Abstract
Objective:
To determine the effect of sildenafil, a phosphodiesterase type 5 inhibitor, on trophoblast invasiveness.
Design:
Laboratory investigation.
Setting:
Academic medical center.
Patient(s):
Placental tissues discarded after first-trimester terminations were obtained from patients with informed consent.
Intervention(s):
A cell line, HTR-8/SVneo, established from first-trimester cytotrophoblast, and villous explants, was treated with or without sildenafil, guanosine 3′,5′-cyclic monophosphate (cGMP) analog, cGMP inhibitor, or L-NAME (NG-nitro-L-arginine methyl ester hydrochloride) and cultured on fibronectin or Matrigel. Integrins α6β4 and α1β1 were detected by immunocytochemistry.
Main Outcome Measure(s):
Trophoblast outgrowth from villous tips, cytotrophoblast cell invasion, and integrin immunostaining were assessed in cytotrophoblast and explant cultures.
Result(s):
Integrin expression in trophoblast cells ex vivo switched from α6 to α1, and invasiveness increased, when exposed to sildenafil or cGMP agonist. Either cGMP antagonist or L-NAME blocked integrin switching and invasion induced by sildenafil. Elevation of nitric oxide pharmacologically induced invasion, but not when cGMP antagonist was present.
Conclusion(s):
Sildenafil altered trophoblast phenotype through a process dependent on nitric oxide availability and cGMP accumulation. In addition to its vasoactivity, sildenafil directly stimulates trophoblast extravillous differentiation, which would be favorable for implantation and reduce risk for adverse pregnancy outcomes. (Fertil Steril® 2015;103: 1587–95. ©2015 by American Society for Reproductive Medicine.)
Keywords: Trophoblast invasion; phosphodiesterase-5 inhibitor; nitric oxide; guanosine 3′,5′-cyclic monophosphate; integrins
Conversion of the endometrium to a receptive state for embryo implantation requires increased vascular permeability, edema, altered membrane fluidity, and programmed epithelial cell death in response to blastocyst adhesion (1). Production of nitric oxide (NO) and guanosine 3′,5′-cyclic monophosphate (cGMP) at the blastocyst implantation site prepares the endometrium for receptivity (2) by inducing vasodilation, immune function, and inflammation (3, 4). Nitric oxide increases the production of cGMP, which activates vascular permeability, edema, and uterine receptivity (5). When NO production is prevented in the rat, cGMP synthesis is suppressed, reducing receptivity of the endometrium and inhibiting embryonic development (2). In pregnant rats, inhibition of NO can reduce the cGMP concentration and generate characteristics of pre-eclampsia (6, 7), suggesting an additional role in placentation. The experimental evidence supports a role for NO signaling in mouse and human trophoblast differentiation (8–10) and survival (4).
Sildenafil is a phosphodiesterase type-5 (PDE5) inhibitor that is in a class of drugs used since 1989 for erectile dysfunction (11). By inhibiting PDE5, cGMP accumulates to elevate blood flow through smooth muscle relaxation. Downstream of cGMP, sildenafil activates the protein kinase G pathway (12). Phosphodiesterase type-5 inhibitors can correct endothelial dysfunction, reduce risk for cardiovascular complications, prevent adverse pregnancy outcomes, and restore adequate numbers of circulating progenitor cells (13). Phosphodiesterase type-5 messenger RNA is expressed in the uterus and placenta (14). Interestingly, recent studies have shown that sildenafil can be used successfully to correct a thin endometrium and reduce recurrent implantation failure (15, 16), and therapeutically for intrauterine growth restriction (IUGR) and pre-eclampsia (17, 18). Others have refuted the suggested benefits of sildenafil as a treatment for pre-eclampsia (19, 20), but the intervention might have been applied too late to reverse endothelial damage and placental dysfunction. Recent studies have examined therapeutic interventions for the treatment of pre-eclampsia in a high-risk population, based on dietary supplementation with l-arginine in food bars, and antioxidant vitamins (21). Endothelial dysfunction, which is due largely to overproduction of soluble fms-like tyrosine kinase (sFLT1) and soluble endoglin (sENG) (22), and vasoconstriction, were prevented with l-arginine, correcting the dysregulation of nitric oxide synthase known to occur in pre-eclampsia. l-Arginine, a precursor of NO, can reduce blood pressure to prevent pre-eclampsia by increasing NO bioavailability and activating the cGMP pathway (23). Nitric oxide donors with or without antioxidants could also suppress other sequelae associated with the disease, including IUGR and preterm delivery (23).
Migrating first-trimester cytotrophoblast cells switch their expression of integrin isoforms as they differentiate in the decidua from a stationary (α6β4) phenotype to a more invasive (α1β1) state (24). Integrin switching initiates normal endovascular invasion during placentation (24). Aberrant trophoblast invasion and failed integrin switching are associated with adverse pregnancy outcomes, including pre-eclampsia and IUGR. As first trimester trophoblast cells differentiate to the invasive extravillous phenotype, an extravillous trophoblast-specific major histocompatibility complex class Ib protein, HLA-G, also accumulates (25).
The potential therapeutic benefits of sildenafil include alleviation of perinatal disorders, such as pre-eclampsia, IUGR, abruption placenta, miscarriage, and implantation failure, which are all linked to poor trophoblast invasion and failure to remodel the uterine spiral arteries (26). Pre-eclampsia and IUGR are associated with disruption of NO and cGMP production (27, 28). Therefore, we hypothesize a link between positive pregnancy outcomes in infertile patients and the direct effect of sildenafil on trophoblast function. The aims of this study were to [1] investigate the ability of sildenafil to induce invasiveness of a human trophoblast cell line and first-trimester villous explant cultures, [2] determine the effect of sildenafil on integrin switching and extravillous differentiation of trophoblast cells, and [3] investigate signaling pathways that mediate the effects of sildenafil on trophoblast function.
MATERIALS AND METHODS
Cell Culture
The HTR-8/SVneo cytotrophoblast cell line was cultured on plastic in T-75 tissue culture flasks (Corning) in Dulbecco’s Modified Eagle Medium (DMEM) and Ham’s F12 (1:1; DMEM/F12) media (Invitrogen) containing 10% fetal bovine serum in a humidified incubator at 5% CO2. Culture medium was replaced with serum-free medium 24 hours before all experiments, as previously described (25).
Villous Explant Culture
Placental tissues were obtained with Wayne State University institutional review board approval and patient informed consent. Specimens were isolated from first-trimester terminations at a Michigan Family Planning Facility. Fresh tissue was placed on ice in phosphate-buffered saline (PBS) and immediately transported to the laboratory. The chorionic villi were dissected into individual villi or villous clusters and cultured on Millicell-CM inserts (12-mm diameter, 0.4-μm pores; Millipore) precoated with 0.2 mL of undiluted Matrigel in a 24-well culture plate for a total of 72 hours (29, 30). The bottom chamber contained 500 μL DMEM/F12 medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin, and the upper chamber contained approximately 25 μL of medium. Chorionic villi were added with 150 μL of medium to the upper chamber, with or without supplementation with 350 ng/mL sildenafil citrate (Sigma), and the culture was continued for 72 hours. The attached villi were fixed in 4% paraformaldehyde, rinsed three times with PBS, and assessed for outgrowth. Extravillous trophoblast migration was determined for each villous tip from digital images captured using a Leica DM IRB epifluorescence microscope, and a Hamamatsu Orca digital camera. Trophoblast outgrowth length from the villous tip at the point of initial attachment to the distal point of migration was measured using Simple PCI (Hamamatsu) imaging software.
Treatments
Cell or villous explant cultures were treated by supplementing culture medium with 35–3,500 ng/mL (52.5 nM–5.25 μM) sildenafil citrate with or without 10 μM N2,2′-O-dibutyryl-guanosine 3′:5′-cyclic monophosphate (cGMP analog), 10 μM 8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphorothioate, Rp-Isomer (cGMP antagonist), 10 μM (±)-S-nitroso-N-acetylpenicillamine (SNAP; NO donor), 10 μM NG-nitro-L-arginine methyl ester, hydrochloride (L-NAME, NO inhibitor), or the control compound D-NAME (all from Sigma).
Immunocytochemistry
Cytotrophoblasts grown in 96-well plates were fixed in PBS containing 4% paraformaldehyde for 30 minutes and washed three times with PBS. Cells were permeabilized for 10 minutes by incubation in PBS containing 0.1% Triton-X100. Permeabilized cells were labeled with 1 μg/mL of mouse monoclonal antibodies against Ki67 (DAKO), the integrin subunit α1, or α6 (Upstate Biotechnology), and primary antibody controls were 10 μg/mL nonimmune mouse IgG (Jackson ImmunoResearch), all prepared in antibody diluent (DAKO). After incubation with primary antibodies, cells were rinsed three times with PBS. To visualize and quantify antigen, an Envision System peroxidase anti-mouse kit (DAKO) was used. Staining was imaged using a Leica DM IRB epifluorescence microscope, and images were captured using a Hamamatsu Orca digital camera. Images were processed to determine gray level using Simple PCI (Hamamatsu) imaging software, as previously described (31, 32). Values obtained with IgG controls were subtracted from each sample. We have found, using matched cell samples, that measurement of antibody stain intensity by image analysis increases linearly with measurement of the corresponding antigen by ELISA (32). Moreover, quantitative immunocytochemistry is comparable to Western blotting for demonstration of integrin switching when using highly specific antibodies (Supplemental Fig. 1, available online).
Invasion Assay
Cytotrophoblast cells (100,000 per well) were cultured for 72 hours on Matrigel (Collaborative Research) in 6.5-mm transwell inserts (Corning), as previously reported (25). Cells that penetrated the Matrigel and populated the lower chamber were detached using trypsin–ethylenediaminetetraacetic acid solution, fixed, and counted.
Statistical Analysis
All assays were conducted in triplicate, and all experiments were repeated at least three times. Data were analyzed statistically using SPSS version 21.0 (IBM) and are reported as mean ± SEM. Independent-sample t tests were used to determine significance in explant outgrowth and integrin switching studies. One-way ANOVA was used to examine the differences among sildenafil concentrations and the efficacy of inhibitors for cGMP and NO pathways. Means were separated using Tukey’s method following significant ANOVA (P<.05).
RESULTS
Sildenafil Stimulates Trophoblast Outgrowth from Villous Explants
To evaluate the direct effect of sildenafil on human trophoblast cells in first-trimester villous explants, culture medium was supplemented with 0 or 350 ng/mL sildenafil, and trophoblast outgrowth on Matrigel was quantified. All villi exposed to sildenafil produced large outgrowths from their tips, whereas control villi had very small, thinly populated areas of trophoblast outgrowth (Fig. 1A and B). The length of villous tips increased significantly after 72 hours of culture in explants treated with sildenafil (97.2 ± 17.9 vs. 443.2 ± 42.9; P<.05; Fig. 1G). To determine whether the increase was due to proliferation or migration, we labeled nuclei with 6-diamino-2-phenylindole in trophoblast outgrowths and determined cell number (Fig. 1C and D). Cell number was not significantly different between outgrowth areas of nontreated and sildenafil-treated villous explants (385 ± 50 vs. 444 ± 47; P=.46). Expression of the nuclear proliferation protein Ki67 was also unaffected by sildenafil treatment (Fig. 1E and F). When the percentage of Ki67-expressing cells in sildenafil-treated and control cells in the explant outgrowths was compared (Fig. 1H), no significant differences were found (14.7 ± 1.6 vs. 15.2 ± 1.8; P=.82). Trophoblast outgrowth stimulation by sildenafil was apparently due to cell migration away from the villous tip and not proliferation of the migrating cells.
FIGURE 1.
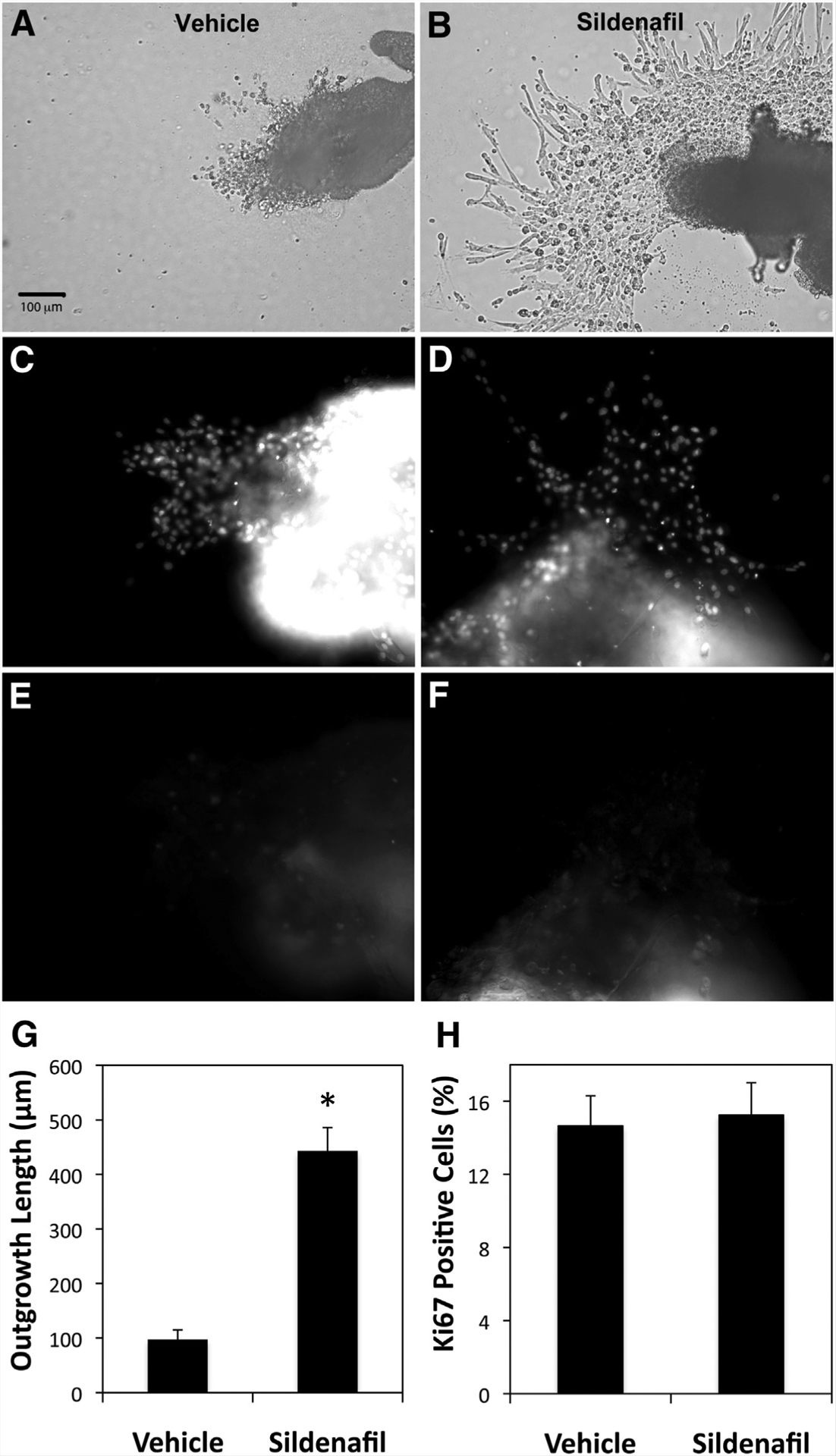
Trophoblast invasion from first-trimester chorionic villous explants. First-trimester villous explants were cultured on Matrigel-coated transwell inserts and treated with vehicle or 350 ng/mL sildenafil for 72 hours before fixation. Examples of outgrowths from villous explants cultured with vehicle (A, C, E) or 350 ng/mL sildenafil (B, E, F) are shown. Bright-field images (A, B) and paired fields labeled with 6-diamino-2-phenylindole (C, D) or anti-Ki67 (E, F) are included. Size bar in (A) applies to all image panels. Outgrowth length and cell proliferation were measured using Simple PCI image analysis software (G, H). The graph in (G) shows outgrowth lengths for triplicate experiments in which 10 villous explants were measured for each treatment. * P<.05 vs. vehicle. The graph in (H) shows the percentage of cell nuclei in the outgrowth regions that were labeled with anti-Ki67 antibody. The differences between vehicle- and sildenafil-treated explants were not significant (n = 8). Error bars represent SEM.
Sildenafil Increases Cell Invasion in Vitro
We examined the sildenafil dose dependency of trophoblast invasion using a Matrigel invasion assay with the human first-trimester cytotrophoblast cell line, HTR-8/SVneo. Sildenafil significantly stimulated (P<.05) trophoblast invasion at or above 350 ng/mL (Fig. 2A). Cytotrophoblast cells cultured with 350 or 3,500 ng/mL sildenafil showed a four-to fivefold increase in invasion, as assessed by penetration through Matrigel.
FIGURE 2.
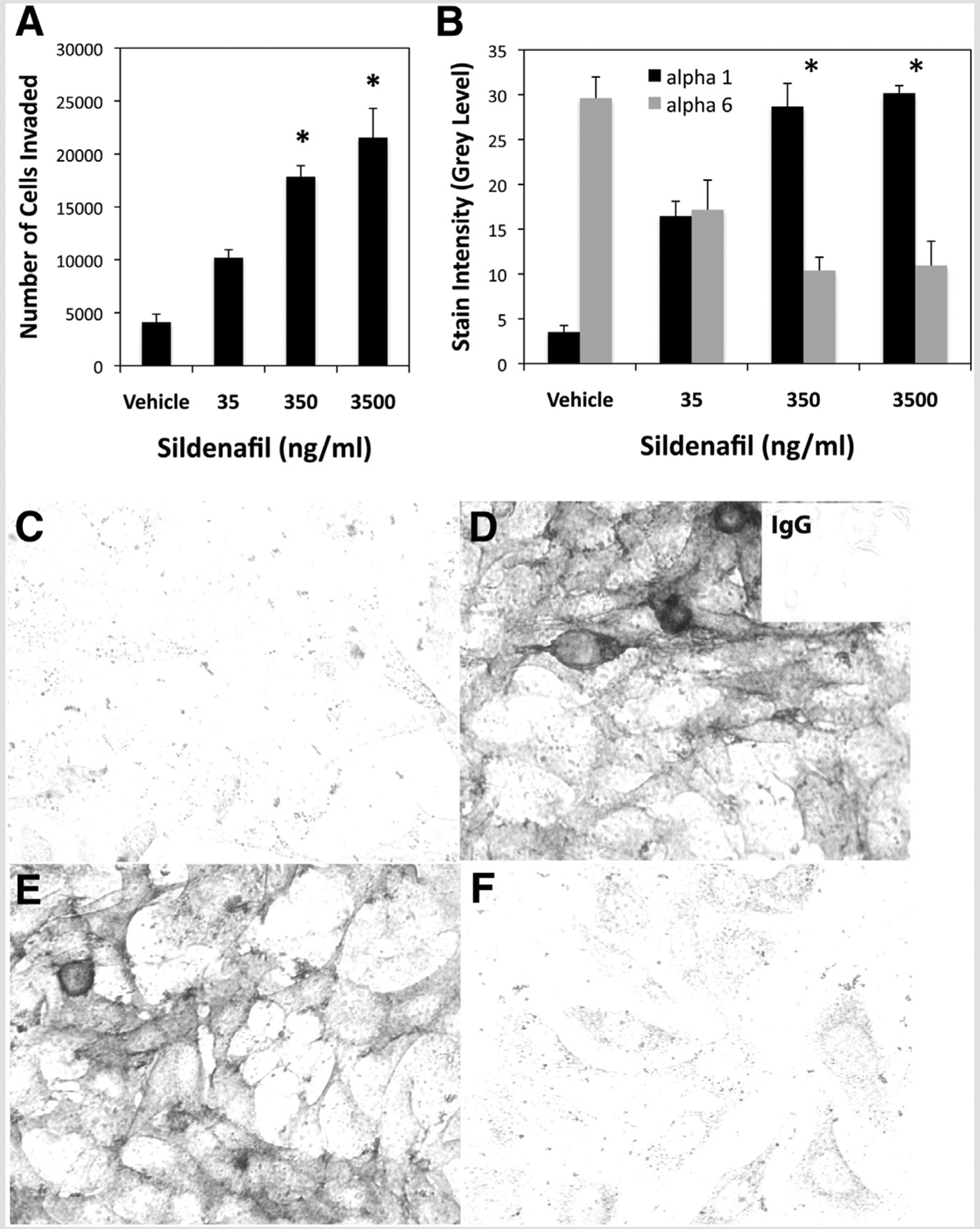
Extravillous trophoblast differentiation and invasion. (A) HTR-8/SVneo cytotrophoblast cells were treated with vehicle or 35–3,500 ng/mL sildenafil for 72 hours during culture on Matrigel-coated transwell inserts. Cells that invaded through the Matrigel were trypsinized from the bottom of the insert, fixed, and counted. (B) Cytotrophoblast cells treated for 24 hours with vehicle or 35–3,500 ng/mL sildenafil were assessed for α1 or α6 integrin subunits by immunocytochemistry and quantitative image analysis. Untreated cells expressed predominantly α6, whereas cells exposed to 35 ng/mL sildenafil showed equivalent α1 and α6 expression. Cells exposed to 350 and 3,500 ng/mL sildenafil demonstrated integrin switching from α6 to α1. * P<.05 vs. vehicle. (C–F) Examples of immunocytochemical staining of α1 (C, E) and α6 (D, F) in confluent cells cultured with vehicle (C, D) or 350 ng/mL sildenafil (E, F). Negative control cells labeled with non-immune IgG are shown in the inset in the upper right corner of (D).
Sildenafil Alters Trophoblast Phenotype
We previously found that HLA-G expression is induced, and integrin expression switches from predominantly α6β4 to α1β1, in HTR-8/SVneo cytotrophoblast cells cultured on Matrigel (25). This is similar to cultures of primary cytotrophoblasts as they become highly invasive, and extravillous trophoblast cells at the base of the anchoring villi in utero, which demonstrate a critical switch in integrin expression from α6β4 to α1β1 (33, 34). Because sildenafil increased trophoblast outgrowth of first-trimester villous explants and invasion of HTR-8/SVneo cells, we determined whether it also induced integrin switching in the cytotrophoblast cell line, comparable to induction by Matrigel (Supplemental Fig. 1). Cytotrophoblast cells cultured without sildenafil expressed high levels of integrin α6 (29.6 ± 2.38), and very low levels of integrin α1 (3.5 ± 0.75), as shown for vehicle treatment in Figure 2B. Cells treated with 35 ng/mL of sildenafil expressed similar levels of both integrins (17.1 ± 3.32 and 16.46 ± 1.67), indicating partial phenotypic change, whereas cells treated with 350 (10.4 ± 1.47 and 28.7 ± 2.59) or 3,500 ng/mL (10.9 ± 2.71 and 30.2 ± 6.84) exhibited a clear switch from integrin α6 to integrin α1 (Fig. 2B and C–F).
Sildenafil Operates through the NO and cGMP Signaling Pathways
Sildenafil inhibits PDE5 to block cGMP turnover; however, the accumulation of cGMP depends on the NO activation of guanylyl cyclase for its synthesis. To examine the involvement of these signaling pathways in sildenafil induction of integrin switching, we evaluated the effects of specific inhibitors and agonists. Both the cGMP analog (N2,2′-O-dibutyrylguanosine 3′:5′-cyclic monophosphate), and the NO donor SNAP induced integrin switching from α6 to α1, similarly to 350 ng/mL sildenafil (Fig. 3A). Moreover, the specific cGMP inhibitor [8-(4-chlorophenylthio)-guanosine 3′,5′-cyclic monophosphorothioate, Rp-Isomer], and the NO antagonist L-NAME each blocked the ability of sildenafil to induce integrin switching in cytotrophoblast cells. As a control for L-NAME, D-NAME did not block the effect of sildenafil. The cGMP inhibitor also blocked integrin switching induced by SNAP, demonstrating that cGMP was the required downstream mediator of NO activity (Fig. 3A). Examples of immunostaining of the α6 and α1 integrin subunits in cells cultured under these various conditions are provided in Supplemental Figure 2.
FIGURE 3.
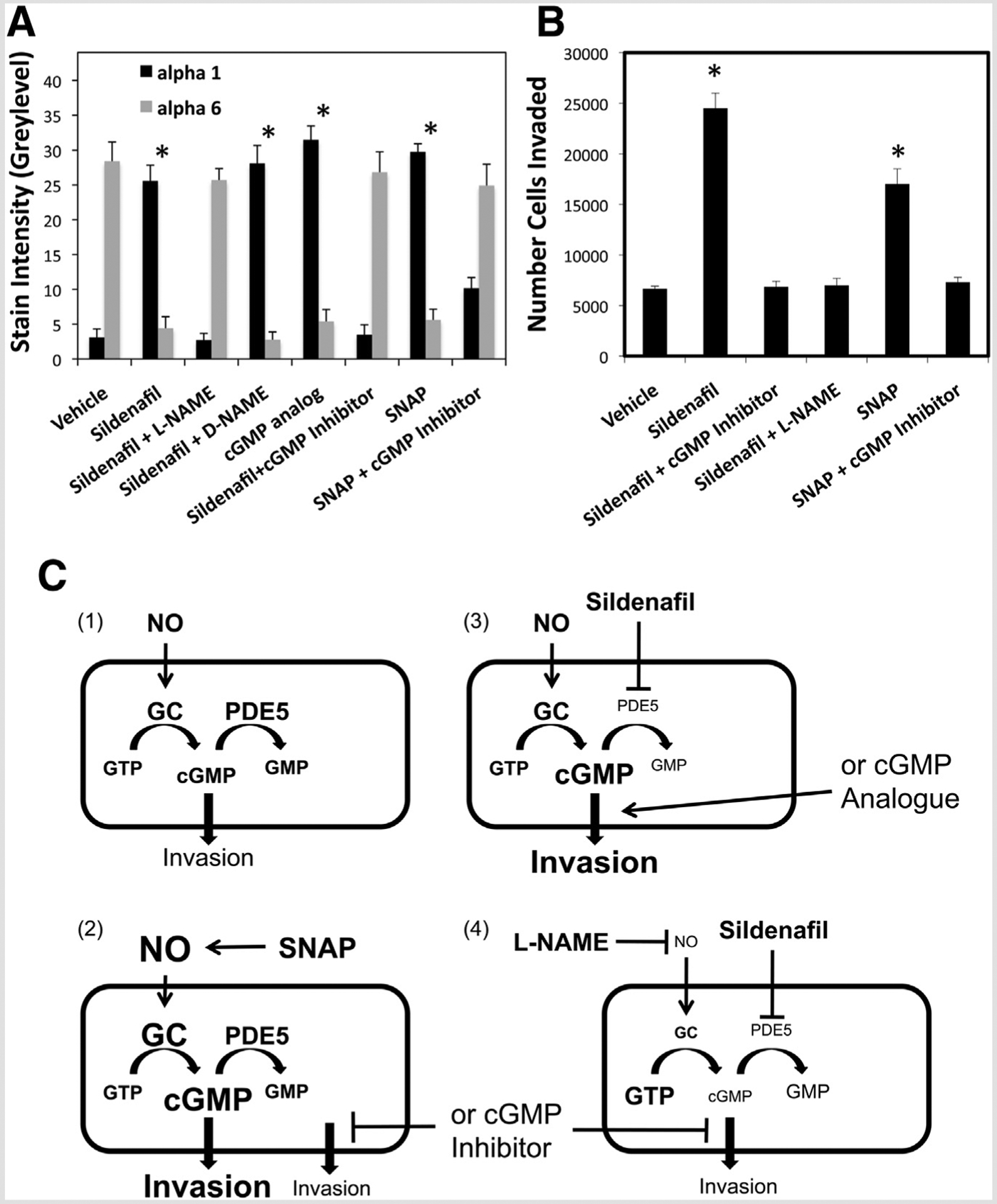
Sildenafil induces trophoblast differentiation through the cGMP and NO signaling pathways. (A) Cytotrophoblast cells were treated during culture for 24 hours with vehicle, 350 ng/mL sildenafil, SNAP, cGMP analogue, or the indicated combinations of sildenafil with L-NAME, D-NAME, or cGMP inhibitor. Expression of the α1 and α6 integrin subunits was determined as in Figure 2B. (B) Invasion was determined as in Figure 2A, for cytotrophoblast cells treated with vehicle, 350 ng/mL sildenafil, or the indicated combinations of sildenafil with cGMP inhibitor or L-NAME. * P<.05 vs. vehicle. (C) Summary of trophoblast invasion mechanisms suggested by the results, assuming the following. [1] Nitric oxide activates guanylyl cyclase (GC) to induce extravillous differentiation, and that PDE5 modulates cGMP levels through conversion to GMP. [2] production of NO by SNAP increases invasion, owing to resulting GC activation and production of cGMP. Inhibition of SNAP-induced invasion by cGMP inhibitor confirms that NO increases invasion specifically through cGMP. [3] Sildenafil inhibition of PGE5 prevents degradation of cGMP, which accumulates to increase invasion, similarly to direct elevation of cGMP signaling by the cGMP analogue. [4] Sildenafil-mediated elevation of cGMP requires continuous NO stimulation of GC and invasion is, thus, repressed by L-NAME inhibition of NO, similarly to inhibition of cGMP signaling by the cGMP inhibitor. Arrows indicate activation, and bars indicate inhibition.
The NO and cGMP signaling pathways were examined with respect to trophoblast invasion using the Matrigel invasion assay. The approximately fourfold increase in invasion induced by sildenafil (P<.05, compared with vehicle) was completely blocked in the presence of either L-NAME or cGMP inhibitor (Fig. 3B). Again, the NO donor SNAP increased invasion above vehicle control, and cGMP inhibitor blocked this effect (Fig. 3B). These findings suggest that sildenafil alters the NO–cGMP signaling axis to convert cytotrophoblasts from a nonmotile, proliferative phenotype to invasive cells required for implantation and proper placentation.
DISCUSSION
This is the first study to demonstrate that sildenafil directly induces trophoblast phenotypic differentiation at the molecular level, indicated by cells switching from α6β4 to α1β1 integrin expression. These changes increased cellular invasive activity in both villous explants and first-trimester cytotrophoblasts. In vitro experiments using the trophoblast cell line demonstrated that both NO and cGMP signaling pathways are critical for integrin switching and stimulation of invasion. The findings strongly support a mechanism (Fig. 3C) in which NO stimulation of guanylyl cyclase activity induces extravillous trophoblast differentiation specifically through downstream cGMP signaling. The potential therapeutic effects of sildenafil arise from its inhibition of PDE5 to block cGMP turnover and produce intracellular cyclic nucleotide accumulation (11). However, NO activation of guanylyl cyclase activity is essential for sildenafil-mediated elevation of cGMP (Fig. 3C). Whereas NO would be the expected initiator of invasive activation of trophoblast cells, cGMP appears to be a requisite downstream mediator of the signal. Potential targets of cGMP that might stimulate trophoblast invasion include protein kinase G, cGMP-gated cation channels, and PDEs (12).
By examining trophoblast cells in the absence of endothelial cells during in vitro culture, evidence was provided that autologous NO production, enhanced by sildenafil, can drive extravillous differentiation. In placental villi, only the syncytiotrophoblast expresses endothelial NO synthase (NOS3), whereas in the decidua, extravillous trophoblast cells also express the enzyme (35). The cellular distribution of NOS3 in cytoplasm is consistent with production of NO for signaling, whereas NOS2 localizes at the front of actively migrating cells, possibly as part of the invasive mechanism (36). Thus, an endogenous NO signaling pathway that regulates extravillous trophoblast differentiation is feasible.
Pre-eclampsia is a complex disorder of pregnancy affecting multiple organ systems that can precipitate serious and deadly complications, including neurologic disorders, liver failure and rupture, pulmonary fluid overload, hemorrhage, stroke, renal complications, and maternal or fetal death (23). It is a major health issue, affecting 2%–8% of all pregnancies, with the ability to cause not only significant maternal morbidity and mortality, but also adverse outcomes in the newborn, including prematurity, IUGR, and fetal death (37). Placentation anomalies are common and central to the manifestations of pre-eclampsia (38). There is a significant paucity of medical interventions for pre-eclampsia and IUGR. Although the exact pathophysiologies are not known, evidence suggests a role for placental abnormalities, including chronic ischemia, vascular endothelial dysfunction, and fibroid necrosis (39). Endothelial dysfunction is found in women with pre-eclampsia and ultimately diminishes NO, as well as cGMP. A detrimental cascade of events can ensue that further decreases trophoblast invasion, leading to inadequate uteroplacental blood flow and growth restriction. At the molecular level, errors in integrin switching accompany a shallow pattern of trophoblast invasion (34) and up to 50% of the invading trophoblast subpopulation in pre-eclamptic placentas is associated with significant programmed cell death (40, 41). Pre-eclamptic patients exhibit high circulating concentrations of sFLT1 and sENG, with a reduction of vascular endothelial growth factor (VEGF) (42, 43). Rapid resolution of clinical symptoms is associated with a reduction in sFLT1 and increase in VEGF.
Sildenafil is a PDE5 inhibitor that potentiates NO downstream signaling by enhancing the accumulation of cGMP through its reduced turnover (23). Nitric oxide is generated in the endothelium, and its production is up-regulated in normal pregnancies. Both NO and cGMP are required for proper placental vasodilation and vascular compliance (44, 45). Improvement in endothelial function has been observed after sildenafil treatment by increasing endothelial progenitor cells, cGMP, growth factors such as VEGF, and matrix metalloproteinase (13, 46, 47). Endothelial progenitor cells develop into mature progenitor cells during repair of endothelial damage and differentiation (48). These cells are involved in the growth, differentiation, remodeling, and repair of vascular endothelial cells throughout the body (49, 50).
Sildenafil has been shown in animal studies to significantly reduce elevated sFLT1 and sENG, increase free VEGF, and reverse oxidative stress (51–53). Sildenafil treatment in pre-eclamptic rat studies has lowered blood pressure, reduced proteinuria, and enhanced placental perfusion (52). Sildenafil has proven to be a palliative remedy in a knockout mouse model of IUGR through a decrease in vascular resistance of the fetal placental unit and increased blood flow in placental ischemia-induced hypertension (54). The use of sildenafil therapeutically for severe IUGR in the human is under investigation (17). In related studies, we found that sildenafil treatment prevents trophoblast apoptosis during oxidative stress (55). Taken together with the present findings, we suggest that sildenafil can improve trophoblast survival and invasion, which are both deficient in uteroplacental insufficiency.
This study suggests potentially useful strategies for intervening in several major categories of pregnancy complications. Adverse pregnancy outcomes, including pre-eclampsia and IUGR, involve trophoblast dysfunction and vascular endothelial anomalies (56). Hypoxic insults arising from trophoblast and endothelial dysfunction can affect placental growth and blood flow to the fetus, resulting in impairment of fetal well-being (38, 56). With an emerging understanding of the mechanism of sildenafil action in the placenta, enthusiasm for its use in treating obstetric disease can be envisioned. The findings of this study demonstrate an important role for NO activation of cGMP in extravillous differentiation of human trophoblast cells. Clinical investigations to assess the risks and benefits of using this PDE5 inhibitor to impede uteroplacental insufficiency are certainly warranted.
Supplementary Material
Acknowledgment:
The authors thank the Northland Family Planning Centers of Michigan for participating in this research study.
This research was supported in part by the Intramural Research Program of The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health grant HD071408, and the W.K. Kellogg Foundation.
Footnotes
J.M.B. has nothing to disclose. B.A.K. has nothing to disclose. A.D.B. has nothing to disclose. M.P.D. has nothing to disclose. M.S. has nothing to disclose. M.H. has nothing to disclose. J.D. has nothing to disclose. D.R.A. has nothing to disclose.
REFERENCES
- 1.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo Implantation. Dev Biol 2000;223:217–37. [DOI] [PubMed] [Google Scholar]
- 2.Duran-Reyes G, Gomez-Melendez MR, Morali-de la Brena G, Mercado-Pichardo E, Medina-Navarro R, Hicks-Gomez JJ. Nitric oxide synthesis inhibition suppresses implantation and decreases cGMP concentration and protein peroxidation. Life Sci 1999;65:2259–68. [DOI] [PubMed] [Google Scholar]
- 3.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–74. [DOI] [PubMed] [Google Scholar]
- 4.Dash PR, Cartwright JE, Baker PN, Johnstone AP, Whitley GS. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic GMP-dependent mechanism and independently of caspase 3 nitrosylation. Exp Cell Res 2003;287:314–24. [DOI] [PubMed] [Google Scholar]
- 5.Vilar-Rojas C, Castro-Osuna G, Hicks JJ. Cyclic AMP and cyclic GMP in the implantation site of the rat. Int J Fertil 1982;27:56–9. [PubMed] [Google Scholar]
- 6.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest 1992;90:278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhimschi I, Yallampalli C, Dong YL, Garfield RE. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol 1995;172: 1577–84. [DOI] [PubMed] [Google Scholar]
- 8.Gagioti S, Scavone C, Bevilacqua E. Participation of the mouse implanting trophoblast in nitric oxide production during pregnancy. Biol Reprod 2000;62:260–8. [DOI] [PubMed] [Google Scholar]
- 9.Lyall F, Jablonka-Shariff A, Johnson RD, Olson LM, Nelson DM. Gene expression of nitric oxide synthase in cultured human term placental trophoblast during in vitro differentiation. Placenta 1998;19:253–60. [DOI] [PubMed] [Google Scholar]
- 10.Cartwright JE, Holden DP, Whitley GS. Hepatocyte growth factor regulates human trophoblast motility and invasion: a role for nitric oxide. Br J Pharmacol 1999;128:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravipati G, McClung JA, Aronow WS, Peterson SJ, Frishman WH. Type 5 phosphodiesterase inhibitors in the treatment of erectile dysfunction and cardiovascular disease. Cardiol Rev 2007;15:76–86. [DOI] [PubMed] [Google Scholar]
- 12.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 2010;62:525–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Vinanzi C, et al. Relationship between vascular damage degrees and endothelial progenitor cells in patients with erectile dysfunction: effect of vardenafil administration and PDE5 expression in the bone marrow. Eur Urol 2007;51: 1411–7. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 14.Coppage KH, Sun X, Baker RS, Clark KE. Expression of phosphodiesterase 5 in maternal and fetal sheep. Am J Obstet Gynecol 2005;193:1005–10. [DOI] [PubMed] [Google Scholar]
- 15.Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril 2010;93: 1851–8. [DOI] [PubMed] [Google Scholar]
- 16.Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril 2002;78:1073–6. [DOI] [PubMed] [Google Scholar]
- 17.von Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, et al. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG 2011;118:624–8. [DOI] [PubMed] [Google Scholar]
- 18.Herraiz S, Pellicer B, Serra V, Cauli O, Cortijo J, Felipo V, et al. Sildenafil citrate improves perinatal outcome in fetuses from pre-eclamptic rats. BJOG 2012; 119:1394–402. [DOI] [PubMed] [Google Scholar]
- 19.Samangaya RA, Wareing M, Skillern L, Baker PN. Phosphodiesterase inhibitor effect on small artery function in preeclampsia. Hypertens Pregnancy 2011;30:144–52. [DOI] [PubMed] [Google Scholar]
- 20.Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy 2009;28:369–82. [DOI] [PubMed] [Google Scholar]
- 21.Vadillo-Ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ 2011;342:d2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–9. [DOI] [PubMed] [Google Scholar]
- 23.Johal T, Lees CC, Everett TR, Wilkinson IB. The nitric oxide pathway and possible therapeutic options in pre-eclampsia. Br J Clin Pharmacol 2014; 78:244–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest 1997;99: 2139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR. Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod 2000;62:739–47. [DOI] [PubMed] [Google Scholar]
- 26.Kovo M, Schreiber L, Bar J. Placental vascular pathology as a mechanism of disease in pregnancy complications. Thromb Res 2013;131(Suppl 1): S18–21. [DOI] [PubMed] [Google Scholar]
- 27.Rutherford RA, McCarthy A, Sullivan MH, Elder MG, Polak JM, Wharton J. Nitric oxide synthase in human placenta and umbilical cord from normal, intrauterine growth-retarded and pre-eclamptic pregnancies. Br J Pharmacol 1995;116:3099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pettersson A, Hedner T, Milsom I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet Gynecol Scand 1998;77: 808–13. [PubMed] [Google Scholar]
- 29.Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology 1997;138:4977–88. [DOI] [PubMed] [Google Scholar]
- 30.Caniggia I, Lye SJ, Cross JC. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 1997;138:3976–86. [DOI] [PubMed] [Google Scholar]
- 31.Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn B, Das SK, et al. Pre-eclampsia and expression of heparin-binding EGF-like growth factor. Lancet 2002;360:1215–9. [DOI] [PubMed] [Google Scholar]
- 32.Leach RE, Jessmon P, Coutifaris C, Kruger M, Myers ER, Ali-Fehmi R, et al. High throughput, cell type-specific analysis of key proteins in human endometrial biopsies of women from fertile and infertile couples. Hum Reprod 2012;27:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol 1995;154:3771–8. [PubMed] [Google Scholar]
- 34.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. Integrin switching regulates normal trophoblast invasion. Development 1994;120:3657–66. [DOI] [PubMed] [Google Scholar]
- 35.Martin D, Conrad KP. Expression of endothelial nitric oxide synthase by extravillous trophoblast cells in the human placenta. Placenta 2000;21: 23–31. [DOI] [PubMed] [Google Scholar]
- 36.Harris LK, McCormick J, Cartwright JE, Whitley GS, Dash PR. S-nitrosylation of proteins at the leading edge of migrating trophoblasts by inducible nitric oxide synthase promotes trophoblast invasion. Exp Cell Res 2008;314: 1765–76. [DOI] [PubMed] [Google Scholar]
- 37.Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag 2011;7: 467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension 2005; 46:1243–9. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DJ. Placental pathology, a survival guide. Arch Pathol Lab Med 2008;132:641–51. [DOI] [PubMed] [Google Scholar]
- 40.DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with wide-spread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol 1999;155:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol 2000;96:271–6. [DOI] [PubMed] [Google Scholar]
- 42.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karumanchi SA, Epstein FH. Placental ischemia and soluble fms-like tyrosine kinase 1: cause or consequence of preeclampsia? Kidney Int 2007; 71:959–61. [DOI] [PubMed] [Google Scholar]
- 44.Khalil RA, Crews JK, Novak J, Kassab S, Granger JP. Enhanced vascular reactivity during inhibition of nitric oxide synthesis in pregnant rats. Hypertension 1998;31:1065–9. [DOI] [PubMed] [Google Scholar]
- 45.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol 2013;305:R397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996;382:833–5. [DOI] [PubMed] [Google Scholar]
- 47.Foresta C, Lana A, Cabrelle A, Ferigo M, Caretta N, Garolla A, et al. PDE-5 inhibitor, vardenafil, increases circulating progenitor cells in humans. Int J Impot Res 2005;17:377–80. [DOI] [PubMed] [Google Scholar]
- 48.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res 2008;58:148–51. [DOI] [PubMed] [Google Scholar]
- 49.Foresta C, De Toni L, Ferlin A, Di Mambro A. Clinical implication of endothelial progenitor cells. Expert Rev Mol Diagn 2010;10:89–105. [DOI] [PubMed] [Google Scholar]
- 50.Aranguren XL, Luttun A, Clavel C, Moreno C, Abizanda G, Barajas MA, et al. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood 2007;109:2634–42. [DOI] [PubMed] [Google Scholar]
- 51.Biyiksiz PC, Filiz S, Vural B. Is sildenafil citrate affect endometrial receptivity? An immunohistochemical study. Gynecol Endocrinol 2011;27:767–74. [DOI] [PubMed] [Google Scholar]
- 52.Ramesar SV, Mackraj I, Gathiram P, Moodley J. Sildenafil citrate decreases sFlt-1 and sEng in pregnant l-NAME treated Sprague-Dawley rats. Eur J Obstet Gynecol Reprod Biol 2011;157:136–40. [DOI] [PubMed] [Google Scholar]
- 53.Koka S, Das A, Salloum FN, Kukreja RC. Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med 2013;60:80–8. [DOI] [PubMed] [Google Scholar]
- 54.Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, et al. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension 2012;59:1021–8. [DOI] [PubMed] [Google Scholar]
- 55.Bolnick JM, Kilburn BA, Bolnick AD, Diamond MP, Singh M, Hertz M, et al. Sildenafil Prevents Apoptosis of Human First-Trimester Trophoblast Cells Exposed to Oxidative Stress: Possible Role for Nitric Oxide Activation of 3’,5’-cyclic Guanosine Monophosphate Signaling. Reprod Sci 2014; manuscript in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592–4. [DOI] [PubMed] [Google Scholar]
- 57.Rout UK, Wang J, Paria BC, Armant DR. alpha5beta1, alphaVbeta3 and the platelet-associated integrin alphaIIbbeta3 coordinately regulate adhesion and migration of differentiating mouse trophoblast cells. Dev Biol 2004; 268:135–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


