Abstract
Purpose
The purpose of this study was to quantify retinal hydration (RH) levels with optical coherence tomography (OCT) and determine the extent of cellular damage resulting from intraretinal fluid alterations.
Methods
We took 6.0 mm sections of the human sensory retina that were excised from 18 fresh (<24 hours) donor eyes. They were either exposed to various osmotic stresses between 90 and 305 mOsm or dehydrated under a laminar flow hood. Change in tissue weight was used to calculate the retinal water content (RWC). Image analyses were conducted on OCT between 0 and 180 minutes to assess retinal thickness (RT) and “optically empty areas” (OEAs) representing intraretinal fluid. Correlations were sought among RWC, OEA, RWC, and RT. The effect of RH on retinal cell viability (RCV) was assessed with the Live-Dead Assay.
Results
RH demonstrated a stronger correlation with the OEA than plain RT measurements (r = 0.99, P < 0.001). RH-RCV interaction fits well to a bell-shaped curve. A significant proportion of retinal cells (>80%) remained viable despite the change in RH ranging between 0.87 and 1.42 times. This “safe zone” was found to be associated with a 22% increase in OEA (r = 0.99, P < 0.01).
Conclusions
OCT has been demonstrated as a valuable tool for assessing RH and can be used for intraretinal fluid content analysis. RH is a better indicator of RCV compared with RT. Computing RH may improve the determination of functional outcome of intravitreal pharmacotherapeutics used for diabetic macular edema and exudative age-related macular degeneration.
Translational Relevance
We link basic research and clinical care by assessing retinal hydration's impact on retinal fluid dynamics, macular edema, and cell viability.
Keywords: optical coherence tomography (OCT), retinal hydration, macular edema, morphometry, cell viability
Introduction
Macular edema is characterized by fluid accumulation in the macular region responsible for central vision.1 Macular edema is a significant cause of visual impairment in many macular diseases, including diabetic retinopathy (DR), age-related macular degeneration (AMD),2 uveitis,3 and retinal vein occlusions.1,3,4 Approximately 500,000 Americans are experiencing moderate visual loss due to macular edema that develops as a complication of DR.5
Macular edema decreases vision by scattering the light rays and defocusing the image on the retina. However, in the later stages, hydrostatic pressure from the interstitial fluid may result in retinal cell loss.6 Microperimetry and multifocal electroretinography have demonstrated that retinal function deteriorates in areas with increased intraretinal fluid.7,8
Advancements in ocular imaging techniques, particularly optical coherence tomography (OCT), have facilitated the identification, monitoring, and quantification of macular edema, enabling more accurate diagnosis and personalized treatment strategies.9 Macular edema detection has traditionally relied on detecting the retinal structural changes induced by increased intraretinal fluid, although some new multimodal retinal imaging modalities have emerged.10 Retinal thickness (RT) change is the most common way to express such changes. Accurate macular thickness measurement has been used to follow disease progression and evaluate treatments11 The introduction of OCT has improved our ability to detect RT alterations as small as a few microns.12,13 However, retinal cellular damage occurs long before any change in RT vascular leakage occurs.1
OCT obtains image information from the backscattered or back-reflected photons as an optical method. The scattering depends on the size and shape of the particles inside the tissue, in other words, the composition of the tissue. Reflection within the retina occurs when light enters from the vitreous into the sensory retina due to its lower water content and higher refractive index. However, the reflection of light within the retina will diminish as it travels through pockets of fluid within the tissue. These areas are represented as black on the linear OCT scan. Thus, comparing the total black area within an OCT scan can give an idea about the relative hydration of the sensory retina.14
In this paper, we aimed to test the validity of this hypothesis. For this purpose, we calculated the retinal water content (RWC) and correlated it with the proportion of optically empty areas (OEAs) on the OCT scan, as well as cell viability and its association with RWC.
Materials and Methods
In this study, our primary objective was to evaluate the capacity of OCT to estimate retinal hydration (RH) levels, identify the role of retinal edema in retinal cell damage, and determine OCT features associated with favorable functional outcomes. We used three primary outcome measures to achieve these goals: calculating the tissue weight-to-RWC ratio to quantify RH, utilizing OCT morphometry to assess RH, and implementing the Live-Dead Assay to evaluate retinal cell viability (RCV). This study was conducted when the corresponding author was a faculty member at University of Louisville and it was approved by the University of Louisville's School of Medicine Institutional Review Board and adhered to the ethical guidelines outlined in the Declaration of Helsinki for conducting research.
Determining RWC
Adult human eyes were obtained within 24 hours postmortem (Lions Eye Bank, Louisville, KY). Sensory retinal strips were carefully dissected in a dry environment, ensuring complete removal of the vitreous from the retina. Subsequently, 6.0-millimeter circular sensory retina explants were trephined using a corneal trephine blade (Rumex, Tampa, FL). The weight of the retinal samples was accurately measured using a microbalance (Mettler-Toledo, AB54-S, Switzerland). The samples were subjected to a range of osmotic stresses, varying from 90 to 305 mOsm. Alternatively, they were dehydrated under a laminar flow hood. This experimental approach allowed assessing retinal tissue OCT features under varying hydration conditions. The samples were then let to dry at room temperature in a tissue culture hood under constant airflow conditions. The difference in tissue weight between wet and dry states was utilized to calculate water influx into the retina. Weight measurements were taken at various time points, ranging from 0 to 180 minutes. No significant change in weight was observed after 3 hours. All the measurements were undertaken at room temperature. RWC at a time point (T) was estimated as:
Determining RH With OCT
After calculating the weight difference, dissected retinas underwent evaluation for OCT imaging. OCT images were acquired and morphometrically analyzed to determine changes in RT and the percent of the area covered by OEA, representing, and corresponding to intraretinal fluid's presence.
The same OCT equipment was used throughout this study. This OCT instrument (Stratus OCT 3000; Zeiss, Meditec, Dublin, CA) uses an 820 nm infrared laser beam from a superluminescent diode to scan the retina, generating its cross-sectional view based on the optical path differences between the object path and a reference path. OCT provides a probe beam of low-coherence light on the retina and allowing a 10- to 15-µm axial image resolution. All B-scan retinal images were composed of 512 consecutive A-scans per scan line acquired through the center of the macula in both vertical and horizontal orientations. We performed 6 radial scans of 6 mm long for each tissue sample at equally spaced angular orientations. The OCT examination was performed simultaneously with weight measurements for up to 3 hours. Specimens were transferred to OCT examination in parafilm-sealed petri dishes at 4°C to avoid iatrogenic dehydration.
The borders of the retina were manually determined in these scans. The inner border of the retina was defined as the inner boundary of the inner hyper-reflective layer. Likewise, the outer border of the retina was identified as the superior edge of the outer hyper-reflective layer, representing the retinal pigment epithelium (RPE)-choriocapillaris hyperreflective complex. Care was paid not to use the automated thickness measurement function of the OCT to avoid incorrect delineation of the outer neural retina boundary.15 The total area of the OEA, appearing black on the OCT scans, was then calculated using an image analysis software (MetaMorph 6.5; Molecular Devices Corporation, Downingtown, PA). During automated standardization of the threshold, the vitreous is assigned a value of 0, whereas Bruch Membrane is assigned a value of 100.
Following the morphometric analysis of the OCT images, which enabled the calculation of both the percentage of RT change and the presence of OEA representing intraretinal fluid, RH per optical section was estimated as:
Correlations were subsequently explored between the amount of RH and the percentage of OEA, and RT data. The average of the six scans was taken as the RH estimate, and the correlation between the RH and measured RWC was sought.
Determining RCV
In another set of experiments, the effect of RH on RCV was evaluated with the Live-Dead Assay. This approach aimed to establish the damage thresholds of RH changes on RCV. All time-dependent measurements were compared on a per-minute basis for advanced calculations.
Viability assessment was conducted using the Live/Dead Viability/Cytotoxicity Kit (Molecular Probes, Eugene, OR), which includes two probes: calcein and ethidium homodimer. The assay is based on intracellular esterase activity in living cells, which cleaves calcein to form a green fluorescent membrane-impermeable product. In dead cells, ethidium can easily penetrate compromised plasma and nuclear membranes, binding to DNA and producing red fluorescence. A minimum of 750 cells were counted under 200× magnification. RCV was expressed as the average ratio of live cells to the total number of cells in three randomly selected areas.
Statistical Evaluation
Data from all experiments were pooled and expressed as mean ± standard deviation. RH estimates from OCT data and RWC measurements were plotted against time, and best-fit curves were derived using linear and exponential regression curves. The correlation between OCT estimates and RWC was determined, and its significance was calculated using a Student's t-test. A P value of > 0.05 is accepted as a statistically significant difference. All analyses were performed using statistical software (SigmaStat; SPSS Inc, Chicago, IL).
Results
The primary findings of this study encompass the changes in OCT parameters due to retinal dehydration, which include RWC, RT, and OEA. Furthermore, we investigated the correlation between RH and OCT parameters, such as RT (%) and OEAs (%), as well as the impact of RH on RCV, expressed as RCV (%).
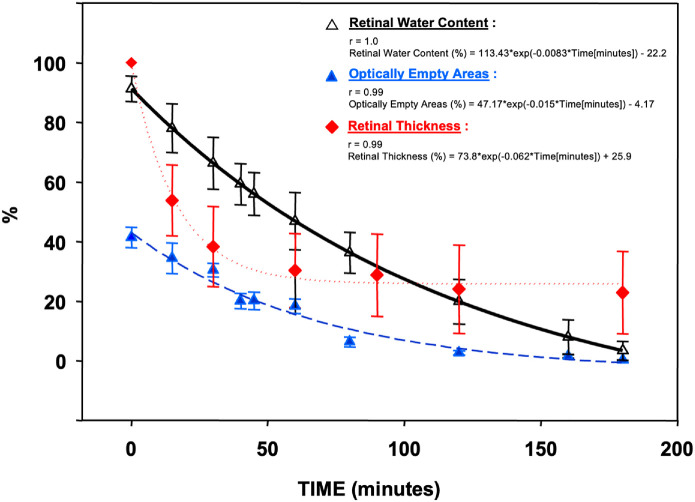
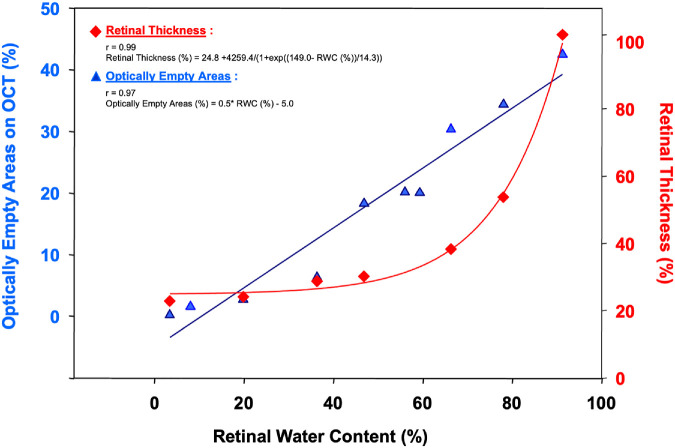
Experiments were conducted using sensory retina samples harvested from 18 donors (mean age = 72 ± 6 years). The water content of the human sensory retina after 16.3 ± 5.8 hours of death was 91.2 ± 3.9%. Keeping the retina at room temperature and under the tissue culture hood airflow decreased its water content exponentially to 3.4 ± 0.1% over 3 hours (r = 1.0; Fig. 1). During this period, the percentage of the total retinal area covered by the OEA on OCT decreased from 41.4 ± 3.4% to 0.2 ± 0.1% (r = 0.99; see Table and Fig. 1). Similarly, the average RT changed from 291.8 ± 22.8 µm to 98.2 ± 53.2 µm (r = 0.99; see Fig. 1). There was a strong linear correlation between RWC and the proportion of OEA on OCT (r = 0.97; Fig. 2), and RT (r = 0.99; see Fig. 2). At each time point, significantly less variation was noted between RWC measurements of different donors (2.1 ± 1.5%) compared with RT (31.6 ± 15.8%, P < 0.001).
Figure 1.
Retinal water content (RWC), optically empty areas (OEAs), and retinal thickness (RT) change over time.
Table.
Optically Empty Areas (OEA) and Retinal Water Content (RWC) Over Time
| Time (Min) | OEA* (%) | RWC† (%) |
|---|---|---|
| 0.00 | 41.36 ± 3.36 | 91.23 ± 4.28 |
| 15.00 | 34.38 ± 5.09 | 77.99 ± 8.20 |
| 30.00 | 30.37 ± 2.28 | 66.29 ± 8.67 |
| 40.00 | 20.03 ± 2.60 | 59.27 ± 6.97 |
| 45.00 | 20.12 ± 2.92 | 55.97 ± 7.23 |
| 60.00 | 18.32 ± 2.41 | 46.84 ± 9.60 |
| 80.00 | 6.32 ± 1.62 | 36.31 ± 6.80 |
| 120.00 | 2.70 ± 0.72 | 19.82 ± 7.48 |
| 160.00 | 1.54 ± 0.51 | 7.98 ± 5.72 |
| 180.00 | 0.19 ± 0.10 | 3.38 ± 3.12 |
OEA: optically empty areas.
RWC: retinal water content.
Figure 2.
Change in retinal thickness (RT) (%) and optically empty areas (OEAs) (%) compared to retinal water content (RWC) (%).
Change in OCT Parameters by Retinal Dehydration
Retinal Water Content
The modeled equation after results were obtained represents an exponential decay model for RWC as a function of time. The equation demonstrates that as time progresses, measured in minutes, the RWC decreases exponentially. RWC (%) is expressed as a function of time, following the equation (see Fig. 1):
Retinal Thickness
The exponential decay model illustrates RT decreases as time elapses, with the rate of decrease gradually slowing. Our results provide insight into the retinal tissue's dynamics under these experimental conditions (see Fig. 1):
Optically Empty Area
The relationship between the percentage of OEA and time using an exponential decay function are given as (see Fig. 1):
In the RWC curve, 113.43 is the initial value at time zero (t = 0). The exponential decay factor −0.0083 indicates the rate at which the RWC declines over time. −22.2 represents an offset value that adjusts the overall range of the RWC percentages. In the RT curve, −0.062 indicates the rate at which the RT declines over time. The OEA curve equation shows how intraretinal fluid represented by OEAs changes over time under experimental conditions. The initial percentage of OEAs at time zero (t = 0) is 47.17. The exponential decay factor, −0.015, signifies the rate at which the ratio of OEAs decreases over time, with the reduction rate gradually slowing. The −4.17 serves as an offset value to adjust the overall range of the OEA percentages (see Fig. 1).
Correlation of RH With OCT Parameters
OEA and RWC
There was a linear relationship between the percentage of OEA and RWC (see Fig. 2):
RWC and RT
With every 1% increase in RWC, there is an increase of 0.5% in the OEAs with a constant term of −5.0. The correlation coefficient (r) of 0.97 signifies a robust positive correlation between the predicted percentage of OEAs. The percentage of RT and RWC fits a sigmoidal relationship (see Fig. 2):
A linear relationship between the percentage of OEA and RWC is shown in Figure 2, whereas there is a sigmoidal relationship between RT and RWC. The inflection point of the RT-RWC curve is at a RWC value of 149.0, and the steepness of the curve is determined as 14.3.
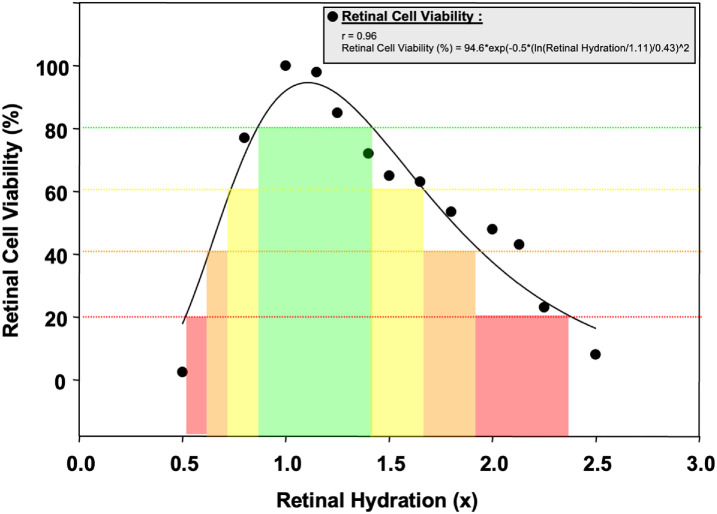
Effect of RH on RCV
The relationship between RCV (%) and RH represents a Gaussian function (Fig. 3):
Figure 3.
Retinal cell viability (RCV) change compared to retinal hydration (RH).
The RCV-RH equation shows how RCV changes with varying levels of RH which fits into a Gaussian bell-shaped curve. The maximum value of RCV when the RH is at the optimal level is 94.6%. The width of this Gaussian curve was 0.43. This curve shows us the limits of the RCV at both ends of the RH (see Fig. 3).
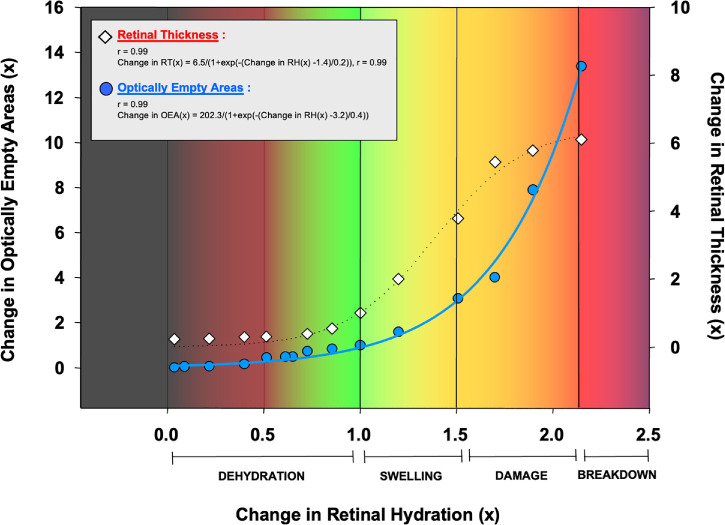
OEA and RH
The relationship between the change in OEA and the change in RH represents a sigmoidal curve, and the equation is below (Fig. 4):
Figure 4.
Change in retinal thickness (RT) and optically empty areas (OEAs) compared to retinal hydration (RH).
RT and RH
Equation showing how variations in RH levels affect changes in RT as shown in (Fig. 4):
Both the RT-RH and OEA-RH functions represent a sigmoidal curve. However, the inflection points of both curves are different (1.4 and 3.2 for RT-RH and OEA-RH, respectively), which indicates these curves reflect anatomic and pathophysiological changes, like dehydration, swelling, damage, and breakdown phases with different orders. The slope of the OEA-RH curve (0.4) is higher than the slope of the RT-RH curve (0.2) which indicates that OEA changes more rapidly than RT, with the alterations in RH (see Fig. 4).
Dimensional Change as a Function of RH
We described the Spatial Equivalence Index (SEI) for the dimensional changes induced by RH changes. In the context of SEI, thickness is represented by the variable “a,” the area is calculated by the product of “a” and “b,” and width is represented by the variable “b.” The equation “b = Area/Thickness” is used to determine the width, which requires dividing the area by the thickness. SEI is the ratio of the dark area observed in OCT to the thickness. An SEI value of 1.0 is equal to a perfect square. SEI represents the dimensional change as a function of RH, and the change is illustrated in Figure 5.
Figure 5.
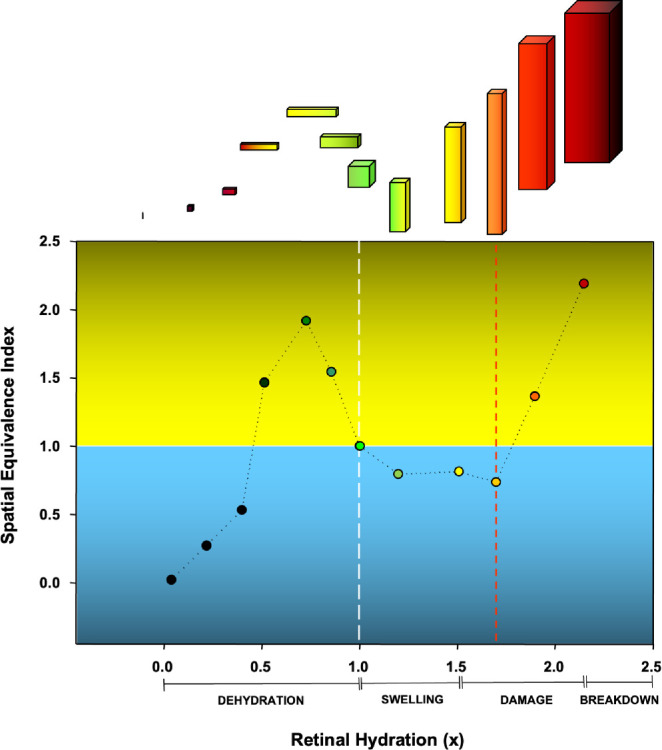
Change in Spatial Equivalence Index (SEI) compared to retinal hydration (RH) change.
The changes in SEI in response to RH follow a triphasic pattern. During the first phase both SEI and RH increase leading to a lateral enlargement of the fluid. In the second phase, RH increases even if the SEI starts to decrease, resulting in a vertical enlargement of the fluid. In the third phase, both SEI and RH increase again, when edema becomes more prominent. These findings suggest that fluid accumulation in the retina is a complex phenomenon that involves both anatomic and physiological processes, but they can be evaluated using RH measurements (see Fig. 5).
Discussion
The precise assessment of RH has become increasingly important in clinical settings to evaluate the efficacy of numerous novel pharmacological interventions targeting the reduction of leakage from insufficient retinal or choroidal vasculature. Typically, foveal thickness or volume measurements in OCT scans or alterations in the foveal profile serve this purpose. However, it is essential to note that normal foveal thickness measurements using OCT can vary depending on race, gender, and age.16 Additionally, the automated software of OCT is susceptible to generating inaccuracies in estimating foveal thickness and volume from OCT images.17 Foveal thickness serves as an indicator of the combined neuroglial tissue and extracellular fluid volume. In various pathological conditions, the disease process or prior treatments can differentially affect the volume of these compartments. For instance, previous focal laser treatments or accelerated retinal neuronal death in diabetes18 may reduce the foveal cellular volume, potentially leading to a false impression of reduced diabetic macular edema in patients with an atrophic fovea compared to those who have not undergone any focal laser treatment. Similarly, the intraretinal fluid in patients with choroidal neovascularization following anti-VEGF treatments may go undetected due to pre-existing geographic atrophy at the lesion site. Similar sources of error may occur in various other macular disorders, such as central serous chorioretinopathy.19 Consequently, there is a need for a more precise and internally standardized method of determining RH.
In this study, our primary objective was to assess RH levels through a novel approach. Our findings revealed a strong correlation between RWC and time, indicating that the exponential decay model accurately demonstrates the relationship between these two variables. A similar observation was made for OEA and RT measurements in relation to time. These results suggest that the proposed model is highly effective in revealing the behavior of intraretinal fluid over time, providing valuable insights into the dynamics of retinal tissue under varying hydration conditions.
Our findings highlighted a linear relationship with a strong correlation between RWC and OEA and a sigmoidal relationship between RWC and RT. These relationships offer valuable insights into understanding the connection between RH and structural changes within the retina.
Furthermore, the high correlation between RH and RCV suggests that the Gaussian function accurately describes this association, providing essential information on how different hydration conditions influence retinal cell health.
In the dimensional change analysis, SEI change reveals that RH initially expands horizontally and subsequently vertically, which can be proposed as a mechanism in many retinal diseases associated with edema.
Some studies aimed to assess RH levels, with one such study evaluating the efficacy of intravitreal triamcinolone (IVTA) in treating diabetic macular edema (DME) by measuring macular hydration.20 The results showed that IVTA injection significantly reduced macular thickness and hydration.20 The findings of this study revealed that IVTA injections resulted in a significant reduction in both macular thickness and hydration. Additionally, the results indicated a significant decrease in macular hydration at 1 month and 3 months following IVTA injection compared to baseline values.20 It appears that macular hydration may serve as a more reliable parameter than macular thickness when evaluating the efficacy of IVTA treatment for a specific subset of patients with DME.20
When focusing on RH other factors should also be considered. The duration of the edema plays a critical role in the severity and appearance of retinal changes. Long-lasting edema may result in more pronounced retinal alterations.21 The elasticity of the retina also can be a confounding factor.22 Age-related changes and comorbidities can affect the retina's ability to expand and contract, possibly impacting the development and resolution of edema.22 This highlights the importance of considering patient-specific factors when assessing RH and edema. Additionally, the fluid constituents can be a significant factor in the clinical characteristics of the edema.23 Understanding the composition of intraretinal fluid may provide valuable insights into the pathophysiology of various macular disorders. Mechanical factors, such as traction forces on the retina resulting from vitreomacular traction or epiretinal membranes, can contribute to developing or exacerbating existing edema, and thus, RH levels.24 Moreover, the clearance capacity of RPE and Müller cells to remove excess fluid from the retina can affect the persistence and resolution of edema.25 Investigating the mechanisms underlying this clearance capacity may yield novel approaches to understanding RH mechanisms.25 Last, concurrent pathologies, such as inflammation, can influence the development and severity of retinal edema.26 This underscores the need for a comprehensive assessment of retinal edema and RH levels to identify co-existing conditions.
The allocation of fluid accumulation within the retina is also a key factor and may vary depending on the etiology and respective characteristics.27,28 RWC consists of intracellular and extracellular components, that contribute to the RH. Intracellular RWC plays a critical role in cellular metabolism, signal transduction, and upkeep of a stable cellular environment.28 Intracellular fluid accumulation can lead to macular edema and changes in intracellular RWC can affect processes, such as ion transport, enzymatic activity, and protein function that affect normal retinal health.28 Changes in extracellular RWC can also influence the retinal microenvironment, affecting their viability and function resulting in water movement between intracellular and extracellular compartments.28 Alongside many diseases, such as diabetes and AMD, elements and factors such as osmotic gradients, ion channels, transporters, and cellular barriers can influence water movement across cell membranes and within extracellular spaces.27,28 Identifying intracellular and extracellular RWC will provide valuable information for understanding the retinal diseases and conditions. A good example is most of the retinal edema is an excess of fluid caused by the breakdown of the blood-retina barrier and is predominantly extracellular.27 Thus, water content which the initial finding of the OEA quantification results could possibly show this extracellular fluid excess and beneficial for disease oriented therapeutical applications.
Targeting therapy to attain an “ideal water content” for optimal RCV could be a promising therapeutic approach. Cell viability findings in this study indicate that retinal cells exhibit lower tolerance toward dehydration compared to hydration. This observation explains why in pioneering days of macular hole repair with vitrectomy and gas-fluid exchange dehydration of the retina resulted in visual-filed defects. Interestingly, the incidence and location of these defects was affected only by the location of the infusion cannula.29 Eventually, use of humidifying devices during gas infusion alleviated this complication.30 These observations unveil previously unrecognized therapeutic implications, suggesting that interventions aimed at maintaining or restoring appropriate RH levels may be crucial for preserving RCV and cell function.
The equations and findings of this study can help us understand how the retina's structure is impacted by hydration levels, which could be valuable information for researchers and clinicians studying retinal disorders and their potential treatments. Artificial intelligence (AI), deep learning, and image processing methodologies have been increasingly used recently to define macular edema and related entities.31,32 Moreover, RH values have the potential to serve as an essential layer for AI-driven analysis, research, and algorithms in the field of ophthalmology. By incorporating RH data, AI-based systems can enhance the precision and accuracy of diagnosing and monitoring various retinal disorders, including macular edema, DR, and AMD. With this approach RH can contribute to improved diagnostic accuracy and a more reliable assessment of prognosis in retinal diseases associated with macular edema. RH assessments may be a critical tool for research purposes, primarily in AI algorithms for large-scale data analysis. This approach can aid researchers in identifying novel biomarkers, investigating the pathophysiology of retinal disorders, and providing a foundation for future studies.
In conclusion, many factors may influence RH and the evaluation of edema, emphasizing the need for a more precise and internally standardized method of determining RH. Further research is warranted to develop and validate such a method, which may improve diagnostic accuracy and treatment efficacy for various macular disorders.
Acknowledgments
Supported in part by an unrestricted grant from Research to Prevent Blindness, Inc, NYC, NY., Foley Research Fund, New York, NY.
Disclosure: O. İnam, None; H.J. Kaplan, None; T.H. Tezel, None
References
- 1. Daruich A, Matet A, Moulin A, et al.. Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018; 63: 20–68. [DOI] [PubMed] [Google Scholar]
- 2. Ting TD, Oh M, Cox TA, Meyer CH, Toth CA.. Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch Ophthalmol. 2002; 120: 731–737. [DOI] [PubMed] [Google Scholar]
- 3. Lardenoye CW, van Kooij B, Rothova A.. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006; 113: 1446–1449. [DOI] [PubMed] [Google Scholar]
- 4. Das UN. Diabetic macular edema, retinopathy and age-related macular degeneration as inflammatory conditions. Arch Med Sci. 2016; 12: 1142–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patz A, Smith RE.. The ETDRS and diabetes 2000. Ophthalmology. 1991; 98: 739–740. [DOI] [PubMed] [Google Scholar]
- 6. Ju WK, Liu Q, Kim KY, et al.. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest Ophthalmol Vis Sci. 2007; 48: 2145–2151. [DOI] [PubMed] [Google Scholar]
- 7. Yamaike N, Kita M, Tsujikawa A, Miyamoto K, Yoshimura N.. Perimetric sensitivity with the micro perimeter 1 and retinal thickness in patients with branch retinal vein occlusion. Am J Ophthalmol. 2007; 143: 342–344. [DOI] [PubMed] [Google Scholar]
- 8. Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F.. Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci. 2006; 47: 3044–3051. [DOI] [PubMed] [Google Scholar]
- 9. Singh SR, Chhablani J.. Optical coherence tomography imaging: advances in ophthalmology. J Clin Med. 2022; 11: 2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Midena E, Bini S.. Multimodal retinal imaging of diabetic macular edema: toward new paradigms of pathophysiology. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 1661–1668. [DOI] [PubMed] [Google Scholar]
- 11. Hannouche RZ, Avila MP.. Retinal thickness measurement and evaluation of natural history of the diabetic macular edema through optical coherence tomography. Arq Bras Oftalmol. 2009; 72: 433–438. [DOI] [PubMed] [Google Scholar]
- 12. Huang D, Swanson EA, Lin CP, et al.. Optical coherence tomography. Science. 1991; 254: 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wojtkowski M, Bajraszewski T, Gorczynska I, et al.. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004; 138: 412–419. [DOI] [PubMed] [Google Scholar]
- 14. Knuttel A, Boehlau-Godau M.. Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography. J Biomed Opt. 2000; 5: 83–92. [DOI] [PubMed] [Google Scholar]
- 15. Costa RA, Calucci D, Skaf M, et al.. Optical coherence tomography 3: automatic delineation of the outer neural retinal boundary and its influence on retinal thickness measurements. Invest Ophthalmol Vis Sci. 2004; 45: 2399–2406. [DOI] [PubMed] [Google Scholar]
- 16. Kashani AH, Zimmer-Galler IE, Shah SM, et al.. Retinal thickness analysis by race, gender, and age using Stratus OCT. Am J Ophthalmol. 2010; 149: 496–502.e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sadda SR, Wu Z, Walsh AC, et al.. Errors in retinal thickness measurements obtained by optical coherence tomography. Ophthalmology. 2006; 113: 285–293. [DOI] [PubMed] [Google Scholar]
- 18. Mizutani M, Kern TS, Lorenzi M.. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996; 97: 2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S.. Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina. 2003; 23: 1–7; quiz 137-138. [DOI] [PubMed] [Google Scholar]
- 20. Sonmez K, Tezel TH, Kaplan HJ.. The role of macular hydration in the evaluation of the effect of intravitreal triamcinolone on visual acuity in eyes with diabetic macular edema. Int Ophthalmol. 2013; 33: 15–25. [DOI] [PubMed] [Google Scholar]
- 21. Yeh WS, Haller JA, Lanzetta P, et al.. Effect of the duration of macular edema on clinical outcomes in retinal vein occlusion treated with dexamethasone intravitreal implant. Ophthalmology. 2012; 119: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 22. Ferrara M, Lugano G, Sandinha MT, Kearns VR, Geraghty B, Steel DHW.. Biomechanical properties of retina and choroid: a comprehensive review of techniques and translational relevance. Eye (Lond). 2021; 35: 1818–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang D, Yan C, Ge L, et al.. Metabolomic analysis of aqueous humor reveals potential metabolite biomarkers for differential detection of macular edema. Eye Vis (Lond). 2023; 10: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Romano MR, Comune C, Ferrara M, et al.. Retinal changes induced by epiretinal tangential forces. J Ophthalmol. 2015; 2015: 372564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nagelhus EA, Mathiisen TM, Bateman AC, et al.. Carbonic anhydrase XIV is enriched in specific membrane domains of retinal pigment epithelium, Muller cells, and astrocytes. Proc Natl Acad Sci USA. 2005; 102: 8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noma H, Mimura T, Yasuda K, Shimura M.. Role of inflammation in diabetic macular edema. Ophthalmologica. 2014; 232: 127–135. [DOI] [PubMed] [Google Scholar]
- 27. Farinha C, Santos T, Marques IP, et al.. OCT-leakage mapping: a new automated method of OCT data analysis to identify and locate abnormal fluid in retinal edema. Ophthalmol Retina. 2017; 1: 486–496. [DOI] [PubMed] [Google Scholar]
- 28. Scholl S, Kirchhof J, Augustin AJ.. Pathophysiology of macular edema. Ophthalmologica. 2010; 224(Suppl 1): 8–15. [DOI] [PubMed] [Google Scholar]
- 29. Welch JC. Dehydration injury as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Am J Ophthalmol. 1997; 124: 698–699. [DOI] [PubMed] [Google Scholar]
- 30. Vote BJ, Russell MK, Newland A, Polkinghorne PJ.. The evaluation of a humidifying device for vitreoretinal surgery. Br J Ophthalmol. 2004; 88: 1582–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Padilla-Pantoja FD, Sanchez YD, Quijano-Nieto BA, Perdomo OJ, Gonzalez FA.. Etiology of macular edema defined by deep learning in optical coherence tomography scans. Transl Vis Sci Technol. 2022; 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freedman IG, Li E, Hui L, Adelman RA, Nwanyanwu K, Wang JC.. The impact of image processing algorithms on optical coherence tomography angiography metrics and study conclusions in diabetic retinopathy. Transl Vis Sci Technol. 2022; 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]