Abstract
Aims
Microsomal prostaglandin E synthase-1 (mPGES-1)/prostaglandin E2 (PGE2) induces angiogenesis through the prostaglandin E2 receptor (EP1–4). Among immune cells, regulatory T cells (Tregs), which inhibit immune responses, have been implicated in angiogenesis, and PGE2 is known to modulate the function and differentiation of Tregs. We hypothesized that mPGES-1/PGE2-EP signalling could contribute to recovery from ischaemic conditions by promoting the accumulation of Tregs.
Methods and results
Wild-type (WT), mPGES-1-deficient (mPges-1−/−), and EP4 receptor-deficient (Ep4−/−) male mice, 6–8 weeks old, were used. Hindlimb ischaemia was induced by femoral artery ligation. Recovery from ischaemia was suppressed in mPges-1−/− mice and compared with WT mice. The number of accumulated forkhead box protein P3 (FoxP3)+ cells in ischaemic muscle tissue was decreased in mPges-1−/− mice compared with that in WT mice. Expression levels of transforming growth factor-β (TGF-β) and stromal cell derived factor-1 (SDF-1) in ischaemic tissue were also suppressed in mPges-1−/− mice. The number of accumulated FoxP3+ cells and blood flow recovery were suppressed when Tregs were depleted by injecting antibody against folate receptor 4 in WT mice but not in mPges-1−/− mice. Recovery from ischaemia was significantly suppressed in Ep4−/− mice compared with that in WT mice. Furthermore, mRNA levels of Foxp3 and Tgf-β were suppressed in Ep4−/− mice. Moreover, the number of accumulated FoxP3+ cells in ischaemic tissue was diminished in Ep4−/− mice compared with that in Ep4+/+ mice.
Conclusion
These findings suggested that mPGES-1/PGE2 induced neovascularization from ischaemia via EP4 by promoting the accumulation of Tregs. Highly selective EP4 agonists could be useful for the treatment of peripheral artery disease.
Keywords: mPGES-1, EP4, Tregs, Angiogenesis, TGF-β
Graphical Abstract
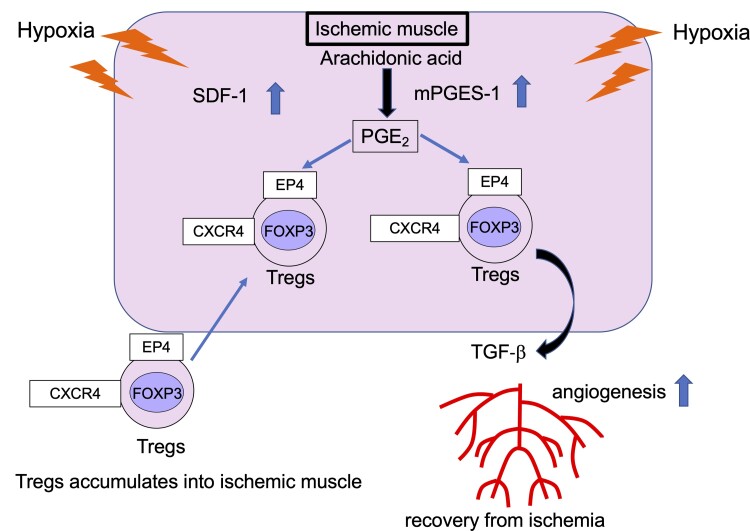
Graphical Abstract.
Time of primary review: 43 daysSee the editorial comment for this article ‘Prostaglandins bet on regulatory T cells to promote therapeutic revascularization’, by J.-S. Silvestre, https://doi.org/10.1093/cvr/cvad051.
1. Introduction
The phenomenon of neovascularization, which includes angiogenesis and vascularization, occurs during tissue repair and remodelling, including that in ischaemic vessel diseases.1,2 These processes are complex and controlled by growth factors and chemokines.3 Many types of immune cells have critical roles in these neovascularization systems.
Lymphocytes are known to be involved in this angiogenic process. For example, the CD4+ subset of lymphocytes, which secretes various cytokines, has been implicated in angiogenesis.4 For instance, the ablation of CD4+ cells impairs blood flow recovery in hindlimb ischaemia.5 A population of CD4+ T cells expresses CD25 and/or folate receptor 4 (FR4), which are the most characterized subsets of regulatory T cells (Tregs). FoxP3 is a specific transcription factor for Tregs and is also used as a specific marker for Tregs.6 Naturally occurring Tregs that develop in the thymus and inducible Tregs generated in the periphery are both involved in immune homeostasis and tolerance and regulate the progression of autoimmune disease.7 Recent reports have indicated that Tregs influence recovery from ischaemia, tumour growth, and metastasis formation, suggesting a potential role for Tregs in ischaemic vascular disease.8,9
Peripheral arterial disease (PAD) affects over 200 million people worldwide and is a common cause of morbidity and mortality due to cardiovascular disease. Although surgical treatment remains the most effective treatment for limb ischaemia, many patients with advanced disease are not suitable for surgical or endovascular management. Numerous treatments have been used for patients at different disease stages. Some patients with chronic kidney disease and diabetes develop severe critical limb ischaemia and have no other option for treatment other than amputation. Among such patients, prostanoids have been proposed as a therapeutic alternative, with the aim of increasing blood supply to the limb with occluded arteries through their vasodilatory, antithrombotic, and anti-inflammatory effects.10
Prostaglandins (PGs) are generated from the conversion of arachidonic acid to PGH2 by the cyclooxygenases (COX)-1 and COX-2, followed by further processing by PG synthases, the terminal enzymes in this process.11 PGE2 is a pro-inflammatory and immunomodulatory lipid mediator formed from PGH2 by microsomal PG E synthase-1 (mPGES-1).12 PGE2 binds four subtype receptors, EP1–4. Previously, we reported that mPGES-1 induces gastric ulcer healing and tumour metastasis by stimulating angiogenesis.13,14 PGE2 has diverse effects on the regulation of CD4+ T-cell activity, including modulation of T-cell proliferation.15 In the tumour microenvironment, elevated COX-2 and PGE2 levels suppress anti-tumour immunity.16 In particular, populations of CD4+CD25+ Tregs are increased by local tumour growth at the tumour site and may be relevant to the progression of systemic disease in the peripheral blood or lymphoid organs.17
Recently, we revealed that COX-2-derived PGE2 induces granulation formation by promoting accumulation of FoxP3+ Tregs.18 Several studies have identified roles for Tregs in the environments in the context of ischaemia in the brain, kidney, and lung.19–21 Others reported that insufficient recruitment of Tregs impaired ventricular remodelling.22
A possible involvement of Tregs has been indicated in recovery from ischaemic conditions. A prior report demonstrated that Tregs induce recovery from hindlimb ischaemia, although the detailed mechanism of action has still been left to be fully addressed.8 Baratelli et al.23 demonstrated that PGE2 could modulate expression of Foxp3 and function in Tregs. These results prompted us to evaluate the precise mechanism of recovery from ischaemia with respect to the mPGES-1/PGE2 axis, especially by focusing on Tregs. In the present study, we demonstrated that the mPGES-1/PGE2 axis enhanced recovery from hindlimb ischaemia and that this effect was mediated by the accumulation of Tregs in ischaemic muscle tissue. We also identified EP receptor subtype that involved the most in ischaemia recovery by analysing the mice deficient for each EP.
2. Methods
Detailed methods are available in Supplementary material online.
2.1. Animals and surgery
All experiments were approved by the Kitasato University Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH, 8th ed., 2011).
Male 8-week-old C57BL/6 mice were obtained from CLEA Japan (Tokyo, Japan). mPges-1−/− mice were developed in a C57BL/6 hybrid background.24Ep4−/− mice were maintained by crossbreeding described previously.25 Mice deficient for recombination activating gene (Rag)-2 in the C57BL/6J background (Rag2−/−) were kindly provided by Dr Yoichi Shinkai (Riken, Advanced Science Institute, Saitama, Japan).
Hindlimb ischaemia was induced as described previously.26 Mice were anaesthetized by intraperitoneal injection of mixed anaesthetic agents (medetomidine 0.75 mg/kg, midazolam 4 mg/kg, and butorphanol 5 mg/kg), and anaesthesia was monitored by confirming absence of the pedal withdrawal reflex throughout the experiments.27 At the end of experiments, animals were euthanized by exsanguination under anaesthesia with mixed anaesthetic agents, followed by cervical dislocation. Aspirin (300 mg/kg per day; Merck, Whitehouse Station, NJ, USA) and celecoxib (100 mg/kg/day: Pfizer, New York, NY, USA) were administrated orally every day.
2.2. Laser Doppler imaging
Blood flow to the right and left hindlimbs was assessed by scanning the lower abdomen and limbs of the mice with laser Doppler imaging (Lisca PIM II, Perimed, Sweden) as described previously.26
2.3. Immunohistochemical analysis
Immunohistochemical analysis was performed as described previously.26
2.4. Flow cytometric analysis
Blood was collected via the tail vein before (Day 0) and 7 days after surgery. Flow cytometric analyses were performed as described previously.28
2.5. Preparation of spleen cells
Single cell suspensions of spleens from wild-type (WT) and Rag2−/−29 were prepared by teasing spleen cells with a pair of frosted slide glasses (Matsunami Glass, Osaka, Japan), and the red blood cells were lysed by suspending the cell pellet in ammonium chloride buffer after centrifugation. The cells were suspended in phosphate-buffered saline (PBS) at a density of 1.0 × 108 cells/mL and 200 µL of the resulting suspension was injected into the tail vein.
2.6. Sorting of thymocytes and adoptive cell transfer of sorted thymocytes
Single-cell suspensions of thymocytes were prepared by grinding thymic in frosted slide glasses. In sorting CD4+ T cells from thymus, apoptotic cells were gated out by 7-AAD (BioLegend, San Diego, CA, USA). Thymocytes were depleted of CD8+ cells to enrich CD4+ CD25+ Tregs, or CD4+ CD25− conventional mature T cells, with PE-labelled anti-CD8 Ab (BioLegend, San Diego, CA, USA), and anti-PE-Ab-coated magnetic beads using the Mojo system (BioLegend, San Diego, CA, USA). Cells were then stained with allophycocyanin-labelled anti-CD4 Ab (BioLegend, San Diego, CA, USA) with or without FITC (fluorescein isothiocyanate)-labelled anti-CD25 Ab (7D4, BD Biosciences, San Jose, CA, USA) to sort Treg-depleted or whole CD4 single positive mature thymocytes, respectively, by FACSAria (BD Bioscience, San Jose, CA, USA). For adoptive transfer experiments, cells were suspended in PBS (5.0 × 106 cells/mL) and 1.0 × 106 cells/mouse were injected intravenously into tail veins.
2.7. Determination of Foxp3, Tgf-β, Cxcr4, Sdf-1, Ep1–4 mRNA levels in ischaemic tissues by real-time reverse-transcription polymerase chain reaction
Transcripts encoding Foxp3, Tgf-β, Cxcr4, Sdf-1, Ep1–4, and Gapdh were quantitated by real-time reverse-transcription–polymerase chain reaction (RT–PCR) analysis as described previously.26 The DNA sequences of the mouse primers used for real-time RT–PCR are described in Supplementary material online, Table S1).
2.8. Quantification of mRNA in Tregs
CD4+/CD25+/CD8− thymocytes were sorted from WT or mPges-1−/− mice, and 5–10 × 104 cells/well were cultured in a well precoated with anti-CD3 antibody (10 µg/mL) or in a well without any treatment, in the presence or absence of an EP4 agonist (ONO-AEI-329, Ono Pharmaceutical Co., Ltd, Osaka Japan). Quantitative real-time RT–PCR was performed as described above. The DNA sequences of the mouse primers used for real-time RT–PCR are summarized in Supplementary material online, Table S1.
2.9. Measurement of transforming growth factor-β protein level
CD4+CD25+ thymocytes from WT or mPges-1−/− mice and 5–10 × 104 cells/well were cultured in wells precoated with anti-CD3 antibody (145-2C11, BioX Cell, Lebanon, NH, USA, 10 µg/mL) or in a well without any treatment for 16–18 h.30 The culture supernatants were harvested and were stored in −20°C until use. The amounts of transforming growth factor (TGF)-β in the samples were measured by murine-specific enzyme-linked immunosorbent assay (DB100C, R&D Systems, Minneapolis, MN, USA). These experiments were performed in duplicate.
2.10. Statistical analyses
Data are expressed as means ± standard deviation. All statistical analyses were performed using GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA, USA). Statistical comparison between two groups was conducted using unpaired two-tailed t-test. Comparisons of more than two groups were conducted using a one-way analysis of variance (ANOVA). Comparisons of time point effects were analysed by repeated-measures ANOVA. If applicable with a repeated measure approach, a Bonferroni post hoc test was performed. A P-value of <0.05 was considered statistically significant.
3. Results
3.1. Reduced recovery from ischaemia in mPges-1−/− mice
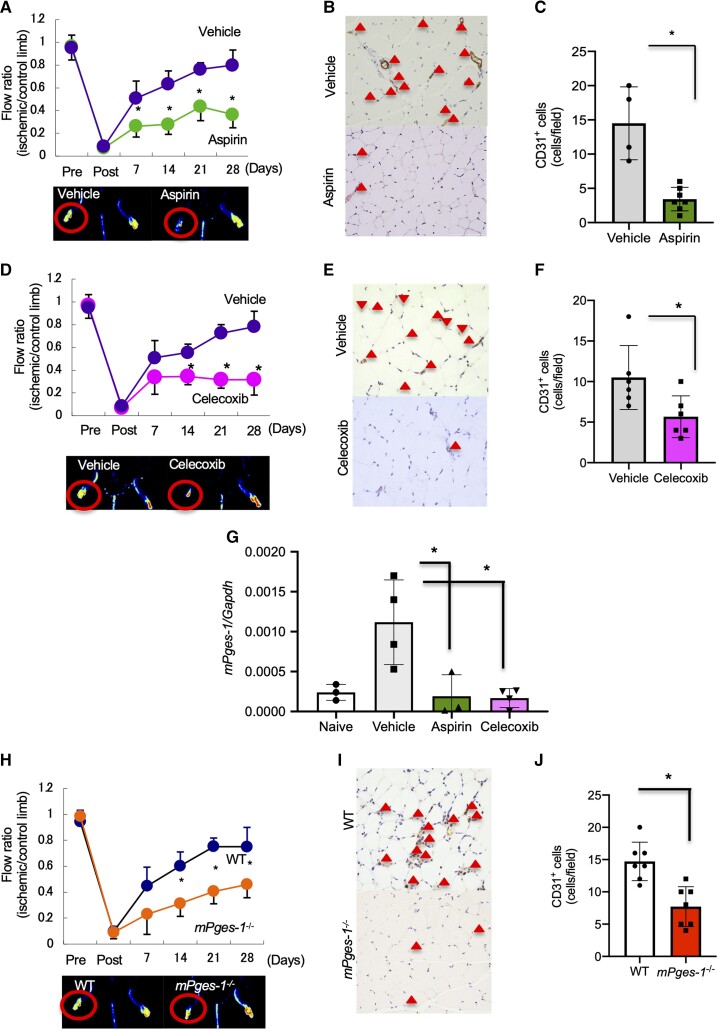
To determine if COX-2-derived PGs enhanced angiogenesis, we first measured recovery from hindlimb ischaemia in mice receiving daily oral administration of aspirin, which inhibits both COX-1 and COX-2. Blood flow recovery on Day 28 after ligation was restored in mice treated with vehicle and was significantly suppressed in mice treated with aspirin (vehicle, 0.80 ± 0.13%; aspirin, 0.37 ± 0.12%; P < 0.05; Figure 1A). Further, the number of CD31+ cells in ischaemic muscle tissue was significantly reduced in aspirin-treated mice compared with that in vehicle-treated mice (vehicle, 14.50 ± 5.32; aspirin, 3.43 ± 1.72; P < 0.05; Figure 1B and C). Next, we analysed the effect of COX-2 in recovery from hindlimb ischaemia. Treatment with celecoxib, a selective COX-2 inhibitor, significantly suppressed recovery from ischaemia (vehicle, 0.78 ± 0.133; celecoxib, 0.32 ± 0.094; P < 0.05; Figure 1D) and decreased the number of CD31+ cells compared with vehicle-treated mice (vehicle, 10.5 ± 0.13; celecoxib, 5.14 ± 2.73; P < 0.05; Figure 1E and F).
Figure 1.
Effects of aspirin, COX-2 inhibitor, and mPGES-1 on recovery from hindlimb ischaemia. (A–G) From the day of surgery, C57Bl/6 mice received oral administration of aspirin, a selective COX-2 inhibitor, or PBS daily. (A) Aspirin impaired blood flow recovery from ischaemia. Data are expressed as means ± standard deviation (SD) of the n = 4–7 mice/group. *P < 0.05 vs. vehicle, repeated-measures ANOVA (upper panel). Representative Doppler images 28 days after ligation (lower panel). (B) CD31 staining of ischaemic limbs 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (C) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. Data are expressed as means ± SD of the n = 4–7 mice/group. *P < 0.05 vs. PBS, Student’s t-test. (D) The selective COX-2 inhibitor, celecoxib, impaired blood flow recovery from ischaemia. Data are means ± SD of the n = 5–6 mice/group. *P < 0.05 vs. vehicle, repeated-measures ANOVA (upper panel). Representative Doppler images 28 days after ligation (lower panel). (E) CD31 staining of the ischaemic limb 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (F) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. Data are means ± SD of the n = 5–6 mice/group. *P < 0.05 vs. PBS, Student’s t-test. (G) mPges-1 mRNA expression in ischaemic muscle tissue. Aspirin- and celecoxib-treated mice exhibited decreased mRNA expression of mPges-1 compared with PBS. Data are expressed means ± SD of the n = 4 mice/group. *P < 0.05, one-way ANOVA. (H) Ischaemia was induced in the left hindlimb of C57Bl/6 mice (wild-type, WT) and mPges-1−/− mice. mPges-1−/− mice ablation impaired blood flow recovery from ischaemia. Data are means ± SD of the n = 5 mice/group. *P < 0.05 vs. WT, repeated-measures ANOVA (upper panel). Representative Doppler images 28 days after ligation (lower panel). (I) Number of CD31+ cells in ischaemic limbs 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (J) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. Data are means ± SD of the n = 7 mice/group. *P < 0.05 vs. PBS, Student’s t-test.
The induction of mPGES-1 expression has been observed in various systems in which COX-2-derived PGE2 is thought to play a critical role, such as in inflammation, tissue repair, and cancer metastasis.13,14,18 After femoral artery ligation, expression of mRNA encoding mPGES-1 in ischaemic muscle tissue was enhanced on Day 3 (see Supplementary material online, Figure S1). That enhancement of mPGES-1 in ischaemic muscle was significantly suppressed by treatment with aspirin and celecoxib (Figure 1G).
To examine the endogenous effect of mPGES-1 on recovery from hindlimb ischaemia, we used mPges-1−/− mice and WT mice. Compared with WT mice, blood flow recovery was significantly delayed on Day 28 in mPges-1−/− mice (WT, 0.75 ± 0.15; mPges-1−/−, 0.46 ± 0.10; P < 0.0001; Figure 1H). The number of CD31+ cells (WT, 14.7 ± 2.98; mPges-1−/−, 7.71 ± 3.09; P < 0.05; Figure 1I and J) and the number of αSMA+ cells (see Supplementary material online, Figure S2) in ischaemic muscle tissue were significantly decreased in mPges-1−/− mice. In addition, murine mPGES-1-overexpressing fibroblasts exhibited enhanced ischaemic recovery compared with empty vector-expressing fibroblasts in WT and mPges-1−/− mice (see Supplementary material online, Figure S3A and B). Despite under celecoxib treatment, murine mPGES-1-overexpressing fibroblast enhanced ischaemic recovery (see Supplementary material online, Figure S3C). Furthermore, under hypoxic conditions, expression of COX-2, mPGES-1, and PGE2 in human umbilical vein endothelial cells (HUVECs) was enhanced compared with normoxic conditions and those were suppressed by specific COX-2 inhibitor treatment, NS-398 (see Supplementary material online, Figure S4A–C). These results suggested that the COX-2/mPGES-1/PGE2 axis plays a critical role in ischaemia-induced angiogenesis.
3.2. Decreased numbers of FoxP3+ Tregs in mPges-1−/− mice
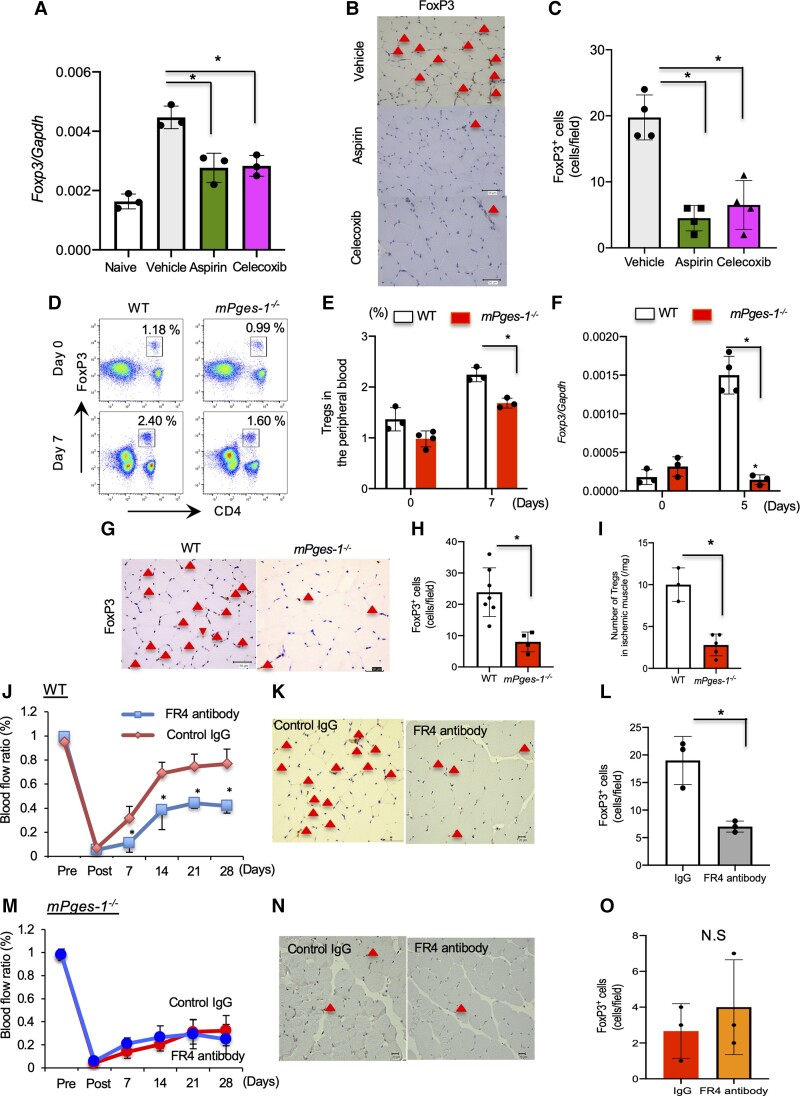
T lymphocytes mediate post-ischaemic neovascularization.5,8 Hence, nude mice or CD4+ T lymphocyte-deficient mice exhibit significant reductions in post-ischaemic vessel growth.5 Expression of Foxp3, a specific transcription factor for Tregs, was enhanced in ischaemic muscle tissue compared with non-ischaemic muscle tissue in WT (see Supplementary material online, Figure S5A and B). As anticipated from the above results, expression of Foxp3 in ischaemic muscle tissue was decreased either in aspirin- or celecoxib-treated mice compared with vehicle-treated mice (Figure 2A). Immunohistochemical analysis of Foxp3 revealed that the number of accumulated FoxP3+ cells in the ischaemic muscle tissue was significantly decreased in aspirin- or celecoxib-treated mice relative to vehicle-treated mice (vehicle, 20.67 ± 3.51; aspirin, 4.02 ± 2.11; celecoxib, 6.51 ± 3.7; P < 0.05; Figure 2B and C). These results indicated that the COX-2/mPGES-1/PGE2 axis induced accumulation of FoxP3+ Tregs after femoral artery ligation.
Figure 2.
Effect of the COX-2/mPGES-1 axis on Treg accumulation in ischaemic muscle tissue. (A) Foxp3 mRNA expression in ischaemic muscle tissue. Aspirin- and celecoxib-treated mice decreased mRNA expression of Foxp3 compared with PBS. Data are means ± SD of the n = 5 mice/group. *P < 0.05 vs. vehicle, one-way ANOVA. (B) Foxp3 staining of ischaemic limbs in aspirin- and celecoxib-treated mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (C) Number of FoxP3+ cells in ischaemic muscle tissue 28 days after ligation. Data are means ± SD of the n = 5 mice/group. *P < 0.05 vs. PBS, one-way ANOVA. (D) Mobilized CD4+CD25+ Tregs isolated from the peripheral blood of WT and mPges-1−/− mice 7 days after induction of hindlimb ischaemia were analysed by two-colour flow cytometry. Mobilization of CD4+CD25+ Tregs was impaired in mPges-1−/− mice compared with WT mice (upper panel). (E) Percentages of CD4+CD25+ Tregs in WT mice and CD4+CD25+ Tregs (lower panel). Data are means ± SD of the n = 5 mice/group. *P < 0.05 vs. mPges-1−/−, one-way ANOVA. (F) Foxp3 mRNA expression in ischaemic muscle tissue. mPges-1−/− mice decreased mRNA expression of Foxp3 relative to WT mice. Data are means ± SD of the n = 5 mice/group. *P < 0.05, one-way ANOVA. (G) FoxP3 staining of the ischaemic limb in WT and mPges-1−/− mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (H) Number of FoxP3+ cells in ischaemic muscle tissue 7 days after ligation. P < 0.05 vs. WT mice, Student’s t-test. (I) Number of accumulated CD4+CD25+Tregs in ischaemic muscle tissue 7 days after ligation. Data are means ± SD of the n = 3–5 mice/group. *P < 0.05 vs. WT mice, Student’s t-test. (J) WT FR4 antibody-treated mice impaired blood flow recovery from ischaemia *P < 0.05, repeated-measures ANOVA (upper panel). (K) FoxP3 staining of ischaemic limb in FR4 antibody-treated WT mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (L) Number of FoxP3+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. IgG, Student’s t-test. (M) In mPges-1−/− mice, FR4 antibody treatment did not affect blood flow recovery from ischaemia. *P < 0.05 vs. IgG, repeated-measures ANOVA (upper panel). (N) FoxP3 staining of ischaemic limb in FR4 antibody-treated mPges-1−/− mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (O) Number of FoxP3+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. IgG, Student’s t-test.
In mPges-1−/− mice, the percent of CD4+ CD25+ Tregs in the peripheral blood on Day 7 was significantly reduced compared with WT mice (Day 0: WT, 1.36 ± 0.29%; mPges-1−/−, 0.98 ± 015%; P < 0.05; Day 7: WT, 2.24 ± 0.14%; mPges-1−/−, 1.58 ± 0.09%; P < 0.05; Figure 2D and E). On Day 5, expression of Foxp3 in ischaemic muscle tissue was also significantly suppressed in mPges-1−/− mice compared with WT mice (P < 0.05; Figure 2F). Furthermore, the number of FoxP3+ Tregs accumulated in ischaemic muscle tissue was decreased in mPges-1−/− mice on Day 7 relative to WT (WT, 23.86 ± 7.78; mPges-1−/−, 8.00 ± 3.16; P < 0.05; Figure 2G and H). FACS analysis revealed that the number of accumulated CD4 + CD25 + Tregs in ischaemic muscle tissue was significantly suppressed in mPges-1−/− mice compared with WT mice (P < 0.05; Figure 2I).
3.3. Depletion of Tregs suppressed recovery from ischaemia
We then examined the effect of Tregs on ischaemic recovery by depleting Tregs in WT mice using anti-CD25 antibody or anti-FR4 antibody. Treatment of WT mice with CD25 antibody significantly suppressed recovery from ischaemia on Day 28 compared with mice treated with control immunoglobulin G (IgG) (see Supplementary material online, Figure S6A). The number of accumulated FoxP3+ Tregs was decreased in CD25 antibody-treated mice (see Supplementary material online, Figure S6B and C) on Day 7. By contrast, in mPges-1−/− mice, treatment with CD25 antibody did not significantly affect recovery from ischaemia (see Supplementary material online, Figure S6D) or number of accumulated FoxP3+ Tregs (see Supplementary material online, Figure S6E and F). FR4 is another Tregs surface marker, expressed differently from CD25 which is found on activated effector T cells as well as on Tregs. WT mice treated with anti-FR4 antibody demonstrated significantly delayed ischaemic recovery compared with IgG-treated mice (IgG, 0.77 ± 0.12; FR4 antibody, 0.42 ± 0.06; P < 0.05; Figure 2I) on Day 28. The number of accumulated FoxP3+ Tregs in ischaemic muscle tissue was diminished in FR4 antibody-treated mice compared with IgG-treated mice (IgG, 19.11 ± 4.36 cells; FR4 antibody, 7.06 ± 1.12 cells; P < 0.01; Figure 2J and K) on Day 7. Contrastingly, in mPges-1−/− mice, there were no significant differences in ischaemic blood flow recovery (IgG, 0.25 ± 0.13; FR4 antibody, 0.32 ± 0.13; P = 0.487; Figure 2L) or number of FoxP3+ Tregs (IgG, 2.67 ± 1.53 cells; FR4 antibody, 4.04 ± 2.64 cells; P = 0.491; Figure 2M and N) on Day 7. These results suggested that accumulation of Tregs in ischaemic muscle tissue is important for recovery from ischaemia and that that was dependent on the mPGES-1/PGE2 axis.
3.4. The mPGES-1/PGE2 axis in Tregs enhanced recovery from ischaemia
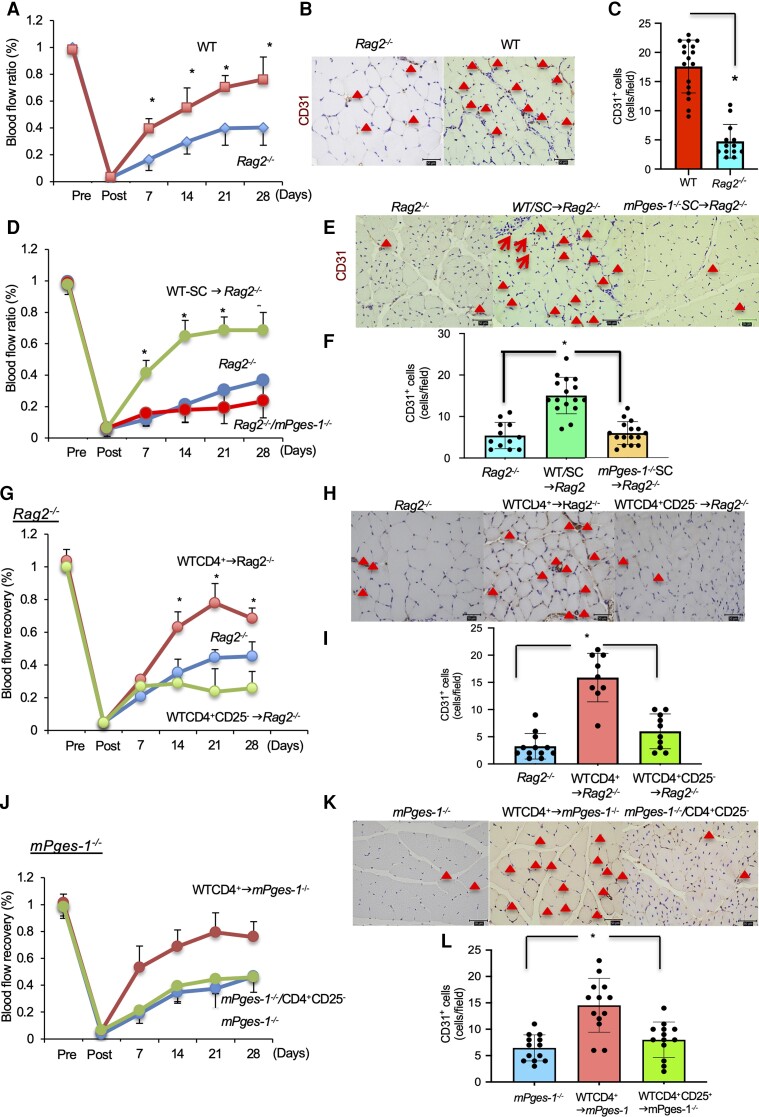
To determine whether mobilization of lymphocytes in ischaemic muscle tissue has an impact on recovery from ischaemia, we used Rag2−/− mice in an ischaemic hindlimb model. Rag2−/− mice lack T and B lymphocytes which require RAG-mediated rearrangement of antigen receptor genes for their development.29 As shown in Figure 3A, the recovery of blood flow after hindlimb ischaemia in Rag2−/− mice was significantly impaired compared with that in WT mice (WT, 0.76 ± 0.17; Rag2−/−, 0.40 ± 0.13; P < 0.05; Figure 3A), and the number of CD31+ cells at ischaemic sites was also reduced (WT, 17.58 ± 4.52 cells; Rag2−/−, 4.76 ± 2.89 cells; P < 0.05; Figure 3B and C) on Day 28. These results indicated that lymphocytes are critically involved in recovery from ischaemia.
Figure 3.
Transplantation of Tregs induced recovery from ischaemia via mPGES-1. (A) Transplantation of WT-derived CD4+ cells into Rag2−/− recipient mice enhanced recovery from ischaemia. (B) CD31 staining of the ischaemic limb 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (C) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. PBS, Student’s t-test. (D) Rag2−/− recipient mice transplanted with mPges-1−/−-derived CD4+ cells delayed blood flow recovery. Data are means ± SD of the n = 4 mice/group. *P < 0.05 vs. WT-SC→Rag2−/−, repeated-measures ANOVA (E) CD31 staining of the ischaemic limb 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (F) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. Rag2−/−/WT, Student’s t-test. (G) Transplantation of Rag2−/− recipient mice with WT-derived CD4+ but not CD4+CD25− T cells enhanced recovery from ischaemia. (H) CD31 staining of the ischaemic limb 28 days after ligation. Red triangles indicate CD31+ cells. Scale bar, 50 μm. (I) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. Rag2−/−/CD4+ all cells, Student’s t-test. (J) Transplantation of mPges-1−/− recipient mice with WT-derived CD4+ but not CD4+CD25− T cells enhanced recovery from ischaemia. (K) CD31 staining of the ischaemic limb 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (L) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. mPges-1−/−/CD4+ all cells, Student’s t-test.
We postulated that the mPGES-1/PGE2 axis might induce recovery from ischaemia; therefore, blood flow recovery from ischaemia was compared among WT, Rag2−/− mice, and mPges-1−/− mice. Adoptive transferring of mPges-1−/−-spleen cells into Rag2−/− mice (mPges-1−/−-SC→Rag2−/− mice) were not conducive to recovery from ischaemia. As expected, blood flow recovery in mPges-1−/−-SC→Rag2−/− mice was attenuated compared with that in WT-SC→ Rag2−/− mice (mPges-1−/−-SC→Rag2−/−, 0.19 ± 0.10; WT-SC→Rag2−/−, 0.68 ± 0.08; P < 0.05, Figure 3D), and was similar to that of Rag2−/− mice (0.32 ± 0.10) on Day 28. In addition, the number of CD31+ cells in the muscle tissue was increased in WT-spleen cells→Rag2−/− (Rag2−/−, 5.42 ± 3.17 cells; WT-SC→Rag2−/− cells, 15.11 ± 4.40; mPges-1−/−-SC→Rag2−/−, 6.02 ± 2.75 cells; P < 0.05; Figure 3E and F).
To specify the cell type necessary for recovery from ischaemia, contribution of Tregs as the source of mPGES-1 was investigated by using Rag2−/− transfer-setting. Because anti-CD25 antibody bound on Tregs may inhibit IL-2 signal, which is critical for Tregs survival, and may also induce ADCC (antibody-dependent cell cytolysis) by NK cells, we compared the effect of whole CD4+ T cells (including Tregs) and CD4+ T cells depleted of CD25+ Treg cells. When Rag2−/− mice were transplanted with whole CD4+ cells containing Tregs, from WT mice (WT CD4+→Rag2−/−), recovery from ischaemia was significantly enhanced compared with Rag2−/− mice, while infusion of Treg-depleted CD4 + CD25− T cells failed to promote recovery from ischaemia (WT CD4+→Rag2−/−, 0.68 ± 0.06; WT CD4 + CD25−→Rag2−/−, 0.26 ± 0.140; P < 0.05, Figure 3G and H). Furthermore, the number of CD31+ cells were increased in whole CD4+-received Rag2−/− mice, but not in mice transplanted with Treg-depleted CD4+ cells (WT CD4+→Rag2−/−, 15.6 ± 4.40 cells; CD4 + CD25−, 7.40 ± 3.21 cells; P < 0.05; Figure 3I) on Day 7. These results again suggested the importance of Tregs in recovery from ischaemic condition.
We also estimated whether accumulation of Tregs in ischaemic muscle tissue depended on mPGES-1/PGE2 signalling. Transplantation of whole CD4+ T cells from WT mice into mPges-1−/− mice (WT CD4+ → mPges-1−/−) enhanced recovery from ischaemia. However, removing CD25+ Tregs from the CD4+ T cell injected population did not restore blood flow. (WT CD4+ → mPges-1−/−, 0.76 ± 0.11; WTCD4 + CD25 mPges-1−/−, 0.46 ± 0.02; P < 0.05; Figure 3J) The same was observed also in blood vessel formation (WT CD4+ → mPges-1−/−, 15.06 ± 4.40; WTCD4 + CD25− → mPges-1−/−, 6.02 ± 2.76; P < 0.05; Figure 3K and L).
3.5. Effect of the stromal cell derived factor-1/CXCR4 axis in mPGES-1/PGE2-induced revascularization
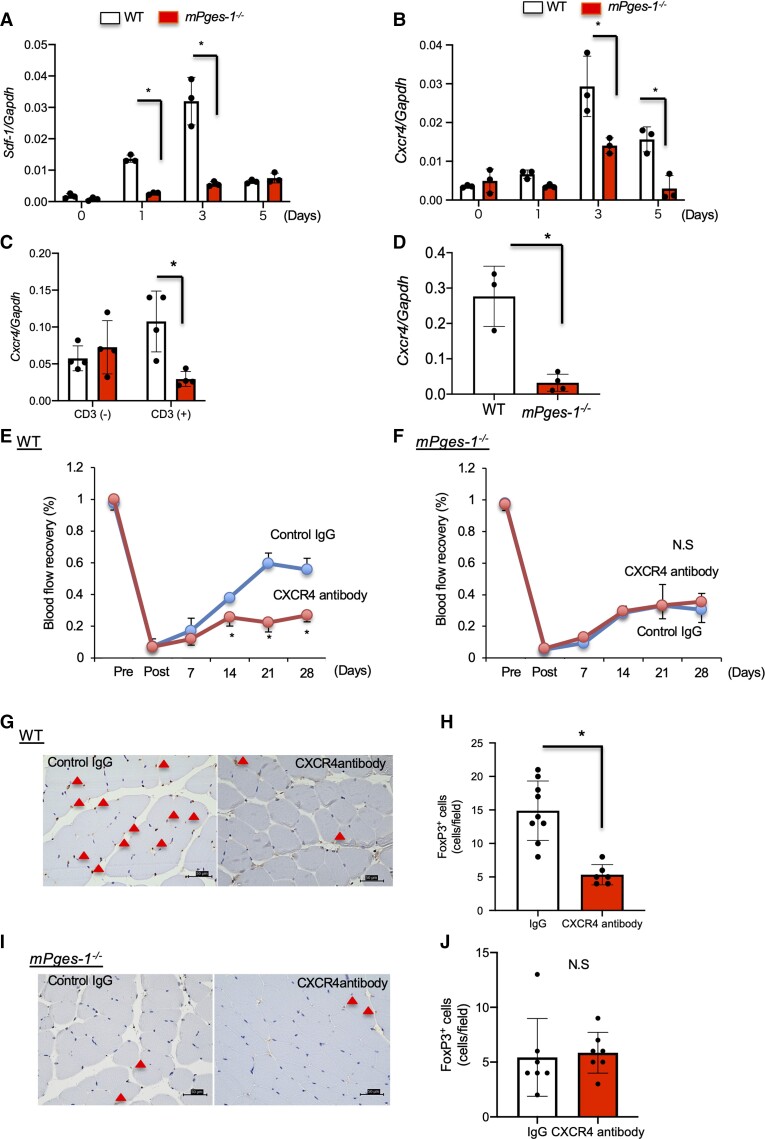
Angiogenesis induces monocyte/macrophage accumulation into the hypoxic area.31 Expression of chemokines and the stromal cell derived factor-1 (SDF-1)/CXCR4 axis has been implicated in Tregs migration in angiogenesis.32 Therefore, we next examined whether or not the SDF-1/CXCR4 axis plays a role in the accumulation of Tregs in angiogenesis. Under hypoxic condition the expression of SDF-1 in HUVECs was enhanced compared to normoxic conditions and those were suppressed by specific COX-2 inhibitor treatment, NS-398 (see Supplementary material online, Figure S7). These results indicated that hypoxia induced SDF-1 expression was related to COX-2-derived PGE2 axis. In ischaemic muscle tissue, expression of Sdf-1 and Cxcr4 mRNA was significantly decreased in mPges-1−/− mice compared with WT mice (Figure 4A and B). In vitro analysis revealed that expression of Cxcr4 mRNA was induced in Tregs from WT mice upon anti-CD3 stimulation but not in Tregs from mPges-1−/− mice (Figure 4C). In accumulated CD4 + CD25+ Tregs in ischaemic muscle tissue, expression of Cxcr4 mRNA was significantly decreased in mPges-1−/− mice compared with WT mice (Figure 4D). In vitro suppression assay by Tregs showed that there was no difference in Treg function between WT mice and mPges-1−/− mice (see Supplementary material online, Figure S8).
Figure 4.
The SDF-1/CXCR4 axis mediated Treg accumulation in mPGES-1-induced ischaemic recovery. (A) Sdf-1 and (B) Cxcr4 mRNA expression in ischaemic muscle tissue. mPges-1−/− mice decreased Sdf-1 and Cxcr4 expression. Data are means ± SD of the n = 3–4 mice/group. *P < 0.05 vs. WT mice, one-way ANOVA. (C) Cxcr4 expression in isolated Tregs was not enhanced by treatment with CD3 antibody in mPges-1−/− Tregs. (D) Cxcr4 mRNA expression in accumulated CD4+CD25+ Tregs 7 days after ligation. Data are means ± SD of the n = 3–4 mice/group. *P < 0.05 vs. WT mice, Student’s t-test. (E) In WT mice, CXCR4 antibody treatment impaired blood flow recovery from ischaemia. *P < 0.05, repeated-measures ANOVA (upper panel). (F) CXCR4 antibody did not affect blood flow recovery from ischaemia in mPges-1−/− mice. *P < 0.05, repeated-measures ANOVA (upper panel). (G) FoxP3 staining of the ischaemic limb in CXCR4 antibody-treated treated WT mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (H) Number of FoxP3+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. IgG, Student’s t-test. (I) FoxP3 staining of the ischaemic limb in CXCR4 antibody-treated mPges-1−/− mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (J) Number of FoxP3+ cells in ischaemic muscle tissue 7 days after ligation. Student’s t-test.
We reasoned that if the SDF-1/CXCR4 axis induces the accumulation of Tregs in ischaemic muscle tissue, treatment of mice with anti-CXCR4 antibody could suppress Tregs accumulation in ischaemic muscle tissue. The administration of anti-CXCR4 antibody in WT mice indeed inhibited recovery from the ischaemic condition, as compared with that in the mice with the control immunoglobulin (IgG) (control IgG, 0.58 ± 0.07; CXCR4 antibody, 0.27 ± 0.04; P < 0.05; Figure 4D). In contrast, there was no significant change in mPges-1−/− mice treated with control IgG vs. CXCR4 antibody (control IgG, 0.35 ± 0.13; CXCR4 antibody, 0.31 ± 0.10; P = 0.61; Figure 4E). The number of FoxP3+ cells accumulated at ischaemic sites were also decreased in WT mice by treating the mice with anti-CXCR4 antibody (control IgG, 14.9 ± 4.43 cells; CXCR4 antibody, 5.33 ± 1.51 cells; P < 0.05; Figure 4F and G) but not in mPges-1−/− mice treated with CXCR4 antibody (control IgG, 5.43 ± 3.55 cells; CXCR4 antibody, 5.85 ± 1.88 cells; P = 0.76; Figure 4H and I).
3.6. The effect of mPGES-1/PGE2-EP4 signalling on the recovery from ischaemia
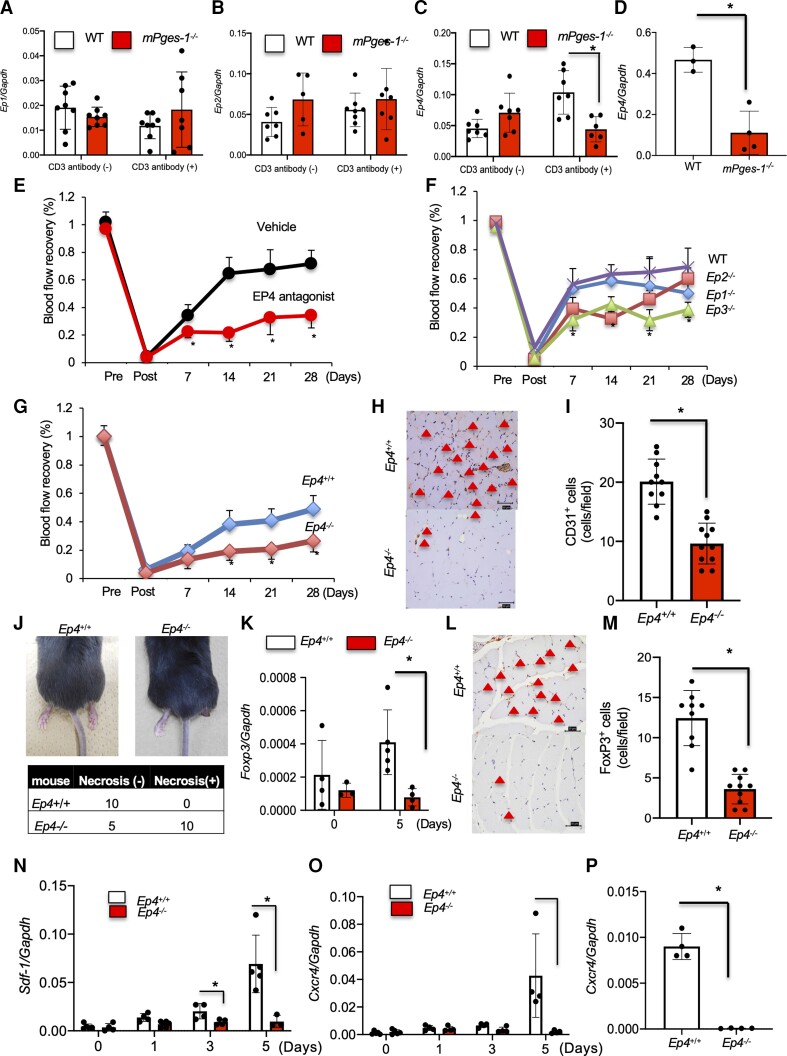
PGE2 binds to the EP receptors EP1–4. Among these receptors, after induction of hindlimb ischaemia, expression of Ep4 mRNA in ischaemic muscle tissue was significantly enhanced on Days 1 and 3 compared with that in non-ischaemic muscle tissue (see Supplementary material online, Figure S9A–D). There were no significant changes in Ep1 or Ep2 mRNA by ischaemia induction. In CD4 + CD25+ Tregs from WT thymus, mRNA expression level of Ep4 but not of Ep1 or Ep2 was elevated by stimulation with anti-CD3 antibody, whereas the increase of EP4 mRNA was not observed in mPges-1−/−-Tregs. We did not detect expression of Ep3 mRNA in CD4 + CD25+ Tregs from the thymus (Figure 5A–C). The expression of Ep4 mRNA in accumulated CD4 + CD25 + Tregs in mPges-1−/− mice was significantly suppressed compared with WT mice (Figure 5D). According to the pattern of specific expression of Ep4 in Tregs after stimulation upon anti-CD3 crosslinking, the treatment of WT mice with a selective EP4 antagonist was first applied to the recovery assay for blood flow and found that suppressed recovery from ischaemia compared with that in vehicle-treated mice (vehicle, 0.72 ± 0.09; EP4 antagonist, 0.34 ± 0.09; P < 0.05; Figure 5D) on Day 28. In contrast, WT mice treated with selective EP4 agonist significantly enhanced recovery from ischaemia (see Supplementary material online, Figure S10). The assays on recovery from ischaemia were then applied to deficient mice for PGE2 receptor subtypes, Ep1−/−–3−/−, in comparison with WT B6 mice, and Ep4−/− mice with Ep4+/+ mice. As anticipated, there were no significant differences among Ep1−/−, Ep2−/−, and WT mice (Ep1−/−, 0.50 ± 0.06; P = 0.09; Ep2−/−, 0.60 ± 0.07; P = 0.39; Figure 5E). It was unexpected that the recovery from ischaemic conditions was prolonged in Ep3−/− mice (WT, 0.68 ± 0.13; Ep3−/−, 0.39 ± 0.05; P < 0.05; Figure 5E). In addition, the number of CD31+ endothelial cells in ischaemic muscle tissue were significantly decreased in Ep3−/− mice compared with that in WT mice (see Supplementary material online, Figure S11A and B). Although the genetic background of Ep4−/− mice was not one of genuine B6, but the mixtures of B6 and 129/Ola mice, the recovery of blood flow from ischaemia between Ep4−/− and Ep4+/+ mice was demonstrated when performed in separate sets of experiments from other EP-deficient mice (Ep4+/+, 0.49 ± 0.09; Ep4−/−, 0.26 ± 0.07; P < 0.001; Figure 5F). The number of CD31+ endothelial cells in ischaemic muscle tissue was significantly decreased in Ep4−/− mice compared with that in Ep4+/+ mice (Ep4+/+, 20.1 ± 3.81; Ep4−/−, 9.63 ± 3.44; P < 0.05; Figure 5G and H). Furthermore, the number of αSMA+ cells in ischaemic muscle was significantly decreased in Ep4−/− mice (see Supplementary material online, Figure S12A and B).
Figure 5.
Effect of EP4 receptors on recovery from hindlimb ischaemia. (A) Ep1, (B) Ep2, and (C) Ep4 mRNA expressions in Tregs from WT and mPges-1−/− mice under CD3 antibody treatment. *P < 0.05, repeated-measures ANOVA. (D) Ep4 mRNA expression in accumulated CD4+CD25+ Tregs 7 days after ligation. Data are means ± SD of the n = 3–4 mice/group. *P < 0.05 vs. WT mice, Student’s t-test. (E) EP4 antagonist-treated WT mice impaired recovery from ischaemia compared with vehicle-treated mice. *P < 0.05, repeated-measures ANOVA (upper panel). Blood flow recovery was impaired in (F) Ep3−/− and (G) Ep4−/− mice relative to WT. *P < 0.05, repeated-measures ANOVA (upper panel). (H) CD31 staining of the ischaemic limb 28 days after ligation. Triangles indicate CD31+ cells. Scale bar, 50 μm. (I) Number of CD31+ cells in ischaemic muscle tissue 28 days after ligation. P < 0.05 vs. Ep4+/+WT mice, Student’s t-test. (J) Typical appearance of an ischaemic footpad (upper panel). The incidence of footpad necrosis was increased in Ep4−/− mice. (K) Foxp3 mRNA expression in ischaemic muscle tissue. Ep4−/− mice decreased mRNA expression of Foxp3 compared with Ep4+/+ mice 5 days after ligation. Data are means ± SD of the n = 3–4 mice/group. *P < 0.05, one-way ANOVA. (L) FoxP3 staining of the ischaemic limb in Ep4−/− mice and Ep4+/+ mice 28 days after ligation. Triangles indicate FoxP3+ cells. Scale bar, 50 μm. (M) Number of FoxP3+ cells in ischaemic muscle tissue 7 days after ligation. P < 0.05 vs. Ep4+/+T mice, one-way ANOVA. (N) Sdf-1 and (O) Cxcr4 mRNA expressions in ischaemic muscle tissue. Ep4−/− mice decreased mRNA expression of Sdf-1 and Cxcr4 compared with Ep4+/+. Data are expressed as means + SD of n = 4 mice/group. *P < 0.05, one-way ANOVA. (P) Cxcr4 mRNA expression in accumulated CD4 + CD25+ Tregs 7 days after ligation. Data are means ± SD of the n = 4 mice/group. *P < 0.05 vs. Ep4+/+ mice, Student’s t-test.
3.7. PGE2-EP4-dependent revascularization is dependent on accumulation of Tregs in ischaemic muscle tissue
Necrosis commonly occurs after prolonged ischaemia in the hindlimb ischaemia model. When we induced hindlimb ischaemia in Ep4−/− mice, two thirds of that cohort developed necrosis in the ligated foot, while no necrotic lesions were observed in Ep4+/+ mice (Figure 5I). Expression of Foxp3 (Figure 5J) and accumulation of FoxP3+ Tregs in ischaemic muscle tissue were suppressed in the Ep4−/− mice (Ep4+/+, 12.4 ± 3.43; Ep4−/−, 3.6 ± 1.83; P < 0.05; Figure 5K and L) on Day 7. In ischaemic muscle, expression of mRNAs encoding Sdf-1 and Cxcr4 were significantly suppressed in Ep4−/− mice compared with Ep4+/+ mice (Figure 5M and N) on Days 3 and 5. Moreover, expression of Cxcr4 in CD4 + CD25+ Tregs were significantly suppressed in Ep4−/− mice compared with Ep4+/+ mice (Figure 5P).
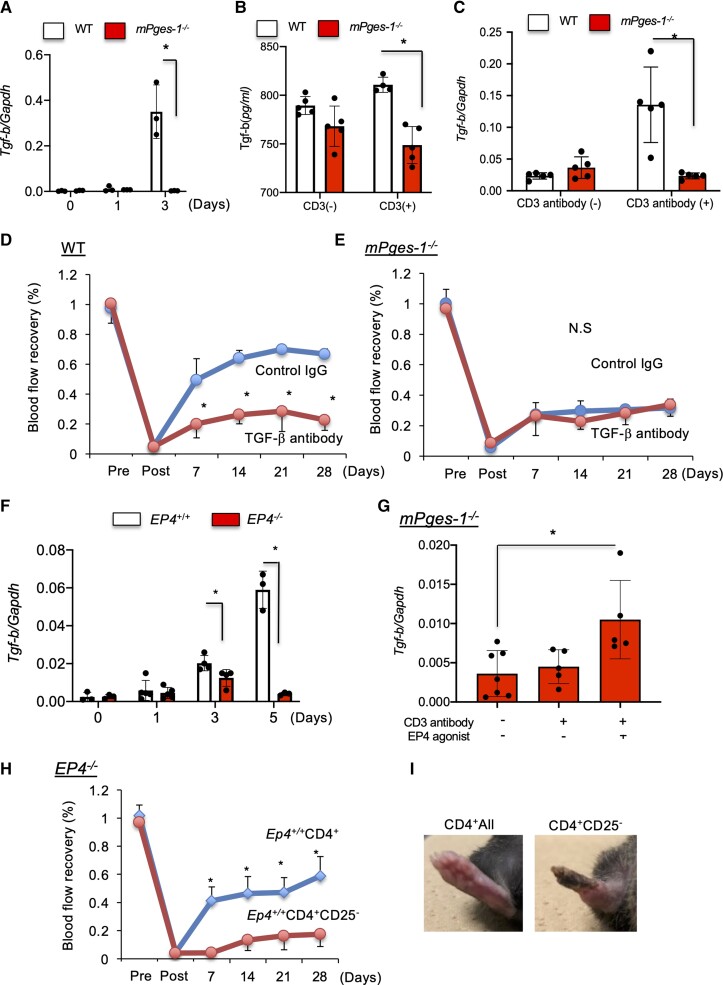
3.8. TGF-β released from Tregs induced blood flow recovery
TGF-β is known not only as an important cytokine secreted from Tregs to regulate immune responses but also as one of the most potent angiogenesis-stimulating factors.33,34 On Day 3 after ischaemia induction, expression of Tgf-β mRNA in ischaemic muscle tissue was significantly suppressed in mPges-1−/− mice relative to WT (Figure 6A). In addition, an in vitro study revealed that Tregs from mPges-1−/− mice expressed significantly reduced level of protein or mRNA of TGF-β compared with WT Tregs (Figure 6B and C). Ischaemic recovery was significantly suppressed in WT mice treated with TGF-β antibody (control IgG, 0.67 ± 0.03; TGF-β antibody, 0.23 ± 0.07; P < 0.05; Figure 6D). On the other hand, in mPges-1−/− mice where ischaemic recovery was already suppressed, the recovery was not further suppressed when treated with TGF-β antibody (control IgG, 0.32 ± 0.06; TGF-β antibody, 0.34 ± 0.08; P = 0.69; Figure 6E) on Day 28. Expression of TGF-β mRNA in ischaemic muscle tissue was significantly lower in Ep4−/− mice than in WT mice on Days 3 and 5 (Figure 6F). TGF-β mRNA expression with anti-CD3 cross-linking in CD4 + CD25+ Tregs from mPges-1−/− mice was restored by the treatment with selective EP4 agonist (Figure 6G). Furthermore, ischaemic blood flow recovery of Ep4−/− mice was significantly improved by transplantation of whole CD4+ cells, containing Tregs, from WT mice, whereas infusion of Treg-depleted CD4+ cells did not restore neovascularization in Ep4−/− mice (Ep4+/+-CD4+ → Ep4−/−, 0.59 ± 0.13; Ep4+/+-CD4 + CD25− → Ep4−/−, 0.17 ± 0.09; P < 0.05; Figure 6H and I). Furthermore, Rag2−/− mice that were transplanted with CD4+ from Ep4+/+ mice significantly improved in terms of ischaemic recovery compared to Rag2−/− mice that were transplanted with CD4+ from Ep4−/− mice (see Supplementary material online, Figure S13). These results suggested that the EP4 receptor on the Tregs is important for recovery from an ischaemic condition.
Figure 6.
The mPGES-1/EP4 axis induced recovery from ischaemia by promoting Treg TGF-β production. (A) Tgf-β mRNA expression in ischaemic muscle tissue. mPges-1−/− mice decreased mRNA expression of Foxp3 relative to WT mice. Data are means ± SD of the n = 3 mice/group. *P < 0.05, one-way ANOVA. (B) Protein level and (C) mRNA level of TGF-β in Tregs from WT and mPges-1−/− mice under CD3 antibody treatment. Expression of Ep4 was enhanced in WT Tregs. *P < 0.05, repeated-measures ANOVA. (D) In WT mice, TGF-β antibody treatment impaired blood flow recovery from ischaemia. *P < 0.05, repeated-measures ANOVA (upper panel). (E) TGF-β antibody treatment did not affect blood flow recovery from ischaemia in mPges-1−/− mice. *P < 0.05, repeated-measures ANOVA (upper panel). (F) mRNA level of Tgf-β in ischaemic muscle tissue. Ep4−/− mice decreased mRNA expression of Tgf-β compared with Ep4+/+. Data are means ± SD of the n = 3 mice/group. *P < 0.05, one-way ANOVA. (G) mRNA level of Tgf-β in Tregs from WT and mPges-1−/− mice under CD3 antibody treatment. Expression of Tgf-β in was enhanced in WT Tregs. *P < 0.05, repeated-measures ANOVA. (H) Transplantation of Ep4+/+-derived CD4+ T cells, but not CD4+CD25− T cells, into Ep4−/− recipient mice enhanced recovery from ischaemia. *P < 0.05, repeated-measures ANOVA. (I) Typical appearance of an ischaemic footpad on Day 28 in Ep4−/− transplanted with Ep4+/+-derived CD4+ T cells and CD4+CD25− T cells.
4. Discussion
The objective of the present study was to investigate the role of the mPGES-1/PGE2 axis in recovery from hindlimb ischaemia. Our findings in mPges-1−/− mice demonstrated that the endogenous mPGES-1/PGE2 axis induced recovery from ischaemia by promoting FoxP3+ Tregs accumulation at the ischaemic site. Furthermore, we suggested that during recovery from ischaemia, endogenous PGE2 binds the EP4 receptor on accumulated Tregs. The mPGES-1/PGE2/EP4 axis up-regulated expression of TGF-β in Tregs and the SDF-1/CXCR4 axis in ischaemic muscle tissue, which eventually induced angiogenesis and recovery from ischaemia.
COX-2-derived PGE2 induces inflammation and cancer growth.35 Aspirin, which inhibits COX-1 and COX-2, suppresses angiogenesis and is currently used for prevention of cancer onset and recurrence.36 However, long-term treatment with COX-2 inhibitors in colon cancer patients increased cardiovascular events.37 In that study, it was concluded that the increase of events was caused by tipping the balance of prostacyclin/thromboxane in favour of thromboxane, a prethrombotic eicosanoid.
Previously, we demonstrated that the mPGES-1/PGE2 axis induces angiogenesis and gastric ulcer healing, leading us to the hypothesis that mPGES-1/PGE2 signalling could be important in recovery from ischaemia.13 To test this hypothesis, mice were treated with aspirin or a selective COX-2 inhibitor after femoral artery ligation, and the recovery from ischaemia was evaluated. Aspirin- and COX-2 inhibitor-treated mice exhibited significantly prolonged recovery from ischaemia. These findings indicated that COX-2-derived PGs induced recovery from ischaemia.
mPGES-1 is a glutathione (GSH)-dependent perinuclear protein belonging to the MPGEG (membrane-associated proteins involved in eicosanoid and GSH metabolism) family. Expression of this enzyme is strongly enhanced by pro-inflammatory stimuli. Induction of mPGES-1 expression has been observed in various systems in which COX-2-derived PGE2 is thought to play a critical role, such as inflammation, fever, pain, tissue repair, and cancer.13,14,38,39 In the present study, muscle tissue mPGES-1 expression was enhanced after femoral artery ligation, which was suppressed by treatment with aspirin and the COX-2 inhibitor. In an in vitro study, exposure of HUVEC to hypoxia enhanced COX-2 and PGE2 expression.40 In the present study, we demonstrated that COX-2 and PGE2 expression was suppressed by COX-2 inhibitor, a specific COX-2 inhibitor. These results indicated that exposure to hypoxic conditions enhanced PGE2 derived from COX-2. In the present study, to investigate the role of mPGES-1 and the endogenous effect of PGE2 in recovery from ischaemia, we used mPges-1−/− mice. We demonstrated that recovery from ischaemia was significantly prolonged in mPges-1−/− mice. Furthermore, immunohistochemical analysis revealed that microvessels in ischaemic muscle tissue were decreased in mPges-1−/− mice compared with WT mice. These results indicated that the COX-2/mPGES-1/PGE2 axis induced recovery from ischaemic condition.
Tregs are well-known to be essential cells in immune tolerance and immune suppression.7 COX-2 has been shown to exert some effects on FoxP3 expression and Tregs function.41 A previous study demonstrated that Tregs contributed to immunosuppression in cancer tissue and inhibited the effect of effector T cells in a COX-2-dependent manner.42 PGE2 production by COX-2 has been reported to have potent immunomodulatory effects in the immune system.
Tregs are important for the induction and maintenance of immune homeostasis, which prevents pathological autoimmunity and inflammation. Tregs are a subpopulation of CD4+ T cells that express CD25 and constitute 5–10% of peripheral CD4+ cells.43 Previous studies show that Tregs levels are increased in response to ischaemia. Brea et al.44 demonstrated that brain ischaemic area was significantly suppressed in Treg-treated mice compared with that in the control mice. Some studies have suggested that Tregs either impair or improve angiogenesis after femoral artery ligation; therefore, the role of Tregs in this context remains controversial.8 In the present study, we demonstrated that the expression of the Tregs marker, FoxP3, was enhanced in ischaemic muscle tissue compared with that in non-ischaemic muscle tissue. Furthermore, expression of mPGES-1 was enhanced in ischaemic muscle tissue, which was suppressed by treatment with aspirin and a COX-2 inhibitor, celecoxib. These findings indicated that the COX-2/mPGES-1/PGE2 pathway is related to expression of FoxP3 and that this interaction was induced by hypoxic stimulation. Immunohistochemical analysis of FoxP3 revealed that the number of FoxP3+ Tregs correlated with the degree of recovery from ischaemia.
In our prior study, we demonstrated that infiltrating Tregs induce angiogenesis in wound healing model.18 In the present study, we demonstrated that treatment with antibodies to CD25 or FR4 which deplete Tregs significantly decreased recovery from ischaemia and the number of accumulated Tregs in ischaemic muscle tissue in the context of hindlimb ischaemia. These findings were suggestive of a correlation between the degree of Tregs infiltration and angiogenesis in recovery from hindlimb ischaemia.
Stabile et al.5 reported that CD4+ T cells induced ischaemic recovery response by recruiting macrophage. They also showed that Cd8−/− mice impaired recovery from ischaemia by reducing recruitment of CD4+ mononuclear cells.45 The present study showed that total B cells, CD8+ cells, CD4+ cells, and Tregs in the ischaemic muscle were significantly suppressed in mPges-1−/− mice compared to WT mice (see Supplementary material online, Figure S14A–C). These results indicated that not only Tregs but also other immune cells, including CD8+ and CD4+, accumulated into ischaemic muscle. These results implying that ischaemic induction may recruit not only Treg cells but also other lymphocytes. Further studies are needed to reveal details.
Rag2−/− mice were viable but fail to produce mature B or T lymphocytes.29 To prove that Tregs directly increased angiogenesis in recovery from ischaemia, we first transplanted CD4+ lymphocytes including Tregs, from WT donor mice, into Rag2−/− recipient mice, and the results indicated that adoptive transfer of whole CD4+ T cells but not Treg-depleted CD4+ T cells enhanced recovery from ischaemia following hindlimb ischaemia. Further, transplantation of CD4+ lymphocytes from WT donor mice into mPges-1−/− recipient mice enhanced recovery from ischaemia; however, injection of WT CD4+ T cells depleted of CD25+ lymphocytes did not improve blood flow recovery. The more important rationale is transplantation of CD4+ lymphocytes from Ep4+/+ donor mice into Rag2−/− enhanced recovery from ischaemia compared with transplantation of CD4+ lymphocytes from Ep4−/− mice donor mice into Rag2−/−. These findings suggested that CD4+CD25+ Tregs are important for ischaemic recovery mediated by the mPGES-1/PGE2-EP4 axis. The suppression assay revealed that Tregs lacking mPGES-1 still exerted comparable activity to suppress T cell proliferation, suggesting that suppressive capacity of Tregs is independent on mPGES-1.
SDF-1 (also designated as, CXCL12) is a chemokine and binds as a specific ligand to the CXCR4 receptor. The SDF-1/CXCR4 axis induces mobilization of bone marrow-derived stem cells.32,46 CXCR4+ Tregs are recruited to the bone marrow where significant levels of SDF-1 are produced.47 SDF-1 is produced by various cell types, including tumour cells, stromal fibroblast cells, and vascular endothelial cells. Tissue ischaemia, such as that in myocardial infarction, induces stabilization of the transcription factor hypoxia inducible factor-1 alpha (HIF-1α), which transcribes SDF-1α to stimulate the migration of endothelial cells and enhance angiogenesis.48 Our previous study showed that SDF-1/CXCR4 axis is needed to accumulate myeloid-derived suppressor cells (MDSCs) in metastasized organs and develop metastasis formation. Kim et al.49 reported that MDSCs enhanced blood recovery after femoral artery ligation.50 Current study revealed that there were no significant changes between WT and mPges-1−/− mice (see Supplementary material online, Figure S15). That result indicated that accumulation of MDSCs is not essential for recovery from ischaemia.
The present study demonstrated that expression of SDF-1 and CXCR4 was significantly suppressed in mPges-1−/− mice subjected to hindlimb ischaemia. Although WT Tregs expressed an augmented level of Cxcr4 mRNA expression by anti-CD3 stimulation, Tregs from mPges-1−/− mice did not. Based on these findings, we hypothesized that CXCR4+ Tregs could accumulate in ischaemic muscle tissue in which SDF-1 expression is up-regulated. Consistent with this hypothesis, CXCR4 antibody treatment prolonged ischaemic recovery in WT but not in mPges-1−/− mice. Furthermore, impaired recovery of blood flow by anti-CXCR4 treatment was accompanied with suppressed accumulation of FoxP3+ Tregs in ischaemic muscle tissue in WT mice. These results demonstrated that mPGES-1/PGE2 may recruit CXCR4+ Tregs to ischaemic muscle tissue where SDF-1 expression is up-regulated.
PGE2 binds four subtype receptors, EP1–EP4. Expression of EP4 was increased in ischaemic muscle tissue on Days 1 and 3 after induction of hindlimb ischaemia. In HUVECs, stimulation with PGE2 induced expression of CXCR4 through EP2 and EP4.46 This was consistent with a previous finding in DSS (dextran sodium sulphate)-induced colitis, in which deficiency of mPGES-1 or PGE2-EP4 signalling abrogated the colitogenic phenotype.51 Furthermore, PGE2 induced muscle regeneration by recruiting muscle-specific stem cells via the EP4 receptor and ablation of this receptor impaired muscle regeneration.52
We demonstrated that purified CD4+CD25+ Tregs from WT mice, but not mPges-1−/− mice, enhanced EP4 expression by anti-CD3 stimulation. EP4 expression in accumulated Tregs in the ischaemia muscle was suppressed in mPges-1−/− mice. Activation of EP4 receptors by PGE2 promoted in vitro tube formation in human neonatal dermal microvascular endothelial cells and ex vivo vessel outgrowth of aortic rings and promoted angiogenesis in an in vivo surgical sponge model.53 To determine if mPGES-1/PGE2-EP4 induced recovery from hindlimb ischaemia, we used a selective EP4 antagonist. Mice treated with an EP4 antagonist exhibited delayed recovery from ischaemia. Furthermore, Ep4−/− mice exhibited delayed recovery from ischaemia and limb necrosis, together with attenuated SDF-1/CXCR4 expression and FoxP3+ Tregs accumulation in ischaemic muscle tissue. Moreover, expression of CXCR4 in accumulated Tregs was significantly suppressed in mPges-1−/− and Ep4−/− mice. These results indicated that mPGES-1/PGE2-EP4 signalling induced recovery from ischaemia by modulating the recruitment of Tregs, which was dependent on the SDF-1/CXCR4 axis. However, other possibilities including the conversion of effector T cells to Tregs and the proliferation of Tregs cannot be excluded.
TGF-β, which is one of the major cytokines secreted from Tregs, acts as a pro-angiogenic factor. In the tumour microenvironment, TGF-β induces suppression of antitumour immunity, promoting expansion of tumour growth and metastasis.54 In a previous study, we observed that expression of TGF-β was enhanced after femoral artery ligation.34 We confirmed that expression of TGF-β was significantly suppressed in mPges-1−/− and Ep4−/− mice. Further, recovery from ischaemia was suppressed by TGF-β antibody treatment. These results suggested that TGF-β is an important factor for recovery from hindlimb ischaemia.
CD3 antibody treatment increases HIF-1 protein stability and activity under hypoxic conditions in human Tregs.55 Our results demonstrated that in purified CD4+CD25+ Tregs activated with CD3 antibody, protein and mRNA levels of TGF-β were decreased in mPges-1−/− cells in comparison with WT cells. Furthermore, TGF-β expression in purified CD4+CD25+ Tregs was enhanced by EP4 agonist treatment.
In Ep4−/− mice, recovery from ischaemia was enhanced by transplantation of CD4+ cells including CD25+ Tregs, from Ep4+/+ mice, while improved recovery was not observed in mice that received CD4+ T cells depleted of CD25+ Tregs. Moreover, the frequency of the foot necrosis was decreased by total CD4+ cell transplantation. These results indicated that under hypoxic conditions, Tregs enhanced TGF-β expression via mPGES-1/PGE2-EP4 signalling. In these experiments, we did not transfer purified Tregs, because CD4+CD25+ cells had to be prepared by sorting using CD25 antibody, which may decrease reactivity to IL-2 and, therefore, the survival of Tregs. Instead, we examined the contribution of Tregs to recovery from hindlimb ischaemia by comparing the relative protective effects of transplantation with whole CD4+ cells and CD4+CD25− cells.
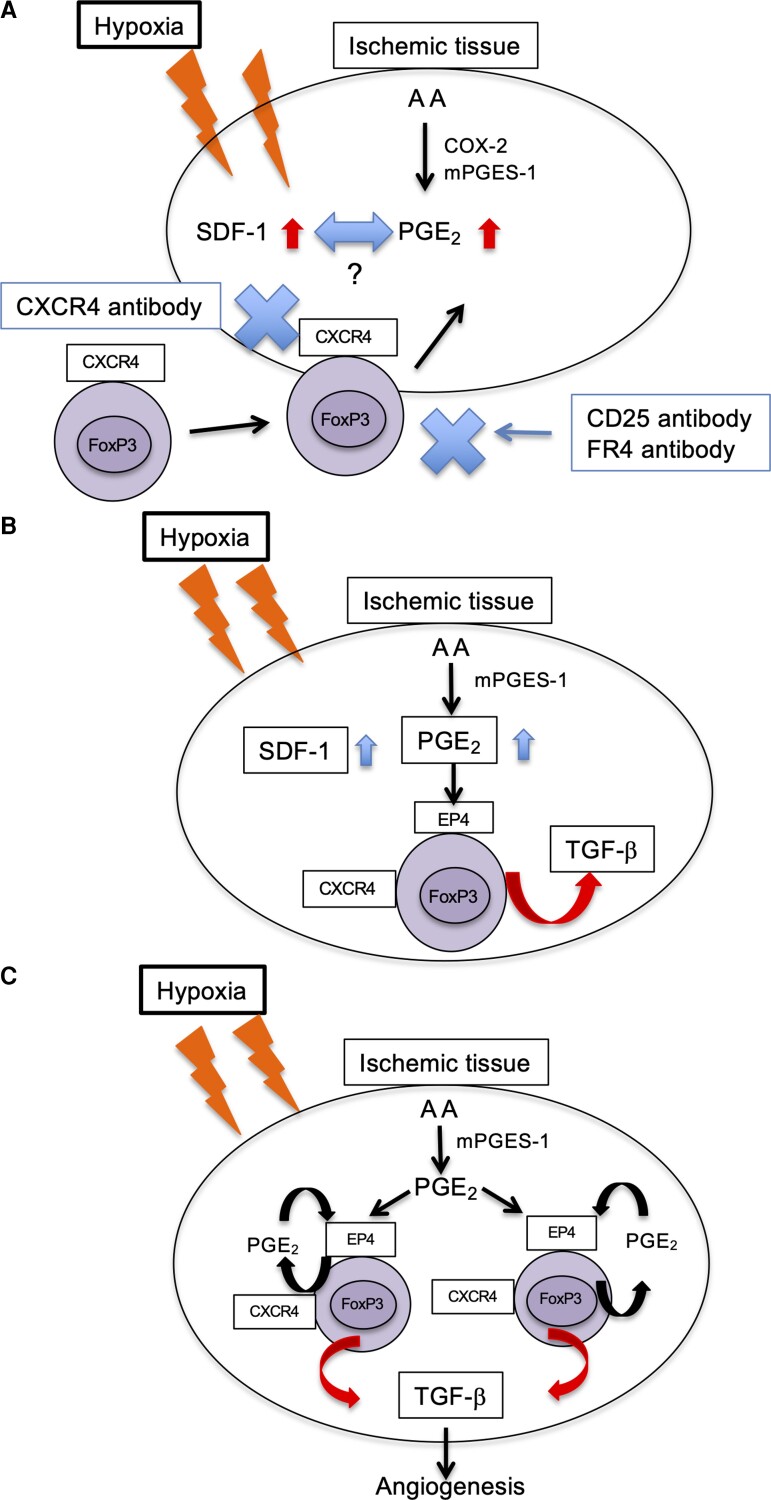
In conclusion, these findings demonstrated that mPGES-1/PGE2-EP4 signalling induced recovery from ischaemia by promoting Tregs recruitment to ischaemic muscle tissue (Figure 7). In PAD, selective EP4 agonist or the transplantation of Tregs could alleviate disease severity in clinical settings.
Figure 7.
Schematic of COX-2/mPGES-1/EP4 axis regulation of recovery from ischaemia. (A) After femoral artery ligation, expression of COX-2/mPGES-1-derived PGE2 and SDF-1 was enhanced. CXCR4+ Tregs accumulated in ischaemic tissue. (B) PGE2 bound to the EP4 receptor on CXCR4+ Tregs and promoted production of TGF-β. (C) Under hypoxic conditions, Tregs enhanced TGF-β expression via mPGES-1/PGE2-EP4 signalling.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
H.A. conceived, designed, and performed the experiments and wrote the manuscript. K.E. and Y.I. conceived, designed, and performed the experiments and wrote the manuscript. M.N., H.K., F.O., and K.H. performed the experiments and provided technical support. K.I., S.U., S.A., and S.N. performed data interpretation, provided technical support, and revised the manuscript critically for intellectual content. M.M. conceived the project and supervised the study. All authors contributed to the article and approved the submitted version.
Supplementary Material
Acknowledgements
We thank Michiko Ogino and Kyoko Yoshikawa for their technical assistance. We also thank Robert E. Brandt, Founder, CEO, and CME of MedEd Japan for editing and formatting the manuscript.
Contributor Information
Hideki Amano, Department of Pharmacology, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan.
Koji Eshima, Department of Immunology, Kitasato University School of Medicine, Kanagawa, Japan.
Yoshiya Ito, Department of Pharmacology, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan.
Masaki Nakamura, Department of Microbiology, Kitasato University School of Allied Health Science, Kanagawa, Japan.
Hidero Kitasato, Department of Microbiology, Kitasato University School of Allied Health Science, Kanagawa, Japan.
Fumihiro Ogawa, Department of Pharmacology, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan.
Kanako Hosono, Department of Pharmacology, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan.
Kazuya Iwabuchi, Department of Immunology, Kitasato University School of Medicine, Kanagawa, Japan.
Satoshi Uematsu, Department of Immunology and Genomics, Osaka City University Graduate School of Medicine, Osaka, Japan.
Shizuo Akira, Laboratory of Host Defense, WPI Immunology Frontier Research Center (IFReC), Osaka University, Osaka, Japan.
Shuh Narumiya, Department of Drug Discovery Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan.
Masataka Majima, Department of Pharmacology, Kitasato University School of Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan; Department of Medical Therapeutics, Kanagawa Institute of Technology, Atsugi, Kanagawa, Japan.
Funding
This work was supported by grants from The Ministry of Education, Culture, Sports, Science and Technology (MEXT; grant nos 19K09250, 15K15056, 80532556, 23116102, 24659119, 26462132, and 26293055).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Translational perspective.
Although surgical treatment for peripheral arterial disease in patients improved, some patients with advanced disease have no other option for treatments other than amputation. In the present study, we revealed that endogenous microsomal prostaglandin E synthase-1/prostaglandin E2-EP4 signalling induced recovery from ischaemia by promoting regulatory T cell (Tregs) accumulation at the ischaemic site. In addition, we showed that selective EP4 agonist, or transplantation of Tregs, induced recovery from ischaemic conditions. These results indicate that the use of a selective EP4 agonist, or cell therapy of Tregs, may be a potential treatment option for severe critical limb ischaemia patients.
References
- 1. Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 2005;112:1813–1824. [DOI] [PubMed] [Google Scholar]
- 2. Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest 1999;103:1231–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sozzani S, Del Prete A, Bonecchi R, Locati M. Chemokines as effector and target molecules in vascular biology. Cardiovasc Res 2015;107:364–372. [DOI] [PubMed] [Google Scholar]
- 4. Schrier SB, Hill AS, Plana D, Lauffenburger DA. Synergistic communication between CD4+ T cells and monocytes impacts the cytokine environment. Sci Rep 2016;6:34942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stabile E, Burnett MS, Watkins C, Kinnaird T, Bachis A, la Sala A, Miller JM, Shou M, Epstein SE, Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 2003;108:205–210. [DOI] [PubMed] [Google Scholar]
- 6. Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect 2004;6:745–751. [DOI] [PubMed] [Google Scholar]
- 7. Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006;212:8–27. [DOI] [PubMed] [Google Scholar]
- 8. Zouggari Y, Ait-Oufella H, Waeckel L, Vilar J, Loinard C, Cochain C, Récalde A, Duriez M, Levy BI, Lutgens E, Mallat Z, Silvestre JS. Regulatory T cells modulate postischemic neovascularization. Circulation 2009;120:1415–1425. [DOI] [PubMed] [Google Scholar]
- 9. Sharir R, Semo J, Shaish A, Landa-Rouben N, Entin-Meer M, Keren G, George J. Regulatory T cells influence blood flow recovery in experimental hindlimb ischaemia in an IL-10-dependent manner. Cardiovasc Res 2014;103:585–596. [DOI] [PubMed] [Google Scholar]
- 10. Vietto V, Franco JV, Saenz V, Cytryn D, Chas J, Ciapponi A. Prostanoids for critical hind limb ischaemia. Cochrane Database Syst Rev 2018;1:CD006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomez I, Foudi N, Longrois D, Norel X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot Essent Fatty Acids 2013;89:55–63. [DOI] [PubMed] [Google Scholar]
- 12. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol 2002;23:144–150. [DOI] [PubMed] [Google Scholar]
- 13. Ae T, Ohno T, Hattori Y, Suzuki T, Hosono K, Minamino T, Sato T, Uematsu S, Akira S, Koizumi W, Majima M. Role of microsomal prostaglandin E synthase-1 in the facilitation of angiogenesis and the healing of gastric ulcers. Am J Physiol Gastrointest Liver Physiol 2010;299:G1139–G1146. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi R, Amano H, Satoh T, Tabata K, Ikeda M, Kitasato H, Akira S, Iwamura M, Majima M. Roles of microsomal prostaglandin E synthase-1 in lung metastasis formation in prostate cancer RM9 cells. Biomed Pharmacother 2014;68:71–77. [DOI] [PubMed] [Google Scholar]
- 15. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol 2012;188:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother 2008;57:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res 2008;14:1032–1040. [DOI] [PubMed] [Google Scholar]
- 18. Inoue Y, Amano H, Asari Y. Roles of regulatory T cells in enhancement of angiogenesis in a sponge implantation model. Kitasato Med J 2018;48:105–117. [Google Scholar]
- 19. Yang B, Hamilton JA, Valenzuela KS, Bogaerts A, Xi X, Aronowski J, Mays RW, Savitz SI. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cells 2017;35:1290–1302. [DOI] [PubMed] [Google Scholar]
- 20. Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, Bai X, Feng Z, Sun S, Chen X. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int 2018;93:814–825. [DOI] [PubMed] [Google Scholar]
- 21. D’Alessio FR, Zhong Q, Jenkins J, Moldobaeva A, Wagner EM. Lung angiogenesis requires CD4(+) forkhead homeobox protein-3(+) regulatory T cells. Am J Respir Cell Mol Biol 2015;52:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaplan A, Altara R, Eid A, Booz GW, Zouein FA. Update on the protective role of regulatory T cells in myocardial infarction: a promising therapy to repair the heart. J Cardiovasc Pharmacol 2016;68:401–413. [DOI] [PubMed] [Google Scholar]
- 23. Baratelli F, Lin Y, Zhu L, Yang SC, Heuzé-Vourc'h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol 2005;175:1483–1490. [DOI] [PubMed] [Google Scholar]
- 24. Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol 2002;168:5811–5816. [DOI] [PubMed] [Google Scholar]
- 25. Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun 1998;246:7–12. [DOI] [PubMed] [Google Scholar]
- 26. Amano H, Ito Y, Eshima K, Kato S, Ogawa F, Hosono K, Oba K, Tamaki H, Sakagami H, Shibuya M, Narumiya S, Majima M. Thromboxane A2 induces blood flow recovery via platelet adhesion to ischaemic regions. Cardiovasc Res 2015;107:509–521. [DOI] [PubMed] [Google Scholar]
- 27. Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Kurosawa T. Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp Anim 2013;62:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eshima K, Suzuki H, Shinohara N. Cross-positive selection of thymocytes expressing a single TCR by multiple major histocompatibility complex molecules of both classes: implications for CD4+ versus CD8+ lineage commitment. J Immunol 2006;176:1628–1636. [DOI] [PubMed] [Google Scholar]
- 29. Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992;68:855–867. [DOI] [PubMed] [Google Scholar]
- 30. Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 1998;10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 31. Abe H, Semba H, Takeda N. The roles of hypoxia signaling in the pathogenesis of cardiovascular diseases. J Atheroscler Thromb 2017;24:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res 2004;64:8451–8455. [DOI] [PubMed] [Google Scholar]
- 33. Ohkura N, Hamaguchi M, Sakaguchi S. FOXP3+ regulatory T cells: control of FOXP3 expression by pharmacological agents. Trends Pharmacol Sci 2011;32:158–166. [DOI] [PubMed] [Google Scholar]
- 34. Mishima T, Ito Y, Hosono K, Tamura Y, Uchida Y, Hirata M, Suzuki T, Amano H, Kato S, Kurihara Y, Kurihara H, Hayashi I, Watanabe M, Majima M. Calcitonin gene-related peptide facilitates revascularization during hindlimb ischemia in mice. Am J Physiol Heart Circ Physiol 2011;300:H431–H439. [DOI] [PubMed] [Google Scholar]
- 35. Amano H, Hayashi I, Endo H, Kitasato H, Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto Y, Murata T, Yoshimura H, Narumiya S, Majima M. Host prostaglandin E(2)-EP3 signaling regulates tumor-associated angiogenesis and tumor growth. J Exp Med 2003;197:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saha S, Mukherjee S, Khan P, Kajal K, Mazumdar M, Manna A, Mukherjee S, De S, Jana D, Sarkar DK, Das T. Aspirin suppresses the acquisition of chemoresistance in breast cancer by disrupting an NFκB-IL6 signaling axis responsible for the generation of cancer stem cells. Cancer Res 2016;76:2000–2012. [DOI] [PubMed] [Google Scholar]
- 37. Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br J Clin Pharmacol 2016;82:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilhelms DB, Kirilov M, Mirrasekhian E, Eskilsson A, Kugelberg UÖ, Klar C, Ridder DA, Herschman HR, Schwaninger M, Blomqvist A, Engblom D. Deletion of prostaglandin E2 synthesizing enzymes in brain endothelial cells attenuates inflammatory fever. J Neurosci 2014;34:11684–11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Isono M, Suzuki T, Hosono K, Hayashi I, Sakagami H, Uematsu S, Akira S, DeClerck YA, Okamoto H, Majima M. Microsomal prostaglandin E synthase-1 enhances bone cancer growth and bone cancer-related pain behaviors in mice. Life Sci 2011;88:693–700. [DOI] [PubMed] [Google Scholar]
- 40. Zhao L, Wu Y, Xu Z, Wang H, Zhao Z, Li Y, Yang P, Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med 2012;16:1840–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahic M, Yaqub S, Johansson CC, Taskén K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol 2006;177:246–254. [DOI] [PubMed] [Google Scholar]
- 42. Brudvik KW, Henjum K, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T-cell-mediated inhibition of antitumor immune responses is associated with clinical outcome in patients with liver metastasis from colorectal cancer. Cancer Immunol Immunother 2012;61:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol 2009;21:1105–1111. [DOI] [PubMed] [Google Scholar]
- 44. Brea D, Agulla J, Rodríguez-Yáñez M, Barral D, Ramos-Cabrer P, Campos F, Almeida A, Dávalos A, Castillo J. Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia. J Cell Mol Med 2014;18:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 2006;113:118–124. [DOI] [PubMed] [Google Scholar]
- 46. Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 2006;12:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoenig MR, Bianchi C, Sellke FW. Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: a unifying hypothesis. Curr Drug Targets 2008;9:422–435. [DOI] [PubMed] [Google Scholar]
- 48. Takahashi R, Amano H, Ito Y, Eshima K, Satoh T, Iwamura M, Nakamura M, Kitasato H, Uematsu S, Raouf J, Jakobsson PJ, Akira S, Majima M. Microsomal prostaglandin E synthase-1 promotes lung metastasis via SDF-1/CXCR4-mediated recruitment of CD11b+Gr1+ MDSCs from marrow. Biomed Pharmacother 2020;121:109581. [DOI] [PubMed] [Google Scholar]
- 49. Kim JA, March K, Chae HD, Johnstone B, Park SJ, Cook T, Merfeld-Clauss S, Broxmeyer HE. Muscle-derived Gr1(dim)CD11b(+) cells enhance neovascularization in an ischemic hind limb mouse model. Blood 2010;116:1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Casós K, Siguero L, Fernández-Figueras MT, León X, Sardá MP, Vila L, Camacho M. Tumor cells induce COX-2 and mPGES-1 expression in microvascular endothelial cells mainly by means of IL-1 receptor activation. Microvasc Res 2011;81:261–268. [DOI] [PubMed] [Google Scholar]
- 51. Maseda D, Banerjee A, Johnson EM, Washington MK, Kim H, Lau KS, Crofford LJ. mPGES-1-mediated production of PGE2 and EP4 receptor sensing regulate T cell colonic inflammation. Front Immunol 2018;9:2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ho ATV, Palla AR, Blake MR, Yucel ND, Wang YX, Magnusson KEG, Holbrook CA, Kraft PE, Delp SL, Blau HM. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc Natl Acad Sci USA 2017;114:6675–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, Daaka Y. PGE2 promotes angiogenesis through EP4 and PKA Cγ pathway. Blood 2011;118:5355–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gigante M, Gesualdo L, Ranieri E. TGF-beta: a master switch in tumor immunity. Curr Pharm Des 2012;18:4126–4134. [DOI] [PubMed] [Google Scholar]
- 55. Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol 2005;174:7592–7599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.