Abstract
A stem-loop structure at the 5′ end of the R region of the long terminal repeat (LTR) of the murine leukemia virus SL3 and other type C mammalian retroviruses is important for maximum levels of expression of a reporter gene under the control of the viral LTR. This element, termed the R region stem-loop (RSL), has a small effect on transcriptional initiation and no effect on RNA polymerase processivity. Its major effect is on posttranscriptional processing of LTR-driven transcripts. Here we tested whether the RSL affected the production of RNAs from a full-length SL3 genome. Mutation of the RSL in the 5′ LTR of SL3 reduced the cytoplasmic levels of full-length viral transcripts but not those of spliced, env mRNA transcripts. Thus, the RSL specifically affected the cytoplasmic levels of the unspliced viral RNA. To test further whether the effect was specific for unspliced transcripts, a system was devised in which the expression of a reporter gene under the control of the viral LTR was tested in the presence or absence of an intron. Mutation of the RSL resulted in only about a twofold decline in the level of reporter gene expression when the transcripts contained an intron. However, when the intron was removed, mutation of the RSL reduced expression of the reporter gene about 10- to 60-fold in various cell lines. The secondary structure of the RSL was essential for its activity on the intronless transcript. Thus, the RSL appears to be important for the cytoplasmic accumulation of unspliced viral RNA and unspliced RNA from chimeric transcription units under the control of the viral LTR.
In addition to their role in reverse transcriptase jumping, sequences that are located within the R regions of the long terminal repeats (LTRs) of retroviruses affect the production of viral RNA transcripts. The steps in viral RNA production that are affected vary among different retroviruses. The best studied example is the TAR element of human immunodeficiency virus type 1 (HIV-1) and related lentiviruses. The virally encoded Tat protein binds to the TAR element in nascent transcripts, resulting in the increased processivity of RNA polymerase (4, 5, 13, 14, 38, 41, 42, 47, 59, 62, 64, 69, 70). Transcription of other retroviruses, including human T-cell leukemia virus, bovine leukemia virus, avian reticuloendotheliosis virus, murine leukemia viruses (MuLVs), and mouse mammary tumor virus (MMTV), is also influenced by elements within LTR R regions (15, 19, 31, 36, 37, 40, 50, 53, 56, 58). The steps in the production of viral RNAs that are affected vary among the different retroviruses.
We previously showed that R region sequences of MuLV SL3 affected expression of a reporter gene under the control of a viral LTR (15, 16). R region sequences from Moloney MuLV or feline leukemia virus could substitute for the SL3 element. Thus, the R region element appears to be a general feature of the mammalian type C genus of retroviruses (16). The crucial sequences were mapped to the first 28 nucleotides of the 68-nucleotide R region of SL3. These sequences were predicted to fold into a stem-loop structure of modest stability. The predicted ΔG of this secondary structure was −7.5 kcal/mol. Phylogenetic analysis indicated that the sequences important for the maintenance of this secondary structure were conserved among mammalian type C retroviruses. Mutations that disrupted secondary structure decreased the level of expression of a reporter gene under the control of viral LTR sequences about 10-fold in transient expression assays and 20-fold in fibroblasts stably transformed with the LTR reporter plasmids (16). A compensatory mutant in which the nucleotide sequence of the stem was different but the predicted secondary structure was maintained had most of the activity of the wild-type LTR (16). Thus, the stem-loop structure was important for the maximum activity of the SL3 LTR and presumably for the LTRs of other type C mammalian retroviruses. For simplicity, we term the R region stem-loop element the RSL.
Primer extension analysis indicated that the RSL affected the levels of cytoplasmic RNA. Nuclear run-on assays indicated that deletion of the RSL had a small effect on transcriptional initiation and no effect on RNA polymerase processivity (15, 16). Thus, the main effect of the RSL was on one or more steps that occurred after the template was transcribed by RNA polymerase. This implied that the main function of the RSL was in RNA processing.
Mutations of the RSL in the full-length MuLV genome.
The studies here were undertaken to ask whether the RSL was important for transcription of the full-length viral genome. Previous studies of the effects of the SL3 R region were performed using plasmids that contained a reporter gene under the control of the viral LTR (15, 16). To test the importance of the RSL in the full-length viral genome, a deletion of the RSL was made in the 5′ LTR of a plasmid clone of the full-length genome (Fig. 1A). The deletion encompassed 28 nucleotides and removed all but the first three nucleotides of the stem-loop structure (Fig. 2A). This deletion was identical to that used in earlier studies with reporter plasmids (15, 16). The experimental design was to transfect the clones of the wild-type and mutated viruses into cultured cells and examine the viral transcripts present in cytoplasmic RNA 48 h later. However, since only the R region in the 5′ LTR was mutated, the intact R region in the 3′ LTR would be capable of reverting the mutation during a single round of viral replication (44). Any jump by reverse transcriptase (RT) during minus-strand synthesis that occurred prior to reaching the deletion in the 5′ R region of the RNA template would result in the formation of reversions in the progeny proviruses. Since we were concerned that this would happen within 48 h in a substantial number of genomes, we felt that it was essential to block the ability of the virus to undergo any replication. One means to accomplish this was to use human cells that are nonpermissive for ecotropic SL3. A second means was to introduce a frameshift mutation by filling in the cohesive ends of a unique XhoI site in the middle of the RT-encoding portion of the pol gene of the full-length viral clones that contained the intact and mutated R regions (Fig. 1A). The latter approach was used for the experiment in mouse cells. The constructs were transfected into human K562 cells, as previously described (15, 16). This line was chosen because earlier work showed that the RSL had a large effect here (15, 16). Dishes (100-mm diameter) containing 106 cells were transfected with 10 μg of plasmid DNA by the DEAE-dextran method as previously described (15, 16). Cells from three plates were pooled and collected 2 days after transfection. Duplicates were performed with parallel sets of three plates each. The cells were lysed with 0.5% Nonidet P-40. Nuclei and microsomes were pelleted by centrifugation at 13,000 × g for 10 min. Supernatants were phenol-chloroform extracted, and RNA was collected by ethanol precipitation. Cytoplasmic RNA (10 μg) were electrophoresed in 1% agarose gels. Blotting and hybridization were performed as described previously (47). The viral probe was a 630-bp BglII fragment from the SU portion of the env gene of SL3. The blots were rehybridized to a mouse β-actin probe (Ambion) to control for the relative amounts of RNA loaded in the different lanes. Hybridization was visualized and quantified by PhosphorImager analysis. Two bands were visible on the Northern blot, corresponding to the full-length transcript and the spliced env mRNA. The results observed in K562 cells are shown in Fig. 1B. Similar results were observed in NIH 3T3 cells (8, 68).
FIG. 1.
(A) Plasmid clone of a full-length SL3 genome. The clone contains a wild-type 3′ LTR and either a wild-type 5′ LTR or the RSL-deleted 5′ LTR. For experiments in mouse cells that were permissive for viral replication, a frameshift mutation was introduced into the unique XhoI site in the portion of pol that encodes RT. (B) Northern blot analysis of transfected K562 cells with viral RNAs transcribed from the full-length viral templates that contained either a wild-type 5′ LTR or the RSL-deleted 5′ LTR. Control, mock-transfected cells. SL3, an intact 5′ LTR. SΔR, a 5′ LTR with a deletion of the RSL. 1 and 2, duplicate transfection of the SL3 or SΔR templates. The slower migrating bands are the unspliced full-length transcripts. The faster migrating bands are the spliced env mRNAs. ΔR shows the position of the RSL deletion relative to wild-type (WT) SL3. Stop indicates a stop codon due to the frameshift mutation introduced at the XhoI site.
FIG. 2.
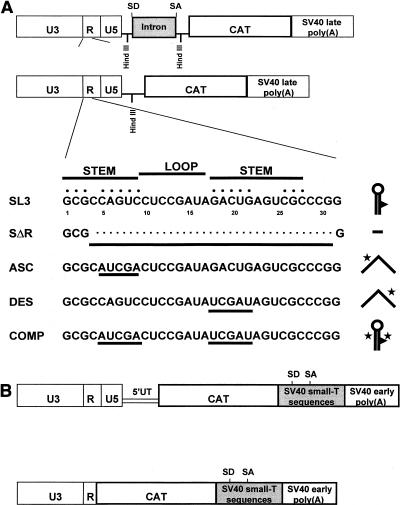
(A) Plasmids used to test the effects of the RSL on spliced and unspliced transcripts. The top diagram shows the pCAT3-Basic plasmid with the SL3 LTR inserted upstream of the intron. SD, splice donor; SA, splice acceptor. The second diagram shows the same plasmid after the intron was deleted by excision of a HindIII fragment. The bottom part of the figure shows the sequences of the first 32 nucleotides of the R region of SL3 and the four mutants that were tested. Names at the left of each line identify the mutations: SΔR, SL3 RSL deletion; ASC, ascending side of the stem; DES, descending side of the stem; COMP, compensatory. The nucleotides that comprise the stem and the loop are indicated. The large dots above the SL3 sequence indicate the nucleotides that are involved in base pairing (15, 16). The underlined, small dots in the SΔR sequence indicate the nucleotides that were deleted. The underlined nucleotides in the ASC, DES, and COMP mutants indicate the positions of the substitutions. The symbols to the right of each sequence represent the structures into which the nucleotides are predicted to fold at the 5′ ends of the transcribed RNAs. Stars indicate the positions of the substitutions of five consecutive nucleotides. (B) Structures of the SL3 CAT plasmids used in earlier studies (15, 16). The top diagram shows the structure of a plasmid that contained U3, R, U5, and 258 nucleotides of 5′ untranslated (5′UT) sequences downstream of the LTR. The bottom diagram shows the structure of a plasmid that contained U3 and R sequences. These plasmids were derived from the CAT plasmids of Gorman et al. (30).
Interestingly, the Northern blot analysis showed that the deletion of the RSL only affected the level of the full-length transcript (Fig. 1B). When corrected for loading by the use of a β-actin probe, the level of the full-length transcript from virus with the RSL deletion was over fivefold lower than that from wild-type SL3. The levels of the spliced envelope transcript were similar in cells transfected with the wild-type construct and in those transfected with RSL-deleted viral constructs. These results showed that the SL3 RSL did function in the context of the full-length viral genome. However, it only affected the cytoplasmic levels of the unspliced full-length transcript.
Use of a CAT reporter plasmid system to test the effect of mutations in the SL3 RSL on spliced versus unspliced transcripts.
We wished to confirm the observation that the SL3 RSL specifically affected the cytoplasmic levels of unspliced mRNAs by using a second experimental approach. Therefore, a reporter gene system was devised to assess the effect of the R region on transcripts that differed by the presence or absence of an intron. This system provided a simple way to compare the effects of a set of key mutations within the RSL on spliced and unspliced RNA. Specifically, we wanted to test whether mutations that disrupted the secondary structure of the RSL specifically affected unspliced transcripts. Chloramphenicol acetyltransferase (CAT) reporter constructs were engineered that were isogenic except for the presence of an intron (Fig. 2A). Transcripts containing the intron should be spliced, whereas transcripts from the template with the deleted intron should not be spliced.
The pCAT3-Basic vector was utilized for this purpose. This plasmid contains a cloning site for promoter sequences and the CAT gene (Fig. 2A). The viral LTR was inserted at the cloning site (Fig. 2A). Between the LTR and the CAT gene, the plasmid contains an intron (Fig. 2A). The intron was composed of the donor site from the first intron of the human β-globin gene and the branch and acceptor sites from the intron of an immunoglobulin gene. The sequences of the donor, acceptor, and branchpoint sites were optimized to match the consensus sequences (9, 63). The intron is flanked by HindIII sites (Fig. 3) and thus was easily removed to generate intronless plasmids. The vector also contained a simian virus 40 (SV40) late polyadenylation sequence 3′ to the CAT reporter gene (Fig. 2A).
FIG. 3.
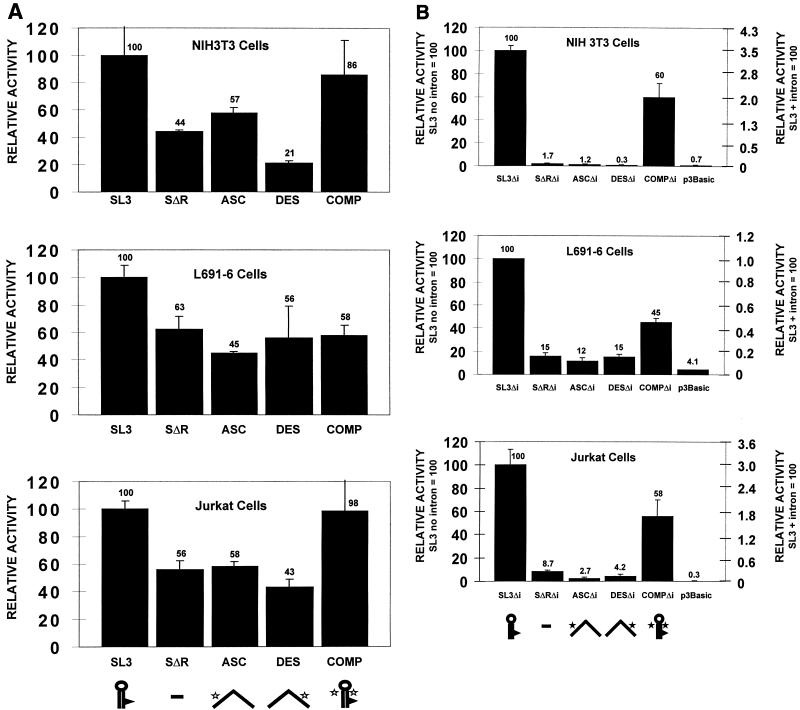
Relative levels of expression of the CAT reporter gene from transfected templates with or without introns in three different cell lines: NIH 3T3 cells, L691 cells, and Jurkat cells. (A) Cellular extracts (20 μg) were tested for constructs containing the intron. The mean values for the actual number readings on the PhosphorImager for the SL3 construct with the intron for different cell lines were 5.06 × 106 (NIH 3T3), 4.20 × 106 (L691), and 2.52 × 106 (Jurkat). (B) Cellular extracts (200 μg) were tested for the intronless (Δi) constructs. The mean values for the actual number readings on the PhosphorImager for the intronless SL3 construct for different cell lines were 1.92 × 106 (NIH 3T3), 3.72 × 105 (L691), and 3.85 × 105 (Jurkat). The y axis in panel A and the y axis on the right in panel B indicate the relative activities of the LTRs normalized to that of the intact SL3 LTR in the plasmid that contained an intron. In panel B, the y axis on the left indicates the relative activities of the LTRs normalized to that of the intact SL3 LTR in the plasmid that lacked an intron. CAT activities of the various mutants are shown compared to that of SL3, which was set at 100%. Error bars indicate 1 standard deviation from the mean. Names and symbols for each structure are as explained in the legend to Fig. 2A.
The wild-type SL3 LTR and LTRs with RSL mutations were introduced into plasmids that contained or lacked the intron (Fig. 2A). The mutations that were tested are shown in Fig. 2A. One mutation, termed SΔR, was the same deletion of 28 nucleotides (positions +4 to +31, inclusive) of the SL3 RSL that was tested in the full-length viral constructs. To test whether the secondary structure of the RSL was important for the effect on the spliced cytoplasmic RNA, mutations that disrupted the stem-loop structure were also tested. These were a mutation of five nucleotides in the upper portion of the ascending side of the stem (ASC) and a mutation in the corresponding five nucleotides in the descending side of the stem (DES). These mutations disrupted most of the base pairs of the original stem-loop structure. The last mutation (COMP) included both the ASC and the DES mutations. These were compensatory mutations that altered the nucleotide sequence of the stem but restored the predicted secondary structure.
Effects of the mutations in the SL3 RSL on spliced and unspliced transcripts.
To test if the mutations of the SL3 RSL had different effects on spliced versus unspliced transcripts, the CAT reporter constructs, with or without the intron, were transiently transfected into cells and CAT activity was determined. Three different cell lines were tested. The first was NIH 3T3, a fibroblast cell line where the original effect of RSL mutations was observed. This line was chosen because it was previously observed that the effect of the RSL mutations was greater in fibroblasts than in any other type of cell tested (15). Since SL3 virus causes T-cell lymphoma, two T-cell lymphoma lines, L691 and Jurkat, were also tested. NIH 3T3 cells were transfected with Lipofectamine Plus (Gibco-BRL). L691 and Jurkat cells were transfected with DEAE-dextran (15, 16). Transfections were performed with 5 μg of LTR-CAT plasmid DNA plus 1 μg of Rous sarcoma virus LTR-luciferase plasmid as a control for transfection efficiency. Each transfection was performed in duplicate on at least two separate occasions. BODIPY FL chloramphenicol (Molecular Probes)-labeled products were resolved by thin-layer chromatography and then visualized and quantified by PhosphorImager (Molecular Dynamics) analysis. CAT activity for each sample was normalized to the luciferase activity of a cotransfected control plasmid to correct for variations in transfection efficiency. Then the means were calculated for the multiple trials of a particular plasmid in each specific cell line. Activities were expressed relative to the wild-type LTR.
When the constructs containing an intron were tested, the RSL was found to have relatively small effects in all three cell lines (Fig. 3A). The LTR with the deletion of the RSL exhibited 44 to 63% of the activity of the wild-type LTR. Mutations in either the ascending or the descending side of the stem resulted in activity similar to that of the LTR with a deletion of the RSL, although in the NIH 3T3 cells, the LTR with the descending stem mutation was only about half as active as SΔR. The LTR with the compensatory mutation in the RSL exhibited activity that ranged between those exhibited by the wild-type and RSL-deleted LTRs. Thus, the RSL had little effect on reporter gene-containing transcripts that also contained an efficiently excised intron.
In contrast, when the intron was not present, the effects of the RSL were quite pronounced (Fig. 3B). Transcripts that did not contain the intron were expected not to be spliced. When the intron was deleted in the construct with the wild-type SL3 LTR, activity was reduced about 30-fold compared to the activity of the intron-containing construct in the different cell lines (Fig. 3). This was consistent with the idea that splicing is generally important for the efficient expression of genes (10–12, 33, 34, 43).
When mutations of the RSL were tested in the intronless template, large effects were observed (Fig. 3B). In NIH 3T3 cells, the LTR with the deletion of the RSL was 60-fold less active than the wild-type LTR. In the other cell lines, the effect was smaller but still very significant. In L691 and Jurkat cells, the LTR with the deletion of the RSL had 15 and 9% as much activity, respectively, as the LTR with an intact RSL. Thus, the RSL had a large effect on the expression of the reporter gene from the unspliced transcript.
We tested whether the effects of the RSL on the intronless transcripts were dependent on the secondary structure of the RSL element. In all cell lines tested, the LTRs with the ASC and DES mutations had effects comparable to the SΔR LTR (Fig. 3B). In NIH 3T3 cells, the activity of the LTR with the disrupted secondary structure was reduced to a level comparable to that of the pCAT3-Basic vector with no promoter driving expression of the CAT gene. This indicated that the RSL was essential for expression of the reporter gene in the unspliced transcript in this cell line.
Restoration of the secondary structure with the COMP mutation restored activity to 45 to 60% of the level of the wild-type LTR in all three cell lines (Fig. 3B). Thus, the secondary structure of the RSL was important for the effect of the RSL on the unspliced transcript.
In summary, the RSL had little effect on spliced transcripts. When splicing was prevented by removal of the intron, the level of expression of the reporter gene was greatly reduced. Thus, expression of the reporter gene in unspliced transcripts was highly dependent on the RSL. In particular, it required the secondary structure formed by the RSL at the 5′ end of the transcript.
Implications for the mechanisms of action of the RSL.
Our experiments provided two lines of evidence that the RSL specifically affected unspliced transcripts. The Northern blot analysis of transcripts of the SL3 genome showed that the RSL was required for cytoplasmic accumulation of the unspliced full-length transcript. The levels of the spliced transcript remained unchanged. The specificity of the RSL for unspliced transcripts was demonstrated further by analyzing its effects on transcripts that were engineered to be either efficiently spliced or not spliced. The RSL had large effects only on the unspliced transcripts from an intronless template. Little to no effect was seen on the efficiently spliced transcripts. Therefore, we conclude that the SL3 RSL plays a role in the cytoplasmic accumulation of unspliced viral transcripts.
An obligatory aspect of the life cycles of all retroviruses is that a fraction of the viral full-length transcripts must be transported to the cytoplasm without being spliced. The unspliced RNA functions both as the mRNA for the gag, pro, and pol genes and as the genome that is packaged into viral particles. In simple retroviruses, a single splice donor is positioned near the 5′ end of the primary transcript and splicing involves joining to acceptor sites positioned downstream in the RNA. One mechanism that results in only a fraction of the primary transcripts being spliced is that the splicing signals of retroviruses are suboptimal (11, 27, 39, 72). Additional sequences in the genomes of simple retroviruses besides the donor, acceptor, and branchpoint elements also affect the levels of spliced and unspliced transcripts (2, 3, 29, 48, 49, 52, 54). Our Northern blot analysis of transcripts from the full-length viral genome showed that the SL3 RSL can increase the fraction of cytoplasmic, viral RNA that is unspliced. Since the equivalent sequences from other type C mammalian retroviruses can substitute for the SL3 RSL and form similar stem-loop structures (15, 16), we hypothesize that the effect of the RSL on unspliced RNA may be a general feature of this genus of retroviruses.
Complex retroviruses control the cytoplasmic accumulation of unspliced and partially spliced RNAs by encoding regulatory proteins such as Rev for HIV-1 and Rex for human T-cell leukemia virus (32, 33, 46). These proteins interact with cis-acting elements in the transcripts and mediate the export of the partially spliced and unspliced RNAs from the nucleus. Due to the presence of suboptimal splicing signals, cellular splicing factors are thought to bind to inefficiently spliced RNAs and prevent them from leaving the nucleus (11, 39, 43). Binding of HIV-1 Rev promotes the transport of the unspliced RNAs out of the nucleus (6, 11, 18, 23–26, 45, 46, 65, 66, 71). In contrast, transcripts containing optimal splicing signals are efficiently exported to the cytoplasm (11, 43). These observations are consistent with the idea that splicing and cytoplasmic transport may be coupled.
Simple retroviruses do not encode proteins that promote nuclear export. However, some have been shown to contain cis regulatory elements that allow the transport of unspliced RNAs to the cytoplasm. Well-studied examples of such elements exist between the env gene and 3′ LTR in Mason-Pfizer monkey virus and simian retrovirus type 1 (7, 22, 33, 67, 74). This element, known as the constitutive transport element, is capable of substituting for the REV response element. Elements with similar functions were identified in hepatitis B virus, woodchuck hepatitis virus, avian leukosis virus, and Rous sarcoma virus (20, 21, 35, 51, 52). Presumably, cellular factors recognize these elements in the viral RNA and mediate nuclear export of the RNA (55).
There are three possible mechanisms by which the RSL of MuLVs might function to increase the cytoplasmic levels of unspliced transcripts. It could play a direct role in the transport of the unspliced transcripts to the cytoplasm in a manner analogous to the constitutive transport element of Mason-Pfizer monkey virus and the RSV element. If the SL3 RSL is indeed a cytoplasmic transport element, then it is located at a different position in the viral genome than the elements identified in other viruses. These are generally located in the 3′ portion of the viral transcripts. It remains to be investigated whether SL3 also contains a 3′ element that affects cytoplasmic levels of unspliced RNAs.
A second possible model is that the secondary structure of the SL3 RSL may interfere with a 5′ exoribonuclease. Stabilization against degradation in the nucleus would then allow the transcripts more chances to be exported to the cytoplasm. There are 5′ exoribonucleases known to exist in Saccharomyces cerevisiae (57, 61). For such an enzyme to account for the effects observed here, it must preferentially degrade unspliced transcripts. This might occur if the unspliced transcripts are detained in the nucleus and the exoribonuclease is specifically localized in the nucleus. The stem-loop structure at the 5′ end of SL3 LTR-driven transcripts might play a role in blocking the activity of such a 5′ exoribonuclease. However, we postulate that it is unlikely that the SL3 RSL functions simply as an RNA structure at the 5′ end of viral transcripts that blocks the progression of a 5′ exoribonuclease. The approximately 600-nucleotide 5′ untranslated sequence of MuLVs is predicted to fold into additional stem-loop structures (1) that the exoribonuclease would also have to overcome. The SL3 RSL has a predicted stability of only −7.5 kcal/mol (15). More stable stem-loop structures could not functionally substitute for the SL3 RSL (17). We hypothesize that it is more likely that the stem-loop structure at the 5′ end of SL3 transcripts is recognized by a cellular factor that acts to block the activity of a 5′ exoribonuclease and/or promote nuclear export of viral RNA.
A third possible mechanism by which the RSL might function is by somehow impeding the splicing process. Perhaps a cellular factor recognizes the RSL and by some mechanism directly interferes with the activity of the cellular splicing apparatus.
In our initial reports, deletion of the SL3 RSL resulted in about a 10-fold decline in the expression of a CAT reporter gene in transient expression assays in NIH 3T3 fibroblasts (15, 16). In the same cells, deletion of the RSL resulted in a 60-fold decline in expression of the CAT reporter gene transcribed from the intronless construct (Fig. 3B). The RSL had only a twofold effect on the reporter gene transcribed from the template that contained an efficiently spliced intron (Fig. 3A). Since the latter two templates were isogenic except for the intron, the intron must account for the difference in activity of the RSL in the two constructs. The intermediate level of activity of the RSL in the constructs used in our earlier studies (15, 16) might also be due to the effects of splicing signals. These constructs contained the SV40 small-t antigen splice donor and acceptor (Fig. 2B). SV40 early RNA is spliced with one of two alternative donors and a common acceptor (28, 73). We hypothesize that this transcript is spliced with an efficiency that is intermediate between those of the efficiently spliced transcripts from the intron-containing pCAT3-Basic plasmid and the unspliced transcripts from the intronless plasmid. The RSL would then function on these transcripts to increase their cytoplasmic levels. Similarly, the fivefold effect of the SL3 RSL on the full-length viral transcript presumably reflected the suboptimal splicing signals known to be present in retroviral transcripts.
Whatever the mechanism by which the SL3 RSL functions, it is clear that the secondary structure of the stem is crucial for the effect. Mutations that disrupted base pairing of the RSL reduced the expression of the reporter gene from the intronless template to a level comparable to that for the deletion of the entire RSL (Fig. 3B). Restoration of base pairing by the compensatory mutation restored most of the activity of the element. We hypothesize that this stem structure is recognized by a cellular factor that mediates the nuclear export of transcripts, protects the transcripts from a nuclear 5′ exoribonuclease, and/or interferes with splicing.
Acknowledgments
This work was supported by NIH grants CA44822 and CA57337 to J.L. A.M.T. was supported by NIH training grant GM7288. Core facilities for oligonucleotide synthesis, PhosphorImager analysis, and DNA sequencing were supported by NIH Cancer Center grant CA13330 and NIH Center for AIDS Research grant AI27741 to the Albert Einstein College of Medicine.
REFERENCES
- 1.Aiyar A, Cobrinik D, Ge Z, Kung H-J, Leis J. Interaction between retroviral U5 RNA and the TψC loop of the tRNATrp primer is required for efficient initiation of reverse transcription. J Virol. 1992;66:2464–2472. doi: 10.1128/jvi.66.4.2464-2472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amendt B A, Simpson S B, Stoltzfus C M. Inhibition of RNA splicing at the Rous sarcoma virus src 3′ splice site is mediated by an interaction between a negative cis element and a chicken embryo fibroblast nuclear factor. J Virol. 1995;69:5068–5076. doi: 10.1128/jvi.69.8.5068-5076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrigo S, Beemon K L. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988;8:4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkhout B. Structural features in TAR RNA of human and simian immunodeficiency viruses: a phylogenetic analysis. Nucleic Acids Res. 1992;20:27–31. doi: 10.1093/nar/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkhout B, Silverman R H, Jeang K. Tat transactivates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 6.Bogerd H P, Fridell R A, Madore S, Cullen B R. Identification of a novel cellular cofactor for the Rev/Rex class of retroviral regulatory proteins. Cell. 1995;82:485–494. doi: 10.1016/0092-8674(95)90437-9. [DOI] [PubMed] [Google Scholar]
- 7.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K T, Rekosh D, Hammarskjold M L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinster R L, Allen J D, Behringer R R, Gelinas R E, Palmiter R D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brondyk B. pCI and pSI mammalian expression vectors. Promega Notes. 1994;49:7–11. [Google Scholar]
- 10.Buchman A R, Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988;8:4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang D D, Sharp P A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 12.Choi T, Huang M, Gorman C, Jaenisch R. A generic intron increases expression in transgenic mice. Mol Cell Biol. 1991;11:3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen B R. The HIV-1 tat protein: an RNA sequence-specific processivity factor? Cell. 1990;63:655–657. doi: 10.1016/0092-8674(90)90129-3. [DOI] [PubMed] [Google Scholar]
- 15.Cupelli L, Lenz J. Transcriptional initiation and postinitiation effects of murine leukemia virus long terminal repeat R-region sequences. J Virol. 1991;65:6961–6968. doi: 10.1128/jvi.65.12.6961-6968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cupelli L, Okenquist S A, Trubetskoy A, Lenz J. The secondary structure of the R region of a murine leukemia virus is important for stimulation of long terminal repeat-driven gene expression. J Virol. 1998;72:7807–7814. doi: 10.1128/jvi.72.10.7807-7814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cupelli, L. A., and J. Lenz. Unpublished results.
- 18.Daly T J, Cook K S, Gray G S, Maione T E, Rusche J R. Specific binding of HIV-1 recombinant Rev protein to the Rev-responsive element in vitro. Nature. 1989;342:816–819. doi: 10.1038/342816a0. [DOI] [PubMed] [Google Scholar]
- 19.Derse D, Casey J. Two elements in the bovine leukemia virus long terminal repeat that regulate gene expression. Science. 1986;231:1437–1440. doi: 10.1126/science.3006241. [DOI] [PubMed] [Google Scholar]
- 20.Donello J E, Beeche A A, Smith G J, Lucero G R, Hope T J. The hepatitis B virus posttranscriptional regulatory element is composed of two subelements. J Virol. 1996;70:4345–4351. doi: 10.1128/jvi.70.7.4345-4351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donello J E, Loeb J E, Hope T J. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst R K, Bray M, Rekosh D, Hammarskjold M L. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol Cell Biol. 1997;17:135–144. doi: 10.1128/mcb.17.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 24.Fridell R A, Bogerd H P, Cullen B R. Nuclear export of late HIV-1 mRNAs occurs via a cellular protein export pathway. Proc Natl Acad Sci USA. 1996;93:4421–4424. doi: 10.1073/pnas.93.9.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz C C, Green M R. HIV Rev uses a conserved cellular protein export pathway for the nucleocytoplasmic transport of viral RNAs. Curr Biol. 1996;6:848–854. doi: 10.1016/s0960-9822(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 26.Fritz C C, Zapp M L, Green M R. A human nucleoporin-like protein that specifically interacts with HIV Rev. Nature. 1995;376:530–533. doi: 10.1038/376530a0. [DOI] [PubMed] [Google Scholar]
- 27.Fu X D, Katz R A, Skalka A M, Maniatis T. The role of branchpoint and 3′-exon sequences in the control of balanced splicing of avian retrovirus RNA. Genes Dev. 1991;5:211–220. doi: 10.1101/gad.5.2.211. [DOI] [PubMed] [Google Scholar]
- 28.Fu X Y, Colgan J D, Manley J L. Multiple cis-acting sequence elements are required for efficient splicing of simian virus 40 small-t antigen pre-mRNA. Mol Cell Biol. 1988;8:3582–3590. doi: 10.1128/mcb.8.9.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gontarek R R, McNally M T, Beemon K L. Mutation of an RSV intronic element abolishes both U11/U12 snRNP binding and negative regulation of splicing. Genes Dev. 1993;7:1926–1936. doi: 10.1101/gad.7.10.1926. [DOI] [PubMed] [Google Scholar]
- 30.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauber J, Cullen B R. Mutational analysis of the trans-activation-responsive region of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1988;62:673–679. doi: 10.1128/jvi.62.3.673-679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hidaka M, Inoue J, Yoshida M, Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988;7:519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hope T J. Viral RNA export. Chem Biol. 1997;4:335–344. doi: 10.1016/s1074-5521(97)90124-1. [DOI] [PubMed] [Google Scholar]
- 34.Huang M, Gorman C. Intervening sequences increase efficiency of RNA 3′ processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990;18:937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Z M, Yen T S. Hepatitis B virus RNA element that facilitates accumulation of surface gene transcripts in the cytoplasm. J Virol. 1994;68:3193–3199. doi: 10.1128/jvi.68.5.3193-3199.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue J, Yoshida M, Seiki M. Transcriptional (p40x) and posttranscriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci USA. 1987;84:3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones K A, Luciw P A, Duchange N. Structural arrangements of transcription control domains within the 5′-untranslated leader regions of the HIV-1 and HIV-2 promoters. Genes Dev. 1988;2:1101–1114. doi: 10.1101/gad.2.9.1101. [DOI] [PubMed] [Google Scholar]
- 38.Kato H, Sumimoto H, Pognonec P, Chen C H, Rosen C A, Roeder R G. HIV-1 Tat acts as a processivity factor in vitro in conjunction with cellular elongation factors. Genes Dev. 1992;6:655–666. doi: 10.1101/gad.6.4.655. [DOI] [PubMed] [Google Scholar]
- 39.Katz R A, Skalka A M. Control of retroviral RNA splicing through maintenance of suboptimal processing signals. Mol Cell Biol. 1990;10:696–704. doi: 10.1128/mcb.10.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiss-Toth E, Unk I. A downstream regulatory element activates the bovine leukemia virus promoter. Biochem Biophys Res Commun. 1994;202:1553–1561. doi: 10.1006/bbrc.1994.2108. [DOI] [PubMed] [Google Scholar]
- 41.Laspia M F, Rice A P, Mathews M B. HIV-1 tat protein increases transcriptional initiation and stabilizes elongation. Cell. 1989;59:283–292. doi: 10.1016/0092-8674(89)90290-0. [DOI] [PubMed] [Google Scholar]
- 42.Laspia M F, Rice A P, Mathews M B. Synergy between HIV-1 Tat and adenovirus E1A is principally due to stabilization of transcriptional elongation. Genes Dev. 1990;4:2397–2408. doi: 10.1101/gad.4.12b.2397. [DOI] [PubMed] [Google Scholar]
- 43.Legrain P, Rosbash M. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell. 1989;57:573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- 44.Lobel L I, Goff S P. Reverse transcription of retroviral genomes: mutations in the terminal repeat sequences. J Virol. 1985;53:447–455. doi: 10.1128/jvi.53.2.447-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malim M H, Cullen B R. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13:6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malim M H, Hauber J, Le S, Maizel J, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 47.Marciniak R A, Sharp P A. HIV-1 Tat protein promotes formation of more-processive elongation complexes. EMBO J. 1991;10:4189–4196. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNally M T, Beemon K L. Intronic sequences and 3′ splice sites control Rous sarcoma virus RNA splicing. J Virol. 1992;66:6–11. doi: 10.1128/jvi.66.1.6-11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller J T, Stoltzfus C M. Two distant upstream regions containing cis-acting signals regulating splicing facilitate 3′-end processing of avian sarcoma virus RNA. J Virol. 1992;66:4242–4251. doi: 10.1128/jvi.66.7.4242-4251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montagne J, Jalinot P. Characterization of a transcriptional attenuator within the 5′ R region of the human T cell leukemia virus type 1. AIDS Res Hum Retroviruses. 1995;11:1123–1129. doi: 10.1089/aid.1995.11.1123. [DOI] [PubMed] [Google Scholar]
- 51.Ogert R A, Beemon K L. Mutational analysis of the Rous sarcoma virus DR posttranscriptional control element. J Virol. 1998;72:3407–3411. doi: 10.1128/jvi.72.4.3407-3411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogert R A, Lee L H, Beemon K L. Avian retroviral RNA element promotes unspliced RNA accumulation in the cytoplasm. J Virol. 1996;70:3834–3843. doi: 10.1128/jvi.70.6.3834-3843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtani K, Nakamura M, Saito S, Noda T, Ito Y, Sugamura K, Hinuma Y. Identification of two distinct elements in the long terminal repeat of HTLV-I responsible for maximum gene expression. EMBO J. 1987;6:389–395. doi: 10.1002/j.1460-2075.1987.tb04767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oshima M, Odawara T, Hanaki K, Igarashi H, Yoshikura H. cis elements required for high-level expression of unspliced gag-containing message in Moloney murine leukemia virus. J Virol. 1998;72:6414–6420. doi: 10.1128/jvi.72.8.6414-6420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pasquinelli A E, Ernst R K, Lund E, Grimm C, Zapp M L, Rekosh D, Hammarskjold M L, Dahlberg J E. The constitutive transport element (CTE) of Mason-Pfizer monkey virus (MPMV) accesses a cellular mRNA export pathway. EMBO J. 1997;16:7500–7510. doi: 10.1093/emboj/16.24.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierce J, Fee B E, Toohey M G, Peterson D O. A mouse mammary tumor virus promoter element near the transcription initiation site. J Virol. 1993;67:415–424. doi: 10.1128/jvi.67.1.415-424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole T L, Stevens A. Structural modifications of RNA influence the 5′ exoribonucleolytic hydrolysis by XRN1 and HKE1 of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1997;235:799–805. doi: 10.1006/bbrc.1997.6877. [DOI] [PubMed] [Google Scholar]
- 58.Ridgway A A G, Kung H-J, Fujita D J. Transient expression analysis of the reticuloendotheliosis virus long terminal repeat element. Nucleic Acids Res. 1989;17:3199–3215. doi: 10.1093/nar/17.8.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosen C A, Sodroski J, Haseltine W A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985;41:813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- 60.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 61.Schwer B, Mao X, Shuman S. Accelerated mRNA decay in conditional mutants of yeast mRNA capping enzyme. Nucleic Acids Res. 1998;26:2050–2057. doi: 10.1093/nar/26.9.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selby M J, Peterlin B M. Trans-activation by HIV-1 Tat via a heterologous RNA binding protein. Cell. 1990;62:769–776. doi: 10.1016/0092-8674(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 63.Senapathy P, Shapiro M B, Harris N L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and application to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- 64.Southgate C, Zapp M L, Green M R. Activation of transcription by HIV-1 Tat protein tethered to nascent RNA through another protein. Nature. 1990;345:640–642. doi: 10.1038/345640a0. [DOI] [PubMed] [Google Scholar]
- 65.Stutz F, Izaurralde E, Mattaj I W, Rosbash M. A role for nucleoporin FG repeat domains in export of human immunodeficiency virus type 1 Rev protein and RNA from the nucleus. Mol Cell Biol. 1996;16:7144–7150. doi: 10.1128/mcb.16.12.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stutz F, Neville M, Rosbash M. Identification of a novel nuclear pore-associated protein as a functional target of the HIV-1 Rev protein in yeast. Cell. 1995;82:495–506. doi: 10.1016/0092-8674(95)90438-7. [DOI] [PubMed] [Google Scholar]
- 67.Tabernero C, Zolotukhin A S, Valentin A, Pavlakis G N, Felber B K. The posttranscriptional control element of the simian retrovirus type 1 forms an extensive RNA secondary structure necessary for its function. J Virol. 1996;70:5998–6011. doi: 10.1128/jvi.70.9.5998-6011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trubetskoy, A., S. Okenquist, and J. Lenz. Unpublished results.
- 69.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Gold M O, Tang D N, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinase and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zapp M L, Green M R. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature. 1989;342:816–819. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]
- 72.Zhang L, Stoltzfus C M. A suboptimal src 3′ splice site is necessary for efficient replication of Rous sarcoma virus. Virology. 1998;206:1099–1107. doi: 10.1006/viro.1995.1033. [DOI] [PubMed] [Google Scholar]
- 73.Zhuang Y, Leung H, Weiner A M. The natural 5′ splice site of simian virus 40 large T antigen can be improved by increasing the base complementarity to U1 RNA. Mol Cell Biol. 1987;7:3018–3020. doi: 10.1128/mcb.7.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zolotukhin A S, Valentin A, Pavlakis G N, Felber B K. Continuous propagation of RRE(−) and Rev(−)RRE(−) human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J Virol. 1994;68:7944–7952. doi: 10.1128/jvi.68.12.7944-7952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]