Abstract
Aims
Little is known about associations of trimethylamine N-oxide (TMAO), a novel gut microbiota-generated metabolite of dietary phosphatidylcholine and carnitine, and its changes over time with all-cause and cause-specific mortality in the general population or in different race/ethnicity groups. The study aimed to investigate associations of serially measured plasma TMAO levels and changes in TMAO over time with all-cause and cause-specific mortality in a multi-ethnic community-based cohort.
Methods and results
The study included 6,785 adults from the Multi-Ethnic Study of Atherosclerosis. TMAO was measured at baseline and year 5 using mass spectrometry. Primary outcomes were adjudicated all-cause mortality and cardiovascular disease (CVD) mortality. Secondary outcomes were deaths due to kidney failure, cancer, or dementia obtained from death certificates. Cox proportional hazards models with time-varying TMAO and covariates assessed the associations with adjustment for sociodemographics, lifestyles, diet, metabolic factors, and comorbidities. During a median follow-up of 16.9 years, 1704 participants died and 411 from CVD. Higher TMAO levels associated with higher risk of all-cause mortality [hazard ratio (HR): 1.12, 95% confidence interval (CI): 1.08–1.17], CVD mortality (HR: 1.09, 95% CI: 1.00–1.09), and death due to kidney failure (HR: 1.44, 95% CI: 1.25–1.66) per inter-quintile range, but not deaths due to cancer or dementia. Annualized changes in TMAO levels associated with higher risk of all-cause mortality (HR: 1.10, 95% CI: 1.05–1.14) and death due to kidney failure (HR: 1.54, 95% CI: 1.26–1.89) but not other deaths.
Conclusion
Plasma TMAO levels were positively associated with mortality, especially deaths due to cardiovascular and renal disease, in a multi-ethnic US cohort.
Keywords: Trimethylamine N-oxide, Microbiome, Red meat, Cardiovascular disease, Mortality
Structured Graphical Abstract
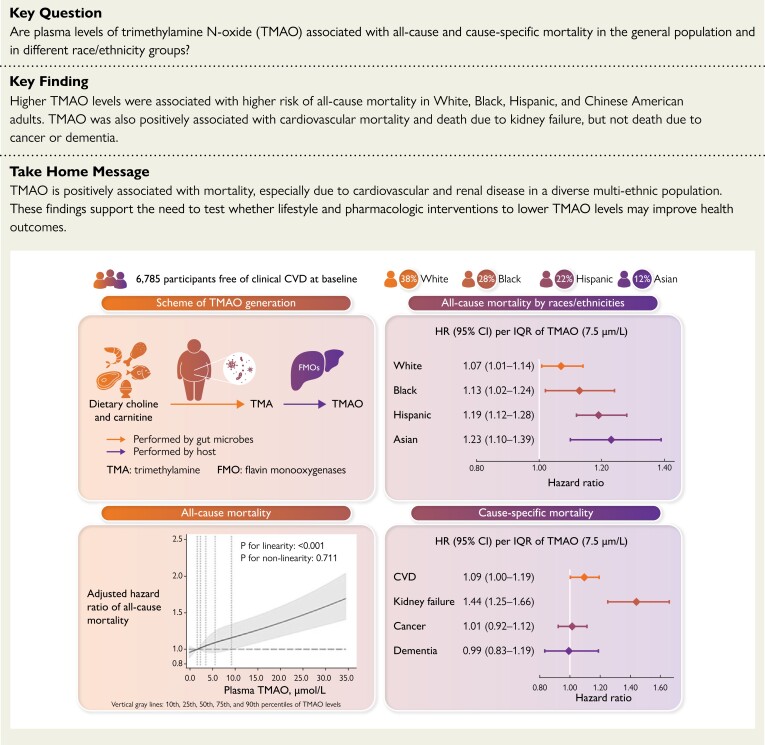
Structured Graphical Abstract.
Plasma TMAO levels are positively associated with mortality, especially deaths due to cardiovascular and renal disease, in a multi-ethnic US cohort. CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; IQR, inter-quintile range, the difference between the midpoint of the first and fifth quintile; TMA, trimethylamine; TMAO, trimethylamine N-oxide.
See the editorial comment for this article ‘They eat what we eat, they digest what we ingest’, by T.F. Lüscher, https://doi.org/10.1093/eurheartj/ehad104.
Introduction
Human gut microbiota are now acknowledged as a novel contributor to host metabolism and health.1,2 Among emerging pathways, trimethylamine N-oxide (TMAO), a gut microbiota-derived metabolite of dietary phosphatidylcholine, choline, and carnitine, rich in animal source foods, may play a role in the pathogenesis of multiple diseases.3–7 Plasma TMAO has been associated with cardiovascular disease (CVD) in clinical and population-based studies,8–14 and enhances atherogenesis and thrombosis in several,3–5,15–17 although not all,18 mechanistic studies. TMAO also appears to contribute to renal tubulointerstitial fibrosis and dysfunction6,19 and hyperglycemia and glucose intolerance.20,21 Elevated TMAO levels have also been hypothesized to contribute to aging-related cognitive dysfunction22 and promote carcinogenesis via inflammatory and oxidative stress pathways.23
In clinical samples of patients with prevalent cardiometabolic and chronic kidney diseases, higher plasma TMAO associates with all-cause mortality.12–14,24 However, to our knowledge, no studies have examined whether TMAO relates to all-cause and cause-specific mortality in well-characterized community-based cohorts using serial TMAO measures. Such studies can provide critical new evidence and address potential bias in prior studies. For example, prevalent diseases can influence dietary and other lifestyle behaviors as well as biologic pathways that alter circulating TMAO levels, leading to reverse causation. Lack of information on sociodemographic, dietary, and other lifestyle factors in prior clinical studies also raises the possibility that observed associations are subject to meaningful residual confounding, especially by education, income, diet, and physical activity. Prior studies also evaluated only a single measure of TMAO at baseline, but TMAO levels are known to vary over time due to changes in diet, microbial composition, and renal clearance.25 Finally, prior findings are based on samples of primarily White participants, and assessment of TMAO and mortality in other races and ethnicities is a priority.
To elucidate whether plasma TMAO levels are associated with incident mortality risk in a diverse general population during long-term follow-up, we examined the relationship between serial measures of plasma TMAO and all-cause and cause-specific mortality in the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective, community-based, multi-racial cohort with deep phenotyping. We hypothesized that higher levels of serially measured plasma TMAO, as well as increases in TMAO over time, would be associated with higher all-cause mortality and CVD mortality. Other cause-specific mortality outcomes were explored as secondary outcomes.
Methods
Study population
MESA is an ongoing multi-center, community-based, prospective cohort study to investigate characteristics and risk factors for onset and progression of subclinical CVD in diverse races/ethnicities.26 Briefly, 6814 adults aged 45–84 years were recruited in 2000–02 from six study sites (Baltimore County, MD; Chicago, IL; Forsyth County, NC; New York, NY; Los Angeles County, CA; St Paul, MN), including 38% White, 28% Black, 22% Hispanic, and 12% Chinese-American adults. All MESA participants were free of clinical CVD at cohort entry. The study was approved by the institutional review board of each participating university, and all participants provided informed written consent. The present study included 6785 individuals with at least one plasma TMAO measurement and follow-up for mortality (99.6% of all enrolled participants).
TMAO measurement
Plasma TMAO concentrations were measured at the Cleveland Clinic Lerner Research Institute using stored frozen (−80˚C) fasting blood samples collected at MESA baseline (2000–02) and exam 4 (2005–07). Among the 6785 participants, 5614 had TMAO measured at both visits, 1162 only at baseline (20% of these patients had died by exam 4), and 9 only at exam 4. Spearman correlation coefficient of baseline and exam 4 TMAO levels was 0.32 (P < 0.001). TMAO was quantified by investigators blinded to sample outcome using a stable-isotope dilution assay coupled with high-performance liquid chromatography, with online electrospray ionization tandem mass spectrometry on a Shimadzu 8050 or 8060 mass spectrometer.27,28 Laboratory coefficients of variation were <6%.
Mortality assessment
A detailed description of MESA event surveillance and classification has been published.26,29 Briefly, participants were contacted by phone every 9–12 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses and procedures, and deaths. Copies of relevant records were requested. In addition, MESA occasionally identified medical encounters through cohort clinic visits, participant call-ins, medical record abstractions, and obituaries. Primary outcomes for the present analysis were all-cause mortality and CVD mortality (death due to coronary heart disease, stroke, other atherosclerotic diseases, or other CVD), adjudicated through 31 December 2018. Secondary outcomes were deaths due to kidney failure, cancer, dementia, and other causes. CVD deaths were centrally adjudicated based on death certificates, hospitalization records, outpatient cardiovascular diagnoses and procedures, and next-of-kin interviews. Underlying cause of non-CVD deaths was obtained from the International Classification of Diseases (ICD)-10 code indicated on the death certificate or state/city vital statistics departments.
Covariates
At each cohort exam, information on demographics, lifestyle, diet, anthropometrics, medical history, medications, and other risk factor including blood biomarkers were collected by questionnaires, physical examinations, and fasting blood draw performed by trained personnel following standardized protocols.26 See Text S1 for full details.
Statistical analysis
Cox proportional hazards models with time-varying TMAO and covariates and cause-specific hazard functions investigated associations of serial measures of TMAO with mortality, with follow-up from the first TMAO measurement until last study contact. The proportional hazards assumption was examined based on Schoenfeld residuals30 and not violated for either TMAO or change in TMAO (see Text S2 and Table S1 for details).
Primary analyses utilized time-varying cumulative averages of serial TMAO measures, with the baseline measure (2000–02) related to mortality risk until exam 4 (2005–07), and the average of measures at baseline and exam 4 related to subsequent risk. For participants with only one TMAO measure, that TMAO measure was carried-forward. We analyzed TMAO linearly per inter-quintile range (IQR, the difference between the midpoint of the first and fifth quintile) and used restricted cubic splines to explore potential non-linear associations. In sensitivity analysis, TMAO measures were assessed using simple updating (i.e. baseline TMAO was related to risk until exam 4; and exam 4 TMAO, to subsequent risk).
Changes in TMAO levels were evaluated among 5614 participants with two serial measures (see Supplementary data online, Table S2). Annualized TMAO changes from baseline to exam 4 were computed and related to mortality risk after exam 4, modeled linearly using similar methods as above.
To minimize confounding, three multivariable models with pre-specified covariates were fitted, with Model 3 defined as our primary model for inference on associations: Model 1, adjusted for age, sex, race/ethnicity, and study site; Model 2, further adjusted for education, income, total energy intake, alternate healthy eating index (AHEI) score, and time-varying smoking status, alcohol intake, physical activity, and antibiotic use; and Model 3, additionally adjusted for time-varying metabolic factors including body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, C-reactive protein (CRP), and diabetes, and comorbidities (for all-cause mortality only). Mediator models further adjusted for factors which are experimentally influenced by TMAO and could be mediators (on the causal pathway) and/or confounders of TMAO-mortality associations,1,6,19 including time-varying estimated glomerular filtration rate (eGFR) and interim CVD events occurring between baseline and exam 4. For changes in TMAO, similar multivariable models with exam 4 covariates were used to assess associations with mortality after exam 4, with further adjustment for baseline TMAO and interim CVD events prior to exam 4. In sensitivity analyses, we used annualized changes in continuous covariates from baseline to exam 4.
To explore whether associations of time-varying TMAO with the primary outcomes (all-cause mortality, CVD mortality) varied by baseline age, sex, race/ethnicity, dietary pattern (AHEI score < vs. ≥ median), and eGFR (< 60 vs. ≥ 60 mL/min/1.73 m2), we included multiplicative interaction terms between TMAO and each variable in separate multivariable models. Statistical significance of these exploratory interactions was Bonferroni adjusted for multiple comparisons (P < 0.005; 5 interaction variables × 2 primary mortality outcomes = 10 comparisons). Statistical significance for other analyses was defined as a two-sided alpha = 0.05. Analyses were performed using Stata 16.1 (Stata Corp, College Station, TX).
Results
Participant characteristics
At baseline, mean (SD) age was 62 (10) years, and 47.2% were males (Table 1). Proportions of White, Black, Hispanic, and Chinese-American adults were 38.5%, 27.7%, 22.0%, and 11.8%, respectively. About 13% of participants were current smokers, 13% had diabetes, 37% were on anti-hypertensive medications, and 16% were on lipid-lowering medications. Three percent had taken antibiotics in the past two weeks. Participants with higher TMAO levels were more likely to be older, male, White, physically inactive, and on anti-hypertensive or lipid-lowering medications. Higher TMAO levels were also associated with diabetes, higher triglycerides and glucose, and lower eGFR.
Table 1.
Characteristics of 6785 participants in MESA, by quintiles of plasma TMAO levels at baselinea
| Q1 | Q2 | Q3 | Q4 | Q5 | Total | |
|---|---|---|---|---|---|---|
| n | 1359 | 1357 | 1355 | 1357 | 1357 | 6785 |
| TMAO range, µmol/L | 0.10–2.21 | 2.22–3.04 | 3.05–4.16 | 4.17–6.29 | 6.30–129.16 | 0.1− 129.16 |
| Sociodemographic | ||||||
| Age, years | 59.0 ± 9.9 | 61.2 ± 9.9 | 62.6 ± 9.9 | 63.5 ± 10.1 | 64.5 ± 10.4 | 62.2 ± 10.2 |
| Male sex | 604 (44.4) | 595 (43.8) | 639 (47.2) | 684 (50.4) | 680 (50.1) | 3202 (47.2) |
| Race | ||||||
| ȃWhite | 402 (29.6) | 523 (38.5) | 534 (39.4) | 580 (42.7) | 575 (42.4) | 2614 (38.5) |
| ȃBlack | 404 (29.7) | 364 (26.8) | 377 (27.8) | 365 (26.9) | 367 (27.0) | 1877 (27.7) |
| ȃHispanic | 336 (24.7) | 313 (23.1) | 293 (21.6) | 290 (21.4) | 259 (19.1) | 1491 (22.0) |
| ȃChinese—American | 217 (16.0) | 157 (11.6) | 151 (11.1) | 122 (9.0) | 156 (11.5) | 803 (11.8) |
| Education | ||||||
| ȃ<High school | 256 (18.8) | 252 (18.6) | 231 (17.0) | 238 (17.5) | 246 (18.1) | 1223 (18.0) |
| ȃHigh school | 212 (15.6) | 254 (18.7) | 254 (18.7) | 262 (19.3) | 251 (18.5) | 1233 (18.2) |
| ȃSome college/technical school/associate degree | 410 (30.2) | 366 (27.0) | 399 (29.4) | 391 (28.8) | 367 (27.0) | 1933 (28.5) |
| ȃBachelor | 253 (18.6) | 258 (19.0) | 213 (15.7) | 231 (17.0) | 217 (16.0) | 1172 (17.3) |
| ȃGraduate school | 228 (16.8) | 227 (16.7) | 258 (19.0) | 235 (17.3) | 276 (20.3) | 1224 (18.0) |
| Annual household income | ||||||
| ȃ<$20 000 | 320 (23.5) | 298 (22.0) | 364 (26.9) | 315 (23.2) | 329 (24.2) | 1626 (24.0) |
| ȃ$20 000–<$50 000 | 508 (37.4) | 493 (36.3) | 488 (36.0) | 535 (39.4) | 493 (36.3) | 2517 (37.1) |
| ȃ$50 000–<$100 000 | 360 (26.5) | 377 (27.8) | 336 (24.8) | 338 (24.9) | 328 (24.2) | 1739 (25.6) |
| ȃ≥$100 000 | 171 (12.6) | 189 (13.9) | 167 (12.3) | 169 (12.5) | 207 (15.3) | 903 (13.3) |
| Lifestyles | ||||||
| Smoking | ||||||
| ȃNever smoked | 713 (52.5) | 707 (52.1) | 665 (49.1) | 668 (49.2) | 659 (48.6) | 3412 (50.3) |
| ȃFormer smoker | 437 (32.2) | 477 (35.2) | 522 (38.5) | 523 (38.5) | 529 (39.0) | 2488 (36.7) |
| ȃCurrent smoker | 209 (15.4) | 173 (12.7) | 168 (12.4) | 166 (12.2) | 169 (12.5) | 885 (13.0) |
| Alcohol (drinks/week) | 0.0 (0.0–3.0) | 0.0 (0.0–3.1) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) | 0.0 (0.0–3.0) |
| Exercise (MET)-minutes/week | 825 (150–2070) | 840 (210–1958) | 840 (105–2048) | 840 (123–2100) | 760 (35–1920) | 825 (105–2028) |
| Alternate healthy eating index (AHEI)b | 41.1 ± 11.5 | 41.3 ± 11.7 | 41.0 ± 11.4 | 41.1 ± 12.0 | 40.6 ± 11.3 | 41.0 ± 11.6 |
| Total energy, kcal/day | 1644 ± 907 | 1606 ± 881 | 1650 ± 896 | 1642 ± 880 | 1577 ± 804 | 1624 ± 875 |
| Metabolic factors | ||||||
| Body mass index (kg/m2) | 27.6 ± 5.6 | 28.2 ± 5.3 | 28.4 ± 5.4 | 28.9 ± 5.6 | 28.6 ± 5.4 | 28.3 ± 5.5 |
| Waist circumference, cm | 95.2 ± 14.6 | 97.4 ± 14.0 | 98.6 ± 14.3 | 100.0 ± 14.1 | 99.6 ± 14.7 | 98.2 ± 14.4 |
| Systolic blood pressure, mmHg | 125 ± 22 | 125 ± 21 | 127 ± 21 | 128 ± 22 | 128 ± 22 | 127 ± 21 |
| Diastolic blood pressure, mmHg | 72 ± 10 | 72 ± 10 | 72 ± 10 | 72 ± 11 | 72 ± 10 | 72 ± 10 |
| HDL cholesterol, mg/dL | 52 ± 14 | 52 ± 16 | 51 ± 14 | 50 ± 15 | 50 ± 15 | 51 ± 15 |
| LDL cholesterol, mg/dL | 118 ± 32 | 118 ± 32 | 118 ± 33 | 115 ± 32 | 115 ± 33 | 117 ± 32 |
| Triglycerides, mg/dL | 105 (75–157) | 110 (77–155) | 113 (81–161) | 115 (79–166) | 112 (77–162) | 111 (78–161) |
| C-reactive protein, mg/L | 1.6 (0.7–4.0) | 2.0 (0.8–4.4) | 1.9 (0.9–4.4) | 2.1 (0.9–4.4) | 2.0 (0.9–4.2) | 1.9 (0.8–4.3) |
| Fasting glucose, mg/dL | 95 ± 29 | 95 ± 27 | 97 ± 27 | 100 ± 34 | 100 ± 33 | 97 ± 30 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 98 ± 15 | 93 ± 15 | 90 ± 17 | 86 ± 18 | 83 ± 22 | 90 ± 18 |
| Medical history c | ||||||
| Diabetes mellitus | 115 (8.5) | 139 (10.2) | 155 (11.4) | 202 (14.9) | 241 (17.8) | 852 (12.6) |
| Cancer (excluding non-melanoma skin cancer) | 54 (4.0) | 69 (5.1) | 94 (6.9) | 102 (7.5) | 83 (6.1) | 402 (5.9) |
| Emphysema | 20 (1.5) | 20 (1.5) | 23 (1.7) | 19 (1.4) | 20 (1.5) | 102 (1.5) |
| Liver disease | 45 (3.3) | 44 (3.2) | 41 (3.0) | 50 (3.7) | 55 (4.1) | 235 (3.5) |
| Anti-hypertensive medication | 365 (26.9) | 434 (32.0) | 508 (37.5) | 593 (43.7) | 623 (45.9) | 2523 (37.2) |
| Lipid-lowering medication | 188 (13.8) | 205 (15.1) | 218 (16.1) | 245 (18.1) | 245 (18.1) | 1101 (16.2) |
| Antibiotics | 50 (3.7) | 33 (2.4) | 38 (2.8) | 41 (3.0) | 43 (3.2) | 205 (3.0) |
Data are mean ± SD or median (interquartile range, 25th – 75th percentile) for continuous variables and n (%) for categorical variables.
Baseline was defined as the first TMAO measurement (n = 6776 at exam 1, n = 5623 at exam 4). All participants were free of CVD at baseline.
Total AHEI score can range from 2.5 (worst) to 87.5 (best).
Diabetes was defined based on treatment with either oral hypoglycemic agents and/or insulin or having a fasting plasma glucose level of ≥ 126 mg/dL. Cancer, emphysema, and liver disease were defined based on self-report. Medications were defined based on use in the past two weeks prior to baseline.
Time-varying cumulative averages of TMAO measures
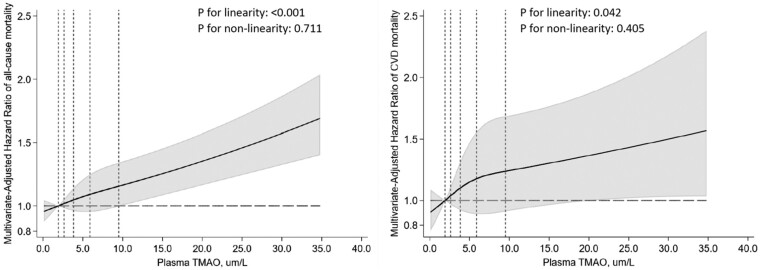
During median follow-up of 16.9 years (maximum, 18.5 years), 1704 deaths occurred, including 411 from CVD. After adjusting for sociodemographics, lifestyles, metabolic factors, and self-reported comorbidities (Model 3), higher plasma TMAO levels associated with 12% higher all-cause mortality (Table 2) [hazard ratio (HR): 1.12, 95% confidence interval (CI): 1.08–1.17, P < 0.001] and 9% higher CVD mortality (HR: 1.09, 95% CI: 1.00–1.19, P = 0.042) per IQR. With further adjustment for eGFR, which could be both a mediator and confounder, the association with all-cause mortality remained statistically significant (HR: 1.09, 95% CI: 1.05–1.13, P < 0.001); while the association with CVD mortality was attenuated and no longer statistically significant (HR: 1.07, 95% CI: 0.98–1.17, P = 0.151). Further adjustment for interim CVD events prior to exam 4 did not change the results appreciably. For both all-cause and CVD mortality, analyses using restricted cubic splines revealed no significant departure from a linear dose–response relationship (Figure 1).
Table 2.
Risk of all-cause and cause-specific mortality associated with time-varying cumulative averages of plasma TMAO levels (per IQR) among 6785 adults in MESA
| All-cause mortality | Adjudicated CVD mortalitya | Underlying cause of death from death certificateb | ||||
|---|---|---|---|---|---|---|
| Kidney failure | Cancer | Dementia | Other | |||
| ICD code | N/A | N/A | N17-N19 | C00-C97 | G30, F00, F01, F03 | N/A |
| No. of deaths | 1704 | 411 | 32 | 496 | 154 | 611 |
| Unadjusted | 1.18 (1.15–1.22) | 1.17 (1.10–1.25) | 1.39 (1.26–1.54) | 1.10 (1.02–1.19) | 1.14 (1.00–1.29) | 1.23 (1.18–1.28) |
| Multivariable: | ||||||
| Model 1 | 1.12 (1.08–1.16) | 1.10 (1.02–1.20) | 1.43 (1.27–1.62) | 1.02 (0.93–1.12) | 0.97 (0.80–1.17) | 1.18 (1.12–1.24) |
| Model 2 | 1.13 (1.09–1.17) | 1.12 (1.03–1.21) | 1.46 (1.27–1.67) | 1.02 (0.93–1.13) | 0.98 (0.82–1.18) | 1.19 (1.13–1.25) |
| Model 3 (primary) | 1.12 (1.08–1.17) | 1.09 (1.00–1.19) | 1.44 (1.25–1.66) | 1.01 (0.92–1.12) | 0.99 (0.83–1.19) | 1.18 (1.12–1.24) |
| Model 3 + eGFRc | 1.09 (1.05–1.13) | 1.07 (0.98–1.17) | 1.22 (0.99–1.51) | 1.00 (0.90–1.11) | 1.00 (0.84–1.20) | 1.12 (1.06–1.19) |
| Model 3 + eGFR + CVDd | 1.09 (1.05–1.14) | 1.07 (0.98–1.17) | 1.21 (0.97–1.50) | 1.00 (0.90–1.11) | 1.00 (0.84–1.19) | 1.12 (1.06–1.19) |
Model 1 adjusted for age, sex, race/ethnicity, and study site.
Model 2 further adjusted for education (<high school, high school, some college, bachelor’s degree, graduate school), household income (<$20,000, $20,000 − < $50,000, $50,000 − < $100,000, ≥ $100 000), alternate healthy eating index, total energy intake, and time-varying smoking status (never, former, and current), alcohol intake(drinks/week), physical activity (MET-MIN per week), and antibiotic use in the past two weeks.
Model 3 further adjusted for time-varying BMI, waist circumference, systolic blood pressure, diastolic blood pressure, LDL-C, HDL-C, triglycerides, CRP, diabetes, anti-hypertensive medication, lipid-lowering mediation, and comorbidities (for all-cause mortality only) including emphysema, liver disease, and cancer (except for non-melanoma skin cancer). Time-varying covariates were updated at the same time of TMAO update using the most recent measures (i.e. simple update).
CVD deaths included those due to atherosclerotic coronary heart disease (CHD) (with sub-classifications of definite fatal MI, definite fatal CHD, and possible fatal CHD), stroke, other atherosclerotic disease, and other cardiovascular disease.
For kidney failure, ICD codes included N17-N19; for cancer, C00-C97; and for dementia, G30, F00, F01, and F03.Other causes of death: deaths due to any causes except for CVD, kidney failure, cancer, and dementia.
eGFR was included as a continuous linear term (mL/min/1.73 m2).
Interim nonfatal CVD events (MI, resuscitated cardiac arrest, angina, stroke) between exam 1 and exam 4, for risk after exam 4.
IQR: inter-quintile range, comparing the midpoints of the first and fifth quintiles (7.5 µmol/L).
Figure 1.
Dose–response relationships of serial measures of plasma TMAO levels with the risk of all-cause mortality (left) and cardiovascular mortality (right). Relationships were evaluated using restricted cubic splines with three knots at the 10th, 50th, and 90th percentiles. Dotted vertical lines represent, from left to right, the 10th, 25th, 50th, 75th, and 90th percentiles of TMAO levels. Variables adjusted were the same as Model 3 (primary model) in Table 2. The top 1% of the TMAO distribution was not shown for better visualization.
Among other causes of death, higher plasma TMAO associated with higher risk of death due to kidney failure (HR 1.44, 95% CI 1.25–1.66, P < 0.001) and miscellaneous causes (HR: 1.18, 95% CI: 1.12–1.24, P < 0.001) (see Supplementary data online, Table S3 for these other causes), but not death due to cancer or dementia.
Annualized changes in TMAO
During a median follow-up of 12.3 years (maximum, 13.3 years) after the second TMAO measure at exam 4, 1166 participants died, including 281 from CVD. Annualized increases in plasma TMAO from baseline to exam 4 associated with 10% higher all-cause mortality (Table 3, HR: 1.10, 95% CI: 1.05–1.14, P < 0.001) per IQR. This association remained significant after further adjusting for eGFR (HR: 1.06, 95% CI: 1.01–1.12, P = 0.011). Changes in TMAO levels were not significantly associated with CVD mortality.
Table 3.
Risk of all-cause and cause-specific mortality associated with annualized changes in plasma TMAO levels (per IQR) among 5614 adults in MESA with two serial TMAO measures
| All-cause mortality | Adjudicated CVD mortalitya | Underlying cause of death from death certificateb | ||||
|---|---|---|---|---|---|---|
| Kidney failure | Cancer | Dementia | Other | |||
| ICD code | N/A | N/A | N17-N19 | C00-C97 | G30, F00, F01, F03 | N/A |
| No. of deaths | 1166 | 281 | 27 | 331 | 112 | 415 |
| Unadjusted | 1.08 (1.03–1.13) | 0.99 (0.87–1.12) | 1.32 (1.19–1.46) | 1.07 (0.97–1.17) | 0.94 (0.77–1.15) | 1.11 (1.03–1.20) |
| Multivariable | ||||||
| Model 1 | 1.10 (1.05–1.14) | 1.04 (0.93–1.15) | 1.43 (1.22–1.68) | 1.05 (0.95–1.17) | 1.00 (0.82–1.21) | 1.13 (1.06–1.20) |
| Model 2 | 1.10 (1.05–1.15) | 1.04 (0.94–1.16) | 1.47 (1.22–1.77) | 1.05 (0.95–1.17) | 0.98 (0.81–1.19) | 1.14 (1.07–1.21) |
| Model 3 (primary) | 1.10 (1.05–1.14) | 1.03 (0.93–1.15) | 1.54 (1.26–1.89) | 1.04 (0.93–1.16) | 1.00 (0.84–1.20) | 1.13 (1.07–1.21) |
| Model 3 + eGFRc | 1.06 (1.01–1.12) | 1.00 (0.89–1.13) | 1.23 (0.98–1.55) | 1.02 (0.91–1.15) | 1.02 (0.86–1.21) | 1.09 (1.02–1.17) |
Model 1 adjusted for age, sex, race/ethnicity, study site, and exam 1 TMAO.
Model 2 further adjusted for education (<high school, high school, some college, bachelor’s degree, graduate school), household income (<$20,000, $20,000 − < $50,000, $50,000 − < $100,000, ≥ $100 000), alternate healthy eating index, total energy intake, and exam 4 smoking status (never, former, and current), alcohol intake(drinks/week), physical activity (MET-MIN per week), and antibiotics use in the past 2 weeks.
Model 3 further adjusted for exam 4 BMI, waist circumference, systolic blood pressure, diastolic blood pressure, LDL-C, HDL-C, triglycerides, CRP, diabetes, interim nonfatal cardiovascular events (MI, resuscitated cardiac arrest, angina, or stroke prior to exam 4), anti-hypertensive medication, lipid-lowering mediation, and comorbidities (for all-cause mortality only) including emphysema, liver disease, and cancer (except for non-melanoma skin cancer).
CVD deaths included those due to atherosclerotic coronary heart disease (CHD) (with sub-classifications of definite fatal MI, definite fatal CHD, and possible fatal CHD), stroke, other atherosclerotic disease, and other cardiovascular disease.
For kidney failure, ICD codes included N17-N19; for cancer, C00-C97; and for dementia, G30, F00, F01, and F03.Other causes of death: deaths due to any causes except for CVD, kidney failure, cancer, and dementia.
eGFR was included as a continuous linear term (mL/min/1.73m2).
IQR, inter-quintile range, comparing the midpoints of the first and fifth quintiles (2.1 µmol/L annualized increase).
Restricted cubic spline analyses identified departures from a linear dose–response relationship for changes in TMAO (see Supplementary data online, Figure S1) and mortality, although these deviations from linearity were no longer statistically significant (P-nonlinearity = 0.439 for all-cause mortality and 0.123 for CVD mortality) in sensitivity analyses removing observations in the top 1% of the distribution of TMAO changes (see Supplementary data online, Figure S2).
Among other causes of death, increases in plasma TMAO over time associated with higher risk of death due to kidney failure (HR: 1.54, 95% CI: 1.26–1.89, P < 0.001) and miscellaneous causes (HR: 1.13, 95% CI: 1.07–1.21, P < 0.001), but not deaths due to cancer or dementia (Table 3).
Sensitivity analyses
Results for the associations of TMAO levels with all-cause and cause-specific mortality were similar but slightly weaker using simple updating of TMAO in place of cumulative updating (see Supplementary data online, Table S4). Results for annualized changes in TMAO were similar when adjusting for changes in continuous covariates from baseline to exam 4 in place of exam 4 values (see Supplementary data online, Table S5).
Effect modification
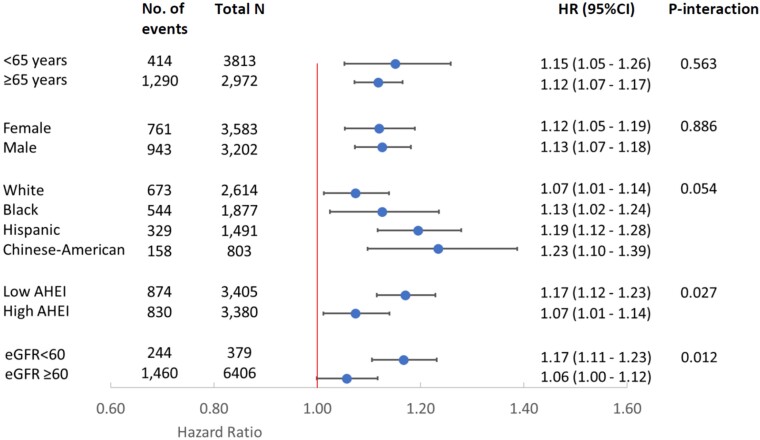
Associations of TMAO with all-cause and CVD mortality were not significantly modified by differences in age, sex, race/ethnicity, AHEI, or eGFR (P-interaction >0.005 each, Figure 2 & Supplementary data online, Table S6). However, there were nonsignificant trends toward effect modification of the TMAO-mortality relationship by eGFR (P-interaction = 0.012) and AHEI (alternate health eating index, P-interaction = 0.027), with potentially stronger associations among those with lower renal function and lower diet quality. The association between TMAO and all-cause mortality was statistically significant in every race/ethnicity group (Figure 2), including among White (HR: 1.07, 95% CI: 1.01–1.14), Black (HR: 1.13, 95% CI: 1.02–1.24), Hispanic, (HR: 1.19, 95% CI: 1.12–1.28) and Chinese-American (HR: 1.23, 95% CI: 1.10–1.39) adults.
Figure 2.
Risk of all-cause mortality associated with serial measures of plasma TMAO levels (per IQR): subgroup analysis. Variables adjusted were the same as Model 3 (primary model) in Table 2. HRs were obtained from the beta coefficients of TMAO and the interaction terms. After Bonferroni’s correction for multiple comparisons, statistical significance was defined as a two-sided P value of <0.005. AHEI was dichotomized based on median value at baseline (low: < 40.4, high ≥ 40.4). IQR: inter-quintile range, comparing the midpoints of the first and fifth quintiles (7.5 µmol/L). AHEI, alternate healthy eating index, with higher score indicating better diet quality. eGFR, estimated glomerular filtration rate, with higher values indicating better renal function.
Discussion
In this large, multi-ethnic, community-based cohort of US adults, higher plasma TMAO levels associated with higher risk of all-cause and CVD mortality. Increases in TMAO over time also associated with higher risk of all-cause, but not CVD, mortality. TMAO and its increases over time also each associated with higher risk of death due to kidney failure, but not due to cancer or dementia. The positive association between TMAO and all-cause mortality was observed in every race/ethnicity group examined including White, Black, Hispanic, and Chinese-American adults (Structured Graphical Abstract). To our knowledge, this is the first study to investigate associations of serial measures of plasma TMAO and its changes over time with mortality in a multi-ethnic, community-based cohort.
In mechanistic and animal studies, TMAO promotes several pathways related to atherosclerosis and thrombosis, including inhibiting reverse cholesterol transport,4 impairing endothelial function,16 and promoting macrophage cholesterol accumulation,3 platelet hyperreactivity and thrombosis potential,5,17 and vascular inflammation and inflammasome activation.15,31 TMAO also induces hyperglycemia by binding to endoplasmic reticulum stress kinase PERK and impairs glucose tolerance.20,21 Furthermore, TMAO is bidirectionally associated with renal function. In animal studies, TMAO causally reduces GFR, and elevates cystatin C levels, microalbuminuria, and renal tubulointerstitial fibrosis,6 and these renal impairments are reduced by targeted inhibition of gut microbial TMAO generation.19 Conversely, renal function also directly impacts TMAO levels, given its renal clearance. Thus, TMAO’s cardiorenal toxicity could be more pronounced in settings of reduced renal function. Evidence for a mechanistic link of TMAO with dementia and cancer is less clear than for cardiovascular and renal disease. Limited animal studies suggest that TMAO may promote aging-related cognitive decline via increased neuroinflammation, oxidative stress, synaptic damage, and astrocyte activation.22,32 TMAO has also been hypothesized to promote carcinogenesis via inflammatory and oxidative stress pathways,23 but direct evidence from mechanistic studies examining carcinogenesis outcome is still lacking.
In the present study of a multi-ethnic, community-based cohort free of clinical CVD at baseline, we observed a 16% higher risk of all-cause mortality per 10 µmol/L of TMAO levels (12% per IQR of 7.5 µmol/L) after adjusting for sociodemographic factors, habitual diet, lifestyles, traditional CVD risk factors, and comorbidities. The dose–response relationship appeared to be linear with no threshold effect. Furthermore, we observed significant associations of TMAO with death due to CVD and kidney failure, but not cancer or dementia. The specificity of associations for deaths due to CVD and kidney failure, rather than other cause-specific deaths, further supports TMAO’s role in atherosclerosis, thrombosis, and renal impairment as seen in multiple mechanistic studies.7
The association between changes in TMAO levels over time and mortality has not been studied. Investigating changes may have implications for developing intervention strategies to modify TMAO levels. We found that annualized changes in TMAO over ∼5-years were positively associated with all-cause mortality and, among specific causes, deaths from kidney failure. In spline analysis, decreases in TMAO levels over time also appeared associated with a lower risk of CVD mortality, while increases over time were not significantly associated with the risk. These novel findings support the hypothesis that reducing TMAO levels by lifestyle or pharmacological interventions may influence mortality risk. Implications of TMAO increases over time are less clear, due to wide CIs at higher levels of positive TMAO changes.
Nearly all previous studies examining associations between plasma TMAO levels and mortality have focused on clinical populations with prevalent CVD, diabetes, and/or chronic kidney disease, and predominantly White patients.12–14,24 These studies also only measured TMAO at baseline and lacked data on major potential confounders including sociodemographic, dietary, and other lifestyle factors. Two prior studies evaluated the risk of all-cause mortality per continuous difference in TMAO levels. One among patients with chronic kidney disease found a 26% (95% CI 13%–40%) higher mortality risk per 10 µmol/L of TMAO;33 and the other among adults from a city in the Netherlands found a 56% (3%–131%) higher mortality risk per 10 µmol/L of TMAO.34 Neither of these studies adjusted for socioeconomic status (e.g. education, income), dietary habits, or physical activity. Three dose–response meta-analyses with varying analysis methods and inclusions of different studies estimated the pooled relative risks of all-cause mortality, with results ranging from 7.6% to 137% higher risk per 10 µmol/L of TMAO.12,24,35 Our findings expand upon and greatly extend these prior results by examining associations in a well-characterized, multi-ethnic, community-based cohort free of clinical CVD at baseline; evaluating serial TMAO measures and changes in TMAO over time; utilizing rigorous control for a range of confounding factors including socioeconomic status, dietary habits, and physical activity; and examining cause-specific mortality.
The association of TMAO with all-cause mortality remained significant following adjustment for eGFR, a measure of renal function. This finding is consistent with prior clinical studies where the association between a single measure of TMAO and all-cause mortality was independent of traditional CVD risk factors and eGFR.6,8,36–39 However, three other studies found TMAO’s association with mortality to be attenuated and no longer statistically significant after adjustment for eGFR or urine albumin.34,40,41 In the present study, associations of TMAO with deaths due to CVD and kidney failure were attenuated and no longer statistically significant following eGFR adjustment. As discussed above, given mechanistically bi-directional pathways between TMAO and renal function, renal function could be both a confounder and an intermediate outcome (i.e. mediator) on the causal pathway between TMAO and mortality. Thus, differences in findings following renal function adjustment might be explained by differing mediation pathways and/or confounding structures for specific outcomes in different populations. Our findings in this multi-ethnic population of US adults, average age 62 years and free of clinical CVD at baseline, suggest that TMAO is a risk factor for all-cause mortality independent of renal function and other risk factors.
The positive associations between TMAO and all-cause and cardiovascular mortality found in the entire population were also observed among those with baseline eGFR < 60 mL/min/1.73 m2, suggesting that TMAO is independently associated with mortality risk among those with impaired renal function. In exploratory analyses, these associations appeared potentially stronger than among those with normal renal function. Similar patterns of effect modification were reported in the PREVEND study34 and the Cardiovascular Health Study.41 Given that renal function could be a downstream product of the exposure TMAO, analyses stratified by eGFR could potentially induce bias (e.g. collider stratification bias) and should be interpreted cautiously.42,43 Our novel findings highlight the need to further investigate the interrelationships between renal function, TMAO, and mortality.
In addition to eGFR, we observed a nonsignificant trend toward a potentially stronger TMAO-mortality association among those with lower diet quality, compared with those with higher diet quality. A similar pattern of effect modification by AHEI was reported in a cohort of mostly White US nurses assessing changes in plasma TMAO levels and incident coronary heart disease.11 Although exploratory, taken together, these findings suggest that a healthier diet may help mitigate TMAO-associated mortality risk.
Notably, we identified significant associations of plasma TMAO with all-cause mortality in each race/ethnicity group in this cohort, including among White, Black, Hispanic, and Chinese-American adults. The magnitude of the association appeared potentially stronger in Chinese-American and Hispanic adults, compared to other race/ethnicity groups, but these differences were not statistically significant. Our study is the first, to our knowledge, to demonstrate positive associations between TMAO and mortality in diverse US racial and ethnic groups.
Our study has several strengths. It was conducted in a large, community-based, multi-ethnic prospective cohort with serial TMAO measures, a broad range of well-measured health phenotypes (including detailed sociodemographic factors, habitual diet, lifestyles, and traditional CVD risk factors and renal function), and a large number of centrally adjudicated mortality outcomes during long-term follow-up. Thus, our findings are less subject to reverse causation, measurement errors, and residual confounding, and have greater statistical power and generalizability than many of the prior studies. Furthermore, the multi-ethnic feature of MESA participants allowed us to examine the associations in each race/ethnicity group including White, Black, Hispanic, and Chinese-American adults, providing unique race/ethnicity-specific data.
Potential limitations should be considered. The observational design cannot exclude residual confounding. However, we adjusted for a broad range of well-measured mortality risk factors, and results were robust except for additional adjustment for eGFR (which could be both a mediator and confounder) for deaths due to CVD and kidney failure. Unlike adjudicated CVD mortality that should have minimal outcome misclassification, other cause-specific mortality outcomes were obtained based on the underlying cause of death from death certificates which is subject to reporting errors.44 However, the differential associations of TMAO with these secondary outcomes consistent with mechanistic evidence in part support their relative accuracy. Statistical power to detect associations for CVD and other cause-specific mortality outcomes may be limited.
Conclusions
In this multi-ethnic, community-based cohort of US adults, higher serial measures of plasma TMAO levels were associated with higher risk of all-cause mortality, CVD mortality, and death due to kidney failure, but not death due to cancer or dementia. Increases in TMAO levels over time were associated with higher risk of all-cause mortality and death due to kidney failure, but not death due to CVD, cancer, or dementia. The positive association between TMAO and all-cause mortality was present in every race/ethnicity group examined including White, Black, Hispanic, and Chinese-American adults, and remained significant after further adjustment for renal function. These findings support the need to test whether lifestyle and pharmacologic interventions to lower TMAO levels may improve clinical outcomes.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The information contained herein was derived in part from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene.
Contributor Information
Meng Wang, Friedman School of Nutrition Science and Policy, Tufts University, 150 Harrison Ave, Boston, MA 02111, USA.
Xinmin S Li, Department of Cardiovascular and Metabolic Sciences, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Center for Microbiome and Human Health, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Zeneng Wang, Department of Cardiovascular and Metabolic Sciences, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Center for Microbiome and Human Health, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Marcia C de Oliveira Otto, Division of Epidemiology, Human Genetics and Environmental Sciences, The University of Texas Health Science Center at Houston (UTHealth) School of Public Health, 1200 Pressler Street, Houston, TX 77030, USA.
Rozenn N Lemaitre, Cardiovascular Health Research Unit, Department of Medicine, University of Washington, 1730 Minor Ave, Suite 1360, Seattle, WA 98101, USA.
Amanda Fretts, Cardiovascular Health Research Unit, Department of Medicine, University of Washington, 1730 Minor Ave, Suite 1360, Seattle, WA 98101, USA; Department of Epidemiology, University of Washington, 3980 15th Ave NE, Seattle, WA 98195, USA.
Nona Sotoodehnia, Cardiovascular Health Research Unit, Department of Medicine, University of Washington, 1730 Minor Ave, Suite 1360, Seattle, WA 98101, USA.
Matthew Budoff, Department of Medicine, Lundquist Institute at Harbor-UCLA Medical Center, 124 West Carson Street, Torrance, CA 90502, USA.
Ina Nemet, Department of Cardiovascular and Metabolic Sciences, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Center for Microbiome and Human Health, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Joseph A DiDonato, Department of Cardiovascular and Metabolic Sciences, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Center for Microbiome and Human Health, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Wai Hong Wilson Tang, Department of Cardiovascular and Metabolic Sciences, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Center for Microbiome and Human Health, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Bruce M Psaty, Cardiovascular Health Research Unit, Department of Medicine, University of Washington, 1730 Minor Ave, Suite 1360, Seattle, WA 98101, USA; Department of Epidemiology, University of Washington, 3980 15th Ave NE, Seattle, WA 98195, USA; Department of Health Systems and Population Health, University of Washington, 3980 15th Ave NE, Seattle, WA 98195, USA.
David S Siscovick, The New York Academy of Medicine, 1216 5th Ave, New York City, NY 10029, USA.
Stanley L Hazen, Department of Cardiovascular and Metabolic Sciences, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Center for Microbiome and Human Health, Lerner Research Institute, 9500 Euclid Ave, Cleveland, OH 44195, USA; Department of Cardiovascular Medicine, Heart, Vascular and Thoracic Institute, Cleveland Clinic, 9500 Euclid Ave, Cleveland, OH 44195, USA.
Dariush Mozaffarian, Friedman School of Nutrition Science and Policy, Tufts University, 150 Harrison Ave, Boston, MA 02111, USA.
Author contributions
MW, XSL, RNL, BMP, DSS, SLH, and DM contributed to the concept and design of this study; DM and SLH led acquisition of funding; MW, XSL, MCO, RNL, AF, NS, BMP, DSS, SLH, and DM contributed to the acquisition, analysis, or interpretation of data; XSL, ZW, IN, JAD, and SLH contributed to laboratory sample analysis; MW contributed to drafting the manuscript and performing statistical analysis. All authors contributed to critical revision of the manuscript for important intellectual content.
Supplementary data
Supplementary data is available at European Heart Journal online.
Data availability
The data underlying this article were provided by the Multi-ethic Study of Atherosclerosis (MESA, https://www.mesa-nhlbi.org) under license/by permission. Data can be shared on request to the corresponding author if permitted by the Multi-ethic Study of Atherosclerosis.
Funding
The authors received support from the National Heart, Lung, and Blood Institute, National Institutes of Health grants R01HL135920, P01 HL147823, and R01 HL103866. MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The funding organizations had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1. Tang WHW, Backhed F, Landmesser U, Hazen SL. Intestinal Microbiota in cardiovascular health and disease: jACC state-of-the-art review. J Am Coll Cardiol 2019;73:2089–2105. 10.1016/j.jacc.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang WH, Kitai T, Hazen SL. Gut Microbiota in cardiovascular health and disease. Circ Res 2017;120:1183–1196. 10.1161/circresaha.117.309715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124. 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang WHW, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015;116:448–455. 10.1161/CIRCRESAHA.116.305360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and cardiovascular disease. Circ Res 2020;127:553–570. 10.1161/circresaha.120.316242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WHW, Li XS, Wu Y, Wang Z, Khaw K-T, Wareham NJ, et al. Plasma trimethylamine N-oxide (TMAO) levels predict future risk of coronary artery disease in apparently healthy individuals in the EPIC-norfolk prospective population study. Am Heart J 2021;236:80–86. 10.1016/j.ahj.2021.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee Y, Nemet I, Wang Z, Lai HTM, de Oliveira Otto MC, Lemaitre RN, et al. Longitudinal plasma measures of trimethylamine N-oxide and risk of atherosclerotic cardiovascular disease events in community-based older adults. J Am Heart Assoc 2021;10:e020646. 10.1161/jaha.120.020646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, et al. Long-Term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J Am Coll Cardiol 2020;75:763–772. 10.1016/j.jacc.2019.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–2956. 10.1093/eurheartj/ehx342 [DOI] [PubMed] [Google Scholar]
- 13. Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota metabolites and risk of Major adverse cardiovascular disease events and death: a systematic review and meta- analysis of prospective studies. J Am Heart Assoc 2017; 6:e004947. doi: 10.1161/JAHA.116.004947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qi J, You T, Li J, Pan T, Xiang L, Han Y, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med 2018;22:185–194. 10.1111/jcmm.13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 2016;5:e002767. 10.1161/jaha.115.002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, et al. Trimethylamine-N-Oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 2020;76:101–112. 10.1161/hypertensionaha.120.14759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine N-oxide from dietary choline is prothrombotic in subjects. Circulation 2017;135:1671–1673. 10.1161/circulationaha.116.025338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aldana-Hernández P, Leonard K-A, Zhao Y-Y, Curtis JM, Field CJ, Jacobs RL. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in ldlr−/− and apoe−/− male mice. J Nutr 2020;150:249–255. 10.1093/jn/nxz214 [DOI] [PubMed] [Google Scholar]
- 19. Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li L, et al. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler Thromb Vasc Biol 2020;40:1239–1255. 10.1161/atvbaha.120.314139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab 2019;30:1141–1151.e5. 10.1016/j.cmet.2019.08.021 [DOI] [PubMed] [Google Scholar]
- 21. Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng 2014;118:476–481. 10.1016/j.jbiosc.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 22. Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018;17:e12768. 10.1111/acel.12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan CWH, Law BMH, Waye MMY, Chan JYW, So WKW, Chow KM. Trimethylamine-N-oxide as one hypothetical link for the relationship between intestinal Microbiota and cancer—where we are and where shall we go? J Cancer 2019;10:5874–5882. 10.7150/jca.31737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farhangi MA. Gut microbiota-dependent trimethylamine N-oxide and all-cause mortality: findings from an updated systematic review and meta-analysis. Nutrition 2020;78:110856. 10.1016/j.nut.2020.110856 [DOI] [PubMed] [Google Scholar]
- 25. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019;40:583–594. 10.1093/eurheartj/ehy799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Levison BS, Hazen JE, Donahue L, Li X-M, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 2014;455:35–40. 10.1016/j.ab.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018;3:e99096. 10.1172/jci.insight.99096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–1345. 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 30. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. 10.2307/2335876 [DOI] [Google Scholar]
- 31. Zhang X, Li Y, Yang P, Liu X, Lu L, Chen Y, et al. Trimethylamine-N-Oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler Thromb Vasc Biol 2020;40:751–765. 10.1161/atvbaha.119.313414 [DOI] [PubMed] [Google Scholar]
- 32. Brunt VE, LaRocca TJ, Bazzoni AE, Sapinsley ZJ, Miyamoto-Ditmon J, Gioscia-Ryan RA, et al. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021;43:377–394. 10.1007/s11357-020-00257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016;27:305–313. 10.1681/ASN.2014111063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruppen EG, Garcia E, Connelly MA, Jeyarajah EJ, Otvos JD, Bakker SJL, et al. TMAO Is associated with mortality: impact of modestly impaired renal function. Sci Rep 2017;7:13781. 10.1038/s41598-017-13739-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li D, Lu Y, Yuan S, Cai X, He Y, Chen J, et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis. Am J Clin Nutr 2022;116:230–243. 10.1093/ajcn/nqac074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017;38:814–824. 10.1093/eurheartj/ehw582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WH, et al. Intestinal Microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal Microbiota in a COURAGE-like patient cohort. J Am Heart Assoc 2016;5:e002816. 10.1161/jaha.115.002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014;64:1908–1914. 10.1016/j.jacc.2014.02.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang WH, Wang Z, Li XS, Fan Y, Li DS, Wu Y, et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes Mellitus. Clin Chem 2017;63:297–306. 10.1373/clinchem.2016.263640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mueller DM, Allenspach M, Othman A, Saely CH, Muendlein A, Vonbank A, et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015;243:638–644. 10.1016/j.atherosclerosis.2015.10.091 [DOI] [PubMed] [Google Scholar]
- 41. Fretts AM HS, Jensen P, Budoff M, Sitlani CM, Wang M, de Oliveira Otto MC, et al. Association of trimethylamine N-oxide and metabolites with mortality in older adults: the cardiovascular health study. JAMA Netw Open 2022;5:e2213242. 10.1001/jamanetworkopen.2022.13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology 2003;14:300–306. 10.1097/01.Ede.0000042804.12056.6c [DOI] [PubMed] [Google Scholar]
- 43. Hernán MA, Robins JM, editors. Causal Inference: What If. 1 ed.: Chapman & Hall/CRC; 2020. [Google Scholar]
- 44. Hoffman RA, Venugopalan J, Qu L, Wu H, Wang MD. Improving validity of cause of death on death certificates. ACM BCB 2018;2018:178–183. 10.1145/3233547.3233581 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the Multi-ethic Study of Atherosclerosis (MESA, https://www.mesa-nhlbi.org) under license/by permission. Data can be shared on request to the corresponding author if permitted by the Multi-ethic Study of Atherosclerosis.