MR imaging is the criterion standard for carotid artery plaque characterization and is best able to characterize features of plaque vulnerability: lipid-rich necrotic core, intraplaque hemorrhage, ulcerations, loss of fibrous integrity, maximum plaque thickness, and plaque enhancement. Many researchers believe that many embolic strokes of undetermined source may originate from nonstenotic ipsilateral carotid plaques with vulnerable features. From the results of many trials, many believe that imaging markers of plaque vulnerability should be considered in the determination of carotid endarterectomy eligibility.
SUMMARY:
MR imaging is well-established as the criterion standard for carotid artery atherosclerosis imaging. The capability of MR imaging to differentiate numerous plaque components has been demonstrated, including those features that are associated with a high risk of sudden changes, thrombosis, or embolization. The field of carotid plaque MR imaging is constantly evolving, with continued insight into the imaging appearance and implications of various vulnerable plaque characteristics. This article will review the most up-to-date knowledge of these high-risk plaque features on MR imaging and will delve into 2 major emerging topics: the role of vulnerable plaques in cryptogenic strokes and the potential use of MR imaging to modify carotid endarterectomy treatment guidelines.
Carotid artery atherosclerosis is a major contributor to ischemic strokes, responsible for up to 20% of strokes and TIAs.1 Historically, carotid artery disease was classified on the basis of the degree to which a plaque narrowed an arterial lumen. However, it is now known that certain histologic characteristics make some plaques more susceptible than others to sudden symptomatic changes. Patients with these “vulnerable” plaque features have a 3 times higher incidence of ipsilateral neurologic ischemic events than those with stable plaques.2
The field of MR imaging of carotid artery atherosclerotic plaques continues to rapidly evolve. Thus, it is crucial that physicians keep up to date on the current applications of such imaging. This review will highlight the most recent developments in different types of high-risk plaque. It will also touch on 2 emerging topics: the use of carotid plaque imaging in the setting of cryptogenic strokes and how plaque imaging may influence future changes in treatment recommendations for carotid atherosclerosis.
Overview of MR Imaging of Carotid Plaque
MR imaging is the criterion standard for carotid artery plaque characterization and is best able to differentiate between “soft” plaque components, such as lipid material, and hemorrhage.3 However, variations in plaque imaging protocols exist, typically based on institutional preference. For example, some institutions elect not to use dedicated carotid surface coils, limiting the ability to evaluate the fibrous cap.4 In general, both pre- and postcontrast sequences are obtained without or with fat saturation.5 Many institutions now use 3D sequences (including 3D TOF, 3D MPRAGE, and 3D FSE; eg, sampling perfection with application-optimized contrasts by using different flip angle evolutions [SPACE sequence; Siemens] and/or Cube; GE Healthcare) to allow multiplanar reformatting.1,6
Regardless of institutional preferences, consensus guidelines on MR imaging of plaque do exist. These include the use of 1.5T or 3T scanners, in-plane resolution of 0.6 mm, and effective blood suppression. At minimum, a plaque protocol should be able to identify intraplaque hemorrhage (IPH), lipid rich necrotic core (LRNC), degree of stenosis, fibrous cap condition (disruption and/or ulceration), and plaque burden and distribution.3
Updates on High-Risk Features
Numerous high-risk carotid artery plaque features have been extensively described. Each of these features increases the risk of a plaque being symptomatic, leading to future ischemic neurologic events or causing accelerated plaque growth. Here, we will review these one by one, with commentary on the histologic features, imaging appearance, and recent insights of each feature.
Lipid-Rich Necrotic Core
An LRNC represents the earliest visible feature of vulnerable plaques. Atherosclerotic plaques begin as lipid streaks, in which lipid material deposits in the intima of arterial walls. Macrophages take up this lipid material, forming so-called “foam cells.” Excessive accumulation of such cells ultimately results in cell lysis and necrosis, leading to the formation of extracellular lipid pools, which eventually coalesce into an LRNC.7
On MR imaging, the LRNC tends to be mildly hypo- to mildly hyperintense to adjacent musculature on fat-suppressed T1-weighted images. LRNCs also lack markedly hyperintense signal on heavily T1-weighted images (namely MPRAGE images), signifying that superimposed plaque hemorrhage is absent (Fig 1). Specifically, LRNCs are slightly hypointense to adjacent muscle on MPRAGE images. Contrast-enhanced images can help distinguish an LRNC from the overlying fibrous cap; fibrous tissue enhances, while LRNCs do not.
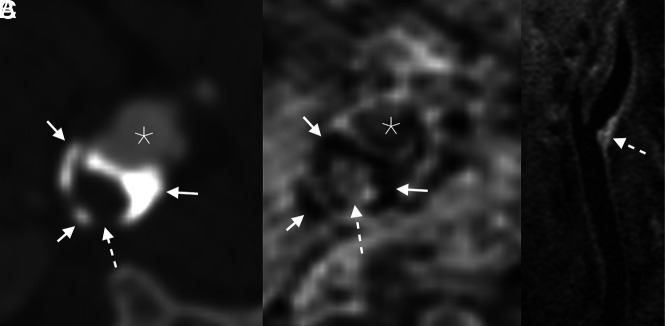
FIG 1.
Example of an LRNC. Axial CTA image (A) demonstrates a peripherally calcified (solid arrows) plaque with a soft interior (dashed arrow). The corresponding axial-reformatted MPRAGE image (B) similarly demonstrates areas of calcifications with markedly low signal (solid arrows); the plaque interior lacks bright signal, ruling out hemorrhage (dashed arrow). T1 fat-saturated Cube image (C) shows hyperintense signal in the plaque interior (dashed arrow), compatible with an LRNC. Asterisks denote the vessel lumen.
In general, LRNC is less concerning than other high-risk plaque features. Nevertheless, LRNCs may be symptomatic. A 2020 meta-analysis, for example, found the hazard ratio (HR) of LRNC to be 2.73 (95% CI, 1.04–7.16) for recurrent stroke or TIA.8 In addition, LRNCs can increase in size or be precursors of higher-risk plaque features.9 Once a LRNC has formed, a plaque may become a higher grade, losing fibrous cap integrity or developing plaque hemorrhage or ulceration.
Treatment with high-intensity statins decreases the size of LRNCs, and this effect can be monitored in vivo using plaque imaging on MR imaging. The degree of expected lipid depletion is dependent on the duration of therapy. After 3 months, modest effects are typically observed.10 After 3 years, however, the LRNC volume and the percentage of overall plaque volume can decrease by as much as 50%.11 This trend has been confirmed with a meta-analysis, in which no significant differences were found after 1–6 months or 7–12 months of therapy, but a significant decrease in LRNC volume was found after 1 year.12
Intraplaque Hemorrhage
IPH is thought to be caused by the breakdown of immature neovasculature, which commonly proliferates along the surface of a plaque. IPH remains the most validated imaging marker of a high-risk carotid artery plaque. It is a significant contributor to plaque growth, is associated with ipsilateral neurologic symptoms, and increases the risk of future strokes.
Historically, IPH was identifiable on T1-weighted MR images; hemorrhage was notably bright given the methemoglobin in the blood products. MPRAGE sequences were later developed to further highlight the T1 intensity within the IPH (Online Supplemental Data). More recently, some institutions have begun using simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) sequences, which provide high contrast between flowing blood and IPH.13
The association between IPH and ipsilateral neurologic ischemic events has been extensively documented.14 Recent studies have supported this evidence, with substantial HRs. A 2020 meta-analysis, for example, found the HR of IPH for recurrent stroke or TIA to be 7.14 (95% CI, 4.32–11.82).8 Another recent meta-analysis found that IPH increased the risk of ipsilateral stroke in both asymptomatic (HR = 7.9; 95% CI, 1.3–47.6) and symptomatic (HR = 10.2; 95% CI, 4.6–22.5) patients.15 Che et al16 found that IPH had a HR of 8.08 (95% CI, 3.65–17.91) for recurrent ischemic events. Some reports suggested that the brighter signal intensity in IPH was associated with increased ipsilateral ischemia.17,18 A more recent study, however, refuted these findings.19
More is now known about when to expect IPH: It is more common in older men, smokers, and patients with hyperlipidemia and hypertension.20 IPH is also more common in the left-sided carotid arteries for reasons that remain unclear.21 Recent studies have confirmed such findings. van Dam-Nolen et al,22 in a cohort of patients with symptomatic plaques causing mild-to-moderate stenosis, found both the presence of IPH (HR = 2.12; 95% CI: 1.02–4.44) and total plaque volume (HR = 1.07; 95% CI, 1.00–1.15) to be associated with recurrent ipsilateral strokes.
In recent years, there has also been a better understanding that plaques with IPH are often symptomatic, even when nonstenotic. For example, Nardi et al20 assessed a cohort of patients that had undergone carotid endarterectomy (CEA) for symptomatic carotid atherosclerosis, subdivided into patients with mild (<50%), moderate (50%–69%), and severe (≥70%) stenosis. The authors found that IPH was significantly more common in patients with mild stenosis (15.7%) than in those with moderate (3.9%) or severe (2.5%) stenosis. Another study found that IPH was associated with ipsilateral ischemia in patients with <30% stenosis (OR = 5.68; 95% CI, 1.49–21.69), but no such association was found in arteries with >30% stenosis.23 Nevertheless, larger plaques are more likely to develop IPH: Increased stenosis is independently associated with IPH on imaging (OR = 1.02; 95% CI, 1.01–1.03).23
The signal related to IPH has drawn continued attention in studies across the years. In general, it seems increasingly clear that the MPRAGE signal related to IPH remains present on follow-up examinations in most patients. van den Bouwhuijsen et al,24 for example, found that 94% of IPH remained present on subsequent examinations. Yamada et al,25 similarly, found that 97% of plaques retained IPH on follow-up MRIs, with no significant change in volume noted with time (Fig 2). It is not clear why abnormal signal at the site of IPH persists across multiple examinations. Takaya et al26 suggested that a relative lack of macrophages in a plaque would delay the degradation of blood products. Others believe that the signal reflects stagnant proteinaceous remnants of lytic blood and/or recurrent hemorrhage.25 Signal characteristics on other sequences may be used to determine the chronicity of blood products. Chu et al,27 for example, found that chronic IPH was hypointense on T1WI, T2WI, and TOF. More recently, quantitative T1 mapping has been used to distinguish acute and chronic IPH, with moderate (κ = 0.40, P = .028) agreement in terms of imaging classification.28
FIG 2.
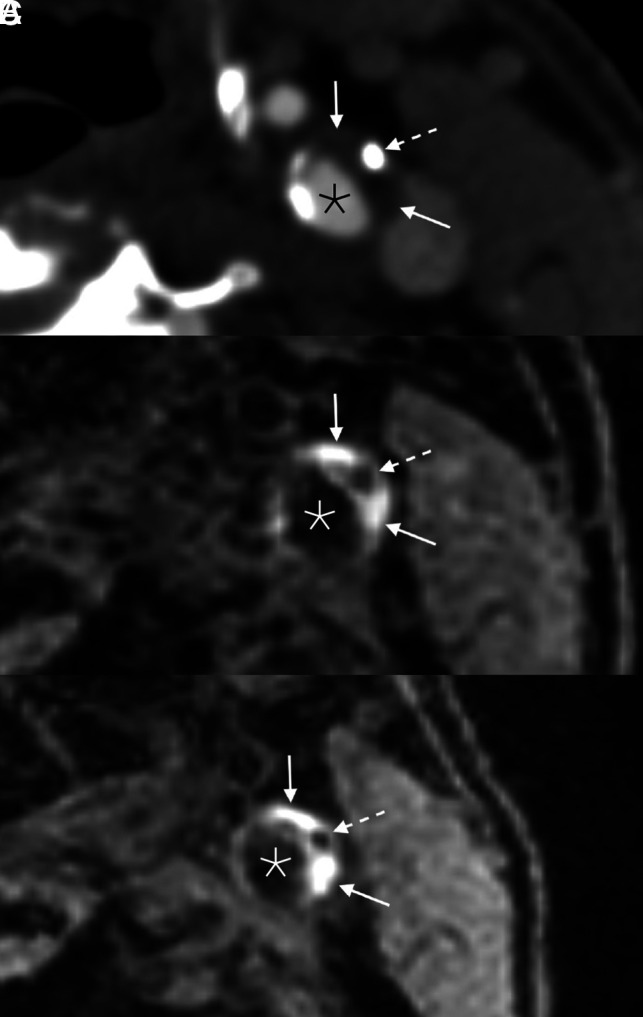
Example of persistent IPH in a 71-year-old man who presented with acute disorientation and unsteady gait. MR imaging of the brain at the time of admission (not shown) demonstrated multiple acute left cerebral infarcts. Axial CTA image (A) shows a mixed calcified (dashed arrow) and soft (solid arrows) plaque in the left ICA. Corresponding MPRAGE image (B) demonstrates IPH throughout the soft plaque components (solid arrows); the focal calcification is also noted (dashed arrow). The patient was started on dual antiplatelet therapy (aspirin and clopidogrel). One year later (C), the appearance of the IPH (solid arrows) and calcification (dashed arrow) was unchanged. Asterisks denote the vessel lumen.
Finally, recent studies have sought to use quantitative susceptibility mapping (QSM) in carotid plaque imaging to better delineate IPH. QSM is able to differentiate between paramagnetic (eg, iron within hemoglobin) and diamagnetic (eg, calcium) materials.29 Volumes of both IPH and calcification detected on QSM have already been shown to agree with findings on conventional plaque MRA techniques.30 In addition, QSM may help distinguish IPH and LRNC, both of which are hyperintense on T1WI. Ikebe et al,31 for example, found that IPH had a significantly higher signal intensity than LRNC, while calcifications demonstrated expectedly low signal intensity.
Ulcerations
A plaque ulceration is a defect in the fibrous cap of a plaque, defined as being an indentation, erosion, or fissuring of the luminal surface of the plaque. These defects are due to weakening of the cap, often due to local inflammation or hemodynamic stress. The result is of substantial clinical concern: Ulcerations expose the inner plaque contents to the arterial blood. This exposure can both rapidly de-stabilize the plaque and allow plaque contents to embolize to the brain.
Although the description of ulceration on imaging varies among studies, most authors define an ulcer as a cavitation into a plaque measuring at least 1–2 mm (Online Supplemental Data).32 The prevalence of plaque ulceration in symptomatic patients is up to 27%. Larger and more stenotic plaques, plaques with higher volumes of LRNC and/or IPH, and plaques with loss of fibrous cap integrity are more likely to develop ulcerations.33 Also, ulcerations are more likely to affect the portion of the plaque proximal to the region of maximum stenosis.34
Historically, ulcerations have been considered one of the main sources of cerebral microemboli. Most recent studies have concurred with this concept, showing that ulcers increase the risk of ipsilateral neurologic ischemic events. A 2017 meta-analysis, for example, found ulcerations to be strongly associated with ipsilateral ischemia, with ORs ranging from 1.5 to 4.9.35 A 2020 study found that patients with plaque ulceration had greater severity of ischemic strokes.36
However, some recent data on the clinical importance of ulcerations have been contradictory. van Dam-Nolen et al,22 also using data from the Plaque At Risk (PARISK) study, found that the presence of plaque ulceration was not a determinant of stroke in symptomatic plaques with <70% stenosis. Fisher et al37 found that the prevalence of ulceration was similar in plaques associated with ipsilateral and contralateral symptoms (34% and 42%, respectively). Nevertheless, the bulk of evidence supports the notion that ulcerated plaques are more likely to cause symptoms and increase a patient’s risk of future strokes.
Loss of Fibrous Cap Integrity
Fibrous caps form early during atherosclerotic plaque development, in which smooth-muscle cells migrate toward the vessel lumen. The cap is functionally protective: It separates the soft plaque components, eg, LRNC and IPH, from blood in the vessel lumen. A thick, well-formed cap can typically withstand pulsatile hemodynamic forces and is a marker of plaque stability, while a thinned or disrupted cap is a high-risk feature that portends future ischemic events. Specifically, cap disruption can lead to fissuring, ulceration, or rupture and can expose the thrombogenic components of a plaque to both platelets and coagulation factors in the bloodstream.
On MR imaging, a fibrous cap is located along the surface of a plaque and is typically hypointense on TOF images, isointense on T1 and T2, and enhances on postcontrast images. In general, assessment of the fibrous cap requires high-resolution carotid plaque surface coils; the accuracy of identifying the fibrous cap with a standard coil is limited.4 Even with high-resolution surface coil imaging, the fibrous cap can be difficult to accurately assess. On imaging, therefore, loss of cap integrity is often combined under the umbrella of thinning or rupture of the fibrous cap (TRFC).
TRFC has been repeatedly shown to be associated with neurologic ischemic events. Recent meta-analyses have confirmed these findings.38 In addition, recent studies have assessed the significance of plaque surface irregularity without specifically looking at TRFC. Li et al,39 for example, found that irregular surfaces were found in more than half of plaques and that irregularities were associated with LRNC and IPH, as well as subsequent vascular events (HR = 11.02; 95% CI, 2.65–45.85).
Plaque Enhancement
Unstable plaques are often characterized by inflammation and/or neoangiogenesis. These often coexist: Inflammatory cells are typically located near regions of fibrous cap disruption, where neovascularization also occurs. Histologic evidence of plaque inflammation—particularly greater numbers of macrophages—is associated with neurologic symptoms. For example, there is a direct correlation between the number of nonlacunar brain infarcts and the quantity of macrophages.40
On imaging, both fibrous tissue (ie, the fibrous cap) and tissue near the vessel adventitial boundary typically demonstrate enhancement in all plaques. Necrotic tissue (ie, the LRNC) is usually nonenhancing. Enhancement superimposed over a LRNC is considered pathologic and a marker of plaque vulnerability (Fig 3).41 Millon et al42 specifically found central enhancement to be a marker of either plaque rupture or loose fibrosis. Other authors have found that pathologic enhancement has been shown to represent either inflamed tissue or areas of neovascularization; thus, enhancement is often grouped together under the nomenclature of “plaque activity.”
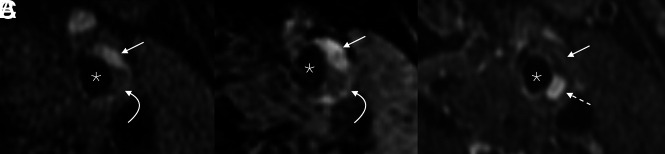
FIG 3.
Example of pathologic plaque enhancement. Axial fat-saturated T1 Cube (A) and MPRAGE (B) images show a plaque in the left ICA, with both hemorrhagic (straight arrows) and nonhemorrhagic LRNC (curved arrows) regions. On the postgadolinium fat-saturated T1 Cube image (C), the LRNC component demonstrates marked enhancement (dashed arrow), while the hemorrhagic component does not (straight arrow). Asterisks denote the vessel lumen.
Historically, studies have focused on establishing plaque enhancement as a high-risk, pathologic finding. It was shown to correspond to multiple histologic markers of vulnerability, including neovascularization, macrophages, and loose fibrosis.42 Enhancement is also more common in symptomatic patients, while its absence is a negative predictor of cerebral ischemic events.43
Recently, many studies have focused on the use of dynamic contrast-enhanced MR imaging to detect and characterize atherosclerotic neovascularization.44 Dynamic contrast images allow the analysis of the intraplaque pharmacokinetic parameter, the volume transfer constant (Ktrans), which is representative of microvascular density, permeability, and flow. Studies have shown, for instance, that Ktrans is associated with plaque types on the basis of the American Heart Association classifications.45 Nevertheless, Ktrans remains an emerging research field and will need to be further refined before being regularly used in clinical practice.
Contrast enhancement in the adventitial layer of carotid plaque has also been associated with an increased risk of stroke. In a study of 58 patients with carotid atherosclerosis, Wasserman46 found that patients with adventitial enhancement had a significantly higher rate of ipsilateral stroke. The presence of adventitial enhancement in carotid plaque may indicate the presence of inflammation or neovascularization, both of which have been linked to plaque instability and an increased risk of stroke. The ability to detect adventitial enhancement on contrast-enhanced MR imaging may thus provide an additional tool for identifying high-risk carotid plaques and guiding appropriate management strategies.
Calcifications
Calcifications are commonly observed in atherosclerotic plaques and are found in up to 90% of atheromas.47 On MR imaging, calcifications are markedly hypointense on all sequences, sometimes described as being “jet black” in appearance (Online Supplemental Data). Unlike the previously described plaque components, calcifications are thought to have beneficial effects on atherosclerosis.48 Hunt et al,49 for example, found that patients with calcified atherosclerotic plaques were more likely to be asymptomatic (P = .042). Larger, bulky calcifications specifically are more likely to be found in asymptomatic patients.50 Thus, plaque calcifications are a marker of plaque stability, representing a more quiescent, low-risk form of atherosclerosis.
Much of the recent data on this presumption have agreed with this hypothesis. The aforementioned PARISK study, for example, found no association between the proportion of calcification and the risk of stroke.22 A recent meta-analysis by Baradaran et al35 found that the patients with calcified carotid plaques had a lower incidence of stroke (OR = 0.5; 95% CI, 0.4–0.7). Zhang et al,51 in another meta-analysis, found calcified plaques to be much less likely to cause strokes than plaques with vulnerable features. Similarly, data from the Rotterdam Study found no association between carotid artery calcifications and stroke.52
However, our understanding of this subject continues to evolve. Increasingly, it is thought that it is insufficient to characterize intraplaque calcifications solely on the basis of their binary presence or absence or total calcification volume.53 Studies that use such simplified assessments failed to recognize the complex relationship between calcific and noncalcific components of atherosclerotic plaques. On CTA, for example, intraplaque calcifications have been categorized on the basis of their imaging appearances.54 Using this classification, scattered microcalcifications can cause vulnerability by acting as an intraplaque stresser.55 Moreover, the so-called rim sign, adventitial calcifications (<2-mm-thick) with a soft plaque component (≥2 mm), has been shown to be associated with intraplaque hemorrhage.56,57
These classifications of intraplaque calcifications have not been validated on MR imaging and remain largely restricted to CT. Nevertheless, calcifications play a more nuanced role in atherosclerosis formation and stability than what was previously thought.
Emerging Trends
Many of the recent developments of carotid plaque imaging are beyond the scope of this review. However, there are 2 major emerging trends in MR imaging of carotid plaque that deserve specific review because they have the potential to substantially impact patient care: the role of vulnerable plaques in embolic strokes of undetermined source (ESUSs) and how MR imaging may influence treatment decisions. Here, we will give a brief review of these topics and discuss how recent literature may guide changes in diagnoses and/or treatment strategies.
ESUSs
ESUSs are defined as being embolic-type ischemic neurologic events in patients without a known etiology. On the basis of the definition established by the Trial of Org 10172 in Acute Stroke Treatment (TOAST),58 “cryptogenic” strokes are restricted to patients with <50% stenosis of the ipsilateral carotid artery (ie, a “nonstenotic plaque”) who have no potential cardiogenic source of emboli and no other known stroke source. The terminology of such strokes varies. Some authors state that ESUSs constitute many of the so-called cryptogenic strokes, while others prefer that the term ESUSs replace the term “cryptogenic.”59,60 ESUSs account for 16%–25% of strokes, are prone to recurrence, and tend to occur in younger patients.59
Increasingly, researchers believe that many ESUSs may originate from nonstenotic ipsilateral carotid plaques with vulnerable features. Coutinho et al60 noted that large-but-nonstenotic plaques were significantly more common in the ipsilateral carotid arteries in patients with ESUSs. Subsequent studies supporting this theory have been primarily based on CTA-based trials. Data from both the Identifying New Approaches to Optimize Thrombus Characterization for Predicting Early Recanalization and Reperfusion With IV Alteplase and Other Treatments Using Serial CT Angiography (INTERRSeCT) trial and the Systematic Evaluation of Patients Treated with Neurothrombectomy Devices for Acute Ischemic Stroke (STRATIS) registry, for example, found that nonstenotic plaques were significantly more common in the ipsilateral carotid artery compared with the contralateral side.61,62
MR imaging data regarding plaque composition in the setting of ESUSs, however, remain sparse. Results from the Carotid Plaque Imaging in Acute Stroke (CAPIAS) study indicated that both a ruptured fibrous cap (HR = 4.91; 95 CI, 1.31–18.45) and IPH (HR = 4.37; 95% CI, 1.20–15.97) were associated with an increased risk of recurrent events in patients with ESUSs.63 Other data from the same study found that high-risk plaque features were significantly more common in the artery ipsilateral to the infarcts compared with the contralateral artery (31% versus 12%, respectively).64 Another study, by Larson et al,65 found that patients with ESUSs and ipsilateral IPH had an annual rate of stroke recurrence of 9.5%; the rate was 2.5% in patients without IPH. Future studies, focusing on the MR imaging characteristics of plaques in patients with ESUSs should yield much more substantial data regarding the etiology of the strokes.
Carotid Plaque Composition and Treatment Guidelines
The decision regarding whether to perform a CEA is typically based on the degree of arterial stenosis and the risk of perioperative complications. Treatment guidelines are based on the results of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST). By means of these studies, eligibility for CEA or stent placement in symptomatic patients depends on the severity of stenosis, with surgery not considered for patients with <50% stenosis.66,67 In the years that followed those trials, however, associations were found between the degree of stenosis and the presence of vulnerable plaque features.68 Thus, the observed successes of the NASCET and ECST trials may have been partly due to treatment of high-risk plaques.
Because it is now known that many symptomatic plaques are nonstenotic by the NASCET criteria (Fig 4), there is a growing call for the modification of treatment guidelines. Specifically, many believe that imaging markers of plaque vulnerability should be considered in the determination of treatment eligibility. The most promising imaging features are IPH, ulceration, and maximum plaque thickness; LRNC, integrity of the fibrous cap, and some categories of intraplaque calcifications also have potential usefulness.55
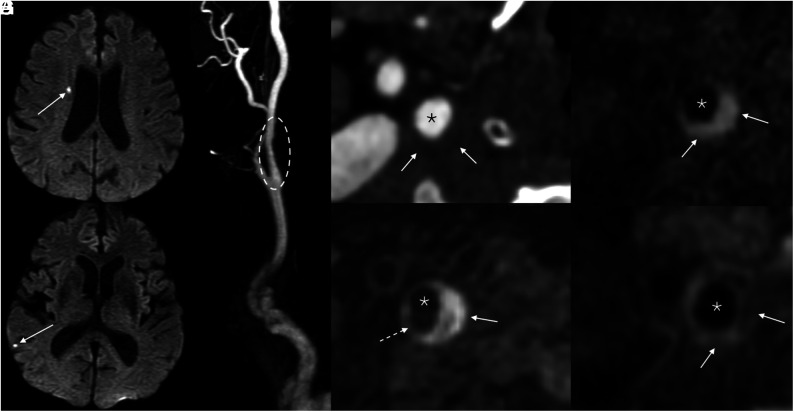
FIG 4.
Example of a symptomatic nonstenotic plaque. Axial DWI (A and B) of the brain demonstrates multiple tiny acute infarcts in the right cerebral hemisphere (arrows). 3D reformatted gadolinium bolus image (C) demonstrates a nonstenotic plaque in the proximal right ICA (dashed oval). Axial CTA image (D) shows a soft, noncalcified plaque. On MRA, MPRAGE image (E) shows that most of this plaque is composed of hemorrhagic material (solid arrow), with a small hypointense component representing a nonhemorrhagic lipid necrotic core (dashed arrow). Pre- (F) and postcontrast (G) T1 Cube images demonstrate a LRNC (solid arrows). Asterisks denote the vessel lumen.
Early data suggest that CEA in patients with relatively small plaques is a viable option. Nardi et al69 reported on a cohort of patients that underwent CEA for nonstenotic (<50%), symptomatic atherosclerotic plaques, 80% of which had IPH. The authors reported no intraoperative complications and an annualized rate of recurrent stroke after CEA of 1.5%. A systematic review of CEAs performed for nonstenotic carotid plaques found that patients had no recurrent ipsilateral ischemic events in any of the 138 studied patients (mean follow-up, 36 months).70 Nevertheless, the issue remains hotly debated. Additional studies are still needed to assess the feasibility, safety, and clinical usefulness of performing CEAs on nonstenotic atherosclerotic lesions.
Carotid plaque MRA can also be used to guide the decision between CEA and carotid artery stent placement (CAS). Although this topic remains in the developing stage, the available data suggest that CEA should be preferred to CAS in the setting of vulnerable plaques because CAS can lead to a higher risk of periprocedural events, including cerebral embolism and restenosis.71-73 A meta-analysis found that patients with IPH had higher composite outcomes of stroke, death, or myocardial infarction within 30 days of stent placement (8.1%) compared with those without IPH (2.1%) (OR = 4.45; 95% CI, 1.61–12.30; P < .01).74
Finally, regarding medical management options, several recent trials have provided evidence strengthening conservative medical treatment of carotid disease, including the protective effects of high-dose statin therapy and anti-inflammatory therapy such as the interleukin-1β innate immunity pathway.75-78 Recent meta-analyses provide evidence that atherosclerosis can be reversed with high-dose lipid-lowering therapy,79 and high-dose statins may shift vulnerable plaque from high lipid content to a more stable calcified plaque.80 Data from natural history studies suggest that IPH may override the beneficial effects of statin therapy, though the statin type and dose were neither randomized nor uniform.81 Currently, no prospective trials exist testing the hypothesis that the effects of IPH can be modified with very intensive lipid-lowering therapy.
Nevertheless, there is still a relative dearth of data on the topic of medical management for vulnerable carotid plaques, and definitive guidelines have yet to be established. Instead, many available conclusions have relied on expert opinion. For example, Holmes et al82 recommended that all patients with ESUSs should be treated with the same medications (high-dose statins and dual antiplatelet therapy for 3 weeks and aspirin for a year) but that MRA should be used to determine further treatment pathways. Specifically, the authors opined that patients with IPH and/or ulceration and repeat strokes should be considered for CEA. Hackam,83 similarly, opined that revascularization for treatment of asymptomatic carotid stenosis should be reserved for some patients with vulnerable plaques, but he did not distinguish between high- and low-risk plaques in his recommendations for medical management.
CONCLUSIONS
MR imaging of carotid artery atherosclerotic plaques is both complex and continually expanding. During recent years, there have been substantial advances in knowledge about many of the well-known plaque features, ranging from high-risk components such as IPH and TRFC to generally stabilizing features such as calcifications. As this field continues to expand, physicians will need to stay informed about how such imaging features may eventually impact management strategies and treatment guidelines.
ABBREVIATIONS:
- CAS
carotid artery stent placement
- CEA
carotid endarterectomy
- ESUS
embolic stroke of undetermined source
- HR
hazard ratio
- IPH
intraplaque hemorrhage
- K trans
volume transfer constant
- LRNC
lipid-rich necrotic core
- QSM
quantitative susceptibility mapping
- TRFC
thinning or rupture of the fibrous cap
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Brinjikji W, Huston J, Rabinstein AA, et al. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016;124:27–42 10.3171/2015.1.JNS142452 [DOI] [PubMed] [Google Scholar]
- 2.Kamtchum-Tatuene J, Wilman A, Saqqur M, et al. Carotid plaque with high-risk features in embolic stroke of undetermined source: systematic review and meta-analysis. Stroke 2020;51:311–14 10.1161/STROKEAHA.119.027272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saba L, Yuan C, Hatsukami TS, et al. ; Vessel Wall Imaging Study Group of the American Society of Neuroradiology. Carotid artery wall imaging: perspective and guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018;39:E9–31 10.3174/ajnr.A5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinjikji W, DeMarco JK, Shih R, et al. Diagnostic accuracy of a clinical carotid plaque MR protocol using a neurovascular coil compared to a surface coil protocol. J Magn Reson Imaging 2018;48:1264–72 10.1002/jmri.25984 [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick LA, Berkovitz N, Dos Santos MP, et al. Vulnerable carotid plaque imaging and histopathology without a dedicated MRI receiver coil. Neuroradiol J 2017;30:120–28 10.1177/1971400916678244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson JC, Cheek H, Aubry MC, et al. Cervical carotid plaque MRI: review of atherosclerosis imaging features and their histologic underpinnings. Clin Neuroradiol 2021;31:295–306 10.1007/s00062-020-00987-y [DOI] [PubMed] [Google Scholar]
- 7.Gui Y, Zheng H, Cao RY. Foam cells in atherosclerosis: novel insights into its origins, consequences, and molecular mechanisms. Front Cardiovasc Med 2022;9:845942 10.3389/fcvm.2022.845942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng F, Mu C, Yang L, et al. Carotid plaque magnetic resonance imaging and recurrent stroke risk: a systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e19377 10.1097/MD.0000000000019377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke 2006;37:818–23 10.1161/01.STR.0000204638.91099.91 [DOI] [PubMed] [Google Scholar]
- 10.Du R, Cai J, Zhao XQ, et al. Early decrease in carotid plaque lipid content as assessed by magnetic resonance imaging during treatment of rosuvastatin. BMC Cardiovasc Disord 2014;14:83 10.1186/1471-2261-14-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao XQ, Dong L, Hatsukami T, et al. MR imaging of carotid plaque composition during lipid-lowering therapy: a prospective assessment of effect and time course. JACC Cardiovasc Imaging 2011;4:977–86 10.1016/j.jcmg.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinjikji W, Lehman VT, Kallmes DF, et al. The effects of statin therapy on carotid plaque composition and volume: a systematic review and meta-analysis. J Neuroradiol 2017;44:234–40 10.1016/j.neurad.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 2013;69:337–45 10.1002/mrm.24254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger MA, Flumignan RL, Sobreira ML, et al. Carotid plaque composition and the importance of non-invasive in imaging stroke prevention. Front Cardiovasc Med 2022;9:885483 10.3389/fcvm.2022.885483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler A, Schinner R, Altaf N, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: meta-analysis of individual patient data. JACC Cardiovasc Imaging 2020;13(2 Pt 1):395–406 10.1016/j.jcmg.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 16.Che F, Mi D, Wang A, et al. Extracranial carotid plaque hemorrhage predicts ipsilateral stroke recurrence in patients with carotid atherosclerosis: a study based on high-resolution vessel wall imaging MRI. BMC Neurol 2022;22:237 10.1186/s12883-022-02758-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Sun J, Zhao X, et al. ; CARE-II Study Investigators. Ipsilateral plaques display higher T1 signals than contralateral plaques in recently symptomatic patients with bilateral carotid intraplaque hemorrhage. Atherosclerosis 2017;257:78–85 10.1016/j.atherosclerosis.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Liu Y, Han Y, et al. Signal of carotid intraplaque hemorrhage on MR T1-weighted imaging: association with acute cerebral infarct. AJNR Am J Neuroradiol 2020;41:836–43 10.3174/ajnr.A6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson AS, Brinjikji W, Kroll NJ, et al. Normalized intraplaque hemorrhage signal on MP-RAGE as a marker for acute ischemic neurological events. Neuroradiol J 2022;35:112–18 10.1177/19714009211029263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nardi V, Benson J, Bois MC, et al. Carotid plaques from symptomatic patients with mild stenosis is associated with intraplaque hemorrhage. Hypertens 2022;79:271–82 10.1161/HYPERTENSIONAHA.121.18128 [DOI] [PubMed] [Google Scholar]
- 21.Larson AS, Brinjikji W, Savastano L, et al. Left-sided carotid arteries have a higher prevalence of intraplaque hemorrhage than right-sided: an asymmetric conundrum. Neuroradiol J 2020;33:494–500 10.1177/1971400920970920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dam-Nolen DH, Truijman MT, van der Kolk AG, et al. ; PARISK Study Group. Carotid plaque characteristics predict recurrent ischemic stroke and TIA: the PARISK (Plaque At RISK) study. JACC Cardiovasc Imaging 2022;15:1715–26 10.1016/j.jcmg.2022.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Larson AS, Brinjikji W, Savastano L, et al. Carotid intraplaque hemorrhage and stenosis: at what stage of plaque progression does intraplaque hemorrhage occur, and when is it most likely to be associated with symptoms? AJNR Am J Neuroradiol 2021;42:1285–90 10.3174/ajnr.A7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Bouwhuijsen QJ, Selwaness M, Tang H, et al. Change in carotid intraplaque hemorrhage in community-dwelling subjects: a follow-up study using serial MR imaging. Radiology 2017;282:526–33 10.1148/radiol.2016151806 [DOI] [PubMed] [Google Scholar]
- 25.Yamada N, Higashi M, Otsubo R, et al. Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events. AJNR Am J Neuroradiol 2007;28:287–92 [PMC free article] [PubMed] [Google Scholar]
- 26.Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 2005;111:2768–75 10.1161/CIRCULATIONAHA.104.504167 [DOI] [PubMed] [Google Scholar]
- 27.Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 2004;35:1079–84 10.1161/01.STR.0000125856.25309.86 [DOI] [PubMed] [Google Scholar]
- 28.Qiao H, Li D, Cao J, et al. Quantitative evaluation of carotid atherosclerotic vulnerable plaques using in vivo T1 mapping cardiovascular magnetic resonaonce: validation by histology. J Cardiovasc Magn Reson 2020;22:38 10.1186/s12968-020-00624-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruetten PP, Cluroe AD, Usman A, et al. Simultaneous MRI water-fat separation and quantitative susceptibility mapping of carotid artery plaque pre- and post-ultrasmall superparamagnetic iron oxide-uptake. Magn Reson Med 2020;84:686–97 10.1002/mrm.28151 [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Zhang Y, Du J, et al. Quantitative susceptibility mapping for characterization of intraplaque hemorrhage and calcification in carotid atherosclerotic disease. J Magn Reson Imaging 2020;52:534–41 10.1002/jmri.27064 [DOI] [PubMed] [Google Scholar]
- 31.Ikebe Y, Ishimaru H, Imai H, et al. Quantitative susceptibility mapping for carotid atherosclerotic plaques: a pilot study. Magn Reson Med Sci 2020;19:135–40 10.2463/mrms.mp.2018-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baradaran H, Gupta A. Extracranial vascular disease: carotid stenosis and plaque imaging. Neuroimaging Clin N Am 2021;31:157–66 10.1016/j.nic.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dilba K, van Dam-Nolen DH, van Dijk AC, et al. Plaque composition as a predictor of plaque ulceration in carotid artery atherosclerosis: the Plaque At RISK study. AJNR Am J Neuroradiol 2021;42:144–51 10.3174/ajnr.A6868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafailidis V, Chryssogonidis I, Tegos T, et al. Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging 2017;8:213–25 10.1007/s13244-017-0543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baradaran H, Al-Dasuqi K, Knight-Greenfield A, et al. Association between carotid plaque features on CTA and cerebrovascular ischemia: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2017;38:2321–26 10.3174/ajnr.A5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao X, Yang Q, Tang Y, et al. Normalized wall index, intraplaque hemorrhage and ulceration of carotid plaques correlate with the severity of ischemic stroke. Atherosclerosis 2020;315:138–44 10.1016/j.atherosclerosis.2020.10.896 [DOI] [PubMed] [Google Scholar]
- 37.Fisher M, Paganini-Hill A, Martin A, et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke 2005;36:253–57 10.1161/01.STR.0000152336.71224.21 [DOI] [PubMed] [Google Scholar]
- 38.Jiang B, He D, Zhang L, et al. Risk prediction of cerebrovascular events with carotid plaque magneitc resonance analysis: a meta-analysis. J Neuroradiol 2019;46:117–23 10.1016/j.neurad.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 39.Li J, Li D, Yang D, et al. Irregularity of carotid plaque surface predicts subsequent vascular event: a MRI study. J Magn Reson Imaging 2020;52:185–94 10.1002/jmri.27038 [DOI] [PubMed] [Google Scholar]
- 40.Saba L, Lai L, Lucatelli P, et al. Association between carotid artery plaque inflammation and brain MRI. J Neuroradiol 2020;47:203–09 10.1016/j.neurad.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 41.Kerwin WS, Hatsukami T, Yuan C, et al. MRI of carotid atherosclerosis. AJR Am J Roentgenol 2013;200:W304–13 10.2214/AJR.12.8665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millon A, Boussel L, Brevet M, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012;43:3023–28 10.1161/STROKEAHA.112.662692 [DOI] [PubMed] [Google Scholar]
- 43.Papini GD, Di Leo G, Bandirali M, et al. Is carotid plaque contrast enhancement on MRI predictive for cerebral or cardiovascular events? A prospective cohort study. J Comput Assist Tomogr 2017;41:321–26 10.1097/RCT.0000000000000506 [DOI] [PubMed] [Google Scholar]
- 44.Cattaneo M, Sun J, Staub D, et al. Imaging of carotid plaque neovascularization by contrast-enhanced ultrasound and dynamic contrast-enhanced magnetic resonance imaging. Cerebrovasc Dis 2019;48:140–48 10.1159/000504042 [DOI] [PubMed] [Google Scholar]
- 45.Ge X, Zhou Z, Zhao H, et al. Evaluation of carotid plaque vulnerability in vivo: correlation between dynamic contrast-enhanced MRI and MRI-modified AHA classification. J Magn Reson Imaging 2017;46:870–76 10.1002/jmri.25637 [DOI] [PubMed] [Google Scholar]
- 46.Wasserman BA. Advanced contrast-enhanced MRI for looking beyond the lumen to predict stroke: building a risk profile for carotid plaque. Stroke 2010;41:S12–16 10.1161/STROKEAHA.110.596288 [DOI] [PubMed] [Google Scholar]
- 47.Ahmed M, McPherson R, Abruzzo A, et al. Carotid artery calcification: what we know so far. Cureus 2021;13:e18938 10.7759/cureus.18938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwee RM. Systematic review on the association between calcification in carotid plaques and clinical ischemic symptoms. J Vasc Surg 2010;51:1015–25 10.1016/j.jvs.2009.08.072 [DOI] [PubMed] [Google Scholar]
- 49.Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke 2002;33:1214–19 10.1161/01.STR.0000013741.41309.67 [DOI] [PubMed] [Google Scholar]
- 50.Shi X, Gao J, Lv Q, et al. Calcification in atherosclerotic plaque vulnerability: friend or foe? Front Physiol 2020;11:56 10.3389/fphys.2020.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Bai Y, Xie J, et al. Carotid plaque components and other carotid artery features associated with risk of stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2022;31:106857 10.1016/j.jstrokecerebrovasdis.2022.106857 [DOI] [PubMed] [Google Scholar]
- 52.Bos D, Arshi B, van den Bouwhuijsen QJ, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol 2021;77:1426–35 10.1016/j.jacc.2021.01.038 [DOI] [PubMed] [Google Scholar]
- 53.Saba L, Nardi V, Cau R, et al. Carotid artery plaque calcifications: lessons from histopathology to diagnostic imaging. Stroke 2022;53:290–97 10.1161/STROKEAHA.121.035692 [DOI] [PubMed] [Google Scholar]
- 54.Saba L, Chen H, Cau R, et al. Impact analysis of different CT configurations of carotid artery plaque calcifications on cerebrovascular events. AJNR Am J Neuroradiol 2022;43:272–79 10.3174/ajnr.A7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bos D, van Dam-Nolen DH, Gupta A, et al. Advances in multimodality carotid plaque imaging: AJR Expert Panel Narrative Review. AJR Am J Roentgenol 2021;217:16–26 10.2214/AJR.20.24869 [DOI] [PubMed] [Google Scholar]
- 56.Benson JC, Nardi V, Madhavan AA, et al. Reassessing the carotid artery plaque “rim sign” on CTA: a new analysis with histopathologic confirmation. AJNR Am J Neuroradiol 2022;43:429–34 10.3174/ajnr.A7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eisenmenger LB, Aldred BW, Kim SE, et al. Prediction of carotid intraplaque hemorrhage using adventitial calcification and plaque thickness on CTA. AJNR Am J Neuroradiol 2016;37:1496–503 10.3174/ajnr.A4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST—Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 59.Hart RG, Diener HC, Coutts SB, et al. ; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–38 10.1016/S1474-4422(13)70310-7 [DOI] [PubMed] [Google Scholar]
- 60.Coutinho JM, Derkatch S, Potvin ARJ, et al. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Neurology 2016;87:665–72 10.1212/WNL.0000000000002978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ospel JM, Singh N, Marko M, et al. Prevalence of ipsilateral nonstenotic carotid plaques on computed tomography angiography in embolic stroke of undetermined source. Stroke 2020;51:1743–49 10.1161/STROKEAHA.120.029404 [DOI] [PubMed] [Google Scholar]
- 62.Singh N, Ospel J, Mayank A, et al. ; STRATIS Investigators. Nonstenotic carotid plaques in ischemic stroke: analysis of the STRATIS Registry. AJNR Am J Neuroradiol 2021;42:1645–52 10.3174/ajnr.A7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopczak A, Schindler A, Sepp D, et al. Complicated carotid artery plaques and risk of recurrent ischemic stroke or TIA. J Am Coll Cardiol 2022;79:2189–99 10.1016/j.jacc.2022.03.376 [DOI] [PubMed] [Google Scholar]
- 64.Kopczak A, Schindler A, Bayer-Karpinska A, et al. Complicated carotid artery plaques as a cause of cryptogenic stroke. J Am Coll Cardiol 2020;76:2212–22 10.1016/j.jacc.2020.09.532 [DOI] [PubMed] [Google Scholar]
- 65.Larson AS, Nasr DM, Rizvi A, et al. Embolic stroke of undetermined source: the association with carotid intraplaque hemorrhage. JACC Cardiovasc Imaging 2021;14:506–08 10.1016/j.jcmg.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 66.Chaturvedi S, Bruno A, Feasby T, et al. ; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Carotid endarterectomy–an evidence-based review: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65:794–801 10.1212/01.wnl.0000176036.07558.82 [DOI] [PubMed] [Google Scholar]
- 67.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–87 10.1016/S0140-6736(97)09292-1 [DOI] [PubMed] [Google Scholar]
- 68.Barnett HJ, Taylor DW, Haynes RB, et al. ; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53 10.1056/NEJM199108153250701 [DOI] [PubMed] [Google Scholar]
- 69.Nardi V, Benson JC, Larson AS, et al. Carotid artery endarterectomy in patients with symptomatic non-stenotic carotid artery disease. Stroke Vasc Neurol 2022;7:251–57 10.1136/svn-2021-000939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Larson A, Nardi V, Brinjikji W, et al. Endarterectomy for symptomatic non-stenotic carotids: a systematic review and descriptive analysis. Stroke Vasc Neurol 2022;7:6–12 10.1136/svn-2021-001122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tokunaga K, Tokunaga S, Hara K, et al. Intraplaque high-intensity signal on time-of-flight magnetic resonance angiography and restenosis after carotid artery stenting. J Neurosurg 2022;136:1029–34 10.3171/2021.4.JNS21546 [DOI] [PubMed] [Google Scholar]
- 72.Yoshimura S, Yamada K, Kawasaki M, et al. High-intensity signal on time-of-flight magnetic resonance angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke 2011;42:3132–37 10.1161/STROKEAHA.111.615708 [DOI] [PubMed] [Google Scholar]
- 73.Yoshimura S, Yamada K, Kawasaki M, et al. Selection of carotid artery stenting or endarterectomy based on magnetic resonance plaque imaging reduced periprocedural adverse events. J Stroke Cerebrovasc Dis 2013;22:1082–87 10.1016/j.jstrokecerebrovasdis.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 74.Brinjikji W, Lehman VT, Huston J, et al. The association between carotid intraplaque hemorrhage and outcomes of carotid stenting: a systematic review and meta-analysis. J Neurointerv Surg 2017;9:837–42 10.1136/neurintsurg-2016-012593 [DOI] [PubMed] [Google Scholar]
- 75.Naylor AR. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol 2012;9:116–24 10.1038/nrcardio.2011.151 [DOI] [PubMed] [Google Scholar]
- 76.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 77.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573–83 10.1161/STROKEAHA.109.556068 [DOI] [PubMed] [Google Scholar]
- 78.Naylor AR, Gaines PA, Rothwell PM. Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals? Eur J Vasc Endovasc Surg 2009;37:625–32 10.1016/j.ejvs.2009.01.026 [DOI] [PubMed] [Google Scholar]
- 79.Ibrahimi P, Jashari F, Bajraktari G, et al. Ultrasound assessment of carotid plaque echogenicity response to statin therapy: a systematic review and meta-analysis. Int J Mol Sci 2015;16:10734–47 10.3390/ijms160510734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mujaj B, Bos D, Selwaness M, et al. Statin use is associated with carotid plaque composition: the Rotterdam Study. Int J Cardiol 2018;260:213–18 10.1016/j.ijcard.2018.02.111 [DOI] [PubMed] [Google Scholar]
- 81.Underhill HR, Yuan C, Yarnykh VL, et al. Arterial remodeling in the subclinical carotid artery disease. JACC Cardiovasc Imaging 2009;2:1381–89 10.1016/j.jcmg.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmes DR, Alkhouli MA, Klaas JP, et al. Change of heart: the underexplored role of plaque hemorrhage in the evaluation of stroke of undetermined etiology. J Am Heart Assoc 2022;11:e025323 10.1161/JAHA.122.025323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hackam DG. Optimal medical management of asymptomatic carotid stenosis. Stroke 2021;52:2191–98 10.1161/STROKEAHA.120.033994 [DOI] [PubMed] [Google Scholar]