This retrospective cohort of patients with observed or suspected CSF-venous fistula identified during standard maximum suspended inspiration CT myelography were rescanned using resisted inspiration and the Valsalva maneuver. Visibility of the CSF-venous fistula was greatest during resisted inspiration in most of the cases.
Abstract
BACKGROUND AND PURPOSE:
CSF-venous fistulas are an important cause of spontaneous intracranial hypotension but are challenging to detect. A newly described technique known as resisted inspiration has been found to augment the CSF-venous pressure gradient and was hypothesized to be of potential use in CSF-venous fistula detection but has not yet been investigated in patients with spontaneous intracranial hypotension. The purpose of this investigation was to determine whether resisted inspiration results in improved visibility of CSF-venous fistulas on CT myelography in patients with spontaneous intracranial hypotension.
MATERIALS AND METHODS:
A retrospective cohort of patients underwent CT myelography from November 2022 to January 2023. Patients with an observed or suspected CSF-venous fistula identified during CT myelography using standard maximum suspended inspiration were immediately rescanned using resisted inspiration and the Valsalva maneuver. The visibility of the CSF-venous fistula among these 3 respiratory phases was compared, and changes in venous drainage patterns between phases were assessed.
RESULTS:
Eight patients with confirmed CSF-venous fistulas who underwent CT myelography using the 3-phase respiratory protocol were included. Visibility of the CSF-venous fistula was greatest during resisted inspiration in 5/8 (63%) of cases. Visibility was optimal with the Valsalva maneuver and maximum suspended inspiration in 1 case each, and it was equivalent in all respiratory phases in 1 case. In 2/8 (25%) cases, the pattern of venous drainage shifted between respiratory phases.
CONCLUSIONS:
In patients with spontaneous intracranial hypotension, resisted inspiration improved visualization of CSF-venous fistulas in most, but not all, cases. Further investigation is needed to determine the impact of this technique on the overall diagnostic yield of myelography in this condition.
CSF-venous fistula (CVF) has been recognized during the past several years as the causative lesion in an increasing proportion of cases of spontaneous intracranial hypotension (SIH).1 Multiple new interventions have been developed to treat CVFs, with relatively high reported success rates.2-5 Use of these techniques depends on accurate anatomic localization of the CVF, which remains challenging in many cases.
A variety of technical innovations have been described to enhance the visibility of CVFs on imaging, to improve detection.6-9 One report found that the appearance of CVFs varied with the respiratory phase, with augmented visibility of CVFs during inspiration compared with expiration or the Valsalva maneuver.8 More recently, Mark et al10 found that a technique of resisted inspiration resulted in decreased pressure within the superior vena cava and increased CSF pressure compared with normal inspiration. Although the investigation did not directly explore the effect of this technique on CVF visualization, the authors hypothesized that it could potentially aid in CVF detection by augmenting the pressure gradient between the CSF and the venous system, thereby promoting transit of contrast through the fistula.
The purpose of this investigation was to determine whether a resisted inspiratory technique resulted in improved subjective visualization of CVFs in patients with SIH compared with other respiratory phases, including maximum suspended inspiration and the Valsalva maneuver, using serial image acquisition of CT myelography (CTM) during 3 separate phases of respiration.
MATERIALS AND METHODS
Subjects
This retrospective cohort included patients with SIH who underwent CTM at our institution between November 2022 and January 2023. The procedure schedule in the institutional electronic medical record was reviewed to identify patients who had undergone postmyelogram CT to evaluate a CSF leak. Patients were included if they satisfied the International Classification of Headache Disorders, 3rd ed, criteria for SIH,11 had a suspected CVF identified on CTM at the time of scan acquisition, and subsequently were imaged with the 3-phase respiratory protocol described below. Patients without an imaging-verified CVF after the work-up was complete were excluded. The investigation was approved by the local institutional review board and is compliant with Health Insurance Portability and Accountability Act.
Myelographic Technique
CTM was performed using an intrathecal injection of 10 mL of iopamidol containing 300 mg/mL of iodine (Isovue-M 300; Bracco) with the patient in lateral decubitus position to increase the density of intrathecal contrast over the nerve roots in the thoracic spine, a technique previously shown to augment detection of CVFs.6
Imaging was performed on a 64–detector row CT scanner (Discovery 750HD; GE Healthcare) or on a photon-counting detector CT scanner (NAEOTOM alpha; Siemens). Intrathecal contrast was injected using either CT fluoroscopy (CTF) or conventional fluoroscopic guidance. In cases in which CTF was used, diagnostic CTM was performed immediately after contrast injection. In cases in which conventional fluoroscopy was used for the intrathecal injection, the patient was transferred to the CT scanner for subsequent diagnostic CTM, and care was taken to maintain the lateral decubitus position during transport to avoid mixing of contrast.
Resisted Inspiration Technique
The initial CTM scan was performed with the patient instructed to breathe in deeply and hold his or her breath (ie, maximum suspended inspiration). This process is equivalent to the “normal inspiration” phase reported by Mark et al.10 Images were reviewed on the CT scanner console by the performing radiologist in real-time to assess a hyperdense paraspinal vein indicating the presence of a CVF.12 If no CVF was seen on the initial scan, the patient was turned to the contralateral decubitus position and repeat scanning of the thoracic spine was performed with maximum suspended inspiration.
In cases in which a CVF was observed or suspected to be present due to the presence of a hyperdense paraspinal vein on immediate review of either decubitus scan by the radiologist, repeat scanning limited to the level of interest was performed using a resisted inspiration technique, followed by a third scan acquired during the Valsalva maneuver. For resisted inspiratory scans, the patient was provided with a 5-mL syringe (Luer-Lock syringe; Becton-Dickinson) with the plunger removed to hold between his or her lips and instructed to breathe in continuously through the syringe beginning immediately before and then continuously throughout image acquisition, similar to the resisted inspiration technique previously reported.10 The syringe was then removed from the patient’s lips, and the limited area of interest was scanned a third time with the patient performing a Valsalva maneuver.
Image Analysis
Cases were retrospectively and independently reviewed by 2 neuroradiologists with 11 and 14 years of experience in treating SIH. Cases were reviewed on the PACS using the thinnest available section thickness (0.625 mm for scans performed on the 64–detector row CT and 0.2 mm on the photon-counting detector CT). Each radiologist assessed which respiratory phase optimally showed the CVF. Disagreement on the initial classification between readers was resolved by consensus.
Images from all 3 phases of respiration were coregistered and reviewed in a 3-plane MPR reformat. Cases were categorized into one of the following groups on the basis of the reader’s subjective analysis: One of the respiratory phases was superior to the other 2 in showing the CVF (pattern A), 2 of the respiratory phases were equally superior to the third (pattern B), or all respiratory phases were equivalent in depicting the CVF (pattern C).
Additionally, cases were categorized as either showing the same distribution of draining veins on all respiratory phases or showing drainage patterns that differed with the respiratory phase. Differing drainage was defined as new drainage into 1 of the following 3 regions of the venous plexus not seen on other respiratory phases: the internal epidural venous plexus, external epidural venous plexus, or basivertebral venous plexus.1
The time of intrathecal contrast injection and of each of the 3 respiratory phase scans was determined from the timestamp of the images stored in the PACS. Time intervals between contrast injection and each of the subsequent 3 scan acquisitions were calculated on the basis of these timestamps. Differences in the time interval from contrast injection to the first CTM scan were compared in subjects for whom CTF was used for intrathecal injection versus those for whom conventional fluoroscopy was used by means of unpaired t tests. P values < .05 were considered statistically significant.
RESULTS
Ninety-one patients who underwent postmyelogram CT were reviewed for eligibility. Of these, 8 subjects were scanned using the 3-phase respiratory protocol and met the inclusion and exclusion criteria. The mean subject age was 56.6 (SD, 9.9) years (range, 44–73 years). Five of 8 (63%) subjects were women. Seven patients reported orthostatic headache as their primary symptom, and 1 patient reported impaired hearing as the primary symptom. In all cases, brain MR imaging with contrast was performed before CTM and showed changes compatible with SIH (at least 1 of the following signs: dural enhancement, venous distension, or brain sagging). In 6/8 cases, the CVF was identified on the first lateral decubitus scan, ipsilateral to the side positioned down during intrathecal contrast injection (left side in 5 cases, right side in 1 case). In the other 2 cases, no CVF was seen on the first scan but was identified after the subject had been rolled onto the contralateral decubitus position (right side in both cases). Spinal levels and laterality of identified CVFs are shown in the Table.
Patient characteristics, CVF location, and behavior of CVF drainage investigated with 3-phase respiratory technique
| Case No. | Age (yr) | Sex | CVF Level | CVF Side | Visibility Patterna | Drainage Pattern Varies with Phaseb | Best Phase for CVF Visualization |
|---|---|---|---|---|---|---|---|
| 1 | 55 | F | T12 | R | A | N | RI |
| 2 | 49 | F | T3 | L | A | N | MI |
| 3 | 67 | M | T3 | L | A | N | V |
| 4 | 60 | F | L1 | R | A | N | RI |
| 5 | 48 | F | T9 | R | A | N | RI |
| 6 | 57 | M | T5 | L | A | Y | RI |
| 7 | 73 | M | T7 | L | C | Y | – |
| 8 | 44 | F | T7 | L | A | N | RI |
Note:—The en dash indicates that no single phase was superior; RI, resisted inspiration; MI, maximum suspended inspiration; V, Valsalva maneuver; M, male; F, female; R, right; L, left; N, no; Y, yes.
The visibility pattern is classified as follows: One respiratory phase is superior to the others (A), 2 phases are equally superior to the third (B), or all respiratory phases are equivalent in depicting the CVF (C).
Y indicates that the drainage pattern shifted to other portions of the epidural venous plexus with different respiratory phases; N indicates no shift.
The mean time interval from intrathecal contrast injection to scan acquisition for the first respiratory phase (ie, maximum suspended inspiration) was 20.5 minutes (range, 7.7–55.9 minutes). The mean time interval between the acquisition of the first respiratory phase and the second phase (ie, resisted inspiration) was 4.5 minutes (range, 2.3–6.5 minutes). The mean time interval between the acquisition of the second respiratory phase and the third phase (ie, the Valsalva maneuver) was 3.5 minutes (range, 1.4–6.2 minutes). In 2 cases (subjects 3 and 4), intrathecal contrast injection was performed using conventional fluoroscopy before the patient was moved to the CT scanner; in one of these cases, dynamic myelography was performed under conventional fluoroscopy before CTM, and in the other case, injection was performed before CTM on the photon-counting detector CT because that scanner was not equipped with a CTF package. The remaining 6 injections were performed using CTF. The time interval between the contrast injection and the first scan was significantly longer for those cases in which conventional fluoroscopy was used for intrathecal contrast injection compared with those cases in which CTF was used (mean time to imaging, 42.3 versus 11.7 minutes; P = .01).
A single respiratory phase was found to best depict the CVF in 7/8 (88%) cases (pattern A) (Figs 1 and 2). Of these 7 cases, the resisted inspiration phase was assessed as the superior phase of respiration in 5 instances. The Valsalva maneuver (Fig 3) and maximum suspended inspiration best depicted the CVF in 1 instance each; both of these CVFs were located on the left at the T3 level. In 1/8 (12%) cases, all respiratory phases resulted in equivalent visualization of the CVF, which was located on the left at T7 (pattern C). There were no cases in which 2 phases were equally superior to the third in visualizing the CVF (pattern B). Thus overall, resisted inspiration best depicted the CVF in 5/8 (63%) total cases in our study.
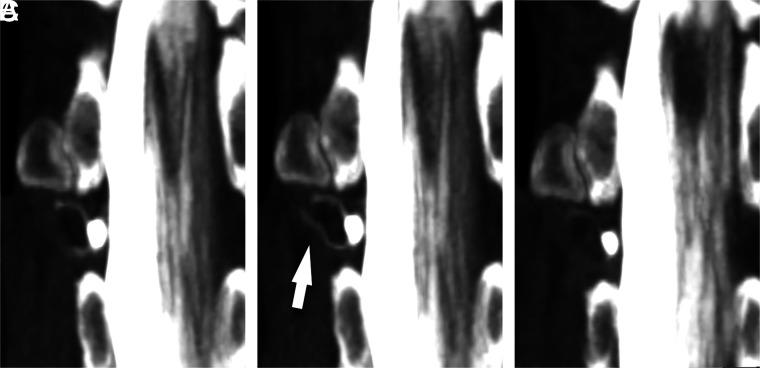
FIG 1.
A right T12 CVF best visualized during resisted inspiration. Coronal MIP images from CTM performed in a patient with SIH are shown during maximum suspended inspiration (A), resisted inspiration (B), and the Valsalva maneuver (C). Although the CVF (arrow) is visible to varying degrees on all phases, visualization is best during resisted inspiration.
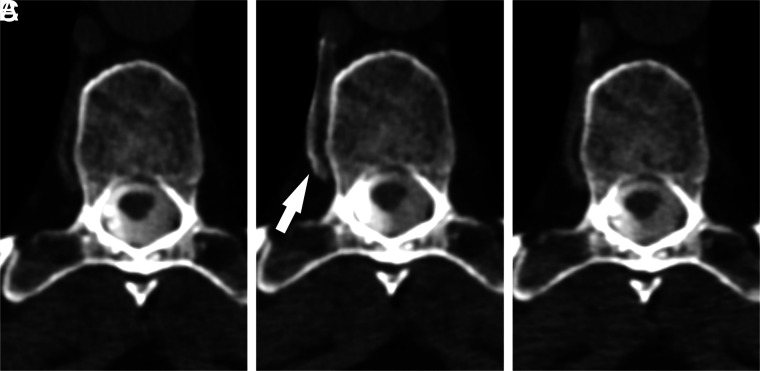
FIG 2.
A right T9 CVF best visualized during resisted inspiration. Axial MIP images from CTM performed on a patient with SIH are shown during maximum suspended inspiration (A), resisted inspiration (B), and the Valsalva maneuver (C). Drainage of the CVF into a segmental vein (arrow) is best seen during resisted inspiration.
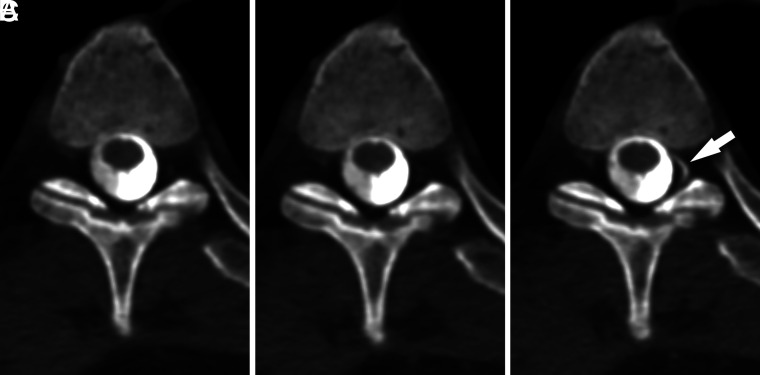
FIG 3.
A left T3 CVF best visualized during the Valsalva maneuver. Axial MIP images from CTM performed on a patient with SIH are shown during maximum suspended inspiration (A), resisted inspiration (B), and the Valsalva maneuver (C). The CVF originating at the T3 level on the left (arrow) is best visualized during the Valsalva maneuver. The presence of a CVF was suspected on the basis of faint hyperdensity in the same location on the initial maximum suspended inspiration image.
A change in the venous drainage pattern was observed between the different respiratory phases in 2/8 (25%) cases. In 1 case (the aforementioned left T7 CVF), drainage was seen into the basivertebral venous plexus on both inspiratory phases, but it partially shifted away from the basivertebral plexus and into the external epidural venous plexus during the Valsalva maneuver (Fig 4). In another case, resisted inspiration resulted in the best visualization of the CVF in the internal epidural venous plexus but also shifted some drainage of the CVF into the external epidural venous plexus.
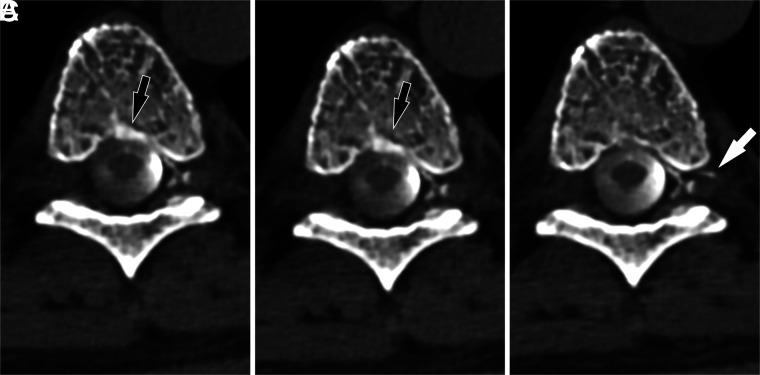
FIG 4.
A left T7 CVF drainage pattern shifts with varying respiratory phases. Axial images from CTM performed on a patient with SIH are shown during maximum suspended inspiration (A), resisted inspiration (B), and the Valsalva maneuver (C). The primary drainage of the CVF is into the internal epidural venous plexus, but during inspiratory phases, a component of drainage is also seen into the basivertebral venous plexus (black arrows). This drainage decreases during the Valsalva maneuver, with new drainage visible into the external epidural venous plexus via an intervertebral vein (white arrow).
DISCUSSION
Our investigation found that in 5/8 (63%) cases, resisted inspiration resulted in superior visualization of the CVF compared with maximum suspended inspiration or the Valsalva maneuver. A recent investigation hypothesized that resisted inspiration might prove superior to maximum suspended inspiration on the basis of venous manometry data obtained during various phases of respiration, but it did not directly test the effect on patients with actual CVFs.10 Our investigation conducted in cases of patients with SIH supports the hypothesis posited by Mark et al10 that resisted inspiration can be useful in enhancing the visibility of a CVF.
Resisted inspiration did not result in improved visibility of CVFs in all cases, however. In 2 cases, the CVF was best visualized on a different phase of inspiration (maximum suspended inspiration and the Valsalva maneuver, respectively). Both of these CVFs were located at T3 on the left. While it is possible that this association is coincidental, venous drainage of the upper left thoracic spine differs from that in the remainder of the thoracic spine. Specifically, the second through fourth thoracic vertebral levels on the left usually drain into the superior intercostal vein, while the rest of the thoracic spine drains into the azygous system.13 It is possible that resisted inspiration may affect augmentation of venous drainage differently in the upper left thoracic spine on the basis of this anatomic difference, though more experience with this technique will be needed to determine if this observation is reproducible.
We also observed that in some cases, the pattern of venous drainage may change on the basis of the respiratory phase. Variations in intrathoracic pressure and the resultant changes in venous pressures during different phases of respiration may influence the route of venous drainage for a single fistula. This possibility could be consequential because some patterns of venous drainage may be more difficult to detect than others. For instance, drainage into the internal epidural venous plexus or basivertebral venous plexus could lie behind the overlying intrathecal contrast column and, therefore, be obscured on planar imaging such as digital subtraction myelography. Similarly, on CTM, drainage into the basivertebral venous plexus could potentially be less conspicuous than drainage into other routes because of the surrounding bone of the vertebral body. With greater experience, it may be possible to predict which phase of respiration is most likely to augment CVF visualization. It is also possible that multiple phases of respiration may be needed during an examination, depending on the vertebral level being imaged.
Some institutions use decubitus Trendelenburg patient positioning coupled with multiple phases of scanning within seconds after intrathecal contrast bolus injection, sometimes referred to as “dynamic CTM,” to visualize CVFs.9,14 We did not use this technique for the CTMs in our study because we have found in our experience that maintaining a dense pool of contrast over the nerve roots is more important than temporal resolution when investigating possible CVFs with CTM, which can be accomplished without a dynamic contrast bolus. Moreover, a dynamic decubitus CTM technique would have made comparison of respiratory phases impossible because the migration of the contrast bolus would not provide adequate time for investigation of multiple respiratory phases. Although we make an effort to scan as quickly as possible after intrathecal contrast injection, our study does show that some delay in scanning does not preclude detection of CVFs as long as dense contrast can be maintained over the spinal nerve roots.
Our study has several limitations. First, this investigation included only CVFs seen or suspected during the initial imaging in maximum suspended inspiration. Although this study design has the potential to bias the results in favor of this particular inspiratory phase, we, nevertheless, found resisted inspiration to be superior to maximum suspended inspiration in most cases. However, because of this study design, the study cannot directly determine whether resisted inspiration will result in greater detection of fistulas in cases in which no fistula is seen at all on maximum suspended inspiration. Although it seems plausible that it might be helpful, the use of resisted inspiration as the sole respiratory phase used during an examination may be difficult to implement in practice in some cases, for several reasons: First, the length of time a patient can maintain inspiration against resistance may be insufficient to allow scanning of all vertebral levels of interest during CTM. When examinations are performed using DSM, resisted inspiration may be difficult to implement because the progressive expansion of the thorax may result in misregistration during image subtraction. Furthermore, resisted inspiration also depends on active cooperation and would not, therefore, be possible if examinations were performed with the patient under general anesthesia, which is used by some practitioners when performing DSM.
A second limitation of the study is that all variables that affect CVF visibility are not currently known; thus, factors unrelated to the respiratory phase (such as changes in contrast density in the thecal sac, differences in the cardiac cycle, or other unknown factors) may serve as confounders. Individual patients being scanned in the same session at very close time intervals may mitigate these effects, but this scenario does not completely exclude such influences. Finally, our study design relied on subjective assessments of CVF visibility by experienced, unblinded readers. Future investigations may consider whether more explicitly structured assessments by readers with varying levels of experience may better reflect the diagnostic performance of this technique across varied clinical practice environments.
CONCLUSIONS
Our investigation found that resisted inspiration resulted in improved visualization of CVFs among actual patients with SIH in most cases. Further work is needed to determine whether this technique can be implemented clinically on a routine basis and whether it increases the overall diagnostic yield of myelography in patients with SIH.
ABBREVIATIONS:
- CTF
CT fluoroscopy
- CTM
CT myelography
- CVF
CSF-venous fistula
- DSM
digital subtraction myelogram
- SIH
spontaneous intracranial hypotension
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Kranz PG, Gray L, Malinzak MD, et al. CSF-venous fistulas: anatomy and diagnostic imaging. AJR Am J Roentgenol 2021;217:1418–29 10.2214/AJR.21.26182 [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Maya M, Prasad RS, et al. Spinal CSF-venous fistulas in morbidly and super obese patients with spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2021;42:397–401 10.3174/ajnr.A6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TY, Karikari IO, Amrhein TJ, et al. Clinical outcomes following surgical ligation of cerebrospinal fluid-venous fistula in patients with spontaneous intracranial hypotension: a prospective case series. Oper Neurosurg (Hagerstown) 2020;18:239–45 10.1093/ons/opz134 [DOI] [PubMed] [Google Scholar]
- 4.Brinjikji W, Savastano LE, Atkinson JLD, et al. A novel endovascular therapy for CSF hypotension secondary to CSF-venous fistulas. AJNR Am J Neuroradiol 2021;42:882–87 10.3174/ajnr.A7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamlouk MD, Shen PY, Sedrak MF, et al. CT-guided fibrin glue occlusion of cerebrospinal fluid-venous fistulas. Radiology 2021;299:409–18 10.1148/radiol.2021204231 [DOI] [PubMed] [Google Scholar]
- 6.Kranz PG, Gray L, Amrhein TJ. Decubitus CT myelography for detecting subtle CSF leaks in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019;40:754–56 10.3174/ajnr.A5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine 2019. Sept 13 [Epub ahead of print] 10.3171/2019.6.SPINE19487 [DOI] [PubMed] [Google Scholar]
- 8.Amrhein TJ, Gray L, Malinzak MD, et al. Respiratory phase affects the conspicuity of CSF-venous fistulas in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2020;41:1754–56 10.3174/ajnr.A6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mamlouk MD, Ochi RP, Jun P, et al. Decubitus CT myelography for CSF-venous fistulas: a procedural approach. AJNR Am J Neuroradiol 2021;42:32–36 10.3174/ajnr.A6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mark IT, Amans MR, Shah VN, et al. Resisted inspiration: a new technique to aid in the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2022;43:1544–47 10.3174/ajnr.A7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headache Classification Committee of the International Headache Society (IHS): the International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 12.Kranz PG, Amrhein TJ, Schievink WI, et al. The “hyperdense paraspinal vein” sign: a marker of CSF-venous fistula. AJNR Am J Neuroradiol 2016;37:1379–81 10.3174/ajnr.A4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg N, Cutsforth-Gregory J, Oushy S, et al. Anatomy of spinal venous drainage for the neurointerventionalist: from puncture site to intervertebral foramen. AJNR Am J Neuroradiol 2022;43:517–25 10.3174/ajnr.A7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madhavan AA, Benson JC, Cutsforth-Gregory JK, et al. Co-existing fast CSF leaks and CSF-venous fistulas on dynamic CT myelography. Radiol Case Rep 2022;17:2968–71 10.1016/j.radcr.2022.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]