Abstract
Background
Infectious diseases and ophthalmology professional societies have disagreed regarding ocular screening in patients with candidemia. We aimed to summarize the current evidence on the prevalence of ocular candidiasis (OC) and Candida endophthalmitis (CE) according to the standardized definitions.
Methods
A literature search was conducted from the inception date through 16 October 2022 using PubMed, Embase, and SCOPUS. Pooled prevalence of ocular complications was derived from generalized linear mixed models (PROSPERO CRD42022326610).
Results
A total of 70 and 35 studies were included in the meta-analysis for OC and concordant CE (chorioretinitis with vitreous involvement), respectively. This study represented 8599 patients with candidemia who underwent ophthalmologic examination. Pooled prevalences (95% CI) of OC, overall CE, concordant CE, and discordant CE were 10.7% (8.4–13.5%), 3.1% (2.1–4.5%), 1.8% (1.3–2.6%), and 7.4% (4.5–12%) of patients screened, respectively. Studies from Asian countries had significantly higher concordant CE prevalence (95% CI) of patients screened (3.6%; 2.9–4.6%) compared with studies from European countries (1.4%; .4–5%) and American countries (1.4%; .9–2.2%) (P <.01). Presence of total parenteral nutrition and Candida albicans was associated with CE, with pooled odds ratios (95% CI) of 6.92 (3.58–13.36) and 3.02 (1.67–5.46), respectively.
Conclusions
Prevalence of concordant CE overall and among Asian countries was 2 and 4 times higher than the prevalence previously reported by the American Academy of Ophthalmology (AAO) of <0.9%, respectively. There is an urgent need to study optimal screening protocols and to establish joint recommendations by the Infectious Diseases Society of America and AAO.
Keywords: candidemia, endophthalmitis, ocular candidiasis
This study demonstrates a higher prevalence of concordant Candida endophthalmitis compared with the previous report by the American Academy of Ophthalmology (1.8% vs <0.9%), with a higher prevalence among studies from Asian countries of 3.6%.
(See the Editorial Commentary by Adriana M. Rauseo and Andrej Spec on pages 1750–2.)
Candidemia is known for high mortality rates of 25–40% despite appropriate treatment [1–3]. Patients with candidemia should be evaluated for metastatic foci, particularly those with persistent candidemia [4]. The 2016 Infectious Diseases Society of America (IDSA) guidelines recommend a dilated eye exam by an ophthalmologist in all patients with candidemia, preferably within 1 week of diagnosis for nonneutropenic patients and delayed until neutrophil recovery among neutropenic patients [5]. Although these recommendations were not based on data from randomized controlled trials, it was thought that the downstream consequences of missing and not appropriately treating patients with Candida endophthalmitis (CE) would be substantial.
However, routine ophthalmologic examination in all patients with candidemia has been questioned, particularly with low cost-effectiveness and low quality of evidence to support this recommendation [5–8]. The American Academy of Ophthalmology (AAO) issued a statement recommending against routine screening for endogenous endophthalmitis in all patients with candidemia and only recommended screening in patients with signs or symptoms suggestive of ocular infection on 19 July 2021 [9]. Due to the disagreements in recommendations by the two professional societies, AAO and IDSA, we conducted this systematic review and meta-analysis to summarize the current evidence on the prevalence of Candida ocular involvement, both ocular candidiasis (OC) and CE, and to exploratorily investigate factors associated with CE.
METHODS
Study Definitions
Candida endophthalmitis was defined as having abnormal ocular findings, including vitritis and chorioretinitis, specifically attributed to Candida infection based on the direct ophthalmologic examination performed by ophthalmologists. Ocular candidiasis included any intraocular abnormalities among patients with candidemia such as vitritis, chorioretinitis, and other nonspecific abnormal retinal findings. The diagnosis of CE was classified according to previous definitions as “concordant” and “discordant” [6]. Patients with concordant CE must meet 1 of the following definitions: (1) Candida chorioretinitis with an extension of the surrounding inflammation into the vitreous or (2) vitreous abscess manifesting as intravitreal fluff balls [10]. Patients who did not meet 1 of the 2 concordant CE criteria, such as patients considered to have CE based on an ophthalmologist's overall impression or patients for whom diagnostic criteria for CE were not explicitly defined, were classified as having discordant CE. We a priori determined variables for potential risk factors associated with CE by incorporating factors that were demonstrated in at least 3 studies for our exploratory meta-analysis. Additional definitions for these parameters are described in Supplementary Methods 1.
Search Strategy
Two authors (K. P. and T. P.) performed this systematic search independently in 3 databases including PubMed, Embase, and SCOPUS from the inception date to 16 October 2022. The complete search terms for each database are available in Supplementary Methods 2. We report this study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. We conducted a manual hand search from reference lists and citation tracking for eligible studies. The International Prospective Register of Systematic Reviews (PROSPERO) registration number is CRD42022326610.
Selection Criteria
The screening process was conducted in the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Two authors (T. P. and K. P.) reviewed the included studies and selected all observational studies in which the prevalence of OC or CE was provided or could be calculated. Conference abstracts, case reports, and case series were excluded due to unavailability of data required for prevalence calculation. We contacted corresponding authors of the recruited studies for methodology and definitions if needed. If more than 1 publication was based on the same cohort or population and reported the same outcomes, only the most recent or comprehensive publication was included [12]. We used the Web-based Google Translate to translate non–English-language abstracts during abstract screening. For the full-text review, non–English-language studies were translated by a professional translation agency (PoliLingua, London, UK).
Data Analysis
Our primary outcome was the prevalence of OC and CE in patients with candidemia. We extracted numbers of patients with candidemia, patients screened for ocular complications, and patients with ocular complications to calculate the pooled prevalence of CE. Crude numbers and unadjusted/adjusted odds ratio (ORs) with 95% confidence intervals (CIs) of each potential risk factor associated with CE were extracted for meta-analysis. We used the risk bias tool by Hoy et al [13] for the quality assessment of papers providing the prevalence of OC and CE. We used the Quality in Prognostic Studies (QUIPS) tool [14] for quality assessment of prognostic studies providing risk factors of concordant CE.
The prevalence of OC and CE was calculated by dividing patients with OC and CE according to the definitions above with patients with candidemia who underwent ophthalmologic screening. We performed meta-analysis using R (R language; R Foundation for Statistical Computing, Vienna, Austria) to calculate the pooled prevalence of OC and CE along with 95% CIs by using generalized linear mixed models (GLMMs) [15]. We performed subgroup analyses to better understand the differences in concordant CE prevalence based on study design, patient population, study continent, risk of bias according to criteria by Hoy et al [13], and proportion of patients with ophthalmologic screening in the study. We then used the chi-square test to determine differences in pooled prevalence between the subgroups. Sensitivity analyses of pooled OC and CE prevalence were performed by removing studies with high risk of bias.
We exploratorily investigated the risk factors associated with CE in patients with candidemia by calculating the pooled OR (pOR) with 95% CIs and using a random-effects model. We directly calculated ORs from raw numerical data provided from the study if the OR was not provided. Heterogeneity of the effect size of each study was assessed using I2 statistics. I2 statistics with a value less than 25% were considered low heterogeneity; a value ranging from 25% to 60% was considered moderate heterogeneity, and an I2 value greater than 60% was considered substantial heterogeneity [16, 17].
RESULTS
Study and Patient Characteristics
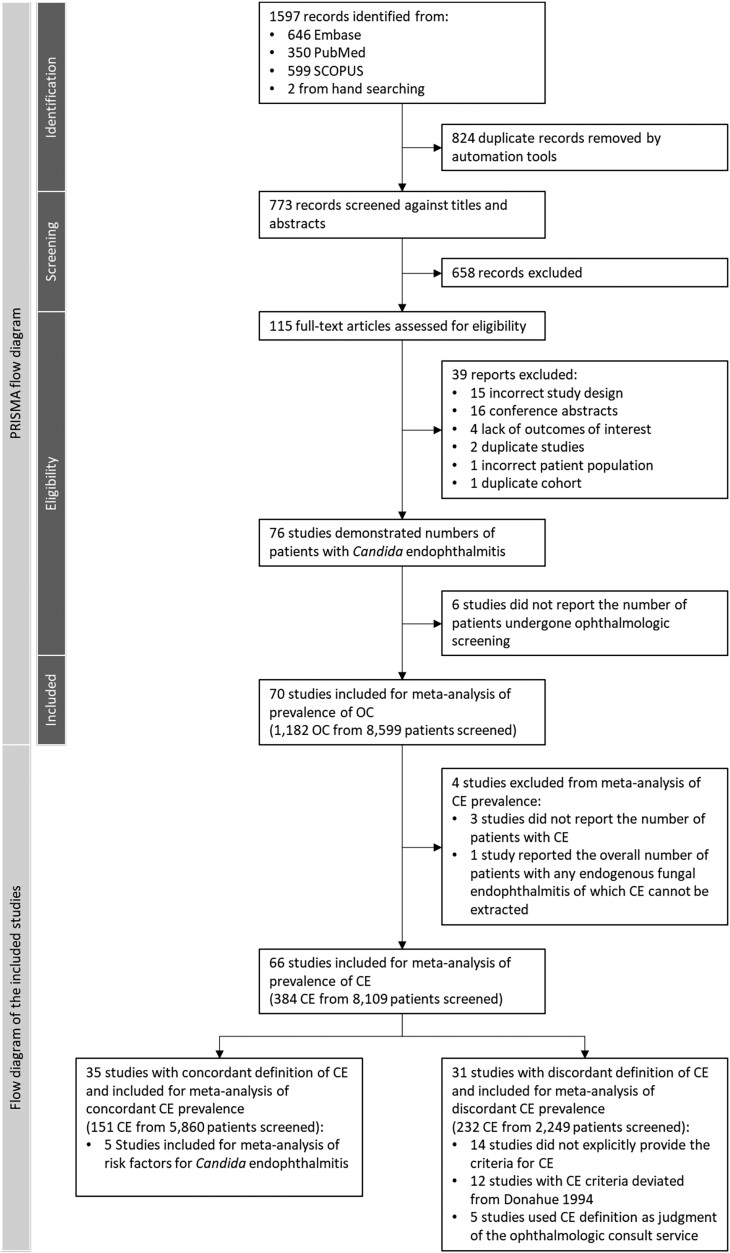
We retrieved 1597 studies from the initial search; 1595 studies were retrieved from the 3 databases, whereas the other 2 studies [3, 18] were identified from the manual search; 824 duplicates were removed, and 658 studies were excluded from title and abstract screening. We performed a full-text review on 115 studies. Thirty-nine were excluded due to incorrect study designs, including case series and case reports, conference abstracts, studies with populations with a fungal infection other than Candida species, studies that did not report the number of patients with ocular complications of candidemia, or those with a duplicate cohort. Six studies did not report the number of patients who underwent ophthalmologic screening. A total of 70 studies [3, 4, 8, 10, 18–83] were included in the systematic review and meta-analysis of OC, of which 35 studies [8, 10, 18, 20, 22, 27, 28, 30, 32, 34, 36, 37, 39, 41–43, 46, 49, 52, 54, 57, 58, 61, 62, 66, 68, 69, 71, 72, 74, 76–78, 80, 82] were incorporated in the meta-analysis and subgroup analysis of prevalence of concordant CE (Figure 1). A total of 5 studies [23, 43, 47, 60, 71] were incorporated in the meta-analysis of risk factors associated with CE. Characteristics of the 70 studies are reported in Supplementary Table 1. There were 8599 patients with candidemia who underwent ophthalmologic screening and were included in the study.
Figure 1.
PRISMA diagram of the included studies for prevalence analysis. Abbreviations: CE, Candida endophthalmitis; OC, ocular candidiasis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Prevalence of Ocular Candidiasis and Candida Endophthalmitis
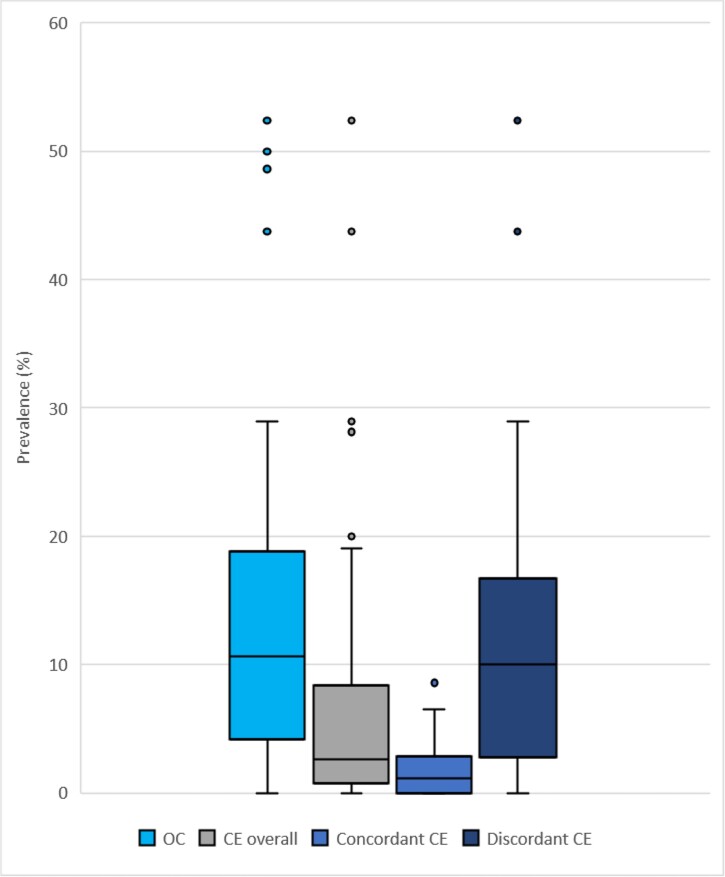
Seventy studies [3, 4, 8, 10, 18–83] reported the number of patients with candidemia who underwent ophthalmologic screening and patients with candidemia who developed OC or CE. The pooled prevalence of OC (95% CI) was 10.70% (8.41–13.51%; I2 = 84%) of patients screened (Supplementary Figure 1). Sixty-six studies [3, 8, 10, 18, 20–54, 56–80, 82, 83] reported the number of patients with candidemia who developed CE. Among 66 studies, 35 studies [8, 10, 18, 20, 22, 27, 28, 30, 32, 34, 36, 37, 39, 41–43, 46, 49, 52, 54, 57, 58, 61, 62, 66, 68, 69, 71, 72, 74, 76–78, 80, 82] used definitions consistent with concordant CE and 31 studies [3, 21, 23–26, 29, 31, 33, 35, 38, 40, 44, 45, 47, 48, 50, 51, 53, 56, 59, 60, 63–65, 67, 70, 73, 75, 79, 83] did not (discordant CE). Characteristics of the 35 concordant CE studies are shown in Table 1. The pooled prevalence of CE (95% CI) (both concordant and discordant definitions) was 3.08% (2.08–4.54%; I2 = 85%) of patients screened (Supplementary Figure 2). The pooled prevalence of concordant CE (95% CI) was 1.83% (1.30–2.57%; I2 = 24%) of patients screened, whereas the pooled prevalence of discordant CE (95% CI) was 7.37% (4.45–11.97%; I2 = 82%) of patients screened (Figure 2 and Supplementary Figure 2). Of the 31 studies reporting discordant CE, 13 studies [3, 21, 25, 29, 31, 35, 38, 40, 45, 48, 50, 65, 79, 83] did not provide the specific criteria for CE diagnosis, 12 studies [23, 26, 33, 47, 51, 53, 56, 60, 63, 64, 67, 70] used criteria that deviated from the definition of concordant CE, and 5 studies [24, 44, 59, 73, 75] used “judgment of ophthalmologic consult service” to determine CE diagnosis.
Table 1.
Evidence Table for Studies With Concordant Candida Endophthalmitis
| First Author, Year [Reference] | Study Design | Data Source | Source Population | Sampling Scheme | Study Population | Criteria for Candida Endophthalmitis | Patients With CBSI, n | Patients Screened, n | Patients With CE, n | Prevalence of CE, % | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adam, 2015 [20] | Retrospective cross-sectional study | Hospital-based study | Pennsylvania, USA | Full census | Patients with +BC | CCR with VE | 213 | 213 | 4 | 1.88 | Low |
| Blennow, 2013 [22] | Retrospective cohort | Hospital-based study | Huddinge, Sweden | Full census | Patients (≥1 year) with +BC | CCR with vitritis or white fluffy lesion with VE | 144 | 60 | 0 | 0 | Low |
| Donahue, 1994 [10] | Prospective observational study | Multicentered hospital-based study | Pennsylvania and North Carolina, USA | Full census | Patients with +BC | CCR with vitritis or white fluffy lesion with VE | 118 | 118 | 0 | 0 | Low |
| Donahue, 2003 [27] | Retrospective review of inpatient hospital consultations | Hospital-based study | Tennessee, USA | Full census | Children with ophthalmologic consultation due to disseminated candidiasis | CCR with vitritis or white fluffy lesion with VE | 24 | 24 | 0 | 0 | Low |
| Dozier, 2011 [8] | Retrospective case series | Hospital-based study | Tennessee, USA | Full census | Patients with +BC | Vitreous abscess manifesting as intravitreal fluff balls | 211 | 211 | 1 | 0.47 | Low |
| El-Abiary, 2018 [28] | Retrospective observational study | Multicentered ICU-based study | Glasgow, Scotland | Full census | Adult patients (≥17 years) with +BC | CCR with vitritis | 168 | 80 | 0 | 0 | Low |
| Feman, 2002 [30] | Retrospective manner, observational case series and case report | Hospital-based study | Missouri, USA | Full census | Patients whose the ophthalmologic consultation record had the words “fungal infection” and “endophthalmitis” or “retinitis” | CCR with vitritis or white fluffy lesion with VE | 82a | 82 | 2 | 2.43 | Low |
| Fierro, 2013 [32] | Retrospective cohort | Hospital-based study | New York and Pennsylvania, USA | Full census | Children with +BC | CCR with vitritis | 378 | 254 | 4 | 1.57 | Low |
| Geraymovych, 2015 [34] | Retrospective review | Hospital-based study | Virginia, USA | Full census | Patients with +BC, without prior ocular surgery or ocular trauma | VE and anterior extension with fluffy vitreous balls, vitreous haze, vitreous abscess, anterior chamber cells, or hypopyon | 132 | 132 | 1 | 0.76 | Low |
| Ghodasra, 2014 [18] | Retrospective case series | Hospital-based study | Pennsylvania, USA | Full census | Adult patients (≥18 years) with +BC | VE with fluff balls, vitreous haze, or vitreous abscess | 326 | 238 | 2 | 0.84 | Moderate |
| Govindaraju, 2022 [82] | Retrospective | Hospital-based study | Michigan, USA | Full census | Adult patients with +BC or elevated beta-D-glucan level | CCR with vitritis or vitreous abscess manifesting as intravitreal fluff balls | 100 | 100 | 3 | 3.00 | Moderate |
| Hautala, 2021 [36] | Retrospective study | Hospital-based study | Oulu, Finland | Full census | Patients with +BC | Vitritis or fluffy lesions with VE | 304 | 271 | 16 | 5.9 | Low |
| Hillenbrand, 2022 [37] | Retrospective study | Hospital-based study | Ohio, USA | Full census | Adult patients with +BC | VE with fluff balls, vitreous haze or vitreous abscess | 226 | 129 | 1 | 0.78 | Low |
| Huynh, 2012 [39] | Retrospective review | Hospital-based study | Massachusetts, USA | Full census | Patients with +BC, sedated or obtunded patients (unable to assess VA) were excluded | CCR with vitritis | 36 | 36 | 0 | 0 | Low |
| Kannangara, 2007 [41] | Prospective study | Hospital-based study | Pennsylvania, USA | Full census | Patients with +BC | CCR with VE | 46 | 46 | 0 | 0 | Moderate |
| Karmisholt, 2008 [42] | Retrospective cohort | Hospital-based study | Aalborg, Denmark | Full census from Bacteremia Research Registry | Patients with +BC | CCR with vitreous inflammation | 203 | 86 | 1 | 1.16 | Moderate |
| Kato, 2018 [43] | Retrospective cohort | Multicentered hospital-based study | Japan | Full census | Adult patients (≥18 years) with +BC | CCR with vitritis | 289 | 174 | 4 | 2.3 | Moderate |
| Khalid, 2014 [46] | Retrospective review | Hospital-based study | Kansas, USA | Full census | Adult patients (>18 years) with +BC | CCR with vitritis | 283 | 144 | 2 | 1.39 | Moderate |
| Krishna, 2000 [49] | Prospective observational study | Hospital-based study | Ohio, USA | Full census | Patients with +BC, no prior fungemia, life expectancy >72 hours | Intravitreal fluff ball or vitreal abscess | 31 | 31 | 0 | 0 | Moderate |
| Mehta, 2007 [52] | Retrospective review | ICU-based study | Mumbai, India | Full census | Patients with +BC | CCR with vitritis | 12a | 12 | 0 | 0 | Moderate |
| Nagao, 2012 [54] | Retrospective study | Multicentered hospital-based study | Kyoto, Japan | Full census | Patients with +BC | Vitritis or white fluffy lesion with VE | 220 | 204 | 10 | 4.9 | Low |
| Noyola, 2001 [57] | Retrospective review | NICU-based study | Texas, USA | Full census | Infants in NICU with Candida spp. isolated from any sterile sites or with histopathological evidence of invasive candidal dermatitis | CCR with vitreous involvement | 86 | 67 | 1 | 1.49 | Moderate |
| Oude Lashof, 2011 [58] | Prospective study | Hospital-based study | Maastricht, The Netherlands | Full census | Patients (≥12 years) with +BC | Vitritis or white fluffy lesion with VE | 370 | 370 | 6 | 1.62 | Moderate |
| Paulus, 2016 [61] | Prospective cohort study | Hospital-based study | California, USA | Full census | Adult patients (≥18 years) with +BC | CCR with vitritis | 125 | 125 | 2 | 1.6 | Moderate |
| Popovich, 2007 [62] | Retrospective review | Hospital-based study | Michigan, USA | Full census | Adult patients (≥18 years) with +BC, patients those died within 48 hours after candidemia were excluded | CCR with vitritis or white fluffy lesion with VE | 100 | 80 | 3 | 3.75 | Low |
| Rodriguez-Adrián, 2003 [66] | Prospective study | ICU-based study | Caracas, Venezuela | Full census | Patients (≥13 years) with +BC, those with ANC <500/mm3 or major immunodeficiencies were excluded | CCR with VE | 206 | 180 | 2 | 1.11 | Moderate |
| Sakai, 2021 [68] | Retrospective observational study | Hospital-based study | Kobe, Japan | Full census | Patients with +BC | Vitritis or white fluffy lesion with VE | 58 | 56 | 0 | 0 | Low |
| Sakamoto, 2022 [69] | Retrospective cohort study | Hospital-based study | Fukuoka, Japan | Full census | Adult patients (≥18 years) with +BC | Vitritis or white fluffy lesion with VE | 138 | 108 | 7 | 6.48 | Moderate |
| Seidelman, 2021 [71] | Retrospective chart review | Multicentered hospital-based study | North Carolina and Virginia, USA | Full census | Adult patients (≥18 years) with +BC | CCR with vitritis | 771 | 771 | 30 | 3.89 | Moderate |
| Shah, 2008 [72] | Retrospective review | Hospital-based study | Pennsylvania, USA | Full census | Patients with +BC | CCR with vitreous cells of “fluff balls” | 38 | 38 | 0 | 0 | Low |
| Siddiqui, 2022 [74] | Retrospective review | Hospital-based study | Arkansas, USA | Full census | Adult patients (≥18 years) with +BC | CCR with vitritis or white fluffy lesion with VE | 290 | 161 | 4 | 2.48 | Low |
| Son, 2019 [76] | Retrospective review | Hospital-based study | Seoul, Korea | Full census | Patients with +BC | Vitritis or white fluffy lesion with VE | 438 | 275 | 8 | 2.91 | Moderate |
| Ueda, 2019 [77] | Retrospective study | Multicentered hospital-based study | Japan | Full census | Nonneutropenic adult patients (>17 years) with +BC | CCR with VE | 1089 | 781 | 32 | 4.1 | Moderate |
| Vena, 2017 [78] | Retrospective study | Multicentered hospital-based study | Spain | Full census | Adult patients (>16 years) with +BC | Vitritis or fluffy lesion with VE | 365 | 168 | 2 | 1.19 | Moderate |
| Zakhem, 2021 [80] | Retrospective review | Hospital-based study | Beirut, Lebanon | Full census | Patients with +BC | Vitreous inflammation | 193 | 35 | 3 | 8.57 | Moderate |
Abbreviations: ANC, absolute neutrophil count; +BC, positive blood culture for Candida species; CBSI, Candida bloodstream infection; CCR, Candida chorioretinitis; CE, Candida endophthalmitis; ICU, intensive care unit; NICU, neonatal intensive care unit; VE, vitreal extension or extension to vitreous.
Feman et al recruited patients with positive fungal culture from any of these sites: blood, bronchial wash, deep abscess, abdominal paracentesis, or thoracentesis, whereas Mehta et al recruited patients in whom Candida spp. can be isolated from the tracheal secretions, peritoneal fluid, the central line tip, urine cultures, or blood cultures.
Figure 2.
Prevalence of ocular involvement/complications in patients with candidemia who underwent ophthalmologic screening: OC, CE, concordant CE, and discordant CE were retrieved from 70, 66, 35, and 31 studies, respectively. The boxed area represents the 25th to the 75th percentile of the data. The horizontal line inside each box represents the median value. Error bars show the minimum and maximum values with filled circles as outliers above the plot. Abbreviations: CE, Candida endophthalmitis; OC, ocular candidiasis.
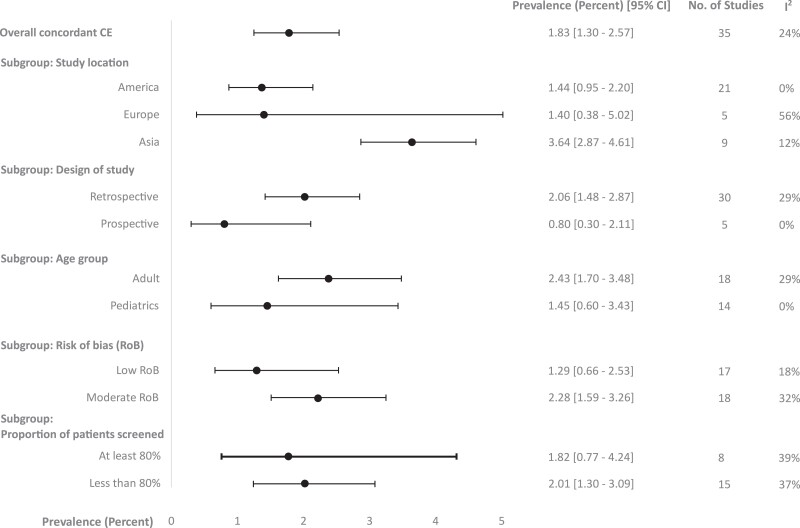
In subgroup analyses of concordant CE prevalence, studies from Asian countries [43, 52, 54, 68, 69, 76–78, 80] showed a significantly higher concordant CE prevalence of patients screened (95% CI) of 3.64% (2.87–4.61%; I2 = 12%) compared with studies from European countries [22, 28, 36, 42, 58] of 1.40% (.38–5.02%; I2 = 56%) and American countries [8, 10, 18, 20, 27, 30, 32, 34, 37, 39, 41, 46, 49, 57, 61, 62, 66, 71, 72, 74] of 1.44% (.95–2.20%; I2 = 0%) (P < .01). The prevalence of concordant CE in adults [18, 19, 28, 36, 37, 39, 43, 46, 49, 61, 62, 66, 69, 71, 74, 76, 77, 82] was not significantly different compared with the prevalence in pediatric populations [27, 32, 57] (2.43% [95% CI: 1.70–3.48%; I2 = 29%] in adult vs 1.45% [95% CI: .60–3.43%; I2 = 0%] in pediatric populations; P = .28). In subgroup analysis of concordant CE based on the proportion of ophthalmologic examination, there was no significant difference in prevalence of concordant CE (95% CI) among the studies with a proportion of 80% or more [10, 36, 41, 54, 58, 62, 66, 68] vs less than 80% [18, 22, 28, 32, 37, 42, 43, 46, 57, 69, 74, 76–78, 80] ophthalmologic examinations (1.82 [.77–4.27; I2 = 39%] vs 2.01 [1.30–3.09; I2 = 37%], respectively; P = .84). Among 35 studies reporting concordant CE prevalence, 12 only included people who were screened for ocular complications and were excluded from the subgroup analysis based on the proportion of ophthalmologic examinations [8, 20, 27, 30, 34, 39, 49, 52, 61, 71, 72, 82]. Subgroup analyses did not show significant differences in concordant CE prevalence by study design and risk of bias (Figure 3, Supplementary Figure 3).
Figure 3.
Subgroup analyses of prevalence of concordant Candida endophthalmitis among patients with candidemia who underwent ophthalmologic screening. Abbreviations: CE, Candida endophthalmitis; CI, confidence interval.
Of the 70 studies included for meta-analysis of OC and CE prevalence, there were 16 studies with high risk of bias, 37 studies with moderate risk of bias, and 17 studies with low risk of bias (Supplementary Tables 2–4). No studies included for concordant CE were considered high risk. We then performed sensitivity analyses by removing studies with high risk of bias for OC and discordant CE prevalence calculation. The pooled prevalences (95% CI) of OC and discordant CE of patients screened after removing studies with high risk of bias were 12.53% (10.03–15.55%; I2 = 83%) and 11.81% (7.76–17.58%; I2 = 75%), respectively (Supplementary Figure 4).
Factors Associated With Candida Endophthalmitis
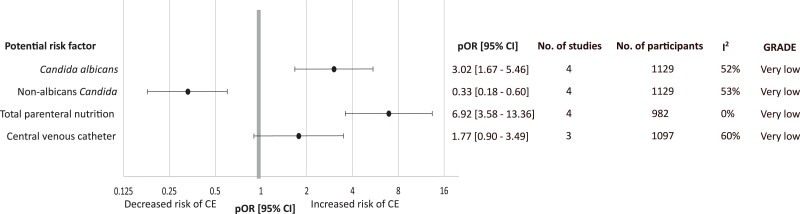
Data extraction of all potential factors associated with CE and Grading of Recommendations Assessment, Development and Evaluation (GRADE) for potential factors associated with CE are available in Supplementary Tables 5 and 6. However, only 4 factors, which were C. albicans, non–albicans Candida, presence of central venous catheter (CVC), and presence of total parenteral nutrition (TPN), were reported in at least 3 studies and for which meta-analysis could be performed. We found that C. albicans candidemia was associated with CE with a pOR of 3.02 (95% CI: 1.67–5.46; P < .01; I2 = 52%) [23, 43, 47, 71], whereas patients with non–albicans candidemia were less likely to have CE with a pOR of .33 (95% CI: .18–.60; P < .01; I2 = 53%) [23, 43, 47, 71]. The presence of TPN was associated with CE with a pOR of 6.92 (95% CI: 3.58–13.36; P < .01; I2= 0%) [23, 47, 60, 71], whereas the presence of CVCs was not significantly associated with CE (Figure 4, Supplementary Figure 5). Subgroup analysis among risk factors associated with CE could not be performed due to the small number of included studies. Risk of bias of the studies included for risk factor analysis is shown in Supplementary Table 7. Of 5 studies used in risk factor analysis, 3 studies [23, 47, 60] were low risk and 2 studies [43, 71] were moderate risk.
Figure 4.
Pooled odd ratios of potential risk factors associated with Candida endophthalmitis. Abbreviations: CE, Candida endophthalmitis; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation; pOR, pooled odds ratio.
DISCUSSION
We conducted an updated systematic review and meta-analysis to summarize the prevalence of ocular findings in patients with candidemia, namely ocular candidiasis (OC) and Candida endophthalmitis (CE), and exploratorily investigated risk factors for CE. With our extensive search from multiple databases without language restrictions, we found that the pooled prevalence of concordant CE (95% CI), the most stringently defined and most concerning of Candida ocular complications, was 1.8% (1.3–2.6%) of patients screened, which is 2 times higher than the previously published systematic analysis [6]. Of 70 included studies included in our review, 48 studies [3, 8, 10, 18, 20, 22–24, 26, 27, 29–35, 38, 39, 41, 42, 44, 46, 48, 49, 51–62, 64–67, 70, 72, 78, 79, 81, 84, 85] were published before 2018, of which 10 [29, 31, 44, 52, 55, 57, 59, 79, 81, 84] were not included in the previous study by Breazzano et al in 2019 [6]. If re-calculating the prevalence of concordant CE from the studies that were published before 2018 [8, 10, 18, 20, 22, 27, 30, 32, 34, 39, 41, 42, 46, 49, 52, 54, 57, 58, 61, 62, 66, 72, 78] by using generalized linear mixed models, the prevalence of concordant CE (95% CI) would be 1.3% (.9–2%), not less than 1%.
In our study, we observed a huge difference in the prevalence of ocular complications based on definitions and high heterogeneity was noted among OC and discordant CE studies, which stresses the importance of understanding terminology when applying these terms in clinical care. The prevalence of OC (any abnormal ocular findings in patients with candidemia) was as high as 11%. However, this high prevalence of OC is possibly attributed to underlying comorbidities rather than solely from Candida infection. Previous prospective studies [22, 32, 66] reported that underlying diseases were confounders in the increased prevalence of abnormal ocular findings in patients with candidemia. We suspect that high heterogeneity in OC and discordant CE could possibly be explained by the nature of their wide range of definitions. Hence, clinicians should be mindful that not all abnormal ophthalmologic findings in patients with candidemia are attributed to Candida infection.
Among studies reporting concordant CE, we found a significantly higher prevalence of concordant CE among studies from Asian countries (3.6%), compared with studies from European countries and American countries. The cause of higher concordant CE prevalence among Asian countries is unclear, but we hypothesize that there could possibly be a component of genetic predisposition favoring more invasive fungal infections, in additional to differences in methods applied to diagnostic screening, fungal epidemiology, and antifungal prophylaxis/treatment [86–88]. Although the necessity of universal ophthalmologic screening is debated, our findings raise concerns that limiting ophthalmologic screening only in symptomatic patients, particularly in populations with a higher prevalence, may lead to underdiagnosis. Our study also identified the scarcity of CE prevalence data from Africa, South America, and Australia. Investigations on this important clinical topic should be encouraged from these continents.
In our exploratory analysis, we identified 2 risk factors associated with CE in patients with candidemia including TPN use and infection with C. albicans. Candida albicans has been well known for high virulence and more severe complications than other Candida species. Candida albicans, which is the most intrinsically virulent Candida species, causes more bloodstream infections, can induce inflammatory cytokines and neutrophil recruitment, and gives rise to metastatic foci such as CE [19]. Although we recognize that the identified risk factors associated with CE should be interpreted with caution due to the limited number of studies included for analysis and pORs mainly calculated from unadjusted ORs, these results highlight the importance of recommending TPN when truly indicated and achieving source control [5, 9], a practice supported by both the IDSA guidelines [5] and the AAO statement [9]. In reality, these goals are not always immediately feasible due to other clinical conditions or diagnostic uncertainty. The critical question remains whether ocular symptom screening alone is sufficient for patients with source control issues.
Limitations
We reported pooled prevalence per patient screened to be consistent with previous publications; however, this could potentially overestimate the true prevalence of Candida ocular complications. Although the pooled prevalence of concordant CE was only derived from studies with low to moderate risk of bias, some studies with OC and discordant CE prevalence calculations were considered high risk and should be interpreted with caution. Due to our study design and unavailability of data, we are not able to adjust for confounders related to CE among patients with candidemia and we are not able to perform a wide range of subgroup analyses to understand the differences in concordant CE prevalence based on host immune status or antifungal therapy. We do not have data from the included studies regarding duration of candidemia prior to hospitalization, treatment, specifics of treatment including dosing and susceptibility, and the presence of ocular symptoms. We recognized a lack of fully systematic screening in some studies, a lack of longitudinal screening, and differing times to ophthalmologic examination. It is also important to note that the significance of chorioretinitis in patients with candidemia is not well established. We propose that multicenter, international, prospective studies should be performed to understand the real burden of Candida endophthalmitis to inform guideline development and clinical practices.
Conclusions
This systematic review of 70 studies and meta-analysis of 35 studies of concordant Candida endophthalmitis in patients with candidemia show a higher prevalence of concordant CE compared with the prevalence cited by the recent AAO positional paper [9] (1.8% vs <0.9%), with a prevalence of 3.6% among studies from Asian countries. To develop optimal screening protocols, we must weigh the risks to the patient from a missed or delayed diagnosis against any risks associated with the evaluation, while also considering outside demands on the resources required for screening. Screening patients with candidemia for ocular involvement requires a dilated eye examination and ophthalmologists’ time and expertise, resources that are limited. Neither professional group can fully address this challenge alone. There is an urgent need for more nuanced, evidence-based screening protocols to detect Candida ocular involvement. A joint statement from both the infectious diseases and ophthalmology professional societies is called for.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kasidis Phongkhun, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Thananop Pothikamjorn, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Karan Srisurapanont, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Kasama Manothummetha, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Anawin Sanguankeo, Department of Preventive and Social Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Achitpol Thongkam, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Nipat Chuleerarux, Department of Medicine, Jackson Memorial Hospital/University of Miami, Miami, Florida, USA.
Surachai Leksuwankun, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Tanaporn Meejun, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Jaedvara Thanakitcharu, Faculty of Medicine, Srinakharinwirot University, Nakhon Nayok, Thailand.
Morgan Walker, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Critical Care Medicine Department, National Institutes of Health, Bethesda, Maryland, USA.
Shilpa Gopinath, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Pattama Torvorapanit, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Nattapong Langsiri, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Navaporn Worasilchai, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok, Thailand.
Chatphatai Moonla, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Rongpong Plongla, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Olivia S Kates, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Saman Nematollahi, Department of Medicine, University of Arizona College of Medicine, Tucson, Arizona, USA.
Nitipong Permpalung, Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Notes
Author Contributions. K. P., T. P., and N. P.: study design, literature search, data extraction, quality assessment of the studies, data analysis, manuscript writing, and critical review. K. M. and A. S.: study design, data analysis, manuscript writing, and critical review. K. S.: data analysis, manuscript writing, and critical review. A. T., N. C., S. L., T. M., J. T., M. W., S. G., P. T., N. L., N. W., C. M., R. P., O. S. K., and S. N.: manuscript writing and critical review.
Acknowledgments. The authors thank the librarians at Chulalongkorn University and Johns Hopkins University School of Medicine for their contribution in retrieving full papers of studies that were not available on the databases.
Data sharing. K. P. and N. P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The data for our systematic review and meta-analysis are publicly available based on the study design. Study protocol and dataset can be requested at npermpa1@jhmi.edu.
References
- 1. Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54:1110–22. [DOI] [PubMed] [Google Scholar]
- 2. Cornely FB, Cornely OA, Salmanton-García J, et al. Attributable mortality of candidemia after introduction of echinocandins. Mycoses 2020; 63:1373–81. [DOI] [PubMed] [Google Scholar]
- 3. Fraser VJ, Jones M, Dunkel J, Storfer S, Medoff G, Dunagan WC. Candidemia in a tertiary care hospital: epidemiology, risk factors, and predictors of mortality. Clin Infect Dis 1992; 15:414–21. [DOI] [PubMed] [Google Scholar]
- 4. Agnelli C, Valerio M, Bouza E, et al. Persistent Candidemia in adults: underlying causes and clinical significance in the antifungal stewardship era. Eur J Clin Microbiol Infect Dis 2019; 38:607–14. [DOI] [PubMed] [Google Scholar]
- 5. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1––e50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breazzano MP, Day HR Jr, Bloch KC, et al. Utility of ophthalmologic screening for patients with Candida bloodstream infections: a systematic review. JAMA Ophthalmol 2019; 137:698–710. [DOI] [PubMed] [Google Scholar]
- 7. Vinikoor MJ, Zoghby J, Cohen KL, Tucker JD. Do all candidemic patients need an ophthalmic examination? Int J Infect Dis 2013; 17:e146–e8. [DOI] [PubMed] [Google Scholar]
- 8. Dozier CC, Tarantola RM, Jiramongkolchai K, Donahue SP. Fungal eye disease at a tertiary care center: the utility of routine inpatient consultation. Ophthalmology 2011; 118:1671–6. [DOI] [PubMed] [Google Scholar]
- 9. Breazzano MP, Bond JB III, Bearelly S, et al. American Academy of Ophthalmology recommendations on screening for endogenous Candida endophthalmitis. Ophthalmology 2022; 129:73–6. [DOI] [PubMed] [Google Scholar]
- 10. Donahue SP, Greven CM, Zuravleff JJ, et al. Intraocular candidiasis in patients with candidemia: clinical implications derived from a prospective multicenter study. Ophthalmology 1994; 101:1302–9. [DOI] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao G, Lopez-Jimenez F, Boyd J, et al. Methodological standards for meta-analyses and qualitative systematic reviews of cardiac prevention and treatment studies: a scientific statement from the American heart association. Circulation 2017; 136:e172–e94. [DOI] [PubMed] [Google Scholar]
- 13. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65:934–9. [DOI] [PubMed] [Google Scholar]
- 14. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158:280–6. [DOI] [PubMed] [Google Scholar]
- 15. Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010; 29:3046–67. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane Reviews. Cross-sectional study. BMC Med Res Methodol 2011; 11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghodasra DH, Eftekhari K, Shah AR, VanderBeek BL. Outcomes, impact on management, and costs of fungal eye disease consults in a tertiary care setting. Ophthalmology 2014; 121:2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abe M, Kinjo Y, Ueno K, et al. Differences in ocular complications between Candida albicans and non-albicans Candida infection analyzed by epidemiology and a mouse ocular candidiasis model. Front Microbiol 2018; 9:2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adam MK, Vahedi S, Nichols MM, et al. Inpatient ophthalmology consultation for fungemia: prevalence of ocular involvement and necessity of funduscopic screening. Am J Ophthalmol 2015; 160:1078–83, e2. [DOI] [PubMed] [Google Scholar]
- 21. Bartoletti M, Rinaldi M, Pasquini Z, et al. Risk factors for candidaemia in hospitalized patients with liver cirrhosis: a multicentre case–control–control study. Clin Microbiol Infect 2021; 27:276–82. [DOI] [PubMed] [Google Scholar]
- 22. Blennow O, Tallstedt L, Hedquist B, Gårdlund B. Duration of treatment for candidemia and risk for late-onset ocular candidiasis. Infection 2013; 41:129–34. [DOI] [PubMed] [Google Scholar]
- 23. Brooks RG. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch Intern Med 1989; 149:2226–8. [PubMed] [Google Scholar]
- 24. Bross J, Talbot GH, Maislin G, Hurwitz S, Strom BL. Risk factors for nosocomial candidemia: a case-control study in adults without leukemia. Am J Med 1989; 87:614–20. [DOI] [PubMed] [Google Scholar]
- 25. Charoo AB, Ashraf Y, Bhat JI, Qazi I. Systemic Candida infection in preterm babies: experience from a tertiary care hospital of North India. J Clin Neonatology 2019; 8:151–4. [Google Scholar]
- 26. Chen J-Y. Neonatal candidiasis associated with meningitis and endophthalmitis. Pediatr Int 1994; 36:261–5. [DOI] [PubMed] [Google Scholar]
- 27. Donahue SP, Hein E, Sinatra RB. Ocular involvement in children with candidemia. Am J Ophthalmol 2003; 135:886–7. [DOI] [PubMed] [Google Scholar]
- 28. El-Abiary M, Jones B, Williams G, Lockington D. Fundoscopy screening for intraocular candida in patients with positive blood cultures-is it justified? Eye (Lond) 2018; 32:1697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Enzenauer RW, Calderwood S, Levin AV, Elder JE, Morin JD. Screening for fungal endophthalmitis in children at risk. Pediatrics 1992; 90:451–7. [PubMed] [Google Scholar]
- 30. Feman SS, Nichols JC, Chung SM, Theobald TA. Endophthalmitis in patients with disseminated fungal disease. Trans Am Ophthalmol Soc 2002; 100:67–70; discussion 70–1. [PMC free article] [PubMed] [Google Scholar]
- 31. Festekjian A, Neely M. Incidence and predictors of invasive candidiasis associated with candidaemia in children. Mycoses 2011; 54:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fierro JL, Prasad PA, Fisher BT, et al. Ocular manifestations of candidemia in children. Pediatr Infect Dis J 2013; 32:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher RG, Gary Karlowicz M, Lall-Trail J. Very low prevalence of endophthalmitis in very low birthweight infants who survive candidemia. J Perinatol 2005; 25:408–11. [DOI] [PubMed] [Google Scholar]
- 34. Geraymovych E, Conduff JH, Braich PS, Leffler CT, Brar VS. Prevalence and factors predictive of intraocular fungal infection in patients with fungemia at an academic urban tertiary care center. Clin Ophthalmol 2015; 9:1853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gluck S, Headdon WG, Tang D, Bastian IB, Goggin MJ, Deane AM. The incidence of ocular candidiasis and evaluation of routine opthalmic examination in critically ill patients with candidaemia. Anaesth Intensive Care 2015; 43:693–7. [DOI] [PubMed] [Google Scholar]
- 36. Hautala N, Köykkä H, Siiskonen M, Saari J, Kauranen J, Hautala T. Effect of first-line antifungal treatment on ocular complication risk in Candida or yeast blood stream infection. BMJ Open Ophthalmol 2021; 6:e000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hillenbrand M, Mendy A, Patel K, et al. The incidence of ocular complications in candidemic patients and implications for the practice of routine eye exams. Open Forum Infect Dis 2022; 9:ofac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirai Y, Asahata S, Ainoda Y, Goto A, Fujita T, Totsuka K. Nosocomial Candida parapsilosis candidaemia: risk factors, antifungal susceptibility and outcome. J Hosp Infect 2014; 87:54–8. [DOI] [PubMed] [Google Scholar]
- 39. Huynh N, Chang HY, Borboli-Gerogiannis S. Ocular involvement in hospitalized patients with candidemia: analysis at a Boston tertiary care center. Ocul Immunol Inflamm 2012; 20:100–3. [DOI] [PubMed] [Google Scholar]
- 40. İnci A, Ünsel M, Kaya E, Salihoğlu Z. Assessment of ocular findings in patients with candidemia in intensive care unit. Klimik Dergisi 2019; 32:315–7. [Google Scholar]
- 41. Kannangara S, Shindler D, Kunimoto DY, Sell B, DeSimone JA. Candidemia complicated by endophthalmitis: a prospective analysis. Eur J Clin Microbiol Infect Dis 2007; 26:839–41. [DOI] [PubMed] [Google Scholar]
- 42. Karmisholt MK, Hjort U, Knudsen LL, Schønheyder HC. Candidaemia and risk of intraocular infection: a Danish hospital-based cohort study. Scand J Infect Dis 2008; 40:241–6. [DOI] [PubMed] [Google Scholar]
- 43. Kato H, Yoshimura Y, Suido Y, et al. Prevalence of, and risk factors for, hematogenous fungal endophthalmitis in patients with Candida bloodstream infection. Infection 2018; 46:635–40. [DOI] [PubMed] [Google Scholar]
- 44. Kazama I, Furukawa K. [A study for candidemia during the six year period from 1993 to 1999 in St. Luke's International Hospital]. Kansenshogaku Zasshi 2003; 77:158–66. [DOI] [PubMed] [Google Scholar]
- 45. Keighley C, Chen SCA, Marriott D, et al. Candidaemia and a risk predictive model for overall mortality: a prospective multicentre study. BMC Infect Dis 2019; 19:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khalid A, Clough LA, Symons RC, Mahnken JD, Dong L, Eid AJ. Incidence and clinical predictors of ocular candidiasis in patients with Candida fungemia. Interdiscip Perspect Infect Dis 2014; 2014:650235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim JH, Suh JW, Kim JY, et al. Prevalence and risk factors for endogenous fungal endophthalmitis in adult patients with candidemia at a tertiary care hospital in the Republic of Korea over 13 years. J Mycol Med 2021; 31:101102. [DOI] [PubMed] [Google Scholar]
- 48. Klein JJ, Watanakunakorn C. Hospital-acquired fungemia. Its natural course and clinical significance. Am J Med 1979; 67:51–8. [DOI] [PubMed] [Google Scholar]
- 49. Krishna R, Amuh D, Lowder CY, Gordon SM, Adal KA, Hall G. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye (Lond) 2000; 14:30–4. [DOI] [PubMed] [Google Scholar]
- 50. Kutlu M, Sayın-Kutlu S, Alp-Çavuş S, et al. Mortality-associated factors of candidemia: a multi-center prospective cohort in Turkey. Eur J Clin Microbiol Infect Dis 2022; 41:597–607. [DOI] [PubMed] [Google Scholar]
- 51. McDonnell PJ, McDonnell JM, Brown RH, Green WR. Ocular involvement in patients with fungal infections. Ophthalmology 1985; 92:706–9. [DOI] [PubMed] [Google Scholar]
- 52. Mehta S, Jiandani P, Desai M. Ocular lesions in disseminated candidiasis. J Assoc Physicians India 2007; 55:483–5. [PubMed] [Google Scholar]
- 53. Menezes AV, Sigesmund DA, Demajo WA, Devenyi RG. Mortality of hospitalized patients with Candida endophthalmitis. Arch Intern Med 1994; 154:2093–7. [PubMed] [Google Scholar]
- 54. Nagao M, Saito T, Doi S, et al. Clinical characteristics and risk factors of ocular candidiasis. Diagn Microbiol Infect Dis 2012; 73:149–52. [DOI] [PubMed] [Google Scholar]
- 55. Nagasako Y, Inagaki K, Serizawa S, et al. Risk factors associated with retinal lesions resulting from widespread systemic infection. Ophthalmol Retina 2017; 1:333–8. [DOI] [PubMed] [Google Scholar]
- 56. Nolla-Salas J, Sitges-Serra A, León C, de la Torre MV, Sancho H. Candida endophthalmitis in non-neutropenic critically ill patients. Eur J Clin Microbiol Infect Dis 1996; 15:503–6. [DOI] [PubMed] [Google Scholar]
- 57. Noyola DE, Fernandez M, Moylett EH, Baker CJ. Ophthalmologic, visceral, and cardiac involvement in neonates with candidemia. Clin Infect Dis 2001; 32:1018–23. [DOI] [PubMed] [Google Scholar]
- 58. Oude Lashof AM, Rothova A, Sobel JD, et al. Ocular manifestations of candidemia. Clin Infect Dis 2011; 53:262–8. [DOI] [PubMed] [Google Scholar]
- 59. Ourives AP, Gonçalves SS, Siqueira RA, et al. High rate of Candida deep-seated infection in patients under chronic hemodialysis with extended central venous catheter use. Rev Iberoam Micol 2016; 33:100–3. [DOI] [PubMed] [Google Scholar]
- 60. Parke DW 2nd, Jones DB, Gentry LO. Endogenous endophthalmitis among patients with candidemia. Ophthalmology 1982; 89:789–96. [DOI] [PubMed] [Google Scholar]
- 61. Paulus YM, Cheng S, Karth PA, Leng T. Prospective trial of endogenous fungal endophthalmitis and chorioretinitis rates, clinical course, and outcomes in patients with fungemia. Retina 2016; 36:1357–63. [DOI] [PubMed] [Google Scholar]
- 62. Popovich K, Malani PN, Kauffman CA, Cinti SK. Compliance with infectious diseases society of America guidelines for ophthalmologic evaluation of patients with candidemia. Infect Dis Clin Pract 2007; 15:254–6. [Google Scholar]
- 63. Price KW, Tsui E, Barbazetto I, Park L. Ocular involvement in patients with fungemia in an urban tertiary care center. Ocul Immunol Inflamm 2019; 27:251–6. [DOI] [PubMed] [Google Scholar]
- 64. Rex JH, Bennett JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med 1994; 331:1325–30. [DOI] [PubMed] [Google Scholar]
- 65. Rodriguez D, Almirante B, Park BJ, et al. Candidemia in neonatal intensive care units: Barcelona, Spain. Pediatr Infect Dis J 2006; 25:224–9. [DOI] [PubMed] [Google Scholar]
- 66. Rodríguez-Adrián LJ, King RT, Tamayo-Derat LG, Miller JW, Garcia CA, Rex JH. Retinal lesions as clues to disseminated bacterial and candidal infections: frequency, natural history, and etiology. Medicine (Baltimore) 2003; 82:187–202. [DOI] [PubMed] [Google Scholar]
- 67. Rose HD. Venous catheter-associated candidemia. Am J Med Sci 1978; 275:265–9. [DOI] [PubMed] [Google Scholar]
- 68. Sakai D, Matsumiya W, Kusuhara S, Nakamura M. Factors associated with the development of ocular candidiasis and ocular prognosis with echinocandin therapy for candidemia. J Ophthalmic Inflamm Infect 2021; 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sakamoto T, Gotoh K, Hashimoto K, Tanamachi C, Watanabe H. Risk factors and clinical characteristics of patients with ocular candidiasis. J Fungi (Basel) 2022; 8:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scherer WJ, Lee K. Implications of early systemic therapy on the incidence of endogenous fungal endophthalmitis. Ophthalmology 1997; 104:1593–8. [DOI] [PubMed] [Google Scholar]
- 71. Seidelman J, Fleece M, Bloom A, et al. Endogenous Candida endophthalmitis: who is really at risk? J Infect 2021; 82:276–81. [DOI] [PubMed] [Google Scholar]
- 72. Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol 2008; 92:466–8. [DOI] [PubMed] [Google Scholar]
- 73. Shin SU, Yu YH, Kim SS, et al. Clinical characteristics and risk factors for complications of candidaemia in adults: focus on endophthalmitis, endocarditis, and osteoarticular infections. Int J Infect Dis 2020; 93:126–32. [DOI] [PubMed] [Google Scholar]
- 74. Siddiqui MZ, Gebhard GM, Ahmad KT, Sallam AB, Rosenbaum ER, Uwaydat SH. Incidence of chorioretinitis and endophthalmitis in hospitalized patients with fungemia. Eye (Lond) 2022; 36:206–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Silvester EJ, Watanabe MMY, Pittet LF, et al. Candidemia in children: a 16-year longitudinal epidemiologic study. Pediatr Infect Dis J 2021; 40:537–43. [DOI] [PubMed] [Google Scholar]
- 76. Son HJ, Kim MJ, Lee S, et al. Risk factors and outcomes of patients with ocular involvement of candidemia. PLoS One 2019; 14:e0222356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ueda T, Takesue Y, Tokimatsu I, et al. The incidence of endophthalmitis or macular involvement and the necessity of a routine ophthalmic examination in patients with candidemia. PLoS One 2019; 14:e0216956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vena A, Muñoz P, Padilla B, et al. Is routine ophthalmoscopy really necessary in candidemic patients? PLoS One 2017; 12:e0183485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yilmaz G, Çiftçioglu A, Gündüz M, Özen M, Saricaoglu EM, Akan H. Evaluation of candida species and risk factors in haematologic cancer patients with candidemia. Klimik Dergisi 2015; 28:117–21. [Google Scholar]
- 80. Zakhem AE, Istambouli R, Alkozah M, et al. Predominance of candida glabrata among non-albicans candida species in a 16-year study of candidemia at a tertiary care center in Lebanon. Pathogens 2021; 10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. Risk factors for disseminated candidiasis in children with candidemia. Pediatr Infect Dis J 2004; 23:635–41. [DOI] [PubMed] [Google Scholar]
- 82. Govindaraju VK, Chao JT, Duvall ER, et al. Incidence of endogenous fungal endophthalmitis in screening dilated exams in patients with elevated beta-D-glucan levels versus positive fungal blood cultures. Clin Ophthalmol 2022; 16:2743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leepattarakit T, Tulyaprawat O, Vongseenin C, et al. EQUAL Candida score, an effective tool for predicting the outcomes of Candida tropicalis candidaemia: a retrospective cohort study. Mycoses 2022; 65:473–80. [DOI] [PubMed] [Google Scholar]
- 84. Sychev D, Maya ID, Allon M. Clinical outcomes of dialysis catheter-related candidemia in hemodialysis patients. Clin J Am Soc Nephrol 2009; 4:1102–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vaziri K, Pershing S, Albini TA, Moshfeghi DM, Moshfeghi AA. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol 2015; 159:498–504. [DOI] [PubMed] [Google Scholar]
- 86. Smeekens SP, van de Veerdonk FL, Kullberg BJ, Netea MG. Genetic susceptibility to Candida infections. EMBO Mol Med 2013; 5:805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jones N, Garcez T, Newman W, Denning D. Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep 2016; 2016:bcr2015214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vaezi A, Fakhim H, Abtahian Z, et al. Frequency and geographic distribution of CARD9 mutations in patients with severe fungal infections. Front Microbiol 2018; 9:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.