ABSTRACT
Non-muscle myosin 2 (NM2) motors are the major contractile machines in most cell types. Unsurprisingly, these ubiquitously expressed actin-based motors power a plethora of subcellular, cellular and multicellular processes. In this Cell Science at a Glance article and the accompanying poster, we review the biochemical properties and mechanisms of regulation of this myosin. We highlight the central role of NM2 in multiple fundamental cellular processes, which include cell migration, cytokinesis, epithelial barrier function and tissue morphogenesis. In addition, we highlight recent studies using advanced imaging technologies that have revealed aspects of NM2 assembly hitherto inaccessible. This article will hopefully appeal to both cytoskeletal enthusiasts and investigators from outside the cytoskeleton field who have interests in one of the many basic cellular processes requiring actomyosin force production.
Keywords: Actin, Contractility, Myosin
Summary: Non-muscle myosin 2 functions broadly as the dominant contractile motor protein in most cells.
Introduction
Experimentation in most, if not all, eukaryotic model organisms has revealed critical roles for non-muscle myosin 2 (NM2) motors in numerous fundamental and specialized cellular processes (note: for simplicity, we use ‘NM2’ throughout, despite discussing key research from species without muscles, where the term ‘non-muscle’ does not apply). Common to most of these NM2-based cellular processes is the ability of the myosin to produce contractile force in cooperation with actin filaments. To accomplish this, NM2 assembles into small bipolar filaments with motor domains at both ends. When these motors engage actin filaments of opposing orientation, they drive the sliding of these actin filaments past each other, resulting in contraction (as in the muscle sarcomere). The beauty of this seemingly simple process is that cells can regulate in space and time where contraction occurs by regulating where and when NM2 monomers are assembled into filaments. As cells and multicellular systems evolved complexity, the contexts in which NM2-based force production functions also multiplied. Here, we briefly summarize decades of NM2 research, highlighting recent advances in the field that have answered existing questions and posed new ones.
Biochemistry and structure
Mechanochemical cycle
The motor activity of myosins involves binding to F-actin, hydrolysis of ATP stimulated by actin binding, and a resulting power stroke. Collectively, this process is referred to as the myosin mechanochemical cycle or the cross-bridge cycle. Characteristics of this cycle have been investigated extensively and reviewed previously for myosins in general and for NM2 motors in particular (De La Cruz and Ostap, 2004; Heissler and Sellers, 2016). In simplest form (see poster), myosins start their ATPase cycle in a nucleotide-free state bound strongly to actin (actin-bound myosin, AM). Upon binding of ATP (to give AM-ATP), myosin enters a weak actin-binding state (in which the myosin can detach from actin, M-ATP) that persists following the hydrolysis of ATP to bound ADP-Pi (as either AM-ADP-Pi or M-ADP-Pi). Subsequent Pi release is coupled to the myosin undergoing its power stroke on actin and generation of the strong actin-binding ADP-bound myosin (AM-ADP). Finally, ADP release regenerates the initial nucleotide-free myosin. The NM2 mechanochemical cycle can be stalled in the weak actin-binding ADP-Pi-bound state by the cell-permeant small molecule blebbistatin and its derivatives (Képiró et al., 2014; Straight et al., 2003; Várkuti et al., 2016).
The kinetics of each step in this cycle can be tuned to drive specificity and function. For instance, the fraction of the total mechanochemical cycle spent in the strong actin-binding state is termed the ‘duty ratio’. Processive myosins that ‘walk’ along filamentous actin (such as myosin V and myosin VI) display a high duty ratio, whereas muscle myosins display low duty ratios, avoiding drag on the system. Unsurprisingly, two of the three mammalian NM2 isoforms (NM2A and NM2C) have duty ratios that are similar to muscle myosin (∼5–10%) (Harris and Warshaw, 1993; Uyeda et al., 1990). However, NM2B has a higher duty ratio (∼25–40%) (Pato et al., 1996; Wang et al., 2003), potentially tuning it for tensile force production. In vitro, NM2B, and to a lesser extent NM2A, can move processively on actin (Melli et al., 2018) despite each individual monomer being a non-processive motor. Finally, resistive loads on myosins may dramatically alter equilibrium constants measured in solution (Kovacs et al., 2007). This range and tunability in duty ratio may prove functionally critical, enabling low duty ratio NM2 to generate rapid contractile forces and high duty ratio NM2 to build tension.
Structures
NM2 is a hexamer consisting of two ∼200 kDa myosin heavy chains (MHCs), two ∼17 kDa essential light chains (ELCs) and two ∼20 kDa regulatory light chains (RLCs) (see poster). This hexamer is referred to as a ‘monomer’ because it self-associates into polymers or filaments. The vast majority of NM2-dependent cellular processes require filaments (for exceptions see Laplante et al., 2016; Shutova et al., 2014). Each MHC consists of (1) the N-terminal motor domain, (2) a short neck region, (3) a long parallel α-helix that dimerizes into a coiled coil and (4) a C-terminal, non-helical tailpiece (Brito and Sousa, 2020).
NM2 exists in a dynamic equilibrium between monomers and filaments. There are two monomer conformations: a folded, autoinhibited ‘10S’ conformation that is mechanically and enzymatically silent, and an extended ‘6S’ conformation that is assembly competent and capable of being activated by F-actin (Suzuki et al., 1978; Trybus et al., 1982) (see poster). Examples of the 10S conformation have been found in evolutionarily distant species (Jung et al., 2008a), suggesting that folding of NM2 plays a central role in controlling its cellular functions. The 10S conformation has two key structural components (Jung et al., 2008b; Trybus et al., 1982; Yang et al., 2020). First, the interacting heads motif (IHM) involves the motor domains folding back onto the N terminus of the coiled-coil tail and asymmetric docking of one head onto the other head (Wendt et al., 1999). This limits actin binding and nucleotide exchange, essentially locking the myosin in an inactive state (Cross et al., 1988). Second, the tail bends twice to wrap around the IHM. Regulating conversion of the 10S conformation to the 6S conformation, accomplished by phosphorylation of conserved residues on the RLCs, is the nexus for regulating NM2 function in cells (Craig et al., 1983; Kendrick-Jones et al., 1987).
Assembly and regulation
Filament assembly
NM2 coiled coils display the traditional heptad repeat, as well as a 28-amino-acid repeat with alternating charge distributions that enable the staggering of tails as they assemble into the bipolar filament (McLachlan and Karn, 1982). In mammalian NM2, the heptad repeat is imperfect, and includes three ‘extra’ amino acids, or ‘skip’ residues. Two of these are located near the bend positions in the tail and potentially destabilize the coiled coil to allow bending into the 10S conformation (Burgess et al., 2007). NM2 tail domains self-associate in both parallel and antiparallel fashion to create ∼300 nm-long bipolar filaments with clusters of motors at opposing ends and the overlapping tail domains in the middle. Once assembled, the motors at opposing ends of the filament engage antiparallel actin filaments and hydrolyze ATP to produce contractile events.
Depending on species and isoform, ∼15–30 NM2 monomers self-associate into the bipolar filament (Billington et al., 2013; Niederman and Pollard, 1975). Assembly might include dimer and tetramer intermediates (Liu et al., 2017; Sinard et al., 1989) and is aided by filamentous actin (Applegate and Pardee, 1992). Cell-based fluorescence recovery after photobleaching (FRAP) experiments demonstrate that monomers exchange into and out of established filaments with half-lives on the order of tens of seconds (Sandquist and Means, 2008). This flux appears to be critical for NM2 function in cells, as mutants that lock NM2 into an assembled state often fail to fully rescue NM2 loss-of-function phenotypes (Beach and Egelhoff, 2009; Egelhoff et al., 1993).
The basic functional model is that folded 10S monomers are free to diffuse throughout the cell until a spatially restricted signaling event driving RLC phosphorylation produces 6S monomers, which then readily self-associate to form bipolar filaments and drive contractile events (see poster). While this model represents a fundamental aspect of NM2 biology, recent live-cell imaging studies have provided mechanistic insight into the assembly of higher-order NM2 structures in cells. Specifically, live-cell structured-illumination microscopy (SIM) has revealed nascent NM2 filaments appearing and then undergoing a partitioning process to produce NM2 clusters (Beach et al., 2017). Similar clusters are observed in fixed imaging of contractile ring formation (Henson et al., 2017), and other studies have used high-resolution imaging to observe the expansion of initial filaments into sarcomere-like stacks (Fenix et al., 2016). Individual NM2 filaments and small clusters are typically seen in regions with a high ratio of filamentous actin to filamentous NM2. As dynamic partitioning and expansion produce more NM2 filaments and the density increases, crosslinking (in collaboration with other actin-binding proteins) and force production drive the higher-order acto-NM2 organization. Notably, in silico experiments have become powerful tools to explore principles of actomyosin self-organization (Linsmeier et al., 2016; Rubinstein and Mogilner, 2017).
Regulation of filament assembly and motor activity
RLC phosphorylation activates NM2 by disrupting key intramolecular interactions that stabilize the 10S conformation. Functionally, this phosphorylation serves two purposes. First, it undocks the motor domains in the IHM to allow actin binding, nucleotide exchange and the resumption of the mechanochemical cycle. Second, it shifts the equilibrium towards the extended 6S monomer, thereby promoting filament assembly (Cremo et al., 1995; Ikebe and Hartshorne, 1985).
The efficacy of RLC phosphorylation on NM2 places the kinases and phosphatase front and center in controlling NM2 function. Two kinases have a dominant role: RhoA-activated Rho-associated coiled-coil kinases (ROCKs) and Ca2+/calmodulin-activated myosin light chain kinases (MLCKs) (Adelstein and Klee, 1981; Amano et al., 1996). ROCK kinases also inactivate myosin phosphatases (Kimura et al., 1996), thereby enhancing NM2 activity. Thus, two paramount signaling pathways (RhoA and Ca2+) are funneled to actomyosin through RLC phosphorylation.
Phosphorylation of other residues near the N terminus of the RLC by protein kinase C (at Ser1, Ser2 and Thr9, and possibly at Thr7 and Thr10) all appear to inhibit the activation of NM2 by reducing its actin-activated ATPase activity or by inhibiting the interaction between the RLC and activating kinases, such as MLCKs (Naka et al., 1983; Nishikawa et al., 1983). This inhibitory RLC phosphorylation is critical for mesenchymal chemotactic migration by preventing NM2 activation at the leading edge and enabling asymmetric force production (Asokan et al., 2014), similar to other leading-edge myosin inhibitory pathways (Steimle et al., 2001).
Perhaps because RLC phosphorylation potently promotes the assembly and activation of mammalian NM2 isoforms, regulation of NM2 by MHC phosphorylation has not received as much attention, despite being known for decades (Muhlrad and Oplatka, 1977). In single-cell organisms, such as Acanthamoeba and Dictyostelium, MHC rather than RLC phosphorylation is the primary modulator of assembly, with phosphorylation of the tail domains inhibiting rather than promoting filament assembly (Egelhoff et al., 1993; Liu et al., 2013; Redowicz et al., 1994). Similarly, in mammalian systems, in vitro (Dulyaninova et al., 2005) and cellular studies (Dulyaninova et al., 2007) suggest that tail phosphorylation also shifts the equilibrium towards the monomeric form. However, in contrast to this inhibitory model, MHC phosphorylation might enhance the activity of mammalian NM2 by promoting filament recycling in vivo, providing a source of monomers for nascent filament assembly (Breckenridge et al., 2009). Interestingly, despite these studies of divergent species, experimentation in other model systems (including yeast, worms and flies) has been lacking and should be explored. Importantly, the kinases implicated in MHC phosphorylation (e.g. protein kinase C) further diversify the modulatory inputs acting on NM2 beyond the key RLC phosphorylation pathways, potentially enabling involvement and modulation in a broader range of cellular processes (Clark et al., 2008; Even-Faitelson and Ravid, 2006; Kelley et al., 1991).
Interactors and diversity
Binding partners
Numerous binding partners have been suggested for NM2 (see poster), but many of them have not been carefully verified for direct interactions using purified components. An exception is S100A4 (also known as metastasin), which is a protein upregulated and involved in metastatic and invasive cells (Stewart et al., 2016). Significant biochemical and structural studies support a model in which S100A4 binds to the C terminus of the coiled coil in a Ca2+-dependent manner (potentially binding a single MHC) and disrupts coiled-coil formation and filament assembly (Badyal et al., 2011; Dulyaninova et al., 2005; Ramagopal et al., 2013). Although S100A4 appears to be specific for the NM2A isoform, the extensive and diverse S100 family of proteins may provide unique opportunities for isoform-specific and tissue-specific NM2 regulation (Du et al., 2012).
Isoforms and paralogs
Whereas Dictyostelium, budding yeast, flies and worms possess a single gene encoding NM2 MHC (De Lozanne et al., 1985; Kiehart et al., 1989), fission yeast, zebrafish and other organisms possess multiple copies. Mammals possess three MHC genes (MYH9, MYH10 and MYH14) that produce three NM2 isoforms (NM2A, NM2B and NM2C, respectively) (Leal et al., 2003; Simons et al., 1991; Toothaker et al., 1991). Although these isoforms are differentially expressed in cells and tissues (Otterpohl et al., 2017; Wang et al., 2010), most cells express multiple isoforms. Knockout or replacement studies in mice have demonstrated both unique and redundant roles for these three NM2 isoforms during development (Wang et al., 2011). Recent imaging (Beach et al., 2014) and electron microscopy (EM) (Shutova et al., 2014) studies have shown that NM2A, NM2B and NM2C co-assemble to form heterotypic filaments (see poster). In addition, myosin 18A (MYO18A) has recently been demonstrated to co-assemble with NM2 to make mixed filaments (Billington et al., 2015). MYO18A has many splice variants and protein–protein interaction domains, and is expressed from flies to humans but lacks motor activity (Guzik-Lendrum et al., 2013), possibly potentiating the interaction potential for mixed NM2 filaments. Therefore, cells are not limited to three simple NM2 filament types but rather can form a vast range of filament compositions depending on the isoforms they express. Given that NM2A, NM2B and NM2C (and their splice variants) possess significant differences in enzymatic properties and filament stabilities (Golomb et al., 2004; Kim et al., 2008; Pato et al., 1996; Wang et al., 2003), co-assembly should serve to greatly expand the biophysical, functional and interaction potential of NM2.
Cellular functions
Given the enormous diversity of cellular functions supported by NM2 motors, we cannot discuss all of them here. Instead, we concentrate on processes where NM2 has been extensively investigated.
Cell migration
Cortical actomyosin is a determinant in defining cell shape and transforming cell shape throughout biology. Cell migration provides an intimate example of this. The classic, mesenchymal mode of cell migration involves protrusion of the cell leading edge, establishment of integrin-based adhesions with the substratum, transport of the cell body and nucleus forward and dissolution of posterior adhesions. The force associated with actin retrograde flow alone appears sufficient to open integrins and maintain nascent adhesions and focal contacts independently of NM2 (Choi et al., 2008; Galbraith et al., 2007). NM2 filaments are largely absent from leading-edge lamellipodia where focal contacts are found (Asokan et al., 2014), and they begin to accumulate just behind the lamellipodium in anterior lamella (Verkhovsky and Borisy, 1993), where focal adhesions also begin to form (Wilkinson et al., 2005). As these NM2 filaments move rearward with actin retrograde flow, they coalesce into larger actomyosin structures, such as ventral stress fibers and transverse arcs (Burnette et al., 2014). Importantly, the forces generated by these larger acto-NM2 structures are required for the maturation of focal contacts into focal adhesions, and for the maintenance of focal adhesions (Choi et al., 2008) (see poster). A major mechanism for this is that several key proteins present within focal adhesions (e.g. talins and vinculin) are mechanosensitive and require large forces to become fully active (del Rio et al., 2009; Kuo et al., 2011; Zhou et al., 2017).
In addition to its roles in the anterior of migrating cells, NM2 has important roles at the rear, sides and center of cells. Many of these functions can be extended to other migration modes, such as amoeboid migration. Most polarized cells display a concentration gradient of NM2 filaments, with the filaments enriched towards the rear, driven by actin retrograde flow (Kolega and Taylor, 1993). Enrichment of these posterior structures promotes contraction to drive forward locomotion (Guo and Wang, 2012). Cortical actomyosin at the sides of migrating cells prevents lateral protrusions, enhancing polarity and directional persistence (Doyle et al., 2009; Lo et al., 2004). NM2 in the cell center (in part associated with subnuclear stress fibers) plays a role in nuclear positioning (Gomes et al., 2005; Luxton et al., 2010) and in translocating the nucleus forward (Rujano et al., 2013; Schenk et al., 2009). Although this can be seen in two-dimensional settings (Guo and Wang, 2012), it is especially relevant in three-dimensional and in vivo settings, where cells require more contractile force to squeeze the nucleus through tight spaces (Lämmermann et al., 2008; Thomas et al., 2015). Finally, cells must disassemble their posterior adhesions to enable forward movement (Guo and Wang, 2012). While a role for NM2 in the disassembly of posterior adhesions appears to be at odds with its role in the assembly of anterior adhesions, inhibition of NM2 activity frequently results in a dramatic elongation of the cell posterior due to an inability to release from the substratum.
Cell division
Assembly of the actomyosin contractile ring (CR) following metaphase–anaphase transition is a prerequisite for reliable, efficient cell division in nearly all eukaryotes (De Lozanne and Spudich, 1987; Lord et al., 2005; Straight et al., 2003). In combination with membrane deposition (Dyer et al., 2007) and actin depolymerization (Mendes Pinto et al., 2012), NM2-based contraction is the principal mechanism for physically separating daughter cells (Mabuchi and Okuno, 1977; Straight et al., 2003) (see poster). To drive cell division, the actomyosin CR must assemble, constrict and disassemble with precise spatiotemporal fidelity. Early experimentation (Rappaport, 1961; Wolpert, 1961) showed that signals emanating from the midzone region between the separating chromatids establish CR formation. We now know that the molecular orchestrator of this process is the highly conserved centralspindlin signaling pathway (Yüce et al., 2005). Centralspindlin, a heterotetramer of the kinesin MKLP1 (also known as KIF23) and CYK4 (also known as RACGAP1), localizes to the plus ends of midzone microtubules (Raich et al., 1998) and recruits the RhoGEF ECT2. This leads to RhoA-mediated activation of ROCKs and citron kinase (Madaule et al., 1998) to activate NM2 via RLC phosphorylation (Yamashiro et al., 2003). In addition, RhoA recruits and activates formins, such as mDia1 (also known as DIAPH1), to nucleate actin filaments (Tolliday et al., 2002). In so doing, centralspindlin signaling drives the coordinated assembly of the two main CR components and appears to be the dominant conserved pathway for building a CR.
Several proteins that localize to the furrow also interact with NM2, including supervillin (Chen et al., 2003), anillin (Straight et al., 2005) and septins (Joo et al., 2007). Anillin is a highly conserved cell division scaffold (Sohrmann et al., 1996; Straight et al., 2005). In fission yeast, anillin is an early component of CR nodes and is critical for downstream recruitment of NM2 and signaling components (Akamatsu et al., 2014; Sun et al., 2015). Although anillin is not required to initiate CR assembly or for the initial recruitment of NM2 in mammals, its loss results in the furrow undergoing lateral oscillations (Hickson and O'Farrell, 2008; Piekny and Glotzer, 2008) or being mispositioned (Maddox et al., 2007). Septins are also a highly conserved contributor to cell division. In budding yeast, they are thought to recruit NM2 monomers to specialized nodes with the adaptor protein Bni5 (Fang et al., 2010). In mammalian cells, septins might bind directly to NM2 tail domains to stabilize NM2 filaments at the furrow (Joo et al., 2007).
Finally, actomyosin networks can move laterally along the cortex towards the midzone via cortical flow (Cao and Wang, 1990). Cortical flow is a principal component of the ‘polar relaxation’ model, which posits that inhibitory cues, delivered via astral microtubules, relax cortical tension at the poles. This creates a tension gradient where midzone actomyosin contraction drives both CR ingression and pulls additional cortical material inward (Khaliullin et al., 2018) aided by polar membrane deposition (Gudejko et al., 2012) (see poster).
Cell–cell contacts
Another area of active research revolves around NM2 function at cell–cell contacts within epithelial and endothelial sheets. NM2 supports a wide range of processes in these sheets, including cell extrusion (Rosenblatt et al., 2001), convergent extension (Simões et al., 2014) and epithelial–mesenchymal transitions (Beach and Egelhoff, 2009). Here, we briefly explore the roles of NM2 in steady-state epithelial junctions and in dynamic remodeling during apical constriction.
NM2 filaments are abundant in the actin-rich apicolateral domain of polarized epithelia, where they are typically oriented parallel to the cell–cell contact and sometimes in near-perfect register across the contact (Ebrahim et al., 2013) (see poster). NM2 associates with the two major junctional complexes present in polarized epithelia: tight junctions (TJs) and adherens junctions (AJs). TJs contain claudin and occludin transmembrane proteins and contribute to barrier function, whereas AJs contain the cadherin transmembrane proteins that engage the cytoskeleton through α-catenins, β-catenin (also known as CTNNB1), vinculin and others (Yamada et al., 2005) to mechanically couple neighboring cells. Although NM2 contributions at junctional complexes are still being revealed (Conti et al., 2004; Naydenov et al., 2016; Smutny et al., 2010), both barrier function and cell–cell mechanical coupling can be attenuated in the absence of NM2. As in focal adhesions, some of the adaptor proteins present at TJs and AJs are mechanosensitive and require tension for full activation (Yonemura et al., 2010). It is likely that NM2-based contractility contributes to generating this tension, but this does not exclude roles for myosin-mediated actin crosslinking. NM2 filaments can also be found at the basal domain (Santa-Cruz Mateos et al., 2020), where they likely contribute to integrin-dependent cell–matrix adhesion as described above.
Mechanotransduction
The central role that actomyosin plays in the generation of forces within cells, as well as in the ability of cells to sense these forces (mechanosensation) and convert mechanical information into biochemical and electrochemical information (mechanotransduction), places NM2 at the crux of mechanics across cell biology, as we briefly summarize here.
One mechanism involving NM2 likely operates through focal adhesion maturation and integrin signaling (Kuo et al., 2011). Although many consequences of integrin activation are realized immediately in membrane-proximal regions, significant signaling materializes over longer distances and times, especially at the transcriptional and/or translational level. The exact pathways leading to these longer-term impacts might vary depending on the extracellular matrix, integrin isoform and other factors, but common elements include FAK (also known as PTK2), Src and ILK kinase signaling (reviewed in Harburger and Calderwood, 2009). A second mechanism by which NM2 could contribute to mechanotransduction is through actin interactions and stabilization of filamentous actin (Laevsky and Knecht, 2003). Multiple transcription factors, including YAP (YAP1) and TAZ (WWTR1; referred to collectively as YAP/TAZ) and megakaryocytic acute leukemia/serum response factor (MAL/SRF) (Connelly et al., 2010; Dupont et al., 2011), are thought to be in part modulated by G-actin and F-actin levels. Third, NM2 could contribute through mechanically activated ion channels, as NM2-mediated forces have been implicated in activating the Piezo family of mechanosensitive Ca2+ channels (Piezo1 and Piezo2) (Pathak et al., 2014). Interestingly, Piezo activity might be specifically enhanced at adhesions that generate high traction forces (Ellefsen et al., 2019). Finally, mechanical coupling of actomyosin with the nucleus can alter nucleocytoplasmic transport (Andreu et al., 2022), especially of YAP1 (Elosegui-Artola et al., 2017), or connect to the nuclear lamina via the LINC complex to alter internuclear biochemical processes (reviewed in Athirasala et al., 2017). Consistent with this idea, nuclear lamins supporting the inner nuclear membrane are mechanosensitive and can significantly influence both chromatin remodeling and transcriptional activity (Lammerding et al., 2004; Le et al., 2016).
In addition to generating or transmitting mechanical forces, NM2 motors are likely to be mechanosensitive. In response to resistive load, NM2 may decrease the rate of ADP release, effectively increasing its duty ratio (Kovacs et al., 2007). This would enable NM2 to maintain tensile stress and transmit mechanical forces in tissues. Additionally, the binding of NM2 to F-actin is proposed to be cooperative in a mechanosensitive fashion, such that small conformational changes in the actin filament created by previously bound NM2 promotes additional NM2 binding (Tokuraku et al., 2009; Uyeda et al., 2011). Complicating the matter, modulators of NM2, such as RhoA, are also mechanosensitive and subject to NM2 activity (Munjal et al., 2015; Priya et al., 2015), thereby creating the potential for highly complex feedback systems.
Conclusions
Perhaps the most exciting aspect of current and future studies of NM2 biology is that they require the coalescence of many disciplines to fully understand the contributions of this molecule across multiple scales. For example, how do cells use a motor that takes 7-nm steps and assembles filaments 300 nm long to drive changes in tissue architecture four or five orders of magnitude larger? Only a combination of biophysics, cell biology, imaging, developmental biology, molecular modeling and more will allow us to answer this and other fundamental questions with mechanistic clarity.
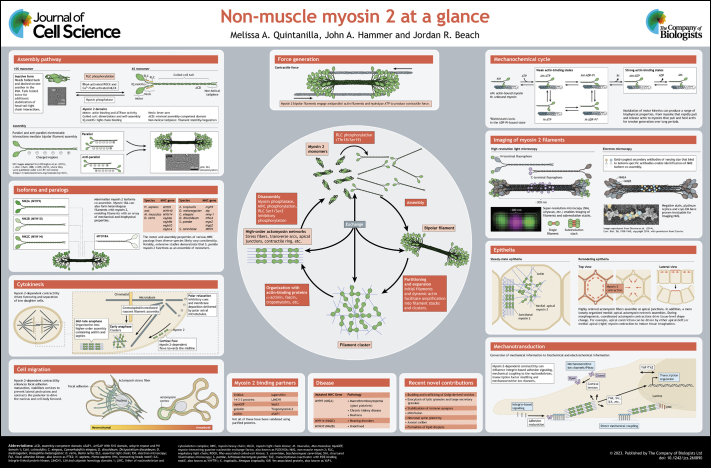
Poster
Panel 1. Formation of actomyosin networks
Panel 2. Force generation
Panel 3. Assembly pathway
Panel 4. Isoforms and paralogs
Panel 5. Mechanochemical cycle
Panel 6. Imaging of myosin 2 filaments
Panel 7. Cytokinesis
Panel 8. Cell migration
Panel 9. Epithelia
Panel 10. Mechanotransduction
Panel 11. Other myosin functions and interaction partners
Acknowledgements
We sincerely apologize to all the myosin researchers that we could not cite.
Footnotes
Funding
Our work in this area is supported by the Maximizing Investigators' Research Award (MIRA) (R35) from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under grant number R35GM138183 to J.R.B and a Graduate Research Fellowship (GRFP) from the National Science Foundation (NSF) under grant number DGE-1842190 to M.A.Q. Deposited in PMC for release after 12 months.
High-resolution poster and poster panels
A high-resolution version of the poster and individual poster panels are available for downloading at https://journals.biologists.com/jcs/article-lookup/doi/10.1242/jcs.260890#supplementary-data.
References
- Adelstein, R. S. and Klee, C. B. (1981). Purification and characterization of smooth muscle myosin light chain kinase. J. Biol. Chem. 256, 7501-7509. 10.1016/S0021-9258(19)68990-8 [DOI] [PubMed] [Google Scholar]
- Akamatsu, M., Berro, J., Pu, K.-M., Tebbs, I. R. and Pollard, T. D. (2014). Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase. J. Cell Biol. 204, 977-988. 10.1083/jcb.201307174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano, M., Ito, M., Kimura, K., Fukata, Y., Chihara, K., Nakano, T., Matsuura, Y. and Kaibuchi, K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246-20249. 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- Andreu, I., Granero-Moya, I., Chahare, N. R., Clein, K., Molina-Jordán, M., Beedle, A. E. M., Elosegui-Artola, A., Abenza, J. F., Rossetti, L., Trepat, X.et al. (2022). Mechanical force application to the nucleus regulates nucleocytoplasmic transport. Nat. Cell Biol. 24, 896-905. 10.1038/s41556-022-00927-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate, D. and Pardee, J. D. (1992). Actin-facilitated assembly of smooth muscle myosin induces formation of actomyosin fibrils. J. Cell Biol. 117, 1223-1230. 10.1083/jcb.117.6.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan, S. B., Johnson, H. E., Rahman, A., King, S. J., Rotty, J. D., Lebedeva, I. P., Haugh, J. M. and Bear, J. E. (2014). Mesenchymal chemotaxis requires selective inactivation of myosin II at the leading edge via a noncanonical PLCγ/PKCα pathway. Dev. Cell 31, 747-760. 10.1016/j.devcel.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athirasala, A., Hirsch, N. and Buxboim, A. (2017). Nuclear mechanotransduction: sensing the force from within. Curr. Opin. Cell Biol. 46, 119-127. 10.1016/j.ceb.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Badyal, S. K., Basran, J., Bhanji, N., Kim, J. H., Chavda, A. P., Jung, H. S., Craig, R., Elliott, P. R., Irvine, A. F., Barsukov, I. L.et al. (2011). Mechanism of the Ca2+-dependent interaction between S100A4 and tail fragments of nonmuscle myosin heavy chain IIA. J. Mol. Biol. 405, 1004-1026. 10.1016/j.jmb.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, J. R. and Egelhoff, T. T. (2009). Myosin II recruitment during cytokinesis independent of centralspindlin-mediated phosphorylation. J. Biol. Chem. 284, 27377-27383. 10.1074/jbc.M109.028316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, J. R., Shao, L., Remmert, K., Li, D., Betzig, E. and Hammer, J. A.3rd. (2014). Nonmuscle myosin II isoforms coassemble in living cells. Curr. Biol. 24, 1160-1166. 10.1016/j.cub.2014.03.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach, J. R., Bruun, K. S., Shao, L., Li, D., Swider, Z., Remmert, K., Zhang, Y., Conti, M. A., Adelstein, R. S., Rusan, N. M.et al. (2017). Actin dynamics and competition for myosin monomer govern the sequential amplification of myosin filaments. Nat. Cell Biol. 19, 85-93. 10.1038/ncb3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington, N., Wang, A., Mao, J., Adelstein, R. S. and Sellers, J. R. (2013). Characterization of three full-length human nonmuscle myosin II paralogs. J. Biol. Chem. 288, 33398-33410. 10.1074/jbc.M113.499848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington, N., Beach, J. R., Heissler, S. M., Remmert, K., Guzik-Lendrum, S., Nagy, A., Takagi, Y., Shao, L., Li, D., Yang, Y.et al. (2015). Myosin 18A coassembles with nonmuscle myosin 2 to form mixed bipolar filaments. Curr. Biol. 25, 942-948. 10.1016/j.cub.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge, M. T., Dulyaninova, N. G. and Egelhoff, T. T. (2009). Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells. Mol. Biol. Cell 20, 338-347. 10.1091/mbc.e08-04-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, C. and Sousa, S. (2020). Non-muscle myosin 2A (NM2A): structure, regulation and function. Cells 9, 1590. 10.3390/cells9071590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S. A., Yu, S., Walker, M. L., Hawkins, R. J., Chalovich, J. M. and Knight, P. J. (2007). Structures of smooth muscle myosin and heavy meromyosin in the folded, shutdown state. J. Mol. Biol. 372, 1165-1178. 10.1016/j.jmb.2007.07.014 [DOI] [PubMed] [Google Scholar]
- Burnette, D. T., Shao, L., Ott, C., Pasapera, A. M., Fischer, R. S., Baird, M. A., Der Loughian, C., Delanoe-Ayari, H., Paszek, M. J., Davidson, M. W.et al. (2014). A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J. Cell Biol. 205(1), 83-96. 10.1083/jcb.201311104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. G. and Wang, Y. L. (1990). Mechanism of the formation of contractile ring in dividing cultured animal cells. II. cortical movement of microinjected actin filaments. J. Cell Biol. 111(5 Pt 1), 1905-1911. 10.1083/jcb.111.5.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Takizawa, N., Crowley, J. L., Oh, S. W., Gatto, C. L., Kambara, T., Sato, O., Li, X. D., Ikebe, M. and Luna, E. J. (2003). F-actin and myosin II binding domains in supervillin. J. Biol. Chem. 278, 46094-46106. 10.1074/jbc.M305311200 [DOI] [PubMed] [Google Scholar]
- Choi, C. K., Vicente-Manzanares, M., Zareno, J., Whitmore, L. A., Mogilner, A. and Horwitz, A. R. (2008). Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 10, 1039-1050. 10.1038/ncb1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, K., Middelbeek, J., Dorovkov, M. V., Figdor, C. G., Ryazanov, A. G., Lasonder, E. and Van Leeuwen, F. N. (2008). The alpha-kinases TRPM6 and TRPM7, but not eEF-2 kinase, phosphorylate the assembly domain of myosin IIA, IIB and IIC. FEBS Lett. 582, 2993-2997. 10.1016/j.febslet.2008.07.043 [DOI] [PubMed] [Google Scholar]
- Connelly, J. T., Gautrot, J. E., Trappmann, B., Tan, D. W.-M., Donati, G., Huck, W. T. S. and Watt, F. M. (2010). Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 12, 711-718. 10.1038/ncb2074 [DOI] [PubMed] [Google Scholar]
- Conti, M. A., Even-Ram, S., Liu, C., Yamada, K. M. and Adelstein, R. S. (2004). Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 279, 41263-41266. 10.1074/jbc.C400352200 [DOI] [PubMed] [Google Scholar]
- Craig, R., Smith, R. and Kendrick-Jones, J. (1983). Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature 302, 436-439. 10.1038/302436a0 [DOI] [PubMed] [Google Scholar]
- Cremo, C. R., Sellers, J. R. and Facemyer, K. C. (1995). Two heads are required for phosphorylation-dependent regulation of smooth muscle myosin. J. Biol. Chem. 270, 2171-2175. 10.1074/jbc.270.5.2171 [DOI] [PubMed] [Google Scholar]
- Cross, R. A., Jackson, A. P., Citi, S., Kendrick-Jones, J. and Bagshaw, C. R. (1988). Active site trapping of nucleotide by smooth and non-muscle myosins. J. Mol. Biol. 203, 173-181. 10.1016/0022-2836(88)90100-3 [DOI] [PubMed] [Google Scholar]
- De La Cruz, E. M. and Ostap, E. M. (2004). Relating biochemistry and function in the myosin superfamily. Curr. Opin. Cell Biol. 16, 61-67. 10.1016/j.ceb.2003.11.011 [DOI] [PubMed] [Google Scholar]
- De Lozanne, A. and Spudich, J. A. (1987). Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236, 1086-1091. 10.1126/science.3576222 [DOI] [PubMed] [Google Scholar]
- De Lozanne, A., Lewis, M., Spudich, J. A. and Leinwand, L. A. (1985). Cloning and characterization of a nonmuscle myosin heavy chain cDNA. Proc. Natl. Acad. Sci. USA 82, 6807-6810. 10.1073/pnas.82.20.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio, A., Perez-Jimenez, R., Liu, R., Roca-Cusachs, P., Fernandez, J. M. and Sheetz, M. P. (2009). Stretching single talin rod molecules activates vinculin binding. Science 323, 638-641. 10.1126/science.1162912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, A. D., Wang, F. W., Matsumoto, K. and Yamada, K. M. (2009). One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481-490. 10.1083/jcb.200810041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, M., Wang, G., Ismail, T. M., Gross, S., Fernig, D. G., Barraclough, R. and Rudland, P. S. (2012). S100P dissociates myosin IIA filaments and focal adhesion sites to reduce cell adhesion and enhance cell migration. J. Biol. Chem. 287, 15330-15344. 10.1074/jbc.M112.349787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulyaninova, N. G., Malashkevich, V. N., Almo, S. C. and Bresnick, A. R. (2005). Regulation of myosin-IIA assembly and mts1 binding by heavy chain phosphorylation. Biochemistry 44, 6867-6876. 10.1021/bi0500776 [DOI] [PubMed] [Google Scholar]
- Dulyaninova, N. G., House, R. P., Betapudi, V. and Bresnick, A. R. (2007). Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol. Biol. Cell 18, 3144-3155. 10.1091/mbc.e06-11-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., Zanconato, F., Le Digabel, J., Forcato, M., Bicciato, S.et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179-183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Dyer, N., Rebollo, E., Domínguez, P., Elkhatib, N., Chavrier, P., Daviet, L., González, C. and González-Gaitán, M. (2007). Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development 134, 4437-4447. 10.1242/dev.010983 [DOI] [PubMed] [Google Scholar]
- Ebrahim, S., Fujita, T., Millis, B. A., Kozin, E., Ma, X., Kawamoto, S., Baird, M. A., Davidson, M., Yonemura, S., Hisa, Y.et al. (2013). NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr. Biol. 23, 731-736. 10.1016/j.cub.2013.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff, T. T., Lee, R. J. and Spudich, J. A. (1993). Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell 75, 363-371. 10.1016/0092-8674(93)80077-R [DOI] [PubMed] [Google Scholar]
- Ellefsen, K., Chang, A., Nourse, J. L., Holt, J. R., Arulmoli, J., Mekhdjian, A., Flanagan, L. A., Dunn, A. R., Parker, I. and Pathak, M. M. (2019). Myosin-II mediated traction forces evoke localized Piezo1-dependent Ca2+ flickers. Commun. Biol. 2, 298. 10.1038/s42003-019-0514-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosegui-Artola, A., Andreu, I., Beedle, A. E. M., Lezamiz, A., Uroz, M., Kosmalska, A. J., Oria, R., Kechagia, J. Z., Rico-Lastres, P., Le Roux, A.-L.et al. (2017). Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397-1410.e14. 10.1016/j.cell.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Even-Faitelson, L. and Ravid, S. (2006). PAK1 and aPKCζ regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol. Biol. Cell 17, 2869-2881. 10.1091/mbc.e05-11-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X., Luo, J., Nishihama, R., Wloka, C., Dravis, C., Travaglia, M., Iwase, M., Vallen, E. A. and Bi, E. (2010). Biphasic targeting and cleavage furrow ingression directed by the tail of a myosin II. J. Cell Biol. 191, 1333-1350. 10.1083/jcb.201005134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenix, A. M., Taneja, N., Buttler, C. A., Lewis, J., Van Engelenburg, S. B., Ohi, R. and Burnette, D. T. (2016). Expansion and concatenation of non-muscle myosin IIA filaments drive cellular contractile system formation during interphase and mitosis. Mol. Biol. Cell 27, 1465-1478. 10.1091/mbc.E15-10-0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, C. G., Yamada, K. M. and Galbraith, J. A. (2007). Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 315, 992-995. 10.1126/science.1137904 [DOI] [PubMed] [Google Scholar]
- Golomb, E., Ma, X., Jana, S. S., Preston, Y. A., Kawamoto, S., Shoham, N. G., Goldin, E., Conti, M. A., Sellers, J. R. and Adelstein, R. S. (2004). Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J. Biol. Chem. 279, 2800-2808. 10.1074/jbc.M309981200 [DOI] [PubMed] [Google Scholar]
- Gomes, E. R., Jani, S. and Gundersen, G. G. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451-463. 10.1016/j.cell.2005.02.022 [DOI] [PubMed] [Google Scholar]
- Gudejko, H. F. M., Alford, L. M. and Burgess, D. R. (2012). Polar expansion during cytokinesis. Cytoskeleton 69, 1000-1009. 10.1002/cm.21078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W.-H. and Wang, Y.-L. (2012). A three-component mechanism for fibroblast migration with a contractile cell body that couples a myosin II-independent propulsive anterior to a myosin II-dependent resistive tail. Mol. Biol. Cell 23, 1657-1663. 10.1091/mbc.e11-06-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik-Lendrum, S., Heissler, S. M., Billington, N., Takagi, Y., Yang, Y., Knight, P. J., Homsher, E. and Sellers, J. R. (2013). Mammalian myosin-18a, a highly divergent myosin. J. Biol. Chem. 288, 9532-9548. 10.1074/jbc.M112.441238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger, D. S. and Calderwood, D. A. (2009). Integrin signalling at a glance. J. Cell Sci. 122, 159-163. 10.1242/jcs.018093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, D. E. and Warshaw, D. M. (1993). Smooth and skeletal muscle myosin both exhibit low duty cycles at zero load in vitro. J. Biol. Chem. 268, 14764-14768. 10.1016/S0021-9258(18)82398-5 [DOI] [PubMed] [Google Scholar]
- Heissler, S. M. and Sellers, J. R. (2016). Kinetic adaptations of myosins for their diverse cellular functions. Traffic 17, 839-859. 10.1111/tra.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson, J. H., Ditzler, C. E., Germain, A., Irwin, P. M., Vogt, E. T., Yang, S., Wu, X. and Shuster, C. B. (2017). The ultrastructural organization of actin and myosin II filaments in the contractile ring: new support for an old model of cytokinesis. Mol. Biol. Cell 28, 613-623. 10.1091/mbc.e16-06-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson, G. R. X. and O'Farrell, P. H. (2008). Rho-dependent control of anillin behavior during cytokinesis. J. Cell Biol. 180, 285-294. 10.1083/jcb.200709005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe, M. and Hartshorne, D. J. (1985). Proteolysis of smooth muscle myosin by Staphylococcus aureus protease: preparation of heavy meromyosin and subfragment 1 with intact 20,000-dalton light chains. Biochemistry 24, 2380-2387. 10.1021/bi00330a038 [DOI] [PubMed] [Google Scholar]
- Joo, E., Surka, M. C. and Trimble, W. S. (2007). Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev. Cell 13, 677-690. 10.1016/j.devcel.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Jung, H. S., Burgess, S. A., Billington, N., Colegrave, M., Patel, H., Chalovich, J. M., Chantler, P. D. and Knight, P. J. (2008a). Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc. Natl. Acad. Sci. USA 105, 6022-6026. 10.1073/pnas.0707846105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H. S., Komatsu, S., Ikebe, M. and Craig, R. (2008b). Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol. Biol. Cell 19, 3234-3242. 10.1091/mbc.e08-02-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, C. A., Kawamoto, S., Conti, M. A. and Adelstein, R. S. (1991). Phosphorylation of vertebrate smooth muscle and nonmuscle myosin heavy chains in vitro and in intact cells. J. Cell Sci. Suppl. 14, 49-54. 10.1242/jcs.1991.Supplement_14.10 [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones, J., Smith, R. C., Craig, R. and Citi, S. (1987). Polymerization of vertebrate non-muscle and smooth muscle myosins. J. Mol. Biol. 198, 241-252. 10.1016/0022-2836(87)90310-X [DOI] [PubMed] [Google Scholar]
- Képiró, M., Várkuti, B. H., Végner, L., Vörös, G., Hegyi, G., Varga, M. and Málnási-Csizmadia, A. (2014). Para-Nitroblebbistatin, the non-cytotoxic and photostable myosin II inhibitor. Angew. Chem. Int. Ed Engl. 53, 8211-8215. 10.1002/anie.201403540 [DOI] [PubMed] [Google Scholar]
- Khaliullin, R. N., Green, R. A., Shi, L. Z., Gomez-Cavazos, J. S., Berns, M. W., Desai, A. and Oegema, K. (2018). A positive-feedback-based mechanism for constriction rate acceleration during cytokinesis in Caenorhabditis elegans. Elife 7, e36073. 10.7554/eLife.36073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart, D. P., Lutz, M. S., Chan, D., Ketchum, A. S., Laymon, R. A., Nguyen, B. and Goldstein, L. S. (1989). Identification of the gene for fly non-muscle myosin heavy chain: Drosophila myosin heavy chains are encoded by a gene family. EMBO J. 8, 913-922. 10.1002/j.1460-2075.1989.tb03452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.-Y., Kawamoto, S., Bao, J., Sellers, J. R. and Adelstein, R. S. (2008). The B2 alternatively spliced isoform of nonmuscle myosin II-B lacks actin-activated MgATPase activity and in vitro motility. Biochem. Biophys. Res. Commun. 369, 124-134. 10.1016/j.bbrc.2007.11.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K., Ito, M., Amano, M., Chihara, K., Fukata, Y., Nakafuku, M., Yamamori, B., Feng, J., Nakano, T., Okawa, K.et al. (1996). Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245-248. 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- Kolega, J. and Taylor, D. L. (1993). Gradients in the concentration and assembly of myosin II in living fibroblasts during locomotion and fiber transport. Mol. Biol. Cell 4, 819-836. 10.1091/mbc.4.8.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, M., Thirumurugan, K., Knight, P. J. and Sellers, J. R. (2007). Load-dependent mechanism of nonmuscle myosin 2. Proc. Natl. Acad. Sci. USA 104, 9994-9999. 10.1073/pnas.0701181104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, J. C., Han, X., Hsiao, C. T., Yates, J. R., III and Waterman, C. M. (2011). Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 13, 383-393. 10.1038/ncb2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laevsky, G. and Knecht, D. A. (2003). Cross-linking of actin filaments by myosin II is a major contributor to cortical integrity and cell motility in restrictive environments. J. Cell Sci. 116, 3761-3770. 10.1242/jcs.00684 [DOI] [PubMed] [Google Scholar]
- Lammerding, J., Schulze, P. C., Takahashi, T., Kozlov, S., Sullivan, T., Kamm, R. D., Stewart, C. L. and Lee, R. T. (2004). Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 113, 370-378. 10.1172/JCI200419670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann, T., Bader, B. L., Monkley, S. J., Worbs, T., Wedlich-Söldner, R., Hirsch, K., Keller, M., Förster, R., Critchley, D. R., Fässler, R.et al. (2008). Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51-55. 10.1038/nature06887 [DOI] [PubMed] [Google Scholar]
- Laplante, C., Huang, F., Tebbs, I. R., Bewersdorf, J. and Pollard, T. D. (2016). Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. Proc. Natl. Acad. Sci. USA 113, E5876-E5885. 10.1073/pnas.1608252113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, H. Q., Ghatak, S., Yeung, C.-Y. C., Tellkamp, F., Günschmann, C., Dieterich, C., Yeroslaviz, A., Habermann, B., Pombo, A., Niessen, C. M.et al. (2016). Mechanical regulation of transcription controls polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 18, 864-875. 10.1038/ncb3387 [DOI] [PubMed] [Google Scholar]
- Leal, A., Endele, S., Stengel, C., Huehne, K., Loetterle, J., Barrantes, R., Winterpacht, A. and Rautenstrauss, B. (2003). A novel myosin heavy chain gene in human chromosome 19q13.3. Gene 312, 165-171. 10.1016/S0378-1119(03)00613-9 [DOI] [PubMed] [Google Scholar]
- Linsmeier, I., Banerjee, S., Oakes, P. W., Jung, W., Kim, T. and Murrell, M. P. (2016). Disordered actomyosin networks are sufficient to produce cooperative and telescopic contractility. Nat. Commun. 7, 12615. 10.1038/ncomms12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Hong, M.-S., Shu, S., Yu, S. and Korn, E. D. (2013). Regulation of the filament structure and assembly of Acanthamoeba myosin II by phosphorylation of serines in the heavy-chain nonhelical tailpiece. Proc. Natl. Acad. Sci. USA 110, E33-E40. 10.1073/pnas.1219727110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Billington, N., Shu, S., Yu, S.-H., Piszczek, G., Sellers, J. R. and Korn, E. D. (2017). Effect of ATP and regulatory light-chain phosphorylation on the polymerization of mammalian nonmuscle myosin II. Proc. Natl. Acad. Sci. USA 114, E6516-E6525. 10.1073/pnas.1702375114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, C.-M., Buxton, D. B., Chua, G. C. H., Dembo, M., Adelstein, R. S. and Wang, Y.-L. (2004). Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol. Biol. Cell 15, 982-989. 10.1091/mbc.e03-06-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, M., Laves, E. and Pollard, T. D. (2005). Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol. Biol. Cell 16, 5346-5355. 10.1091/mbc.e05-07-0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton, G. W. G., Gomes, E. R., Folker, E. S., Vintinner, E. and Gundersen, G. G. (2010). Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 329, 956-959. 10.1126/science.1189072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi, I. and Okuno, M. (1977). The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 74, 251-263. 10.1083/jcb.74.1.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule, P., Eda, M., Watanabe, N., Fujisawa, K., Matsuoka, T., Bito, H., Ishizaki, T. and Narumiya, S. (1998). Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature 394, 491-494. 10.1038/28873 [DOI] [PubMed] [Google Scholar]
- Maddox, A. S., Lewellyn, L., Desai, A. and Oegema, K. (2007). Anillin and the septins promote asymmetric ingression of the cytokinetic furrow. Dev. Cell 12, 827-835. 10.1016/j.devcel.2007.02.018 [DOI] [PubMed] [Google Scholar]
- Mclachlan, A. D. and Karn, J. (1982). Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature 299, 226-231. 10.1038/299226a0 [DOI] [PubMed] [Google Scholar]
- Melli, L., Billington, N., Sun, S. A., Bird, J. E., Nagy, A., Friedman, T. B., Takagi, Y. and Sellers, J. R. (2018). Bipolar filaments of human nonmuscle myosin 2-A and 2-B have distinct motile and mechanical properties. Elife 7, e32871. 10.7554/eLife.32871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes Pinto, I., Rubinstein, B., Kucharavy, A., Unruh, J. R. and Li, R. (2012). Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell 22, 1247-1260. 10.1016/j.devcel.2012.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad, A. and Oplatka, A. (1977). Phosphorylation of fibroblast myosin. FEBS Lett. 77, 37-40. 10.1016/0014-5793(77)80188-9 [DOI] [PubMed] [Google Scholar]
- Munjal, A., Philippe, J.-M., Munro, E. and Lecuit, T. (2015). A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351-355. 10.1038/nature14603 [DOI] [PubMed] [Google Scholar]
- Naka, M., Nishikawa, M., Adelstein, R. S. and Hidaka, H. (1983). Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature 306, 490-492. 10.1038/306490a0 [DOI] [PubMed] [Google Scholar]
- Naydenov, N. G., Feygin, A., Wang, D., Kuemmerle, J. F., Harris, G., Conti, M. A., Adelstein, R. S. and Ivanov, A. I. (2016). Nonmuscle myosin IIA regulates intestinal epithelial barrier in vivo and plays a protective role during experimental colitis. Sci. Rep. 6, 24161. 10.1038/srep24161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederman, R. and Pollard, T. D. (1975). Human platelet myosin. II. In vitro assembly and structure of myosin filaments. J. Cell Biol. 67, 72-92. 10.1083/jcb.67.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, M., Hidaka, H. and Adelstein, R. S. (1983). Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid-dependent protein kinase. The effect on actin-activated MgATPase activity. J. Biol. Chem. 258, 14069-14072. 10.1016/S0021-9258(17)43820-8 [DOI] [PubMed] [Google Scholar]
- Otterpohl, K. L., Hart, R. G., Evans, C., Surendran, K. and Chandrasekar, I. (2017). Nonmuscle myosin 2 proteins encoded by Myh9, Myh10, and Myh14 are uniquely distributed in the tubular segments of murine kidney. Physiol Rep 5, e13513. 10.14814/phy2.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak, M. M., Nourse, J. L., Tran, T., Hwe, J., Arulmoli, J., Le, D. T. T., Bernardis, E., Flanagan, L. A. and Tombola, F. (2014). Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 111, 16148-16153. 10.1073/pnas.1409802111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato, M. D., Sellers, J. R., Preston, Y. A., Harvey, E. V. and Adelstein, R. S. (1996). Baculovirus expression of chicken nonmuscle heavy meromyosin II-B. characterization of alternatively spliced isoforms. J. Biol. Chem. 271, 2689-2695. 10.1074/jbc.271.5.2689 [DOI] [PubMed] [Google Scholar]
- Piekny, A. J. and Glotzer, M. (2008). Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr. Biol. 18, 30-36. 10.1016/j.cub.2007.11.068 [DOI] [PubMed] [Google Scholar]
- Priya, R., Gomez, G. A., Budnar, S., Verma, S., Cox, H. L., Hamilton, N. A. and Yap, A. S. (2015). Feedback regulation through myosin II confers robustness on RhoA signalling at E-cadherin junctions. Nat. Cell Biol. 17, 1282-1293. 10.1038/ncb3239 [DOI] [PubMed] [Google Scholar]
- Raich, W. B., Moran, A. N., Rothman, J. H. and Hardin, J. (1998). Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell 9, 2037-2049. 10.1091/mbc.9.8.2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal, U. A., Dulyaninova, N. G., Varney, K. M., Wilder, P. T., Nallamsetty, S., Brenowitz, M., Weber, D. J., Almo, S. C. and Bresnick, A. R. (2013). Structure of the S100A4/myosin-IIA complex. BMC Struct. Biol. 13, 31. 10.1186/1472-6807-13-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport, R. (1961). Experiments concerning the cleavage stimulus in sand dollar eggs. J. Exp. Zool. 148, 81-89. 10.1002/jez.1401480107 [DOI] [PubMed] [Google Scholar]
- Redowicz, M. J., Martin, B., Zolkiewski, M., Ginsburg, A. and Korn, E. D. (1994). Effects of phosphorylation and nucleotides on the conformation of myosin II from Acanthamoeba castellanii. J. Biol. Chem. 269, 13558-13563. 10.1016/S0021-9258(17)36867-9 [DOI] [PubMed] [Google Scholar]
- Rosenblatt, J., Raff, M. C. and Cramer, L. P. (2001). An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847-1857. 10.1016/S0960-9822(01)00587-5 [DOI] [PubMed] [Google Scholar]
- Rubinstein, B. Y. and Mogilner, A. (2017). Myosin clusters of finite size develop contractile stress in 1D random actin arrays. Biophys. J. 113, 937-947. 10.1016/j.bpj.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujano, M. A., Sanchez-Pulido, L., Pennetier, C., Le Dez, G. and Basto, R. (2013). The microcephaly protein Asp regulates neuroepithelium morphogenesis by controlling the spatial distribution of myosin II. Nat. Cell Biol. 15, 1294-1306. 10.1038/ncb2858 [DOI] [PubMed] [Google Scholar]
- Sandquist, J. C. and Means, A. R. (2008). The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol. Biol. Cell 19, 5156-5167. 10.1091/mbc.e08-05-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Cruz Mateos, C., Valencia-Expósito, A., Palacios, I. M. and Martín-Bermudo, M. D. (2020). Integrins regulate epithelial cell shape by controlling the architecture and mechanical properties of basal actomyosin networks. PLoS Genet. 16, e1008717. 10.1371/journal.pgen.1008717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk, J., Wilsch-Bräuninger, M., Calegari, F. and Huttner, W. B. (2009). Myosin II is required for interkinetic nuclear migration of neural progenitors. Proc. Natl. Acad. Sci. USA 106, 16487-16492. 10.1073/pnas.0908928106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutova, M. S., Spessott, W. A., Giraudo, C. G. and Svitkina, T. (2014). Endogenous species of mammalian nonmuscle myosin IIA and IIB include activated monomers and heteropolymers. Curr. Biol. 24, 1958-1968. 10.1016/j.cub.2014.07.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões, Sde. M., Mainieri, A. and Zallen, J. A. (2014). Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J. Cell Biol. 204, 575-589. 10.1083/jcb.201307070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, M., Wang, M., Mcbride, O. W., Kawamoto, S., Yamakawa, K., Gdula, D., Adelstein, R. S. and Weir, L. (1991). Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ. Res. 69, 530-539. 10.1161/01.RES.69.2.530 [DOI] [PubMed] [Google Scholar]
- Sinard, J. H., Stafford, W. F. and Pollard, T. D. (1989). The mechanism of assembly of Acanthamoeba myosin-II minifilaments: minifilaments assemble by three successive dimerization steps. J. Cell Biol. 109, 1537-1547. 10.1083/jcb.109.4.1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny, M., Cox, H. L., Leerberg, J. M., Kovacs, E. M., Conti, M. A., Ferguson, C., Hamilton, N. A., Parton, R. G., Adelstein, R. S. and Yap, A. S. (2010). Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 12, 696-702. 10.1038/ncb2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrmann, M., Fankhauser, C., Brodbeck, C. and Simanis, V. (1996). The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 10, 2707-2719. 10.1101/gad.10.21.2707 [DOI] [PubMed] [Google Scholar]
- Steimle, P. A., Yumura, S., Côté, G. P., Medley, Q. G., Polyakov, M. V., Leppert, B. and Egelhoff, T. T. (2001). Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr. Biol. 11, 708-713. 10.1016/S0960-9822(01)00182-8 [DOI] [PubMed] [Google Scholar]
- Stewart, R. L., Carpenter, B. L., West, D. S., Knifley, T., Liu, L., Wang, C., Weiss, H. L., Gal, T. S., Durbin, E. B., Arnold, S. M.et al. (2016). S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide. Oncotarget 7, 34630-34642. 10.18632/oncotarget.8969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight, A. F., Cheung, A., Limouze, J., Chen, I., Westwood, N. J., Sellers, J. R. and Mitchison, T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299, 1743-1747. 10.1126/science.1081412 [DOI] [PubMed] [Google Scholar]
- Straight, A. F., Field, C. M. and Mitchison, T. J. (2005). Anillin binds nonmuscle myosin II and regulates the contractile ring. Mol. Biol. Cell 16, 193-201. 10.1091/mbc.e04-08-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L., Guan, R., Lee, I.-J., Liu, Y., Chen, M., Wang, J., Wu, J.-Q. and Chen, Z. (2015). Mechanistic insights into the anchorage of the contractile ring by anillin and Mid1. Dev. Cell 33, 413-426. 10.1016/j.devcel.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, H., Onishi, H., Takahashi, K. and Watanabe, S. (1978). Structure and function of chicken gizzard myosin. J. Biochem. 84, 1529-1542. 10.1093/oxfordjournals.jbchem.a132278 [DOI] [PubMed] [Google Scholar]
- Thomas, D. G., Yenepalli, A., Denais, C. M., Rape, A., Beach, J. R., Wang, Y.-L., Schiemann, W. P., Baskaran, H., Lammerding, J. and Egelhoff, T. T. (2015). Non-muscle myosin IIB is critical for nuclear translocation during 3D invasion. J. Cell Biol. 210, 583-594. 10.1083/jcb.201502039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuraku, K., Kurogi, R., Toya, R. and Uyeda, T. Q. P. (2009). Novel mode of cooperative binding between myosin and Mg2+ -actin filaments in the presence of low concentrations of ATP. J. Mol. Biol. 386, 149-162. 10.1016/j.jmb.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Tolliday, N., Verplank, L. and Li, R. (2002). Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12, 1864-1870. 10.1016/S0960-9822(02)01238-1 [DOI] [PubMed] [Google Scholar]
- Toothaker, L. E., Gonzalez, D. A., Tung, N., Lemons, R. S., Le Beau, M. M., Arnaout, M. A., Clayton, L. K. and Tenen, D. G. (1991). Cellular myosin heavy chain in human leukocytes: isolation of 5’ cDNA clones, characterization of the protein, chromosomal localization, and upregulation during myeloid differentiation. Blood 78, 1826-1833. 10.1182/blood.V78.7.1826.1826 [DOI] [PubMed] [Google Scholar]
- Trybus, K. M., Huiatt, T. W. and Lowey, S. (1982). A bent monomeric conformation of myosin from smooth muscle. Proc. Natl. Acad. Sci. USA 79, 6151-6155. 10.1073/pnas.79.20.6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda, T. Q., Kron, S. J. and Spudich, J. A. (1990). Myosin step size. estimation from slow sliding movement of actin over low densities of heavy meromyosin. J. Mol. Biol. 214, 699-710. 10.1016/0022-2836(90)90287-V [DOI] [PubMed] [Google Scholar]
- Uyeda, T. Q. P., Iwadate, Y., Umeki, N., Nagasaki, A. and Yumura, S. (2011). Stretching actin filaments within cells enhances their affinity for the myosin II motor domain. PLoS One 6, e26200. 10.1371/journal.pone.0026200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várkuti, B. H., Képiró, M., Horváth, I. Á., Végner, L., Ráti, S., Zsigmond, Á., Hegyi, G., Lenkei, Z., Varga, M. and Málnási-Csizmadia, A. (2016). A highly soluble, non-phototoxic, non-fluorescent blebbistatin derivative. Sci. Rep. 6, 26141. 10.1038/srep26141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky, A. B. and Borisy, G. G. (1993). Non-sarcomeric mode of myosin II organization in the fibroblast lamellum. J. Cell Biol. 123, 637-652. 10.1083/jcb.123.3.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., Kovacs, M., Hu, A., Limouze, J., Harvey, E. V. and Sellers, J. R. (2003). Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J. Biol. Chem. 278, 27439-27448. 10.1074/jbc.M302510200 [DOI] [PubMed] [Google Scholar]
- Wang, A., Ma, X., Conti, M. A., Liu, C., Kawamoto, S. and Adelstein, R. S. (2010). Nonmuscle myosin II isoform and domain specificity during early mouse development. Proc. Natl. Acad. Sci. USA 107, 14645-14650. 10.1073/pnas.1004023107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A., Ma, X., Conti, M. A. and Adelstein, R. S. (2011). Distinct and redundant roles of the non-muscle myosin II isoforms and functional domains. Biochem. Soc. Trans. 39, 1131-1135. 10.1042/BST0391131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt, T., Taylor, D., Messier, T., Trybus, K. M. and Taylor, K. A. (1999). Visualization of head-head interactions in the inhibited state of smooth muscle myosin. J. Cell Biol. 147, 1385-1390. 10.1083/jcb.147.7.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, S., Paterson, H. F. and Marshall, C. J. (2005). Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat. Cell Biol. 7, 255-261. 10.1038/ncb1230 [DOI] [PubMed] [Google Scholar]
- Wolpert, L. (1961). The mechanics and mechanism of cleavage. Int. Rev. Cytol. 10, 163-216. [Google Scholar]
- Yamada, S., Pokutta, S., Drees, F., Weis, W. I. and Nelson, W. J. (2005). Deconstructing the cadherin-catenin-actin complex. Cell 123, 889-901. 10.1016/j.cell.2005.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro, S., Totsukawa, G., Yamakita, Y., Sasaki, Y., Madaule, P., Ishizaki, T., Narumiya, S. and Matsumura, F. (2003). Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol. Biol. Cell 14, 1745-1756. 10.1091/mbc.e02-07-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., Tiwari, P., Lee, K. H., Sato, O., Ikebe, M., Padrón, R. and Craig, R. (2020). Cryo-EM structure of the inhibited (10S) form of myosin II. Nature 588, 521-525. 10.1038/s41586-020-3007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura, S., Wada, Y., Watanabe, T., Nagafuchi, A. and Shibata, M. (2010). Alpha-catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533-542. 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- Yüce, O., Piekny, A. and Glotzer, M. (2005). An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol. 170, 571-582. 10.1083/jcb.200501097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, D. W., Lee, T. T., Weng, S., Fu, J. and García, A. J. (2017). Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol. Biol. Cell 28, 1901-1911. 10.1091/mbc.e17-02-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.