of Medicine): Roundtable on translating genomic-based research for health | www.iom.edu/Activities/Research/GenomicBasedResearch.aspx |
10. Future Opportunities and Summary
Informatics remains a cornerstone to the integration of genomics into nursing practice. The ongoing efforts to establish international collaborative networks to build upon the resources and strategies implemented by other countries to learn, support, and collaborate to answer questions and to reduce duplication of effort are vital to realizing the benefits of genomics to health. Informatics experts can play critical roles in adapting successful tools such as Clinical Decision Support for other countries without or with less developed resources. Additionally, efforts to expand the evidence-base and improve the understanding of global genomic variation can facilitate effective genomic implementation.
International collaborative opportunities for IMIA-NI are provided in Table 3. IMIA-NI could expand the International Medical Informatics Association (IMIA) standards activity to engage with other genetic and genomic groups to identify gaps and develop global resources filling those gaps. There are other global needs that include what data to be aggregated for variant/phenotype association, how to define the federated databases for genomic data, and development of a vocabulary to phenotype ontology, standardized phenotype ontology, and inventory of existing phenotype ontologies. Work needs to continue to build on what is already available to disseminate globally, the genomic medicine implementation guidelines, algorithms, CDS, workflows, and pharmacogenomics guidelines.
Table 3.
Opportunities for Collaboration in IMIA-NI
|
There remains a need to collect and disseminate data on different countries on existing nursing workforce prepared in genetics/genomics and/or clinical translation. There is a need to assess the state of genomic competency of the interprofessional workforce and implement strategies to address competency deficits.
There are several opportunities that IMIA NI-WG can engage in educational programs to highlight the importance of genetic and genomic competencies in nursing and other healthcare professionals. IMIA-NI-WG could provide:
Websites that link nurses in all participating countries with competencies, certification and literature resources in genetics and genomics internationally;
Conduct international webinars and through telemedicine links, establish regular educational programs;
Provide links to ongoing educational resources internationally;
Provide links to studies implementing genetic and genomic education into practice, similar to the Magnet® study in the US;
Develop and disseminate a repository to compile and conduct studies comparing certifications at the certificate level, baccalaureate level, and advanced practice level internationally;
Compile and deliver new evidence in pharmacogenomics through the current network of best practice centers developing guidelines throughout the world;
Collaborate with other global nursing organizations such as ICN and ISONG to provide resources for informatics management of genomic content.
This paper has provided an overview of the journey over the past 25 years of the science of genetics and genomics, and of the nursing profession establishing practice standards, competencies for general and advanced practice nurses, and an internationally ISO accepted certification for advanced practice nurses. In addition, some major discoveries with implications for nursing and the integration into the EHR and nursing informatics were provided. A study implementing genetics and genomics into Magnet® Hospitals and the development of a toolkit were discussed. National efforts from around the world were highlighted. Finally, future opportunities for the IMIA-NI working group were described. Most importantly, this review highlights that there are similar needs across the globe that are amenable to international collaborations that can accelerate the integration of genomics into healthcare worldwide to improve health outcomes.
Acknowledgments
The authors acknowledge the contributions of all nurses who have defined the competencies for general nurses, advanced practice nurses, and interprofessional groups. It is only by reaching consensus on common principals that the science of genetics and genomics and the integration into practice to improve quality outcomes and achieve safer care will be provided.
References
- [1].American Nurses Association, Nursing Informatics: Scope & Standards of Practice, 2nd Edition. 2015, American Nurses Association. [Google Scholar]
- [2].Collins FS and Varmus H, A New Initiative on Precision Medicine. N Engl J Med, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Green ED and Guyer MS. Charting a course for genomic medicine from base pairs to bedside. Nature, 2011. 470(7333): p. 204–13. [DOI] [PubMed] [Google Scholar]
- [4].Weitzel KW., et al. , The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genomics, 2016. 9: p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McCormick KA. and Calzone KA. The impact of genomics on health outcomes, quality, and safety. Nurs Manage, 2016. 47(4): p. 23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Williams JK., et al. , Advanced nursing practice and research contributions to precision medicine. Nurs Outlook, 2016. 64(2): p. 117–23. [DOI] [PubMed] [Google Scholar]
- [7].McCormick KA., Calzone KA. , Big Data Initiatives: Genomics and Information Technology for Personalized Health, in Essentials of Nursing informatics, 6th Edition. Saba VK &. McCormick KA Editor. 2015, McGraw-Hill: New York. p. 707–725. [Google Scholar]
- [8].Hoffman JM. et al. , Developing knowledge resources to support precision medicine: principles from the Clinical Pharmacogenetics Implementation Consortium (CPIC). J Am Med Inform Assoc, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clinical Phamacogenetics Implementation Constorium. CPIC Guidelines. 2016. [cited 2016 4/2/2016]; Available from: https://www.pharmgkb.org/view/dosing-guidelines.do?source=CPIC#
- [10].Relling MV. and. Evans WE, Pharmacogenomics in the clinic. Nature, 2015. 526(7573): p. 343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Consensus Panel on Genetic/Genomic Nursing Competencies, Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics. 2006, American Nurses Association: Silver Spring. [Google Scholar]
- [12].Consensus Panel on Genetic/Genomic Nursing Competencies, Essentials of Genetic and Genomic Nursing: Competencies, Curricula Guidelines, and Outcome Indicators, 2nd Edition. 2nd ed. 2009, Silver Spring, MD: American Nurses Association. [Google Scholar]
- [13].Greco KE., Tinley S., Seibert D. Essential genetic and genomic competencies for nurses with graduate degrees. 2012. [cited 2013 8/31/2013]; Available from: http://www.nursingworld.org/MainMenuCategories/EthicsStandards/Genetics-1/Essential-Genetic-and-Genomic-Competencies-for-Nurses-With-Graduate-Degrees.pdf. [DOI] [PubMed]
- [14].Korf BR. et al. Framework for development of physician competencies in genomic medicine: report of the Competencies Working Group of the Inter-Society Coordinating Committee for Physician Education in Genomics. Genet Med, 2014. 16(11): p. 804–9. [DOI] [PubMed] [Google Scholar]
- [15].Consensus Panel on Pharmacist Pharmacogenomic Competencies. Pharmacist Phamacogenomic Competencies 2012. [cited 2016 2/17/2016]; Available from: http://g-2-c-2.org/files/Pharmacist-Comp.pdf.
- [16].Rackover M, Goldgar C, Wolpert C, Healy K, Feiger J, Jenkins J, Establishing essential physician assistant clinical competencies guidelines for genetics and genomics. Journal of Physician Assistant Education, 2007. 18(2): p. 48–49. [Google Scholar]
- [17].Manolio TA. and. Murray MF, The growing role of professional societies in educating clinicians in genomics. Genet Med, 2014. 16(8): p. 571–2. [DOI] [PubMed] [Google Scholar]
- [18].Kirk M, Tonkin E and Skirton H An iterative consensus-building approach to revising a genetics/genomics competency framework for nurse education in the UK. J Adv Nurs, 2014. 70(2): p. 405–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kirk M et al. Genetics-Genomics Competencies and Nursing Regulation. Journal of Nursing Scholarship, 2011. 43(2): p. 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Calzone KA., et al. Introducing a New Competency Into Nursing Practice. J Nurs Regul, 2014. 5(1): p. 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jenkins J et al. Methods of genomic competency integration in practice. J Nurs Scholarsh, 2015. 47(3): p. 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].American Nurses Credentialing Center (ANCC). Advanced Genetic Nursing. 2016. [cited 2016 3/7/2016]; Available from: http://nursecredentialing.org/AdvancedGenetics.
- [23].National Human Genome Research Institute. Genetics/genomics for Patients. 2016. [cited 2016 3/30/2016]; Available from: http://www.Genome.gov/patient.
- [24].Genetic Alliance. Genes in Life. 2016. [cited 2016 3/30/2016]; Available from: http://geneticalliance.org/programs/geneslife.
- [25].Center for Disease Control and Prevention (CDC). Pubic Health Genomics. 2016. [cited 2016 5/18/2016]; Available from: http://www.cdc.gov/genomics.
- [26].Gottesman O et al. The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genet Med, 2013. 15(10): p. 761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pathak J et al. Mapping clinical phenotype data elements to standardized metadata repositories and controlled terminologies: the eMERGE Network experience. J Am Med Inform Assoc, 2011. 18(4): p. 376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pendergrass SA. et al. The use of phenome-wide association studies (PheWAS) for exploration of novel genotype-phenotype relationships and pleiotropy discovery. Genet Epidemiol, 2011. 35(5): p. 410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Herr TM. et al. Practical considerations in genomic decision support: The eMERGE experience. J Pathol Inform, 2015. 6: p. 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].HL7 V3 CG PEDINTEROP, R1 HL7 Version 3 Implementation Guide: Family History/Pedigree Interoperability, Release 1.
- [31].ANSI/HL7 V3 CGPED, R1–2007 HL7 Version 3 Standard: Clinical Genomics; Pedigree, Release 1 7/5/2007 HL7 International.
- [32].Warner JL. et al. SMART precision cancer medicine: a FHIR-based app to provide genomic information at the point of care. J Am Med Inform Assoc, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mandel JC. et al. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pharmacogenomics for Every Nation Initiative (PGENI). Pharmacogenomics for Every Nation Initiative (PGENI) 2016. [cited 2016 4/1/2016]; Available from: http://www.pgeni.org/.
- [35].Genomic Nursing State of the Science Advisory Panel, C., Jenkins KA, Bakos J, Cashion AD, Donaldson AK, Feero N, Feetham WG, Grady S, Hinshaw PA, Knebel AS, Robinson AR, Ropka N, Seibert ME, Stevens D, Tully KR, Webb LA A Blueprint for Genomic Nursing Science. Journal of Nursing Scholarship, 2013. 45(1): p. 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Manolio TA., et al. Global implementation of genomic medicine: We are not alone. Sci Transl Med, 2015. 7(290): p. 290ps13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].International Society for Nurses in Genetics. Mission. 2016. [cited 2016 5/21/2016]; Available from: http://www.isong.org/.
- [38].Lawler M et al. All the World’s a Stage: Facilitating Discovery Science and Improved Cancer Care through the Global Alliance for Genomics and Health. Cancer Discov, 2015. 5(11): p. 1133–6. [DOI] [PubMed] [Google Scholar]
- [39].Baxter K and Terry SF. International Rare Disease Research Consortium commits to aggressive goals. Genet Test Mol Biomarkers, 2011. 15(7–8): p. 465. [DOI] [PubMed] [Google Scholar]
- [40].European Observatory on Health Systems and Policies. European Observatory on Health Systems and Policies 2016. [cited 2016 3/7/2016]; Available from: http://www.euro.who.int/en/about-us/partners/observatory


