Abstract
Background
Limited data are available to guide effective antibiotic durations for hospitalized patients with complicated urinary tract infections (cUTIs).
Methods
We conducted an observational study of patients ≥18 years at 24 US hospitals to identify the optimal treatment duration for patients with cUTI. To increase the likelihood patients experienced true infection, eligibility was limited to those with associated bacteremia. Propensity scores were generated for an inverse probability of treatment weighted analysis. The primary outcome was recurrent infection with the same species ≤30 days of completing therapy.
Results
1099 patients met eligibility criteria and received 7 (n = 265), 10 (n = 382), or 14 (n = 452) days of therapy. There was no difference in the odds of recurrent infection for patients receiving 10 days and those receiving 14 days of therapy (aOR: .99; 95% CI: .52–1.87). Increased odds of recurrence was observed in patients receiving 7 days versus 14 days of treatment (aOR: 2.54; 95% CI: 1.40–4.60). When limiting the 7-day versus 14-day analysis to the 627 patients who remained on intravenous beta-lactam therapy or were transitioned to highly bioavailable oral agents, differences in outcomes no longer persisted (aOR: .76; 95% CI: .38–1.52). Of 76 patients with recurrent infections, 2 (11%), 2 (10%), and 10 (36%) in the 7-, 10-, and 14-day groups, respectively, had drug-resistant infections (P = .10).
Conclusions
Seven days of antibiotics appears effective for hospitalized patients with cUTI when antibiotics with comparable intravenous and oral bioavailability are administered; 10 days may be needed for all other patients.
Keywords: UTI, gram-negative bacteremia, duration, antibiotics, E. coli
In a multicenter study of 1099 hospitalized adults with complicated urinary tract infections and associated bacteremia, 7 days of antibiotics was sufficient for patients who remained on intravenous antibiotics or were transitioned to highly bioavailable oral agents.
(See the Editorial Commentary by Jason C. Gallagher on pages 1613–4.)
Urinary tract infections (UTIs) are the most common bacterial infections worldwide and are a frequent indication for antibiotic therapy [1]. Complicated UTIs (cUTIs) are a subset of UTIs that occur in the setting of pre-existing structural or functional abnormalities of the urinary tract, resulting from a broad range of clinical syndromes [1]. The clinical outcomes of patients with cUTI are generally poorer than with uncomplicated UTIs because of the underlying patient complexity, the diversity of pathogens involved, and residual reservoirs that present a nidus for recurrent infections such as catheters, stents, or urinary stasis [2]. Identifying the shortest but still effective duration of antibiotic therapy is of critical importance for patients prone to cUTI as they may remain at an elevated risk for UTIs for the remainder of their lives. Moreover, their future UTIs are often increasingly drug-resistant and progressively more challenging to treat [3].
A clinical trial of outpatient afebrile males with cUTI demonstrated that 7 days of antibiotic therapy is generally sufficient for the resolution of symptoms [4]. The optimal duration of therapy for patients with cUTI warranting hospitalization remains undefined. Our objective was to determine the shortest, effective duration of therapy for the treatment of hospitalized adults with cUTI.
METHODS
Design, Setting, and Participants
A retrospective cohort study of all unique, consecutive patients 18 years of age or older with gram-negative cUTIs and associated bloodstream infections (BSIs) hospitalized at any of 24 US hospitals in the calendar year 2019 was conducted. The 24 sites consisted of a mixture of academic medical centers, community hospitals, and Veterans Affairs hospitals with locations in the United States as follows: Northeast (14), Midwest (6), Mountain West (3), and West (1).
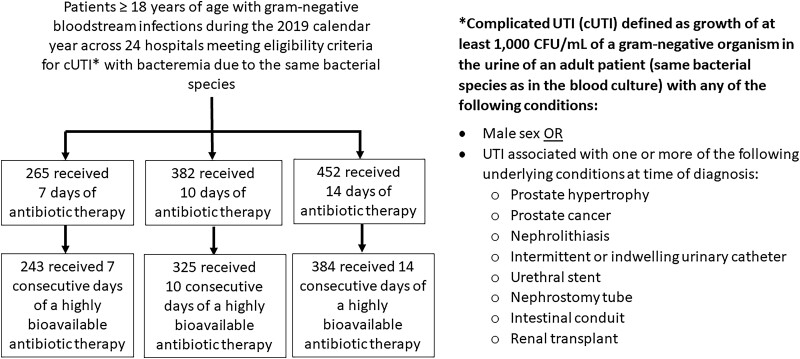
Complicated UTIs were defined as UTIs due to structural or functional abnormalities of the genitourinary tract or any UTI in a male patient (Figure 1). Distinguishing colonization from infection for patients with structural or functional genitourinary tract abnormalities is challenging as patients may have difficulty reporting urinary symptoms (eg, dysuria or flank pain in patients with paraplegia syndromes) and because of ambiguity surrounding the diagnostic criteria (ie, uncertain threshold for urinary white blood cell counts to qualify as infections). Therefore, to increase the likelihood that the cUTI represented true infection and not colonization, eligibility was limited to patients with associated BSI (ie, bacteremia with the same bacterial species causing the cUTI).
Figure 1.
Overall cohort. Abbreviations: CFU, colony-forming units; UTI, urinary tract infection.
Exposures
The primary exposure was the duration of antibiotic therapy, dichotomized to 10 (±1) days and 14 (±1) days. Ten days and 14 days were selected after exploratory analysis indicated that the administered durations of therapy most frequently congregated at these 2 values (Supplementary Figure 1). Durations of therapy were categorized rather than included as continuous variables to improve clinical applicability. Discrete durations (10 vs 14 days) were selected instead of less than 10 days vs 11 or more days, as differences in outcomes between a 10-day and 11-day course may be negligible compared with 10-day versus 14-day courses. All inpatient and outpatient antibiotic therapy prescribed from the day of culture collection to completion of therapy was included in the total duration, based on electronic prescription data at hospital discharge.
Because of uncertainty surrounding the minimum duration of effective therapy for hospitalized patients with cUTI, it was decided a priori that the analysis would be repeated comparing 7 (±1) days versus 14 (±1) days of antibiotic therapy. Furthermore, if 7 days of therapy was associated with an increased odds of recurrent infection compared with 14 days, additional analysis was planned comparing 7 versus 14 days of antibiotics in a subgroup of patients who received intravenous (IV) beta-lactam therapy for the entire treatment duration or were transitioned to an oral agent expected to have adequate and consistent renal parenchyma/serum concentrations for the entire treatment course (henceforth, termed “highly bioavailable agents”). Agents included in the highly bioavailable agent category included the following: (1) ciprofloxacin 500–750 orally every 12 hours, (2) levofloxacin 500–750 orally every 24 hours, (3) trimethoprim-sulfamethoxazole [TMP-SMX] ≥5 mg/kg per day, (4) amoxicillin 1000 mg orally every 8 hours, (5) amoxicillin-clavulanate 875–1000 mg orally every 8 hours, or (6) cephalexin 1000 mg orally every 6 hours. Although data informing optimal dosing of oral beta-lactams are limited, suggested optimal dosing for oral antibiotic agents for the treatment of gram-negative BSIs were previously developed based on consensus from a group of infectious diseases pharmacists [5]; this dosing guidance was applied for the current study.
Outcomes
The primary outcome was recurrent infection up to 30 days after the discontinuation of antibiotic therapy for the index infection. To meet criteria for “recurrent infection” the following had to be met: (1) a positive urine culture with the same bacterial species as the index infection, (2) receipt of a course of antibiotics for the recurrent positive culture, and (3) at least 2 of the following—new fever or hypothermia, new hypotension, new mental status changes, new dysuria, or new flank pain. The time period of 30 days after discontinuation of antibiotics (as opposed to 30 days after the day of culture collection) was selected to ensure that the number of days for which patients were “at risk” for the outcome was similar between the 2 treatment groups. Patients had to survive until at least 1 day after antibiotics were discontinued to be eligible for inclusion so that they had the “opportunity” to be evaluated for treatment failure (ie, to limit the impact of immortal time bias). For example, a patient receiving 10 days of antibiotics had to be alive on day 11 and a person receiving 14 days of antibiotics had to be alive on day 15.
The secondary outcome was development of a subsequent antibiotic-resistant infection with the same bacterial species within 30 days of completing treatment. Antibiotic-resistant infections were defined as organisms with antibiotic minimum inhibitory concentration (MIC) increases of 4-fold or greater for an antibiotic used as treatment for the initial infection, comparing the index and subsequent culture results. As an example, if an initial Escherichia coli cUTI was treated with cefepime and the initial E. coli cefepime MIC was 2 µg/mL, a subsequent E. coli cUTI with a cefepime MIC of 8 µg/mL or higher qualified as an incident antibiotic-resistant infection.
Data Collection
Data collection across the 24 hospitals was performed by infectious diseases physicians, infectious diseases pharmacists, or trainees closely supervised by infectious diseases specialists. Data were collected via medical record review following a standardized electronic data collection form and data dictionary. The Institutional Review Boards of each participating site approved the study, with waivers of informed consent. Johns Hopkins University served as the central site to oversee data collection and analysis, after data-use agreements between Johns Hopkins University and participating sites were in place. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for comparative effectiveness research were followed [6].
Definitions
Extended-spectrum beta-lactamase (ESBL)–producing infections were defined as cUTIs meeting any of the following criteria based on testing performed at local microbiology laboratories: (1) blaCTX-M gene identified using a molecular platform (eg, Verigene Gram-Negative Blood Culture Nucleic Acid Test); (2) ESBL phenotypic confirmatory testing; or (3) E. coli, Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis exhibiting ceftriaxone MICs of 2 µg/mL or higher [7]. Source control was defined as receipt of a procedure expected to lead to the resumption of normal urinary flow (eg, transurethral resection of the prostate, stent placement to relieve obstruction) or removal of all existing urinary catheters/hardware or renal stones by the end of the antibiotic course.
Statistical Analysis
To ensure the exposed (ie, patients who received 10 days of therapy) and unexposed (ie, patients who received 14 days of therapy) groups were as similar as possible at the time of antibiotic administration, inverse probability of treatment weighting (IPTW) incorporating propensity scores was used because more ill and/or complex patients are likely to receive prolonged courses of therapy compared with relatively well-appearing patients and those with less medical complexity. The following covariates were included in generating propensity scores: age 65 years or older, male sex, weight, body mass index of 30 kg/m2 or greater, severe immune compromise, intensive care unit admission on day 1, Pitt bacteremia score of 4 or higher, Charlson Comorbidity Index of 5 or greater, diabetes, cerebrovascular disease, renal replacement therapy, documented urologic conditions/devices on day 1 (as opposed to males with cUTIs without known underlying conditions/devices), active empiric therapy, Pseudomonas aeruginosa infection, ESBL-producing infection, and source control.
Patients in the exposed group were weighted by the inverse of the propensity score and patients in the unexposed group were weighted by the inverse of 1 minus the propensity score (ie, each person was weighted by the inverse probability of receiving the treatment [s]he actually received) [8]. A new weighted pseudo-population was created in which individuals in the exposed and unexposed groups were up-weighted or down-weighted to ensure that, at baseline, both groups were as similar as possible on all variables, except for the duration of antibiotic therapy prescribed. Standardized mean differences were calculated in the new pseudo-population to ensure similarity between exposed and unexposed groups for each included variable. The same variables and an identical approach were used for the exploratory analysis of patients receiving 7 versus 14 days of therapy.
Baseline data were compared using the Pearson chi-square test for categorical variables or the Wilcoxon rank-sum test for continuous variables. Odds ratios (ORs) and 95% confidence intervals (CIs) for recurrent infections were estimated using weighted regression, adjusting for variables with standardized mean differences greater than 10%, indicating suboptimal balance between exposed and unexposed groups for that specific variable. A 2-sided P value less than .05 was considered statistically significant for all tests. Statistical analysis was completed using R statistical software, version 4.1.3 (R Foundation for Statistical Learning, Austria).
RESULTS
Overall Cohort
In total, 1099 patients met the eligibility criteria and received either 7 days (n = 265), 10 days (n = 382), or 14 days (n = 452) of antibiotic therapy (Figure 1). The most commonly identified pathogens were as follows: E. coli (59%), K. pneumoniae (16%), P. mirabilis (8%), and P. aeruginosa (6%) (Table 1). Overall, 147 (17%) patients were infected with ESBL-producing organisms (when limiting the analysis to E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis).
Table 1.
Gram-Negative Organisms Recovered From 1099 Patients With Complicated Urinary Tract Infection and Associated Bloodstream Infections Receiving 7, 10, or 14 Days of Antibiotic Therapy
| Organism | 7 Days (n = 265) | 10 Days (n = 382) | 14 Days (n = 452) |
|---|---|---|---|
| Acinetobacter baumannii | 1 | 0 | 2 |
| Citrobacter freundii | 1 | 3 | 2 |
| Citrobacter koseri | 4 | 7 | 5 |
| Citrobacter youngae | 0 | 2 | 0 |
| Enterobacter cloacae | 6 | 8 | 15 |
| Escherichia coli | 160 | 247 | 240 |
| Klebsiella aerogenes | 4 | 1 | 5 |
| Klebsiella oxytoca | 6 | 8 | 9 |
| Klebsiella pneumoniae | 41 | 47 | 85 |
| Morganella morganii | 3 | 4 | 4 |
| Proteus mirabilis | 19 | 26 | 40 |
| Proteus vulgaris | 0 | 1 | 1 |
| Providencia stuartii | 1 | 1 | 4 |
| Pseudomonas aeruginosa | 13 | 20 | 34 |
| Serratia marcescens | 6 | 7 | 6 |
Outcomes of 10 Days Versus 14 Days of Therapy
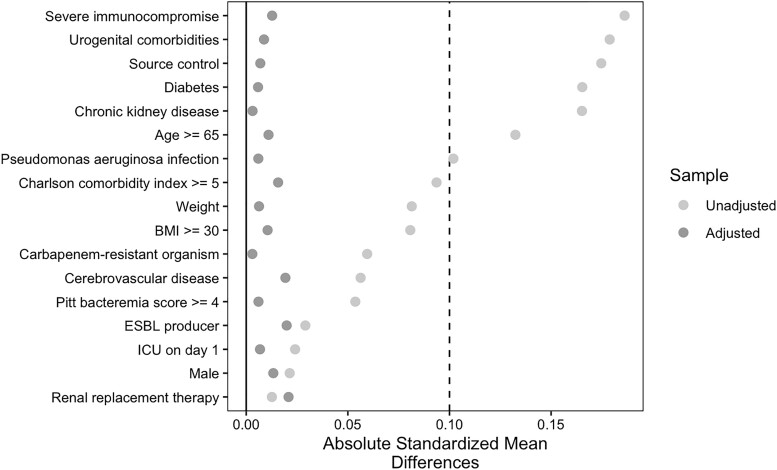
Of the 834 patients included in this cohort, 382 (46%) received 10 days of therapy and 452 (54%) received 14 days of therapy. The 10-day and 14-day groups were generally similar in baseline characteristics, with few differences (Table 2). Patients who received 10 days of therapy were less likely to have severe immunocompromise, chronic kidney disease, or urologic conditions/devices on day 1 and were more likely to have source control. Inverse probability of treatment weighting yielded 2 well-balanced groups without any residual differences in all measured baseline characteristics. The standardized mean difference for all variables was less than 0.10 (Figure 2). Forty-eight patients (6%) out of 834 achieved the primary outcome of recurrent infection. More specifically, 20 (5%) patients in the 10-day arm and 28 (6%) patients in the 14-day arm experienced subsequent identification of the same bacterial species in a clinical specimen within 30 days of completing antibiotic therapy, warranting additional antibiotic therapy. In the IPTW cohort, patients receiving 10 days of therapy had no increased odds of recurrent infection compared with patients receiving 14 days of antibiotic therapy (adjusted OR [aOR]: .99; 95% CI: .52–1.87; P = .99).
Table 2.
Baseline Characteristics of 834 Adults With Complicated Urinary Tract Infections With Associated Bloodstream Infections, Before and After Inverse Probability of Treatment Weighting, Comparing 10 Days With 14 Days of Antibiotic Therapy
| Full Cohort | Inverse Probability Weighted Cohort | |||||
|---|---|---|---|---|---|---|
| Variable | 10 Days (n = 382; 46%) |
14 Days (n = 452; 54%) |
P | 10 Days (%) |
14 Days (%) |
Standardized Mean Difference |
| Age in years, median (IQR) | 70 (60–80) | 68 (56–77) | .04 | … | … | … |
| ȃAge ≥65 years | 246 (64%) | 263 (58%) | .08 | 64.4 | 64.9 | 0.011 |
| Male sex, n (%) | 244 (64%) | 293 (65%) | .83 | 63.9 | 64.5 | 0.013 |
| Weight, median (IQR), kg | 78 (66–94) | 82 (68–96) | .09 | 78 (66–94) | 80 (68–93) | 0.007 |
| Body mass index ≥30 kg/m2 | 137 (36%) | 178 (39%) | .33 | 35.9 | 35.4 | 0.011 |
| Race/ethnicity, n (%) | ||||||
| ȃWhite | 212 (55%) | 238 (53%) | .45 | … | … | … |
| ȃBlack | 76 (20%) | 110 (24%) | .15 | … | … | … |
| ȃAsian | 15 (3.9%) | 17 (3.8%) | .99 | … | … | … |
| ȃHispanic | 53 (14%) | 60 (13%) | .88 | … | … | … |
| Severe immunocompromise,a n (%) | 71 (19%) | 117 (26%) | .015 | 18.6 | 18.1 | 0.013 |
| Intensive care unit on day 1, n (%) | 107 (28%) | 120 (27%) | .69 | 28.0 | 27.7 | 0.007 |
| Pitt bacteremia score ≥4 on day 1, n (%) | 52 (14%) | 70 (15%) | .51 | 13.6 | 13.8 | 0.006 |
| Charlson Comorbidity Index ≥5, n (%) | 58 (15%) | 84 (19%) | .23 | 15.2 | 15.7 | 0.016 |
| Diabetes, n (%) | 116 (30%) | 172 (38%) | .02 | 30.4 | 30.6 | 0.006 |
| Cerebrovascular disease, n (%) | 61 (16%) | 63 (14%) | .47 | 16.0 | 15.3 | 0.019 |
| Chronic kidney disease, n (%) | 95 (25%) | 143 (32%) | .04 | 24.9 | 25.0 | 0.003 |
| Renal replacement therapy, n (%) | 11 (2.9%) | 14 (3.1%) | .99 | 2.9 | 3.2 | 0.020 |
| Urologic conditions/devices on day 1, n (%) | 208 (54%) | 288 (64%) | .008 | 54.5 | 54.0 | 0.009 |
| ȃRenal transplant | 13 (3.4%) | 42 (9.3%) | .001 | … | … | … |
| ȃProstate hypertrophy | 45 (12%) | 55 (12%) | .95 | … | … | … |
| ȃNephrostomy tube | 19 (5%) | 36 (8%) | .11 | … | … | … |
| ȃUreteral stent | 9 (2.4%) | 6 (1.3%) | .39 | … | … | … |
| ȃIleostomy | 5 (1.3%) | 7 (1.5%) | .99 | … | … | … |
| ȃSuprapubic catheter | 3 (0.8%) | 16 (3.5%) | .015 | … | … | … |
| ȃIntermittent urinary catheterization | 19 (5%) | 25 (5.5%) | .84 | … | … | … |
| ȃFoley catheter | 55 (14%) | 56 (12%) | .45 | … | … | … |
| ȃProstate cancer | 12 (3.1%) | 18 (4%) | .64 | … | … | … |
| ȃNephrolithiasis | 70 (18%) | 96 (21%) | .34 | … | … | … |
| Active empiric therapy, n (%) | 348 (91%) | 407 (90%) | .61 | 90 | 90 | 0.008 |
| Pseudomonas aeruginosa, n (%) | 20 (5.2%) | 34 (7.5%) | .23 | 5.2 | 5.4 | 0.006 |
| ESBL-producing Enterobacterales, n (%) | 51 (13%) | 55 (12%) | .68 | 13.4 | 14.0 | 0.019 |
| Carbapenem-resistant organism, n (%) | 4 (1%) | 2 (0.4%) | .54 | … | … | … |
| Source control by end of antibiotic therapy, n (%) | 65 (17%) | 50 (11%) | .03 | 11.3 | 11.5 | 0.009 |
Abbreviations: ESBL, extended-spectrum beta-lactamase; HIV, human immunodeficiency virus; IQR, interquartile range.
Defined by at least 1 of the following: (1) hematopoietic stem cell transplantation within the previous 12 months or active treatment for graft-versus-host disease, (2) active chemotherapy within the prior 6 months, (3) previous solid-organ transplantation, (4) HIV infection with a CD4 count <200 cells/mm3, (5) absolute neutrophil count <500 cells/mm3 at the time of or within 7 days after blood culture collection, or (6) receipt of corticosteroids at a dose equivalent to 10 mg daily of prednisone for ≥14 days or other immunosuppressive therapy.
Figure 2.
Standardized mean differences across variables for patients receiving 10 days versus 14 days of antibiotics for cUTI. Abbreviations: BMI, body mass index; ESBL, extended-spectrum beta-lactamase; ICU, intensive care unit.
Outcomes of 7 Days Versus 14 Days of Therapy
There were 265 (37%) patients who received 7 days of therapy and 452 (63%) who received 14 days of therapy (Table 3). The 452 patients in the 14-day group are the same patients included in the 10- versus 14-day analysis. In an IPTW cohort where standardized mean differences for all variables were less than 0.10, patients receiving 7 days of therapy had an approximately 2.5 increase in the odds of recurrent infection compared with patients receiving 14 days of therapy (aOR: 2.54; 95% CI: 1.40–4.60; P = .002).
Table 3.
Baseline Characteristics of 717 Adults With Complicated Urinary Tract Infections With Associated Bloodstream Infections, Before and After Inverse Probability of Treatment Weighting, Comparing 7 Days With 14 Days of Antibiotic Therapy
| Full Cohort | Inverse Probability Weighted Cohort | |||||
|---|---|---|---|---|---|---|
| Variable | 7 Days (n = 265; 37%) |
14 Days (n = 452; 63%) |
P | 7 Days (%) |
14 Days (%) |
Standardized Mean Difference |
| Age in years, median (IQR) | 72 (63–81) | 68 (56–77) | <.001 | … | … | … |
| ȃAge ≥65 years | 192 (72%) | 263 (58%) | <.001 | 72.7 | 72.6 | 0.003 |
| Male sex, n (%) | 188 (71%) | 293 (65%) | .094 | 71.2 | 71.8 | 0.014 |
| Weight, median (IQR), kg | 78 (67–94) | 82 (68–96) | .16 | 80 (68–93) | 78 (67–94) | 0.003 |
| Body mass index ≥30 kg/m2 | 84 (32%) | 178 (39%) | .052 | 31.8 | 31.7 | 0.002 |
| Race/ethnicity, n (%) | ||||||
| ȃWhite | 157 (59%) | 238 (53%) | .37 | … | … | … |
| ȃBlack | 54 (20%) | 110 (24%) | .37 | … | … | … |
| ȃAsian | 12 (4.5%) | 17 (3.8%) | .25 | … | … | … |
| ȃHispanic | 21 (8.0%) | 60 (13%) | .059 | … | … | … |
| Severe immunocompromise,a n (%) | 43 (16%) | 117 (26%) | .004 | 16.3 | 16 | 0.007 |
| Intensive care unit on day 1, n (%) | 72 (27%) | 120 (27%) | .90 | 27.3 | 26.4 | 0.019 |
| Pitt bacteremia score ≥4 on day 1, n (%) | 33 (12%) | 70 (15%) | .32 | 12.5 | 11.5 | 0.032 |
| Charlson Comorbidity Index ≥5, n (%) | 30 (11%) | 84 (19%) | .015 | 11.4 | 11.5 | 0.004 |
| Diabetes, n (%) | 103 (39%) | 172 (38%) | .86 | 38.6 | 36.4 | 0.046 |
| Cerebrovascular disease, n (%) | 41 (15%) | 63 (14%) | .64 | 15.5 | 14.7 | 0.022 |
| Chronic kidney disease, n (%) | 81 (31%) | 143 (32%) | .86 | 30.3 | 27.9 | 0.052 |
| Renal replacement therapy, n (%) | 6 (2.3%) | 14 (3.1%) | .68 | 2.3 | 2.4 | 0.009 |
| Urologic conditions/devices on day 1, n (%) | 112 (42%) | 288 (64%) | <.001 | 42.4 | 42 | 0.008 |
| ȃRenal transplant | 4 (1.5%) | 42 (9.3%) | <.001 | … | … | … |
| ȃProstate hypertrophy | 21 (8.0%) | 55 (12%) | .10 | … | … | … |
| ȃNephrostomy tube | 12 (4.5%) | 36 (8.0%) | .11 | … | … | … |
| ȃUreteral stent | 2 (0.8%) | 6 (1.3%) | .74 | … | … | … |
| ȃIleostomy | 6 (2.3%) | 7 (1.5%) | .68 | … | … | … |
| ȃSuprapubic catheter | 7 (2.6%) | 16 (3.5%) | .67 | … | … | … |
| ȃIntermittent urinary catheterization | 17 (6.4%) | 25 (5.5%) | .74 | … | … | … |
| ȃFoley catheter | 36 (14%) | 56 (12%) | .71 | … | … | … |
| ȃProstate cancer | 4 (1.5%) | 18 (4%) | .10 | … | … | … |
| ȃNephrolithiasis | 18 (6.8%) | 96 (21%) | <.001 | … | … | … |
| Active empiric therapy, n (%) | 239 (90%) | 407 (90%) | .53 | 90 | 90 | 0.008 |
| Pseudomonas aeruginosa, n (%) | 13 (4.9%) | 34 (7.5%) | .23 | 4.9 | 5.1 | 0.008 |
| ESBL-producing Enterobacterales, n (%) | 41 (15%) | 55 (12%) | .25 | 15.5 | 16.1 | 0.016 |
| Carbapenem-resistant organism, n (%) | 2 (0.8%) | 2 (0.4%) | .98 | … | … | … |
| Source control by end of antibiotic therapy, n (%) | 16 (6%) | 50 (11%) | <.001 | 6.1 | 6.3 | 0.008 |
Abbreviations: ESBL, extended-spectrum beta-lactamase; HIV, human immunodeficiency virus; IQR, interquartile range.
Defined by at least 1 of the following: (1) hematopoietic stem cell transplantation within the previous 12 months or active treatment for graft-versus-host disease, (2) active chemotherapy within the prior 6 months, (3) previous solid-organ transplantation, (4) HIV infection with a CD4 count <200 cells/mm3, (5) absolute neutrophil count <500 cells/mm3 at the time of or within 7 days after blood culture collection, or (6) receipt of corticosteroids at a dose equivalent to 10 mg daily of prednisone for ≥14 days or other immunosuppressive therapy.
Outcomes of Patients Receiving 7 Days of Therapy With Highly Bioavailable Agents
Although approximately 10 days of antibiotic therapy appears to be necessary for most patients with gram-negative cUTIs and associated BSIs, we sought to determine if 7 days of therapy may be sufficient for patients receiving highly bioavailable agents for their entire treatment course. A total of 627 patients in the 7-day (n = 243; 39%) and 14-day (n = 384; 61%) groups either received IV beta-lactams for the complete treatment course or were transitioned to highly bioavailable agents. Commonly administered dosages of oral antibiotics are described in Supplementary Table 1. Unfortunately, as only 5 patients were transitioned to amoxicillin 1 g orally every 8 hours and no patients were transitioned to other oral beta-lactam agents that were considered optimally dosed, we were very limited in our ability to include optimally dosed oral beta-lactam agents in the highly bioavailable agent subanalysis. Comparing patients who completed their treatment course with IV antibiotics or were transitioned to highly bioavailable agents in the IPTW cohort, there was no difference in the odds of recurrent infection between patients receiving 7 days versus 14 days of antibiotics (aOR: .76; 95% CI: .38–1.52).
Development of Subsequent Antibiotic-Resistant Infections
Of the 1099 patients who received 7, 10, or 14 days of antibiotics, 76 (7%) had a recurrent infection from any source within the subsequent 30 days. Of these, 14 (18%) had subsequent antibiotic-resistant infections. More specifically, 2 (11%), 2 (10%), and 10 (36%) in the 7-, 10-, and 14-day groups, respectively, had subsequent infections with antibiotic-resistant organisms (P = .10).
DISCUSSION
In adults with cUTI and associated BSIs and hospitalized at any of 24 hospitals across the United States, 10 days and 14 days of antibiotic therapy yielded similar clinical outcomes. Although not achieving statistical significance, potentially because only 7% of patients had recurrent infections within 30 days, differences in the emergence of subsequent antibiotic-resistant isolates were nevertheless notable at 11% versus 10% versus 36% in the 7-day, 10-day, and 14-day groups, respectively. Interestingly, 7 days of antibiotic therapy was associated with more clinical failures compared with 14 days, except in the subgroup of patients receiving highly bioavailable agents. Regrettably, as suboptimal dosages were used for 96% of patients transitioned to oral beta-lactam agents, an investigation into whether 7 days of therapy would be sufficient for patients with bacteremic cUTI transitioned to optimally dosed oral beta-lactams could not be performed.
Over the past 2 decades, a growing number of clinical trials have underscored that treatment durations shorter than frequently prescribed are sufficient for common bacterial infections in hospitalized patients predominantly caused by gram-negative pathogens including BSIs [9–11], intra-abdominal infections [12], pyelonephritis [13–17], and ventilator-associated pneumonia [18, 19]. Few comparative effectiveness studies, however, have investigated the optimal duration of therapy for cUTI, either in the inpatient or outpatient setting [4, 20–22]. Drekonja and colleagues [4] conducted a placebo-controlled pragmatic trial in which 272 afebrile male outpatients were randomly assigned to 7 days or 14 days of therapy with either ciprofloxacin or TMP-SMX. Symptom resolution was successfully achieved in over 90% of patients in both treatment arms, with no differences observed between the 2 groups.
Our findings suggest that 7 days of antibiotics may also be sufficient for bacteremic patients with cUTI when IV beta-lactams are used for the entire treatment course or when antibiotics are transitioned to highly bioavailable agents. The high bioavailability and sustained serum concentrations of oral fluoroquinolones [23, 24] and TMP-SMX [25] support the basis for these findings. Furthermore, clinical trials have demonstrated more favorable outcomes of patients receiving oral fluoroquinolones or TMP-SMX for the treatment of UTIs, compared with the outcomes of patients transitioned to oral beta-lactams [26, 27]. These differences in outcomes are thought to be due to the persistent urogenital colonization of pathogenic bacteria observed with oral beta-lactam treatment [26]. An important caveat to these findings, however, is that oral beta-lactams are commonly suboptimally dosed both in clinical trials and in clinical practice. As an example, in 1 trial, amoxicillin-clavulanate was dosed as 500/125 mg twice daily, which would not provide comparable serum or urinary concentrations to IV beta-lactam counterparts [26]; more aggressive dosing may have led to different outcomes. In our cohort, there was significant heterogeneity in the dosages of oral beta-lactams prescribed and almost all patients received suboptimally dosed oral beta-lactams, preventing us from further analysis of appropriate durations of therapy when optimally dosed oral beta-lactam agents are administered. Of note, the vast majority of patients received TMP-SMX as 8–10 mg/kg/day (based on the TMP component) divided twice daily, precluding evaluation of whether a lower dosage of TMP-SMX (eg, 1 double-strength tablet twice daily) would have led to similar outcomes.
Due to increasing antibiotic resistance in urinary isolates, the likelihood of identifying bacterial isolates susceptible to ciprofloxacin, levofloxacin, or TMP-SMX continues to decline. Data from recent years indicate that approximately 20–75% of uropathogens are resistant to fluoroquinolones [28–31], whereas 23–76% of isolates are resistant to TMP-SMX [28, 30, 32]. Although fluoroquinolones and TMP-SMX are associated with notable adverse events [33–37], they nonetheless represent convenient oral options that can enable hospitalized patients to continue care in the outpatient setting and avoid complications from peripherally inserted central catheters [38]. An investigation into the role of urinary susceptibility criteria (ie, urinary breakpoints) for fluoroquinolones and TMP-SMX, both of which are primarily excreted in the urine [23–25], may increase the percentage of susceptible isolates without compromising clinical outcomes. For patients with isolates not susceptible to fluoroquinolones or TMP-SMX, the decision to continue IV beta-lactam therapy versus transition to an oral beta-lactam and potentially extend the treatment duration needs to be carefully weighed.
This study had several limitations. Infectious diseases specialists and closely supervised trainees at 24 US hospitals collected all clinical data. Although standardized data-collection forms with detailed data dictionaries were provided and regular virtual meetings were conducted to provide a forum for clarifications needed during data collection, the lack of the data-management and statistical analysis center's (ie, Johns Hopkins University) access to protected health information across all sites precluded the ability to ensure the integrity of antibiotic-use data submitted from participating hospitals. Second, due to the retrospective nature of the study, some variables that impact the decision of how long to treat an infection (eg, the decision to extend therapy because of a new fever on day 4 of antibiotics) remain unaccounted for. In clinical trials, the planned treatment duration is determined at a set time point, generally precluding ad hoc duration of therapy decisions that evolve over time based on changes in the clinical status of patients, spurious laboratory values, or clinician preferences with rotating medical teams. The use of IPTW incorporating propensity scores mitigates—but does not eliminate—baseline differences between treatment groups in observational studies; however, differences between treatment groups beyond day 1, which likely influence final treatment durations, are generally not addressed. Additionally, although sites were encouraged to access Epic Care Everywhere and other resources that enabled them to identify subsequent medical encounters, the possibility of missing data remains, making the number of recurrent infections a likely underestimate. However, we have no reason to believe that missing outcomes data disproportionately impacted 1 treatment arm over the other.
Our study suggests that, for patients receiving IV beta-lactams or fluoroquinolones/TMP-SMX for the entire treatment course, 7 days of antibiotic therapy is likely sufficient; 10 days may be needed for other patients. More data are needed to determine if patients treated with oral beta-lactam agents administered at dosages and frequencies that mimic IV beta-lactam agents can also be successfully treated with 7 days of antibiotics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
John McAteer, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jae Hyoung Lee, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Sara E Cosgrove, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Kathryn Dzintars, Department of Pharmacy, The Johns Hopkins Hospital, Baltimore, Maryland, USA.
Suiyini Fiawoo, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Emily L Heil, Department of Practice, Science, and Health Outcomes Research, University of Maryland School of Pharmacy, Baltimore, Maryland, USA.
Ronald E Kendall, Department of Pharmacy, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, Michigan, USA.
Ted Louie, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA.
Anurag N Malani, Department of Medicine, Trinity Health St. Joseph Mercy, Ann Arbor, Michigan, USA.
Priya Nori, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York, USA.
Kelly M Percival, Department of Pharmaceutical Care, University of Iowa Hospitals & Clinics, Iowa City, Iowa, USA.
Pranita D Tamma, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Notes
Financial support. J. M. is funded by a National Institutes of Health grant (grant number T32-AI052071).
References
- 1. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015; 13:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:625–63. [DOI] [PubMed] [Google Scholar]
- 3. Tenney J, Hudson N, Alnifaidy H, Li JTC, Fung KH. Risk factors for acquiring multidrug-resistant organisms in urinary tract infections: a systematic literature review. Saudi Pharm J 2018; 26:678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drekonja DM, Trautner B, Amundson C, Kuskowski M, Johnson JR. Effect of 7 vs 14 days of antibiotic therapy on resolution of symptoms among afebrile men with urinary tract infection: a randomized clinical trial. JAMA 2021; 326:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heil EL, Bork JT, Abbo LM, et al. Optimizing the management of uncomplicated gram-negative bloodstream infections: consensus guidance using a modified Delphi process. Open Forum Infect Dis 2021; 8:ofab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18:800–4. [DOI] [PubMed] [Google Scholar]
- 7. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America 2022 guidance on the treatment of extended-spectrum beta-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 2022; 75:187–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amoah J, Stuart EA, Cosgrove SE, et al. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin Infect Dis 2020; 71:e497–e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019; 69:1091–8. [DOI] [PubMed] [Google Scholar]
- 10. von Dach E, Albrich WC, Brunel AS, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA 2020; 323:2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Molina J, Montero-Mateos E, Praena-Segovia J, et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by Enterobacterales: a randomized, controlled trial. Clin Microbiol Infect 2022; 28:550–7. [DOI] [PubMed] [Google Scholar]
- 12. Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med 2015; 372:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klausner HA, Brown P, Peterson J, et al. A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr Med Res Opin 2007; 23:2637–45. [DOI] [PubMed] [Google Scholar]
- 14. Dinh A, Davido B, Etienne M, et al. Is 5 days of oral fluoroquinolone enough for acute uncomplicated pyelonephritis? The DTP randomized trial. Eur J Clin Microbiol Infect Dis 2017; 36:1443–8. [DOI] [PubMed] [Google Scholar]
- 15. Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA 2000; 283:1583–90. [DOI] [PubMed] [Google Scholar]
- 16. Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet 2012; 380:484–90. [DOI] [PubMed] [Google Scholar]
- 17. Peterson J, Kaul S, Khashab M, Fisher AC, Kahn JB. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology 2008; 71:17–22. [DOI] [PubMed] [Google Scholar]
- 18. Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 2003; 290:2588–98. [DOI] [PubMed] [Google Scholar]
- 19. Capellier G, Mockly H, Charpentier C, et al. Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS One 2012; 7:e41290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ulleryd P, Sandberg T. Ciprofloxacin for 2 or 4 weeks in the treatment of febrile urinary tract infection in men: a randomized trial with a 1 year follow-up. Scand J Infect Dis 2003; 35:34–9. [DOI] [PubMed] [Google Scholar]
- 21. van Nieuwkoop C, van der Starre WE, Stalenhoef JE, et al. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med 2017; 15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dow G, Rao P, Harding G, et al. A prospective, randomized trial of 3 or 14 days of ciprofloxacin treatment for acute urinary tract infection in patients with spinal cord injury. Clin Infect Dis 2004; 39:658–64. [DOI] [PubMed] [Google Scholar]
- 23. Chien SC, Rogge MC, Gisclon LG, et al. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother 1997; 41:2256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lettieri JT, Rogge MC, Kaiser L, Echols RM, Heller AH. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob Agents Chemother 1992; 36:993–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chin TW, Vandenbroucke A, Fong IW. Pharmacokinetics of trimethoprim-sulfamethoxazole in critically ill and non-critically ill AIDS patients. Antimicrob Agents Chemother 1995; 39:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 2005; 293:949–55. [DOI] [PubMed] [Google Scholar]
- 27. Nicolle LE, Madsen KS, Debeeck GO, et al. Three days of pivmecillinam or norfloxacin for treatment of acute uncomplicated urinary infection in women. Scand J Infect Dis 2002; 34:487–92. [DOI] [PubMed] [Google Scholar]
- 28. Rank EL, Lodise T, Avery L, et al. Antimicrobial susceptibility trends observed in urinary pathogens obtained from New York state. Open Forum Infect Dis 2018; 5:ofy297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah A, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. Application of fluoroquinolone resistance score in management of complicated urinary tract infections. Antimicrob Agents Chemother 2017; 61:e02313-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bosch-Nicolau P, Falco V, Vinado B, et al. A cohort study of risk factors that influence empirical treatment of patients with acute pyelonephritis. Antimicrob Agents Chemother 2017; 61:e01317-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park KH, Oh WS, Kim ES, et al. Factors associated with ciprofloxacin- and cefotaxime-resistant Escherichia coli in women with acute pyelonephritis in the emergency department. Int J Infect Dis 2014; 23:8–13. [DOI] [PubMed] [Google Scholar]
- 32. Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ 2016; 352:i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Food and Drug Administration . FDA updates warnings for fluoroquinolone antibiotics. Available at: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics. Accessed 2 September 2022.
- 34. Baggio D, Ananda-Rajah MR. Fluoroquinolone antibiotics and adverse events. Aust Prescr 2021; 44:161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tanne JH. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008; 337:a816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Butler AM, Durkin MJ, Keller MR, Ma Y, Powderly WG, Olsen MA. Association of adverse events with antibiotic treatment for urinary tract infection. Clin Infect Dis 2022; 74:1408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keller SC, Cosgrove SE, Kohut M, et al. Hazards from physical attributes of the home environment among patients on outpatient parenteral antimicrobial therapy. Am J Infect Control 2019; 47:425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.