Abstract
In the later part of the 1980s, the time was ripe for identifying genes controlling flower development. In that pregenomic era, the easiest way to do this was to induce random mutations in seeds by chemical mutagens (or irradiation) and to screen thousands of plants for those with phenotypes specifically defective in floral morphogenesis. Here, we discuss the results of premolecular screens for flower development mutants in Arabidopsis thaliana, carried out at Caltech and Monash University, emphasizing the usefulness of saturation mutagenesis, multiple alleles to identify full loss-of-function, conclusions based on multiple mutant analyses, and from screens for enhancer and suppressor modifiers of original mutant phenotypes. One outcome was a series of mutants that led to the ABC floral organ identity model (AP1, AP2, AP3, PI, and AG). In addition, genes controlling flower meristem identity (AP1, CAL, and LFY), floral meristem size (CLV1 and CLV3), development of individual floral organ types (CRC, SPT, and PTL), and inflorescence meristem properties (TFL1, PIN1, and PID) were defined. These occurrences formed targets for cloning that eventually helped lead to an understanding of transcriptional control of the identity of floral organs and flower meristems, signaling within meristems, and the role of auxin in initiating floral organogenesis. These findings in Arabidopsis are now being applied to investigate how orthologous and paralogous genes act in other flowering plants, allowing us to wander in the fertile fields of evo-devo.
Keywords: Arabidopsis, flower development, mutant screen, homeotic mutants, ABC model, flower meristem identity, inflorescence development
Thirty-five years ago, the field set out to understand how genes controlled flower morphogenesis, relying on chemical mutagenesis-based forward genetic screens in Arabidopsis since molecular tools were not yet available. Here, Smyth reviews these screens, which led to the ABC model of floral organ identity; the uncovering of later sepal, petal and carpel developmental control; understanding how inflorescence and floral meristem identity are conferred; and identifying new signaling pathways. Now, molecular understanding of how these genes function is extending these early findings across Angiosperms.
Introduction
Mendel (1866) established the transmission behavior of genes (determinants of traits) firstly as alternative forms of one gene and secondly in combinations of more than one gene. He succeeded because he chose to follow traits with alternative forms (variants) rather than traits that show continuous variation. In addition, he started out with true breeding lines that behaved predictably, generating only one form of a trait unless intercrossed with other lines.
Classical genetics relied at first on serendipity to provide variants to interbreed and follow down the generations. In the 1920s, it was discovered that variants (mutants) could be induced by treating organisms with X-irradiation (Muller 1927) or later by specific chemicals (Auerbach et al. 1947). Even though the recovery of variants was much increased over spontaneous levels, the production of specific variants was unpredictable and varied stochastically. In ground-breaking studies on the nature of the gene, Seymour Benzer in the 1950s focused on plaque morphology in bacteriophage T4 following chemical mutagenesis. He obtained hundreds of variants within 2 individual genes and showed that each gene (he called them cistrons) was mutable at many internal sites, either as point mutants or as deletions of adjoining sites (Benzer 1959). Later analysis of extensive mutation collections in bacteriophage T4 revealed that the structure of the mature phage was controlled by the sequential action of genes in branched linear pathways (Wood 1980), an early use of saturation mutagenesis to identify all genes involved in a specific morphogenetic process.
In Drosophila melanogaster, similar approaches were used to reveal how genes controlled morphogenesis. A successful example was that of Ed Lewis, who in the 1950s and 1960s, collected mutants that disrupted segmentation of the adult body. This led him to uncover several adjacent genes in the Bithorax complex that determined the identity of thoracic and abdominal segments. Their role was inferred because mutants resulted in changes in body segment identity, known as homeotic mutants (Lewis 1978). Subsequent large-scale mutation hunts in the 1970s focused on changes in embryo and larval segmentation (loss or identity changes) (Nüsslein-Volhardt and Wieschaus 1980; Irion and Nüsslein-Volhard 2022) and led to an understanding of how the sequential activity of the wild-type genes controlled each step. Similar screens in the nematode Caenorhabditis elegans had shown the way (Brenner 1974).
Meanwhile in plants, morphogenesis had been meticulously described at the macroscopic and cellular levels. But understanding the genetic control of these processes relied on rare sporadic mutants. Significant collections of mutants had been obtained especially in crop plants subject to intimate and long-term observation such as maize (Neuffer et al. 1968; Scandalios 1982; Sheridan 1988) and tomato (Stevens and Rick 1986). Large-scale collections in floricultural species including snapdragon (Stubbe 1966) and petunia (Cornu and Maizonnier 1983) were also curated. But the following were needed to help uncover the mechanism of the action of genes controlling morphogenesis: (1) allelic series indicating the consequences of full loss-of-function in recurring strongly affected alleles, (2) saturation mutagenesis to reveal most or all genes involved in specific developmental pathways, (3) multiple mutant combinations to deduce gene interactions (Table 1), and (4) second-site screens for enhancers and suppressors of the original mutant phenotype.
Table 1.
How the respective roles of developmental genes X and Y can be deduced from their double loss-of-function mutant phenotype.
| Class of interaction | Double mutant phenotype | Deduction | |
|---|---|---|---|
| Additive |

|
Double mutant combines phenotypes of single mutants | X and Y have different roles |
| Overlapping |

|
Double mutant is more disrupted than the additive phenotype | X and Y share part function |
| Epistatic |

|
Double and X mutant the same, Y mutant different | X acts upstream of Y |
| Shared |

|
Double mutant the same as both X and Y single mutants | Both X and Y are required |
| Redundant |
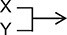
|
Double mutant is disrupted, X and Y single mutants wild type | Either X or Y is required |
Examples, especially involving organ identity (ABCE) mutants, include: additive: ap3 and ag; pi and ag; overlapping: ap1 and cal; spt and crc; epistatic: ag and crc; ag and spt; shared: pi and ap3; clv1/2/3; redundant: sep1/2/3/4.
A plant model species with facile genetics analogous to D. melanogaster was needed. In the mid 1980s, the wall cress Arabidopsis thaliana (family Brassicaceae) was chosen by many with the expectation that genes could be identified and cloned relatively easily (Leutwiler et al. 1984; Meyerowitz and Pruitt 1985; Estelle and Somerville 1986; Meyerowitz 1987). Morphological mutants were induced in early studies, although their genetic analysis was not followed up (Reinholz 1946; Röbbelen 1962; McKelvie 1962). Various groups then set out to focus on the development of embryos (Mayer et al. 1991), roots (Benfey et al. 1993), trichomes (Hülskamp et al. 1994), and flowers (Komaki et al. 1988) by collecting morphologically defective mutants specifically disrupted in each process using forward genetic screens. At this time, the molecular nature of developmental genes in plants was unknown, and it was anticipated that assemblies of mutants targeting specific processes would provide targets for cloning, leading to an understanding of molecular mechanisms.
Several homeotic genetic disruptions of flower morphogenesis had already been obtained in A. thaliana by Koornneef (1983) at Wageningen, the Netherlands. In these, one type of floral organ was replaced by another usually found at a different site within the flower. Such homeotic mutants had already shown their worth in revealing organ identity genes in Drosophila (see above), and we set about studying their genetics and developmental biology in Arabidopsis. At first, we relied on Koornneef's mutants agamous (ag), apetala1 (ap1), ap2, ap3, and pistillata (pi), each single alleles of their respective wild-type genes. None of these had disruptions elsewhere in the plant indicating flower-specific action (Bowman et al. 1989). But a fuller understanding of flower morphogenesis required further mutants of both these genes (Bowman et al. 1991) and others specifically affecting other processes in floral development. This paper reports the results of mutant screens of Arabidopsis carried out in 1988 at Caltech and several years subsequently at Monash University that focused on flower morphogenesis and how they helped build a foundation for our understanding of this process.
Methods
Dry seed of Landsberg erecta background was treated with 40-mM aqueous ethyl methane sulphonate (EMS) with stirring at room temperature for 8 hours. At this dose, it is expected that each locus has about one chance in 1,000 of being mutated per treated cell (Koornneef et al. 1982). Seeds were washed in distilled water overnight and blotted dry before planting 9 seeds per 65 mm square pot. The seeds were incubated at ∼25°C in a growth room under continuous Cool White fluorescent light. Once the M1 plants bore mature seeds generated by self-pollination in up to 10 siliques on the primary inflorescence (∼6–8 weeks), whole plants were collected individually into paper bags and dried. These steps were carried out at the California Institute of Technology by Leslie Leutwiler and Bob Pruitt. These growth conditions illustrate the advantages of Arabidopsis in the ability to grow many plants in a small space with rapid production of seeds.
In 1988, M2 seeds of 555 individual M1 plants [arising from ∼1,110 treated meristematic cells in the embryo, assuming the inflorescence of an adult plant is derived from two cells per seed (Koornneef et al. 1982)] were planted out. Plants were observed at weekly intervals for 6 weeks from germination until seed set. For most families, 24 or 25 seeds were planted, yielding 16.21 ± 0.012 SE flowering progeny per family on average (64%, range 2–24). This is consistent with many seeds carrying homozygous recessive mutations resulting in embryonic lethality. Overall 9,142 plants were screened at the flowering stage. Seeds of mutant M2 plants, or, if sterile, at least 5 of their phenotypically normal sibs, were collected. Individual mutants were given an isolation number corresponding to the M2 family number (prefixed “S”).
The effectiveness of the mutagenesis was shown by the occurrence of bleached cotyledons (either white or yellow) in M2 families (Li and Rédei 1969). Among 302 of the families screened for this trait, 45 (14.9%) had at least one bleached seedling (17 white families, 28 yellow). These were likely to have arisen from mutation events in the many nuclear genes required for photosynthesis.
Mutant lines of interest (all recessive) were successively back-crossed to Landsberg erecta to segregate out second-site mutations. Allelism tests between new mutants and mutants of known genes were then carried out by scoring the phenotypes of F1 progeny (if mutant, allelism was inferred).
This screen was extensive but unlikely to have approached saturation. We scanned M2 plants derived from about 1,110 M1 cells and estimated that the mutation rate per gene was around 1 in 1,000 cells (see above). Thus, by chance many genes may not have been mutated even once. For this reason, further mutagenesis screens of similar design were carried out from 1989 by John Alvarez at Monash University, Melbourne. Differences included using 25-mM EMS for 13 hours rather than 40 mM for 8 hours, and 1 of the 4 batches of treated seeds was of Columbia background rather than Landsberg erecta. Also, an additional batch of Landsberg erecta seeds was mutagenized with 25 krad of gamma irradiation from a 60Co source.
Results
Homeotic floral organ (ABCE) mutants: ap1, ap2, ap3, pi, ag, and sep
Our initial study of homeotic mutants was limited to one recessive mutant allele each of AG, AP2, AP3, and PI genes kindly provided by Maarten Koornneef (Koornneef 1983; Bowman et al. 1989). Based on single and double mutant phenotypes, these were sufficient to deduce that the wild-type genes act in combination to “allow cells to determine their place in the developing flower and thus to differentiate appropriately”, and that the 4 genes may act “in setting up or responding to concentric, overlapping fields within the flower primordium” (Bowman et al. 1989). On obtaining further alleles in the mutant hunt (Table 2), it was possible to deduce the full loss-of-function mutant phenotype (a strong phenotype shared by more than one allele) (Bowman et al. 1991). Interactions between homeotic mutants of all 4 genes, especially null mutants, were also studied by generating all gene combinations that were essential in developing the ABC model (Bowman et al. 1991, 2012).
Table 2.
Flower morphogenetic mutants identified in EMS screens.
| Gene | Mutant Allele No.a | Encoded Proteinb | References |
|---|---|---|---|
| AGAMOUS (AG) | 3, [4] | MADS TF (C) |
Bowman et al. (1991), Sieburth et al. (1995) |
| APETALA1 (AP1) | [2–5, 7] | MADS TF (A) | Bowman et al. (1993) |
| APETALA2 (AP2) | 2, 8, 9 | AP2 TF (A) | Bowman et al. (1991) |
| APETALA3 (AP3) | None obtained | MADS TF (B) | Bowman et al. (1991) |
| CAULIFLOWER (CAL) | [1c] | MADS TF | Bowman et al. (1993) |
| CLAVATA1 (CLV1) | S292, S368 | LRR-RLK | unpublished |
| CLAVATA3 (CLV3) | 1, [2d] | CLE signaling peptide | Clark et al. (1995) |
| CRABS CLAW (CRC) | 1, [2d] | YABBY TF |
Alvarez and Smyth (1999, 2002), Bowman and Smyth (1999) |
| LEAFY (LFY) | 3, 4 [5, 6, 10e, 11] | LEAFY TF | Weigel et al. (1992) |
| LEUNIG (LUG) | 8, 9 | TUP1 co-repressor | Liu and Meyerowitz (1995) |
| PETAL LOSS (PTL) | [1e, 3–5] | Trihelix TF |
Griffith et al. (1999), Brewer et al. (2004) |
| PETAL LOSS MODIFIER (PMD) | [1f] | unknown | Griffith et al. (1999) |
| PIN FORMED1 (PIN1) | [3, 5] | Auxin efflux carrier | Bennett et al. (1995) |
| PINOID (PID) | 1, [2, 3e, 4, 7] | S/T protein kinase | Bennett et al. (1995) |
| PISTILLATA (PI) | 2, 3 | MADS TF (B) | Bowman et al. (1991) |
| SEPALLATA1-4 (SEP1-4) | None obtained | MADS TF (E) | |
| SPATULA (SPT) | 1, [2, 3] | bHLH TF |
Alvarez and Smyth (1999, 2002), Heisler et al. (2001) |
| SUPERMAN (SUP) | [4e] | C2H2 ZnF TF | Bowman et al. (1992) |
| TERMINAL FLOWER1 (TFL1) | 2–5, [6, 7, 8e] | CETS | Alvarez et al. (1992) |
| UNUSUAL FLORAL ORGANS (UFO) | [3–5] | F-box | Levin and Meyerowitz (1995) |
Obtained at Caltech in 1988, or at Monash University in 1989–1992 (square brackets).
Determined subsequently. TF—transcription factor; A, B, C, E—floral organ identity class.
In Ws background.
Induced by gamma irradiation.
Induced in Col background, all others in Ler background.
Present as a dominant modifier in Ler background.
For instance, an additional allele of AG, ag-3, had a strong phenotype identical to ag-1 indicating the full loss-of-function phenotype (Fig. 1b). A partial loss-of-function allele, ag-4, was later useful in separating out components of AG function in the third and fourth floral whorls (Sieburth et al. 1995). Also, later generation of second-site mutants in the ag-4 background led to the discovery that microRNAs regulate the lifetime of mRNA transcripts of some of the interacting genes (Chen and Meyerowitz 1999).
Fig. 1.
Inflorescences of wild-type and ABC mutants. a) Wild-type (Landsberg erecta). b) ag-3 mutant (C function), closely similar to ag-1 and thus likely to reflect full loss-of-function. c) ap2-2 mutant (A function), an atypical allele with leaf-like sepals. d) ap2-2 mutant with more severe disruptions same as those of several other alleles. e) ap3-1 mutant (B function), with a weak phenotype. f) pi-1 mutant (B function), with a strong phenotype. a–e) modified from Meyerowitz et al. (1989); f) modified from Bowman et al. (1989).
For AP2, the original allele ap2-1 was clearly atypical when further alleles were obtained by us [ap2-2, 8, and 9, Bowman et al. (1991)] (Fig. 1c and d), and others [ap2-3 and 4, Okada et al. (1991) and ap2-5, 6, and 7, Kunst et al. (1989)]. ap2-2 and ap2-8 shared the same strong abnormal phenotype pointing to the full loss-of-function phenotype.
For PI, 2 new mutant alleles pi-2 and pi-3 were less abnormal than pi-1 (Fig. 1f) indicating that they, at least, had only partially lost PI function (Bowman et al. 1991). The weakest pi allele, pi-3, resembled ap3-1 (Fig. 1e) suggesting these 2 genes had a shared function (Bowman et al. 1991) (Table 1). This was confirmed subsequently when 3 further ap3 alleles were obtained by others that had the same strong phenotype as each other and pi-1 (Jack et al. 1992), presumably resulting from full loss of AP3 or PI function.
The AP1 gene was followed up later when an allelic series of 5 mutants was obtained (Bowman et al. 1993) (Table 2). The phenotype of one of these, ap1-7, matched the strong phenotype of the original allele, ap1-1 (Koornneef 1983), likely indicating full loss-of-function. Going by mutant combinations, we proposed that one function of AP1 was to confer A function in combination with AP2 in defining the identity of the 2 outer floral whorls (Bowman et al. 1993).
The identity of all 4 floral organ types was shown subsequently by Marty Yanofsky's group to be controlled by 4 paralogous SEPALLATA (SEP) genes (Pélaz et al. 2000; Ditta et al. 2004), thus defining E function. These were identified using a reverse genetics approach. [Reverse genetics requires knowledge of the molecular nature of a gene (and its relatives if appropriate) before manipulating its function. Forward genetics involves modifying the function of a gene, usually by mutation, as a precursor to identifying its molecular nature]. In this case, 4 close relatives of AG from the MADS transcription factor family were each mutated by insertions and identified using a transgenic approach. Single mutants were phenotypically normal, and only quadruple mutants (sep1 sep2 sep3 sep4) had leaf-like organs in all floral whorls. Presumably, this redundancy (Table 1) accounts for why no mutants of SEP genes were identified in the forward genetics screens.
Further mutants affected in organ identity: sup, and lug
The identity of fourth whorl organs as carpels is lost in superman (sup) mutants (Bowman et al. 1992), and termination of the floral meristem is delayed. This results in many additional stamens in place of carpels. One sup allele was obtained in our screen, sup-4, with a phenotype similar to the other 3 known at that stage (Table 2). It was proposed that SUP represses AP3 and PI (B) function in the fourth whorl, consistent with the localization of transcripts to the inside of the whorl 3/4 boundary (Prunet et al. 2017). Thus, SUP is not a primary organ identity gene, but one that blocks B function in a spatially defined region of the floral meristem. It is also involved along with AG in terminating the flower meristem after 2 carpel primordia have been generated. Later studies of weaker sup mutant alleles uncovered the general role of DNA methylation in epigenetic silencing of gene expression in plants (Jacobsen et al. 2000).
Other mutants were also obtained with less clear-cut effects on floral organ identity, and with additional defects elsewhere in the plant. Two were alleles of a gene named LEUNIG (LUG) (lug-8 and 9) after Michael Leunig, the creator of the cartoon character “Mr Curly” (Table 2). This reflected the curled apical extension of the carpels characteristic of such mutants [including lug-10 (Fl89 of Komaki et al. 1988)]. Additional alleles were subsequently isolated as enhancers of identity defects in sepals and petals in the weak A function mutant ap2-1 (Liu and Meyerowitz 1995). lug single mutants had similar but less severe identity changes to sepals and petals, leading the authors to propose that LUG constrained AG function somewhat in these whorls.
Flower meristem identity mutants: ap1, cal, lfy, and ufo
AP1 is also involved earlier in defining the identity of the flower meristem itself. It does this partly in combination with a closely related MADS paralog CAULIFLOWER (CAL) (Bowman et al. 1993; Kempin et al. 1995) (Table 2). Double mutants of ap1 and cal produce inflorescence primordia that generate further inflorescence primordia on their flanks rather than flower primordia, and so resemble dwarf “cauliflowers”. Mutants of the cal gene alone are phenotypically normal, so its loss-of-function is only revealed if AP1 function is also lost. That is, AP1 wild-type function fully overlaps with that of CAL, but not vice versa (Table 1). The ap1 cal double mutant proved useful in obtaining synchronized samples of developing flower buds from the earliest stages for transcriptional profiling. By incorporating an inducible AP1 construct, the many newly arising meristems can be switched to develop as flower meristems rather than as inflorescence meristems (Wellmer et al. 2006).
Another key gene that defines flower meristem identity together with AP1 and CAL encodes the transcription factor LEAFY (LFY) (Weigel et al. 1992; Bowman et al. 1993). An allelic series of 6 lfy mutant alleles was obtained with a strengthening range of abnormal phenotypes [2 weak alleles (lfy-5 and 10), 2 intermediate (lfy-3 and 4), and 2 strong (lfy-6 and 11)] (Table 2). The phenotypes of the strong alleles were closely similar to lfy-1 (Schultz and Haughn 1991), presumably revealing the consequences of full loss-of-function. Again, multiple mutant combinations of lfy with ap1 and cal allowed their overlapping roles to be deduced (Weigel and Meyerowitz 1993).
A further gene with a role in defining floral meristem identity was UNUSUAL FLORAL ORGANS (UFO) (Wilkinson and Haughn 1995). Three mutants obtained here (ufo-3, 4, and 5) (Table 2) were closely similar in phenotype to each other and to 5 of 6 other independent mutant occurrences studied by Levin and Meyerowitz (1995). UFO is involved in conferring sepal and petal identity, boundary formation and determinacy of the flower meristem, sharing the first function at least with LFY (Levin and Meyerowitz 1995).
Floral organogenesis mutants: spt, crc, and ptl
One category of mutant was identified as being defective in the development of one floral organ type. Gynoecium-only mutants were chosen as potentially acting downstream of the carpel identity gene AG (Alvarez and Smyth 1999, 2002). One of these had siliques wider in the medial plane, especially at the apex, and the gene was named SPATULA (SPT) after the laboratory and kitchen utensil (Table 2; Fig. 2a–d). Three alleles were obtained, with the stronger spt-2 and spt-3 alleles having carpels unfused at the apex and lacking a transmitting tract.
Fig. 2.
Mature flowers of wild type (wt), spatula-2 and crabs claw-1 mutants. a) The gynoecia of spt-2 flowers are longer than wild type, with unfused carpels at the apex and no transmitting tract in the reduced septum. Those of crc-1 mutants are shorter, wider, and the 2 unfused carpels are bent inward at the apex like a crab's claw (arrows). b) A wild-type silique with one valve removed showing seeds and septum. c) Two spt-2 siliques showing reduced seed set and the septum absent (left) or much reduced (right). Bars represent 500 μm. d) A spatula after, which the gene was named. e) A YABBY, an Australian freshwater crayfish and a relative of the crab, after which the YABBY transcription factor family was named (Bowman and Smyth 1999). a–c) modified from Alvarez and Smyth (2002).
Another mutant class had shorter siliques with the 2 carpels unfused at the apex and bending inwards; hence, it was named CRABS CLAW (CRC) (Fig. 2a). Two alleles were identified, the stronger crc-1 allele having more pronounced defects in the gynoecia that were shorter and wider than normal, and also lacked nectaries (Table 2). Additional alleles were identified later and revealed that crc-1 was the strongest loss-of-function phenotype (Bowman and Smyth 1999). When spt-2 and crc-1 were combined, the gynoecia were abnormal in a nonadditive fashion, indicating that they have partially overlapping roles (Table 1). In addition, when combined as quadruple mutants with ap2 ag mutants (lacking A and C functions), the residual carpelloid properties of ap2 ag double mutants were lost, indicating that both SPT and CRC have a role in controlling aspects of carpel structure independently of AG (Alvarez and Smyth 1999).
A second category of floral organ mutants affected only sepals and petals, although not their identity. The most obvious phenotype was loss of petals, and hence, the gene was named PETAL LOSS (PTL) (Griffith et al. 1999) (Table 2). However, sepals were also affected in being wider than normal and sometimes fused at the base. Subsequent studies (Brewer et al. 2004) revealed that PTL acts as a boundary gene in the sepal whorl, dampening growth between sepals. When extra intersepal growth occurs in ptl loss-of-function mutants, petal initiation nearby is disrupted (Lampugnani et al. 2012). This was related to auxin dynamics through a second-site mutagenesis screen of ptl single mutants that uncovered the auxin efflux gene AUXIN1 as a supporter of petal initiation (Lampugnani et al. 2013).
A modifier of the ptl mutant phenotype, PETAL LOSS MODIFIER (PMD), was uncovered in un-mutagenized Landsberg erecta background that resulted in additional petals rather than fewer, with these petals arising with disrupted orientation within the flower meristem (Griffith et al. 1999). This variant was unique in being dominant, and its molecular nature has not yet been uncovered.
Flower meristem size mutants: clv1, and clv3
One class of phenotypic mutants had flowers with increased numbers of all organs, especially carpels. In previous studies, this phenotype was named clavata (clv) after the club-shaped fruit with swelling at the apex (McKelvie 1962). Allelism tests showed that 2 occurrences obtained here were allelic with the clv1-1 mutant already described (Koornneef 1983). Two further occurrences were shown not to be allelic with either clv1-1 (Clark et al. 1993) or clv2-1 (Kayes and Clark 1998), and were named clv3-1 and clv3-2 (Clark et al. 1995) (Table 2). Mutants of these 3 genes, CLV1, 2, and 3, have closely similar single and multiple mutant phenotypes indicating that they share the same ultimate function (Table 1).
Inflorescence meristem mutants: tfl1
One category of gene with a mutant phenotype with abnormal flowers was named TERMINAL FLOWER1 (TFL1) (Shannon and Meeks-Wagner 1991; Alvarez et al. 1992). This arose 4 times in the original hunt at Caltech, and thrice more subsequently at Monash University (Table 2). The 7 alleles showed a spectrum of increasingly severe abnormalities, but all shared the consumption of the inflorescence meristem sooner or later by floral organs. This was accelerated at higher temperatures. It was proposed that TFL1 is required to maintain inflorescence meristem identity by preventing it from taking on floral meristem identity (Alvarez et al. 1992). We speculated that TFL1 may play a role in the evolution of determinate inflorescence shoots.
Auxin response mutants: pin1 and pid
A final category of flower development mutants mostly lacked flowers on the pin-like inflorescence shoot. However, occasional flowers did form that were characteristically abnormal in the number and morphology of all floral organs (Bennett et al. 1995) (Table 2). Two mutants were allelic with pin formed1-1 (pin1-1) already-characterized (Okada et al. 1991). pin1 mutants have pleiotropic defects in cotyledon number (reduced) and leaf number (decreased). One allele obtained in the screen, pin1-5, had a weaker phenotype and has been useful in revealing the consequences of partial loss of PIN1 function (e.g. Sohlberg et al. 2006). Also, 2 pin1 mutant alleles were recently used to assay the ancient conserved transport function of PIN in nonvascular plants (Fisher et al. 2023).
At the same time, 5 other mutants sharing pin-like inflorescences contrarily had increased cotyledon numbers and increased numbers of leaves. A series of allelism tests revealed that a second locus was involved, and the gene was named PINOID (PID), meaning “pin-like” (Bennett et al. 1995). An allelic series of increasing severity was obtained, and double mutants with an auxin resistant1 (axr1) mutant showed that PID, like PIN1, is associated with auxin function.
Discussion
Most mutants obtained were in flower specific genes
By focusing our screening on mutants that changed only floral properties, we were mostly able to avoid genes with wider and more general functions in plant growth and development, including house-keeping genes. For example, genes controlling cell structure and cell division were not revealed. These were more often obtained in screens of earlier developmental events, especially during embryogenesis when wild-type gene products derived maternally may be active for some time after fertilization. When eventually no activity remains, the disruption could lead to seed lethality (Meinke and Sussex 1979) or sometimes embryo patterning defects (Mayer et al. 1991).
It is of interest that all the mutants obtained here were recessive. This is in contrast with collections of maize morphological mutants (Richardson and Hake 2022). However, the maize collection includes many defective plants arising in crop conditions where dominant mutants would be immediately obvious. Also, in many cases, we now know that the dominant maize mutants often result from mis-expression of genes rather than their loss-of-function. As here, screens of EMS mutants in maize yield mainly recessive mutations, as expected from single base changes predominantly in transcribed regions (Neuffer and Sheridan 1980).
Predominant recovery of transcription factor genes
In 1988, we had no knowledge of the molecular nature of flower development genes. Possible roles for hormones had been considered (Meyerowitz et al. 1989), but upon subsequent cloning, the majority of genes were found to encode transcription factors. In hindsight, this was perhaps unsurprising given precedents from the cloning of animal developmental genes, especially those with homeotic mutational changes. Differences between homeotic gene classes in animals (encoding homeodomain family members of the HOX subfamily) and plants (encoding mostly MADS family members) were revealed, including their genomic organization (clustered in animals, dispersed in plants), their expression patterns (sequential along a physical gradient in animals, coincidental in concentric overlapping fields in plants), and the multimeric structure of their functional products (mono- or di-meric HOX proteins in animals, hetero-tetrameric MADS proteins in plants). But a common function is the regulation of target genes, still being defined. For example, CRC expression is indeed a direct target of AG action (Ó’Maoiléidigh et al. 2013), as proposed in the initial screen.
Apart from the MADS family, founder members of plant-specific families such as the AP2 family (Jofuku et al. 1994) and the YABBY family (Bowman and Smyth 1999) (Fig. 2e) were revealed. Many members of these families control morphogenetic processes, suggesting that they have arisen from neofunctionalization following duplication of an early common ancestor with such a role. Other genes encoding trihelix proteins (Brewer et al. 2004) and bHLH proteins (Heisler et al. 2001) occurred too (Table 2), but members of these large families have widely divergent roles suggesting more recent neo-functionalization leading to a floral role. On the other hand, the plant-specific LFY gene usually occurs as the sole member of its family (Moyroud et al. 2010), indicating that newly duplicated copies are unlikely to coexist because of mal-adaptation.
As an illustration of how our screen has led to a wider understanding of plant development, CRC was a founding member of the YABBY family (Bowman and Smyth 1999) along with FILAMENTOUS FLOWER (FIL) (Sawa et al. 1999). In addition to its role in fine-tuning carpel development, CRC was also revealed to be required for nectary development and to function with AG in promoting floral meristem determinacy. The strategy of screening for second-site mutants was also exploited in crc mutants (Page and Grossniklaus 2002) and led to uncovering a role for YABBY family members in defining top-bottom polarity of lateral organs, including leaves and carpels, and to identifying other gene families involved in this process (Eshed et al. 1999; Siegfried et al. 1999). Later studies have revealed other roles of CRC in supporting leaf midrib development in grasses (Yamaguchi et al. 2004), and in conferring sexual dimorphism in flowers of melons and cucumbers (Zhang et al. 2022), for example.
New signaling genes
One important plant-specific signaling mechanism was uncovered in the clavata mutants. These are now known to limit the spread of stem cell identity from the organizing center of flower meristems as well as shoot and inflorescence meristems (Schoof et al. 2000). In particular, a novel entry point to signaling was provided by CLV3 (Fletcher et al. 1999; Brand et al. 2000), a founding member of the CLE family of mobile peptide signaling molecules, now known to be a predominant mode of signaling between plant cells (Willoughby and Nimchuk 2021).
Another signaling process is controlled by TFL1, a phosphatidylethanolamine binding protein (Bradly et al. 1997) that represses the floral nature of the inflorescence meristem. It does this antagonistically with the florigen FLOWERING LOCUS T (FT) from the same family. The ft mutant phenotype is late flowering [tfl1 is early, Shannon and Meeks-Wagner (1991)] but ft flowers themselves are normal, and so mutants of this gene, and many other occurrences of late flowering mutants (e.g. Rédei 1962; Koornneef et al. 1991), were not followed up here. We proposed that TFL1 plays a role in the evolutionary switching of the inflorescence meristems between determinate and indeterminate growth forms (Alvarez et al. 1992), now being borne out by observation, for example in the woodland strawberry Fragaria vesca (Lembinen et al. 2023).
Our focus on abnormal flowers meant that we included pin1 and pid mutant plants in which monstrous flowers sometimes arose on otherwise naked inflorescences. We discovered the PID gene through genetically separating pid from pin1 mutants. This ultimately led to the discovery that the PID kinase phosphorylates PIN1 (Michniewicz et al. 2007), and to a continuing expansion of knowledge of auxin signaling.
Conclusion
Forward genetic screens have been instrumental in providing an initial spectrum of genes for follow up by cloning and molecular characterization. In Arabidopsis, chemical mutagenesis methods were joined by transposon tagging (Sundaresan et al. 1995), activation tagging (Weigel et al. 2000), antisense RNA knock down (Chuang and Meyerowitz 2000), and large-scale T-DNA insertional mutagenesis (Feldmann et al. 1989; Alonso et al. 2003) as a source of developmental genes. These could generate mutant phenotypes per se, or changes in reporter gene expression. Once the DNA sequence of candidate genes was known, large-scale libraries of such resources could be screened, in pools if needed, to obtain additional mutant versions of the gene of interest. One disadvantage of insertional mutagenesis was the predominance of loss-of-function mutant alleles, but this was remedied to some extent by screening of Targeted Induced Local Lesions in Genomes populations (McCallum et al. 2000). A recent revolution in obtaining knock outs of genes of known sequence has been provided by CRISPR-Cas9 methods (Zhang et al. 2019), although recovery of partial loss-of-function alleles is not yet straightforward. In general, genomic resources are now widespread and accessible, but our understanding of a gene's function still depends to a large extent on modifying its expression.
Developmental gene discovery has been very successful in Arabidopsis. Once cloned, the role of each gene and its paralogs has been followed up in Arabidopsis using expression analysis, multiple mutant studies, and reverse genetics approaches, especially in the large gene families characteristic of many transcription factors. Together with further regulatory and signaling genes uncovered by us and others, gene regulatory networks of flower development in Arabidopsis are now one of the most complete developmental pathways yet defined for flowering plants (Chen et al. 2018; Thomson and Wellmer 2019; Zúñiga-Mayo et al. 2019).
Also, findings have been extended to both orthologs and paralogs in other species, leading to the burgeoning field of evo-devo (Smyth 2018; Kramer 2019). One satisfying result of initially focusing on one model species, Arabidopsis, has been the finding that the role of core morphogenetic genes is often conserved across flowering plants. This early assumption has fortunately borne fruit. Even so, exceptions are to be treasured as helping build a more complete and balanced picture of exactly how the exceptional diversity of floral forms has evolved.
Acknowledgments
It was a pleasure to work with Elliot Meyerowitz while on sabbatical leave at Caltech in 1988. John Alvarez generated many additional mutants at Monash University, as well as many ideas and interpretations. John Bowman played an active and influential role, both at Caltech and later at Monash University. I thank them for their collegiality and insight and for comments on the manuscript. I gratefully acknowledge Bob Pruitt and Leslie Leutwiler for carrying the initial mutagenesis and M1 seed collection. Tori and Ryan Somerville, and Rob and Kerryn Smyth and families, helped obtain the YABBY image.
Funding
Research at Monash University was supported by grants from the Australian Research Council (A18831996 and A19131181).
Literature cited
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301(5633):653–657. doi: 10.1226/science.1086391. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu X-H, Smyth DR. Terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992;2(1):103–116. doi: 10.1111/j.1365.-313X.1992.00103.x. [DOI] [Google Scholar]
- Alvarez J, Smyth DR. CRABS CLAW and SPATULA, two Arabidopsis genes controlling carpel development in parallel with AGAMOUS. Development. 1999;126(11):2377–2386. doi: 10.1242/dev.126.11.2377. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Smyth DR. CRABS CLAW and SPATULA genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. Int J Plant Sci. 2002;163(1):17–41. doi: 10.1086/324178. [DOI] [Google Scholar]
- Auerbach C, Robson JM, Carr JG. The chemical production of mutations. Science. 1947;195(2723):243–247. doi: 10.1126/science.195.2723.243. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Lindstead PJ, Roberts K, Schielfelbein JW, Hauser M-T, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119(1):57–70. doi: 10.1242/dev.119.1.57. [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8(4):505–520. doi: 10.1046/j.1365-313X.1995.8040505.x. [DOI] [Google Scholar]
- Benzer S. On the topology of the genetic fine structure. Proc Natl Acad Sci U S A. 1959;45(11):1607–1620. doi: 10.1073/pnas.45.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development. 1993;119(3):721–743. doi: 10.1242/dev.119.3.721. [DOI] [Google Scholar]
- Bowman JL, Sakai H, Jack T, Weigel D, Mayer U, Meyerowitz EM. SUPERMAN, a regulator of floral homeotic genes in Arabidopsis. Development. 1992;114(3):599–615. doi: 10.1242/dev.114.3.599. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126(11):2387–2396. doi: 10.1242/dev.126.11.2387. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. Genes directing flower development in Arabidopsis. Plant Cell. 1989;1(1):37–52. doi: 10.1105/tpc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. Genetic interactions among floral homeotic genes. Development. 1991;112(1):1–20. doi: 10.1242/dev.112.1.1. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. The ABC model of flower development: then and now. Development. 2012;139(22):4095–4098. doi: 10.1242/dev.083972. [DOI] [PubMed] [Google Scholar]
- Bradly D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275(5296):80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. Dependence of stem cell fate in Arabidopsis on a feedback loop generated by CLV3 activity. Science. 2000;289(5479):617–619. doi: 10.1126/science.289.5479.617. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1534/genetics.77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Howles PA, Dorian K, Griffith ME, Ishida T, Kaplan-Levy RN, Kilinc A, Smyth DR. PETAL LOSS, a trihelix transcription factor gene, regulates perianth architecture in the Arabidopsis flower. Development. 2004;131(16):4035–4045. doi: 10.1242/dev.01279. [DOI] [PubMed] [Google Scholar]
- Chen X, Meyerowitz EM. HUA1 and HUA2 are two members of the floral homeotic AGAMOUS pathway. Mol Cell. 1999;3(3):349–360. doi: 10.1016/S1097-2765(00)80462-1. [DOI] [PubMed] [Google Scholar]
- Chen D, Yan W, Fu L-Y, Kaufmann K. Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat Commun. 2018;9(4534):1–13. doi: 10.1038/s43467-018-06772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C-F, Meyerowitz EM. Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2000;97(9):4985–4990. doi: 10.1073/pnas.060034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development. 1993;119(2):397–418. doi: 10.1242/dev.119.2.397. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development. 1995;121(7):2057–2067. doi: 10.1242/dev.121.7.2057. [DOI] [Google Scholar]
- Cornu A, Maizonnier D. 1983. The genetics of petunia. In: Janick J, editor. Plant Breed Reviews. Vol. 1. Boston: Springer; 1983. p. 11–58. doi: 10.1007/978-1-4684-8896-8_2. [DOI] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S. Yanofsky MF the SEP4 gene in Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol. 2004;14(21):1935–1940. doi: 10.1016/j.cub.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Baum SF, Bowman JL. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell. 1999;99(2):199–209. doi: 10.1016/s0092-8674(00)81651-7. [DOI] [PubMed] [Google Scholar]
- Estelle M, Somerville CR. The mutants of Arabidopsis. Trends Genet. 1986;2(4):89–93. doi: 10.1016/0168-9525(86)90190-3. [DOI] [Google Scholar]
- Feldmann KA, Marks MD, Christianson ML, Quatrano RS. A dwarf mutant of Arabidopsis generated by T-DNA insertion mutagenesis. Science. 1989;243(4896):1351–1354. doi: 10.1126/science.243.4896.1351. [DOI] [PubMed] [Google Scholar]
- Fisher TJ, Flores-Sandoval E, Alvarez JP, Bowman JL. PIN-FORMED is required for shoot phototropism/gravitropism and facilitates meristem formation in Marchantia polymorpha. New Phytol. 2023;238(4):1498–1515. doi: 10.1111/nph.18854. [DOI] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science. 1999;283(5409):1911–1914. doi: 10.1126/science.283.5409.1911. [DOI] [PubMed] [Google Scholar]
- Griffith ME, da Silva Conceição A, Smyth DR. PETAL LOSS gene regulates initiation and orientation of second whorl organs in the Arabidopsis flower. Development. 1999;126(24):5635–5644. doi: 10.1242/dev.126.24.5635. [DOI] [PubMed] [Google Scholar]
- Heisler MGB, Atkinson A, Bylstra YH, Walsh R, Smyth DR. SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development. 2001;128(7):1089–1098. doi: 10.1242/dev.128.7.1089. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Miséra S, Jürgens G. Genetic dissection of trichome cell development in Arabidopsis. Cell. 1994;76(3):555–566. doi: 10.1016/0092-8674(94)90118-X. [DOI] [PubMed] [Google Scholar]
- Irion U, Nüsslein-Volhard C. Developmental genetics with model organisms. Proc Natl Acad Sci U S A. 2022;119(30):e2122148119. doi: 10.1073/pnas.2122148119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T, Brockman LL, Meyerowitz EM. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell. 1992;68(4):683–697. doi: 10.1016/0092-8674(92)90144-2. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Sakai H, Finnegan EJ, Cao X, Meyerowitz EM. Ectopic methylation of flower-specific genes in Arabidopsis. Curr Biol. 2000;10(4):179–186. doi: 10.1016/S.0960-9822(00)00324-9. [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer DG, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6(9):1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayes JM, Clark SE. CLAVATA2: a regulator of organ and meristem development in Arabidopsis. Development. 1998;125(19):3843–3851. doi: 10.1242/dev.125.19.3843. [DOI] [PubMed] [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF. Molecular basis of the cauliflower phenotype in Arabidopsis. Science. 1995;267(5197):522–525. doi: 10.1126/science.7824951. [DOI] [PubMed] [Google Scholar]
- Komaki MK, Okada K, Nishino E, Shimura Y. Isolation and characterisation of novel mutants of Arabidopsis thaliana defective in flower development. Development. 1988;104(2):195–203. doi: 10.1242/dev.104.2.195. [DOI] [Google Scholar]
- Koornneef M. Linkage map of Arabidopsis thaliana. J Hered. 1983;74(4):265–272. doi: 10.1093/oxfordjournals.jhered.a109781. [DOI] [Google Scholar]
- Koornneef M, Dellaert LWM, van der Veen JH. EMS- and radiation-induced mutation frequencies at individual loci in Arabidopsis thaliana (L.) Heynh. Mutat Res. 1982;93(1):109–123. doi: 10.1016/0027-5107(82)91029-4. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229(1):57–66. doi:10/1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Kramer E. Plus ça change, plus c'est la même chose: the developmental evolution of flowers. In: Grossniklaus U, editor. Plant Development and Evolution, Current Topics in Developmental Biology. Vol. 31. Cambridge MA: Academic Press; 2019. p. 211–238. doi: 10.1016/bs.ctdb.2018.11.015. [DOI] [PubMed] [Google Scholar]
- Kunst L, Klenz JE, Martinez-Zapater J, Haughn GW. AP2 gene determines the identity of perianth organs in Arabidopsis thaliana. Plant Cell. 1989;1(12):1195–1208. doi: 10.1105/tpc.1.12.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani ER, Kilinc A, Smyth DR. PETAL LOSS is a boundary gene that inhibits growth between developing sepals in Arabidopsis thaliana. Plant J. 2012;71(5):724–735. doi: 10.1111/j.1365-313X.2012.05023.x. [DOI] [PubMed] [Google Scholar]
- Lampugnani ER, Kilinc A, Smyth DR. Auxin controls petal initiation in Arabidopsis. Development. 2013;140(1):185–194. doi: 10.1242/dev.084582. [DOI] [PubMed] [Google Scholar]
- Lembinen S, Cieslak M, Zhang T, Mackenzie K, Elomaa P, Prusinkiewicsi P, Hytönen T. Diversity of woodland strawberry inflorescences arises from heterochrony regulated by TERMINAL FLOWER1 and FLOWERING LOCUS T. Plant Cell. 2023;35(6):2079–2094. doi: 10.1093/plcell/koad086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutwiler LS, Hough-Evans BR, Meyerowitz EM. The DNA of Arabidopsis thaliana. Mol Gen Genet. 1984;194(1-2):15–23. doi: 10.1007/BF00383491. [DOI] [Google Scholar]
- Levin JZ, Meyerowitz EM. UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell. 1995;7(5):529–548. doi: 10.1105/tpc.7.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EB. Gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Li SL, Rédei GP. Estimation of mutation rate in autogamous diploids. Radiat Bot. 1969;9(2):125–131. doi: 10.1016/S0033-7560(69)80079-7. [DOI] [Google Scholar]
- Liu Z, Meyerowitz EM. LEUNIG Regulates AGAMOUS expression in Arabidopsis flowers. Development. 1995;121(4):975–991. doi: 10.1242/dev.121.4.975. [DOI] [PubMed] [Google Scholar]
- Mayer U, Torres-Ruiz RA, Berleth T, Miséra S, Jürgens G. Mutations affecting body organisation in the Arabidopsis embryo. Nature. 1991;353(6343):402–407. doi: 10.1038/353402a0. [DOI] [Google Scholar]
- McCallum CM, Comai L, Green EA, Henikoff S. Targeted screening for induced mutations. Nat Biotechnol. 2000;18(4):455–457. doi: 10.1038/74542. [DOI] [PubMed] [Google Scholar]
- McKelvie AD. A list of mutant genes in Arabidopsis thaliana (L.) Heynh. Radiat Bot. 1962;1:233–241. doi: 10.1016/S0033-7560(61)80032-X. [DOI] [Google Scholar]
- Meinke DW, Sussex IM. Embryo lethal mutants of Arabidopsis thaliana: a model system for plant embryo development. Dev Biol. 1979;72(1):50–61. doi: 10.1016/0012-1606(79)90097-6. [DOI] [PubMed] [Google Scholar]
- Mendel G. Versuche über pflanzen-hybriden. Verhandlungen des Naturforschenden Vereins in Brünn. 1866;4:3–47. https://www.biodiversitylibrary.org/item/124139#page/133/mode/1up. [Google Scholar]
- Meyerowitz EM. Arabidopsis thaliana. Annu Rev Genet. 1987;21(1):93–111. doi: 10.1146/annurev.ge.21.120187.000521. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM, Pruitt RE. Arabidopsis thaliana and plant molecular genetics. Science. 1985;229(4719):1214–1218. doi: 10.1126/science.229.4719.1214. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM, Smyth DR, Bowman JL. Abnormal flowers and pattern formation in floral development. Development. 1989;106(2):209–217. doi: 10.1242/dev.106.2.209. [DOI] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130(6):1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Moyroud E, Kusters E, Monniaux M, Koes R, Parcy F. LEAFY blossoms. Trends Plant Sci. 2010;15(6):346–352. doi: 10.1016/tplants.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Muller HJ. Artificial transmutation of the gene. Science. 1927;66(1699):84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- Neuffer MG, Jones L, Zuber MS. The Mutants of Maize. Madison: Crop Science Society of America; 1968. 74pp. [Google Scholar]
- Neuffer MG, Sheridan WF. Defective kernel mutants of maize. Genetics. 1980;95(4):925–944. doi: 10.1093/genetics/95.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhardt C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Kimura Y. Requirement of the auxin transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3(7):677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó'Maoiléidigh DS, Wuest ST, Rae L, Raganelli A, Ryan PT, Kwaśniewska K, Das P, Lohan AJ, Loftus B, Graciet E, et al. Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell. 2013;25(7):2482–2503. doi: 10.1105/tpc.113.113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DR, Grossniklaus U. The art and design of genetic screens; Arabidopisis thaliana. Nat Rev Genet. 2002;3(2):124–136. doi: 10.1058/nrg730. [DOI] [PubMed] [Google Scholar]
- Pélaz S, Ditta GS, Baumann E, Wisman E, Yanofsky M. B and C organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405(6783):200–203. doi:10.1038.35012103. [DOI] [PubMed] [Google Scholar]
- Prunet N, Yang W, Das P, Meyerowitz EM, Jack TP. SUPERMAN Prevents class B gene expression and promotes stem cell termination in the fourth whorl of Arabidopsis thaliana flowers. Proc Natl Acad Sci U S A. 2017;114(27):7166–7171. doi: 10.1073/pnas.1705977114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rédei GP. Supervital mutants of Arabidopsis. Genetics. 1962;47(4):443–460. doi: 10.1093/genetics/47.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholz E 1946. X-ray mutations of Arabidopsis thaliana L. (Heynh.) and their significance for plant breeding and the theory of evolution. Field Information Agency, Technical, Office for Military Government, Germany (US). 1946; 1006: 1–60. [Google Scholar]
- Richardson AE, Hake S. The power of classic maize mutants; driving forward our fundamental understanding of plants. Plant Cell. 2022;34(7):2505–2517. doi: 10.1093/plcell/koac08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röbbelen G. Wirkungsvergleich zwischen Äthylmethansulfonat und Röngenstrahlen in Mutationsversuch mit Arabidopsis thaliana. Naturwissenschaften. 1962;49:65. doi: 10.1007/BF00595396. [DOI] [Google Scholar]
- Sawa S, Watanabe K, Goto K, Liu Y-G, Shibata D, Kanaya E, Morita EH, Okada K. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis encodes a protein with a zinc finger and HMG-related domains. Genes Dev. 1999;13(9):1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Developmental genetics of maize. Annu Rev Genet. 1982;16(1):85–112. doi: 10.1146/annurev.ge.16.120182.000505. [DOI] [PubMed] [Google Scholar]
- Schoof H, Leonard M, Haecker A, Meyer KFX, Jügens G, Laux T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100(6):635–644. doi: 10.1016/S0095-86749(00)80700-X. [DOI] [PubMed] [Google Scholar]
- Schultz EA, Haughn GW. LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell. 1991;3(8):771–781. doi: 10.1105/tpc.3.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. A mutation in the Arabidopsis TFL1 gene affects inflorescence development. Plant Cell. 1991;3(9):877–892. doi: 10.1105/tpc.3.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan WF. Maize developmental genetics: genes of morphogenesis. Annu Rev Genet. 1988;22(1):353–385. doi: 10.1146/annurev.ge.22.120188.002033. [DOI] [PubMed] [Google Scholar]
- Sieburth LE, Running MP, Meyerowitz EM. Genetic separation of third and fourth whorl functions of AGAMOUS. Plant Cell. 1995;7(8):1249–1258. doi: 10.1105/tpc.7.8.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development. 1999;126(18):4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- Smyth DR. Evolution and genetic control of the floral ground plan. New Phytol. 2018;220(1):70–86. doi: 10.1111/nph.15282. [DOI] [PubMed] [Google Scholar]
- Sohlberg JJ, Myrenás M, Kuusk S, Lagerkrantz U, Kowalczyk M, Sandberg G, Sundberg E. STY1 Regulates auxin homeostasis and affect apical-basal patterning in the Arabidopsis gynoecium. Plant J. 2006;47(1):112–123. doi: 10.1111/j.1365.313X.2006.02775.x. [DOI] [PubMed] [Google Scholar]
- Stevens MA, Rick CM. Genetics and breeding. In: Atherton JG, Rudich J. editors. The Tomato Crop. London: Chapman Hall; 1986. p. 35–109 [Google Scholar]
- Stubbe H. Genetik und Zytologie von Antirrhinum L Sect. Antirrhinum. Jena: VEB Gustav Fischer Verlag; 1966. [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JDJ, Dean C, Ma H, Martienssen R. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 1995;9(5-6):1797–1810. doi: 10.1101/gad.9.14.1797. [DOI] [PubMed] [Google Scholar]
- Thomson B, Wellmer F. Molecular regulation of flower development. In: Grossniklaus U. editor. Plant Development and Evolution, Current Topics in Developmental Biology. Vol. 131. Cambridge MA: Academic Press; 2019. p. 185–210. doi: 10.1016/bs.ctdb.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blázquez MA, Borevitz JO, Christensen SA, Fankhauser C, Férrandiz C, Kardailsky I, Malancharuvil EJ, Neff MN, et al. Activation tagging in Arabidopsis. Plant Physiol. 2000;122(4):1003–1014. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. LEAFY Controls floral meristem identity in Arabidopsis. Cell. 1992;69(5):843–859. doi: 10.1016/0092-8674(92)90295-N. [DOI] [PubMed] [Google Scholar]
- Weigel D, Meyerowitz EM. Activation of floral homeotic genes in Arabidopsis. Science. 1993;261(5129):1723–1726. doi: 10.1126/science.261.5129.1723. [DOI] [PubMed] [Google Scholar]
- Wellmer F, Alves-Ferreira M, Dubois A, Riechmann JL, Meyerowitz EM. Genome-wide analysis of gene expression during early Arabidopsis flower development. PLoS Genet. 2006;2(7):e117. doi: 10.1371/journal.pgen.0020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M, Haughn GW. UNUSUAL FLORAL ORGANS controls meristem identity and organ primordia fate in Arabidopsis. Plant Cell. 1995;7(9):1485–1499. doi: 10.1105/tpc.7.9.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby AC, Nimchuk ZL. WOX Going on: CLE peptides in plant development. Curr Opin Plant Biol. 2021;63:102056. doi: 10.1016/j.pbi.2021.102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WB. Bacteriophage T4 as a model for the assembly of subcellular substructure. Q Rev Biol. 1980;55(4):353–367. doi: 10.1086/411980. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano H-Y. The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell. 2004;16(2):500–509. doi: 10.1105/tpc.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Malzahn AA, Sretenovic S, Qi Y. The emerging and uncultivated potential of CRISPR technology in plant science. Nat Plants. 2019;5(8):788–794. doi: 10.1038/s41477-019-0461-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Tan F-Q, Chung C-H, Slavkovic F, Devani RS, Troadec C, Marcel F, Morin H, Camps C, Gomez Roldan MV, et al. The control of carpel determinacy pathway leads to sex determination in cucurbits. Science. 2022;378(6619):543–549. doi: 10.1126/science.add4250. [DOI] [PubMed] [Google Scholar]
- Zúñiga-Mayo VM, Goméz-Felipe A, Herrera-Ulbaldo H, de Folter S. Gynoecium development: networks in Arabidopsis and beyond. J Exp Bot. 2019;70(5):1447–1460. doi: 10.1093/jxb/erz026. [DOI] [PubMed] [Google Scholar]




